Abstract
The extract of the sea hare, Aplysia kurodai, showed cytotoxicity against HeLa cells and antibacterial activity against Staphylococcus aureus. Bioassay-guided purification afforded four active compounds, which were identified to be laurinterol, laurinterol acetate, debromolaurinterol, and debromolaurinterol acetate by spectroscopic analysis. Although the acetates were derived from laurinterol and debromolaurinterol before, this is the first isolation of the acetates from natural sources.
Introduction
It is well known that the content of sea hare digestive glands is derived from the diet that this animal ingests [1–4]. A large number of cognate compounds have been isolated from both the red alga of the genus Laurencia and the sea hare that preys on the red alga. Metabolites isolated from sea hares exhibit various biological activities, for example, cytotoxic [5–7], algicidal [6], ichthyotoxic [8], antifungal [6, 8], and neurotrophic [9] activities. In the course of our search for new biologically active compounds from marine organisms [9–12], we succeeded in isolating four compounds exhibiting cytotoxic and antibacterial activities from sea hare collected from Toyama Bay in the Japan Sea. Here we report the isolation, structure determination, and the biological activities of the four compounds including two new derivatives.
Results and Discussion
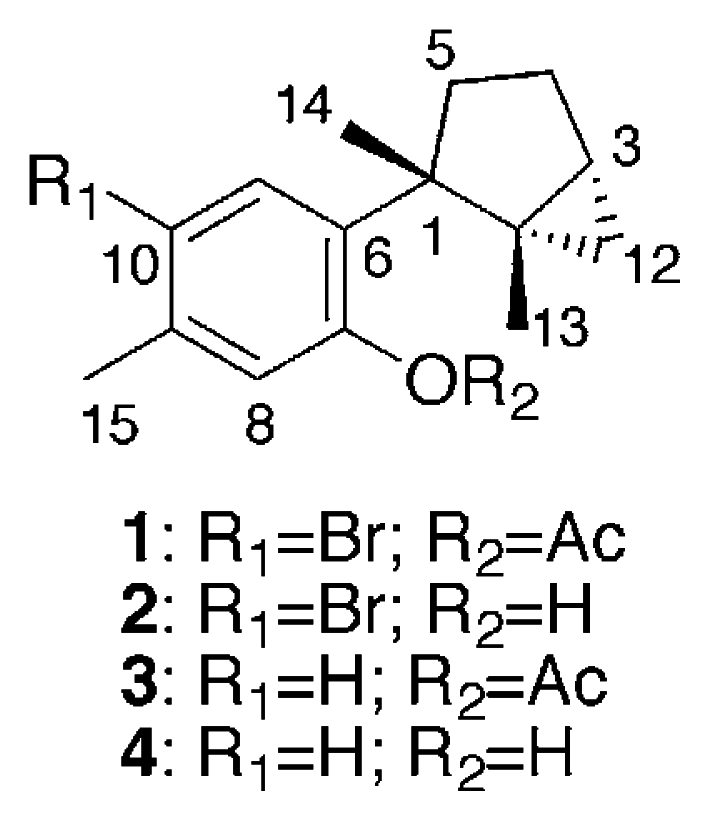
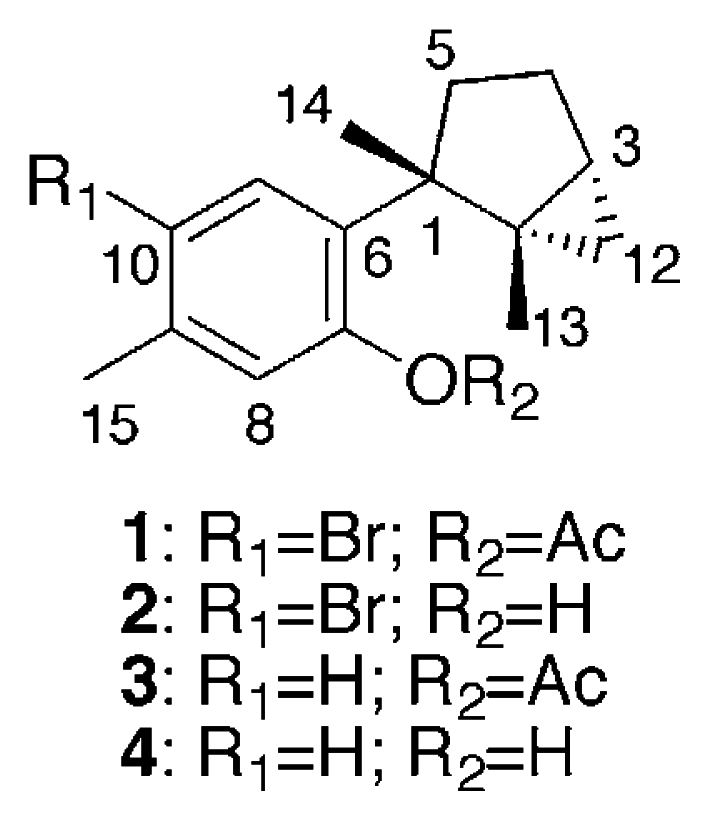
Specimens of Aplysia kurodai were collected by hand from Toyama Bay in the Japan Sea and kept frozen until extraction with MeOH. The MeOH extract was partitioned between EtOAc and water. The EtOAc layer, which showed cytotoxic and antibacterial activity against Staphylococcus aureus, was fractionated by a combination of silica gel chromatography, silica gel HPLC, and gel permeation on Sephadex LH-20 to afford laurinterol acetate (1), laurinterol (2) [13, 14], debromolaurinterol acetate (3), and debromolaurinterol (4) [13, 14] in yields of 2.3 × 10−3, 3.8 × 10−3, 2.6 × 10−4, and 3.9 × 10−4%, respectively.
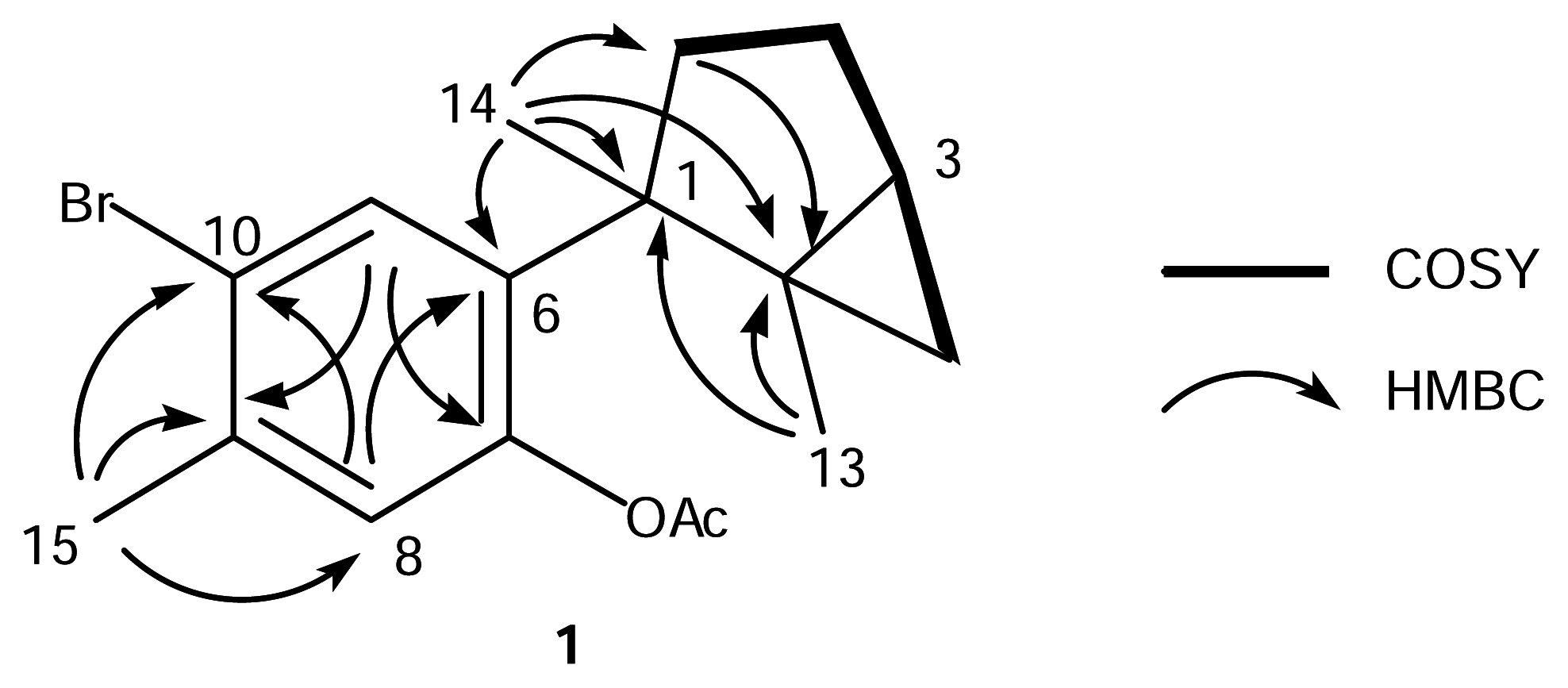
The EIMS of 1 showed 1:1 doublet ion peaks at m/z 336 and 338 [M]+, indicating the presence of a bromine atom in the molecule, and a molecular formula of C17H21O2Br was established by HREIMS and 13C NMR data. The IR spectrum of 1 showed absorption at 1762 and 1600 cm−1, which indicated the presence of a conjugated ester group and an aromatic ring. The 1H NMR spectrum of 1 (Table 1) revealed two characteristic high-field signals assignable to a trisubstituted cyclopropane ring at δ 0.50 (t, J = 4.6 Hz) and 0.55 (dd, J = 4.6, 8.8 Hz), two singlet