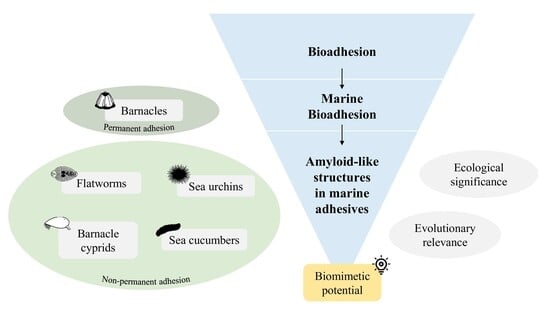
Amyloid-like Structures in Marine Adhesive Proteins
Abstract
1. Introduction
2. Environmental Pressures Favoring the Development of Amyloid-like Structures in Marine Adhesives
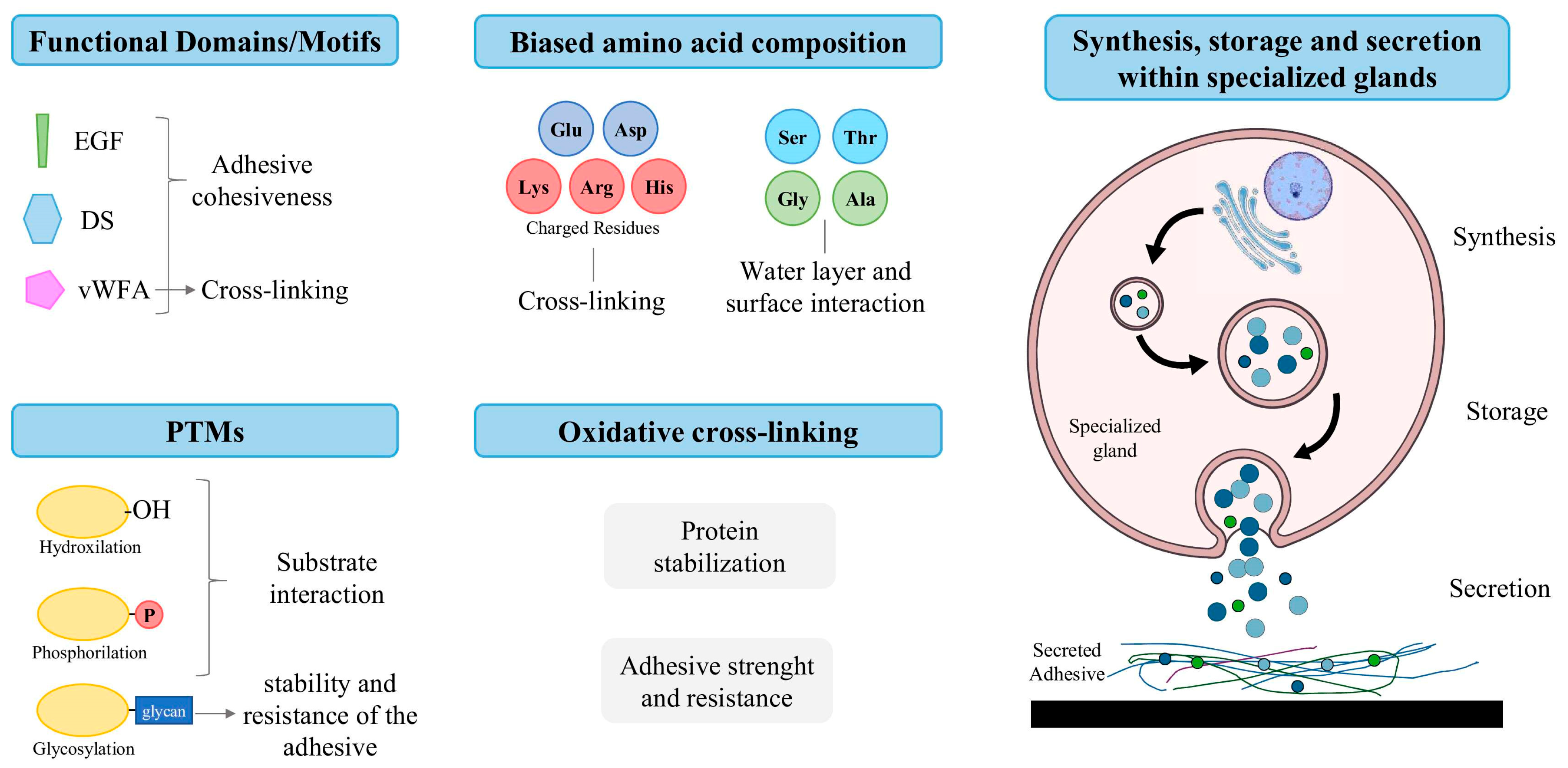
- Functional domains/motifs, such as epidermal growth factor (EGF) domains, which possess metal ion binding sites, and phosphotyrosine residues, which are associated with protein–metal and protein–protein interactions, as well as the cohesion of adhesive secretions [15,31,32,33]; von Willebrand Factor type A (vWFA) domains, thought to be responsible for cohesiveness and cross-linking [15,32,34,35]; and discoidin (DS)-like domains, linked to protein–protein and protein–carbohydrate interactions [36].
- Post-translational modifications (PTMs), such as hydroxylation, phosphorylation and glycosylation [15]. Hydroxylated residues allow proteins to form hydrogen bonds with the substrate [15,37,38]; phosphorylated residues allow for the formation of ionic bonds with mineral and charged surfaces [15,39,40,41]; and glycosylation is thought to stabilize the conformation of adhesive proteins and confer resistance to proteolytic degradation [15,42,43].
- Biased amino acid composition, such as the presence of charged residues (i.e., Lys, Arg, Glu, Asp and His), involved in protein cross-linking; and the abundance of Ser-, Thr-, Ala- and Gly-rich proteins, which are responsible for the interactions between substrate surfaces and the aqueous layer [15,19,23]. This is especially critical, as submerged surfaces are coated with a stable hydration layer that must be removed to allow for direct contact, given that this barrier tends to repel adhesives. Even after making contact, the presence of interfacial residual water can reduce its effectiveness by limiting the contact area between the adhesive and the substrate [44,45,46,47,48,49].
- The occurrence of oxidative cross-linking, involving the formation of covalent disulfide bridges (–S–S–) between thiol (–SH) groups of cysteine residues, often mediated by enzymes like peroxidases [50,51,52]. The formation of these cross-links stabilizes the protein structure, enhancing adhesive strength, insolubility and resistance to degradation [25,39,53,54,55].
- Amyloid structures are known to possess high cohesiveness and mechanical strength (comparable to that of steel [23]), due to their modular nature [57] and the so-called “sacrificial bonds” [58]. These are weaker bonds between structural modules (i.e., β-sheets) of the amyloid-like structures that are preferentially broken when the fibril is subjected to any outside mechanical stress, preventing the backbone of the fibril from being exposed to stress, and potentially breaking and damaging the adhesive [57,58]. This structural characteristic is extremely important in the context of marine adhesion as it allows for the preservation of the integrity of the adhesive in dynamic environments.
- Since amyloids self-assemble, it is thought that an amyloid-based adhesive can rapidly repair itself if damaged [58], which means that the sacrificial bonds mentioned above are likely replaced before any damage can come to the fibril backbone, allowing it to maintain the adhesion even when exposed to extreme circumstances, such as strong tides or predators.
- Additionally, amyloids are known to be highly stable in water and degradation-resistant, which is a desirable trait for wet adhesives, where the constant presence of water could cause the deterioration of the adhesive due to increased permeability (plasticization), which could lead to their swelling, erosion and degradation [1,59,60].
- The insolubility of amyloid-like fibrils, as they are composed of highly ordered packed β-sheets resistant to dissolution, can also be advantageous to marine adhesives. The adhesive will likely rapidly polymerize once secreted due to a difference in pH and/or ionic strength between the content of the secretory granules and seawater (pH 8), stimulating self-assembly of cross-β sheet structures that will not be dissolved and leading to a fast adhesion process [54,61], a characteristic often associated with marine adhesives.
