Phycobiliprotein Extract from Arthrospira platensis Boosts Immune Function in Pacific Oysters (Magallana gigas)
Abstract
1. Introduction
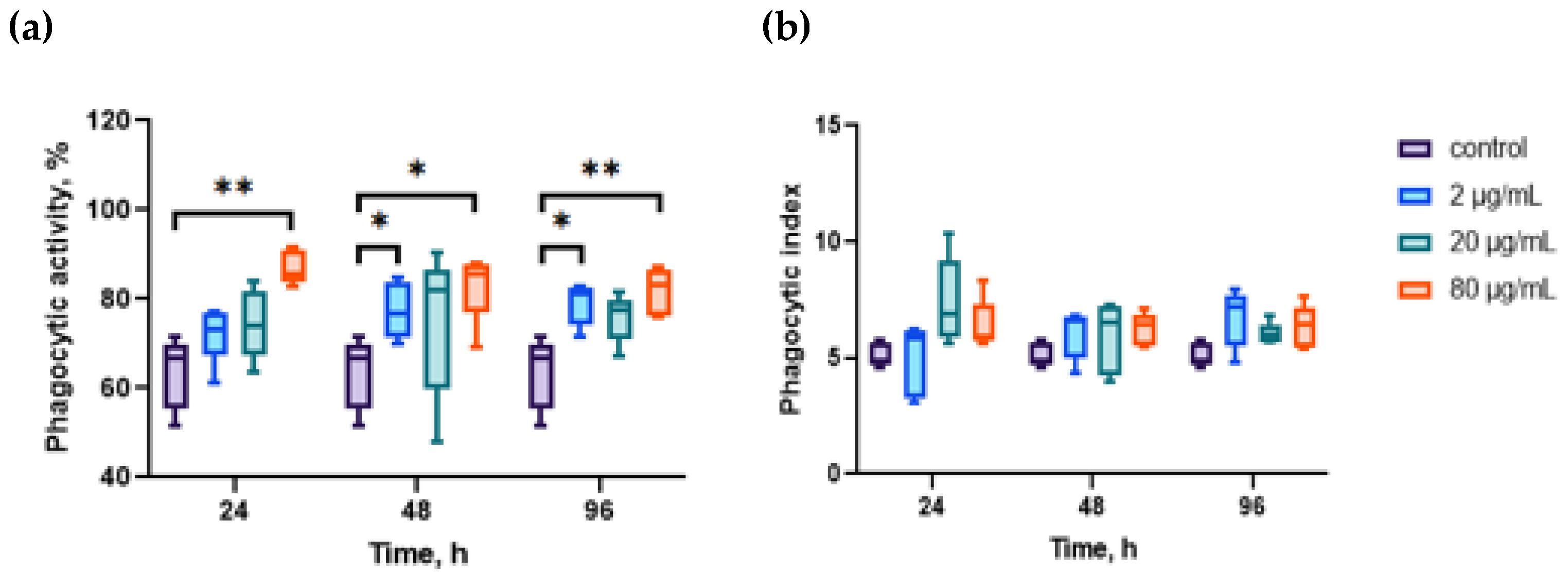
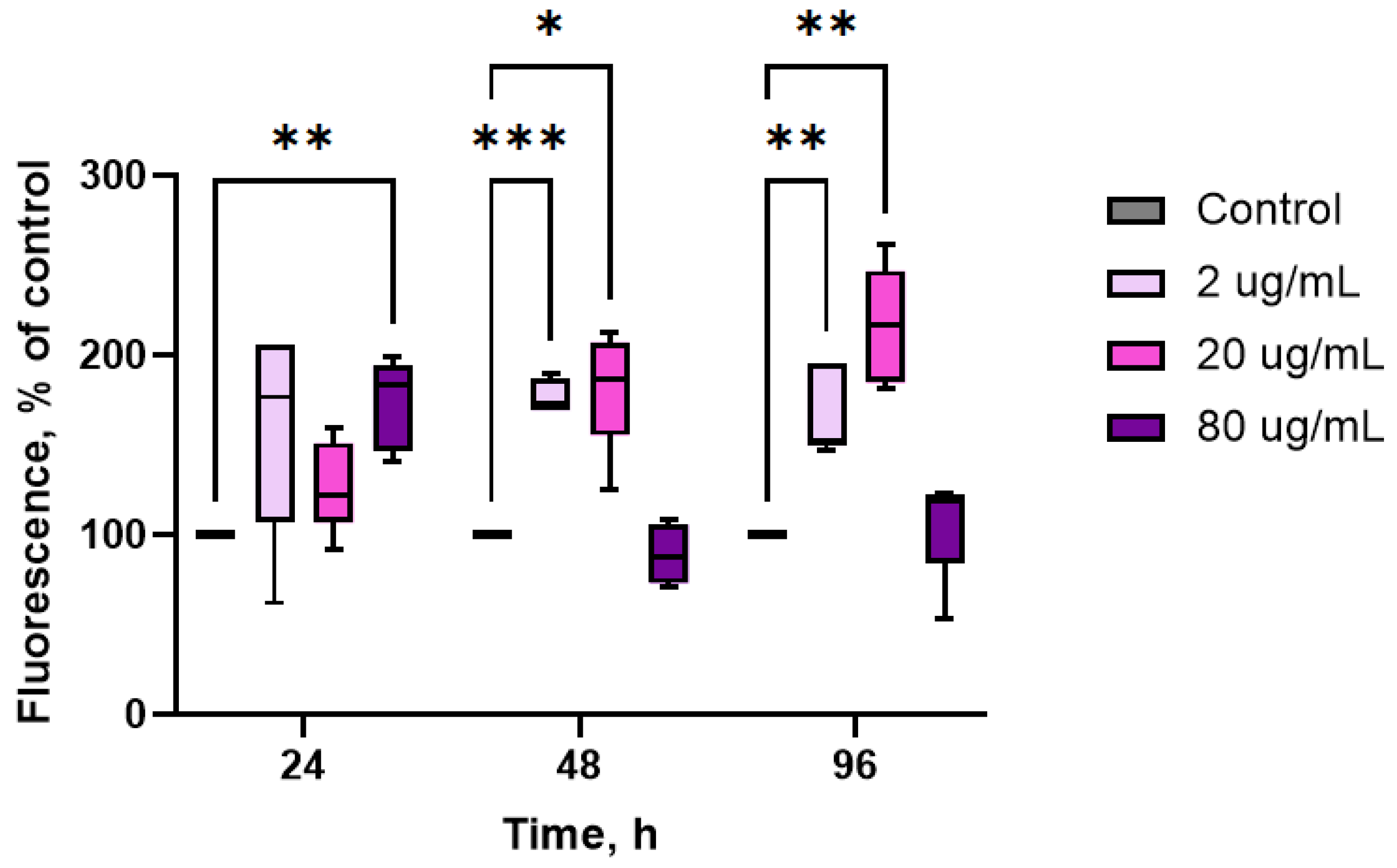
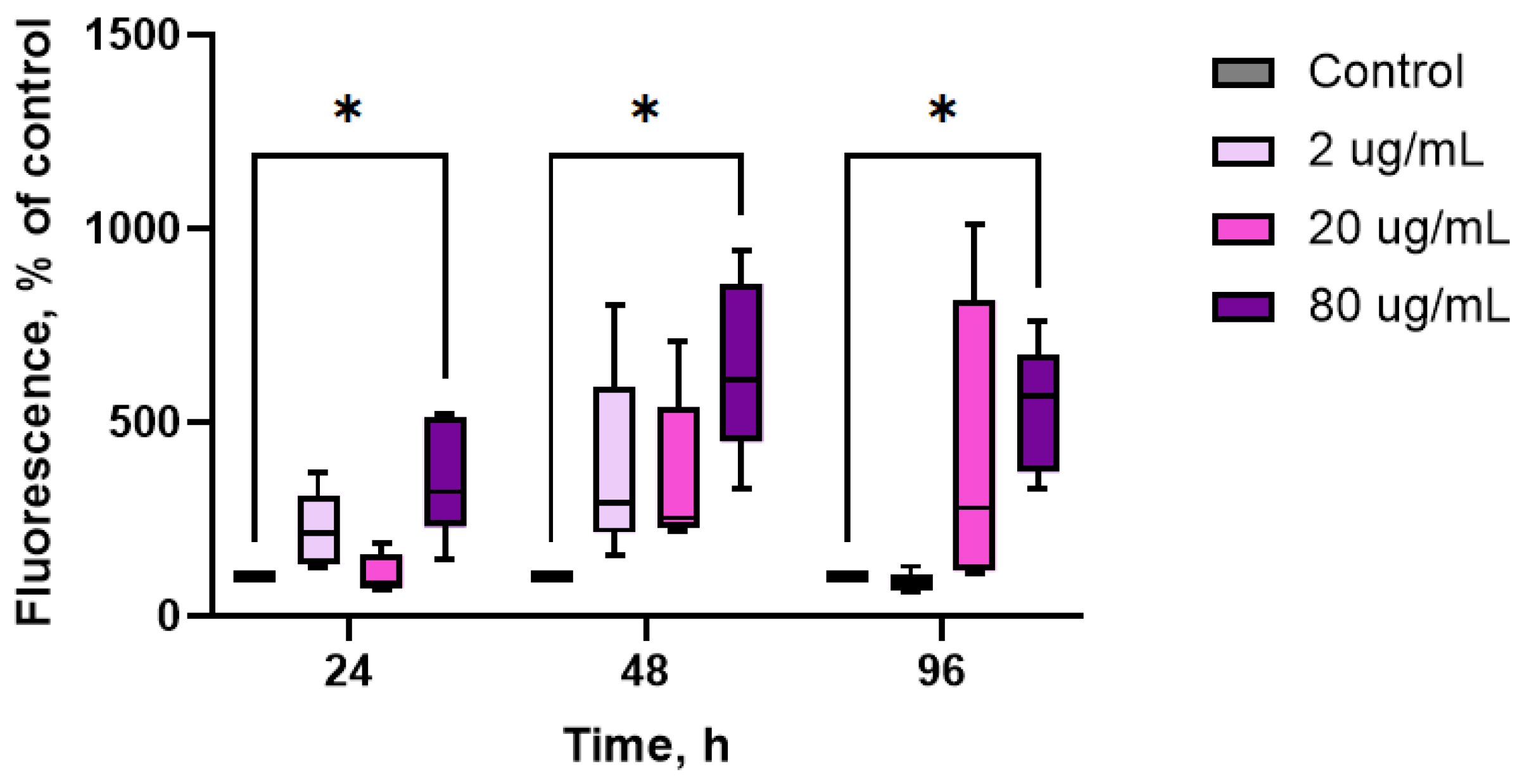
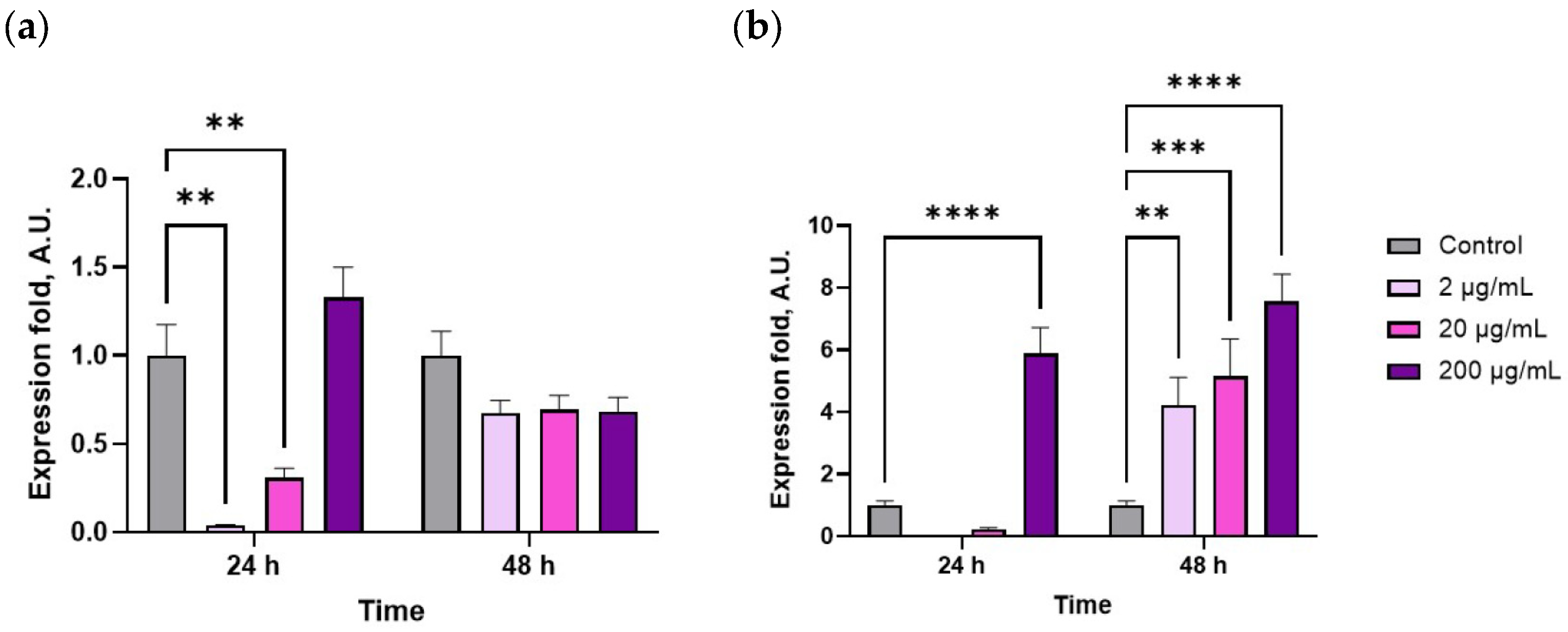
2. Results
3. Discussion
4. Materials and Methods
4.1. Test Substance
4.2. Experimental Animals
4.3. Feeding Experiment
4.4. Investigated Parameters
4.4.1. Flow Cytometric Analysis
4.4.2. Microscopic Observations
4.5. RNA Extraction and qRT-PCR Analysis of Gene Expression
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Demann, F.; Wegner, K.M. Infection by invasive parasites increases susceptibility of native hosts to secondary infection via modulation of cellular immunity. J. Anim. Ecol. 2019, 88, 427–438. [Google Scholar] [CrossRef]
- Dieudonne, J.; Carroll, J.M. The impacts of boring sponges on oyster health across multiple sites and tidal heights. Estuaries Coasts 2022, 45, 213–224. [Google Scholar] [CrossRef]
- Maulu, S.; Hasimuna, O.J.; Haambiya, L.H.; Monde, C.; Musuka, C.G.; Makorwa, T.H.; Nsekanabo, J.D. Climate change effects on aquaculture production: Sustainability implications, mitigation, and adaptations. Front. Sustain. Food Syst. 2021, 5, 609097. [Google Scholar] [CrossRef]
- Byers, J.E. Marine parasites and disease in the era of global climate change. Annu. Rev. Mar. Sci. 2021, 13, 397–420. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K. Neutrophils and aquatic pathogens. Parasite Immunol. 2022, 44, e12915. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Zhang, H.; Zheng, H. Selective breeding of edible bivalves and its implication of global climate change. Rev. Aquac. 2020, 12, 2559–2572. [Google Scholar] [CrossRef]
- Nascimento-Schulze, J.C.; Bean, T.P.; Houston, R.D.; Santos, E.M.; Sanders, M.B.; Lewis, C.; Ellis, R.P. Optimizing hatchery practices for genetic improvement of marine bivalves. Rev. Aquac. 2021, 13, 2289–2304. [Google Scholar] [CrossRef]
- Lodeiros, C.; Rodríguez-Pesantes, D.; Márquez, A.; Revilla, J.; Chávez-Villalba, J.; Sonnenholzner, S. Suspended cultivation of the Pacific oyster Crassostrea gigas in the Eastern Tropical Pacific. Aquac. Int. 2018, 26, 337–347. [Google Scholar] [CrossRef]
