Extraction of Marine Bioactive Compounds from Seaweed: Coupling Environmental Concerns and High Yields
Abstract
1. Introduction
Literature Search Procedure
2. Conventional Extractive Routes and Techniques
2.1. Main Conventional Routes
2.1.1. The Conventional Approach
2.1.2. Conventional Thermal Pre-Treatments
2.1.3. Conventional Mechanical and Other Pre-Treatments
2.2. Conventional Extractive Techniques
2.2.1. General Overview
2.2.2. Specific Case of Seaweed Polysaccharides
2.2.3. Utilization of Conventional Extraction Techniques at an Industrial Scale
2.2.4. Optimization of Conventional Extraction Techniques
3. Innovative Extractive Routes and Techniques
3.1. Biorefinery, Wet Route, and Other Innovative Approaches
3.1.1. The Current Challenge
3.1.2. The Biorefinery Approach
Biorefinery in Red Seaweed Species
Biorefinery in Green Seaweed Species
Biorefinery in Brown Seaweed Species
Biorefinery in Seaweed
3.1.3. Wet Route and Other Sustainable Approaches
Wet Route
Application of ‘Green’ Solvents
3.2. Novel Extractive Techniques
3.2.1. General Overview
3.2.2. Technologies Based on Physical Processes
Microwave-Assisted Extraction
Ultrasound-Assisted Extraction
Pulsed Electric Field
High Hydrostatic Pressure
Pressurized Liquid Extraction
Sub-Critical Water Extraction
Supercritical Fluid Extraction
Other Physical Extraction Technologies
3.2.3. Technologies Based on Chemical Processes
Solid-Phase Extraction
pH-Shift Extraction
Assessment of Technologies Based on Chemical Processes Applied to Seaweed
3.2.4. Technologies Based on Enzymes and Biological Systems
Enzyme-Assisted Extraction
Fermentation of Seaweed
3.2.5. Challenges in Novel Extractive Techniques: ‘Green’ Solvents, Optimization, Combination, and Biorefinery
4. Conclusions and Future Challenges
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-Bis(3-ethylbenzoThiazoline-6-Sulfonic acid |
| AlE | Alkali Extraction |
| APCI | Antioxidant Potency Composite Index |
| BaM | Ball Milling |
| BBD | Box–Behnken Design |
| BeM | Bead Milling |
| BMIA | 1-Butyl-3-MethylImidazolium Acetate |
| BMIC | 1-Butyl-3-MethylImidazolium Chloride |
| BMIDP | 1-Butyl-3-MethylImidazolium Dibutyl Phosphate |
| BMITFB | 1-Butyl-3-MethylImidazolium TetraFluoroBorate |
| ChC | Choline Chloride |
| CPAE | Cold Plasma-Assisted Extraction |
| DES | Deep Eutectic Solvent |
| DPPH | 2,2-DiPhenyl-1-PicrylHydrazyl |
| DR | Dry Route |
| DW | Dry Weight |
| EAE | Enzyme-Assisted Extraction |
| EC50 | Half Maximal Effective Concentration |
| EMIDP | 1-Ethyl-3-Methyl-Imidazolium Dibutyl Phosphate |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalent |
| HaM | Hammer Milling |
| HDC | HydroDynamic Cavitation |
| HHP | High Hydrostatic Pressure |
| HMF | 5-hydroxyMethylFurfural |
| HPCD | High-Pressure Cell Disruption |
| HSH | High Speed Homogenization |
| ICPD/DIC | Instant Controlled Pressure Drop/‘Détente Instantanée Contrôlée’ |
| IL | Ionic Liquid |
| IMTA | Integrated Multi-Trophic Aquaculture |
| LA | Lactic Acid |
| LCA | Life Cycle Analysis |
| MAE | Microwave-Assisted Extraction |
| NADES | Natural Deep Eutectic Solvent |
| OL | Osmotic Lysis |
| ORAC | Oxygen Radical Absorbance Capacity |
| PE | Percolation |
| PEF | Pulsed Electric Field |
| PGE | PhloroGlucinol Equivalent |
| PHB | PolyHydroxyButyrate |
| pHE | pH-shift Extraction |
| PLE | Pressurized Liquid Extraction |
| RE | Reflux Extraction |
| RSM | Response Surface Methodology |
| SFE | Supercritical Fluid Extraction |
| SLE | Solid–Liquid Extraction |
| SP | Screw Pressing |
| SPE | Solid-Phase Extraction |
| SPME | Solid-Phase MicroExtraction |
| SWE | Sub-critical Water Extraction |
| TBTDPC | TriButylTetraDecylPhosphonium Chloride |
| TMAH | TetraMethylAmmonium Hydroxide |
| UAE | Ultrasound-Assisted Extraction |
| WR | Wet Route |
References
- Abdel-Kareem, M.S.M.; ElSaied, A.A.F. Global seaweeds diversity, Chapter Two. In Handbook of Algal Fuels—Aspects of Cultivation, Conversion, and Biorefinery; El-Sheekh, M., El-Fatah Abomohra, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 39–55. [Google Scholar]
- Ashraf, M.T.; Fang, C.; Bochenski, T.; Cybulska, I.; Alassali, A.; Sowunmi, A.; Farzanah, R.; Brudecki, G.P.; Chaturvedi, T.; Haris, S.; et al. Estimation of bioenergy potential for local biomass in the United Arab Emirates. Emir. J. Food Agric. 2016, 28, 99–106. [Google Scholar] [CrossRef]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.H.; Ishak, W.F.W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Serralheiro, M.; Bandarra, N.; Afonso, C. Seaweed bioactives potential as nutraceuticals and functional ingredients: A review. J. Food Compos. Anal. 2024, 133, 106453. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, R. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef]
- Blikra, M.J.; Altintzoglou, T.; Løvdal, T.; Rognså, G.; Skipnes, D.; Skåra, T.; Sivertsvik, M.; Fernández, E.N. Seaweed products for the future: Using current tools to develop a sustainable food industry. Trends Food Sci. Technol. 2021, 118 Pt B, 765–776. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Pressurized liquid extraction for the recovery of bioactive compounds from seaweeds for food industry application: A review. Antioxidants 2023, 12, 612. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.; Zhang, W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017, 70, 20–33. [Google Scholar] [CrossRef]
- Reboleira, J.; Ganhão, R.; Mendes, S.; Adão, P.; Andrade, M.; Vilarinho, F.; Sanches-Silva, A.; Sousa, D.; Mateus, A.; Bernardino, S. Optimization of extraction conditions for Gracilaria gracilis extracts and their antioxidative stability as part of microfiber food coating additives. Molecules 2020, 25, 4060. [Google Scholar] [CrossRef]
- Gunathilake, T.; Akanbi, T.O.; Suleria, H.A.R.; Nalder, T.D.; Francis, D.S.; Barrow, C.J. Seaweed phenolics as natural antioxidants, aquafeed additives, veterinary treatments and cross-linkers for microencapsulation. Mar. Drugs 2022, 20, 445. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—Cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Sharma, S.; Sahu, S.; Arya, S.K. Pretreatment and fractionation of algae biomass for value-added extraction. In Value Added Products from Bioalgae Based Biorefineries: Opportunities and Challenges; Arya, S.K., Khatri, M., Singh, G., Eds.; Springer: Singapore, 2024. [Google Scholar]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A critical comparison of the advanced extraction techniques applied to obtain health-promoting compounds from seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hawboldt, K.; MacQuarrie, S. Extraction of bioactive compounds from beachcast brown algae: A review on accelerated solvent extraction and subcritical water extraction. RSC Sustain. 2024, 2, 2069–2091. [Google Scholar] [CrossRef]
- Didion, Y.P.; Tjalsma, T.G.; Su, Z.; Malankowska, M.; Pinelo, M. What is next? The greener future of solid liquid extraction of biobased compounds: Novel techniques and solvents overpower traditional ones. Sep. Purif. Technol. 2023, 320, 124147. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; Food and Agricultural Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Bordoloi, A.; Goosen, N. Green and integrated processing approaches for the recovery of high-value compounds from brown seaweeds, Chapter Eleven. In Seaweeds Around the World: State of Art and Perspectives—Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press (Elsevier): Cambridge, MA, USA, 2020; Volume 95. [Google Scholar]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.-S.F. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef]
- Irianto, I.; Naryaningsih, A.; Trisnawati, N.W.; Astuti, A.; Komariyah, K.; Qomariyah, L.; Chaidir, C.; Saputri, A.; Wulandari, R.; Rizkiyah, D.N.; et al. From sea to solution: A review of green extraction approaches for unlocking the potential of brown algae. S. Afr. J. Chem. Eng. 2024, 48, 1–21. [Google Scholar] [CrossRef]
- Bhadange, Y.A.; Carpenter, J.; Saharan, V.K. A comprehensive review on advanced extraction techniques for retrieving bioactive components from natural sources. ACS Omega 2024, 9, 31274–31297. [Google Scholar] [CrossRef]
- Naseem, S.; Rizwan, M.; Durrani, A.I.; Munawar, A.; Gillani, S.R. Innovations in cell lysis strategies and efficient protein extraction from blue food (Seaweed). Sustain. Chem. Pharm. 2024, 39, 101586. [Google Scholar] [CrossRef]
- Sacramento, M.M.A.; Borges, J.; Correia, F.J.S.; Calado, R.; Rodrigues, J.M.M.; Patrício, S.G.; Mano, J.F. Green approaches for extraction, chemical modification and processing of marine polysaccharides for biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 1041102. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral utilization of red seaweed for bioactive production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef]
- Carpena, M.; Garcia-Perez, P.; Garcia-Oliveira, P.; Chamorro, F.; Otero, P.; Lourenço-Lopes, C.; Cao, H.; Simal-Gandara, J.; Prieto, M.A. Biological properties and potential of compounds extracted from red seaweeds. Phytochem. Rev. 2023, 22, 1509–1540. [Google Scholar] [CrossRef]
- Jacobsen, C.; Sørensen, A.-D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, extraction, characterization, and applications of novel antioxidants from seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.-M.; Kim, D.-H.; Kim, S.-K.; Jeong, G.-T. Production of sugars from macroalgae Gracilaria verrucosa using combined process of citric acid-catalyzed pretreatment and enzymatic hydrolysis. Algal Res. 2016, 13, 293–297. [Google Scholar] [CrossRef]
- Khoo, C.G.; Dasan, Y.K.; Lam, M.K.; Lee, K.T. Algae biorefinery: Review on a broad spectrum of downstream processes and products. Bioresour. Technol. 2019, 292, 121964. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Viñas, M.; Flórez-Fernández, N.; Dolores Torres, M.; Domínguez, H. Successful approaches for a red seaweed biorefinery. Mar. Drugs 2019, 17, 620. [Google Scholar] [CrossRef]
- van Hal, J.W.; Huijgen, W.J.J.; López-Contreras, A.M. Opportunities and challenges for seaweed in the biobased economy. Trends Biotechnol. 2014, 32, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, G.C. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Martins, A.; Silva, J.; Alves, C.; Pinteus, S.; Félix, C.; Augusto, A.; Pedrosa, R.; Mestre, A.S.; Santos, R.M.; Carvalho, A.P.; et al. Towards a zero-waste sustainable biorefinery of Codium sp. seaweed: From bioactives application to soil enhancement materials. J. Clean. Prod. 2024, 453, 142191. [Google Scholar] [CrossRef]
- Choudhary, P.; G, V.S.; Khade, M.; Savant, S.; Musale, A.; Kumar, R.K.; Chelliah, M.S.; Dasgupta, S. Empowering blue economy: From underrated ecosystem to sustainable industry. J. Environ. Manag. 2021, 291, 112697. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hosano, N.; Hosano, H. Recovering microalgal bioresources: A review of cell disruption methods and extraction technologies. Molecules 2022, 27, 2786. [Google Scholar] [CrossRef]
- Pantis, A.; Nikoloudakis, C.; Tsoutsos, T. A critical review of macroalgae exploitation pathways implemented under the scope of Life Cycle Assessment. Chemengineering 2024, 8, 74. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Qiu, H.-M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef]
- Nunes, N.; Leça, J.M.; Pereira, A.C.; Pereira, V.; Ferraz, S.; Barreto, M.C.; Marques, J.C.; de Carvalho, M.P. Evaluation of fucoxanthin contents in seaweed biomass by vortex-assisted solid-liquid microextraction using high-performance liquid chromatography with photodiode array detection. Algal Res. Biomass Biofuels Bioprod. 2019, 42, 101603. [Google Scholar] [CrossRef]
- Philippsen, A.; Wild, P.; Rowe, A. Energy input, carbon intensity and cost for ethanol produced from farmed seaweed. Renew. Sustain. Energy Rev. 2014, 38, 609–623. [Google Scholar] [CrossRef]
- Ceaser, R.; Bedzo, O.K.K.; Donkor, K.O. Biorefinery approach to producing polysaccharides from seaweed: A focus on hydrocolloids and nanocellulose. Biomass Convers. Biorefinery 2025. [Google Scholar] [CrossRef]
- Tedesco, S.; Barroso, T.M.; Olabi, A. Optimization of mechanical pre-treatment of Laminariaceae spp. biomass-derived biogas. Renew. Energy 2014, 62, 527–534. [Google Scholar] [CrossRef]
- Shah, F.A.; Mahmood, Q.; Rashid, N.; Pervez, A.; Raja, I.A.; Shah, M.M. Co-digestion, pretreatment and digester design for enhanced methanogenesis. Renew. Sustain. Energy Rev. 2015, 42, 627–642. [Google Scholar] [CrossRef]
- Manns, D.; Andersen, S.K.; Saake, B.; Meyer, A.S. Brown seaweed processing: Enzymatic saccharification of Laminaria digitata requires no pre-treatment. J. Appl. Phycol. 2016, 28, 1287–1294. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.S.; Um, B.H.; Oh, K. Pretreatment of Laminaria japonica for bioethanol production with extremely low acid concentration. Renew. Energy 2013, 54, 196–200. [Google Scholar] [CrossRef]
- Kerton, F.M.; Yan, N. Fuels, Chemicals and Materials from the Oceans and Aquatic Sources; JohnWiley & Sons Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Sappati, P.K.; Nayak, B.; van Walsum, G.P. Effect of glass transition on the shrinkage of sugar kelp (Saccharina latissima) during hot air convective drying. J. Food Eng. 2017, 210, 50–61. [Google Scholar] [CrossRef]
- Ringeisen, B.M.; Barrett, D.; Stroeve, P. Concentrated solar drying of tomatoes. Energy Sustain. Dev. 2014, 19, 47–55. [Google Scholar] [CrossRef]
- Murthy, M.V.R. A review of new technologies, models and experimental investigations of solar driers. Renew. Sustain. Energy Rev. 2009, 13, 835–844. [Google Scholar] [CrossRef]
- Pangan, R.; Ampo, M.V.; Barredo, Y. Design, development and evaluation of seaweed drying technology for village level operation. Philipp. J. Agric. Biosyst. Eng. 2021, 17, 13–25. [Google Scholar] [CrossRef]
- Gupta, S.; Cox, S.; Abu-Ghannam, N. Effect of different drying temperatures on the moisture and phytochemical constituents of edible Irish brown seaweed. LWT—Food Sci. Technol. 2011, 44, 1266–1272. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Jakubczyk, E. Effect of hot air temperature on mechanical properties of dried apples. J. Food Eng. 2004, 64, 307–314. [Google Scholar] [CrossRef]
- Santhoshkumar, P.; Yoha, K.; Moses, J. Drying of seaweed: Approaches, challenges and research needs. Trends Food Sci. Technol. 2023, 138, 153–163. [Google Scholar] [CrossRef]