3. Evidence of Amyloid-like Structures in Marine Adhesives
3.1. Amyloid-like Structures in Permanent Adhesives
3.2. Amyloid-like Structures in Non-Permanent Adhesives
4. Biomimetic Amyloid-like Marine Adhesive Proteins as an Inspiration for Adhesive Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| ATR-FTIR | Attenuated total reflectance–Fourier transform infrared |
| CD | Circular dichroism |
| CO | Cuvierian organ |
| COOLPs | Cuvierian organ outer-layer proteins |
| CP | Barnacle cement protein |
| Cryo-EM | Cryo-electron microscopy |
| DLS | Dynamic light scattering |
| DOPA | Dihydroxyphenylalanine |
| DS | Discoidin domain |
| EGF | Epidermal growth factor domain |
| FTIR | Fourier transform infrared |
| MD | Molecular dynamics |
| PFT-AFM | Peak force tapping–atomic force microscopy |
| PTMs | Post-translational modifications |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| SEM + EDX | Scanning electron microscopy–energy dispersive X-ray spectroscopy |
| ssNMR | Solid-state nuclear magnetic resonance |
| TEM | Transmission electron microscopy |
| ThT | Thioflavin-T |
| vWFA | von Willebrand factor type A domain |
| vWFD | von Willebrand factor type D domain |
References
- Fowler, D.M.; Koulov, A.V.; Balch, W.E.; Kelly, J.W. Functional amyloid—From bacteria to humans. Trends Biochem. Sci. 2007, 32, 217–224. [Google Scholar] [CrossRef]
- Levkovich, S.A.; Gazit, E.; Laor Bar-Yosef, D. Two Decades of Studying Functional Amyloids in Microorganisms. Trends Microbiol. 2021, 29, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Scheres, S.H.W.; Zhang, W.; Falcon, B.; Goedert, M. Cryo-EM structures of tau filaments. Curr. Opin. Struct. Biol. 2020, 64, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Meisl, G.; Xu, C.K.; Taylor, J.D.; Michaels, T.C.T.; Levin, A.; Otzen, D.; Klenerman, D.; Matthews, S.; Linse, S.; Andreasen, M.; et al. Uncovering the universality of self-replication in protein aggregation and its link to disease. Sci. Adv. 2022, 8, eabn6831. [Google Scholar] [CrossRef]
- Peña-Díaz, S.; Olsen, W.P.; Wang, H.; Otzen, D.E. Functional Amyloids: The Biomaterials of Tomorrow? Adv. Mater. 2024, 36, 2312823. [Google Scholar] [CrossRef]
- Evans, M.L.; Chapman, M.R. Curli biogenesis: Order out of disorder. Biochim. Biophys. Acta 2014, 1843, 1551–1558. [Google Scholar] [CrossRef]
- Mostaert, A.S.; Higgins, M.J.; Fukuma, T.; Rindi, F.; Jarvis, S.P. Nanoscale mechanical characterisation of amyloid fibrils discovered in a natural adhesive. J. Biol. Phys. 2006, 32, 393–401. [Google Scholar] [CrossRef]
- Iconomidou, V.A.; Vriend, G.; Hamodrakas, S.J. Amyloids protect the silkmoth oocyte and embryo. FEBS Lett. 2000, 479, 141–145. [Google Scholar] [CrossRef]
- Buchanan, J.A.; Varghese, N.R.; Johnston, C.L.; Sunde, M. Functional Amyloids: Where Supramolecular Amyloid Assembly Controls Biological Activity or Generates New Functionality. J. Mol. Biol. 2023, 435, 167919. [Google Scholar] [CrossRef]
- Barlow, D.E.; Dickinson, G.H.; Orihuela, B.; Kulp, J.L., 3rd; Rittschof, D.; Wahl, K.J. Characterization of the adhesive plaque of the barnacle Balanus amphitrite: Amyloid-like nanofibrils are a major component. Langmuir 2010, 26, 6549–6556. [Google Scholar] [CrossRef] [PubMed]
- Phang, I.Y.; Aldred, N.; Clare, A.S.; Callow, J.A.; Vancso, G.J. An in situ study of the nanomechanical properties of barnacle (Balanus amphitrite) cyprid cement using atomic force microscopy (AFM). Biofouling 2006, 22, 245–250. [Google Scholar] [CrossRef]
- Viana, A.S.; Santos, R. Nanoscale characterization of the temporary adhesive of the sea urchin Paracentrotus lividus. Beilstein J. Nanotechnol. 2018, 9, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ren, C.; Wong, N.K.; Yan, A.; Sun, C.; Fan, D.; Luo, P.; Jiang, X.; Zhang, L.; Ruan, Y.; et al. The Holothuria leucospilota genome elucidates sacrificial organ expulsion and bioadhesive trap enriched with amyloid-patterned proteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2213512120. [Google Scholar] [CrossRef] [PubMed]
- So, C.R.; Yates, E.A.; Estrella, L.A.; Fears, K.P.; Schenck, A.M.; Yip, C.M.; Wahl, K.J. Molecular Recognition of Structures Is Key in the Polymerization of Patterned Barnacle Adhesive Sequences. ACS Nano 2019, 13, 5172–5183. [Google Scholar] [CrossRef]
- Li, X.; Li, S.G.; Huang, X.A.; Chen, Y.Y.; Cheng, J.W.; Zhan, A.B. Protein-mediated bioadhesion in marine organisms: A review. Mar. Env. Res. 2021, 170, 105409. [Google Scholar] [CrossRef]
- Liu, J.; Song, J.; Zeng, L.; Hu, B. An Overview on the Adhesion Mechanisms of Typical Aquatic Organisms and the Applications of Biomimetic Adhesives in Aquatic Environments. Int. J. Mol. Sci. 2024, 25, 7994. [Google Scholar] [CrossRef] [PubMed]
- Ditsche, P.; Summers, A.P. Aquatic versus terrestrial attachment: Water makes a difference. Beilstein J. Nanotechnol. 2014, 5, 2424–2439. [Google Scholar] [CrossRef]
- Federle, W.; Labonte, D. Dynamic biological adhesion: Mechanisms for controlling attachment during locomotion. Phil. Trans. R. Soc. 2019, 374, 20190199. [Google Scholar] [CrossRef]
- Delroisse, J.; Kang, V.; Gouveneaux, A.; Santos, R.; Flammang, P. Convergent Evolution of Attachment Mechanisms in Aquatic Animals. Preprints 2022. [Google Scholar] [CrossRef]
- Hennebert, E.; Gregorowicz, E.; Flammang, P. Involvement of sulfated biopolymers in adhesive secretions produced by marine invertebrates. Biol. Open 2018, 7, bio037358. [Google Scholar] [CrossRef]
- Flammang, P.; Santos, R. The Temporary Adhesion of Echinoderm Tube Feet. In Frontiers in Invertebrate Physiology: A Collection of Reviews; Saleuddin, A.S.M., Wilkie, I., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2024. [Google Scholar]
- Naldrett, M.J.; Kaplan, D.L. Characterization of barnacle (Balanus eburneus and B. cenatus) adhesive proteins. Mar. Biol. 1997, 127, 629–635. [Google Scholar] [CrossRef]
- Smith, A.M. The Biochemistry and Mechanics of Gastropod Adhesive Gels. In Biological Adhesives; Smith, A.M., Callow, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 167–182. [Google Scholar]
- Davies, M.S.; Jones, H.D.; Hawkins, S.J. Seasonal-Variation in the Composition of Pedal Mucus from Patella vulgata L. J. Exp. Mar. Biol. Ecol. 1990, 144, 101–112. [Google Scholar] [CrossRef]
