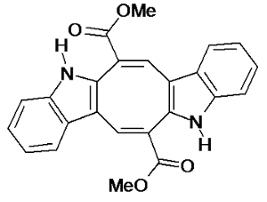
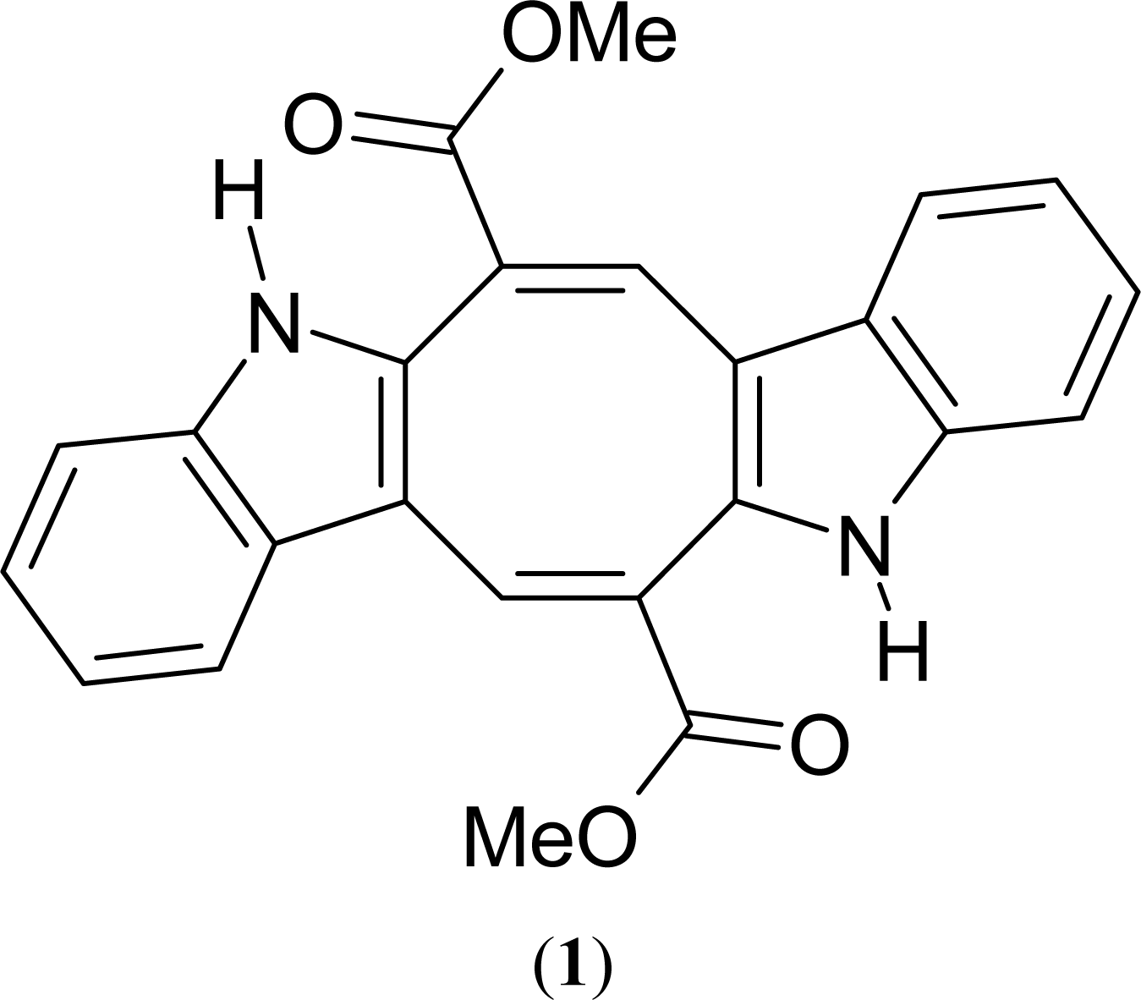
The Antinociceptive and Anti-Inflammatory Activities of Caulerpin, a Bisindole Alkaloid Isolated from Seaweeds of the Genus Caulerpa
Abstract
:1. Introduction
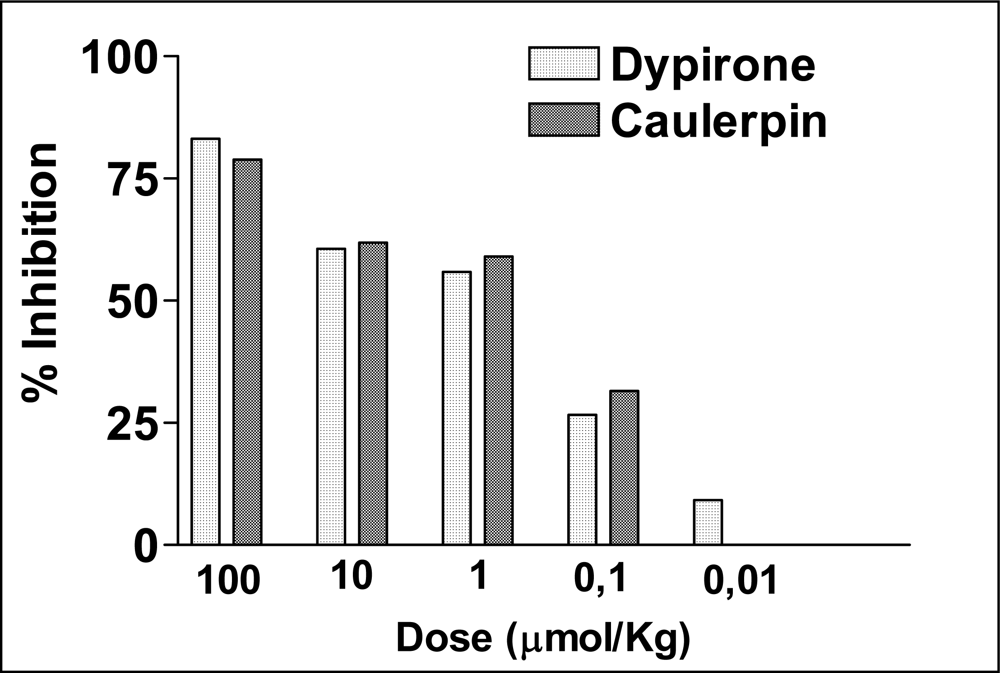
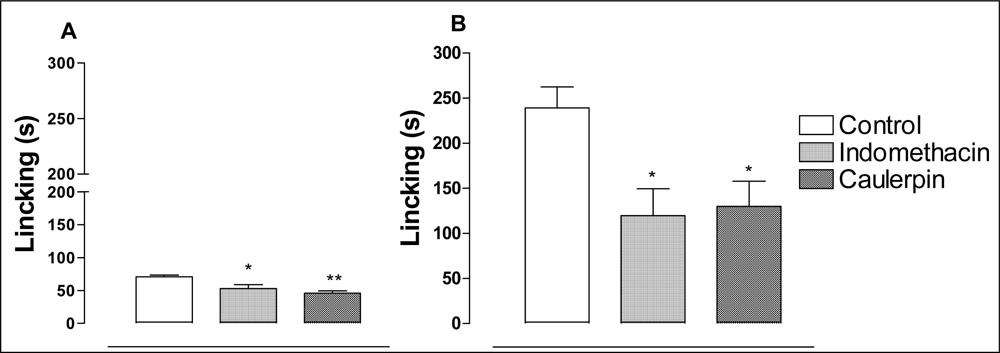
2. Results and Discussion
3. Conclusions
4. Experimental
4.1. Extraction and Isolation
4.2. Biological Activity Tests
Drugs and reagents
Animals
The Writhing test
The Hot-Plate Test
The Rotarod Test
Formalin-Induced Pain in Mice
Capsaicin-Induced Ear Edema in Mice
Carrageenan-Induced Peritonitis in Mice
4.3. Statistical Analysis
Acknowledgments
References
- Barbosa-Filho, JM; Cunha, EVL; Gray, AI. Alkaloids of the Menispermaceae. In The Alkaloids; Cordell, GA, Ed.; Academic Press: Illinois, USA, 2000; Volume 54, pp. 1–199. [Google Scholar]
- Barbosa-Filho, JM; Sette, IMF; Cunha, EVL; Guedes, DN; Silva, MS. Protoberberine alkaloids. In The Alkaloids; Cordell, GA, Ed.; Elsevier Inc: San Diego, CA, USA, 2005; Volume 62, pp. 1–75. [Google Scholar]
- Conserva, LM; Pereira, CAB; Barbosa-Filho, JM. Alkaloids of the Hernandiaceae: Occurrence and a Compilation of their Biological Activities. In The Alkaloids; Cordell, GA, Ed.; Elsevier: San Diego, CA, USA, 2005; Volume 62, pp. 175–243. [Google Scholar]
- Falcão, HS; Leite, JA; Barbosa-Filho, JM; Athayde-Filho, PF; Chaves, MCO; Moura, MD; Ferreira, AL; Almeida, ABA; Souza-Brito, ARM; Diniz, MFFM; Batista, LM. Gastric and duodenal antiulcer activity of alkaloids: A review. Molecules 2008, 13, 3198–3223. [Google Scholar]
- Cunha, EVL; Barbosa-Filho, JM. Alcalóides derivados do núcleo isoquinolínico. In Química de Produtos Naturais, Novos Fármacos e a Moderna Farmacognosia, 2a ed; Yunes, RA, Cechinel-Filho, V, Eds.; Univali Editora: Itajaí-PR, Brazil, 2009; pp. 279–319. [Google Scholar]
- Williams, JE. Review of antiviral and immunomodulating properties of plants of the Peruvian rainforest with a particular emphasis on uña de gato and sangre de grado. Altern Med Rev 2001, 6, 567–579. [Google Scholar]
