Abstract
Inflammation plays a central role in various pathological conditions, necessitating the search for safer and more effective anti-inflammatory agents. This study investigates the anti-inflammatory activity of caulerpin, a bisindolic alkaloid isolated from Caulerpa racemosa. In vitro assays demonstrated that caulerpin significantly reduced nitric oxide, TNF-α, IL-6, and IL-12 levels in macrophages stimulated with LPS + IFN-γ, without affecting cell viability. In silico toxicity predictions using Protox 3.0 reinforce a favorable safety profile of caulerpin. Molecular docking and molecular dynamics simulations revealed its high-affinity binding to the glucocorticoid receptor ligand-binding domain (GR-LBD), suggesting a mechanism of action similar to dexamethasone. The involvement of the glucocorticoid receptor was confirmed by the partial reversal of caulerpin’s effects upon RU486 treatment. In vivo, caulerpin exhibited a favorable safety profile, with no signs of acute toxicity at an oral dose of 100 mg/kg. Moreover, in a mouse model of endotoxic shock, caulerpin administration significantly improved survival rates in a dose-dependent manner, providing complete protection at 4 mg/kg. These findings highlight caulerpin as a promising candidate for the development of novel anti-inflammatory therapies. Further studies are warranted to explore its pharmacokinetics, optimize its structure, and evaluate its efficacy in chronic inflammatory diseases.
1. Introduction
Inflammation is a complex biological process triggered by the immune system in response to various stimuli, including tissue damage, infections, toxins, and physical or psychological stressors [1]. Its primary function is to restore homeostasis by eliminating the cause of cellular injury, removing damaged cells, and initiating tissue repair [2]. Inflammation is typically classified as either acute or chronic. Acute inflammation is a rapid and short-term response that resolves within minutes to days, whereas chronic inflammation arises from unresolved acute phases, persisting for months or years and contributing to the development of neoplasms, degenerative disorders, and organ dysfunction [3,4,5].
Although the treatment of inflammatory diseases often relies on nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids, their prolonged use is associated with severe adverse effects, including gastrointestinal damage, renal impairment, cardiovascular complications, bone marrow suppression, and Cushing’s syndrome [6]. This highlights the need for new anti-inflammatory agents that offer both efficacy and improved safety profiles. In this context, caulerpin emerges as a promising alternative.
Caulerpin is a bisindolic alkaloid predominantly found in species of the genus Caulerpa (family Caulerpaceae), which includes approximately 97 species such as C. cylindracea, C. mexicana, C. peltata, and C. racemosa [7,8]. The edible green macroalga Caulerpa racemosa is traditionally harvested and cultivated in the Indo-Pacific region [9,10], and caulerpin is among its most abundant secondary metabolites [11,12]. This compound has been reported to exhibit a broad spectrum of biological activities, including anticancer, antitubercular, antioxidant, antidiabetic, and antiviral effects—particularly against SARS-CoV-2 [13,14,15]. Additionally, caulerpin displays notable anti-inflammatory properties, with experimental studies demonstrating its protective effects in murine models of peritonitis and colitis [16].
Although previous studies have reported the anti-inflammatory properties of caulerpin, important knowledge gaps remain regarding its safety profile and molecular mechanism of action. To the best of our knowledge, this is the first study to evaluate the acute toxicity of caulerpin and its therapeutic effects in a murine model of endotoxemic shock. Furthermore, this work provides novel evidence—supported by both in silico and in vitro approaches—implicating the glucocorticoid receptor (GR) as a key molecular target involved in caulerpin’s immunomodulatory activity. Therefore, this study aims to advance the pharmacological understanding of caulerpin and support its potential application in the treatment of inflammatory disorders.
2. Results and Discussion
Initially, the in silico toxicity assessment of caulerpin (Figure 1) was performed across various organs and toxicological categories, using a 0.7 probability threshold to define significant predictions [17].
Figure 1.
Chemical structure of caulerpin.
As shown in Table 1, caulerpin did not exhibit a significant probability for causing deleterious effects, such as hepatotoxicity, neurotoxicity, and nephrotoxicity, among others, suggesting a favorable safety profile in these aspects. Conversely, caulerpin demonstrated a high probability (>0.7) of being non-cardiotoxic, non-immunotoxic, and non-cytotoxic, further supporting its potential as a safe compound.

Table 1.
Predicted toxicity of caulerpin across different organs and biological categories, along with the associated probability for each prediction.
Using PROTOX 3.0, we also evaluated the interaction of caulerpin with cytochrome P450 (CYP450) enzymes (Figure 2). Among the analyzed CYPs, only CYP2E1 was predicted to be inactive; this enzyme primarily functions in the metabolism of xenobiotics. Caulerpin showed significant interaction with CYP2E1, an enzyme associated with the activation of pro-carcinogenic and hepatotoxic compounds. This suggests a lower risk of oxidative stress and liver injury, thereby supporting a favorable safety profile. Since CYP2E1 generates reactive oxygen species (ROS) that contribute to liver disease, its inactivity in response to caulerpin may reduce hepatic toxicity and the formation of toxic metabolites [18,19].
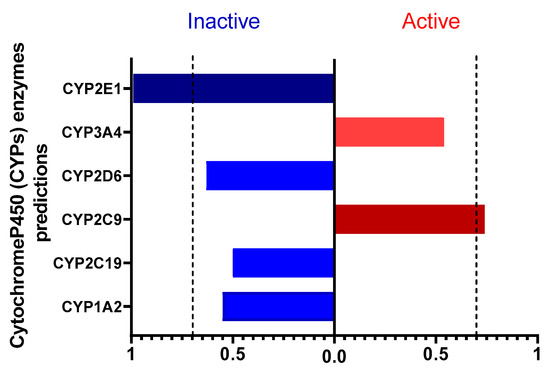
Figure 2.
Prediction of caulerpin interaction with cytochrome P450 enzymes. The chart shows the enzymes predicted as active (red) or inactive (blue) in the metabolism of the compound. Values above 0.7 indicate that caulerpin may activate or inhibit certain CYP450 enzymes. A probability threshold of 0.7 was set for significance.
Caulerpin interacts with CYP2C9, a key enzyme involved in the metabolism of anticoagulants, NSAIDs, and oral hypoglycemics. This interaction suggests a favorable metabolic profile by enhancing drug biotransformation, reducing plasma concentrations, and minimizing adverse effects from drug accumulation [20]. CYP2C9 activation is particularly relevant for NSAID metabolism (e.g., ibuprofen, diclofenac, naproxen), potentially optimizing therapeutic efficacy while lowering gastrointestinal and renal risks [21,22]. Additionally, it may facilitate the formation of active metabolites with anti-inflammatory properties, thereby supporting the management of chronic inflammation and autoimmune diseases [23].