- Flammang, P.; Michel, A.; Cauwenberge, A.V.; Alexandre, H.; Jangoux, M. A study of the temporary adhesion of the podia in the sea star asterias rubens (Echinodermata, asteroidea) through their footprints. J. Exp. Biol. 1998, 201 Pt 16, 2383–2395. [Google Scholar] [CrossRef]
- Santos, R.; da Costa, G.; Franco, C.; Gomes-Alves, P.; Flammang, P.; Coelho, A.V. First insights into the biochemistry of tube foot adhesive from the sea urchin Paracentrotus lividus (Echinoidea, Echinodermata). Mar. Biotechnol. 2009, 11, 686–698. [Google Scholar] [CrossRef]
- Smith, A.M.; Quick, T.J.; St Peter, R.L. Differences in the Composition of Adhesive and Non-Adhesive Mucus from the Limpet Lottia limatula. Biol. Bull. 1999, 196, 34–44. [Google Scholar] [CrossRef]
- Whittington, I.D.; Cribb, B.W. Adhesive secretions in the Platyhelminthes. Adv. Parasitol. 2001, 48, 101–224. [Google Scholar] [CrossRef]
- Gohad, N.V.; Aldred, N.; Hartshorn, C.M.; Jong Lee, Y.; Cicerone, M.T.; Orihuela, B.; Clare, A.S.; Rittschof, D.; Mount, A.S. Synergistic roles for lipids and proteins in the permanent adhesive of barnacle larvae. Nat. Commun. 2014, 5, 4414. [Google Scholar] [CrossRef]
- Kamino, K. Mini-review: Barnacle adhesives and adhesion. Biofouling 2013, 29, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.S.; Zeng, H.; Masic, A.; Harrington, M.J.; Israelachvili, J.N.; Waite, J.H. Protein- and metal-dependent interactions of a prominent protein in mussel adhesive plaques. J. Biol. Chem. 2010, 285, 25850–25858. [Google Scholar] [CrossRef] [PubMed]
- Hennebert, E.; Wattiez, R.; Demeuldre, M.; Ladurner, P.; Hwang, D.S.; Waite, J.H.; Flammang, P. Sea star tenacity mediated by a protein that fragments, then aggregates. Proc. Natl. Acad. Sci. USA 2014, 111, 6317–6322. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.; Lee, Y.; Hwang, D.S. Sticky organisms create underwater biological adhesives driven by interactions between EGF- and GlcNAc- containing polysaccharides. Nat. Commun. 2025, 16, 233. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Chen, Y.; Li, X.; Zhan, A. Identification and characterization of proteins involved in stolon adhesion in the highly invasive fouling ascidian Ciona robusta. Biochem. Biophys. Res. Commun. 2019, 510, 91–96. [Google Scholar] [CrossRef]
- Sun, C.; Lucas, J.M.; Waite, J.H. Collagen-binding matrix proteins from elastomeric extraorganismic byssal fibers. Biomacromolecules 2002, 3, 1240–1248. [Google Scholar] [CrossRef]
- Flammang, P.; Demeuldre, M.; Hennebert, E.; Santos, R. Adhesive Secretions in Echinoderms: A Review. In Biological Adhesives; Smith, A.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 193–222. [Google Scholar]
- Waite, J.H.; Andersen, N.H.; Jewhurst, S.; Sun, C. Mussel Adhesion: Finding the Tricks Worth Mimicking. J. Adhes. 2005, 81, 297–317. [Google Scholar] [CrossRef]
- Lu, Q.; Danner, E.; Waite, J.H.; Israelachvili, J.N.; Zeng, H.; Hwang, D.S. Adhesion of mussel foot proteins to different substrate surfaces. J. R. Soc. Interface 2013, 10, 20120759. [Google Scholar] [CrossRef] [PubMed]
- Flammang, P. Adhesive Secretions in Echinoderms: An Overview. In Biological Adhesives; Smith, A.M., Callow, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 183–206. [Google Scholar]
- Stewart, R.J.; Weaver, J.C.; Morse, D.E.; Waite, J.H. The tube cement of Phragmatopoma californica: A solid foam. J. Exp. Biol. 2004, 207, 4727–4734. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Strickland, J.V.; Ye, Z.; Wu, W.-J.; Hu, B.; Rittschof, D. Biochemistry of Barnacle Adhesion: An Updated Review. J. Front. Mar. Sci. 2019, 6, 565. [Google Scholar] [CrossRef]
- Roth, Z.; Yehezkel, G.; Khalaila, I. Identification and quantification of protein glycosylation. Int. J. Carbohydr. Chem. 2012, 2012, 640923. [Google Scholar] [CrossRef]
- Hennebert, E.; Maldonado, B.; Ladurner, P.; Flammang, P.; Santos, R. Experimental strategies for the identification and characterization of adhesive proteins in animals: A review. Interface Focus. 2015, 5, 20140064. [Google Scholar] [CrossRef]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef]
- Israelachvili, J.N. 17—Adhesion and Wetting Phenomena. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 415–467. [Google Scholar]
- Li, X.; Wang, Z.; Li, W.; Sun, J. Superstrong Water-Based Supramolecular Adhesives Derived from Poly(vinyl alcohol)/Poly(acrylic acid) Complexes. ACS Mater. Lett. 2021, 3, 875–882. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, C.; Gao, S.; Zhang, B.; Sun, J.; Kai, J.-J.; Wang, B.; Wang, Z. Instant and Strong Underwater Adhesion by Coupling Hygroscopicity and In Situ Photocuring. Chem. Mater. 2021, 33, 8822–8830. [Google Scholar] [CrossRef]
- Raviv, U.; Klein, J. Fluidity of Bound Hydration Layers. Science 2002, 297, 1540–1543. [Google Scholar] [CrossRef]
- Akdogan, Y.; Wei, W.; Huang, K.-Y.; Kageyama, Y.; Danner, E.W.; Miller, D.R.; Martinez Rodriguez, N.R.; Waite, J.H.; Han, S. Intrinsic Surface-Drying Properties of Bioadhesive Proteins. Chem. Int. 2014, 53, 11253–11256. [Google Scholar] [CrossRef]
- Lebesgue, N.; da Costa, G.; Ribeiro, R.M.; Ribeiro-Silva, C.; Martins, G.G.; Matranga, V.; Scholten, A.; Cordeiro, C.; Heck, A.J.; Santos, R. Deciphering the molecular mechanisms underlying sea urchin reversible adhesion: A quantitative proteomics approach. J. Proteom. 2016, 138, 61–71. [Google Scholar] [CrossRef]
- Hennebert, E.; Leroy, B.; Wattiez, R.; Ladurner, P. An integrated transcriptomic and proteomic analysis of sea star epidermal secretions identifies proteins involved in defense and adhesion. J. Proteom. 2015, 128, 83–91. [Google Scholar] [CrossRef]
- So, C.R.; Scancella, J.M.; Fears, K.P.; Essock-Burns, T.; Haynes, S.E.; Leary, D.H.; Diana, Z.; Wang, C.; North, S.; Oh, C.S.; et al. Oxidase Activity of the Barnacle Adhesive Interface Involves Peroxide-Dependent Catechol Oxidase and Lysyl Oxidase Enzymes. ACS Appl. Mater. Interfaces 2017, 9, 11493–11505. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K.; Nakano, M.; Kanai, S. Significance of the conformation of building blocks in curing of barnacle underwater adhesive. FEBS J. 2012, 279, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K. Underwater adhesive of marine organisms as the vital link between biological science and material science. Mar. Biotechnol. 2008, 10, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Urushida, Y.; Nakano, M.; Uchiyama, S.; Kamino, K. Calcite-specific coupling protein in barnacle underwater cement. FEBS J. 2007, 274, 6436–6446. [Google Scholar] [CrossRef]