- Fechine, IM; Navarro, VR; Cunha, EVL; Silva, MS; Maia, JGS; Barbosa-Filho, JM. Alkaloids and volatile constituents from Duguetia flagellaris. Biochem Syst Ecol 2002, 30, 267–269. [Google Scholar]
- Melo, PS; Cavalcante, HMM; Barbosa-Filho, JM; Diniz, MFFM; Medeiros, IA; Haun, M. Warifteine and milonine, alkaloids isolated from Cissampelos sympodialis Eichl: cytotoxicity on rat hepatocyte culture and in V79 cells. Toxicol Lett 2003, 142, 143–151. [Google Scholar]
- Cano, A; Alcaraz, O; Arnao, MB. Free radical-scavenging activity of indolic compounds in aqueous and ethanolic media. Anal Bioanal Chem 2003, 376, 33–37. [Google Scholar]
- St-Pierre, B; Vazquez-Flota, FA; de Luca, V. Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell 1999, 11, 887–900. [Google Scholar]
- Wall, ME; Wani, M; Cook, CE; Palmer, KH; Mc Phail, AT; Sim, GA. Plant antitumor agents I. The isolation and structure elucidation of Camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca cuminata. J Am Chem Soc 1966, 88, 3888–3890. [Google Scholar]
- Shaari, K; Ling, KC; Rashid, ZM; Jean, TP; Abas, F; Raof, SM; Zainal, Z; Lajis, NH; Mohamad, H; Ali, AM. Cytotoxic aaptamines from Malaysian Aaptos aaptos. Mar Drugs 2009, 7, 1–8. [Google Scholar]
- Wu, WH; Hasumi, K; Peng, H; Hu, XW; Wang, XC; Bao, B. Fibrinolytic compounds isolated from a brown alga Sargassum fulvellum. Mar Drugs 2009, 7, 85–94. [Google Scholar]
- Genovese, G; Tedone, L; Hamann, MT; Morabito, M. The Mediterranean red alga Asparagopsis: A source of compounds against leishmania. Mar Drugs 2009, 7, 361–366. [Google Scholar]
- Walsh, CJ; Leggett, SR; Strohbehn, K; Pierce, RH; Sleasman, J. Effects of in vitro brevetoxin exposure on apoptosis and cellular metabolism in a leukemic T cell line (Jurkat). Mar Drugs 2008, 6, 291–307. [Google Scholar]
- Courtois, A; Simon-Colin, C; Boisset, C; Berthou, C; Deslandes, E; Guezennec, J; Bordron, A. Floridoside extracted from the red alga Mastocarpus stellatus is a potent activator of the classical complement pathway. Mar Drugs 2008, 6, 407–417. [Google Scholar]
