Design and Synthesis of Marine-Inspired Itampolin A Derivatives to Overcome Chemoresistance in NSCLC via Cholesterol Homeostasis Modulation
Abstract
1. Introduction
2. Results
2.1. Fragment-Based Drug Design
2.2. Synthesis
2.3. Inhibitory Activities of (-)-Itampolin A Skeleton Brominated Tyrosine Derivatives
2.4. Evaluation of the Biological Activity of Compound 4l Against Drug-Resistant NSCLC
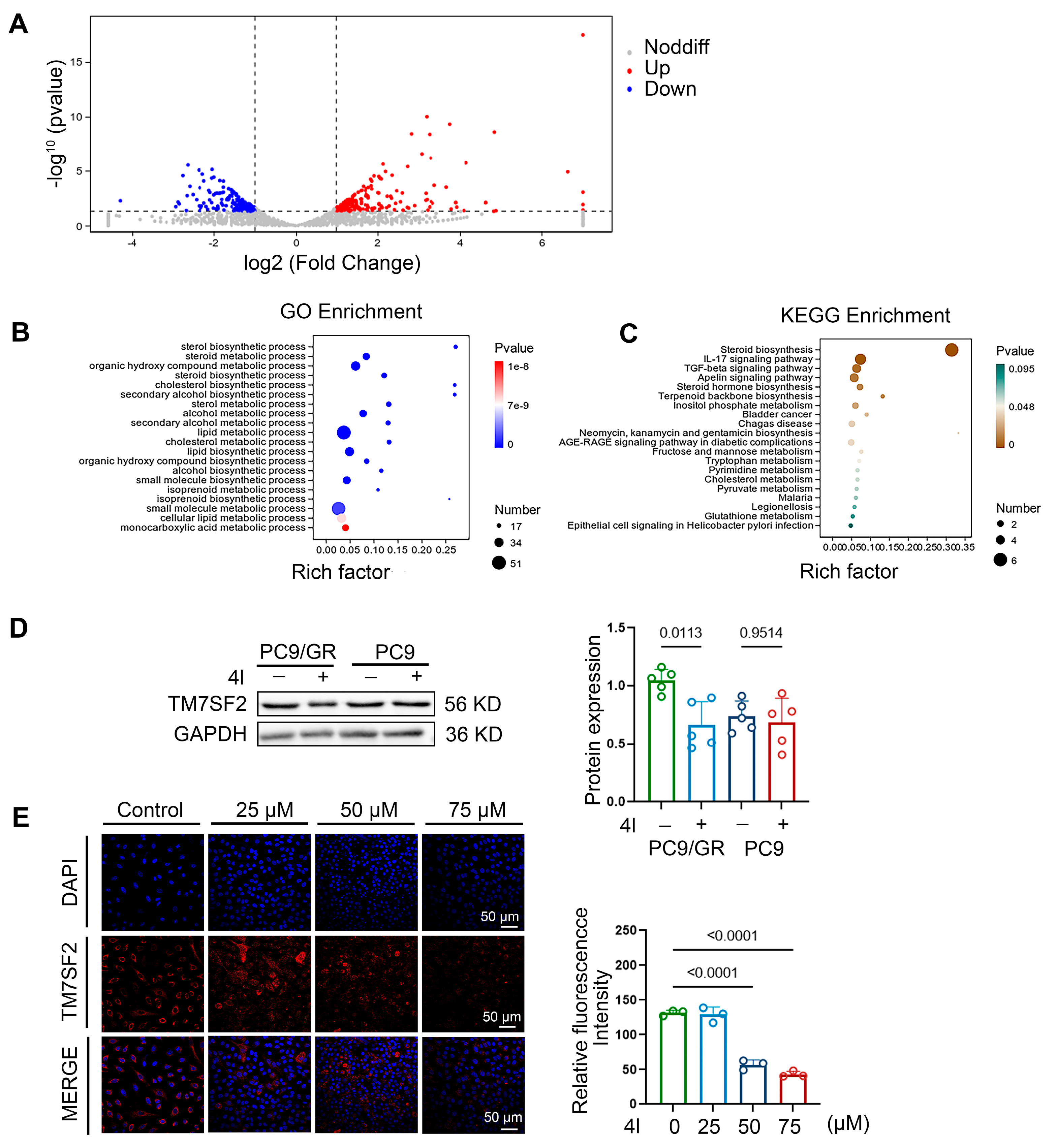
2.5. Screening of Target Genes for 4l Antiresistant NSCLC Proliferation Based on Transcriptomics
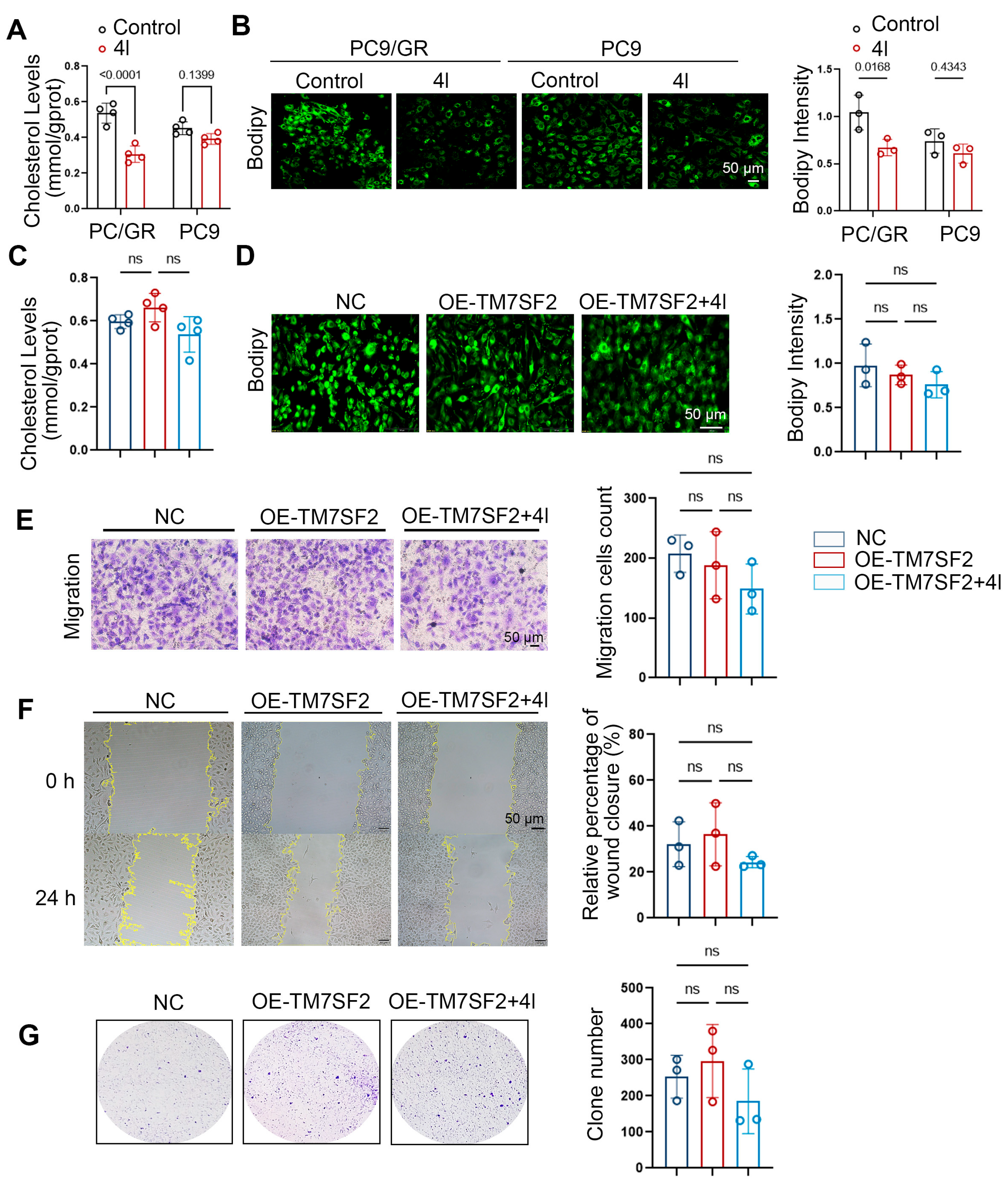
2.6. Compound 4l Reverses NSCLC Chemoresistance by TM7SF2 Mediated Modulating Cholesterol Homeostasis
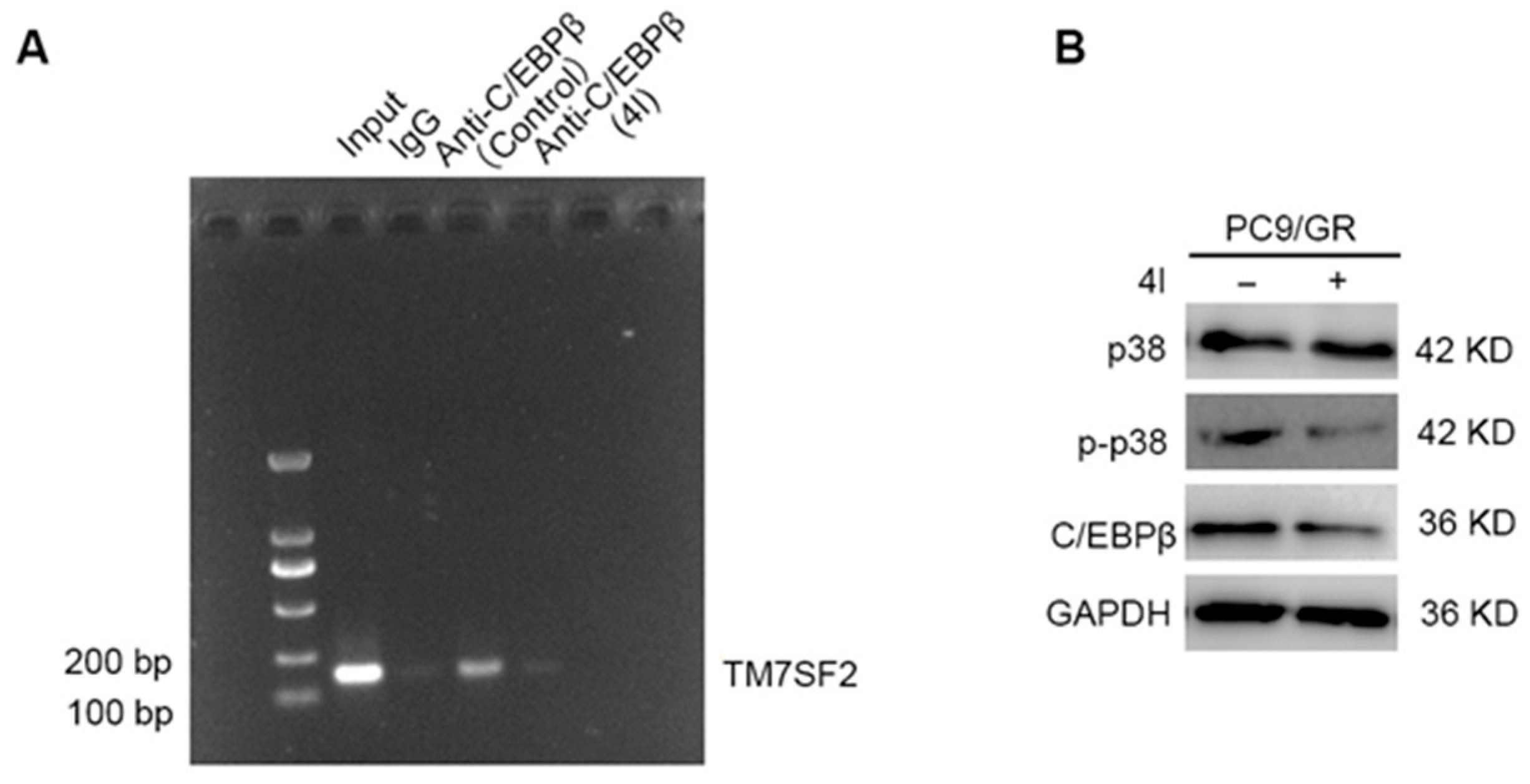
2.7. 4l Downregulates the Expression of TM7SF2 Through the Transcription Factor C/EBPβ
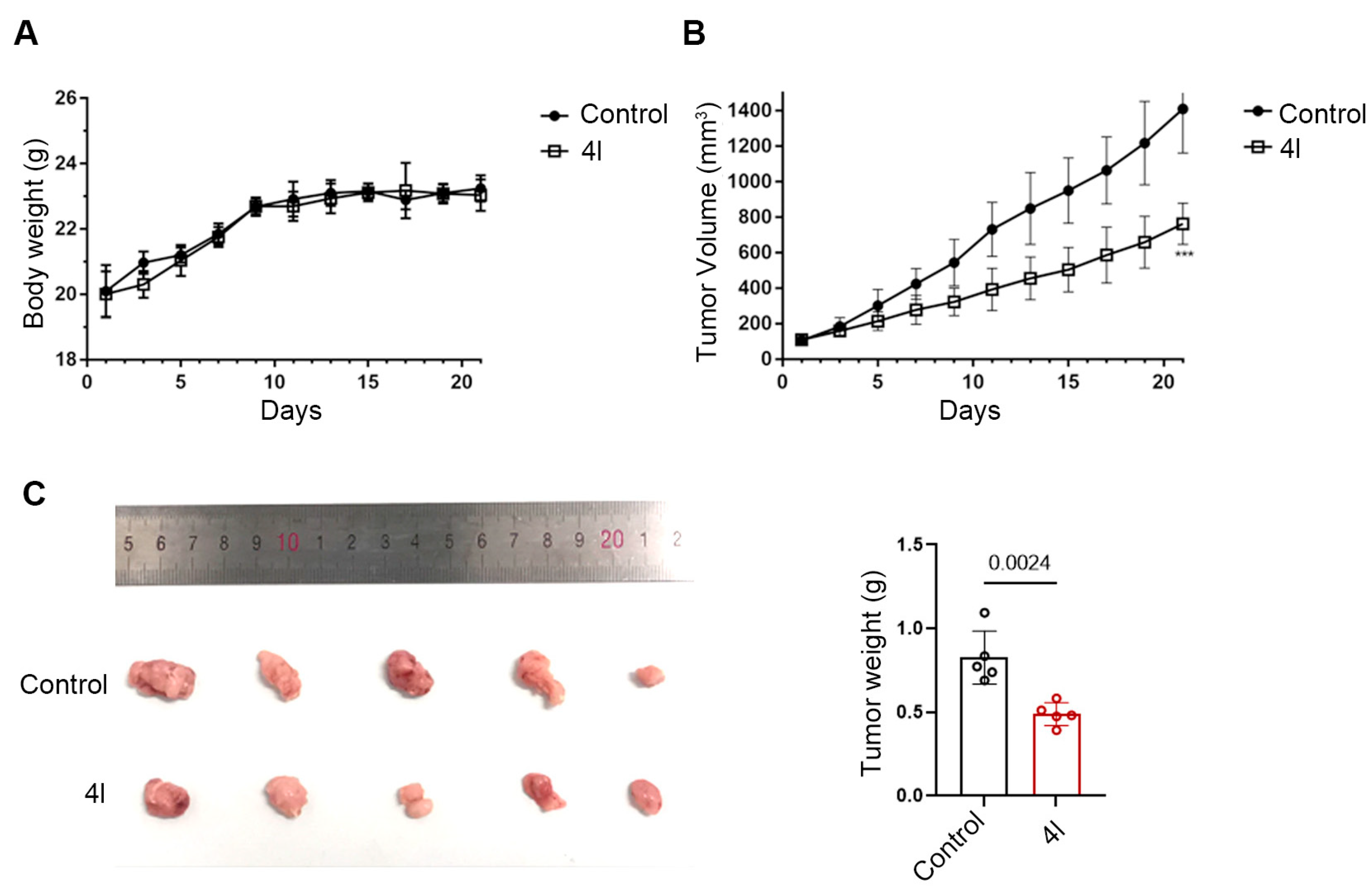
2.8. 4l Significantly Inhibits Tumorigenicity of Drug-Resistant Strains In Vivo
3. Discussion
4. Materials and Methods
4.1. Drug Design
4.2. Chemistry
4.3. Cell Culture and Reagents
4.4. Cell Survival and Growth Assays
4.5. Cell Transfection and Plasmid Construction
4.6. Wound Healing Assay
4.7. Cell Migration Assay
4.8. Colony Formation Assay