- Pirkova, A.V.; Ladygina, L.V.; Kholodov, V.I. Biologicheskie i Biotekhnicheskie Aspekty Organizatsii i Funktsionirovaniya Ustrichnogo Pitomnika na Chernom More (Biological and Biotechnical Aspects of Organization and Functioning of Oyster Hatchery in the Black Sea); FITs InBYuM: Sevastopol, Ukraine, 2020. [Google Scholar]
- Guo, X.; Ford, S.E. Infectious diseases of marine molluscs and host responses as revealed by genomic tools. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150206. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Nguyen, X.T.; Tran, D.M.T.; Vu, Q.T.; Nguyen, V.H.; Nguyen, D.T.; Do, M.T.; Nguyen, T.L.; Lien Ly, T.N.; Ngoc, T. Adsorption ability for toxic chromium (VI) ions in aqueous solution of some modified oyster shell types. Bioinorg. Chem. Appl. 2020, 2020, 2435777. [Google Scholar] [CrossRef]
- Adzigbli, L.; Hao, R.; Jiao, Y.; Deng, Y.; Du, X.; Wang, Q.; Huang, R. Immune response of pearl oysters to stress and diseases. Rev. Aquac. 2020, 12, 513–523. [Google Scholar] [CrossRef]
- Pourmozaffar, S.; Tamadoni Jahromi, S.; Rameshi, H.; Sadeghi, A.; Bagheri, T.; Behzadi, S.; Gozari, M.; Zahedi, M.R.; Abrari Lazarjani, S. The role of salinity in physiological responses of bivalves. Rev. Aquac. 2020, 12, 1548–1566. [Google Scholar] [CrossRef]
- Huiping, Y. Immunological assays of hemocytes in molluscan bivalves as biomarkers to evaluate stresses for aquaculture. In Proceedings of the 47th UJNR. Bulletin of Japan Fisheries Research and Education Agency; Japan Fisheries Research and Education Agency: Yokohama, Japan, 2021; pp. 31–45. [Google Scholar]
- Coates, C.J.; Söderhäll, K. The stress–immunity axis in shellfish. J. Invertebr. Pathol. 2021, 186, 107492. [Google Scholar] [CrossRef] [PubMed]
- Söderhäll, K. Invertebrate immunology–some thoughts about past and future research. Dev. Comp. Immunol. 2024, 161, 105256. [Google Scholar] [CrossRef] [PubMed]
- Kamermans, P.; Saurel, C. Interacting climate change effects on mussels (Mytilus edulis and M. galloprovincialis) and oysters (Crassostrea gigas and Ostrea edulis): Experiments for bivalve individual growth models. Aquat. Living Resour. 2022, 35, 1. [Google Scholar] [CrossRef]