- Baunbk, D; Trinkler, N; Ferandin, Y; Lozach, O; Ploypradith, P; Rucirawat, S; Ishibashi, F; Iwao, M; Meijer, L. Anticancer alkaloid lamellarins inhibit protein kinases. Mar Drugs 2008, 6, 514–527. [Google Scholar]
- Kijjoa, A; Wattanadilok, R; Campos, N; Nascimento, MSJ; Pinto, M; Herz, W. Anticancer activity evaluation of kuanoniamines A and C isolated from the marine sponge Oceanapia sagittaria, collected from the gulf of Thailand. Mar Drugs 2007, 5, 6–22. [Google Scholar]
- Oda, T; Wang, WF; Ukai, K; Nakazawa, T; Mochizuki, M. A sesquiterpene quinone, 5-epi-smenospongine, promotes TNF-alpha production in LPS-stimulated RAW 264.7 cells. Mar Drugs 2007, 5, 151–156. [Google Scholar]
- Bao, B; Zhang, P; Lee, Y; Hong, J; Lee, CO; Jung, JH. Monoindole alkaloids from a marine sponge Spongosorites sp. Mar Drugs 2007, 5, 31–39. [Google Scholar]
- Souza, ET; Queiroz, AC; Miranda, GEC; Lorenzo, VP; Silva, EF; Freire-Dias, TLM; Cupertino-Silva, YK; Melo, GMA; Santos, BVO; Chaves, MCO; Alexandre-Moreira, MS. Antinociceptive activities of crude methanolic extract and phases, n-butanolic, chloroformic and ethyl acetate from Caulerpa racemosa (Caulerpaceae). Braz J Pharmacogn 2009, 19, 115–120. [Google Scholar]
- Ayyad, SEN; Badria, FA. Caulerpin, an antitumor índole alkaloid from Caulerpa racemosa, Alex. J Pharm Sci 1994, 8, 217. [Google Scholar]
- Xu, XH; Su, JG. The separation, identification and bioassay of caulerpin. Zhongshan Daxue Xuebao Ziran Kexueban 1996, 35, 64–66. [Google Scholar]
- Raub, MF; Cardellina, JH, II; Schwede, JG. The green algal pigment caulerpin as a plant growth regulator. Phytochemistry 1987, 26, 619–620. [Google Scholar]
- Vidal, JP; Laurent, D; Kabore, A; Rechencg, E; Boucard, M; Girard, JP; Escale, R; Rossi, JC. Caulerpin, caulerpicin, Caulerpa scalpelliformis: comparative acute toxicity study. Bot Mar 1984, 27, 533–537. [Google Scholar]
- Du, J; Yu, Y; Ke, Y; Wang, C; Zhu, L; Qian, ZM. Ligustilide attenuates pain behavior induced by acetic acid or formalin. J Ethnopharmacol 2007, 112, 211–214. [Google Scholar]