- Thunyawanichnondh, J.; Suebsiri, N.; Leartamonchaikul, S.; Pimolsri, W.; Jittanit, W.; Charoensiddhi, S. Potential of green seaweed Ulva rigida in Thailand for healthy snacks. J. Fish. Environ. 2020, 44, 29–39. [Google Scholar]
- Silva, A.F.R.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Effect of oven-drying on the recovery of valuable compounds from Ulva rigida, Gracilaria sp. and Fucus vesiculosus. Mar. Drugs 2019, 17, 90. [Google Scholar] [CrossRef]
- Susanti, D.; Ruslan, F.S.; Shukor, M.I.; Nor, N.M.; Aminudin, N.I.; Taher, M.; Khotib, J. Optimisation of vitamin B12 extraction from green edible seaweed (Ulva lactuca) by applying the central composite design. Molecules 2022, 27, 4459. [Google Scholar] [CrossRef] [PubMed]
- Uribe, E.; Pardo-Orellana, C.M.; Vega-Gálvez, A.; Ah-Hen, K.S.; Pastén, A.; García, V.; Aubourg, S.P. Effect of drying methods on bioactive compounds, nutritional, antioxidant, and antidiabetic potential of brown algae Durvillaea antarctica. Dry. Technol. 2020, 38, 1915–1928. [Google Scholar] [CrossRef]
- Obluchinskaya, E.; Daurtseva, A. Effects of air drying and freezing and long-term storage on phytochemical composition of brown seaweeds. J. Appl. Phycol. 2020, 32, 4235–4249. [Google Scholar] [CrossRef]
- Firdayanti, L.; Yanti, R.; Rahayu, E.S.; Hidayat, C. Carrageenan extraction from red seaweed (Kappaphycopsis cottonii) using the bead mill method. Algal Res. 2023, 69, 102906. [Google Scholar] [CrossRef]
- Teo, H.L.; Wahab, R.A. Towards an eco-friendly deconstruction of agro-industrial biomass and preparation of renewable cellulose nanomaterials: A review. Int. J. Biol. Macromol. 2020, 161, 1414–1430. [Google Scholar] [CrossRef]
- Montalescot, V.; Rinaldi, T.; Touchard, R.; Jubeau, S.; Frappart, M.; Jaouen, P.; Bourseau, P.; Marchal, L. Optimization of bead milling parameters for the cell disruption of microalgae: Process modeling and application to Porphyridium cruentum and Nannochloropsis oculata. Bioresour. Technol. 2015, 196, 339–346. [Google Scholar] [CrossRef]
- Garcia, E.S.; Miranda, C.F.; Cesario, M.T.; Wijffels, R.H.; Berg, C.v.D.; Eppink, M.H.M. Ionic liquid-assisted selective extraction and partitioning of biomolecules from macroalgae. ACS Sustain. Chem. Eng. 2023, 11, 1752–1762. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van den Berg, C.; Eppink, M. Combined bead milling and enzymatic hydrolysis for efficient fractionation of lipids, proteins, and carbohydrates of Chlorella vulgaris microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef]
- Vanegas, C.; Hernon, A.; Bartlett, J. Influence of chemical, mechanical, and thermal pretreatment on the release of macromolecules from two Irish seaweed species. Sep. Sci. Technol. 2014, 49, 30–38. [Google Scholar] [CrossRef]
- Hjorth, M.; Gränitz, K.; Adamsen, A.P.; Møller, H.B. Extrusion as a pretreatment to increase biogas production. Bioresour. Technol. 2011, 102, 4989–4994. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Balboa, E.M.; Domínguez, H. Extraction and purification of fucoidan from marine sources. In Encyclopedia of Marine Biotechnology; Kim, S.K., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 1093–1125. [Google Scholar]
- Poole, C.F. New trends in solid-phase extraction. Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and innovative approaches for the extraction of bioactive compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Warmiński, K.; Stolarski, M.J.; Gil, Ł.; Krzyżaniak, M. Willow bark and wood as a source of bioactive compounds and bioenergy feedstock. Ind. Crops Prod. 2021, 171, 113976. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal proteins: Extraction, application, and challenges concerning production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Overview of neoteric solvents as extractants in food industry: A focus on phenolic compounds separation from liquid streams. Int. Food Res. J. 2020, 136, 109558. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef]
- Wang, Y.; Eilertsen, K.; Elvevoll, E.O.; Walquist, M.J. Assessing the efficiency of ethyl acetate for lipid extraction as an alternative to the Folch method in selected marine low-trophic species. J. Am. Oil Chem. Soc. 2025, 102, 871–883. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Cequier-Sánchez, E.; Rodríguez, C.; Ravelo, Á.G.; Zárate, R. Dichloromethane as a solvent for lipid extraction and assessment of lipid classes and fatty acids from samples of different natures. J. Agric. Food Chem. 2008, 56, 4297–4303. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.G.-B.; Herrero, M.; Señoráns, F.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M. Enzyme-assisted extraction of Nizamuddinia zanardinii for the recovery of sulfated polysaccharides with anticancer and immune-enhancing activities. J. Appl. Phycol. 2019, 31, 1391–1402. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Yang, H.; Li, H.; Xu, W.; Chen, G.; Zhu, H. Physicochemical characterization, antioxidant and immunostimulatory activities of sulfated polysaccharides extracted from Ascophyllum nodosum. Molecules 2018, 23, 1912. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimum conditions of microwave-assisted extraction for phenolic compounds and antioxidant capacity of the brown alga Sargassum vestitum. Sep. Sci. Technol. 2018, 53, 1711–1723. [Google Scholar] [CrossRef]
- Dinh, T.V.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Harb, T.B.; Pereira, M.S.; Cavalcanti, M.I.L.; Fujii, M.T.; Chow, F. Antioxidant activity and related chemical composition of extracts from Brazilian beach-cast marine algae: Opportunities of turning a waste into a resource. J. Appl. Phycol. 2021, 33, 3383–3395. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant activity and phenolic content of pressurised liquid and solid–liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound assisted extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Santos, F.; Soares, C.; Morais, S.L.; Neves, C.; Grosso, C.; Ramalhosa, M.J.; Vieira, M.; Delerue-Matos, C.; Domingues, V.F. Optimized extraction protocols for bioactive antioxidants from commercial seaweeds in Portugal: A comparative study of techniques. Foods 2025, 14, 453. [Google Scholar] [CrossRef]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of bioactive properties in brown algae from the Northwest Iberian Peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef]
- Singkoh, M.F.; Katili, D.Y.; Rumondor, M.J. Phytochemical screening and antibacterial activity of brown algae (Padina australis) from Atep Oki Coast, East Lembean of Minahasa Regency. Aquac. Aquar. Conserv. Legis. 2021, 14, 455–461. [Google Scholar]
- Sivagnanam, S.P.; Yin, S.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar. Drugs 2015, 13, 3422–3442. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Sunarwidhi, A.L.; Hernawan, A.; Frediansyah, A.; Widyastuti, S.; Martyasari, N.W.R.; Abidin, A.S.; Padmi, H.; Handayani, E.; Utami, N.W.P.; Maulana, F.A.; et al. Multivariate analysis revealed ultrasonic-assisted extraction improves anti-melanoma activity of non-flavonoid compounds in indonesian brown algae ethanol extract. Molecules 2022, 27, 7509. [Google Scholar] [CrossRef]
- Terme, N.; Boulho, R.; Kendel, M.; Kucma, J.-P.; Wielgosz-Collin, G.; Bourgougnon, N.; Bedoux, G. Selective extraction of lipid classes from Solieria chordalis and Sargassum muticum using supercritical carbon dioxide and conventional solid–liquid methods. J. Appl. Phycol. 2017, 29, 2513–2519. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’doherty, J.; O’donnell, C.; Rajauria, G. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar. Drugs 2020, 18, 250. [Google Scholar] [CrossRef]
- Al Monla, R.; Dassouki, Z.; Kouzayha, A.; Salma, Y.; Gali-Muhtasib, H.; Mawlawi, H. The cytotoxic and apoptotic effects of the brown algae Colpomenia sinuosa are mediated by the generation of reactive oxygen species. Molecules 2020, 25, 1993. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M. Microcrystalline cellulose from Posidonia oceanica brown algae: Extraction and characterization. Int. J. Biol. Macromol. 2019, 138, 837–845. [Google Scholar] [CrossRef]
- Praveen, M.A.; Parvathy, K.K.; Balasubramanian, P.; Jayabalan, R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci. Technol. 2019, 92, 46–64. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Rajauria, G.; O’Doherty, J.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99 Pt 3, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Jan, K.; Bashir, K. Extraction and characterization of bioactive compounds from different sources. In Bioactive Components—A Sustainable System for Good Health and Well-Being; Thakur, M., Belwal, T., Eds.; Springer Nature: Singapore, 2023; Chapter 8; pp. 121–141. [Google Scholar]
- Rioux, L.-E.; Turgeon, S.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ko, C.-I.; Ahn, G.; You, S.; Kim, J.-S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.-J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Dias, P.F.; Siqueira, J.M., Jr.; Maraschin, M.; Ferreira, A.G.; Gagliardi, A.R.; Ribeiro-do-Valle, R.M. A polysaccharide isolated from the brown seaweed Sargassum stenophyllum exerts antivasculogenic effects evidenced by modified morphogenesis. Microvasc. Res. 2008, 75, 34–44. [Google Scholar] [CrossRef]
- Saravana, P.S.; Cho, Y.-J.; Park, Y.-B.; Woo, H.-C.; Chun, B.-S. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef]
- Das, I.J.; Bal, T. Exploring carrageenan: From seaweed to biomedicine—A comprehensive review. Int. J. Biol. Macromol. 2024, 268 Pt 2, 131822. [Google Scholar] [CrossRef]
- Alves, A.; Caridade, S.G.; Mano, J.F.; Sousa, R.A.; Reis, R.L. Extraction and physico-chemical characterization of a versatile biodegradable polysaccharide obtained from green algae. Carbohydr. Res. 2010, 345, 2194–2200. [Google Scholar] [CrossRef]
- Hilliou, L.; Larotonda, F.; Abreu, P.; Ramos, A.; Sereno, A.; Gonçalves, M. Effect of extraction parameters on the chemical structure and gel properties of κ/ι-hybrid carrageenans obtained from Mastocarpus stellatus. Biomol. Eng. 2006, 23, 201–208. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to Seaweed Industry; FAO Fisheries and Aquaculture Department: Rome, Italy, 2003. [Google Scholar]
- Debbarma, J.; Viji, P.; Rao, B.M.; Ravishankar, C.N. Seaweeds: Potential applications of the aquatic vegetables to augment nutritional composition, texture, and health benefits of food and food products, Chapter 1. In Sustainable Global Resources of Seaweeds, Volume 2 (Food, Pharmaceutical and Health Applications); Rao, A.R., Ravishankar, G.A., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 3–54. [Google Scholar]
- Kılınç, B.; Cirik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for food and industrial applications. In Food Industry; Muzzalupo, I., Ed.; InTechOpen: London, UK, 2013; pp. 735–748. [Google Scholar]
- Abdul Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Fazita, M.R.N.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed hydrocolloid production: An update on enzyme assisted extraction and modification technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Murillo-Álvarez, J.I.; Muñoz-Ochoa, M.; Hernández-Carmona, G. Effect of alkali treatment time and extraction time on agar from Gracilaria vermiculophylla. J. Appl. Phycol. 2008, 20, 515–519. [Google Scholar] [CrossRef]
- Distantina, S.; Wiratni, W.; Fahrurrozi, M.; Rochmadi, R. Carrageenan properties extracted from Eucheuma cottonii, Indonesia. World Acad. Sci. Eng. Technol. 2011, 78, 738–742. [Google Scholar]
- Kumar, V.; Fotedar, R. Agar extraction process for Gracilaria cliftonii. Carbohydr. Polym. 2009, 78, 813–819. [Google Scholar] [CrossRef]
- Ummat, V.; Sivagnanam, S.P.; Rajauria, G.; O’Donnell, C.; Tiwari, B.K. Advances in pre-treatment techniques and green extraction technologies for bioactives from seaweeds. Trends Food Sci. Technol. 2021, 110, 90–106. [Google Scholar] [CrossRef]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. Novel technologies for seaweed polysaccharides extraction and their use in food with therapeutically applications—A Review. Foods 2022, 11, 2654. [Google Scholar] [CrossRef]
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, N.; Jalsa, N.K.; Lee, K.-Y.; Ward, K. Multistage extraction and purification of waste Sargassum natans to produce sodium alginate: An optimization approach. Carbohydr. Polym. 2018, 198, 109–118. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, G.; Zhao, G.; Hu, X. Optimization of microwave-assisted extraction of astaxanthin from Haematococcus pluvialis by response surface methodology and antioxidant activities of the extracts. Sep. Sci. Technol. 2009, 44, 243–262. [Google Scholar] [CrossRef]
- Dunford, N.T.; Irmak, S.; Jonnala, R. Pressurised solvent extraction of policosanol from wheat straw, germ and bran. Food Chem. 2010, 119, 1246–1249. [Google Scholar] [CrossRef]
- Li, B.; Smith, B.; Hossain, M. Extraction of phenolics from citrus peels: I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Zygler, A.; Słomińska, M.; Namieśnik, J. Soxhlet extraction and new developments such as Soxtec. In Comprehensive Sampling and Sample Preparation—Analytical Techniques for Scientists, Volume 2, Theory of Extraction Techniques; Pawliszyn, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 65–82. [Google Scholar]
- Gutiérrez, R.; Núñez, R.; Quintana, L.; Valdés, O.; González, K.; Rodríguez, M.; Hernández, Y.; Ortiz, E. Optimization of the extraction process of phenolic compounds from the brown algae Sargassum fluitans Børgesen (Børgesen). Biotecnol. Apl. 2017, 34, 3301–3304. [Google Scholar]
- Matos, G.S.; Pereira, S.G.; Genisheva, Z.A.; Gomes, A.M.; Teixeira, J.A.; Rocha, C.M.R. Advances in extraction methods to recover added-value compounds from seaweeds: Sustainability and functionality. Foods 2021, 10, 516. [Google Scholar] [CrossRef]
- Zollmann, M.; Robin, A.; Prabhu, M.; Polikovsky, M.; Gillis, A.; Greiserman, S.; Golberg, A. Green technology in green macroalgal biorefineries. Phycologia 2019, 58, 516–534. [Google Scholar] [CrossRef]
- European Commission. Communication from the European Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on a New Approach for Sustainable Blue Economy in the EU Transforming the EU’s Blue Economy for a Sustainable Future. COM(2021) 240, Final: Brussels, Belgium. 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52021DC0240 (accessed on 13 September 2025).