aromatic signals at δ 6.88 (s) and 7.82 (s), four singlet methyls at δ 1.30 (3H, s), 1.34 (3H, s), 2.30 (3H, s), and 2.39 (3H, s). The analysis of COSY and HMBC spectra of 1 suggested the presence of a dimethylbicyclo[3.1.0]hexane group and a 1,2,4,5-tetrasubstituted aromatic ring (Fig. 1). The HMBC cross-peak, δH 1.34 (H3-14)/δC 139.7 (C-6), showed the connection between C-1 of the bicyclohexane ring and C-6 of the aromatic ring. The HMBC cross-peak, δH 2.39 (H3-15)/δC 136.1 (C-9), revealed the methyl signal was attached to a carbon at C-9, which was supported by the NOE correlation between H3-15 and H-8 (δ 6.88, s). The magnitude of carbon chemical shifts of δ 148.3 (C-7) and 121.2 (C-10) indicated that an acetate and a bromine group were substituted at C-7 and C-10, resepectively. These data suggested that 1 was an acetate of laurinterol (2) [13, 14]. The acetylation of 2 afforded its monoacetate, of which the 1H NMR spectrum showed the appearance of an additional acetate signal at δ 2.30 (3H, s) instead of the disappearence of a hydroxy signal at δ 5.39 (s, 7-OH) in 2. The spectral data of the monoacetate were all identical with those of 1 including the specific rotation, which implied that 1 was laurinterol acetate.

Table 1.
NMR Data for 1 in CDCl3.
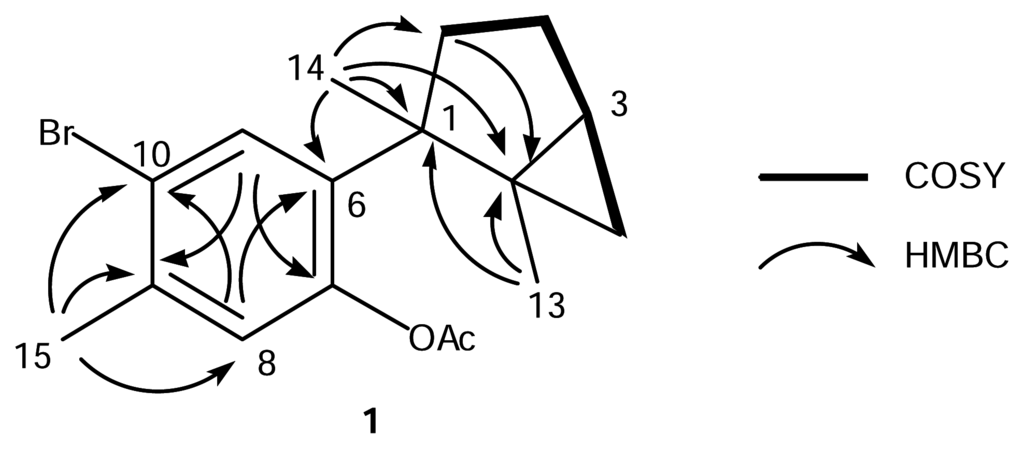
Figure 1.