In addition to isoenzymes, PROTOX assesses the activation or inhibition of cellular receptors, transcription factors, and enzymes involved in metabolism, inflammation, hormonal modulation, and signaling pathways [24]. The heatmap in Figure S1 shows that caulerpin predominantly appears in the blue range, suggesting a favorable safety profile regarding toxic pathway activation. The inactivity of key biomarkers such as AhR [25], p53 [26], and PPAR-gamma [27] indicates a low potential for oxidative stress and cell cycle dysregulation, reducing carcinogenicity and cytotoxicity risks. Additionally, the low activity of ER and AR receptors suggests minimal endocrine interference [28]. Overall, these findings indicate a low toxicity profile, with few significant interactions with critical toxicity biomarkers, highlighting the need for further experimental validation to confirm caulerpin’s safety in therapeutic applications.
Regarding the biological results, initially, the cytotoxicity of caulerpin (10, 20, and 40 µM) was evaluated in peritoneal macrophage cultures. The compound showed no significant cytotoxicity at any of the tested concentrations, even in the presence of LPS and IFN-γ. Similar results were observed with dexamethasone, the standard drug used in immunomodulation assays (Figure 3A). Our findings align with those of Cuomo and colleagues (2021) [29], who also reported non-cytotoxic activity of caulerpin in gastric adenocarcinoma epithelial cells at concentrations up to 45 µM, with cell viability remaining above 80%. Likewise, Mert-Ozupek and collaborators (2022) [11] demonstrated that caulerpin does not exhibit cytotoxic effects on HDF (human dermal fibroblasts) and NIH-3T3 (mouse embryonic fibroblasts) cell lines.
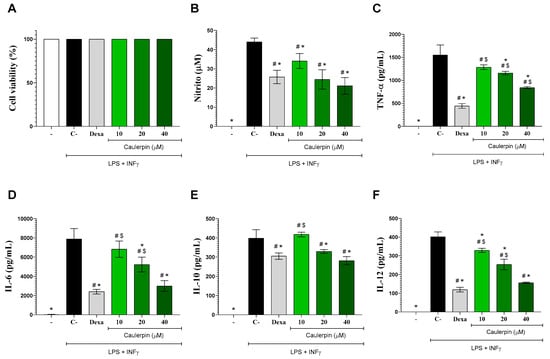
Figure 3.
Effects of caulerpin on macrophages in vitro. Mouse peritoneal exudate macrophages stimulated or not with LPS + IFN-γ were cultured in the absence or presence of caulerpin (10, 20, or 40 µM) or dexamethasone (Dexa; 10 µM). (A) Cell viability was determined by the Alamar Blue method. Cell-free supernatants were collected for nitrite (B), TNF-α (C), IL-6 (D), IL-10 (E), and IL-12 (F) quantification. “−” refers to the group of untreated and unstimulated cells. C– refers to the group of untreated cells stimulated with LPS + IFN-γ. Data are expressed as the mean ± standard deviation (S.D.) of nine replicates obtained from three independent experiments. * p < 0.05 compared to stimulated and untreated cells; # p < 0.05 compared to unstimulated and untreated cells; $ p < 0.05 compared to dexamethasone-treated cells.
The anti-inflammatory effects of caulerpin were subsequently assessed in macrophage cultures stimulated with LPS + IFN-γ. Nitrite levels were initially measured as an indicator of nitric oxide (NO) production. NO is a critical mediator of the inflammatory response, playing a key role in pathogen elimination by macrophages. However, excessive NO production can lead to cytotoxicity and tissue damage [30]. As shown in Figure 3B, LPS + IFN-γ stimulation significantly increased nitrite production (p < 0.05). However, treatment with caulerpin at concentrations of 20 and 40 µM resulted in a significant reduction in nitrite levels (p < 0.05), with inhibition rates of 44.5% and 52%, respectively. These findings align with those reported by Lee and colleagues (2012) [22], which demonstrated that fucoidan polysaccharides extracted from Ecklonia cava inhibited NO production in RAW 264.7 macrophages stimulated with lipopolysaccharide (LPS).
To further characterize the immunomodulatory effects of caulerpin, cytokine production by activated macrophages was quantified by ELISA. Stimulation with LPS + IFN-γ induced a marked increase in IL-6, IL-10, IL-12, and TNF-α levels (Figure 3C–F). However, treatment with caulerpin significantly reduced the production of these cytokines (p < 0.05). Under the same experimental conditions, dexamethasone (10 μM) also led to a significant decrease in cytokine levels. These findings are in agreement with those of Cuomo and colleagues (2021) [29], who showed that pre-treatment with caulerpin (15 μM) significantly downregulated the production of pro-inflammatory cytokines, including IL-1β, IL-6, IL-8, and TNF-α, by macrophages stimulated with Helicobacter pylori, a Gram-negative bacterium with an LPS-containing cell wall. These results are further supported by a study in infectious models, in which Sidrônio and colleagues (2025) [31] demonstrated that caulerpin at 25 and 50 μM significantly reduced IL-1β and TNF-α production in RAW 264.7 macrophages infected with Mycobacterium smegmatis or M. tuberculosis. The authors also reported NLRP3 inflammasome modulation and the absence of cytotoxicity in Vero E6 and HepG2 cells, reinforcing the compound’s immunomodulatory potential and favorable safety profile across different cell systems.
Similarly, Bitencourt and colleagues (2015) [32] demonstrated the in vitro anti-inflammatory properties of aqueous and methanolic extracts from Caulerpa mexicana, which reduced IL-6, IL-12, and TNF-α production in peritoneal macrophages stimulated with LPS. In murine models of zymosan-induced peritonitis and DSS-induced ulcerative colitis, Lucena and colleagues (2018) [16] further reported that caulerpin treatment reduced TNF-α, IFN-γ, IL-17, and IL-6 levels while suppressing NF-κB transcription factor activity. Notably, caulerpin also increased IL-10 levels and reduced leukocyte migration into the peritoneal cavity. Consistently, Bitencourt and colleagues (2015) [32] demonstrated that methanolic extracts of Caulerpa mexicana also lowered IFN-γ, IL-6, IL-12, and TNF-α concentrations in colonic culture supernatants from mice with dextran sulfate sodium-induced colitis.
In order to investigate the mechanism of action of caulerpin, docking and molecular dynamics simulations were performed. In this study, the macromolecular target was the glucocorticoid receptor ligand-binding domain (GR-LBD).
The results of the molecular dynamics simulations conducted in this study revealed key structural insights. The radius of gyration (Rg) of the sampled structures was analyzed as a function of time (Figure 4A). Rg represents the mass distribution relative to the center of mass of the protein, providing insight into how well-packed (folded) the protein structure remains over time. As shown in Figure 4A, none of the systems exhibited significant deviations from their mean values: free GR-LBD (average Rg = 1.82 nm), caulerpin–GR-LBD complex (average Rg = 1.85 nm), and dexamethasone–GR-LBD complex (average Rg = 1.84 nm).