- Baghel, R.S.; Reddy, C.; Jha, B. Characterization of agarophytic seaweeds from the biorefinery context. Bioresour. Technol. 2014, 159, 280–285. [Google Scholar] [CrossRef]
- Nilsson, A.E.; Bergman, K.; Barrio, L.P.G.; Cabral, E.M.; Tiwari, B.K. Life cycle assessment of a seaweed-based biorefinery concept for production of food, materials, and energy. Algal Res. 2022, 65, 102725. [Google Scholar] [CrossRef]
- Nakhate, P.; van der Meer, Y. A systematic review on seaweed functionality: A sustainable bio-based material. Sustainability 2021, 13, 6174. [Google Scholar] [CrossRef]
- Jiang, R.; Ingle, K.N.; Golberg, A. Macroalgae (seaweed) for liquid transportation biofuel production: What is next? Algal Res. 2016, 14, 48–57. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Gadkari, S.; Martinez-Hernandez, E.; Ng, K.S.; Shemfe, M.; Torres-Garcia, E.; Lynch, J. Novel macroalgae (seaweed) biorefinery systems for integrated chemical, protein, salt, nutrient and mineral extractions and environmental protection by green synthesis and life cycle sustainability assessments. Green Chem. 2019, 21, 2635–2655. [Google Scholar] [CrossRef]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Meireles, F.; Abreu, H.T.; Ribeiro-Claro, P.J. A comparative analysis of carrageenans produced by underutilized versus industrially utilized macroalgae (Gigartinales Rhodophyta). In Marine Algae Extracts Processes Products and Applications; Kim, S.-K., Chojnacka, K., Eds.; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, Germany, 2015; pp. 277–294. [Google Scholar]
- Sudhakar, M.; Merlyn, R.; Arunkumar, K.; Perumal, K. Characterization, pretreatment and saccharification of spent seaweed biomass for bioethanol production using baker’s yeast. Biomass Bioenergy 2016, 90, 148–154. [Google Scholar] [CrossRef]
- Baghel, R.S.; Trivedi, N.; Gupta, V.; Neori, A.; Reddy, C.R.K.; Lali, A.; Jha, B. Biorefining of marine macroalgal biomass for production of biofuel and commodity chemicals. Green Chem. 2015, 17, 2436–2443. [Google Scholar] [CrossRef]
- Baghel, R.S.; Trivedi, N.; Reddy, C. A simple process for recovery of a stream of products from marine macroalgal biomass. Bioresour. Technol. 2016, 203, 160–165. [Google Scholar] [CrossRef]
- Ingle, K.; Vitkin, E.; Robin, A.; Yakhini, Z.; Mishori, D.; Golberg, A. Macroalgae biorefinery from Kappaphycus alvarezii: Conversion modeling and performance prediction for India and Philippines as examples. BioEnergy Res. 2018, 11, 22–32. [Google Scholar] [CrossRef]
- Shanmugam, M.; Seth, A. Recovery ratio and quality of an agricultural bio-stimulant and semi-refined carrageenan co-produced from the fresh biomass of Kappaphycus alvarezii with respect to seasonality. Algal Res. 2018, 32, 362–371. [Google Scholar] [CrossRef]
- El-Gendy, N.S.; Nassar, H.N.; Ismail, A.R.; Ali, H.R.; Ali, B.A.; Abdelsalam, K.M.; Mubarak, M. Fully integrated biorefinery process for the valorization of Ulva fasciata into different green and sustainable value-added products. Sustainability 2023, 15, 7319. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Lens, P.N.L. Sustainable biorefining and bioprocessing of green seaweed (Ulva spp.) for the production of edible (ulvan) and non-edible (polyhydroxyalkanoate) biopolymeric films. Microb. Cell Factories 2023, 22, 140. [Google Scholar] [CrossRef]
- Polikovsky, M.; Gillis, A.; Steinbruch, E.; Robin, A.; Epstein, M.; Kribus, A.; Golberg, A. Biorefinery for the co-production of protein, hydrochar and additional co-products from a green seaweed Ulva sp. with subcritical water hydrolysis. Energy Convers. Manag. 2020, 225, 113380. [Google Scholar] [CrossRef]
- Paone, E.; Mauriello, F. From bio-based furanics to biodegradable plastics. Chem 2022, 8, 897–899. [Google Scholar] [CrossRef]
- Mo’o, F.R.C.; Wilar, G.; Devkota, H.P.; Wathoni, N. Ulvan, a polysaccharide from macroalga Ulva sp.: A review of chemistry, biological activities and potential for food and biomedical applications. Appl. Sci. 2020, 10, 5488. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel bioactive compounds from marine sources as a tool for functional food development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- Silva, A.; Cassani, L.; Grosso, C.; Garcia-Oliveira, P.; Morais, S.L.; Echave, J.; Carpena, M.; Xiao, J.; Barroso, M.F.; Simal-Gandara, J.; et al. Recent advances in biological properties of brown algae-derived compounds for nutraceutical applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 1283–1311. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.E.; Su, P.; Puri, M.; Raston, C.L.; Zhang, W. Optimisation of biorefinery production of alginate, fucoidan and laminar infrom brown seaweed Durvillaea potatorum. Algal Res. 2019, 38, 101389. [Google Scholar] [CrossRef]
- Birgersson, P.S.; Oftebro, M.; Strand, W.I.; Aarstad, O.A.; Sætrom, G.I.; Sletta, H.; Arlov, Ø.; Aachmann, F.L. Sequential extraction and fractionation of four polysaccharides from cultivated brown algae Saccharina latissima and Alaria esculenta. Algal Res. 2023, 69, 102928. [Google Scholar] [CrossRef]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C. Seaweed biorefinery: A sustainable process for valorising the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Han, Y.; Tan, X.; Fitzpatrick, S.; Lyons, H.; Zhu, X.; Tiwari, B.K. Impact of pre-treatment strategies for enhance conversion of Irish brown seaweed into high value ingredients using a biorefinery approach. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Kostas, E.T.; White, D.A.; Cook, D.J. Development of a bio-refinery process for the production of speciality chemical, biofuel and bioactive compounds from Laminaria digitata. Algal Res. 2017, 28, 211–219. [Google Scholar] [CrossRef]
- Sasaki, C.; Tamura, S.; Suzuki, M.; Etomi, K.; Nii, N.; Hayashi, J.; Kanemaru, K. Continuous microwave-assisted step-by-step extraction of bioactive water-soluble materials and fucoidan from brown seaweed Undaria pinnatifida waste. Biomass Convers. Biorefinery 2024, 14, 7673–7682. [Google Scholar] [CrossRef]
- Sequeira, R.A.; Pereira, M.M.; Vaghela, P.; Bhayani, A.; Dhimmar, A.; Maru, D.; Ghosh, A.; Dineshkumar, R.; Shinde, P.B.; Ray, S.; et al. Sustainable production of quaternary ammonium seaweed polysaccharide salts and their evaluation for seed dressing in agricultural applications. ACS Agric. Sci. Technol. 2021, 1, 674–683. [Google Scholar] [CrossRef]
- Ummat, V.; Sivagnanam, S.P.; Rameshkumar, S.; Pednekar, M.; Fitzpatrick, S.; Rai, D.K.; Padamati, R.B.; O’DOnnell, C.; Tiwari, B.K. Sequential extraction of fucoidan, laminarin, mannitol, alginate and protein from brown macroalgae Ascophyllum nodosum and Fucus vesiculosus. Int. J. Biol. Macromol. 2024, 256, 128195. [Google Scholar] [CrossRef]
- Zhu, X.; Healy, L.; Das, R.S.; Bhavya, M.; Karuppusamy, S.; Sun, D.-W.; O’DOnnell, C.; Tiwari, B.K. Novel biorefinery process for extraction of laminarin, alginate and protein from brown seaweed using hydrodynamic cavitation. Algal Res. 2023, 74, 103243. [Google Scholar] [CrossRef]
- Hjelland, F.; Andersen, A.H.; Yang, H.S. Process for Isolating Fucoidan and Laminarin from Live, Harvested Seaweed. U.S. Patents US20120302742A1, 17 March 2020. [Google Scholar]
- Bikker, P.; Blaauw, R.; Brandenburg, W.; van den Burg, S.; Dijkstra, W.; van Duren, L.; van Hal, J.; Harmsen, P.; Huijgen, W.; Jak, R.; et al. North-Sea-Weed-Chain Sustainable Seaweed from the North Sea; An Exploration of the Value Chain; Groenendijk, F., Ed.; IMARES Report C055/16; Wageningen Marine Research: Wageningen, The Netherlands, 2016. [Google Scholar]
- Juul, L.; Nissen, S.H.; Bruhn, A.; Alexi, N.; Jensen, S.K.; Hammershøj, M.; Dalsgaard, T.K. Ulva species: A critical review on the green seaweed as a source of food protein. Trends Food Sci. Technol. 2024, 149, 104534. [Google Scholar] [CrossRef]
- Juul, L.; Danielsen, M.; Nebel, C.; Steinhagen, S.; Bruhn, A.; Jensen, S.; Undeland, I.; Dalsgaard, T. Ulva fenestrata protein—Comparison of three extraction methods with respect to protein yield and protein quality. Algal Res. 2021, 60, 102496. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; CRC Press: London, UK, 2008. [Google Scholar]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.-G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) JV Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef]
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Gajaria, T.K.; Suthar, P.; Baghel, R.S.; Balar, N.B.; Sharnagat, P.; Mantri, V.A.; Reddy, C. Integration of protein extraction with a stream of byproducts from marine macroalgae: A model forms the basis for marine bioeconomy. Bioresour. Technol. 2017, 243, 867–873. [Google Scholar] [CrossRef]
- Magnusson, M.; Glasson, C.R.; Vucko, M.J.; Angell, A.; Neoh, T.L.; de Nys, R. Enrichment processes for the production of high-protein feed from the green seaweed Ulva ohnoi. Algal Res. 2019, 41, 101555. [Google Scholar] [CrossRef]
- Harrysson, H.; Konasani, V.R.; Toth, G.B.; Pavia, H.; Albers, E.; Undeland, I. Strategies for improving the protein yield in pH-shift processing of Ulva lactuca Linnaeus: Effects of ulvan lyases, pH-exposure time, and temperature. ACS Sustain. Chem. Eng. 2019, 7, 12688–12691. [Google Scholar] [CrossRef]
- Trigo, J.P.; Engström, N.; Steinhagen, S.; Juul, L.; Harrysson, H.; Toth, G.B.; Pavia, H.; Scheers, N.; Undeland, I. In vitro digestibility and Caco-2 cell bioavailability of sea lettuce (Ulva fenestrata) proteins extracted using pH-shift processing. Food Chem. 2021, 356, 129683. [Google Scholar] [CrossRef]
- Postma, P.R.; Cerezo-Chinarro, O.; Akkerman, R.J.; Olivieri, G.; Wijffels, R.H.; Brandenburg, W.A.; Eppink, M.H.M. Biorefinery of the macroalgae Ulva lactuca: Extraction of proteins and carbohydrates by mild disintegration. J. Appl. Phycol. 2018, 30, 1281–1293. [Google Scholar] [CrossRef]
- Juel, N.; Juul, L.; Tanambell, H.; Dalsgaard, T.K. Extraction and purification of seaweed protein from Ulva sp.—Challenges to overcome. LWT 2024, 198, 115944. [Google Scholar] [CrossRef]
- Boakye, P.; Sewu, D.; Woo, H.C.; Choi, J.H.; Lee, C.W.; Woo, S.H. Extraction of inorganic materials from fresh and dried alga Saccharina japonica. J. Environ. Chem. Eng. 2017, 5, 4454–4461. [Google Scholar] [CrossRef]
- Yanik, J.; Stahl, R.; Troeger, N.; Sinag, A. Pyrolysis of algal biomass. J. Anal. Appl. Pyrolysis 2013, 103, 134–141. [Google Scholar] [CrossRef]
- Ali, Z.; Subeshan, B.; Alam, M.A.; Asmatulu, E.; Xu, J. Recent progress in extraction/transesterification techniques for the recovery of oil from algae biomass. Biomass Convers. Biorefinery 2023, 13, 2553–2569. [Google Scholar] [CrossRef]
- Lajoie, L.; Fabiano-Tixier, A.S.; Chemat, F. Water as green solvent: Methods of solubilisation and extraction of natural products—Past, present and future solutions. Pharmaceuticals 2022, 15, 1507. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green extraction techniques of bioactive compounds: A state-of-the-art review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef]
- Bouizgma, K.; Rabbah, N.; Abbas, Z.; Abourriche, A. Unlocking sustainable extraction of natural antioxidants: Green solvents, smart technologies, scalability and future directions. Sep. Sci. Technol. 2025, 60, 657–683. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Garrigós, M.C. Il-based advanced techniques for the extraction of value-added compounds from natural sources and food by-products. TrAC Trends Anal. Chem. 2019, 119, 115616. [Google Scholar] [CrossRef]
- Yogesh, R.; Srivastava, N.; Mulik, B.M. Efforts to replace methylene chloride in pharmaceutical process chemistry. Macromol. Symp. 2023, 407, 2100502. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Allcca-Alca, E.E.; Nina, F.H.; León-Calvo, N.C.; Vilca, F.Z.; Chura, Y.L.V. Pressurized liquid extraction of antioxidant and α-amylase-inhibitory compounds from red seaweed using water–ethanol mixtures. Molecules 2024, 29, 5018. [Google Scholar] [CrossRef]
- Nunes, N.; Valente, S.; Ferraz, S.; Barreto, M.C.; de Carvalho, M.P. Constructing ethanol-derived bioactive extracts using the brown seaweed Zonaria tournefortii (J.V.Lamouroux) Montagne performed with Timatic extractor by means of response surface methodology (RSM). J. Appl. Phycol. 2020, 32, 2321–2333. [Google Scholar] [CrossRef]
- Hamiche, S.; Bouzidi, N.; Daghbouche, Y.; Badis, A.; Garrigues, S.; de la Guardia, M.; El Hattab, M. Eucalyptol-based green extraction of brown alga Zonaria tournefortii. Sustain. Chem. Pharm. 2018, 10, 97–102. [Google Scholar] [CrossRef]