COSY and HMBC correlations for 1.
The EI mass spectrum of compound 3 exhibited an ion peak at m/z 258 [M]+, which matched a formula of C17H22O2. The 1H NMR spectrum of 3 was similar to that of 1, except for the presence of two doublet aromatic protons at δ 6.98 (1H, d, J = 7.8 Hz, H-10) and 7.57 (1H, d, J = 7.8 Hz, H-11) and the absence of a singlet aromatic proton at δ 7.82 (1H, s, H-11) in 1. These spectral features indicated that 3 was an acetate of debromolaurinterol (4) [13, 14]. The acetylation of 4 afforded a monoacetate and its spectral data were identical with those of 3. Therefore, compound 3 was determined as debromolaurinterol acetate.
The antibacterial activity using Staphylococcus aureus (Table 2) and cytotoxicity (Table 3) were tested for 1–4. Compared with acetates (1 and 3) and their original metabolites (2 and 4), the latter exhibited more potent antibacterial activity than the acetates. Compounds 1, 2, and 4 showed moderate cytotoxicity against HeLa cells.

Table 2.
Growth-Inhibitory Activity of 1–4 against Staphylococcus aureus.

Table 3.
Cytotoxicity of 1–4 against HeLa cells.
Conclusion
We have isolated laurinterol acetate (1), laurinterol (2) [13, 14], debromolaurinterol acetate (3), and debromolaurinterol (4) [13, 14] from the sea hare, Aplysia kurodai. Isolation of 2 and 4 was reported from the red alga, Laurencia intermedia Yamada, and their acetates 1 and 3 were derived from 2 and 4 by acetylation, respectively [13, 14]. However, this is the first isolation of 1 and 3 from natural sources. Among the four metabolites, 1, 2, and 4 showed moderate cytotoxicity against tumor cells, and 2 and 4 exhibited more potent antibacterial activity against Staphylococcus aureus than 1 and 3. It is implied that the content of sea hare digestive glands is derived from the red alga of the genus Laurencia that this animal ingests [1–4] and that the metabolites are used for the sake of their self-defense. Although isolation of acetates 1 and 3 from red alga has not been reported so far, the acetates could be derived from the red alga ingested as diet or transformed from 2 and 4 in the digestive gland of the sea hare.
Experimental
General
Optical rotations were determined with a HORIBA SEPA-300 high sensitive polarimeter. UV spectra were measured on a Shimadzu UV-1600 UV-visible spectrometer. IR spectra were recorded on a SHIMADZU IR-460 infrared spectrophotometer. NMR spectra were recorded on a JEOL GSX500 in CDCl3. All chemical shifts were reported with respect to the residual solvent peaks (δH 7.26 amd δC 77.0). Mass spectra were measured on a JEOL SX-102 mass spectrometer.
Extraction and Isolation
The frozen material (1.7 kg, wet wt) was extracted with MeOH. The extract was concentrated under reduced pressure and extracted with EtOAc. The EtOAc layer (1.4 g) was subjected to silica gel chromatography with a stepwise gradient of hexane/EtOAc to give two cytotoxic and antibacterial fractions, Fr. 1 (642 mg) eluted with hexane/EtOAc (19:1) and Fr. 2 (354 mg) eluted with hexane/EtOAc (9:1). After purification by silica gel chromatography (hexane/EtOAc) followed by silica gel HPLC (hexane/EtOAc), Fr. 1 gave laurinterol acetate (1, 38.7 mg, 2.3 × 10−3%) and debromolaurinterol acetate (3, 4.5 mg, 2.6 × 10−4%). Fr. 2 was purified by silica gel chromatography (hexane/EtOAc) followed by Sephadex LH-20 gel filtration (hexane/EtOAc) to give laurinterol (2, 64.8 mg, 3.8 × 10−3%) [13, 14] and debromolaurinterol (4, 6.8 mg, 3.9 × 10−4%) [13, 14].
Laurinterol acetate (1): [α]23D +1.7° (c 0.064, CHCl3); UV (MeOH) λmax (log ɛ) 269 (3.5) and 279 nm (3.4); IR (film) νmax 3060, 2930, 1762, 1600, 1544, 1482, 1364, 1203 cm−1; 1H and 13C NMR (CDCl3), see Table 1; EIMS m/z 336/338 (intensity, 1:1) [M]+; HREIMS m/z 336.0707(calcd for C17H21O279Br, 336.0725).