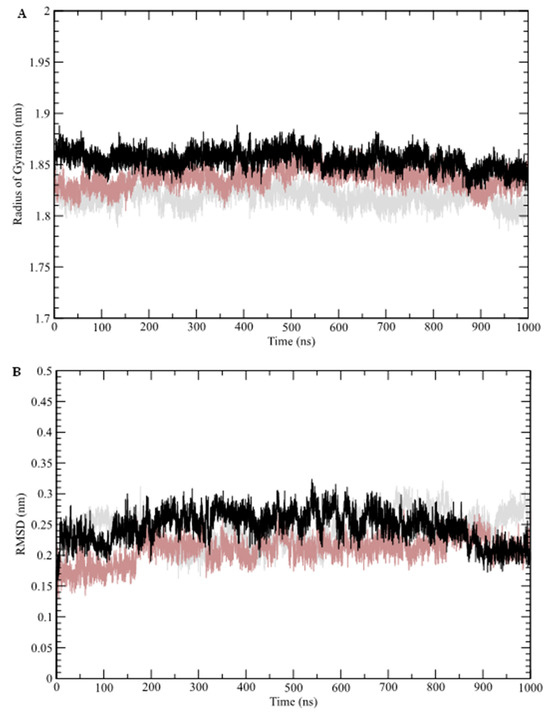
Figure 4.
Molecular dynamics simulation analysis. (A) Radius of gyration (Rg); (B) root mean square deviation (RMSD). Molecular dynamics data for the free GR-LBD are represented by gray lines, the dexamethasone–GR-LBD complex by brown lines, and the caulerpin–GR-LBD complex by black lines.
The structural stability of the GR-LBD complexes was further assessed through root mean square deviation (RMSD) analysis, which monitors conformational changes over time (Figure 4B). This analysis indicates that the systems reached equilibrium after approximately 70 ns. The calculated average RMSD values for the free GR-LBD, dexamethasone–GR-LBD complex, and caulerpin–GR-LBD complex were 0.24 nm, 0.21 nm, and 0.25 nm, respectively. These results suggest that while both complexes exhibit structural stability, the dexamethasone–GR-LBD complex appears to be slightly more stable than the caulerpin–GR-LBD complex. Additionally, root mean square fluctuations (RMSF) were analyzed to evaluate residue-level flexibility by measuring the fluctuation of Cα atoms relative to the average structure sampled during the simulation (Figure 5A). The frequency of hydrogen bond formation along the simulation trajectory was also examined (Figure 5B). As shown, dexamethasone predominantly interacts with GR-LBD through at least two hydrogen bonds throughout the entire simulation, whereas caulerpin primarily engages with GR-LBD via a single hydrogen bond. Notably, between approximately 520 ns and 850 ns, the frequency of hydrogen bonds stabilizing caulerpin within the binding site increased significantly.
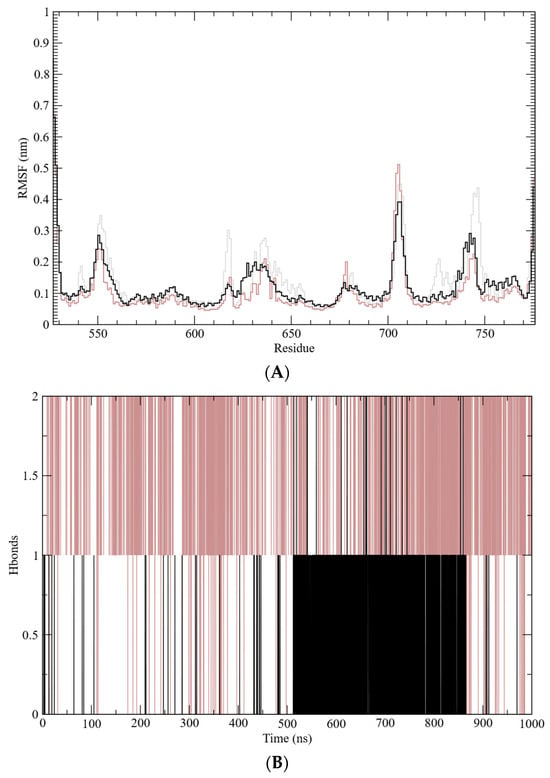
Figure 5.
Molecular dynamics simulation analysis. (A) Root mean square fluctuation (RMSF); (B) hydrogen bond (H-bond) frequency. Molecular dynamics data for the free GR-LBD are represented by gray lines, the dexamethasone–GR-LBD complex by brown lines, and the caulerpin–GR-LBD complex by black lines.
To obtain representative ligand–GR-LBD interaction poses, a cluster analysis of the molecular dynamics trajectories was performed (Figure 6). A time window of 930 ns was used for pose sampling. The simulation of the dexamethasone–GR-LBD complex revealed a single dominant cluster, whereas the simulation of the caulerpin–GR-LBD complex identified two major clusters. These results suggest that caulerpin binds to the target protein binding site in more than one energetically favorable conformation, while dexamethasone adopts a single dominant binding mode. Representative intermolecular poses for the dexamethasone–GR-LBD and caulerpin–GR-LBD complexes are shown in Figure 6 and Figure 7, respectively.
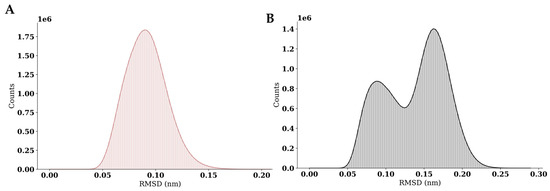
Figure 6.
Cluster analysis of GR-LBD complexes with dexamethasone and caulerpin. (A) RMSD distribution for the dexamethasone–GR-LBD complex; (B) RMSD distribution for the caulerpin–GR-LBD complex.
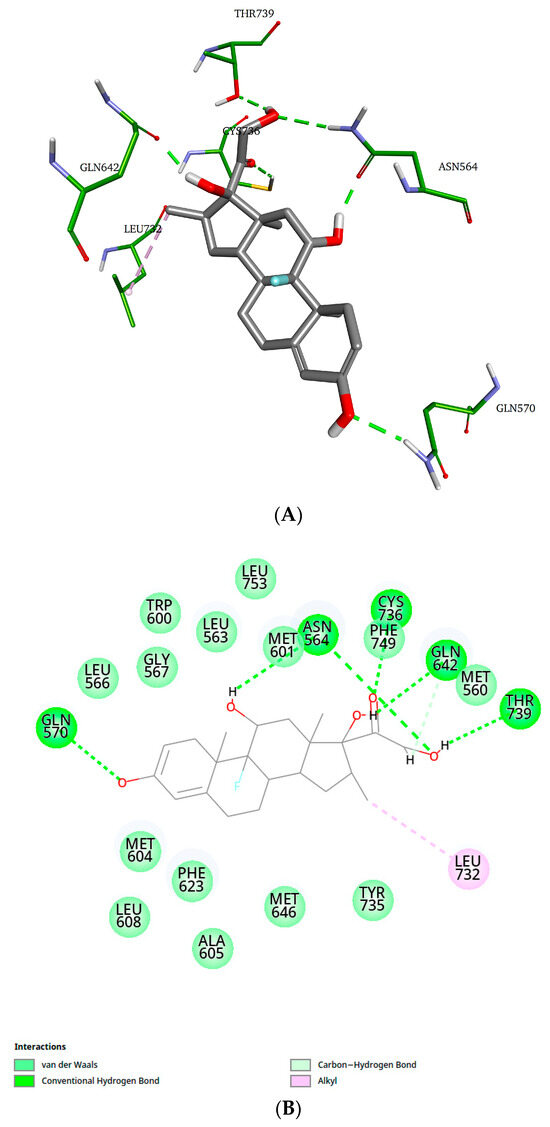
Figure 7.