- Zeashana, H; Amresha, G; Raoa, CV; Singhb, S. Antinociceptive activity of Amaranthus spinosus in experimental animals. J Ethnopharmacol 2009, 122, 492–496. [Google Scholar]
- Choi, EM. Antinociceptive and antiinflammatory activities of pine (Pinus densiflora) pollen extract. Phytother Res 2007, 21, 471–475. [Google Scholar]
- Collier, HOJ; Dinneen, JC; Johnson, CA; Schneider, C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Brit J Pharm Chemoth 1968, 32, 295–310. [Google Scholar]
- Alexandre-Moreira, MS; Piuvezam, MR; Araújo, CC; Thomas, G. Studies on the anti-inflammatory and analgesic activity of Curatella americana L. J Ethnopharmacol 1999, 67, 171–177. [Google Scholar]
- Miranda, FGG; Vilar, JC; Alves, IAN; Cavalcanti, SCH; Antoniolli, AR. Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol 2001, 1, 1–16. [Google Scholar]
- Moñoa, J; Moscatelli, V; Hnatyszyn, O; Gorzalczany, S; Acevedo, C; Ferraro, G. Antinociceptive and antiinflammatory activities of Artemisia copa extracts. Pharmacol Res 2004, 50, 59–63. [Google Scholar]
- García, MD; Fernández, MA; Alvarez, A; Saenz, MT. Antinociceptive and anti-inflammatory effect of the aqueous extract from leaves of Pimenta racemosa var. ozua (Mirtaceae). J Ethnopharmacol 2004, 91, 69–73. [Google Scholar]
- Ojewole, JAO. Antinociceptive, anti-inflammatory and antidiabetic properties of Hypoxis hemerocallidea Fisch. & C.A. Mey. (Hypoxidaceae) corm [‘African Potato’] aqueous extract in mice and rats. J Ethnopharmacol 2006, 103, 126–134. [Google Scholar]
- Ballou, L; Botting, RM; Goorha, S; Zhang, J; Vane, JR. Nociception in cyclooxygenase isozyme-deficient mice. Proc Natl Acad Sci USA 2000, 97, 10272–10276. [Google Scholar]
- Clunie, M; Crone, LA; Klassen, L; Yip, R. Psychiatric side effects of indomethacin in parturients. Can J Anesth 2003, 50, 586–588. [Google Scholar]
- Tjølsen, A; Berger, OG; Hunskaar, S; Rosland, JH; Hole, K. The formalin test: evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar]