4.9. Apoptosis Detection
4.10. Intracellular Cholesterol Quantification
4.11. Molecular Interaction and Expression Profiling
4.12. Tumor Growth and Response Monitoring
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C/EBPβ | CCAAT-enhancer-binding proteins |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Gene and Genomes |
| NSCLC | Non-small cell lung cancer |
References
- Yuan, Y.; Lei, Y.; Xu, M.; Zhao, B.; Xu, S. Bioactive Terpenes from Marine Sponges and Their Associated Organisms. Mar. Drugs 2025, 23, 96. [Google Scholar] [CrossRef]
- Cai, C.; Yang, D.; Cao, Y.; Peng, Z.; Wang, Y.; Xi, J.; Yan, C.; Li, X. Anticancer potential of active alkaloids and synthetic analogs derived from marine invertebrates. Eur. J. Med. Chem. 2024, 279, 116850. [Google Scholar] [CrossRef]
- Santhiravel, S.; Dave, D.; Shahidi, F. Bioactives from marine resources as natural health products: A review. Pharmacol. Rev. 2025, 77, 100006. [Google Scholar] [CrossRef]
- Strickland, S.A.; Vey, N. Diagnosis and treatment of therapy-related acute myeloid leukemia. Crit. Rev. Oncol. Hematol. 2022, 171, 103607. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar. Drugs 2014, 12, 255–278. [Google Scholar] [CrossRef]