- Stewart, R.J.; Ransom, T.C.; Hlady, V. Natural Underwater Adhesives. J. Polym. Sci. B Polym. Phys. 2011, 49, 757–771. [Google Scholar] [CrossRef]
- Smith, B.L.; Schäffer, T.E.; Viani, M.; Thompson, J.B.; Frederick, N.A.; Kindt, J.; Belcher, A.; Stucky, G.D.; Morse, D.E.; Hansma, P.K. Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature 1999, 399, 761–763. [Google Scholar] [CrossRef]
- Fukuma, T.; Mostaert, A.S.; Jarvis, S.P. Explanation for the mechanical strength of amyloid fibrils. Tribol. Lett. 2006, 22, 233–237. [Google Scholar] [CrossRef]
- Frantzis, P. Durability of Adhesive Joints Made Underwater. J. Mater. Civ. Eng. 2008, 20, 635–639. [Google Scholar] [CrossRef]
- Nguyen, T.; Byrd, W.E.; Alshed, D.; Chin, J.; Clerici, C.; Martin, J. Relationship Between Interfacial Water Layer Adhesion Loss of Silicon/Glass Fiber–Epoxy Systems: A Quantitative Study. J. Adhes. 2007, 83, 587–610. [Google Scholar] [CrossRef]
- Nakano, M.; Kamino, K. Amyloid-like conformation and interaction for the self-assembly in barnacle underwater cement. Biochemistry 2015, 54, 826–835. [Google Scholar] [CrossRef]
- Shewmaker, F.; McGlinchey, R.P.; Wickner, R.B. Structural insights into functional and pathological amyloid. J. Biol. Chem. 2011, 286, 16533–16540. [Google Scholar] [CrossRef]
- Gomes, C.M.; Faísca, P.F.N. Protein Folding: An Introduction. In Protein Folding: An Introduction; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–63. [Google Scholar]
- Sawaya, M.R.; Sambashivan, S.; Nelson, R.; Ivanova, M.I.; Sievers, S.A.; Apostol, M.I.; Thompson, M.J.; Balbirnie, M.; Wiltzius, J.J.; McFarlane, H.T.; et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 2007, 447, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Thirumalai, D.; Reddy, G.; Straub, J.E. Role of water in protein aggregation and amyloid polymorphism. Acc. Chem. Res. 2012, 45, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002, 16, 77–83. [Google Scholar] [CrossRef]
- Gade Malmos, K.; Blancas-Mejia, L.M.; Weber, B.; Buchner, J.; Ramirez-Alvarado, M.; Naiki, H.; Otzen, D. ThT 101: A primer on the use of thioflavin T to investigate amyloid formation. Amyloid 2017, 24, 1–16. [Google Scholar] [CrossRef]
- Bancroft, J.D. Theory and Practice of Histological Techniques; Bancroft, J.D., Gamble, M., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; p. 725. [Google Scholar]
- Bouchard, M.; Zurdo, J.; Nettleton, E.J.; Dobson, C.M.; Robinson, C.V. Formation of insulin amyloid fibrils followed by FTIR simultaneously with CD and electron microscopy. Protein Sci. 2000, 9, 1960–1967. [Google Scholar] [CrossRef]
- Cristóvão, J.S.; Henriques, B.J.; Gomes, C.M. Biophysical and Spectroscopic Methods for Monitoring Protein Misfolding and Amyloid Aggregation. In Protein Misfolding Diseases: Methods and Protocols; Gomes, C.M., Ed.; Springer: New York, NY, USA, 2019; pp. 3–18. [Google Scholar]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.O.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-beta spine of amyloid-like fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef]
- Gremer, L.; Schölzel, D.; Schenk, C.; Reinartz, E.; Labahn, J.; Ravelli, R.B.G.; Tusche, M.; Lopez-Iglesias, C.; Hoyer, W.; Heise, H.; et al. Fibril structure of amyloid-β(1–42) by cryo–electron microscopy. Science 2017, 358, 116–119. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.P.; Falcon, B.; He, S.; Murzin, A.G.; Murshudov, G.; Garringer, H.J.; Crowther, R.A.; Ghetti, B.; Goedert, M.; Scheres, S.H.W. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017, 547, 185–190. [Google Scholar] [CrossRef]
- Li, B.; Ge, P.; Murray, K.A.; Sheth, P.; Zhang, M.; Nair, G.; Sawaya, M.R.; Shin, W.S.; Boyer, D.R.; Ye, S.; et al. Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat. Commun. 2018, 9, 3609. [Google Scholar] [CrossRef] [PubMed]
- Van Melckebeke, H.; Wasmer, C.; Lange, A.; Ab, E.; Loquet, A.; Bockmann, A.; Meier, B.H. Atomic-resolution three-dimensional structure of HET-s(218-289) amyloid fibrils by solid-state NMR spectroscopy. J. Am. Chem. Soc. 2010, 132, 13765–13775. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ma, B.; McElheny, D.; Parthasarathy, S.; Long, F.; Hoshi, M.; Nussinov, R.; Ishii, Y. Abeta(1-42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat. Struct. Mol. Biol. 2015, 22, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Matos, A.I.; Abrantes, L.M.; Viana, A.S.; Jin, G. Antibody Oriented Immobilization on Gold using the Reaction between Carbon Disulfide and Amine Groups and Its Application in Immunosensing. Langmuir 2012, 28, 17718–17725. [Google Scholar] [CrossRef]
- Marquês, J.T.; de Almeida, R.F.M.; Viana, A.S. Biomimetic membrane rafts stably supported on unmodified gold. Soft Matter 2012, 8, 2007–2016. [Google Scholar] [CrossRef]
- Hennebert, E.; Viville, P.; Lazzaroni, R.; Flammang, P. Micro- and nanostructure of the adhesive material secreted by the tube feet of the sea star Asterias rubens. J. Struct. Biol. 2008, 164, 108–118. [Google Scholar] [CrossRef]
- Mostaert, A.S.; Crockett, R.; Kearn, G.; Cherny, I.; Gazit, E.; Serpell, L.C.; Jarvis, S.P. Mechanically functional amyloid fibrils in the adhesive of a marine invertebrate as revealed by Raman spectroscopy and atomic force microscopy. Arch. Histol. Cytol. 2009, 72, 199–207. [Google Scholar] [CrossRef]
- Harrington, M.J.; Masic, A.; Holten-Andersen, N.; Waite, J.H.; Fratzl, P. Iron-clad fibers: A metal-based biological strategy for hard flexible coatings. Science 2010, 328, 216–220. [Google Scholar] [CrossRef]
- Melanitis, N.; Galiotis, C.; Tetlow, P.L.; Davies, C.K.L. Interfacial shear stress distribution in model composites: The effect of fibre modulus. Composites 1993, 24, 459–466. [Google Scholar] [CrossRef]
- Barlow, D.E.; Dickinson, G.H.; Orihuela, B.; Rittschof, D.; Wahl, K.J. In situ ATR–FTIR characterization of primary cement interfaces of the barnacle Balanus amphitrite. Biofouling 2009, 25, 359–366. [Google Scholar] [CrossRef]
- Sullan, R.M.; Gunari, N.; Tanur, A.E.; Chan, Y.; Dickinson, G.H.; Orihuela, B.; Rittschof, D.; Walker, G.C. Nanoscale structures and mechanics of barnacle cement. Biofouling 2009, 25, 263–275. [Google Scholar] [CrossRef]