- Inoue, H; Nagata, N; Koshihara, Y. Profile of capsaicin-induced mouse ear oedema as neurogenic inflammatory model: comparison with arachidonic acid-induced ear oedema. Brit J Pharmacol 1993, 110, 1614–1620. [Google Scholar]
- Tang, MLY; Haas, DA; Hu, JW. Capsaicin-induced joint inflammation is not blocked by local anesthesia. Anesth Prog 2004, 51, 2–9. [Google Scholar]
- Grant, AD; Gerard, NP; Brain, SD. Evidence of a role for NK1 and CGRP receptors in mediating neurogenic vasodilatation in the mouse ear. Brit J Pharmacol 2002, 135, 356–362. [Google Scholar]
- Szallasia, A; Blumbergb, PM. Vanilloid receptors: New insights enhance potential as a therapeutic. Pain 1996, 68, 195–208. [Google Scholar]
- Chizh, BA; Aylott, MC; Bullman, JN; Gray, EJ; Lai, RY; Williams, PM; Appleby, JM. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptormediated activity and inflammatory hyperalgesia in humans. Pain 2007, 132, 132–141. [Google Scholar]
- Foster, SJ; McCormick, ME; Howarth, A; Aked, D. Leukocyte recruitment in the subcutaneous sponge implant model of acute inflammation in the rat is not mediated by leukotriene B4. Biochem Pharmacol 1986, 35, 1709–1717. [Google Scholar]
- Yong-Fang, HU; Yong-Jie, WU. Effect of recombinant human basic fibroblast growth factor on acute inflammation in mice and rats. Acta Pharmacol Sin 2001, 22, 357–379. [Google Scholar]
- Oktar, BK; Yuksel, M; Alican, I. The role of cyclooxygenase inhibition in the effect of a-melanocyte-stimulating hormone on reactive oxygen species production by rat peritoneal neutrophils. Prostag Leukot Essent Fatty Acids 2004, 71, 1–5. [Google Scholar]
- Calixto, JB; Beirith, A; Ferreira, J; Santos, ARS; Cechinel-Filho, V; Yunes, RA. Naturally occurring antinociceptive substances from plants. Phytother Res 2000, 14, 401–418. [Google Scholar]
- Radwan, MAA; Ragab, EA; Sabry, NM; El-Shenawy, SM. Synthesis and biological evaluation of new 3-substituted indole derivatives as potential anti-inflammatory and analgesic agents. Biol Med Chem 2007, 15, 3832–3841. [Google Scholar]