Representative structure of the dexamethasone–GR-LBD complex (cluster 1). (A) 3D representation; (B) 2D representation of dexamethasone–GR-LBD interactions.
As shown in Figure 7, dexamethasone interacts with residues Asn564, Gln570, Gln642, Cys736, and Thr739 through hydrogen bonds, and with residue Leu732 via an alkyl-type interaction. Additionally, the 2D diagram illustrates van der Waals interactions that are not represented in the 3D structure.
Figure 8A,B illustrate the interaction of caulerpin with GR-LBD, as sampled from cluster 1 (Figure 6B). As shown, caulerpin forms a conventional hydrogen bond with Met604, a carbon–hydrogen bond with Asn564 and Gln642, and π–π stacked and T-shaped interactions with Phe749 and Tyr735, respectively. Additionally, it engages in π–alkyl interactions with Ala605, Met646, Leu732, and Phe749; alkyl interactions with Met560 and Ile747; π–sulfur interactions with Met604 and Cys736; and a π-donor hydrogen bond with Asn564. Van der Waals interactions are also depicted in the 2D diagram.
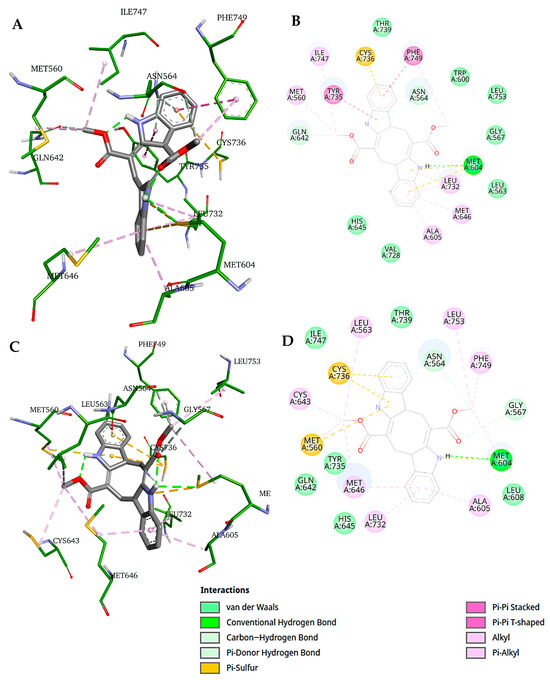
Figure 8.
Representative structures of the caulerpin–GR-LBD complex (clusters 1 and 2). (A) 3D representation and (B) 2D representation of caulerpin–GR-LBD interactions for the representative structure from cluster 1; (C) 3D representation and (D) 2D representation of caulerpin–GR-LBD interactions for the representative structure from cluster 2.
Figure 8C,D depict the intermolecular interactions sampled from cluster 2. In this conformation, caulerpin forms a conventional hydrogen bond with Met604 and carbon–hydrogen bonds with Asn564 and Gly567. It also interacts via π–alkyl interactions with Ala605, Met646, Leu732, and Phe749; alkyl interactions with Met560, Leu563, Cys643, Met646, and Leu753; and π–sulfur interactions with Met560 and Cys736. Van der Waals interactions are also represented in the 2D diagram.
Two key differences can be observed between the intermolecular poses sampled from clusters 1 and 2: (a) caulerpin does not form a π–sulfur interaction with Met560 in cluster 1, and (b) caulerpin does not establish π–π interactions with Phe749 and Tyr735 in cluster 2. Despite binding to the protein in a similar conformation in both clusters, caulerpin exhibits a different spatial orientation.
Throughout the molecular dynamics simulations, the binding free energies of caulerpin and dexamethasone to the GR-LBD binding site were calculated. Caulerpin exhibited an estimated binding free energy of −24.07 kcal/mol, whereas dexamethasone showed a binding free energy of −32.64 kcal/mol. These results indicate that both molecules have a significant affinity for the GR-LBD binding site, with dexamethasone displaying a stronger interaction.
To further investigate the role of glucocorticoid receptors in the immunomodulatory activity of caulerpin, the potential antagonistic effect of RU486 was assessed in stimulated macrophage cultures. The addition of RU486 (10 µM) to macrophage cultures stimulated with LPS + IFN-γ partially reversed the inhibitory effect of caulerpin (40 µM) on nitrite (Figure 9A) and completely reversed the production of IL-6 (Figure 9B) and TNF-α (Figure 9C). As expected, RU486 also blocked the inhibitory effect of dexamethasone (10 µM) on nitrite and cytokine levels, further supporting the involvement of glucocorticoid receptors in the anti-inflammatory activity of caulerpin.
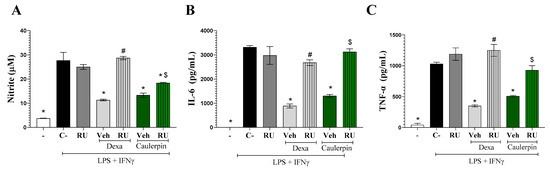
Figure 9.
Involvement of glucocorticoid receptor in the immunomodulatory effect of caulerpin. (A) Nitrite concentrations, (B) IL-6 levels, and (C) TNF-α levels were measured in macrophages stimulated with LPS + IFN-γ. Cells were treated with caulerpin (40 µM) or dexamethasone (Dexa; 10 µM). In some cultures, cells were treated with these compounds in the presence of RU486 (glucocorticoid receptor antagonist, RU; 10 µM). “−” refers to the group of untreated and unstimulated cells. C– refers to the group of untreated cells stimulated with LPS + IFN-γ. Veh = Vehicle. Data are expressed as the mean ± S.D. of nine replicates obtained from three independent experiments. * p < 0.05 compared to stimulated and untreated cells; # p < 0.05 compared to stimulated and treated with dexamethasone-treated cells; $ p < 0.05 compared to caulerpin-treated cells.
Following the in vitro findings, we evaluated the potential toxicity of a single oral dose of caulerpin (100 mg/kg) in female BALB/c mice. Administration of caulerpin at this dose did not result in mortality or any observable signs of toxicity in the treated animals (Table 2). Additionally, no significant differences in body weight were observed between caulerpin-treated and vehicle-treated groups (Table 3). Consistent with our findings of no acute toxicity in mice, Russo and colleagues (2024) [33] reported that caulerpin did not induce toxicological alterations in Mytilus galloprovincialis, even when combined with caffeine. Notably, the study suggests a potential protective role of caulerpin against xenobiotic-induced toxicity, possibly via activation of peroxisome proliferator-activated receptors involved in detoxification pathways. These results reinforce caulerpin’s safety profile across different biological systems.