- So, C.R.; Fears, K.P.; Leary, D.H.; Scancella, J.M.; Wang, Z.; Liu, J.L.; Orihuela, B.; Rittschof, D.; Spillmann, C.M.; Wahl, K.J. Sequence basis of Barnacle Cement Nanostructure is Defined by Proteins with Silk Homology. Sci. Rep. 2016, 6, 36219. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.L.; von Byern, J.; Flammang, P.; Klepal, W.; Power, A.M. Unusual adhesive production system in the barnacle Lepas anatifera: An ultrastructural and histochemical investigation. J. Morphol. 2012, 273, 1377–1391. [Google Scholar] [CrossRef]
- Kamino, K.; Inoue, K.; Maruyama, T.; Takamatsu, N.; Harayama, S.; Shizuri, Y. Barnacle cement proteins. Importance of disulfide bonds in their insolubility. J. Biol. Chem. 2000, 275, 27360–27365. [Google Scholar] [CrossRef]
- Nakano, M.; Shen, J.R.; Kamino, K. Self-assembling peptide inspired by a barnacle underwater adhesive protein. Biomacromolecules 2007, 8, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Mohanram, H.; Kumar, A.; Verma, C.S.; Pervushin, K.; Miserez, A. Three-dimensional structure of Megabalanus rosa Cement Protein 20 revealed by multi-dimensional NMR and molecular dynamics simulations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190198. [Google Scholar] [CrossRef]
- Tilbury, M.A.; McCarthy, S.; Domagalska, M.; Ederth, T.; Power, A.M.; Wall, J.G. The expression and characterization of recombinant cp19k barnacle cement protein from Pollicipes pollicipes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190205. [Google Scholar] [CrossRef]
- Rocha, M.; Antas, P.; Castro, L.F.C.; Campos, A.; Vasconcelos, V.; Pereira, F.; Cunha, I. Comparative Analysis of the Adhesive Proteins of the Adult Stalked Goose Barnacle Pollicipes pollicipes (Cirripedia: Pedunculata). Mar. Biotechnol. 2019, 21, 38–51. [Google Scholar] [CrossRef]
- Phang, I.Y.; Aldred, N.; Ling, X.Y.; Huskens, J.; Clare, A.S.; Vancso, G.J. Atomic force microscopy of the morphology and mechanical behaviour of barnacle cyprid footprint proteins at the nanoscale. J. R. Soc. Interface 2010, 7, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Phang, I.Y.; Aldred, N.; Ling, X.Y.; Tomczak, N.; Huskens, J.; Clare, A.S.; Vancso, G.J. Chemistry-Specific Interfacial Forces Between Barnacle (Semibalanus Balanoides) Cyprid Footprint Proteins and Chemically Functionalised AFM Tips. J. Adhes. 2009, 85, 616–630. [Google Scholar] [CrossRef]
- Burden, D.K.; Spillmann, C.M.; Everett, R.K.; Barlow, D.E.; Orihuela, B.; Deschamps, J.R.; Fears, K.P.; Rittschof, D.; Wahl, K.J. Growth and development of the barnacle Amphibalanus amphitrite: Time and spatially resolved structure and chemistry of the base plate. Biofouling 2014, 30, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Fears, K.P.; Orihuela, B.; Rittschof, D.; Wahl, K.J. Acorn Barnacles Secrete Phase-Separating Fluid to Clear Surfaces Ahead of Cement Deposition. Adv. Sci. 2018, 5, 1700762. [Google Scholar] [CrossRef]
- He, Y.; Sun, C.; Jiang, F.; Yang, B.; Li, J.; Zhong, C.; Zheng, L.; Ding, H. Lipids as integral components in mussel adhesion. Soft Matter 2018, 14, 7145–7154. [Google Scholar] [CrossRef]
- Kamino, K. Barnacle Underwater Attachment. In Biological Adhesives; Smith, A.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 153–176. [Google Scholar]
- Kamino, K.; Odo, S.; Maruyama, T. Cement proteins of the acorn barnacle, Megabalanus rosa. Biol. Bull. 1996, 190, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K. Barnacle Underwater Attachment. In Biological Adhesives; Smith, A.M., Callow, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 145–166. [Google Scholar]
- Davey, P.A.; Power, A.M.; Santos, R.; Bertemes, P.; Ladurner, P.; Palmowski, P.; Clarke, J.; Flammang, P.; Lengerer, B.; Hennebert, E.; et al. Omics-based molecular analyses of adhesion by aquatic invertebrates. Biol. Rev. Camb. Philos. Soc. 2021, 96, 1051–1075. [Google Scholar] [CrossRef]
- Urushida, Y.; Nakano, M.; Matsuda, S.; Inoue, N.; Kanai, S.; Kitamura, N.; Nishino, T.; Kamino, K. Identification and functional characterization of a novel barnacle cement protein. FEBS J. 2007, 274, 4336–4346. [Google Scholar] [CrossRef] [PubMed]
- Fears, K.P.; Barnikel, A.; Wassick, A.; Ryou, H.; Schultzhaus, J.N.; Orihuela, B.; Scancella, J.M.; So, C.R.; Hunsucker, K.Z.; Leary, D.H.; et al. Adhesion of acorn barnacles on surface-active borate glasses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190203. [Google Scholar] [CrossRef]
- Gan, K.; Liang, C.; Bi, X.; Wu, J.; Ye, Z.; Wu, W.; Hu, B. Adhesive Materials Inspired by Barnacle Underwater Adhesion: Biological Principles and Biomimetic Designs. Front. Bioeng. Biotechnol. 2022, 10, 870445. [Google Scholar] [CrossRef]
- Kamino, K. Novel barnacle underwater adhesive protein is a charged amino acid-rich protein constituted by a Cys-rich repetitive sequence. Biochem. J. 2001, 356, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, G.H.; Vega, I.E.; Wahl, K.J.; Orihuela, B.; Beyley, V.; Rodriguez, E.N.; Everett, R.K.; Bonaventura, J.; Rittschof, D. Barnacle cement: A polymerization model based on evolutionary concepts. J. Exp. Biol. 2009, 212, 3499–3510. [Google Scholar] [CrossRef]
- Kamino, K. Molecular Design of Barnacle Cement in Comparison with Those of Mussel and Tubeworm. J. Adhes. 2010, 86, 96–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, A.; DeBenedictis, E.P.; Keten, S. Bending energy penalty enhances the adhesive strength of functional amyloid curli to surfaces. Nanotechnology 2017, 28, 464002. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating aqueous solubility directly from molecular structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Yachdav, G.; Kloppmann, E.; Kajan, L.; Hecht, M.; Goldberg, T.; Hamp, T.; Hönigschmid, P.; Schafferhans, A.; Roos, M.; Bernhofer, M.; et al. PredictProtein—An open resource for online prediction of protein structural and functional features. Nucleic Acids Res. 2014, 42, W337–W343. [Google Scholar] [CrossRef]
- Xiong, H.; Buckwalter, B.L.; Shieh, H.M.; Hecht, M.H. Periodicity of polar and nonpolar amino acids is the major determinant of secondary structure in self-assembling oligomeric peptides. Proc. Natl. Acad. Sci. USA 1995, 92, 6349–6353. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Ye, Z.; Xue, B.; Zeng, L.; Wu, W.; Zhong, C.; Cao, Y.; Hu, B.; Messersmith, P.B. Self-Assembled Nanofibers for Strong Underwater Adhesion: The Trick of Barnacles. ACS Appl. Mater. Interfaces 2018, 10, 25017–25025. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Xu, B.; Wei, J.; Xiao, Y.; Huang, F. Adsorption of intrinsically disordered barnacle adhesive proteins on silica surface. Appl. Surf. Sci. 2018, 427, 942–949. [Google Scholar] [CrossRef]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef]