- Esposito, R.; Federico, S.; Bertolino, M.; Zupo, V.; Costantini, M. Marine Demospongiae: A Challenging Treasure of Bioactive Compounds. Mar. Drugs 2022, 20, 244. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.W.; Li, X.Y.; He, X.; Sun, Q.; Zhang, T.J.; Meng, F.H. (+)- and (-)-Itampolin A: First Total Synthesis, Anticancer Effect Through Inhibition of Phospho p38 Expression. Curr. Org. Synth. 2017, 14, 912–917. [Google Scholar] [CrossRef]
- Liang, J.W.; Wang, M.Y.; Wang, S.; Li, X.Y.; Meng, F.H. Fragment-Based Structural Optimization of a Natural Product Itampolin A as a p38α Inhibitor for Lung Cancer. Mar. Drugs 2019, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of non-small cell lung cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and future development in lung cancer diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef]
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol. Cancer 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Broderick, S.R. Adjuvant and Neoadjuvant Immunotherapy in Non–small Cell Lung Cancer. Thorac. Surg. Clin. 2020, 30, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Russo, A.; Raez, L.E.; Cervetto, C.R. Adjuvant therapy in non-small cell lung cancer: Is targeted therapy joining the standard of care? Expert Rev. Anticancer. Ther. 2021, 21, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lakshmanan, J.; Motameni, A.; Harbrecht, B.G. MicroRNA-203 suppresses proliferation in liver cancer associated with PIK3CA, p38 MAPK, c-Jun, and GSK3 signaling. Mol. Cell. Biochem. 2018, 441, 89–98. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, H. MAP17 contributes to non-small cell lung cancer progression via suppressing miR-27a-3p expression and p38 signaling pathway. Cancer Biol. Ther. 2021, 22, 19–29. [Google Scholar] [CrossRef]
- Sorek, H.; Rudi, A.; Aknin, M.; Gaydou, E.; Kashman, Y. Itampolins A and B, new brominated tyrosine derivatives from the sponge Iotrochota purpurea. Tetrahedron Lett. 2006, 47, 7237–7239. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Huber, R.; Pietsch, D.; Panterodt, T.; Brand, K. Regulation of C/EBPβ and resulting functions in cells of the monocytic lineage. Cell. Signal. 2012, 24, 1287–1296. [Google Scholar] [CrossRef]