- Savira, A.D.R.; Amin, M.N.G.; Alamsjah, M.A. The effect of different type of solvents on the antioxidant activity of fucoxanthin extract from brown seaweed Sargassum duplicatum. IOP Conf. Ser. Earth Env. Sci. 2021, 718, 012010. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Afraz, M.T.; Yılmaz, B.B.; Adil, M.; Arshad, N.; Goksen, G.; Ali, M.; Zeng, X.-A. Recent progress in natural seaweed pigments: Green extraction, health-promoting activities, techno-functional properties and role in intelligent food packaging. J. Agric. Food Res. 2024, 15, 100991. [Google Scholar] [CrossRef]
- Ktari, L.; Mdallel, C.; Aoun, B.; Ajjabi, L.C.; Sadok, S. Fucoxanthin and phenolic contents of six Dictyotales from the Tunisian Coasts with an emphasis for a green extraction using a supercritical CO2 method. Front. Mar. Sci. 2021, 8, 647159. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.; FitzGerald, R.J.; Vila-Soler, A.; Mendiola, J.; Ibáñez, E.; Brunton, N. Comparison of extraction methods for selected carotenoids from macroalgae and the assessment of their seasonal/spatial variation. Innov. Food Sci. Emerg. Technol. 2016, 37, 221–228. [Google Scholar] [CrossRef]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycol. Res. 2017, 66, 52–57. [Google Scholar] [CrossRef]
- Malihan, L.B.; Mittal, N.; Nisola, G.M.; Weldemhret, T.G.; Kim, H.; Chung, W. Macroalgal biomass hydrolysis using dicationic acidic ionic liquids. J. Chem. Technol. Biotechnol. 2017, 92, 1290–1297. [Google Scholar] [CrossRef]
- Martins, M.; Vieira, F.A.; Correia, I.; Ferreira, R.A.S.; Abreu, H.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of phycobiliproteins from the red macroalga: Gracilaria sp. using ionic liquid aqueous solutions. Green Chem. 2016, 18, 4287–4296. [Google Scholar] [CrossRef]
- Martins, M.; Fernandes, A.P.; Torres-Acosta, M.A.; Collén, P.N.; Abreu, M.H.; Ventura, S.P. Extraction of chlorophyll from wild and farmed Ulva spp. using aqueous solutions of ionic liquids. Sep. Purif. Technol. 2021, 254, 117589. [Google Scholar] [CrossRef]
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.S.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.-S. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 2018, 196, 309–317. [Google Scholar] [CrossRef]
- Moradiya, K.; Pereira, M.M.; Prasad, K. Aqueous solution of ionic liquid is an efficient substituting solvent system for the extraction of alginate from Sargassum tenerrimum. Sustain. Chem. 2024, 5, 116–129. [Google Scholar] [CrossRef]
- Das, A.K.; Sharma, M.; Mondal, D.; Prasad, K. Deep eutectic solvents as efficient solvent system for the extraction of κ-carrageenan from Kappaphycus alvarezii. Carbohydr. Polym. 2016, 136, 930–935. [Google Scholar] [CrossRef]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Santos, J.M.; Jesus, B.C.; Ribeiro, H.; Martins, A.; Marto, J.; Fitas, M.; Pinto, P.; Alves, C.; Silva, J.; Pedrosa, R.; et al. Extraction of macroalgae phenolic compounds for cosmetic application using eutectic solvents. Algal Res. 2024, 79, 103438. [Google Scholar] [CrossRef]
- van Breda, D.; Lufu, R.; Goosen, N.J. Optimisation of cellulase--assisted extraction of laminarin from the brown seaweed Ecklonia maxima, using response surface methodology. Biomass Convers. Biorefinery 2023, 13, 10399–10412. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Mai, N.L.; Ahn, K.; Koo, Y.-M. Methods for recovery of ionic liquids—A review. Process. Biochem. 2014, 49, 872–881. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Zhu, X.; Healy, L.; Zhang, Z.; Maguire, J.; Sun, D.-W.; Tiwari, B.K. Novel postharvest processing strategies for value-added applications of marine algae. J. Sci. Food Agric. 2021, 101, 4444–4455. [Google Scholar] [CrossRef]
- Hamamouche, K.; Elhadj, Z.; Khattabi, L.; Zahnit, W.; Djemoui, B.; Kharoubi, O.; Boussebaa, W.; Bouderballa, M.; Kallouche, M.E.M.; Attia, S.M.; et al. Impact of ultrasound- and microwave-assisted extraction on bioactive compounds and biological activities of Jania rubens and Sargassum muticum. Mar. Drugs 2024, 22, 530. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviðsson, G.O.; Karlsson, E.N. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Krishnan, L.; Ravi, N.; Mondal, A.K.; Akter, F.; Kumar, M.; Ralph, P.; Kuzhiumparambil, U. Seaweed-based polysaccharides—Review of extraction, characterization, and bioplastic application. Green Chem. 2024, 26, 5790–5823. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 1901–1929. [Google Scholar] [CrossRef]
- Thanh, T.T.T.; Ngo, Q.V.; Nguyen, T.T.; Nguyen, A.N.; Quach, T.T.M.; Dang, L.V.; Nguyen, T.Q.; Do, X.T.T. Ulvan from green seaweed Ulva lactuca: Optimization of ultrasound-assisted extraction, structure, and cytotoxic activity. J. Carbohydr. Chem. 2023, 42, 92–111. [Google Scholar] [CrossRef]
- Touhamia, Y.; Aamiri, A.; Baghel, R.S.; Bellahcen, T.O. Exploring sulfated polysaccharides from seaweeds: An extensive review on structures, extraction and biorefining processes, applications, and biological functions. SSRN 2023. [Google Scholar] [CrossRef]
- Wassie, T.; Niu, K.; Xie, C.; Wang, H.; Xin, W. Extraction techniques, biological activities and health benefits of marine algae Enteromorpha prolifera polysaccharide. Front. Nutr. 2021, 8, 747928. [Google Scholar] [CrossRef]
- André, J.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Microwave-assisted extraction of Ulva spp. including a stage of selective coagulation of ulvan stimulated by a bio-ionic liquid. Int. J. Biol. Macromol. 2023, 225, 952–963. [Google Scholar] [CrossRef]
- Dhaouafi, J.; Nedjar, N.; Jridi, M.; Romdhani, M.; Balti, R. Extraction of protein and bioactive compounds from Mediterranean red algae (Sphaerococcus coronopifolius and Gelidium spinosum) using various innovative pretreatment strategies. Foods 2024, 13, 1362. [Google Scholar] [CrossRef]
- Bāliņa, K.; Ivanovs, K.; Romagnoli, F.; Blumberga, D. Comprehensive literature review on valuable compounds and extraction technologies: The Eastern Baltic Sea seaweeds. Environ. Clim. Technol. 2020, 24, 178–195. [Google Scholar] [CrossRef]
- Kazemi, M.; Fathi, M.; Jahanbin, K.; Taghdir, M.; Abbaszadeh, S. Optimization of ultrasonic-assisted hot acidic solvent extraction of ulvan from Ulva intestinalis of the Persian Gulf: Evaluation of structural, techno-functional, and bioactivity properties. Food Hydrocoll. 2023, 142, 108837. [Google Scholar] [CrossRef]
- Belhadj, R.N.A.; Mellinas, C.; Jiménez, A.; Bordehore, C.; Garrigós, M.C. Invasive seaweed Rugulopteryx okamurae: A potential source of bioactive compounds with antioxidant activity. Antioxidants 2024, 13, 1298. [Google Scholar] [CrossRef]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujar, P.C. Ultrasound assisted extraction of selected edible macroalgae: Effect on antioxidant activity and quantitative assessment of polyphenols by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Algal Res. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Lee, Z.J.; Xie, C.; Duan, X.; Ng, K.; Suleria, H.A.R. Optimization of ultrasonic extraction parameters for the recovery of phenolic compounds in brown seaweed: Comparison with conventional techniques. Antioxidants 2024, 13, 409. [Google Scholar] [CrossRef]
- Teixeira-Guedes, C.; Gomes-Dias, J.S.; Cunha, S.A.; Pintado, M.E.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M. Enzymatic approach for the extraction of bioactive fractions from red, green and brown seaweeds. Food Bioprod. Process. 2023, 138, 25–39. [Google Scholar] [CrossRef]
- Rodríguez-González, I.; Díaz-Reinoso, B.; Domínguez, H. Intensification strategies for the extraction of polyunsaturated fatty acids and other lipophilic fractions from seaweeds. Food Bioprocess Technol. 2022, 15, 978–997. [Google Scholar] [CrossRef]
- Kostas, E.T.; Adams, J.M.; Ruiz, H.A.; Durán-Jiménez, G.; Lye, G.J. Macroalgal biorefinery concepts for the circular bioeconomy: A review on biotechnological developments and future perspectives. Renew. Sustain. Energy Rev. 2021, 151, 111553. [Google Scholar] [CrossRef]
- Yong, W.T.L.; Thien, V.Y.; Misson, M.; Chin, G.J.W.L.; Hussin, S.N.I.S.; Chong, H.L.H.; Yusof, N.A.; Ma, N.L.; Rodrigues, K.F. Seaweed: A bioindustrial game-changer for the green revolution. Biomass Bioenergy 2024, 183, 107122. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Pantoa, T.; Roytrakul, S.; Praiboon, J.; Kosawatpat, P.; Tamtin, M.; Thongdang, B. Ultrasonic-assisted extraction and antioxidant potential of valuable protein from Ulva rigida macroalgae. Life 2023, 13, 86. [Google Scholar] [CrossRef]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of microwave-assisted extraction of polysaccharides from Ulva pertusa and evaluation of their antioxidant activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef]
- Tirado-Kulieva, V.A.; Sánchez-Chero, M.; Yarlequé, M.V.; Aguilar, G.F.V.; Carrión-Barco, G.; Cruz, A.G.Y.S.; Sánchez-Chero, J. An overview on the use of Response Surface Methodology to model and optimize extraction processes in the food industry. Curr. Res. Nutr. Food Sci. J. 2021, 9, 745–754. [Google Scholar] [CrossRef]
- Pappou, S.; Dardavila, M.M.; Savvidou, M.G.; Louli, V.; Magoulas, K.; Voutsas, E. Extraction of bioactive compounds from Ulva lactuca. Appl. Sci. 2022, 12, 2117. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.S.; Donnay-Moreno, C.; Bergé, J.P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Michel, G.; Czjzek, M. Polysaccharide-degrading enzymes from marine bacteria. In Marine Enzymes for Biocatalysis; Trincone, A., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 429–464. [Google Scholar]
- Hung, Y.-H.R.; Chen, G.-W.; Pan, C.-L.; Lin, H.-T.V. Production of ulvan oligosaccharides with antioxidant and angiotensin-converting enzyme-inhibitory activities by microbial enzymatic hydrolysis. Fermentation 2021, 7, 160. [Google Scholar] [CrossRef]
- Malvis Romero, A.; Picado Morales, J.J.; Klose, L.; Liese, A. Enzyme-Assisted Extraction of ulvan from the green macroalgae Ulva fenestrata. Molecules 2023, 28, 6781. [Google Scholar] [CrossRef] [PubMed]
- Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M.; Gomaa, M. Upgrading the antioxidant properties of fucoidan and alginate from Cystoseira trinodis by fungal fermentation or enzymatic pretreatment of the seaweed biomass. Food Chem. 2018, 269, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Oktarina, N.; Chen, P.-T.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Fermentative lactic acid production from seaweed hydrolysate using Lactobacillus sp. and Weissella sp. Bioresour. Technol. 2022, 344 Pt A, 126166. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring ultrasound, microwave and ultrasound-microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef]
- Toan, T.Q.; Phong, T.D.; Tien, D.D.; Linh, N.M.; Anh, N.T.M.; Minh, P.T.H.; Nhut, P.T. Optimization of microwave-assisted extraction of phlorotannin from Sargassum swartzii (Turn.) C. Ag. with ethanol/water. Nat. Prod. Commun. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Torabi, P.; Hamdami, N.; Keramat, J. Microwave-assisted extraction of sodium alginate from brown macroalgae Nizimuddinia zanardinii, optimization and physicochemical properties. Sep. Sci. Technol. 2022, 57, 872–885. [Google Scholar] [CrossRef]
- Tsubaki, S.; Oono, K.; Hiraoka, M.; Onda, A.; Mitani, T. Microwave-assisted hydrothermal extraction of sulfated polysaccharides from Ulva spp. and Monostroma latissimum. Food Chem. 2016, 210, 311–316. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D.J. Microwave assisted acid hydrolysis of brown seaweed Ascophyllum nodosum for bioethanol production and characterization of alga residue. ACS Sustain. Chem. Eng. 2015, 3, 1359–1365. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef]
- Zayed, A.; Finkelmeier, D.; Hahn, T.; Rebers, L.; Shanmugam, A.; Burger-Kentischer, A.; Ulber, R. Characterization and cytotoxic activity of microwave-assisted extracted crude fucoidans from different brown seaweeds. Mar. Drugs 2023, 21, 48. [Google Scholar] [CrossRef]
- Li, G.-Y.; Luo, Z.-C.; Yuan, F.; Yu, X.-B. Combined process of high-pressure homogenization and hydrothermal extraction for the extraction of fucoidan with good antioxidant properties from Nemacystus decipiens. Food Bioprod. Process. 2017, 106, 35–42. [Google Scholar] [CrossRef]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Supercritical carbon dioxide extraction of fucoxanthin from Undaria pinnatifida. J. Agric. Food Chem. 2013, 61, 5792–5797. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Getachew, A.T.; Cho, Y.-J.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J. Supercrit. Fluids 2017, 120, 295–303. [Google Scholar] [CrossRef]