- Nott, J.A. Settlement of barnacle larvae: Surface structure of the antennular attachment disc by scanning electron microscopy. Mar. Biol. 1969, 2, 248–251. [Google Scholar] [CrossRef]
- Nott, J.A.; Foster, B.; Crisp, D.J. On the structure of the antennular attachment organ of the cypris larva of Balanus balanoides (L.). Philos. Trans. R. Soc. Lond. B Biol. Sci. 1969, 256, 115–134. [Google Scholar] [CrossRef]
- Aldred, N.; Scardino, A.; Cavaco, A.; de Nys, R.; Clare, A.S. Attachment strength is a key factor in the selection of surfaces by barnacle cyprids (Balanus amphitrite) during settlement. Biofouling 2010, 26, 287–299. [Google Scholar] [CrossRef]
- Yap, F.C.; Wong, W.-L.; Maule, A.G.; Brennan, G.P.; Chong, V.C.; Lim, L.H.S. First evidence for temporary and permanent adhesive systems in the stalked barnacle cyprid, Octolasmis angulata. Sci. Rep. 2017, 7, 44980. [Google Scholar] [CrossRef]
- Chaw, K.C.; Birch, W.R. Quantifying the exploratory behaviour of Amphibalanus amphitrite cyprids. Biofouling 2009, 25, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Phang, I.Y.; Aldred, N.; Clare, A.S.; Vancso, G.J. Towards a nanomechanical basis for temporary adhesion in barnacle cyprids (Semibalanus balanoides). J. R. Soc. Interface 2008, 5, 397–401. [Google Scholar] [CrossRef]
- Phang, I.Y.; Chaw, K.C.; Choo, S.S.H.; Kang, R.K.C.; Lee, S.S.C.; Birch, W.R.; Teo, S.L.M.; Vancso, G.J. Marine biofouling field tests, settlement assay and footprint micromorphology of cyprid larvae of Balanus amphitrite on model surfaces. Biofouling 2009, 25, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Puniredd, S.R.; Jańczewski, D.; Lee, S.S.C.; Teo, S.L.M.; He, T.; Zhu, X.; Vancso, G.J. Barnacle Larvae Exploring Surfaces with Variable Hydrophilicity: Influence of Morphology and Adhesion of “Footprint” Proteins by AFM. ACS Appl. Mater. Interfaces 2014, 6, 13667–13676. [Google Scholar] [CrossRef]
- Andersson, O.; Ekblad, T.; Aldred, N.; Clare, A.S.; Liedberg, B. Novel application of imaging surface plasmon resonance for in situ studies of the surface exploration of marine organisms. Biointerphases 2009, 4, 65–68. [Google Scholar] [CrossRef]
- Walker, G.; Yule, A.B. Temporary adhesion of the barnacle cyprid: The existence of an antennular adhesive secretion. J. Mar. Biol. Assoc. 1984, 64, 679–686. [Google Scholar] [CrossRef]
- Clare, A.S.; Freet, R.K.; McClary, M. On the antennular secretion of the cyprid of Balanus amphitrite amphitrite, and its role as a settlement pheromone. J. Mar. Biol. Assoc. 1994, 74, 243–250. [Google Scholar] [CrossRef]
- Matsumurad, K.; Nagano, M.; Kato-Yoshinaga, Y.; Yamazaki, M.; Clare, A.S.; Fusetani, N. Immunological studies on the settlement–inducing protein complex (SIPC) of the barnacle Balanus amphitrite and its possible involvement in larva–larva interactions. Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 1825–1830. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Jańczewski, D.; Lee, S.S.C.; He, T.; Teo, S.L.M.; Vancso, G.J. Measuring protein isoelectric points by AFM-based force spectroscopy using trace amounts of sample. Nat. Nanotechnol. 2016, 11, 817–823. [Google Scholar] [CrossRef]
- Lengerer, B.; Hennebert, E.; Flammang, P.; Salvenmoser, W.; Ladurner, P. Adhesive organ regeneration in Macrostomum lignano. BMC Dev. Biol. 2016, 16, 20. [Google Scholar] [CrossRef]
- Wunderer, J.; Lengerer, B.; Pjeta, R.; Bertemes, P.; Kremser, L.; Lindner, H.; Ederth, T.; Hess, M.W.; Stock, D.; Salvenmoser, W.; et al. A mechanism for temporary bioadhesion. Proc. Natl. Acad. Sci. USA 2019, 116, 4297–4306. [Google Scholar] [CrossRef] [PubMed]
- Hamwood, T.E.; Cribb, B.W.; Halliday, J.A.; Kearn, G.C.; Whittington, I.D. Preliminary characterisation and extraction of anterior adhesive secretion in monogenean (platyhelminth) parasites. Folia Parasitol. 2002, 49, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Barreto, Â.; Franco, C.; Coelho, A.V. Mapping sea urchins tube feet proteome—A unique hydraulic mechano-sensory adhesive organ. J. Proteom. 2013, 79, 100–113. [Google Scholar] [CrossRef]
- Lengerer, B.; Ladurner, P. Properties of temporary adhesion systems of marine and freshwater organisms. J. Exp. Biol. 2018, 221, jeb182717. [Google Scholar] [CrossRef]
- Lengerer, B.; Algrain, M.; Lefevre, M.; Delroisse, J.; Hennebert, E.; Flammang, P. Interspecies comparison of sea star adhesive proteins. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190195. [Google Scholar] [CrossRef]
- Pjeta, R.; Lindner, H.; Kremser, L.; Salvenmoser, W.; Sobral, D.; Ladurner, P.; Santos, R. Integrative Transcriptome and Proteome Analysis of the Tube Foot and Adhesive Secretions of the Sea Urchin Paracentrotus lividus. Int. J. Mol. Sci. 2020, 21, 946. [Google Scholar] [CrossRef]
- Toubarro, D.; Gouveia, A.; Ribeiro, R.M.; Simões, N.; Costa, G.; Cordeiro, C.; Santos, R. Cloning, Characterization, and Expression Levels of the Nectin Gene from the Tube Feet of the Sea Urchin Paracentrotus Lividus. Mar. Biotechnol. 2016, 18, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Simão, M.; Moço, M.; Marques, L.; Santos, R. Characterization of the glycans involved in sea urchin Paracentrotus lividus reversible adhesion. Mar. Biol. 2020, 167, 125. [Google Scholar] [CrossRef]
- Gaspar, L.; Flammang, P.; Jose, R.; Luis, R.; Ramalhosa, P.; Monteiro, J.; Nogueira, N.; Canning-Clode, J.; Santos, R. Interspecific Analysis of Sea Urchin Adhesive Composition Emphasizes Variability of Glycans Conjugated With Putative Adhesive Proteins. Front. Mar. Sci. 2021, 8, 737886. [Google Scholar] [CrossRef]
- Ventura, I.; Harman, V.; Beynon, R.J.; Santos, R. Glycoproteins Involved in Sea Urchin Temporary Adhesion. Mar. Drugs 2023, 21, 145. [Google Scholar] [CrossRef]
- Hamel, J.F.; Mercier, A. Cuvierian tubules in tropical holothurians: Usefulness and efficiency as a defence mechanism. Mar. Freshw. Behav. Physiol. 2000, 33, 115–139. [Google Scholar] [CrossRef]
- Becker, P.T.; Flammang, P. Unravelling the Sticky Threads of Sea Cucumbers—A Comparative Study on Cuvierian TubuleMorphology and Histochemistry. In Biological Adhesive Systems: From Nature to Technical and Medical Application; von Byern, J., Grunwald, I., Eds.; Springer: Vienna, Austria, 2010; pp. 87–98. [Google Scholar]