- Skelton, B.M.; Jeffs, A.G. An assessment of the use of macroalgae to improve the retention of Greenshell™ mussel (Perna canaliculus) spat in longline culture. Aquac. Int. 2021, 29, 1683–1695. [Google Scholar] [CrossRef]
- Yusoff, F.M.; Banerjee, S.; Nagao, N.; Imaizumi, Y.; Shariff, M.; Toda, T. Use of Microalgae Pigments in Aquaculture. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M., Zepka, L., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar] [CrossRef]
- Vijayaram, S.; Sun, Y.Z.; Zuorro, A.; Ghafarifarsani, H.; Van Doan, H.; Hoseinifar, S.H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol. 2022, 130, 294–308. [Google Scholar] [CrossRef]
- Bahi, A.; Ramos-Vega, A.; Angulo, C.; Monreal-Escalante, E.; Guardiola, F.A. Microalgae with immunomodulatory effects on fish. Rev. Aquac. 2023, 15, 1522–1539. [Google Scholar] [CrossRef]
- Vijayaram, S.; Ringø, E.; Ghafarifarsani, H.; Hoseinifar, S.H.; Ahani, S.; Chou, C.C. Use of algae in aquaculture: A review. Fishes 2024, 9, 63. [Google Scholar] [CrossRef]
- Biabani Asrami, M.; Sudagar, M.; Shahraki, N.; Vahdat, S. Effect of extracted phycocyanin from Spirulina platensis on growth parameters, colorations, digestive enzymes and body chemical compositions of guppy fish (Poecilia reticulata). J. Surv. Fish. Sci. 2019, 6, 21–34. [Google Scholar] [CrossRef]
- Nonwachai, T.; Purivirojkul, W.; Limsuwan, C.; Chuchird, N.; Velasco, M.; Dhar, A.K. Growth, nonspecific immune characteristics, and survival upon challenge with Vibrio harveyi in Pacific white shrimp (Litopenaeus vannamei) raised on diets containing algal meal. Fish Shellfish Immunol. 2010, 29, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Maliwat, G.C.; Velasquez, S.; Robil, J.L.; Chan, M.; Traifalgar, R.F.; Tayamen, M.; Ragaza, J.A. Growth and immune response of giant freshwater prawn Macrobrachium rosenbergii postlarvae fed diets containing Chlorella vulgaris. Aquac. Res. 2017, 48, 1666–1676. [Google Scholar] [CrossRef]