- Yin, S.; Niu, L.; Shibata, M.; Liu, Y.; Hagiwara, T. Optimization of fucoxanthin extraction obtained from natural by-products from Undaria pinnatifida stem using supercritical CO2 extraction method. Front. Nutr. 2022, 9, 981176. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; You, S.; Mariatti, F.; Cravotto, G. Subcritical water extraction as an efficient technique to isolate biologically-active fucoidans from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 128, 244–253. [Google Scholar] [CrossRef]
- Bordoloi, A.; Goosen, N.J. A greener alternative using subcritical water extraction to valorize the brown macroalgae Ecklonia maxima for bioactive compounds. J. Appl. Phycol. 2020, 32, 2307–2319. [Google Scholar] [CrossRef]
- Gan, A.; Baroutian, S. Subcritical water extraction for recovery of phenolics and fucoidan from New Zealand Wakame (Undaria pinnatifida) seaweed. J. Supercrit. Fluids 2022, 190, 105732. [Google Scholar] [CrossRef]
- Saravana, P.S.; Tilahun, A.; Gerenew, C.; Tri, V.D.; Kim, N.H.; Kim, G.-D.; Woo, H.-C.; Chun, B.-S. Subcritical water extraction of fucoidan from Saccharina japonica: Optimization, characterization and biological studies. J. Appl. Phycol. 2018, 30, 579–590. [Google Scholar] [CrossRef]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Hellencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef]
- Casas, M.P.; Conde, E.; Domínguez, H.; Moure, A. Ecofriendly extraction of bioactive fractions from Sargassum muticum. Process. Biochem. 2019, 79, 166–173. [Google Scholar] [CrossRef]
- Robin, A.; Kazir, M.; Sack, M.; Israel, A.; Frey, W.; Mueller, G.; Livney, Y.D.; Golberg, A. Functional protein concentrates extracted from the green marine macroalga Ulva sp., by high voltage pulsed electric fields and mechanical press. ACS Sustain. Chem. Eng. 2018, 6, 13696–13705. [Google Scholar] [CrossRef]
- Kashyap, M.; Ghosh, S.; Levkov, K.; Livney, Y.D.; Israel, Á.; Golberg, A. High-voltage pulsed electric fields and pH shift process for protein and solute release from Gracilaria sp., red edible seaweed. Food Bioprocess Technol. 2024, 17, 5273–5284. [Google Scholar] [CrossRef]
- Vilge, V.J.; Undeland, I. pH-driven solubilization and isoelectric precipitation of proteins from the brown seaweed Saccharina latissima —Effects of osmotic shock, water volume and temperature. J. Appl. Phycol. 2017, 29, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Fernández, N.; López-García, M.; González-Muñoz, M.J.; Vilariño, J.M.L.; Domínguez, H. Ultrasound-assisted extraction of fucoidan from Sargassum muticum. J. Appl. Phycol. 2017, 29, 1553–1561. [Google Scholar] [CrossRef]
- Leonelli, C.; Veronesi, P.; Cravotto, G. Microwave-assisted extraction: An introduction to dielectric heating. In Microwave-Assisted Extraction for Bioactive Compounds; Chemat, F., Cravotto, G., Eds.; Food Engineering Series; Springer: Boston, MA, USA, 2012. [Google Scholar]
- Dobrinčić, A.; Balbino, S.; Zorić, Z.; Pedisić, S.; Kovačević, D.B.; Garofulić, I.E.; Dragović-Uzelac, V. Advanced technologies for the extraction of marine brown algal polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef]
- Cao, C.; Huang, Q.; Zhang, B.; Li, C.; Fu, X. Physicochemical characterization and in vitro hypoglycemic activities of polysaccharides from Sargassum pallidum by microwave-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018, 109, 357–368. [Google Scholar] [CrossRef]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. The comparative influence of novel extraction technologies on in vitro prebiotic-inducing chemical properties of fucoidan extracts from Ascophyllum nodosum. Food Hydrocoll. 2019, 90, 462–471. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Shang, X.-C.; Chu, D.; Zhang, J.-X.; Zheng, Y.-F.; Li, Y. Microwave-assisted extraction, partial purification and biological activity in vitro of polysaccharides from bladder-wrack (Fucus vesiculosus) by using deep eutectic solvents. Sep. Purif. Technol. 2021, 259, 118169. [Google Scholar] [CrossRef]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Remya, R.R.; Samrot, A.V.; Kumar, S.S.; Mohanavel, V.; Karthick, A.; Chinnaiyan, V.K.; Umapathy, D.; Muhibbullah, M.; Nassar, A. Bioactive potential of brown algae. Adsorpt. Sci. Technol. 2022, 2022, 9104835. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of Ultrasound-Assisted Extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.; Simal-Gandara, J. Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Meullemiestre, A.; Breil, C.; Abert-Vian, M.; Chemat, F. Microwave, ultrasound, thermal treatments, and bead milling as intensification techniques for extraction of lipids from oleaginous Yarrowia lipolytica yeast for a biojetfuel application. Bioresour. Technol. 2016, 211, 190–199. [Google Scholar] [CrossRef]
- Wang, S.-H.; Chen, C.-Y.; Chang, C.-C.; Huang, C.-Y.; Dong, C.-D.; Chang, J.-S. Isolation and purification of brown algae fucoidan from Sargassum siliquosum and the analysis of anti-lipogenesis activity. Biochem. Eng. J. 2021, 165, 107798. [Google Scholar] [CrossRef]
- Brain-Isasi, S.; Correa, S.; Amado-Hinojosa, J.; Buschmann, A.H.; Camus, C.; Lienqueo, M.E. Combined extraction methodology for simultaneous recovery of phycobiliproteins and agar from the red alga Gracilaria chilensis CJ Bird, McLachlan & EC Oliveira. Algal. Res. 2022, 67, 102821. [Google Scholar]
- Hmelkov, A.B.; Zvyagintseva, T.N.; Shevchenko, N.M.; Rasin, A.B.; Ermakova, S.P. Ultrasound-assisted extraction of polysaccharides from brown alga Fucus evanescens. Structure and biological activity of the new fucoidan fractions. J. Appl. Phycol. 2018, 30, 2039–2046. [Google Scholar] [CrossRef]
- Rahimi, F.; Tabarsa, M.; Rezaei, M. Ulvan from green algae Ulva intestinalis: Optimization of ultrasound-assisted extraction and antioxidant activity. J. Appl. Phycol. 2016, 28, 2979–2990. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Q.; Di, X.; Li, S.; Barba, F.J.; Koubaa, M.; Roohinejad, S.; Xiong, X.; He, J. Multistage recovery process of seaweed pigments: Investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod. Process. 2017, 104, 40–47. [Google Scholar] [CrossRef]
- Nie, J.; Chen, D.; Ye, J.; Lu, Y.; Dai, Z. Optimization and kinetic modeling of ultrasonic-assisted extraction of fucoxanthin from edible brown algae Sargassum fusiforme using green solvents. Ultrason. Sonochem. 2021, 77, 105671. [Google Scholar] [CrossRef] [PubMed]
- La, H.-J.; Choi, G.-G.; Cho, C.; Seo, S.-H.; Srivastava, A.; Jo, B.-H.; Lee, J.-Y.; Jin, Y.-S.; Oh, H.-M. Increased lipid productivity of Acutodesmus dimorphus using optimized pulsed electric field. J. Appl. Phycol. 2016, 28, 931–938. [Google Scholar] [CrossRef]
- Zayed, A.; El-Seadawy, H.M.; Attia, E.Z.; Rushdi, M.I.; Abdelmohsen, U.R. Adopting biorefinery and a circular bioeconomy for extracting and isolating natural products from marine algae. Front. Nat. Prod. 2024, 3, 1425242. [Google Scholar] [CrossRef]
- Robin, A.; Golberg, A. Pulsed electric fields and electroporation technologies in marine macroalgae biorefineries. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer: Cham, Switzerland, 2016; pp. 1–16. [Google Scholar]
- Polikovsky, M.; Fernand, F.; Sack, M.; Frey, W.; Müller, G.; Golberg, A. Towards marine biorefineries: Selective proteins extractions from marine macroalgae Ulva with pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2016, 37, 194–200. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field permeabilization and extraction of phycoerythrin from Porphyridium cruentum. Algal Res. 2019, 37, 51–56. [Google Scholar] [CrossRef]
- Castejón, N.; Thorarinsdottir, K.A.; Einarsdóttir, R.; Kristbergsson, K.; Marteinsdóttir, G. Exploring the potential of Icelandic seaweeds extracts produced by aqueous pulsed electric fields-assisted extraction for cosmetic applications. Mar. Drugs 2021, 19, 662. [Google Scholar] [CrossRef]
- Golberg, A.; Sack, M.; Teissie, J.; Pataro, G.; Pliquett, U.; Saulis, G.; Stefan, T.; Miklavcic, D.; Vorobiev, E.; Frey, W. Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnol. Biofuels 2016, 9, 94. [Google Scholar] [CrossRef]
- Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; Avdović, E.H.; Radulović, M.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Seaweed protein hydrolysates and bioactive peptides: Extraction, purification, and applications. Mar. Drugs 2021, 19, 500. [Google Scholar] [CrossRef]
- Connor, J.O.; Meaney, S.; Williams, G.A.; Hayes, M. Extraction of protein from four different seaweeds using three different physical pre-treatment strategies. Molecules 2020, 25, 2005. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Queirós, R.; Rocha-Santos, T.A.; Saraiva, J.A.; Gomes, A.M.; Duarte, A.C. Bioactive polysaccharide extracts from Sargassum muticum by High Hydrostatic Pressure. J. Food Process. Preserv. 2017, 41, e12977. [Google Scholar] [CrossRef]
- Suwal, S.; Perreault, V.; Marciniak, A.; Tamigneaux, É.; Deslandes, É.; Bazinet, L.; Jacques, H.; Beaulieu, L.; Doyen, A. Effects of high hydrostatic pressure and polysaccharidases on the extraction of antioxidant compounds from red macroalgae, Palmaria palmata and Solieria chordalis. J. Food Eng. 2019, 252, 53–59. [Google Scholar] [CrossRef]
- Otero, P.; Gutierrez-Docio, A.; del Hierro, J.N.; Reglero, G.; Martin, D. Extracts from the edible insects Acheta domesticus and Tenebrio molitor with improved fatty acid profile due to ultrasound assisted or pressurized liquid extraction. Food Chem. 2020, 314, 126200. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Cho, Y.-N.; Woo, H.-C.; Chun, B.-S. Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J. Clean. Prod. 2018, 198, 1474–1484. [Google Scholar] [CrossRef]
- Fayad, S.; Nehmé, R.; Tannoury, M.; Lesellier, E.; Pichon, C.; Morin, P. Macroalga Padina pavonica water extracts obtained by pressurized liquid extraction and microwave-assisted extraction inhibit hyaluronidase activity as shown by capillary electrophoresis. J. Chromatogr. A 2017, 1497, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; García-Risco, M.R. Pressurized Liquid Extraction (PLE) as an innovative green technology for the effective enrichment of Galician algae extracts with high quality fatty acids and antimicrobial and antioxidant properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Herrero, M.; Mendiola, J.; Ibáñez, E. Subcritical water extraction of bioactive components from algae, Chapter 16. In Functional Ingredients from Algae for Foods and Nutraceuticals; Dominguez, H., Pereira, L., Kraan, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 534–560. [Google Scholar]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Majeed, T.; Shabir, I.; Srivastava, S.; Maqbool, N.; Dar, A.H.; Jan, K.; Pandey, V.K.; Shams, R.; Bashir, I.; Dash, K.K.; et al. Valorization of food wastes by implementation of subcritical water extraction: A comprehensive review. Trends Food Sci. Technol. 2023, 143, 104316. [Google Scholar] [CrossRef]
- Na, H.S.; Kim, J.Y.; Park, J.S.; Choi, G.C.; Yang, S.I.; Lee, J.H.; Cho, J.Y.; Ma, S.J. Characteristics of marine algae extracts using subcritical water extract method. Korean J. Food Preserv. 2014, 21, 62–68. [Google Scholar] [CrossRef]
- Gondo, T.F.; Jönsson, M.; Karlsson, E.N.; Sandahl, M.; Turner, C. Extractability, selectivity, and comprehensiveness in supercritical fluid extraction of seaweed using ternary mixtures of carbon dioxide, ethanol, and water. J. Chromatogr. A 2023, 1706, 464267. [Google Scholar] [CrossRef]
- Gondo, T.F. Advancing Selectivity in Extraction and Analysis of Bioactive Compounds in Seaweed and Plant-Based Foods. Doctoral Thesis, Lund University, Lund, Sweden, 2024. [Google Scholar]
- Honda, M.; Murakami, K.; Takasu, S.; Goto, M. Extraction of fucoxanthin isomers from the edible brown seaweed Undaria pinnatifida using supercritical CO2: Effects of extraction conditions on isomerization and recovery of fucoxanthin. J. Oleo Sci. 2022, 71, 1097–1106. [Google Scholar] [CrossRef]
- Ospina, M.; Castro-Vargas, H.I.; Parada-Alfonso, F. Antioxidant capacity of Colombian seaweeds: 1. Extracts obtained from Gracilaria mammillaris by means of supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 314–322. [Google Scholar] [CrossRef]