Laurinterol (2): 1H NMR (CDCl3) δ 0.54 (1H, dd, J = 8.8, 4.6 Hz, H-12), 0.57 (1H, t, J = 4.6 Hz, H-12), 1.13 (1H, dt, J = 8.8, 4.6 Hz, H-3), 1.27 (1H, m, H-5), 1.31 (3H, s, H3-13), 1.40 (3H, s, H3-14), 1.65 (1H, dd, J = 13.2, 8.8 Hz, H-4), 1.94 (1H, m, H-4), 2.10 (1H, dd, J = 13.2, 8.8 Hz, H-5), 2.28 (3H, s, H3-15), 5.39 (1H, br s, 7-OH), 6.61 (1H, s, H-8), 7.60 (1H, s, H-11); EIMS m/z 294/296 (intensity, 1:1) [M]+.
Debromolaurinterol acetate (3): [α]23D −4.7° (c 0.092, CHCl3); UV (MeOH) λmax (log ɛ) 265 (3.5) and 275 nm (3.4); IR (film) νmax 2920, 2850, 1730, 1460, 1177, 1038 cm−1; 1H NMR (CDCl3) δ 0.50 (2H, H2-2), 1.09 (1H, dt, J = 8.8, 4.6 Hz, H-3), 1.28 (1H, m, H-5), 1.30 (3H, s, H3-13), 1.34 (3H, s, H3-14), 1.65 (1H, dd, J = 13.2, 8.8 Hz, H-4), 1.83 (1H, dd, J = 13.2, 8.8 Hz, H-5), 1.94 (1H, m, H-4), 2.31 (3H, s, 7-OAc), 2.33 (3H, s, H3-15), 6.81 (1H, s, H-8), 6.98 (1H, d, J = 7.8 Hz, H-10), 7.57 (1H, d, J = 7.8 Hz, H-11); EIMS m/z 258 [M]+; HREIMS m/z 258.1625 (calcd for C17H22O2, 258.1620).
Debromolaurinterol (4): 1H NMR (CDCl3) δ 0.51 (1H, dd, J = 7.8, 4.4 Hz, H-12), 0.58 (1H, t, J = 4.4 Hz, H-12), 1.13 (1H, dt, J = 7.8, 4.4 Hz, H-3), 1.32 (1H, m, H-5), 1.33 (3H, s, H3-13), 1.43 (3H, s, H3-14), 1.66 (1H, dd, J = 13.2, 7.8 Hz, H-4), 1.95 (1H, m, H-4), 2.11 (1H, dd, J = 13.2, 7.8 Hz, H-5), 2.27 (3H, s, H3-15), 5.05 (1H, br s, 7-OH), 6.55 (1H, s, H-8), 6.68 (1H, d, J = 7.8 Hz, H-10), 7.39 (1H, d, J = 7.8 Hz, H-11); 13C NMR (CDCl3) δ 16.6 (C-12), 19.1 (C-13), 20.9 (C-15), 23.9 (C-14), 24.6 (C-3), 25.6 (C-4), 30.0 (C-2), 36.4 (C-5), 48.2 (C-1), 117.6 (C-8), 121.1 (C-10), 129.1 (C-11), 131.7 (C-6), 137.0 (C-9), 154.3 (C-7); EIMS m/z 216 [M]+.
Cytotoxicity Test
Cytotoxicity test was carried out with HeLa cells. HeLa cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, penicillin (50 units/mL), and streptomycin (50 μg/mL) under a humidified atmosphere of 5% CO2 at 37 °C. The cells were seeded into 96-well microplates (3×103 cells/well) and pre-cultured for a day. The medium was replaced with that containing test compounds at various concentrations and the cells were further cultured at 37 °C for 3 days. The medium was then replaced with 50 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (0.2 mg/mL in medium) and the cells were incubated under the same conditions for 4 h. After addition of 200 μL of DMSO, the optical density at 570 nm was measured with a microplate reader.
Antibacterial Test
Growth-inhibitory activity was determined by the paper disk method. Paper disk (φ 6 mm) with sample (50, 25, or, 12.5 μg) was incubated on an agar plate containing bacterium at 37 °C.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture, and Technology of Japan.