- Nagappan, S.; Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Mahata, C.; Al-Jabri, H.; Vatland, A.K.; Kumar, G. Potential of microalgae as a sustainable feed ingredient for aquaculture. J. Biotechnol. 2021, 341, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.; Rathi, D.N.G.; Mustar, S.; Lee, J.C. Microalgae as an Eco-Friendly and Functional Ingredient for Sustainable Aquafeed. Aquac. J. 2025, 5, 14. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.A.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Kizhakkekarammal, S.P.; Angel, J.R.J.; Thirugnanamurthy, S.; Suresh, S.; Nathamuni, S.; Raja, R.A.; Kumar, S.; Tomy, S.; Syama Dayal, J.; Paran, B.C.; et al. Effect of dietary C-phycocyanin on growth, survival, haematology, immune response, gut microbiome and disease resistance of Pacific white shrimp, Penaeus vannamei. Aquac. Res. 2022, 53, 6292–6309. [Google Scholar] [CrossRef]
- Thazeem, B.; Umesh, M.; Sarojini, S.; Vyas, G.A.; Sankar, S.A.; Sapthami, K.; Suresh, S.; Stanly, L.M. Developments in feeds in aquaculture sector: Contemporary aspects. In Aquaculture Science and Engineering; Springer Nature: Singapore, 2022; pp. 35–78. [Google Scholar] [CrossRef]
- Rosas, V.T.; Poersch, L.H.; Romano, L.A.; Tesser, M.B. Feasibility of the use of Spirulina in aquaculture diets. Rev. Aquac. 2019, 11, 1367–1378. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, S.C.; Li, Y.L.; Li, J.; Liu, H.P. Hemocyte-mediated phagocytosis in crustaceans. Front. Immunol. 2020, 11, 268. [Google Scholar] [CrossRef]
- Anisimova, A.A. Morphofunctional parameters of hemocytes in the assessment of the physiological status of bivalves. Russ. J. Mar. Biol. 2013, 39, 381–391. [Google Scholar] [CrossRef]
- Mao, T.K.; Van de Water, J.; Gershwin, M.E. Effect of Spirulina on the secretion of cytokines from peripheral blood mononuclear cells. J. Med. Food 2000, 3, 135–140. [Google Scholar] [CrossRef]
- Khan, Z.; Bhadouria, P.; Bisen, P.S. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Khafaga, A.F.; Abd El-Hack, M.E.; Al-Gabri, N.A.; Abukhalil, M.H.; Alfwuaires, M.A.; Bin-Jumah, M.; Alkahtani, S.; Abdel-Daim, M.M.; Aleya, L.; et al. Spirulina platensis ameliorates the sub-chronic toxicities of lead in rabbits via anti-oxidative, anti-inflammatory, and immune stimulatory properties. Sci. Total Environ. 