- Rudke, A.R. The Biorefinery Concept Applied to the Red Macroalgae Kappaphyccus alvarezii: Green Extraction Technologies. Doctoral Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2024. [Google Scholar]
- Turner, C. From supercritical carbon dioxide to gas expanded liquids in extraction and chromatography of lipids. Lipid Technol. 2015, 27, 275–277. [Google Scholar] [CrossRef]
- Pilařová, V.; Al Hamimi, S.; Cunico, L.P.; Nováková, L.; Turner, C. Extending the design space in solvent extraction—From supercritical fluids to pressurized liquids using carbon dioxide, ethanol, ethyl lactate, and water in a wide range of proportions. Green Chem. 2019, 21, 5427–5436. [Google Scholar] [CrossRef]
- Machado, N.D.; Verano-Naranjo, L.; Cejudo-Bastante, C.; Mantell-Serrano, C.; Casas-Cardoso, L. Technical evaluation of supercritical fluid impregnation scaling-up of olive leaf extract: Froml to pilot scale. J. CO2 Util. 2024, 88, 102932. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.-Y. Enhancement of phenolic compounds extraction from grape pomace by high voltage atmospheric cold plasma. LWT 2020, 133, 109970. [Google Scholar] [CrossRef]
- Pliego-Cortés, H.; Boy, V.; Bourgougnon, N. Instant Controlled Pressure Drop (DIC) as an innovative pre-treatment for extraction of natural compounds from the brown seaweed Sargassum muticum (Yendo) Fensholt 1955 (Ochrophytina, Fucales). Algal Res. 2024, 83, 103705. [Google Scholar] [CrossRef]
- Schultz-Jensen, N.; Thygesen, A.; Leipold, F.; Thomsen, S.T.; Roslander, C.; Lilholt, H.; Bjerre, A.B. Pretreatment of the macroalgae Chaetomorpha linum for the production of bioethanol—Comparison of five pretreatment technologies. Bioresour. Technol. 2013, 140, 36–42. [Google Scholar] [CrossRef]
- Leipold, F.; Schultz-Jensen, N.; Kusano, Y.; Bindslev, H.; Jacobsen, T. Decontamination of objects in a sealed container by means of atmospheric pressure plasmas. Food Control 2011, 22, 1296–1301. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Alañón, M.E.; Villegas-Aguilar, M.d.C.; Fernández-Ochoa, Á.; Rojas-García, A.; Fernández-Moreno, P.; Arráez-Román, D.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. Clean and green analytical techniques. In Marine Phenolic Compounds: Science and Engineering; Pérez-Correa, J.R., Mateos, R., Domínguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–45. [Google Scholar]
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent developments and applications of Solid Phase Microextraction (SPME) in food and environmental analysis—A review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Onofrejová, L.; Vašíčková, J.; Klejdus, B.; Stratil, P.; Mišurcová, L.; Kráčmar, S.; Kopecký, J.; Vacek, J. Bioactive phenols in algae: The application of pressurized-liquid and solid-phase extraction techniques. J. Pharm. Biomed. Anal. 2010, 51, 464–470. [Google Scholar] [CrossRef]
- Omena, E.; Oenning, A.L.; Merib, J.; Richter, P.; Rosero-Moreano, M.; Carasek, E. A green and simple sample preparation method to determine pesticides in rice using a combination of SPME and rotating disk sorption devices. Anal. Chim. Acta 2019, 1069, 57–65. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Herath, K.; Kim, Y.-S.; Jeon, Y.-J.; Kim, S.-K. Enzyme-assisted extraction of bioactive compounds from seaweeds and microalgae. TrAC Trends Anal. Chem. 2023, 167, 117266. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of protein from the macroalga Palmaria palmata. LWT—Food. Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Yaich, H.; Ben Amira, A.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir Coast. Int. J. Biol. Macromol. 2017, 105 Pt 2, 1430–1439. [Google Scholar] [CrossRef]
- Nova, P.; Cunha, S.A.; Costa-Pinto, A.R.; Gomes, A.M. Chemical and antioxidant properties of solvent and enzyme-assisted extracts of Fucus vesiculosus and Porphyra dioica. Mar. Drugs 2024, 22, 319. [Google Scholar] [CrossRef]
- Choulot, M.; Jabbour, C.; Burlot, A.-S.; Jing, L.; Welna, M.; Szymczycha-Madeja, A.; Le Guillard, C.; Michalak, I.; Bourgougnon, N. Application of enzyme-assisted extraction on the brown seaweed Fucus vesiculosus Linnaeus (Ochrophyta, Fucaceae) to produce extracts for a sustainable agriculture. J. Appl. Phycol. 2025, 37, 1325–1340. [Google Scholar] [CrossRef]
- Hammed, A.M.; Jaswir, I.; Amid, A.; Alam, Z.; Asiyanbi-H, T.T.; Ramli, N. Enzymatic hydrolysis of plants and algae for extraction of bioactive compounds. Food Rev. Int. 2013, 29, 352–370. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Puspita, M.; Déniel, M.; Widowati, I.; Radjasa, O.K.; Douzenel, P.; Marty, C.; Vandanjon, L.; Bedoux, G.; Bourgougnon, N. Total phenolic content and biological activities of enzymatic extracts from Sargassum muticum (Yendo) Fensholt. J. Appl. Phycol. 2017, 29, 2521–2537. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of enzyme- and ultrasound-assisted extraction methods on biological properties of red, brown, and green seaweeds from the central west coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-derived biofuel: A review of methods of energy extraction from seaweed biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Robin, A.; Chavel, P.; Chemodanov, A.; Israel, A.; Golberg, A. Diversity of monosaccharides in marine macroalgae from the eastern Mediterranean Sea. Algal Res. 2017, 28, 118–127. [Google Scholar] [CrossRef]
- Suraiya, S.; Lee, J.M.; Cho, H.J.; Jang, W.J.; Kim, D.-G.; Kim, Y.-O.; Kong, I.-S. Monascus spp. fermented brown seaweeds extracts enhance bio-functional activities. Food Biosci. 2018, 21, 90–99. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Martera, G.; Goñi, I.; Villanueva-Suárez, M.-J.; Redondo-Cuenca, A. Chemical structure and molecular weight influence the in vitro fermentability of polysaccharide extracts from the edible seaweeds Himathalia elongata and Gigartina pistillata. Food Hydrocoll. 2018, 83, 348–354. [Google Scholar] [CrossRef]
- Sudhakar, M.; Dharani, G. Evaluation of seaweed for the production of lactic acid by fermentation using Lactobacillus plantarum. Bioresour. Technol. Rep. 2022, 17, 100890. [Google Scholar] [CrossRef]
- Tabacof, A.; Calado, V.; Pereira, N. The macroalga Kappaphycus alvarezii as a potential raw material for fermentation processes within the biorefinery concept: Challenges and perspectives. Fermentation 2024, 10, 283. [Google Scholar] [CrossRef]
- Cheng, J. Biomass to Renewable Energy Processes; CRC Press: Boca Raton, MA, USA, 2017; 437p. [Google Scholar]
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Olfat, A.; Mostaghim, T.; Shahriari, S.; Salehifar, M. Extraction of bioactive compounds of Hypnea flagelliformis by ultrasound-assisted extraction coupled with natural deep eutectic solvent and enzyme inhibitory activity. Algal Res. 2024, 78, 103388. [Google Scholar] [CrossRef]
- Steinbruch, E.; Wise, J.; Levkov, K.; Chemodanov, A.; Israel, Á.; Livney, Y.D.; Golberg, A. Enzymatic cell wall degradation combined with pulsed electric fields increases yields of water-soluble-protein extraction from the green marine macroalga Ulva sp. Innov. Food Sci. Emerg. Technol. 2022, 84, 103231. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Le Guillard, C.; Bergé, J.-P.; Donnay-Moreno, C.; Cornet, J.; Ragon, J.-Y.; Fleurence, J.; Dumay, J. Optimization of R-phycoerythrin extraction by Ultrasound-Assisted Enzymatic hydrolysis: A comprehensive study on the wet seaweed Grateloupia turuturu. Mar. Drugs 2023, 21, 213. [Google Scholar] [CrossRef] [PubMed]
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.-Y.; Fleurence, J.; Bergé, J.-P. Ultrasound-Assisted Extraction of R-phycoerythrin from Grateloupia turuturu with and without enzyme addition. Algal Res. 2015, 12, 522–528. [Google Scholar] [CrossRef]
- Das, R.S.; Tiwari, B.K.; Selli, S.; Kelebek, H.; Garcia-Vaquero, M. Exploring pilot scale ultrasound-microwave assisted extraction of organic acids and phytochemicals from brown seaweed Alaria esculenta. Algal Res. 2025, 86, 103896. [Google Scholar] [CrossRef]
- Barragán, J.A.H.; Olivieri, G.; Boboescu, I.; Eppink, M.; Wijffels, R.; Kazbar, A. Enzyme assisted extraction for seaweed multiproduct biorefinery: A techno-economic analysis. Front. Mar. Sci. 2022, 9, 948086. [Google Scholar] [CrossRef]
- Cardoso, C.; Almeida, J.; Coelho, I.; Delgado, I.; Gomes, R.; Quintã, R.; Bandarra, N.; Afonso, C. Farming a wild seaweed and changes to its composition, bioactivity, and bioaccessibility: The Saccorhiza polyschides case study. Aquaculture 2023, 566, 739217. [Google Scholar] [CrossRef]
- Prabhu, M.; Chemodanov, A.; Gottlieb, R.; Kazir, M.; Nahor, O.; Gozin, M.; Israel, A.; Livney, Y.D.; Golberg, A. Starch from the sea: The green macroalga Ulva ohnoi as a potential source for sustainable starch production in the marine biorefinery. Algal Res. 2019, 37, 215–227. [Google Scholar] [CrossRef]
- Saravana, P.S.; Ummat, V.; Bourke, P.; Tiwari, B.K. Emerging green cell disruption techniques to obtain valuable compounds from macro and microalgae: A review. Crit. Rev. Biotechnol. 2023, 43, 904–919. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
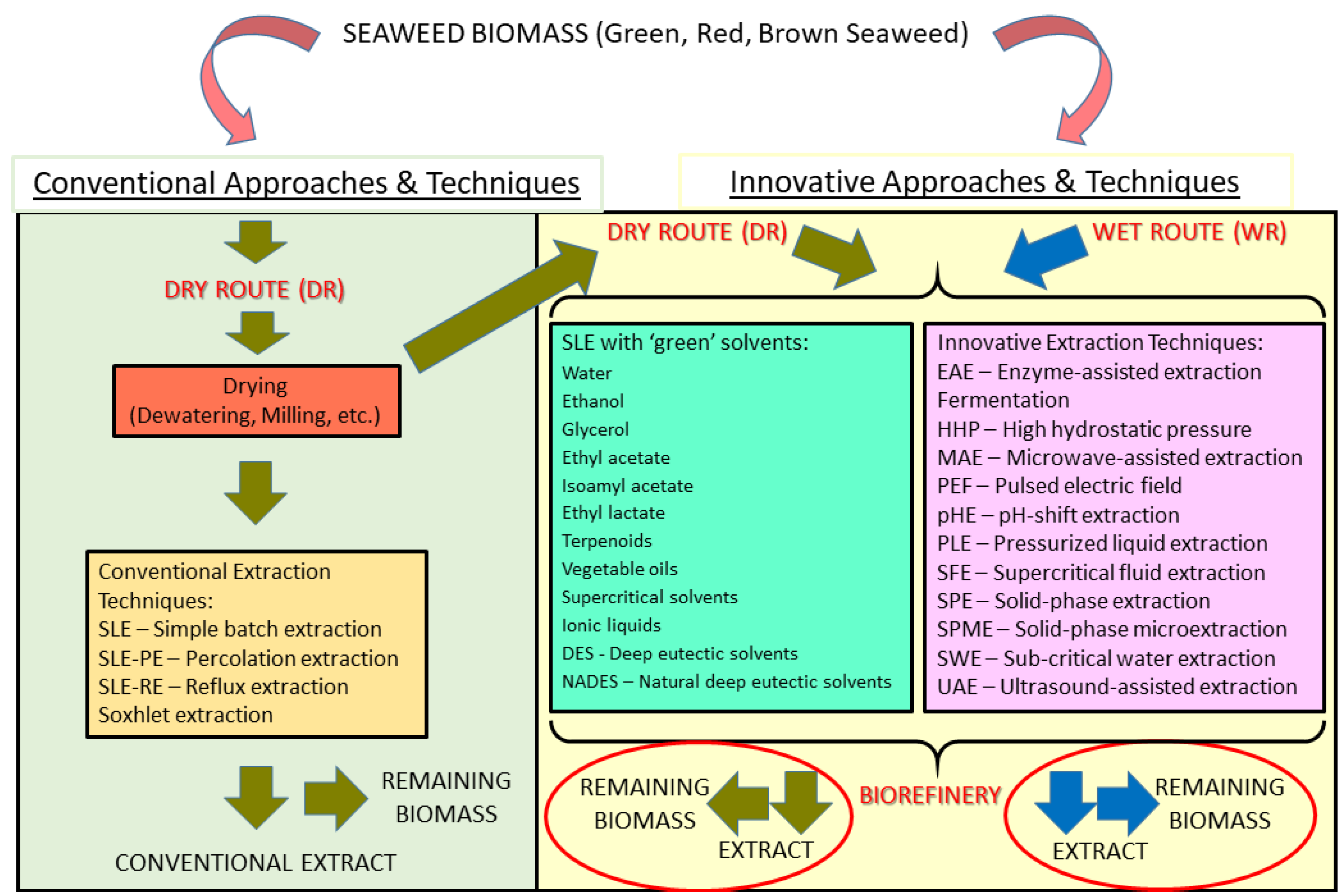
| Approach/Technique | Operational Conditions | Study Outcome | Reference |
|---|---|---|---|
| DR/SLE | Brown seaweed N. zanardinii was extracted with water using a 1:20 biomass/solvent ratio and stirring for 6 h at 65 °C | Fucoidan extraction yield was 5.2% | [78] |
| Brown seaweed A. nodosum was extracted with ethanol/water (8:2, v/v) three times for 36 h and then with hot water | Polysaccharide extraction yield of 9.3% when extracted with hot water at 83.9 °C for 4.3 h and a dry biomass/water ratio of 1:26.9 (w/v) | [79] | |
| Brown seaweed S. vestitum was extracted with ethanol/water (7:3, v/v) at 30 °C for 12 h using 1:50 (w/v) biomass/solvent ratio | Total polyphenol extraction was 40.3 mg GAE/g dw | [80] | |
| Brown seaweed S. japonica was extracted with either water, ethanol, acetone, dichloromethane or diethyl ether using a 1:32 biomass/solvent ratio, 24 h extraction time, and 500 rpm stirring velocity at room temperature | Phenolic extraction yield was optimal with water, reaching 2.4 mg PhloroGlucinol Equivalent (PGE)/g dw; other conventional solvents did not surpass 0.6 mg PGE/g dw | [81] | |
| 13 species (4 brown, 8 red, and 1 green) extracted with hexane, CH2Cl2, ethyl acetate, methanol, and water in a 1:30 (w/v) ratio; for all solvents except water: triple extraction at room temperature for 24 h each; for water: triple extraction at 80 °C for 3 h each | Aqueous extraction led to better yields: from 9.5% in D. polypodioides to 58.5% in Gracilaria domingensis | [82] | |
| 4 seaweed species extracted with methanol/water (7:3, v/v), ethanol/water (8:2, v/v), cold water, and hot water in a 1:20 (w/v) ratio for pure aqueous and 1:10 (w/v) in the other extractions and at room temperature for 24 h, with exception of hot water (60 °C for 24 h) | Cold water presented the highest extraction yields, ranging from 35.9% in F. serratus to 39.5% in L. digitata and 48.2% in C. fragile, with exception of G. gracilis, whose highest yield was attained with methanol/water (7:3, v/v), 29.2% | [83] | |
| Brown seaweed species A. nodosum and L. hyperborea were extracted with either water or 0.1 M HCl at 70 °C for 2.5 h using a 1:20 (w/v) biomass/solvent ratio | Laminarin content in the extracts was highest with water than with 0.1 M HCl, 4.6 vs. 4.3% in A. nodosum and 4.4 vs. 3.2% in L. hyperborea | [84] | |
| 2 green seaweed (C. tomentosum and U. lactuca), 4 red seaweed (C. crispus, G. gracilis, P. palmata, and P. dioica), and 4 brown seaweed species (E. bicyclis, F. vesiculosus, H. elongata, and U. pinnatifida) were extracted by SLE and a BBD/RSM was applied for parameter optimization: time (1, 3, 5 h), temperature (25, 50, 75 °C), and biomass/water ratio (1:25, 1:50, 1:75, w/v) | The highest ABTS in relation to the Antioxidant Potency Composite Index (APCI) was attained for P. palmata, 10.1% vs. 9.1%, respectively, with SLE at 25 °C for 3 h and a biomass/water ratio of 1:75, w/v, but still lower than the SWE technique, 12.2–14.4% | [85] | |
| 9 brown seaweed species extracted with hexane, chloroform, ethyl acetate, acetone, and ethanol in a 1:33 (w/v) ratio at 50 °C for 24 h using 150 rpm stirring | Extraction yield increased partially with polarity, and ethanol enabled the highest yields, ranging from 14.6% in F. spiralis to 24.1% in Bifurcaria bifurcata, 27.0% in H. elongata, and 38.8% in U. pinnatifida | [86] | |