- Bhati, SK; Kumar, A. Synthesis of new substituted azetidinoyl and thiazolidinoyl-1,3,4-thiadiazino (6,5-b) indoles as promising anti-inflammatory agents. Eur J Med Chem 2008, 43, 2323–2330. [Google Scholar]
- Hata, AN; Lybrand, TP; Marnett, LJ; Breyer, RM. Structural determinants of arylacetic acid nonsteroidal anti-inflammatory drugs necessary for binding and activation of the prostaglandin D2 receptor CRTH2. Mol Pharmacol 2005, 67, 640–647. [Google Scholar]
- Touhey, S; O’Connor, R; Plunkett, S; Maguire, A; Clynes, M. Structure-activity relationship of indomethacin analogues for MRP-1, COX-1 and COX-2 inhibition: identification of novel chemotherapeutic drug resistance modulators. Eur J Cancer 2002, 38, 1661–1670. [Google Scholar]
- Stole, S. Indole derivatives as neuroprotectants. Life Sci 1999, 65, 1943–1950. [Google Scholar]
- Kang, K; Park, S; Kim, YS; Lee, LS; Back, K. Biosynthesis and biotechnological production of serotonin derivatives. Appl Microbiol Biotechnol 2009, 83, 27–34. [Google Scholar]
- Maffei-Facino, R; Carini, M; Aldini, G; Saibene, L; Macciocchi, A. Antioxidant profile of nimesulide, indomethacin and diclofenac in phosphatidylcholine liposomes (PCL) as membrane model. Int J Tissue React 1993, 15, 225–234. [Google Scholar]
- Zimmermann, M. Ethical guidelines for investigation of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar]
- Koster, R; Anderson, M; De-Beer, EJ. Acetic acid analgesic screen. Fed Proc 1959, 18, 418–420. [Google Scholar]
- Eddy, NB; Leimbach, D. Synthetic analgesics. II. Dithienylbutenyland dithienylbutylamines. JPET 1953, 107, 385–393. [Google Scholar]
- Godoy, MCM; Figueira, MR; Flores, AE; Rubin, MA; Oliveira, MR; Zanatta, N; Martins, MAP; Bonacorso, HG; Mello, CF. α2-Adrenoceptors and 5-HT receptors mediate the antinociceptive effect of new pyrazoles, but not of dipyrone. Eur J Pharmacol 2004, 496, 93–97. [Google Scholar]
- Hunskaar, S; Hole, K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar]
- Tjolsen, A; Hole, K. 1997; Animal models of analgesia. In The Pharmacology of Pain; Dickenson, A, Besson, J, Eds.; Springer Verlag: Berlin, Germany, 1997; Volume 130, pp. 1–20.