2020, 701, 134879. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.; Huang, J.; Huang, C.Y.; Liu, Z.X.; Yeh, H.Y.; Huang, H.T.; Chen, L.L.; Nan, F.; Lee, M.C. Phycoerythrin from Colaconema sp. has immunostimulatory effects on the whiteleg shrimp Litopenaeus vannamei and increases resistance to Vibrio parahaemolyticus and white spot syndrome virus. Animals 2021, 11, 2371. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Chew, P.F.; Soh, B.S.; Tham, L.Y. Enhancing phagocytic activity of hemocytes and disease resistance in the prawn Penaeus merguiensis by feeding Spirulina platensis. J. Appl. Phycol. 2003, 15, 279–287. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, W.X. Lysosomal Cu (I)/Cu (II) dependence of antimicrobial ability of oyster hemocytes and regulation of phagolysosomal system. Environ. Sci. Technol. 2023, 57, 20219–20227. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, J.C.; Tayag, C.M.; Li, H.F.; Putra, D.F.; Kuo, Y.H.; Bai, J.C.; Chang, Y.H. Spirulina elicits the activation of innate immunity and increases resistance against Vibrio alginolyticus in shrimp. Fish Shellfish Immunol. 2016, 55, 690–698. [Google Scholar] [CrossRef]
- Elabd, H.; Wang, H.P.; Shaheen, A.; Matter, A. Nano Spirulina dietary supplementation augments growth, antioxidative and immunological reactions, digestion, and protection of Nile tilapia Oreochromis niloticus against Aeromonas veronii and some physical stressors. Fish Physiol. Biochem. 2020, 46, 2143–2155. [Google Scholar] [CrossRef]
- Abdo, S.E.; El-Nahas, A.F.; Abdellatif, R.E.; Mohamed, R.; Helal, M.A.; Azzam, M.M.; Cerbo, A.D.; El-Kassas, S. Combined dietary Spirulina platensis and Citrus limon essential oil enhances the growth, immunity, antioxidant capacity and intestinal health of Nile tilapia. Vet. Sci. 2024, 11, 474. [Google Scholar] [CrossRef]
- Donaghy, L.; Kraffe, E.; Le Goïc, N.; Lambert, C.; Volety, A.K.; Soudant, P. Reactive oxygen species in unstimulated hemocytes of the Pacific oyster Crassostrea gigas: A mitochondrial involvement. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 162, 235–243. [Google Scholar] [CrossRef]