| Brown seaweed Padina australis was subjected to cold maceration SLE at room temperature for 24 h (repeated three times) using a 1:2 (w/v) biomass/ethanol ratio | SLE extracts contained alkaloids, flavonoids, steroids, saponins, and tannins, exhibiting antibacterial activity | [87] | |
| Hexane, ethanol, and acetone:methanol (1:1, v/v) were used on S. japonica and Sargassum horneri in a 1:20 (w/v) ratio at 25 °C for 20 h with 300 rpm stirring | Oil extraction yields: 1.2% in S. japonica and 1.3–1.4% in S. horneri | [88] | |
| Brown seaweed L. japonica was subjected to acid, water, and alkali extraction at 80 °C and twice using a 1:50 (w/v) ratio; acid (1%, w/v, citric acid, pH 2.0) and alkali (1%, w/v, NaOH, pH 10.0) extractions took 4 h, and water extraction only 2 h | Polysaccharide extraction yield varied from 10.3% with water to 44.6% in the case of alkali extraction | [89] | |
| 4 brown seaweed species (Sargassum aquifolium, S. cristaefolium, Sargassum polycystum, T. ornata) were subjected to cold maceration SLE at room temperature for 24 h (repeated three times) using a 1:10 (w/v) biomass/ethanol ratio | Extraction yield and total flavonoid contents were 2–3% and 400–600 mg quercetin/g dw, respectively | [90] | |
| Red seaweed S. chordalis and brown seaweed S. muticum were extracted with chloroform:methanol (1:1, v/v) in a 1:3 (w/v) biomass/solvent ratio at room temperature and for 12 h (repeated three times); S. muticum was also extracted with chloroform in a 1:5 (w/v) biomass/solvent ratio at room temperature and for 12 h | The highest lipid recovery was achieved in S. muticum with chloroform:methanol (1:1, v/v), 3.2% lipid yield; for the same conditions, lipid yield was 3.0% in S. chordalis | [91] | |
| 11 brown seaweed species (A. esculenta, A. nodosum, F. serratus, F. spiralis, F. vesiculosus, Halidrys siliquosa, H. elongata, L. digitata, L. hyperborea, L. saccharina, and Pelvetia caniculata) were extracted with ethanol/water (5:5, v/v) using a biomass/solvent ratio of 1:15, w/v, at 20 °C for 4 h | Extraction yield ranged between 10.5% in F. serratus and 19.3% in L. hyperborea | [92] | |
| S. latissima dry biomass:anhydrous sodium sulfate:ethyl acetate in 4:11:20 (w/w/v) ratio, 60 min at 1373 rpm shaking | Relative lipid yield from S. latissima: 27.3% | [73] | |
| DR/Soxhlet | Brown seaweed Colpomenia sinuosa was extracted alternatively with cyclohexane, CH2Cl2:methanol (1:1, v/v), methanol, and water in a 1:10 (w/v) biomass/solvent ratio for 72 h at room temperature; Soxhlet extractions were performed with the same 4 solvents for 6 h | The most potent extract was obtained by Soxhlet using CH2Cl2:methanol (1:1. v/v) solvent, and it displayed anti-tumoral effects | [93] |
| Brown seaweed Posidonia oceanica was extracted with ethanol:toluene (1:2, v/v) in a 1:22 (w/v) biomass/solvent ratio for 6 h in a Soxhlet system | Polysaccharide (cellulose) extraction yield was 32.5% with ethanol:toluene (1:2, v/v) | [94] |
| Approach/Technique | Operational Conditions | Study Outcome | Reference |
|---|---|---|---|
| DR/EAE | Brown seaweed N. zanardinii was extracted by EAE using four alternative enzymatic treatments: (i) Alcalase (5%, v/v), pH 8.0 at 50 °C for 24 h; (ii) Celluclast (5%, w/v), pH 4.5 at 50 °C for 24 h; (iii) Viscozyme (5%, v/v), pH 4.5 at 50 °C for 24 h; and (iv) Flavourzyme (5%, v/v), pH 7.0 at 50 °C for 24 h | Fucoidan extraction yield varied from 4.3% with Viscozyme treatment to 4.8% with Celluclast and to 5.6% with Alcalase and encompassing 4.4% with Flavourzyme treatment | [78] |
| Green seaweed U. fenestrata was extracted by EAE using a 1:20, w/v, biomass/solvent ratio; two cellulase blends, Viscozyme L and Cellulysin, in 0.1 M sodium acetate buffer, and two proteases, Neutrase 0.8 L and Flavourzyme, in 0.1 M Tris HCl buffer, were tested; Viscozyme L was tested at pH 5 and 50 °C, Cellulysis at pH 5 and 40 °C, Neutrase 0.8 L at pH 7 and 60 °C, and Flavourzyme at pH 5 and 50 °C; four different extraction times were assayed (3, 6, 17, and 20 h) | Ulvan extraction yield reached a maximum of ~14%, w/dw, with Cellulysis, after 20 h of cellulase treatment; the comparable yield for Neutrase 0.8 L was ~13%, w/dw, and for Viscozyme L and Flavourzyme was ~12%, w/dw; higher extraction time led to higher yields, thereby ranging from 3 to 6%, w/dw, after 3 h, to 12–14%, w/dw, after 20 h | [228] | |
| Brown (F. vesiculosus), green (U. rigida), and red (G. vermiculophylla and P. dioica) seaweed species were extracted by EAE; parameter optimization was carried out for G. vermiculophylla applying sequentially cellulolytic (Viscozyme at pH 4.5) and proteolytic enzymes (Flavourzyme and papain at pH 7.0) at 50 °C; firstly, cellulolytic enzyme concentration (3.1–61.9 U/g seaweed), biomass/solvent ratio (1:9 to 1:100, w/v), and time (7.9–28.1 h) were optimized; and secondly, optimal proteolytic enzyme concentration (1378.7–5621.3 U/g seaweed) and time (1.2–6.8 h) were established | For G. vermiculophylla, while cellulolytic yield varied from 17.6% with the lowest Viscozyme level (3.1 U/g), 1:17, w/v, biomass/solvent ratio, and 18 h to 42.4% with 50 U/g, 1:33, w/v, biomass/solvent ratio, and 24 h, proteolytic yield ranged from 36.9 to 38.2% with 3500 U/g and only 1.2 h to 48.9–51.8% with 5621 U/g and 4 h (the lower end of the intervals results from Flavourzyme and the upper end from papain); sequential EAE improved overall extraction yield by 30–160% in comparison to conventional SLE; for all tested species with exception of F. vesiculosus, yield was enhanced by adding a proteolytic stage to the cellulolytic one; for all seaweed species, yield was increased with a cellulolytic treatment prior to proteolysis | [217] | |
| DR/Fermentation | Brown seaweed C. trinodis was subjected to a semi-solid fermentation for fucoidan and alginate extraction purposes; both seaweed biomass and water were sterilized by autoclaving at 121 °C for 20 min; fungal inocula of Aspergillus niger, Chaetomium funicola, Dendryphiella arenaria, Emericella nidulans, Eurotium chevalieri, and Stachybotrys chartarum were attained from 3 seaweed species; 1:20, w/v, biomass/water ratio was used and 1%, v/v, inoculum was added to the biomass–water mixture, being fermentation carried out at 28 °C and with continuous shaking (120 rpm) for 3 days | Fucoidan extraction yield was higher with the fungal inoculum from C. funicola, 4.4%, than with all other inocula, 3.4–3.9%; alginate extraction yield was higher with the fungal inoculum from E. chevalieri, 21.8%, than with all other inocula, 17.4–18.9% | [229] |
| Green (Ulva sp.), red (Gracilaria sp.), and brown (S. cristaefolium) seaweed species were studied as feedstocks for LA fermentation; previous acid thermal hydrolysis (<5% sulfuric acid, 121 °C, 20 min); fermentation with Lactobacillus sp., and Weissella sp. | Maximum reducing sugar recovery, 0.39 g/g seaweed, and LA yield, 0.94 g/g, was achieved with Gracilaria sp.; for S. cristaefolium, 0.36 g/g seaweed in reducing sugar and 0.81 g/g LA yield; for Ulva sp., 0.21 g/g seaweed in reducing sugar and 0.85 g/g LA yield (note however the very low sugar recovery for LA production) | [230] | |
| DR/MAE | Brown seaweed S. vestitum was extracted with ethanol/water using 1:50 (w/v) biomass/solvent ratio and MAE frequency of 2450 MHz; a BBD in an RSM was applied for parameter optimization: MAE power (60, 80, 100% of 1200 W), ethanol/water proportion (3:7, 5:5, 7:3, v/v), and time (25, 50, 75 s) | Best total polyphenol extraction (58.2 mg GAE/g dw) and more favorable antioxidant properties were attained with the following optimal MAE conditions: 960 W power (80% of 1200 W), 7:3, v/v, ethanol/water, and 75 s irradiation | [80] |
| Brown seaweed A. nodosum was extracted by either MAE or UAE-MAE with 0.1 M HCl, using a biomass/solvent ratio of 1:10, w/v, at 37.7–92 °C in MAE and 36.2–98 °C in UAE-MAE, for 2 or 5 min, and applying variable microwave power (250, 600, 1000 W) at 2450 MHz | Applying 1000 W microwave power for 5 min increased total soluble carbohydrate yield from 0.5 to 1.5 to 3.2 g glucose equivalent/100 g dw; maximum yields were attained with UMAE: total soluble carbohydrates (10.4 g glucose equivalent/100 g dw), fucose-sulfated polysaccharides (3.5 g fucose/100 g dw), and phenolic compounds (2.6 GAE/100 g dw) | [231] | |
| Brown seaweed Sargassum swartzii was extracted with ethanol/water; an RSM was applied for parameter optimization: MAE power (174, 240, 400, 560, 626 W), biomass/solvent ratio (1:23, 1:25, 1:30, 1:35, 1:37, w/v), ethanol/water percentage (31.7, 40.0, 60.0, 80.0, 88.3%, v/v), and time (23.8, 30, 45, 60, 66.2 min) | Optimal phlorotannin recovery of 5.59 mg Phlorotannin Equivalent/g dw was achieved with 613 W MAE power, 1:33, w/v, biomass/solvent ratio, 52.0%, v/v, ethanol/water percentage, and 65 min | [232] | |
| Brown seaweed N. zanardinii was extracted with HCl aqueous solution; a BBD in an RSM was used for parameter optimization: temperature (45, 60, 75 °C), time (10, 20, 30 min), MAE power (300, 400, 500 W), and biomass/solvent ratio (1:10, 1:20, 1:30, w/v) | Alginate extraction yield was 31.4% for the optimized conditions: 67 °C, 19 min, 400 W, and 1:29, w/v, biomass/solvent ratio | [233] | |
| Green seaweed species (Ulva spp. and Monostroma latissimum) were extracted with water, and the effect of temperature (100, 120, 140, 160, 180 °C) on yields and properties was studied; a 1:20 biomass/water ratio was combined with a maximal microwave power of 1000 W at 2450 MHz for 10 min | Solubilization rate increased from ~30–50% to ~70–90% when temperature increased from 100 to 180 °C; while ulvan yields were 37–40% from Ulva spp. subjected to MAE at 160 °C, rhamnan sulfate yield was 53% from M. latissimum at 140 °C | [234] | |
| Brown seaweed A. nodosum was extracted with aqueous acid solutions and a MAE parameter optimization was carried out: acid concentration (0.01, 0.05, 0.1, 0.2, 0.4 M H2SO4), temperature (120, 150, 180 °C), biomass/solvent ratio (1:18, 1:32, 1:159, w/v), and time (1, 5, 10, 20, 30 min) | Monosaccharide extraction yield of 12.7% was achieved with 0.4 M H2SO4, 150 °C, 1:32 biomass/solvent ratio, and 1 min MAE treatment; monosaccharide extraction yield increased with H2SO4 concentration and it was lower at 120 and 180 °C | [235] | |
| Green seaweed U. prolifera was extracted with aqueous acid solutions, and a MAE parameter optimization was performed for acid concentration (0.01, 0.05, 0.1 M HCl) and temperature (90, 120, 150 °C), while 1:20, w/v, biomass/solvent ratio, microwave power of 500 W at 2450 MHz, and 15 min time were constant | Total solubilization rate reached ~70% for 0.1 M HCl MAE at 150 °C, thereby increasing with acid concentration and temperature; polysaccharide yield was ~35% for 0.01 M HCl MAE at 120 °C, decreasing with acid concentration and temperatures higher than 120 °C; however, polysaccharides extracted with 0.1 M HCl MAE and 150 °C displayed higher antioxidant activity | [236] | |
| 3 brown seaweed species (F. vesiculosus, F. spiralis, and L. saccharina) were extracted by MAE with 0.1 M HCl aqueous solution (containing 2 M calcium chloride, CaCl2) and parameter optimization was carried out: MAE power (240, 560 W), biomass/solvent ratio (1:10, 1:25, w/v), and time (60. 120 s) | A 1:25, w/v, biomass/solvent ratio led to better results, being maximal yield, 12.3%, achieved with 240 W and 60 s, and optimal fucoidan recovery, 0.77 mg/mg extract, with 560 W and 120 s | [237] | |
| DR/PLE | 4 seaweed species extracted with methanol/water (7:3, v/v), ethanol/water (8:2, v/v), and water; methanol/water PLE was carried out at 90 °C and under 69 bar for 25 min; ethanol/water PLE was carried out at 100 °C and under 69 bar for 25 min; water PLE was carried out at 120 °C and under 104 bar for 25 min | Water PLE presented the highest extraction yields, varying from 26.9% in G. gracilis to 33.4% in F. serratus | [83] |
| Brown seaweed Nemacystus decipiens was extracted by PLE with water (biomass/water ratio of 1:15, w/v) at ~35 °C and under 400, 700 or 1000 bar for one to three consecutive cycles | Fucoidan extraction yield was 16.7% for the best PLE conditions, 700 bar and two consecutive cycles, thereby presenting a value lower than the SLE yield with water, 18.1%; however, PLE fucoidan displayed higher antioxidant activity than SLE fucoidan | [238] | |