Table 2.
Effect of caulerpin on behavioral and general appearance of female BALB/c mice.

Table 3.
Body weight of female BALB/c mice treated with caulerpin.
Finally, the protective effect of caulerpin was evaluated in a murine model of endotoxic shock induced by a lethal dose of LPS. As shown in Figure 10, caulerpin treatment improved survival rates in a dose-dependent manner compared to the vehicle-treated group. Administration of 2.5 mg/kg resulted in a 40% survival rate, whereas the 5 mg/kg dose significantly extended survival (p < 0.05), reaching 80% survival by the end of the experiment. Notably, treatment with 10 mg/kg of caulerpin provided complete protection, ensuring 100% survival. Similarly, dexamethasone (2.5 mg/kg) also conferred full protection throughout the observation period.
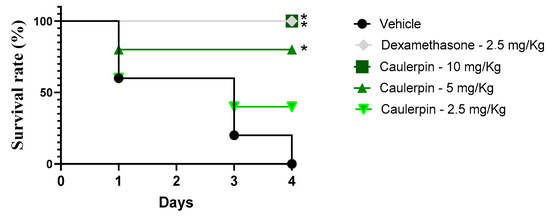
Figure 10.
Survival curve of mice treated with caulerpin and subjected to endotoxic shock. Mice were orally treated with caulerpin (2.5, 5, or 10 mg/kg) or dexamethasone (2.5 mg/kg). Animals in the vehicle-treated group received saline solution containing 5% DMSO. Survival was monitored for four days following LPS challenge. Data represent results from two independent experiments. * p < 0.05, ** p < 0.01 compared to the vehicle-treated group. Statistical analysis was performed using the log-rank (Mantel–Cox) test.
The endotoxemic shock model is crucial for evaluating and confirming anti-inflammatory effects, particularly those targeting macrophages, as monocytes and macrophages are the primary sources of cytokines involved in sepsis and the resulting damage to target organs [34]. Thus, this finding reinforces previous results demonstrating the inhibition of key inflammatory mediators produced by macrophages.
Furthermore, other in vivo models involving caulerpin have been used in previous studies, supporting these findings related to sepsis. In the carrageenan-induced peritonitis model, Swiss albino mice treated with caulerpin were evaluated. Intraperitoneal pretreatment with caulerpin at a dose of 100 μmol/kg reduced leukocyte migration into the peritoneal cavity by approximately 48% [35].
Taken together, our results provide new insights into the pharmacological potential of caulerpin as a selective anti-inflammatory agent. Notably, the identification of the glucocorticoid receptor as a key molecular target represents a novel mechanistic contribution, differentiating our study from previous reports that merely described caulerpin’s anti-inflammatory effects without exploring its mode of action. While prior studies demonstrated the protective effects of caulerpin in murine models of colitis and peritonitis [16,35], none investigated its interaction with GR-LBD or employed molecular dynamics simulations and receptor antagonism assays (e.g., RU486) to confirm target engagement.
The dual binding modes observed in our cluster analysis suggest that caulerpin exhibits greater conformational flexibility than dexamethasone, which could be advantageous or detrimental depending on the biological context. This hypothesis warrants further investigation through crystallographic studies or extended MD simulations in different cellular environments.
Despite these promising findings, some limitations must be acknowledged. First, although RU486 experiments suggest GR involvement, definitive validation through genetic approaches (e.g., GR knockout or siRNA silencing) was not performed. Second, the in vivo efficacy of caulerpin was only evaluated in an acute model of endotoxic shock. Future studies should explore its effects in models of chronic inflammation, autoimmune diseases, or glucocorticoid resistance. Additionally, although in silico data point to a favorable safety profile, pharmacokinetic and metabolism studies are still necessary to assess oral bioavailability, half-life, and potential drug–drug interactions.
3. Materials and Methods
3.1. Drugs
Dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), a synthetic glucocorticoid, was used as a positive control in immunomodulatory assays. Mifepristone (RU 486; Sigma-Aldrich), an antagonist of glucocorticoid receptors, was used in mechanism assays. Caulerpin was isolated from Caulerpa racemosa as previously described [36] (Figure 1). All compounds were initially solubilized in dimethyl sulfoxide (DMSO; Synth, São Paulo, SP, Brazil) and subsequently diluted in Dulbecco’s Modified Eagle Medium (DMEM; Life Technologies, Carlsbad, CA, USA) for in vitro assays. The final DMSO concentration was maintained below 0.1% in all experiments. For in vivo assays, compounds were prepared in a vehicle solution consisting of 5% DMSO and 95% saline.
3.2. Animals
BALB/c mice aged 4 to 8 weeks were obtained from the animal breeding facility of the Gonçalo Moniz Institute, Salvador, Brazil. Animals were housed in sterilized cages under controlled environmental conditions, including a temperature of 22 ± 2 °C and humidity of 55 ± 10%. They were provided water ad libitum and a nutritionally balanced rodent diet. All experimental procedures were reviewed and approved by the Institutional Committee on the Ethical Use of Laboratory Animals (approval number: L-IGM-019/24).
3.3. In Silico Toxicity Prediction of Caulerpin Using PROTOX 3.0
The toxicity profile of caulerpin was assessed in silico using the PROTOX 3.0 online tool (http://tox.charite.de/protox_II/; accessed on 30 August 2024). The chemical structure of caulerpin, defined by its SMILES notation (COC(=O)/C/1=C/C2=C(NC3=CC=CC=C23)/C(=C\C4=C1NC5=CC=CC=C45)/C(=O)OC), was entered into the platform to predict various toxicity parameters. These included acute toxicity classification, LD50 values, hepatotoxicity, immunotoxicity, mutagenicity, carcinogenicity, and cytotoxicity. Predictions were generated using the tool’s advanced machine learning models, trained on extensive toxicological datasets. The results provided insights into the potential safety profile of caulerpin, supporting subsequent experimental validation efforts.
3.4. Cytotoxicity Assay in Mammalian Cells
Peritoneal macrophages were isolated from BALB/c mice 4–5 days after intraperitoneal injection of 1.5 mL of 3% thioglycolate solution (Sigma-Aldrich) in saline. The harvested cells were seeded into 96-well plates at a density of 2 × 105 cells/well in DMEM supplemented with 10% fetal bovine serum (FBS; GIBCO, Thermo Fisher Scientific, Waltham, MA, USA) and 50 µg/mL gentamicin (Life Technologies). The macrophages were incubated for 24 h at 37 °C in a humidified atmosphere containing 5% CO2_22. Subsequently, the cells were stimulated with LPS (500 ng/mL; Sigma-Aldrich) and IFN-γ (5 ng/mL; Sigma-Aldrich) and treated with caulerpin at concentrations of 10, 20, or 40 µM. After 72 h of incubation, 20 µL of Alamar Blue reagent (Invitrogen, Carlsbad, CA, USA) was added to each well, followed by an additional 4 h incubation. Colorimetric readings were performed using a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA, USA). Three independent experiments were conducted, each performed in triplicate.