- Ye, J.; Zhou, F.; Al-Kareef, A.M.; Wang, H. Anticancer agents from marine sponges. J. Asian Nat. Prod. Res. 2015, 17, 64–88. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, H.; Halasz, M.; Herak Bosnar, M. Sponges: A Reservoir of Genes Implicated in Human Cancer. Mar. Drugs 2018, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Mukai, H.; Kubota, T.; Aoyama, K.; Mikami, Y.; Fromont, J.; Kobayashi, J. Tyrokeradines A and B, new bromotyrosine alkaloids with an imidazolyl-quinolinone moiety from a Verongid sponge. Bioorganic Med. Chem. Lett. 2009, 19, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1358–1375. [Google Scholar] [CrossRef]
- Bonney, E.A. Mapping out p38 MAPK. Am. J. Reprod. Immunol. 2017, 77, e12652. [Google Scholar] [CrossRef]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef]
- Gatticchi, L.; Petricciuolo, M.; Scarpelli, P.; Macchioni, L.; Corazzi, L.; Roberti, R. Tm7sf2 gene promotes adipocyte differentiation of mouse embryonic fibroblasts and improves insulin sensitivity. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118897. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef]
- Salotti, J.; Johnson, P.F. Regulation of senescence and the SASP by the transcription factor C/EBPβ. Exp. Gerontol. 2019, 128, 110752. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Luo, H.; Zhu, L.; Zhao, Y.; Tian, H.; Wang, R.; Shang, P.; Zhao, Y. Microgravity activates p38 MAPK-C/EBPβ pathway to regulate the expression of arginase and inflammatory cytokines in macrophages. Inflamm. Res. 2015, 64, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the wheels of the cancer machine: The role of lipid metabolism in cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X. Nanomedicine solutions to intricate physiological-pathological barriers and molecular mechanisms of tumor multidrug resistance. J. Control. Release 2020, 323, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Bhave, R.; Vijayaraghavalu, S.; Stine, A.; Kooijman, E.; Labhasetwar, V. Drug Resistance in Breast Cancer Cells: Biophysical Characterization of and Doxorubicin Interactions with Membrane Lipids. Mol. Pharm. 2010, 7, 2334–2348. [Google Scholar] [CrossRef]
- Vijayaraghavalu, S.; Peetla, C.; Lu, S.; Labhasetwar, V. Epigenetic modulation of the biophysical properties of drug-resistant cell lipids to restore drug transport and endocytic functions. Mol. Pharm. 2012, 9, 2730–2742. [Google Scholar] [CrossRef]
- Henriques Palma, G.B.; Kaur, M. Cholesterol Depletion Modulates Drug Resistance Pathways to Sensitize Resistant Breast Cancer Cells to Tamoxifen. Anticancer Res. 2022, 42, 565. [Google Scholar] [CrossRef]