| H. elongata extracted at 4 different temperatures (50 °C, 100 °C, 150 °C, 200 °C) for 20 min using hexane, ethanol or water | Extraction yield from H. elongata increased with temperature and solvent polarity: 3.4–7.6% (hexane); 8.3–36.9% (ethanol); 9.5–51.6% (water) | [77] | |
| DR/SFE | Supercritical CO2 (0.17:1/min, v/w, flow rate) was used on U. pinnatifida at 40 °C and under 400 bar for 3 h | Total extraction yield of 1.2% and recovered fucoxanthin reached nearly 80 mg/g of extract in U. pinnatifida, being the latter inversely correlated with temperature | [239] |
| Supercritical CO2 (0.27:1/min, w/w, flow rate) and several co-solvents (sunflower oil, soybean oil, canola oil, ethanol, water) were applied to S. japonica using three sets of conditions for 4 h: (i) 0.50% co-solvent at 45 °C and under 200 bar; (ii) 1.25% co-solvent at 50 °C and under 250 bar; (iii) 2.00% co-solvent at 55 °C and under 300 bar; | Carotenoid extraction yield: 2.4 mg of total carotenoids per g dw with canola oil and sunflower oil using 2.00% co-solvent at 55 °C and under 300 bar | [240] | |
| Supercritical CO2 (0.27:1/min, w/w, flow rate) and co-solvent ethanol (0.01:1/min, v/w, flow rate) were used on S. japonica and Sargassum horneri at 45 °C and under 250 bar for 2 h | Oil extraction yields: 1.1% in S. japonica and 1.3–1.4% in S. horneri | [88] | |
| Supercritical CO2 (0.33:1/min, w/w, flow rate) with and without ethanol as co-solvent was used on S. chordalis and supercritical CO2 (0.33:1/min, w/w, flow rate) only was used on S. muticum, all performed at 45 °C and under 290 bar | The lipid recovery through supercritical CO2 and co-solvent ethanol (2%, w/w) was 41% of the initial lipid content in S. chordalis, but only 25% in S. muticum | [91] | |
| Supercritical CO2 and co-solvent ethanol were used on U. pinnatifida and a BBD in an RSM was applied for parameter optimization: flow rate (0.2:1, 0.5:1, 0.8:1/min, v/w), ethanol amount (1.250, 3.125, 5.000 mL), particle size (100, 450, 800 μm), temperature (20, 50, 80 °C), pressure (69, 241, 414 bar), and time (30, 135, 240 min) | Total flavonoid content of 31.76 mg/g and fucoxanthin content of 20.42 mg/g were reached with optimized conditions: 0.8:1/min, v/w, CO2 flow rate, 3.0 mL ethanol, 100 μm particle size, 48 °C, 400 bar, and 230 min | [241] | |
| DR/SWE | Brown seaweed N. zanardinii was extracted by SWE, and a BBD/RSM was applied for parameter optimization: time (10, 20, 30 min), temperature (90, 120, 150 °C), and biomass/water ratio (1:20, 1:30, 1:40, w/v) | Fucoidan extraction yield was 26.0% for the optimized conditions: 29 min, 150 °C, and 1:21, w/v, biomass/water ratio, which compares to 5.2% yield with conventional DR/SLE applied to the same seaweed species | [242] |
| Brown seaweed E. maxima was extracted by SWE and a central composite experimental design (RSM) was applied for parameter optimization: time (5, 10, 15, 20, 30 min), temperature (100, 120, 140, 160, 180 °C), and biomass/water ratio (1:10, 1:20, 1:30, 1:40, 1:50, w/v) | Whereas optimal phenolic extract yield was ~76% for 180 °C, 23.75 min, and water/biomass ratio of 1:30, w/v, optimal polysaccharide yield was ~58% for 120 °C, 5 min, and water/biomass ratio of 1:30, w/v | [243] | |
| Brown seaweed S. japonica was extracted by SWE with water or a 0.5 M IL, 1-Butyl-3-MethylImidazolium TetraFluoroBorate (BMITFB), aqueous solution, thereby keeping a 1:32 biomass/solvent ratio, a 50 bar pressure, and a 5 min extraction time and varying temperature (100, 125, 150, 175, 200, 225, 250 °C); alternative BMITFB concentrations in aqueous solution were also tested: 0.25, 0.75, and 1.0 M | Phenolic extraction yield was optimal with 0.25 M BMITFB aqueous solution and 175 °C, reaching ~60 mg PGE/g dw; 0.5 M BMITFB aqueous solution was only better than water at 150 and 250 °C; while, for water, optimal temperature range was 175–200 °C, for 0.5 M BMITFB aqueous solution, optimal temperature was between 150 and 175 °C; higher BMITFB concentration led to lower total phenolic content in the extracts, reaching ~20 mg PGE/g dw for 1 M BMITFB; conventional extractions did not surpass 2.4 mg PGE/g dw | [81] | |
| Brown seaweed U. pinnatifida was extracted by SWE, and different operational parameters were tested: time (5, 10, 15, 20, 30 min), temperature (120, 150, 180, 210 °C), and biomass/water ratio (1:50, 1:100, w/v) | Fucoidan extraction yield was maximal (46 mg/g dw) with a SWE combination of 5 min, 120 °C, and 1:50, w/v, biomass/water ratio | [244] | |
| 2 green seaweed (C. tomentosum and U. lactuca), 4 red seaweed (C. crispus, G. gracilis, P. palmata, and P. dioica), and 4 brown seaweed species (E. bicyclis, F. vesiculosus, H. elongata, and U. pinnatifida) were extracted by SWE using 140 °C and 20 bar or 190 °C and 30 bar, always for 30 min; biomass/water ratio was 1:75, w/v, with exception of U. lactuca and U. pinnatifida, which required 1:100, w/v | APCI reached a maximal value of 46% for E. bicyclis, being the highest ABTS and FRAP attained with SWE at 190 °C and 30 bar applied to E. bicyclis, thereby surpassing an optimized SLE and UAE techniques | [85] | |
| Brown seaweed S. japonica was extracted by SWE and a BBD/RSM was used for parameter optimization: time (5, 10, 15 min), temperature (100, 140, 180 °C), pressure (20, 50, 80 bar), agitation speed (100, 200, 300 rpm), and biomass/water ratio (1:11, 1:15, 1:25, w/v) | Fucoidan extraction yield was 13.6% for the optimized conditions: 12 min, 127 °C, 80 bar, 300 rpm, and 1:21, w/v, biomass/water ratio | [245] | |
| DR/UAE | Brown seaweed S. vestitum was extracted with ethanol/water (7:3, v/v) at 30 °C for 60 min using a 1:50 (w/v) biomass/solvent ratio | Total polyphenol extraction was 48.5 mg GAE/g dw | [80] |
| Brown seaweed A. nodosum was extracted by either UAE or UAE-MAE with 0.1 M HCl, using a biomass/solvent ratio of 1:10, w/v, at room temperature in UAE and up to 98 °C in UAE-MAE, for 2 or 5 min, and applying variable ultrasonic amplitude (20, 50, 100% of 500 W) and microwave power (250, 600, 1000 W) | Applying 50% ultrasonic amplitude for 5 min increased total soluble carbohydrate yield from 1.5 to 2.0 to 2.5 g glucose equivalent/100 g dw; maximum yields were attained with UMAE: total soluble carbohydrates (10.4 g glucose equivalent/100 g dw), fucose-sulfated polysaccharides (3.5 g fucose/100 g dw), and phenolic compounds (2.6 GAE/100 g dw) | [231] | |
| Brown seaweed species A. nodosum and L. hyperborea were extracted with either water or 0.1 M HCl for 15 min using a 1:20 (w/v) biomass/solvent ratio, 20 kHz frequency, and 35.61 W/cm2 ultrasonic intensity | Laminarin content in the extracts was highest with 0.1 M HCl solvent, reaching 5.8% in A. nodosum and 6.2% in L. hyperborea; SLE did not surpass 5% | [84] | |
| 2 green seaweed (C. tomentosum and U. lactuca), 4 red seaweed (C. crispus, G. gracilis, P. palmata, and P. dioica), and 4 brown seaweed species (E. bicyclis, F. vesiculosus, H. elongata, and U. pinnatifida) were extracted with water at 20 °C for 10 or 20 min | ABTS varied between 51 (20 min) and 58% (10 min) when UAE was applied to E. bicyclis, thus exceeding optimized SLE, but not SWE | [85] | |
| 4 brown seaweed species (Sargassum aquifolium, S. cristaefolium, Sargassum polycystum, T. ornata) were subjected to UAE at room temperature for 30 min (repeated three times) using a 1:10 (w/v) biomass/ethanol ratio and 30 kHz frequency | Extraction yield and total flavonoid contents were 5–8% and 400–700 mg quercetin/g dw, respectively, thus surpassing DR/SLE yields | [90] | |
| 11 brown seaweed species (A. esculenta, A. nodosum, F. serratus, F. spiralis, F. vesiculosus, Halidrys siliquosa, H. elongata, L. digitata, L. hyperborea, L. saccharina, and Pelvetia caniculata) were extracted with ethanol/water (3:7, 5:5 or 7:3, v/v) using a biomass/solvent ratio of 1:10, w/v, at room temperature for 10 or 30 min and subjected to either 35 or 130 kHz | The optimal conditions for F. vesiculosus were applied to all other species: 5:5, v/v, ethanol/water for 30 min and using 35 kHz; extraction yield varied between 20.4% in F. serratus and 36.9% in L. hyperborea, being UAE yield improved by 1.5–2.2 fold in comparison to SLE in all tested seaweeds | [92] | |
| 2 red seaweed species (E. denticulatum and K. alvarezii) and 2 brown seaweed species (S. binderi and T. ornata) were extracted by UAE and a parameter optimization was carried out: temperature (50, 70, 90 °C), pH (8–12), biomass/water ratio (1:33, 1:50, 1:100, w/v), and ultrasound power (75, 120, 150 W) | Polysaccharide extraction yield of 55% of the initial dry biomass weight was achieved in 15–30 min, while only 27% yield was attained after 2 h processing in a conventional extraction | [246] | |
| WR/EAE | Brown seaweed S. muticum was extracted with 0.1 M phosphate buffer or 0.1 M acetate buffer at 40–60 °C using a 1:50 (w/v) biomass/solvent ratio and several alternative enzymes (Alcalase, Amylase, Celluclast, Protamex, Rapidase, Viscozyme) | In the concentration of 2–5% enzyme, solubilization yield was ~50% of the initial dry material, and also maximal phenolic content and antioxidant activity were achieved; Celluclast led to the highest solubilization yields | [247] |
| WR/PEF | Green seaweed Ulva sp. was extracted by PEF using water as solvent, variable voltage (20, 35, 50 kV), and variable number of 4–6 μs pulses (10, 20, 30, 40, 50) at 0.5 Hz frequency; a control extract with no voltage was also prepared | Compared to control, protein extraction yield increased by a factor of ~7 with PEF’s following conditions: 50 pulses of 50 kV, 247 kJ/kg fresh seaweed, and 70.3 mm electrode gap | [248] |
| WR/pHE | Red seaweed Gracilaria sp. was extracted by pHE in combination with PEF (3 Hz frequency, 200 pulses, 50 μs duration of the pulses, 1 kV), testing alternative sequential pH treatments, from 12 to 1 and from 1 to 12; PEF without pHE, pHE without PEF, and water-mediated extraction were also performed | Total solute release of ~10% with a combination of PEF and pHE, which did not improve with respect to pHE, but in comparison to PEF (~7%) and water-mediated extraction (~7%); however, protein yield was improved with respect to pHE, PEF, and water-mediated extraction, ~25% vs. ~15%, ~8%, and ~8%, respectively | [249] |
| Brown seaweed S. latissima protein was extracted by pHE, being pH adjusted in a 2–13 range and optimization performed: temperature (4, 20, 50 °C), ratio between biomass and volume of water in the osmoshock step and alkaline extraction (1:20, 1:40, 1:60, w/v), osmotic shock duration (0, 1, 2, 16 h) | Maximum protein extraction, 34% of total protein, was reached at pH 12 and with 1:20, w/v, overall biomass/solvent; after a pH-shift combining alkaline extraction and acid precipitation, ~16% of seaweed protein recovery was achieved; osmoshocking significantly increased the yield | [250] | |
| WR/UAE | Brown seaweed S. muticum was extracted with 0.1 M phosphate buffer or 0.1 M acetate buffer at 40–60 °C using a 1:50 (w/v) biomass/solvent ratio and 24 kHz frequency | Extraction yield varied between 21 and 40% of the initial dry material | [247] |
| Brown seaweed S. muticum was extracted with water at 25 °C for 5, 10, 15, 20, 25, or 30 min using a 1:20 (w/v) biomass/solvent ratio, 40 kHz frequency, and 150 W power | Fucoidan extraction by UAE for 15 min resulted in a maximal sulfate content of 39.5 mg/g extract | [251] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, C.; Matos, J.; Afonso, C. Extraction of Marine Bioactive Compounds from Seaweed: Coupling Environmental Concerns and High Yields. Mar. Drugs 2025, 23, 366. https://doi.org/10.3390/md23090366
Cardoso C, Matos J, Afonso C. Extraction of Marine Bioactive Compounds from Seaweed: Coupling Environmental Concerns and High Yields. Marine Drugs. 2025; 23(9):366. https://doi.org/10.3390/md23090366
Chicago/Turabian StyleCardoso, Carlos, Joana Matos, and Cláudia Afonso. 2025. "Extraction of Marine Bioactive Compounds from Seaweed: Coupling Environmental Concerns and High Yields" Marine Drugs 23, no. 9: 366. https://doi.org/10.3390/md23090366
APA StyleCardoso, C., Matos, J., & Afonso, C. (2025). Extraction of Marine Bioactive Compounds from Seaweed: Coupling Environmental Concerns and High Yields. Marine Drugs, 23(9), 366. https://doi.org/10.3390/md23090366