3.5. Macrophage Cultures
Peritoneal macrophages (2 × 105 cells/well) were seeded into 96-well plates in DMEM supplemented with 10% FBS and 50 µg/mL gentamicin. Cells were incubated in triplicate under different conditions, including stimulation with LPS (500 ng/mL) and IFN-γ (5 ng/mL), and treated with caulerpin at various concentrations (10, 20, and 40 µM) or dexamethasone (10 µM). In some experiments, RU486 (10 µM), a glucocorticoid receptor antagonist, was added to the cultures to investigate the mechanism of action of caulerpin. After 4 h, cell-free supernatants were collected for TNF-α measurement, and after 24 h, supernatants were analyzed for IL-6, IL-10, IL-12, and nitrite levels. All samples were stored at −80 °C until analysis.
3.6. Cytokines and Nitric Oxide Production
Cytokine levels, including IL-6, IL-10, IL-12, and TNF-α, were measured in culture supernatants using enzyme-linked immunosorbent assay (ELISA) DuoSet kits (R&D Systems, Bio-Techne, Minneapolis, MN, USA), according to the manufacturer’s instructions. Nitric oxide production was quantified in macrophage supernatants using the Griess reaction, with nitrite concentrations serving as an indicator [37].
3.7. Acute Toxicity in Mice
Female BALB/c mice (6–8 weeks old) were randomly assigned to two groups and administered a single oral dose of caulerpin (100 mg/kg) or vehicle (5% DMSO in saline). Animals were observed daily for 14 days to assess clinical signs of toxicity, including alterations in the eyes, fur, and skin, as well as symptoms such as tremors, salivation, convulsions, diarrhea, lethargy, and coma. Body weights were recorded on days 0, 7, and 14 to monitor potential effects on growth and overall health [38].
3.8. LPS-Induced Endotoxin Shock
Male BALB/c mice (4 weeks old) were treated intraperitoneally with caulerpin (2.5, 5, or 10 mg/kg), dexamethasone (2 mg/kg), or vehicle. Ninety minutes after treatment, the mice were challenged intraperitoneally with 600 µg of lipopolysaccharide (LPS; serotype 0111:B4, Escherichia coli; Sigma-Aldrich) dissolved in saline. Survival rates were monitored daily over a period of 4 days.
3.9. Dexamethasone and Caulerpin Structures
The structure of caulerpin was obtained from the PubChem database under the identification code 5326018 [39]. The structure of dexamethasone, which was found co-crystallized within the glucocorticoid receptor (PDB ID: 1P93), was also used. Both ligands were energy-minimized using the DFT LC-BLYP/def2-TZVPP quantum method, implemented in the ORCA 5.0.4 package [40].
3.10. Molecular Docking
Molecular docking simulation was performed with GOLD 2024.2.0, a protein–ligand docking software based on a genetic algorithm (GA) [41]. Considering the GR-LBD binding site, a sphere with a radius equal to 10 Å was defined, with the geometric center coordinates set as: x = 45.320 Å; y = 12.050 Å; and z = 17.100 Å. The scoring function used was ChemPLP [42]. Default GA parameters GOLD were applied for sampling the docked poses.
3.11. Dynamics Simulations
Molecular dynamics simulations were carried out using GROMACS 2024.2 software for the free GR-LBD structure, as well as for the dexamethasone–GR-LBD and caulerpin–GR-LBD complexes [43]. The CHARMM36 force field was used [44], and ligand parameterization was performed using the CHARMM General Force Field (CGenFF) method [45]. A dodecahedral simulation box was created using the TIP3P water model, and sodium ions were added to neutralize the systems electronically. Periodic boundary conditions were applied. Each system was subjected to energy minimization using the steepest descent algorithm, with 50,000 steps and a convergence criterion of less than 2.39 kcal/mol. Subsequently, two 100 ps equilibration simulations were performed for both the free protein and the protein–ligand complexes. The first simulation used the NVT ensemble, followed by the NPT ensemble. In both cases, the temperature was maintained at 300 K. During the NPT simulation, pressure was kept constant at 1 bar. After equilibration, 1 μs (1000 ns) production molecular dynamics simulations were performed to calculate the binding free energies of each ligand with GR-LBD. Simulations were run under the NPT ensemble, with temperature control using the V-rescale implementation of Berendsen’s thermostat [46] and pressure control using the Parrinello–Rahman barostat [47]. The particle mesh Ewald (PME) method was used for long-range electrostatics [48]. Trajectory frames were sampled every 100 ps.
For binding free energy estimation, the time interval from 70 ns to 1000 ns was selected. Calculations were performed using the MM/PBSA method implemented in the gmx_MMPBSA tool [49]. For intermolecular interaction analysis, structural clustering was performed using the GROMOS method implemented in GROMACS, with an RMSD cutoff of 0.1 nm to group similar structures [50].
3.12. Visualization Tools and Plots
Molecular visualization was performed using BIOVIA Discovery Studio 2021 [51], and graphical analyses were conducted using the Grace plotting tool [52].
3.13. Statistical Analysis
The significance of differences between groups was evaluated using one-way ANOVA, followed by the Newman–Keuls multiple comparison post-test. Analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA). Results were considered statistically significant when p-values were less than 0.05 (p < 0.05).
4. Conclusions
In this study, we demonstrated that caulerpin, a bisindolic alkaloid isolated from Caulerpa racemosa, exhibits potent and selective anti-inflammatory activity. In vitro, it significantly reduced nitric oxide, TNF-α, IL-6, and IL-12 levels in LPS + IFN-γ-stimulated macrophages without affecting cell viability. In silico toxicity predictions using PROTOX 3.0 support a favorable safety profile for caulerpin. Its mechanism of action involves high-affinity binding to the glucocorticoid receptor ligand-binding domain (GR-LBD), as revealed by molecular docking and dynamics simulations. This was corroborated by the partial reversal of its activity upon treatment with RU486, a glucocorticoid receptor antagonist. In vivo, caulerpin also exhibited a favorable safety profile, with no signs of acute toxicity at an oral dose of 100 mg/kg. Furthermore, caulerpin administration significantly improved survival rates in a murine model of endotoxic shock, providing complete protection at 4 mg/kg, further supporting its therapeutic potential in inflammatory conditions. Taken together, these findings position caulerpin as a promising candidate for the development of novel anti-inflammatory therapies, warranting further investigation into its pharmacokinetics, structural optimization, and efficacy in chronic inflammatory diseases.
Supplementary Materials
The following Supporting Information can be downloaded at: https://www.mdpi.com/article/10.3390/md23060232/s1, Figure S1: Heat map representing the activity of different biomarkers for toxicity pathways.