- Mantione, CR; Rodrigues, RA. Bradykinin (BK)1-receptor antagonist blocks capsaicin-induced ear inflammation in mice. Brit J Pharmacol 1990, 99, 516–518. [Google Scholar]
- Ferrándiz, ML; Alcaraz, MJ. Antiinflammatory activity and inhibition of arachidonic acidmetabolism by flavonoids. Inflamm Res 1991, 32, 283–288. [Google Scholar]
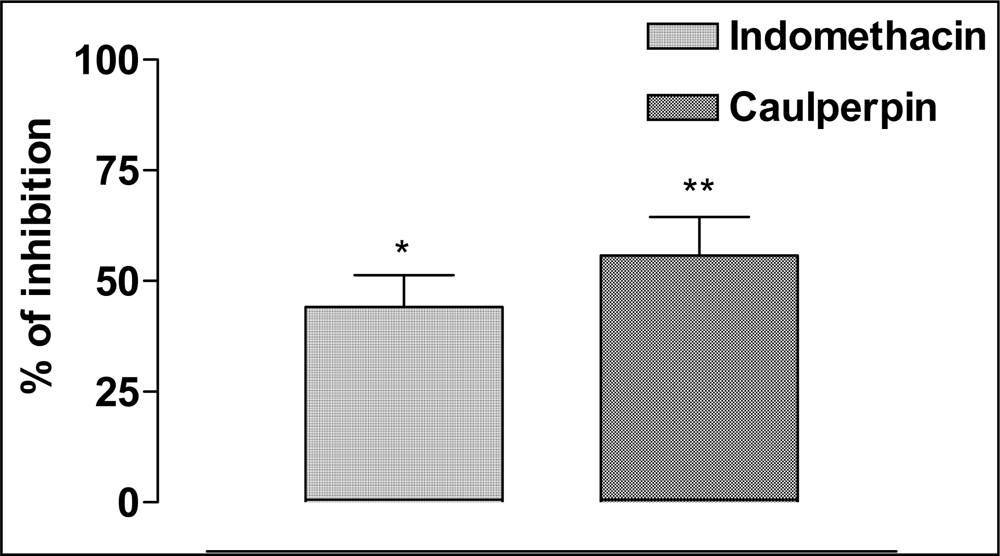
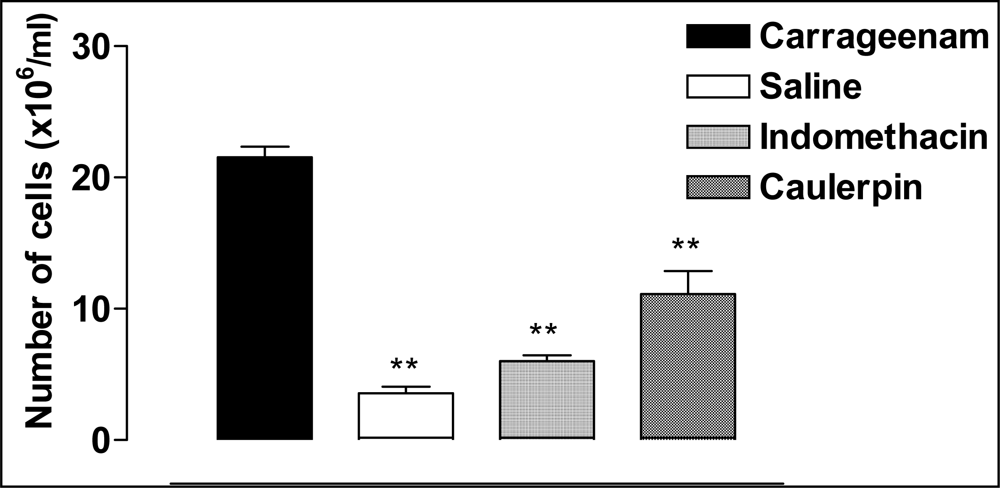
| Animal Group | Dose (μmol/kg) | Pretreatmenta | Post-treatment (min)a | ||||
|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | 150 min | ||
| Control | --- | 1.4 ± 0.3 | 2.2 ± 0.6 | 1.8± 0.2 | 3.2 ± 0.3 | 2.8 ± 0.3 | 2.6 ± 0.5 |
| Morphine | 15 | 6.9 ± 0.4 | 5.9 ± 0.3 | 12.8 ± 0.4** | 10.3 ± 0.8** | 9.7 ± 0.7** | 9.7 ± 0.9** |
| Caulerpin | 100 | 1.9 ± 0.2 | 2.3 ± 0.6 | 3.3 ± 0.5 | 4.6 ± 0.6** | 3.8 ± 0.5* | 4.0 ± 0.5* |
| Treatment (μmol/kg) | Fall latency (s) | Number of falls (s) | ||
|---|---|---|---|---|
| 0.5 h | 1 h | 0.5 h | 1 h | |
| Saline | 198.0 ± 28.1 | 221.0 ± 19.0 | 0.4 ± 0.3 | 0.2 ± 0.2 |
| Caulerpin | 182.3 ± 37.3 | 240.0 ± 0 | 0.9 ± 0.4 | 0 ± 0 |
| Diazepan | 2.7 ± 0.6** | 109.9 ± 36.7** | 9.2 ± 2.2** | 3.9 ± 1.4** |
| Treatment | Percentage of cells (%) | |||
|---|---|---|---|---|
| Polymorphonuclear | Lymphocyte | Monocyte | Basophil | |
| Saline | 15.5 | 80.0* | 1.5 | 3.0 |
| Carrageenan | 34.5 | 44.0 | 20.5 | 1.0 |
| Indomethacin + Carrageenan | 7.0* | 68.0* | 21.0 | 4.0 |
| Caulerpin + Carrageenan | 6.0* | 63.0* | 31.0* | 0.0 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Souza, É.T.; Pereira de Lira, D.; Cavalcanti de Queiroz, A.; Costa da Silva, D.J.; Bezerra de Aquino, A.; Campessato Mella, E.A.; Prates Lorenzo, V.; De Miranda, G.E.C.; De Araújo-Júnior, J.X.; De Oliveira Chaves, M.C.; et al. The Antinociceptive and Anti-Inflammatory Activities of Caulerpin, a Bisindole Alkaloid Isolated from Seaweeds of the Genus Caulerpa. Mar. Drugs 2009, 7, 689-704. https://doi.org/10.3390/md7040689