- Gostyukhina, O.L.; Kladchenko, E.S.; Chelebieva, E.S.; Tkachuk, A.A.; Lavrichenko, D.S.; Andreyeva, A.Y. Short-time salinity fluctuations are strong activators of oxidative stress in Mediterranean mussel (Mytilus galloprovincialis). Ecol. Montenegrina 2023, 63, 46–58. [Google Scholar] [CrossRef]
- Andreyeva, A.; Gostyukhina, O.; Gavruseva, T.; Sigacheva, T.; Tkachuk, A.; Podolskaya, M.; Chelebieva, E.; Kladchenko, E. Mediterranean mussels (Mytilus galloprovincialis) under salinity stress: Effects on antioxidant capacity and gill structure. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2025, 343, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Ardia, D.R.; Gantz, J.E.; Schneider, B.; Strebel, S. Costs of immunity in insects: An induced immune response increases metabolic rate and decreases antimicrobial activity. Funct. Ecol. 2012, 26, 732–739. [Google Scholar] [CrossRef]
- Bajgar, A.; Kucerova, K.; Jonatova, L.; Tomcala, A.; Schneedorferova, I.; Okrouhlik, J.; Dolezal, T. Extracellular adenosine mediates a systemic metabolic switch during immune response. PLoS Biol. 2015, 13, e1002135. [Google Scholar] [CrossRef]
- Brokordt, K.; Defranchi, Y.; Esposito, I.; Cárcamo, C.; Schmitt, P.; Mercado, L.; Fuente-Ortega, E.; Rivera-Ingraham, G.A. Reproduction–immunity trade-off in a mollusk: Hemocyte energy metabolism underlies cellular and molecular immune responses. Front. Physiol. 2019, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Xu-Bo, C.H.E.N.; Zi-Qiang, T.A.N.G.; Yang, Z.H.A.O.; Xiao-Sheng, H.U.A.N.G.; Zi-Ming, L.I.U. Dietary supplementation with Spirulina platensis on the growth, antioxidant, immune function, and heat shock protein expression of Opsariichthys bidens under mercury stress. J. Hydrobiol. Shuisheng Shengwu Xuebao 2025, 49, 1–12. [Google Scholar] [CrossRef]
- Werner, I.; Hinton, D.E. Field validation of hsp70 stress proteins as biomarkers in Asian clam (Potamocorbula amurensis): Is downregulation an indicator of stress? Biomarkers 1999, 4, 473–484. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.S. A review on the involvement of heat shock proteins (extrinsic chaperones) in response to stress conditions in aquatic organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution a l’Etude d’Une Cyanobacterie: Influence de Divers Facteurs Physiqueset Chimiques sur la Croissance et la Photosynthese de Spirulina Maxima. Ph.D. Thesis, University of Paris, Paris, France, 1966; 138p. [Google Scholar]