Author Contributions
J.S.P.d.S.: Investigation, conceptualization, formal analysis, writing—original draft; D.K.C.S.: methodology, formal analysis; V.d.S.O.: methodology, formal analysis, S.S.S.J.: formal analysis, writing—original draft; E.d.S.R.: methodology, formal analysis; C.V.C.d.S.: methodology, conceptualization, writing—review and editing; S.T.M.: conceptualization, writing—review and editing; O.A.S.-F.: conceptualization, resources, writing—review and editing, supervision; C.S.M.: conceptualization, formal analysis, writing—review and editing, supervision, funding acquisition; M.B.P.S.: conceptualization, writing—review and editing, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from FAPESB (grant number PPP0027/2024). M.B.P.S is a recipient of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) fellowship. E.d.S.R. is grateful for the PhD scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível superior—Brasil (CAPES-Finance Code 001).
Institutional Review Board Statement
The study was approved, in July 2024, by the Institutional Ethics Committee for Animal Care and Use of FIOCRUZ (IGM 019/24).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| BBB | blood–brain barrier |
| CYP | cytochrome P450 |
| CGenFF | CHARMM General Force Field |
| DFT | density functional theory |
| Dexa | dexamethasone |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| DSS | dextran sulfate sodium |
| ELISA | enzyme-linked immunosorbent assay |
| FBS | fetal bovine serum |
| GA | genetic algorithm |
| GR | glucocorticoid receptor |
| GR-LBD | glucocorticoid receptor ligand-binding domain |
| IFN-γ | interferon gamma |
| IL | interleukin |
| LD50 | lethal dose 50% |
| LPS | lipopolysaccharide |
| MD | molecular dynamics |
| MM/PBSA | molecular mechanics/Poisson–Boltzmann surface area |
| NF-κB | nuclear factor kappa B |
| NO | nitric oxide |
| NSAIDs | nonsteroidal anti-inflammatory drug |
| NVT | constant number of particles, volume, and temperature ensemble |
| NPT | constant number of particles, pressure, and temperature ensemble |
| ORP | oxidation-reduction potential |
| PBMC | peripheral blood mononuclear cells |
| PME | particle mesh Ewald |
| PROTOX | in silico toxicity prediction tool |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| RMSD | root mean square deviation |
| RMSF | root mean square fluctuation |
| ROS | reactive oxygen species |
| TNF-α | tumor necrosis factor alpha |
References
- Singh, N.; Baby, D.; Rajguru, J.; Patil, P.; Thakkannavar, S.; Pujari, V. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Kohler, O.; Krogh, J.; Mors, O.; Benros, M.E. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, E.; Kumar, S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Savin, I.A.; Zenkova, M.A.; Sen’kova, A.V. Pulmonary Fibrosis as a Result of Acute Lung Inflammation: Molecular Mechanisms, Relevant In Vivo Models, Prognostic and Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 14959. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Mehra, R.; Bhushan, S.; Bast, F.; Singh, S. Marine macroalga Caulerpa: Role of Its Metabolites in Modulating Cancer Signaling. Mol. Biol. Rep. 2019, 46, 3545–3555. [Google Scholar] [CrossRef]
- Kumar, J.G.S.; Umamaheswari, S.; Kavimani, S.; Ilavarasan, R. Pharmacological Potential of Green Algae Caulerpa: A Review. Int. J. Pharm. Sci. Res. 2019, 10, 1014–1024. [Google Scholar]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef]
- Gaillande, C.; Payri, C.; Remoissenet, G.; Zubia, M. Caulerpa Consumption, Nutritional Value and Farming in the Indo-Pacific Region. J. Appl. Phycol. 2017, 29, 2249–2266. [Google Scholar] [CrossRef]
- Mert-Özüpek, N.; Calibaşi-Kocal, G.; Olgun, N.; Başbınar, Y.; Cavas, L.; Ellidokuz, H. An Efficient and Quick Analytical Method for the Quantification of an Algal Alkaloid Caulerpin Showed In-Vitro Anticancer Activity against Colorectal Cancer. Mar. Drugs 2022, 20, 757. [Google Scholar] [CrossRef] [PubMed]
- Ornano, L.; Donno, Y.; Sanna, C.; Ballero, M.; Serafini, M.; Bianco, A. Phytochemical Study of Caulerpa racemosa (Forsk.) J. Agarth, an Invading Alga in the Habitat of La Maddalena Archipelago. Nat. Prod. Res. 2014, 28, 1795–1799. [Google Scholar] [CrossRef] [PubMed]
- Abílio, G.M.F.; Camilo, C.J.; Coutinho, H.D.M.; da Costa, J.G.M.; Pena, L.J.; Silva-Júnior, A.; do Nascimento, Y.M.; Barbosa-Filho, J.M.; de Oliveira Santos, B.V.; de Luna Freire, K.R. Cytotoxic and Anti-HSV-1 Effects of Caulerpin Derivatives. Mar. Drugs 2024, 22, 3859. [Google Scholar] [CrossRef] [PubMed]
- Canché Chay, C.I.; Gómez Cansino, R.; Espitia Pinzón, C.I.; Torres-Ochoa, R.O.; Martínez, R. Synthesis and Anti-Tuberculosis Activity of the Marine Natural Product Caulerpin and Its Analogues. Mar. Drugs 2014, 12, 1757–1772. [Google Scholar] [CrossRef]
- Dissanayake, I.H.; Bandaranayake, U.; Keerthirathna, L.R.; Manawadu, C.; Silva, R.M.; Mohamed, B.; Ali, R.; Peiris, D.C. Integration of in vitro and in-silico analysis of Caulerpa racemosa against antioxidant, antidiabetic, and anticancer activities. Sci. Rep. 2022, 12, 20848. [Google Scholar] [CrossRef]
- Lucena, A.; Souza, C.; Jales, J.; Guedes, P.; Miranda, G.D.; Moura, A.; Araújo-Júnior, J.; Nascimento, G.; Scortecci, K.; Santos, B. The Bisindole Alkaloid Caulerpin, from Seaweeds of the Genus Caulerpa, Attenuated Colon Damage in a Murine Colitis Model. Mar. Drugs 2018, 16, 318. [Google Scholar] [CrossRef]
- Ferreira, D.A.; de Oliveira, G.C.S.L.; de Freitas, M.E.G.; de Araújo, D.A.M.; Scavone, C.; de Souza, T.A.; Villar, J.A.F.P.; Barbosa, L.A.; Mendonça-Junior, F.J.B.; Rodrigues-Junior, V.S.; et al. Evaluation of Anti-Inflammatory Activity of the New Cardiotonic Steroid γ-Benzylidene Digoxin 8 (BD-8) in Mice. Cells 2024, 13, 1568. [Google Scholar] [CrossRef]
- Massart, J.; Zoller, H.; Milosevic, I.; Haag, L.; Müller, T.; Haybaeck, J.; Oberkofler, H.; Stadlbauer, V. Role of Mitochondrial Cytochrome P450 2E1 in Healthy and Diseased Liver. Cells 2022, 11, 288. [Google Scholar] [CrossRef]