De Souza ÉT, Pereira de Lira D, Cavalcanti de Queiroz A, Costa da Silva DJ, Bezerra de Aquino A, Campessato Mella EA, Prates Lorenzo V, De Miranda GEC, De Araújo-Júnior JX, De Oliveira Chaves MC, et al. The Antinociceptive and Anti-Inflammatory Activities of Caulerpin, a Bisindole Alkaloid Isolated from Seaweeds of the Genus Caulerpa. Marine Drugs. 2009; 7(4):689-704. https://doi.org/10.3390/md7040689
Chicago/Turabian StyleDe Souza, Éverton Tenório, Daysianne Pereira de Lira, Aline Cavalcanti de Queiroz, Diogo José Costa da Silva, Anansa Bezerra de Aquino, Eliane A. Campessato Mella, Vitor Prates Lorenzo, George Emmanuel C. De Miranda, João Xavier De Araújo-Júnior, Maria Célia De Oliveira Chaves, and et al. 2009. "The Antinociceptive and Anti-Inflammatory Activities of Caulerpin, a Bisindole Alkaloid Isolated from Seaweeds of the Genus Caulerpa" Marine Drugs 7, no. 4: 689-704. https://doi.org/10.3390/md7040689
APA StyleDe Souza, É. T., Pereira de Lira, D., Cavalcanti de Queiroz, A., Costa da Silva, D. J., Bezerra de Aquino, A., Campessato Mella, E. A., Prates Lorenzo, V., De Miranda, G. E. C., De Araújo-Júnior, J. X., De Oliveira Chaves, M. C., Barbosa-Filho, J. M., Filgueiras de Athayde-Filho, P., De Oliveira Santos, B. V., & Alexandre-Moreira, M. S. (2009). The Antinociceptive and Anti-Inflammatory Activities of Caulerpin, a Bisindole Alkaloid Isolated from Seaweeds of the Genus Caulerpa. Marine Drugs, 7(4), 689-704. https://doi.org/10.3390/md7040689