- Yao, T.; Huang, J.; Su, B.; Wei, L.; Zhang, A.H.; Zhang, D.F.; Zhou, Y.; Ma, G. Enhanced phycocyanin production of Arthrospira maxima by addition of mineral elements and polypeptides using response surface methodology. Front. Mar. Sci. 2022, 9, 1057201. [Google Scholar] [CrossRef]
- Stadnichuk, I.N. Phycobiliproteins; Mir Publishers: Moscow, Russia, 1990; p. 196. [Google Scholar]
- Soni, B.; Visavadiya, N.P.; Dalwadi, N.; Madamwar, D.; Winder, C.; Khalil, C. Purified C-phycoerythrin: Safety studies in rats and protective role against permanganate-mediated fibroblast-DNA damage. J. Appl. Toxicol. 2010, 30, 542–550. [Google Scholar] [CrossRef]
- Hamza-Chaffai, A. Usefulness of bioindicators and biomarkers in pollution biomonitoring. Int. J. Biotechnol. Wellness Ind. 2014, 3, 19–26. [Google Scholar] [CrossRef]
- Won, E.J.; Kim, K.T.; Choi, J.Y.; Kim, E.S.; Ra, K. Target organs of the Manila clam Ruditapes philippinarum for studying metal accumulation and biomarkers in pollution monitoring: Laboratory and in-situ transplantation experiments. Environ. Monit. Assess. 2016, 188, 478. [Google Scholar] [CrossRef]
- Fabbri, E.; Valbonesi, P.; Franzellitti, S. HSP expression in bivalves. Invertebr. Surviv. J. 2008, 5, 135–161. [Google Scholar]
- Andreyeva, A.Y.; Kladchenko, E.S.; Vyalova, O.Y.; Kukhareva, T.A. Functional characterization of the Pacific oyster, Crassostrea gigas (Bivalvia: Ostreidae), hemocytes under normoxia and short-term hypoxia. Turk. J. Fish. Aquat. Sci. 2021, 21, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Bayne, C.J. Bivalve mollusc hemocyte behaviors: Characterization of hemocyte aggregation and adhesion and their inhibition in the California mussel (Mytilus californianus). Biol. Bull. 1995, 188, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, B.; Dougherty, J.A.; Ponnalagu, D.; Singh, H.; Angelos, M.; Chen, C.A.; Khan, M. Measurement of oxidative stress markers in vitro using commercially available kits. Meas. Oxid. Oxidative Stress Biol. Syst. 2020, 39–60. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, S.Y.; Kim, P.J.; Dahms, H.U.; Han, K.N. Bio-effect-monitoring of long-term thermal wastes on the oyster, Crassostrea gigas, using heat shock proteins. Mar. Pollut. Bull. 2017, 119, 359–364. [Google Scholar] [CrossRef]