- Demeuldre, M.; Chinh Ngo, T.; Hennebert, E.; Wattiez, R.; Leclère, P.; Flammang, P. Instantaneous adhesion of Cuvierian tubules in the sea cucumber Holothuria forskali. Biointerphases 2014, 9, 029016. [Google Scholar] [CrossRef] [PubMed]
- DeMoor, S.; Waite, H.J.; Jangoux, M.J.; Flammang, P.J. Characterization of the Adhesive from Cuvierian Tubules of the Sea Cucumber Holothuria forskali (Echinodermata, Holothuroidea). Mar. Biotechnol. 2003, 5, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Flammang, P.; Lambert, A.; Bailly, P.; Hennebert, E. Polyphosphoprotein-Containing Marine Adhesives. J. Adhes. 2009, 85, 447–464. [Google Scholar] [CrossRef]
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polym. Sci. 2012, 37, 1031–1050. [Google Scholar] [CrossRef]
- Bhagat, V.; Becker, M.L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, M.; Yang, J. Design Strategies and Applications of Tissue Bioadhesives. Macromol. Biosci. 2013, 13, 271–288. [Google Scholar] [CrossRef]
- Tarafder, S.; Park, G.Y.; Felix, J.; Lee, C.H. Bioadhesives for musculoskeletal tissue regeneration. Acta Biomater. 2020, 117, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Sierra, D.H.; Eberhardt, A.W.; Lemons, J.E. Failure characteristics of multiple-component fibrin-based adhesives. J. Biomed. Mater. Res. 2002, 59, 1–11. [Google Scholar] [CrossRef]
- Strausberg, R.L.; Link, R.P. Protein-based medical adhesives. Trends Biotechnol. 1990, 8, 53–57. [Google Scholar] [CrossRef]
- Farrar, D.F. Bone adhesives for trauma surgery: A review of challenges and developments. Int. J. Adhes. Adhes. 2012, 33, 89–97. [Google Scholar] [CrossRef]
- Heiss, C.; Kraus, R.; Schluckebier, D.; Stiller, A.-C.; Wenisch, S.; Schnettler, R. Bone Adhesives in Trauma and Orthopedic Surgery. Eur. J. Trauma. 2006, 32, 141–148. [Google Scholar] [CrossRef]
- Burke, K.A.; Roberts, D.C.; Kaplan, D.L. Silk Fibroin Aqueous-Based Adhesives Inspired by Mussel Adhesive Proteins. Biomacromolecules 2016, 17, 237–245. [Google Scholar] [CrossRef]
- Sogawa, H.; Ifuku, N.; Numata, K. 3,4-Dihydroxyphenylalanine (DOPA)-Containing Silk Fibroin: Its Enzymatic Synthesis and Adhesion Properties. ACS Biomater. Sci. Eng. 2019, 5, 5644–5651. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Gurry, T.; Cheng, A.A.; Downey, J.; Deng, Z.; Stultz, C.M.; Lu, T.K. Strong underwater adhesives made by self-assembling multi-protein nanofibres. Nat. Nanotechnol. 2014, 9, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Jiang, Y.; Liu, Y.; Ren, Y.; Xu, Z.; Li, Z.; Zhao, Y.; Wu, X.; Ren, J. Marine-inspired molecular mimicry generates a drug-free, but immunogenic hydrogel adhesive protecting surgical anastomosis. Bioact. Mater. 2021, 6, 770–782. [Google Scholar] [CrossRef]
- Diaz, C.; Missirlis, D. Amyloid-Based Albumin Hydrogels. Adv. Healthc. Mater. 2023, 12, 2201748. [Google Scholar] [CrossRef]
- Hu, B.; Shen, Y.; Adamcik, J.; Fischer, P.; Schneider, M.; Loessner, M.J.; Mezzenga, R. Polyphenol-Binding Amyloid Fibrils Self-Assemble into Reversible Hydrogels with Antibacterial Activity. ACS Nano 2018, 12, 3385–3396. [Google Scholar] [CrossRef]
- Ke, P.C.; Zhou, R.; Serpell, L.C.; Riek, R.; Knowles, T.P.J.; Lashuel, H.A.; Gazit, E.; Hamley, I.W.; Davis, T.P.; Fandrich, M.; et al. Half a century of amyloids: Past, present and future. Chem. Soc. Rev. 2020, 49, 5473–5509. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Wei, S.; Sun, C.; Zhong, C. Natural and bio-inspired underwater adhesives: Current progress and new perspectives. APL Mater. 2017, 5, 116102. [Google Scholar] [CrossRef]
- Stewart, R.J. Protein-based underwater adhesives and the prospects for their biotechnological production. Appl. Microbiol. Biotechnol. 2011, 89, 27–33. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A. Chapter 16 Tagging for Protein Expression. In Methods in Enzymology; Burgess, R.R., Deutscher, M.P., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 463, pp. 239–258. [Google Scholar]
- Ding, D.; Guerette, P.A.; Hoon, S.; Kong, K.W.; Cornvik, T.; Nilsson, M.; Kumar, A.; Lescar, J.; Miserez, A. Biomimetic Production of Silk-Like Recombinant Squid Sucker Ring Teeth Proteins. Biomacromolecules 2014, 15, 3278–3289. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.R. Bacillus subtilis and its relatives: Molecular biological and industrial workhorses. Trends Biotechnol. 1992, 10, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Papov, V.V.; Diamond, T.V.; Biemann, K.; Waite, J.H. Hydroxyarginine-containing polyphenolic proteins in the adhesive plaques of the marine mussel Mytilus edulis. J. Biol. Chem. 1995, 270, 20183–20192. [Google Scholar] [CrossRef]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef]
- Waite, J.H. The formation of mussel byssus: Anatomy of a natural manufacturing process. Results Probl. Cell Differ. 1992, 19, 27–54. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, C.; Stewart, R.J.; Waite, J.H. Cement proteins of the tube-building polychaete Phragmatopoma californica. J. Biol. Chem. 2005, 280, 42938–42944. [Google Scholar] [CrossRef]
- Maugh, K.J.; Anderson, D.M.; Strausberg, R.; Strausberg, S.L. Method of Producing Bioadhesive Protein. U.S. Patent 5,202,236, 1993. [Google Scholar]
- Deepankumar, K.; Lim, C.; Polte, I.; Zappone, B.; Labate, C.; De Santo, M.P.; Mohanram, H.; Palaniappan, A.; Hwang, D.S.; Miserez, A. Supramolecular β-Sheet Suckerin–Based Underwater Adhesives. Adv. Funct. Mater. 2020, 30, 1907534. [Google Scholar] [CrossRef]
- Raman, S.; Malms, L.; Utzig, T.; Shrestha, B.R.; Stock, P.; Krishnan, S.; Valtiner, M. Adhesive barnacle peptides exhibit a steric-driven design rule to enhance adhesion between asymmetric surfaces. Colloids Surf. B Biointerfaces 2017, 152, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Fujii, D.; Takase, K.; Takagi, A.; Kamino, K.; Hirano, Y. Design of RGDS Peptide-Immobilized Self-Assembling β-Strand Peptide from Barnacle Protein. Int. J. Mol. Sci. 2021, 22, 1240. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, C.; Zhang, X.; Li, J.; Huang, J.; Zeng, L.; Ye, Z.; Hu, B.; Wu, W. Amyloid fibril aggregation: An insight into the underwater adhesion of barnacle cement. Biochem. Biophys. Res. Commun. 2017, 493, 654–659. [Google Scholar] [CrossRef]