- Sample availability: Not available.
References and Notes
- Erickson, K. L. Constituents of Laurencia. In Marine Natural Products; Scheuer, P. J., Ed.; Academic; New York, 1983; Volume V, pp. 132–257. [Google Scholar]
- Stallard, M. O.; Faulkner, D. J. Chemical Constituents of the Digestive Gland of the Sea Hare Aplysia californica. I. Importance of Diet. Comp. Biochem. Physiol. B 1974, 49B, 25–35. [Google Scholar]
- Stallard, M. O.; Faulkner, D. J. Chemical Constituents of the Digestive Gland of the Sea Hare Aplysia californica. II. Chemical Transformations. Comp. Biochem. Physiol. B 1974, 49B, 37–41. [Google Scholar]
- Ireland, C.; Stallard, M. O.; Faulkner, D. J. Some Chemical Constituents of the Digestive Gland of the Sea Hare Aplysia californica. J. Org. Chem 1976, 41, 2461–2465. [Google Scholar]
- Yamada, K.; Ojika, M.; Ishigaki, T.; Yoshida, Y.; Ekimoto, H.; Arakawa, M. Aplyronine A, a Potent Antitumor Substance, and the Congeners Aplyronines B and C Isolated from the Sea Hare Aplysia kurodai. J. Am. Chem. Soc 1993, 115, 11020–11021. [Google Scholar]
- Wessels, M.; Konig, G. M.; Wright, A. D. New Natural Product Isolation and Comparison of the Secondary Metabolite Content of Three Distinct Samples of the Sea Hare Aplysia dactylomela from Tenerife. J. Nat. Prod 2000, 63, 920–928. [Google Scholar]
- Schmitz, F. J.; Michaud, D. P.; Schmidt, P. G. Marine Natural Products: Parguerol, Deoxyparguerol, and Isoparguerol. New Brominated Diterpenes with Modified Pimarane Skeletons from the Sea Hare Aplysia dactylomela. J. Am. Chem. Soc 1982, 104, 6415–6423. [Google Scholar]
- Ichiba, T.; Higa, T. New Cuparene-derived Sesquiterpenes with Unprecedented Oxygenation Patterns from the Sea Hare Aplysia dactylomela. J. Org. Chem 1986, 51, 3364–3366. [Google Scholar]
- Tsukamoto, S.; Yamashita, Y.; Yoshida, T.; Ohta, T. Parguerol and Isoparguerol Isolated from the Sea Hare, Aplysia kurodai, Induce Neurite Outgrowth in PC-12 Cells. Mar. Drugs 2004, 2, 170–175. [Google Scholar]
- Tsukamoto, S.; Tatsuno, M.; van Soest, R. W. M.; Yokosawa, H.; Ohta, T. New Polyhydroxy Sterols: Proteasome Inhibitors from a Marine Sponge Acanthodendrilla sp. J. Nat. Prod 2003, 66, 1181–1185. [Google Scholar]
- Tsukamoto, S.; Miura, S.; Yamashita, Y.; Ohta, T. Aspermytin A: A New Neurotrophic Polyketide Isolated from a Marine-derived Fungus of the Genus Aspergillus. Bioorg. Med. Chem. Lett 2004, 14, 417–420. [Google Scholar]
- Tsukamoto, S.; Hirota, H.; Imachi, M.; Fujimuro, M.; Onuki, H.; Ohta, T.; Yokosawa, H. Himeic Acid A: A New Ubiquitin-Activating Enzyme Inhibibtor Isolated from a Marine-Derived Fungus, Aspergillus sp. Bioorg. Med. Chem. Lett 2005, 15, 191–194. [Google Scholar]
- Irie, T.; Suzuki, M.; Kurosawa, E.; Masamune, T. Laurinterol and Debromolaurinterol, Constituents from Laurencia intermedia. Tetrahedron Lett. 1966, 1837–1840. [Google Scholar]
- Irie, T.; Suzuki, M.; Kurosawa, E.; Masamune, T. Laurinterol, Debromolaurinterol and Isolaurinterol, Constituents of Laurencia intermedia Yamada. Tetrahedron 1970, 26, 3271–3277. [Google Scholar]
© 2005 by MDPI Reproduction is permitted for noncommercial purposes.