- Xu, J.; Ye, Y.; Huang, F.; Chen, G.; Pan, Z.; Yao, P. The Role of Human Cytochrome P450 2E1 in Liver Inflammation and Fibrosis. Hepatol. Commun. 2017, 1, 1043–1057. [Google Scholar] [CrossRef]
- Daly, A.K.; Rettie, A.E.; Fowler, D.M.; Miners, J.O. Pharmacogenomics of CYP2C9: Functional and Clinical Considerations. J. Pers. Med. 2017, 8, 1. [Google Scholar] [CrossRef]
- Yee, D.L.; O’Brien, S.H.; Young, G. Pharmacokinetics and Pharmacodynamics of Anticoagulants in Paediatric Patients. Clin. Pharmacokinet. 2013, 52, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Ko, C.-I.; Ahn, G.; You, S.; Kim, J.-S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.-J. Molecular Characteristics and Anti-Inflammatory Activity of the Fucoidan Extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Rakateli, L.; Huchzermeier, R.; van der Vorst, E.P.C. AhR, PXR and CAR: From Xenobiotic Receptors to Metabolic Sensors. Cells 2023, 12, 2752. [Google Scholar] [CrossRef]
- Dvorak, Z. Involvement of Aryl Hydrocarbon Receptor (AhR) in Polyphenol Inhibition of Benzo[a]pyrene-Induced Oxidative Stress and Neoplastic Transformation. Food Chem. Toxicol. 2017, 107, 523–525. [Google Scholar] [CrossRef]
- Shi, T.; van Soest, D.M.K.; Polderman, P.E.; Burgering, B.M.T.; Dansen, T.B. DNA Damage and Oxidant Stress Activate p53 through Differential Upstream Signaling Pathways. Free. Radic. Biol. Med. 2021, 172, 298–311. [Google Scholar] [CrossRef]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Tu, L.; Chen, J.; Zhou, L. Interference Mechanisms of Endocrine System and Other Systems of Endocrine-Disrupting Chemicals in Cosmetics—In Vitro Studies. Cells 2023, 12, 2752. [Google Scholar] [CrossRef]
- Cuomo, P.; Medaglia, C.; Allocca, I.; Montone, A.M.I.; Guerra, F.; Cabaro, S.; Mollo, E.; Eletto, D.; Papaianni, M.; Capparelli, R. Caulerpin Mitigates Helicobacter pylori-Induced Inflammation via Formyl Peptide Receptors. Int. J. Mol. Sci. 2021, 22, 13154. [Google Scholar] [CrossRef]
- Lim, E.H.; Mun, S.-K.; Kim, J.-J.; Chang, D.-J.; Yee, S.-T. Anti-Inflammatory Effects of Phlebia sp. Extract in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. BioMed Res. Int. 2022, 20, 2717196. [Google Scholar] [CrossRef]
- Sidrônio, T.S.; dos Santos, L.L.; Costa, A.B.; Macêdo, J.R.O.; Silva, M.F.; Freitas, M.L.; Costa, D.M.; Fuly, A.L.; Scotti, L.; Soares, M.B.P.; et al. Host-mediated antimicrobial effects and NLRP3 inflammasome modulation by caulerpin and its derivatives in macrophage models of mycobacterial infections. Microorganisms 2025, 13, 561. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, M.A.; Silva, H.M..; Abílio, G.M.; Miranda, G.E.; Moura, A.M.; Araújo-Júnior, J.X.D.; Silveira, E.J.; Santos, B.V.; Souto, J.T. Anti-Inflammatory Effects of Methanolic Extract of Green Algae Caulerpa mexicana in a Murine Model of Ulcerative Colitis. Braz. J. Pharmacogn. 2015, 25, 667–682. [Google Scholar] [CrossRef]
- Russo, T.; Coppola, F.; Paris, D.; De Marchi, L.; Meucci, V.; Motta, A.; Carbone, M.; Di Cosmo, A.; Soares, A.M.V.M.; Pretti, C.; et al. Exploring toxicological interactions in a changing sea: The case of the alkaloids caffeine and caulerpin. Sci. Total Environ. 2024, 912, 169190. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Q.; Li, M.; Lao, J.; Tang, H.; Ming, S.; Wu, M.; Gong, S.; Li, L.; Liu, L.; et al. SLAMF7 Regulates the Inflammatory Response in Macrophages During Polymicrobial Sepsis. J. Clin. Investig. 2023, 133, e150224. [Google Scholar] [CrossRef]
- de Souza, É.T.; Ferreira, E.; Almeida-Lima, J.; Costa, L.S.; Tronchini, E.A.; Lima, M.E.; Rocha, H.A.; Leite, E.L. The Antinociceptive and Anti-Inflammatory Activities of Caulerpin, a Bisindole Alkaloid Isolated from Seaweeds of the Genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef]
- Cantarino, S.J.; Coutinho, R.; Soares, A.R.; Duarte, H.M.; Martinez, S.T. Microwave irradiation is a suitable method for caulerpin extraction from the green algae Caulerpa racemosa (Chlorophyta, Caulerpaceae). Nat. Prod. Res. 2022, 36, 2149–2153. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N]Nitrate in Biological Fluids. Anal. Biochem. 1982, 121, 431–439. [Google Scholar] [CrossRef]
- Silva, L.P.; Santos, I.P.; Silva, D.K.C.; dos Reis, B.P.Z.C.; Meira, C.S.; de Souza Castro, M.V.B.; dos Santos Filho, J.M.; de Araujo-Neto, J.H.; Ellena, J.A.; da Silveira, R.G.; et al. Molecular Hybridization Strategy on the Design, Synthesis, and Structural Characterization of Ferrocene-N-acyl Hydrazones as Immunomodulatory Agents. Molecules 2022, 27, 8343. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2025 Update. Nucleic Acids Res. 2025, 53, D1516–D1525. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and Validation of a Genetic Algorithm for Flexible Docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Korb, O.; Stützle, T.; Exner, T.E. Empirical Scoring Functions for Advanced Protein−Ligand Docking with PLANTS. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef] [PubMed]
- GROMACS Development Team. GROMACS Documentation: Release 2024.2; GROMACS Development Team: 2024. Available online: https://manual.gromacs.org/2024.2/manual-2024.2.pdf (accessed on 1 September 2024).
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-Like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single Crystals: A New Molecular Dynamics Method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef]
- Daura, X.; Gademann, K.; Jaun, B.; Seebach, D.; Van Gunsteren, W.F.; Mark, A.E. Peptide Folding When Simulation Meets Experiment. Angew. Chem. 1999, 111, 245–249. [Google Scholar] [CrossRef]
- BIOVIA, Dassault Systèmes 21.1.0. Discovery Studio Visualizer. Dassault Systèmes: San Diego, CA, USA, 2021.
- Grace. Available online: https://plasma-gate.weizmann.ac.il/Grace/ (accessed on 2 February 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).