- Murugan, V.K.; Mohanram, H.; Budanovic, M.; Latchou, A.; Webster, R.D.; Miserez, A.; Seita, M. Accelerated corrosion of marine-grade steel by a redox-active, cysteine-rich barnacle cement protein. npj Mater. Degrad. 2020, 4, 20. [Google Scholar] [CrossRef]
- Zeng, L. Expression and Functional Characterization of the 52kDa Cement Protein in Megabalanus rosa; National University of Defense Technology Changsha: Changsha, China, 2016. [Google Scholar]
- Estrella, L.A.; Yates, E.A.; Fears, K.P.; Schultzhaus, J.N.; Ryou, H.; Leary, D.H.; So, C.R. Engineered Escherichia coli Biofilms Produce Adhesive Nanomaterials Shaped by a Patterned 43 kDa Barnacle Cement Protein. Biomacromolecules 2021, 22, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, J.; Zhang, J.; Liu, S.; Cui, M.; An, B.; Wang, X.; Pu, J.; Zhao, T.; Fan, C.; et al. Engineered Bacillus subtilis biofilms as living glues. Mater. Today 2019, 28, 40–48. [Google Scholar] [CrossRef]
- Lefevre, M.; Flammang, P.; Aranko, A.S.; Linder, M.B.; Scheibel, T.; Humenik, M.; Leclercq, M.; Surin, M.; Tafforeau, L.; Wattiez, R.; et al. Sea star-inspired recombinant adhesive proteins self-assemble and adsorb on surfaces in aqueous environments to form cytocompatible coatings. Acta Biomater. 2020, 112, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Ederth, T.; Masai, T.; Wattiez, R.; Leclere, P.; Flammang, P.; Hennebert, E. Disentangling the Roles of Functional Domains in the Aggregation and Adsorption of the Multimodular Sea Star Adhesive Protein Sfp1. Mar. Biotechnol. 2021, 23, 724–735. [Google Scholar] [CrossRef] [PubMed]
| Organism | Methodological Approach | Amyloid-like Characteristics | Reference | |
|---|---|---|---|---|
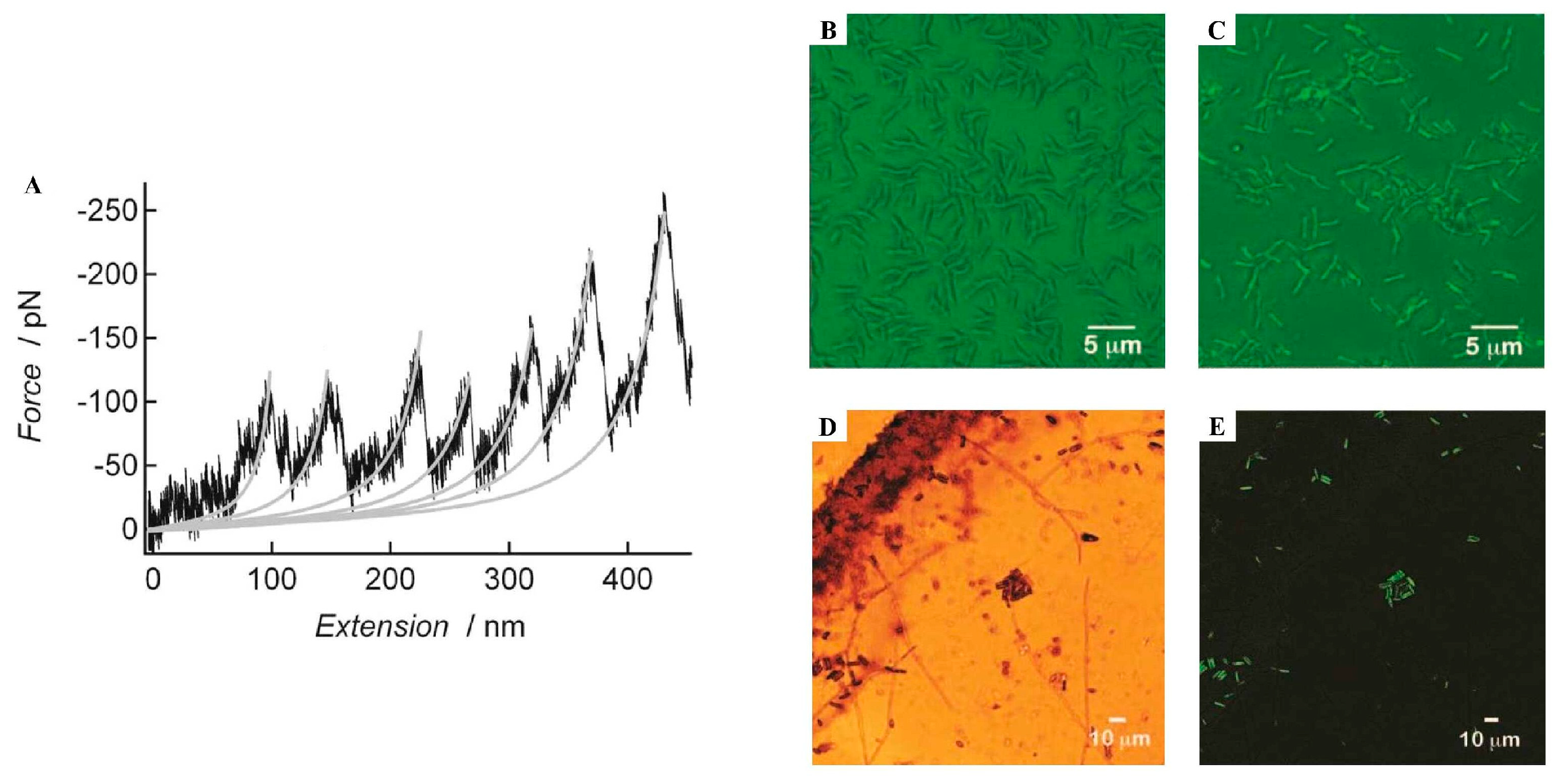
| Permanent Adhesives | Barnacle Amphibalanus amphitrite  | in situ ATR-FTIR | Cement containing mostly β-sheet (~50%) content, with minor α-helix, turned and unordered components; Detection of cross β-sheets and amyloid content in the cement. | [83] |
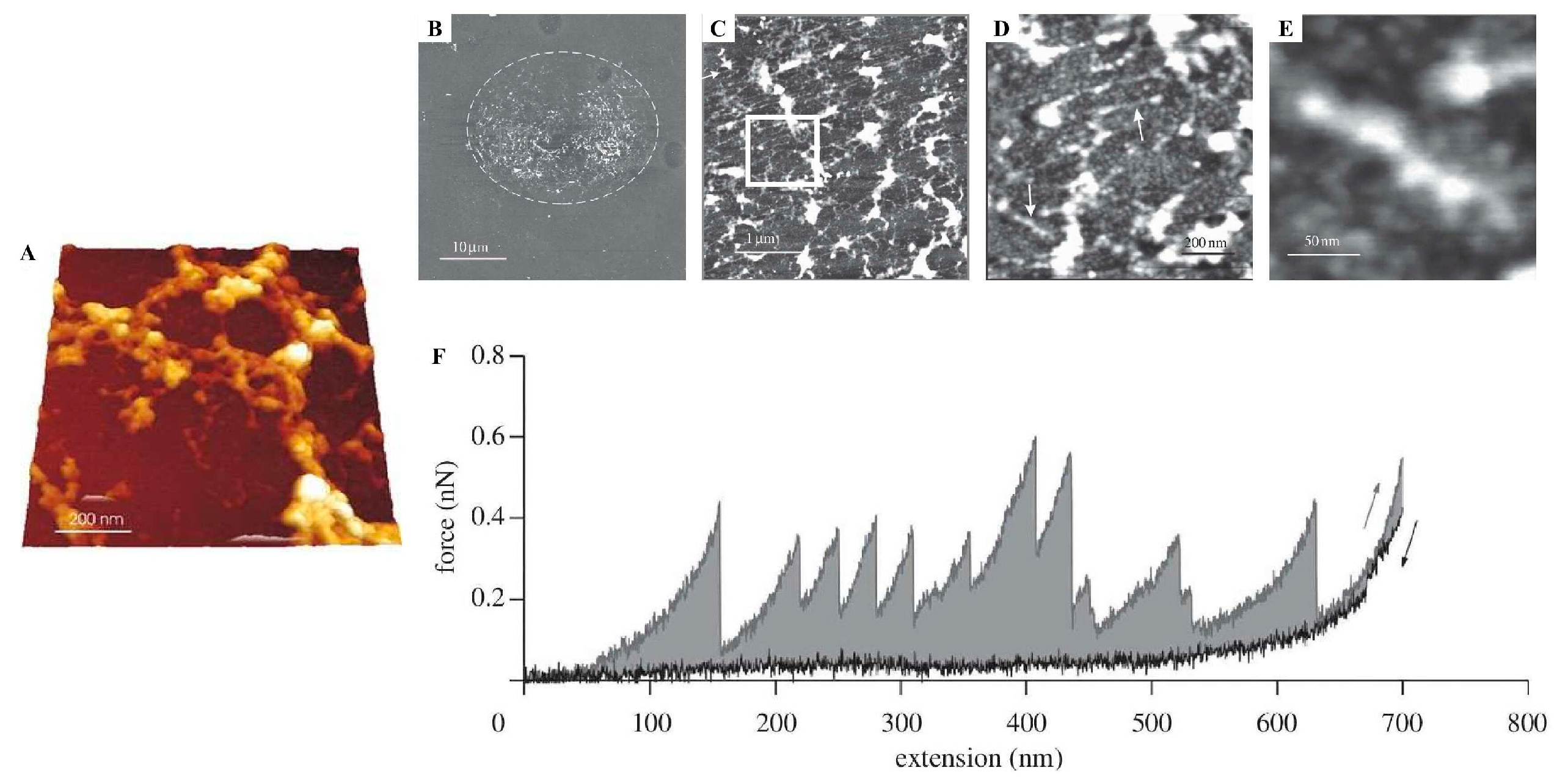
| AFM SEM + EDX ThT stain Congo Red stain FTIR | Elastic cement with rod- and globular-shaped morphologies (nanoscale) of organic nature (AFM, SEM + EDX); the rod-shaped structures were positively stained by Thioflavin-T (ThT) and Congo Red, indicating 5% amyloid content. The cement contained both β-sheet and random coil content (FTIR) and showed a periodic sawtooth force–extension curve (AFM), characteristic of amyloid structures. | [84] | ||
| Far-UV CD FTIR ThT stain AFM | Cement with both β-sheet (~30%) and disordered regions (~40%) (CD); composed of amyloid-like and globular structures (FTIR), thought to have around 28% amyloid content. The cement was positively stained by ThT and there was detection of nanofibrillar structures (AFM). | [10] | ||
| SEM | Cement composed of a network of dense nanofibrillar structures. | [85] | ||
| ThT stain | Cement positively stained by ThT. | [14] | ||
| Barnacle Lepas anatifera  | ThT stain | Adhesive glands and cement were positively stained by ThT. | [86] | |
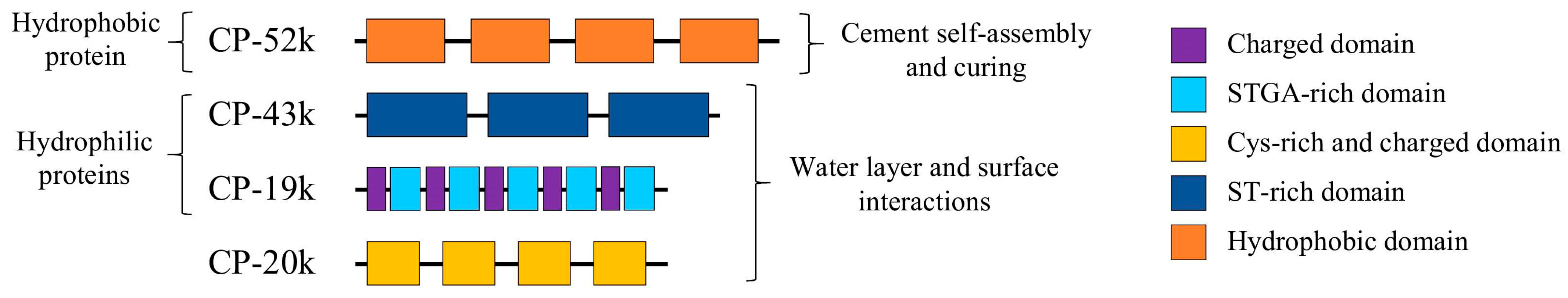
| Barnacle Megabalanus rosa  | Bioinformatic tools | CP-100k protein was predicted to form amyloid-like β-sheets. | [87] | |
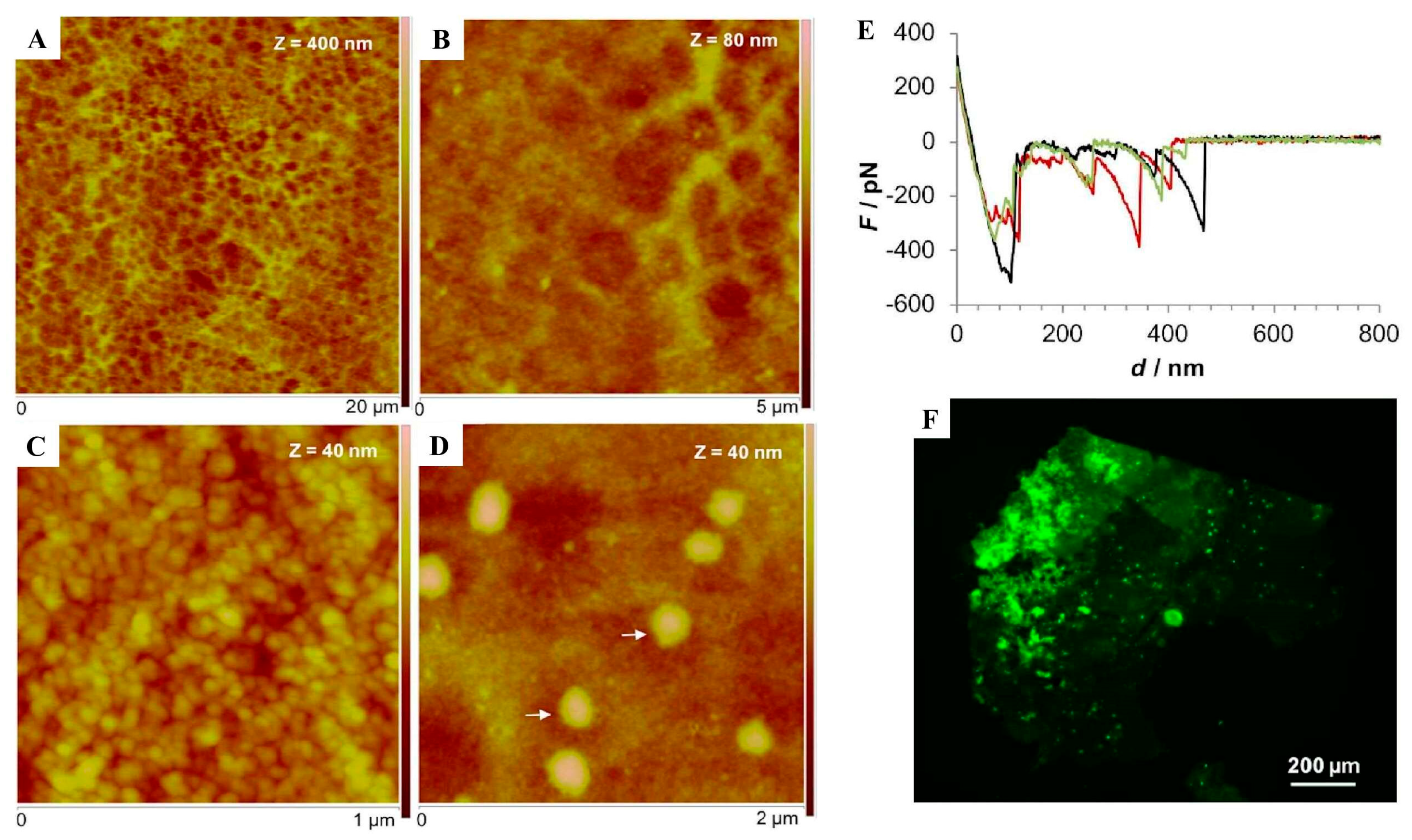
| DLS AFM SEM CD | Recombinant CP-20k peptides self-assembled in a pH and salt-dependent manner (DLS). There was formation of fibers made of bundles of nanofilaments (AFM, SEM). Changes in pH led to irreversible changes in secondary structure, with a possible increase in β-sheet content (CD). | [88] | ||
| ThT stain CD AFM | Identification of amyloidogenic motifs (ThT) in recombinant CP-52k peptides that formed fibrillar entanglements and amyloid-like fibrils. Peptide conformation changed in response to pH and ionic strength increase (CD, ThT, AFM). | [61] | ||
| NMR CD Bioinformatic tools | Recombinant CP-20k presented β-sheet and α-helix structures (CD, NMR). A highly stable and conserved motif—possible seed for fibrillization—was identified through MD simulations. | [89] | ||
| Barnacle Pollicipes pollicipes  | Bioinformatic tools | CP-19k was predicted to self-assemble into amyloid plaques under the appropriate environmental triggers. | [90] | |
| Bioinformatic tools | CP-19k has a long low-complexity Gly-rich region, which can be associated with β-sheet formation in amyloid development. | [91] | ||
| Non-Permanent Adhesives | Barnacle cyprids Amphibalanus amphitrite  | AFM | A footprint, porous in nature, with bundles of fibrils and individual nanofibrils. It showed sawtooth force–extension curves, characteristic of amyloid structures. | [92] |
| Barnacle cyprids Semibalanus balanoides  | AFM | A footprint with an aggregated fibrillar structure. It showed a sawtooth force–extension curve, characteristic of amyloid structures. | [93] | |
| Flatworm Entobdella solea  | Raman spectroscopy AFM ThT stain | A footprint containing intermolecular β-sheets and strong intermolecular H-bonds (Raman spectroscopy) showed periodic sawtooth force–extension curves (AFM), characteristic of amyloid structures, and was positively stained by ThT. | [80] | |
| Sea urchin Paracentrotus lividus  | AFM ThT stain | A footprint presented a honeycomb-like meshwork of interconnected threads of globular nanostructures and periodic sawtooth force–extension curves (AFM), characteristic of amyloid structures. The footprint was positively stained by ThT. | [12] | |
| Sea cucumber Holothuria leucospilota  | SEM Congo Red stain Bioinformatic tools | Detection of amyloid-like fibrils on the surface of the Cuvierian tubules (CO, adhesive organs) (Congo Red stain). Several COOLPs (Hl-25083, Hl-25084, Hl-25088, Hl-30757) were predicted to have a secondary structure composed of full intramolecular β-sheets. | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.R.; Henriques, B.J.; Santos, R. Amyloid-like Structures in Marine Adhesive Proteins. Mar. Drugs 2025, 23, 363. https://doi.org/10.3390/md23090363
Santos MR, Henriques BJ, Santos R. Amyloid-like Structures in Marine Adhesive Proteins. Marine Drugs. 2025; 23(9):363. https://doi.org/10.3390/md23090363
Chicago/Turabian StyleSantos, Mariana Rodrigues, Bárbara Joana Henriques, and Romana Santos. 2025. "Amyloid-like Structures in Marine Adhesive Proteins" Marine Drugs 23, no. 9: 363. https://doi.org/10.3390/md23090363
APA StyleSantos, M. R., Henriques, B. J., & Santos, R. (2025). Amyloid-like Structures in Marine Adhesive Proteins. Marine Drugs, 23(9), 363. https://doi.org/10.3390/md23090363