- Sun, Q.; Liang, J.W.; Zhang, Q.; Wang, X.; Zhao, N.; Meng, F. Pharmacokinetics and Tissue Distribution of Itampolin A following Intragastric and Intravenous Administration in Rats Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2024, 29, 2652. [Google Scholar] [CrossRef]

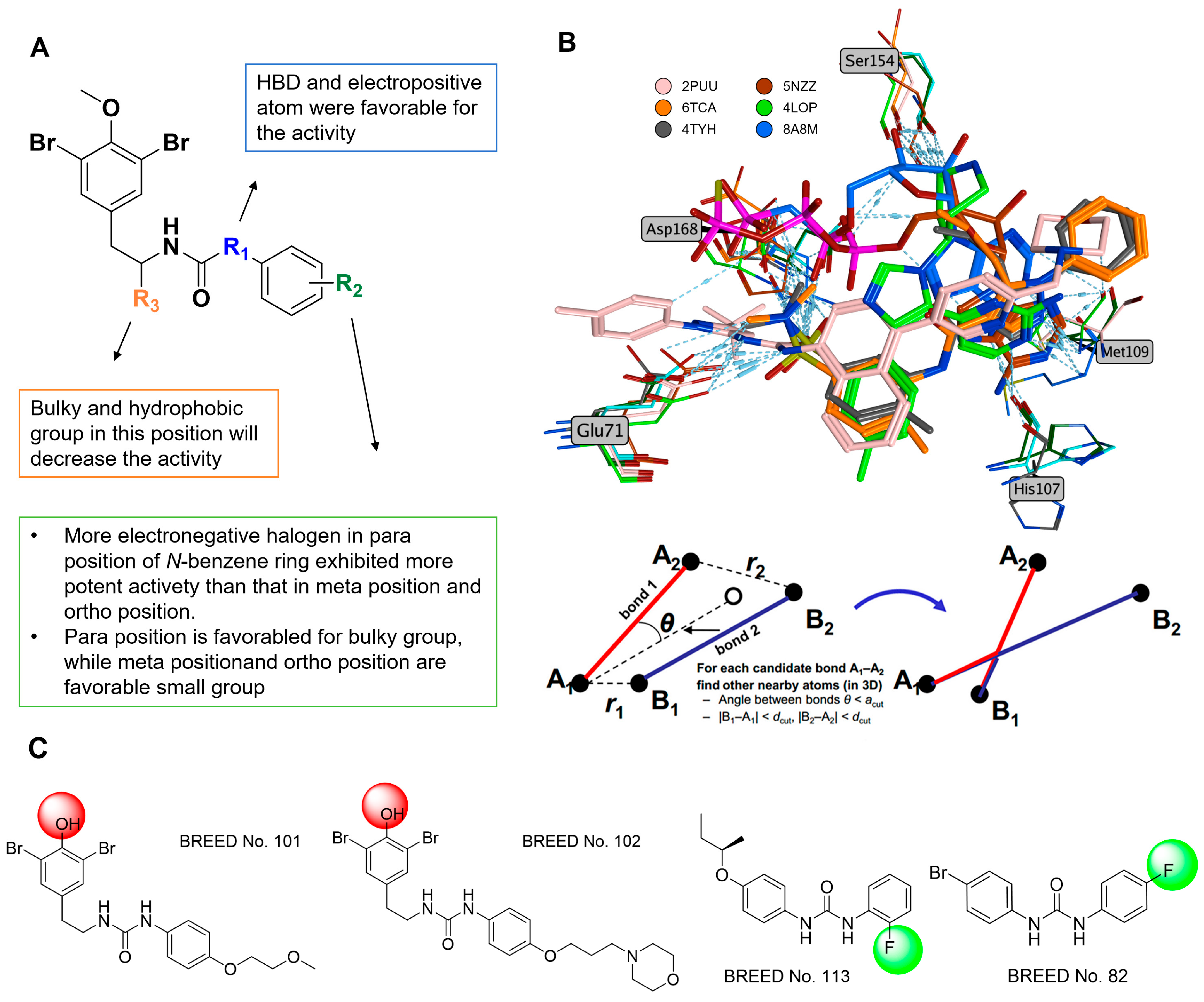
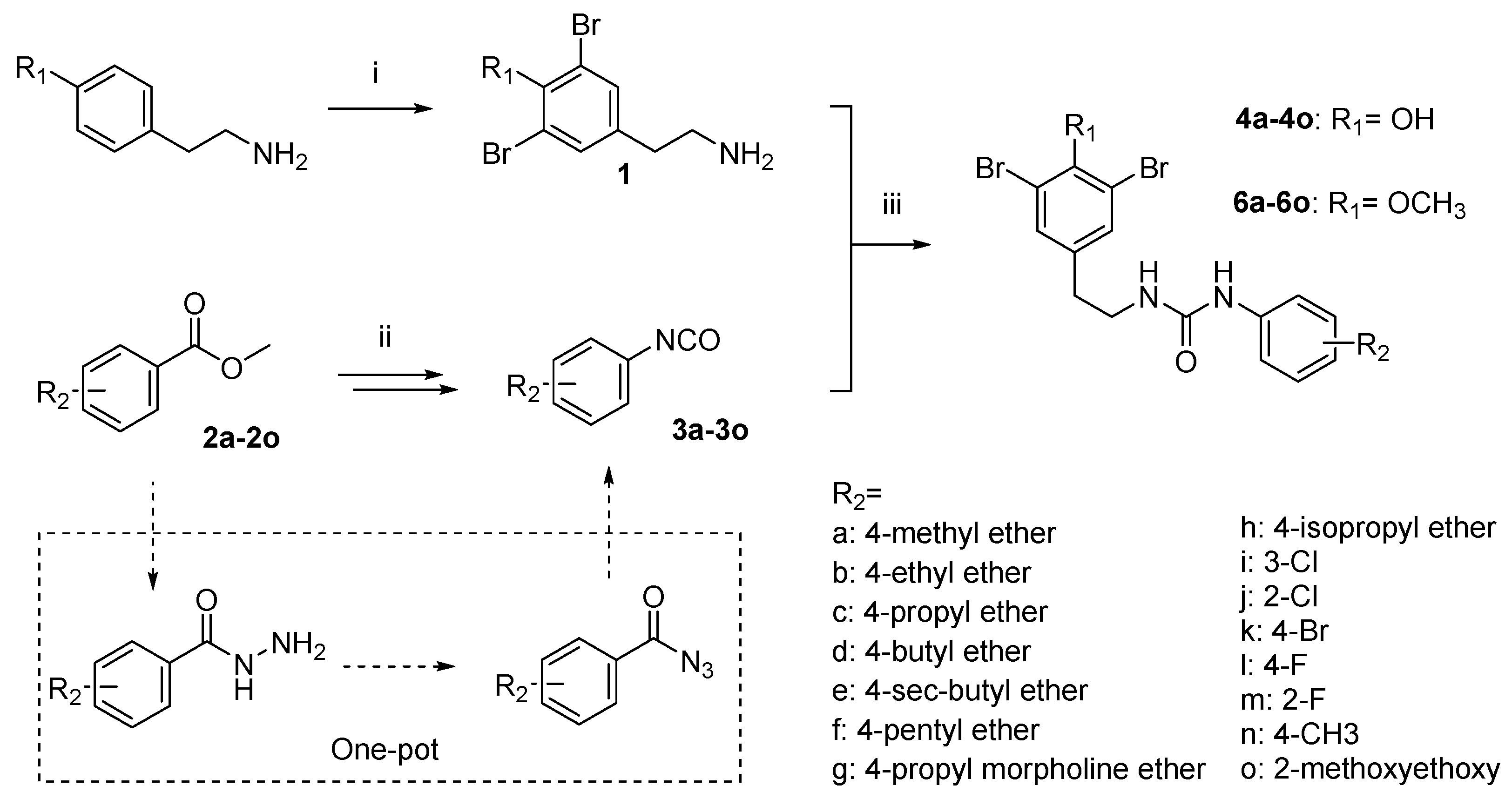

| No. | IC50 (nM) | No. | IC50 (nM) |
|---|---|---|---|
| 4a | 265.5 ± 20.7 | 6aa | 262.7 ± 3.3 |
| 4b | 170.0 ± 12.1 | 6b | 221.4 ± 4.2 |
| 4c | 296.6 ± 17.5 | 6c | 701.4 ± 7.4 |
| 4d | 914.5 ± 44.3 | 6d | - |
| 4e | - | 6e | 920.3 ± 6.1 |
| 4f | - | 6f | - |
| 4g | 167.4 ± 13.6 | 6g | 11.7 ± 3.0 |
| 4h | 849.5 ± 32.7 | 6h | 329.4 ± 44.2 |
| 4i | 13.9 ± 2.7 | 6i | 17.5 ± 2.4 |
| 4j | 74.4 ± 13.0 | 6j | 71.2 ± 4.5 |
| 4k | 51.1 ± 9.5 | 6k | 169.0 ± 2.6 |
| 4l | 9.7 ± 1.4 | 6l | 21.5 ± 4.6 |
| 4m | 11.3 ± 26.2 | 6m | 13.6 ± 3.0 |
| 4n | 38.7 ± 8.1 | 6n | 299.6 ± 11.7 |
| 4o | 10.2 ± 2.3 | 6o | 7.9 ± 1.7 |