- Cao, R.; Liu, Y.; Wang, Q.; Zhang, Q.; Yang, D.; Liu, H.; Qu, Y.; Zhao, J. The impact of ocean acidification and cadmium on the immune responses of Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2018, 81, 456–462. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Gene Name | Nucleotide Sequence (5′–3′) | Efficiency (%) | Genbank Accession Number | |
|---|---|---|---|---|
| Elongation factor 1 alpha (EF1α) (reference) | F | AGTCACCAAGGCTGCACAGAAAG | 99.8 | AB122066.1 |
| R | TCCGACGTATTTCTTTGCGATGT | |||
| HSP70 | F | AACGGTATCCTGAATGTGTC | 101.1 | AF144646 |
| R | CTTCTCGTCTTCCTGCTTG | |||
| HSP90 | F | CGAGGAAGCAGAAGCAGAG | 98.8 | AF144646 |
| R | ATGTCACCAGACGGTTAGATAC | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreyeva, A.; Kukhareva, T.; Tkachuk, A.; Podolskaya, M.; Chelebieva, E.; Borovkov, A. Phycobiliprotein Extract from Arthrospira platensis Boosts Immune Function in Pacific Oysters (Magallana gigas). Mar. Drugs 2025, 23, 355. https://doi.org/10.3390/md23090355
Andreyeva A, Kukhareva T, Tkachuk A, Podolskaya M, Chelebieva E, Borovkov A. Phycobiliprotein Extract from Arthrospira platensis Boosts Immune Function in Pacific Oysters (Magallana gigas). Marine Drugs. 2025; 23(9):355. https://doi.org/10.3390/md23090355
Chicago/Turabian StyleAndreyeva, Aleksandra, Tatyana Kukhareva, Anastasiya Tkachuk, Maria Podolskaya, Elina Chelebieva, and Andrey Borovkov. 2025. "Phycobiliprotein Extract from Arthrospira platensis Boosts Immune Function in Pacific Oysters (Magallana gigas)" Marine Drugs 23, no. 9: 355. https://doi.org/10.3390/md23090355
APA StyleAndreyeva, A., Kukhareva, T., Tkachuk, A., Podolskaya, M., Chelebieva, E., & Borovkov, A. (2025). Phycobiliprotein Extract from Arthrospira platensis Boosts Immune Function in Pacific Oysters (Magallana gigas). Marine Drugs, 23(9), 355. https://doi.org/10.3390/md23090355