| BIRB-796 | 11.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.-Y.; Ji, S.-C.; Xie, S.-H.; Chen, Y.; Lin, C.-X.; Huang, X.; Wang, Y.-Q.; Liang, J.-W.; Liu, Y. Design and Synthesis of Marine-Inspired Itampolin A Derivatives to Overcome Chemoresistance in NSCLC via Cholesterol Homeostasis Modulation. Mar. Drugs 2025, 23, 357. https://doi.org/10.3390/md23090357
Zhang H-Y, Ji S-C, Xie S-H, Chen Y, Lin C-X, Huang X, Wang Y-Q, Liang J-W, Liu Y. Design and Synthesis of Marine-Inspired Itampolin A Derivatives to Overcome Chemoresistance in NSCLC via Cholesterol Homeostasis Modulation. Marine Drugs. 2025; 23(9):357. https://doi.org/10.3390/md23090357
Chicago/Turabian StyleZhang, Hai-Ying, Shun-Chang Ji, Si-Hua Xie, Yu Chen, Cai-Xia Lin, Xu Huang, Yi-Qiao Wang, Jing-Wei Liang, and Yan Liu. 2025. "Design and Synthesis of Marine-Inspired Itampolin A Derivatives to Overcome Chemoresistance in NSCLC via Cholesterol Homeostasis Modulation" Marine Drugs 23, no. 9: 357. https://doi.org/10.3390/md23090357
APA StyleZhang, H.-Y., Ji, S.-C., Xie, S.-H., Chen, Y., Lin, C.-X., Huang, X., Wang, Y.-Q., Liang, J.-W., & Liu, Y. (2025). Design and Synthesis of Marine-Inspired Itampolin A Derivatives to Overcome Chemoresistance in NSCLC via Cholesterol Homeostasis Modulation. Marine Drugs, 23(9), 357. https://doi.org/10.3390/md23090357





