Marine Algal Metabolites as Cellular Antioxidants: A Study of Caulerpin and Caulerpinic Acid in Saccharomyces cerevisiae
Abstract
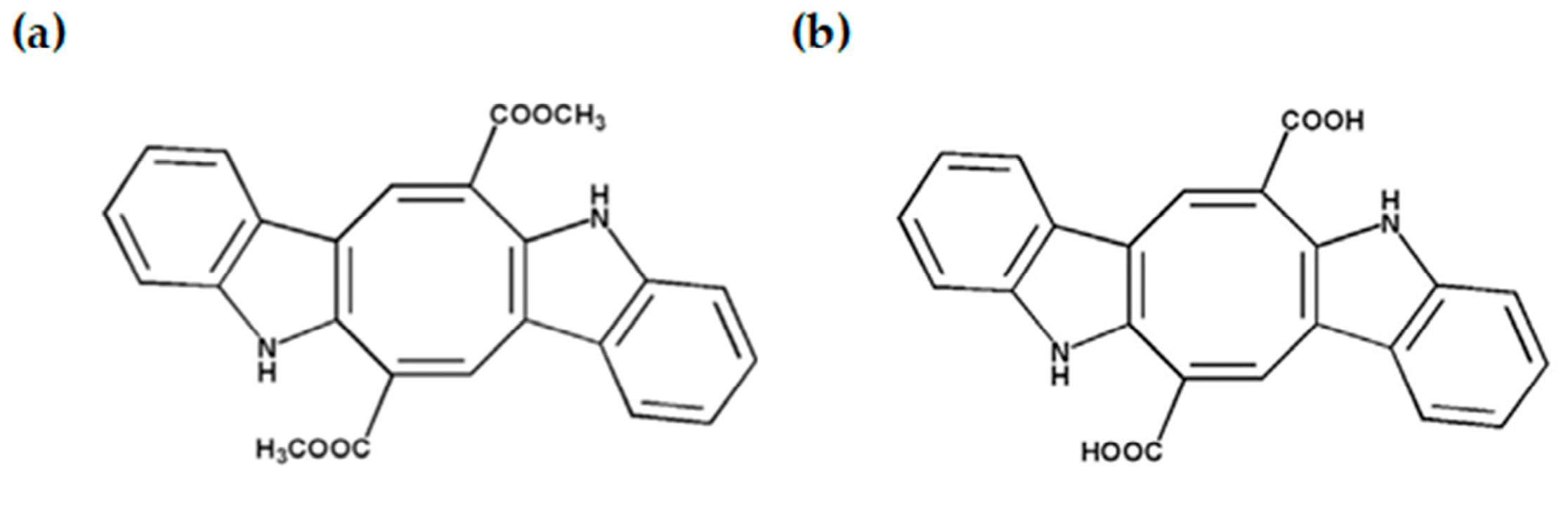
1. Introduction
2. Results
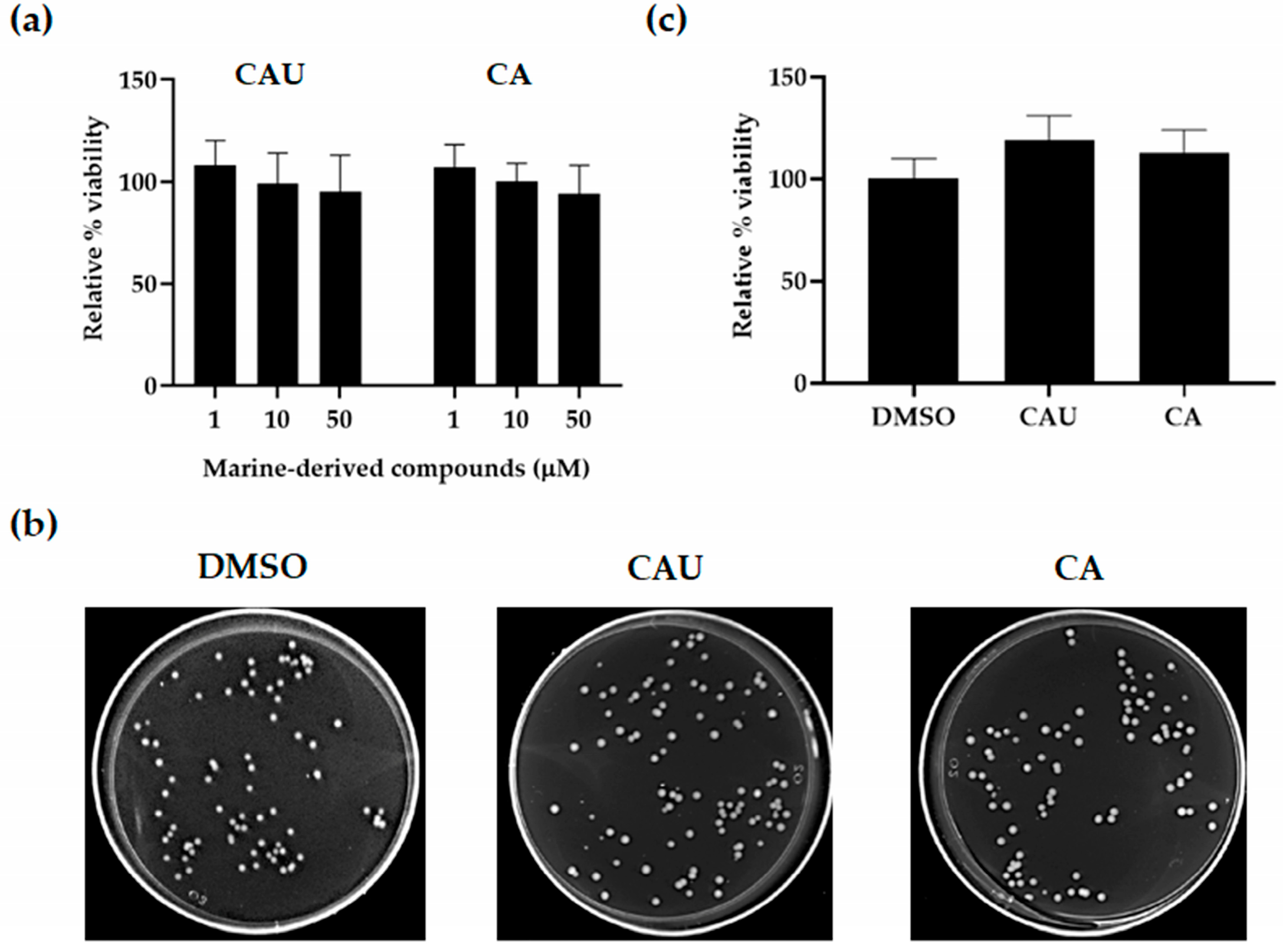
2.1. Effects of Marine-Derived Compounds on Yeast Cell Viability
2.2. Antioxidant Properties of Marine-Derived Compounds
2.3. Reduction of Intracellular ROS and Oxidative Damage by Marine-Derived Compounds
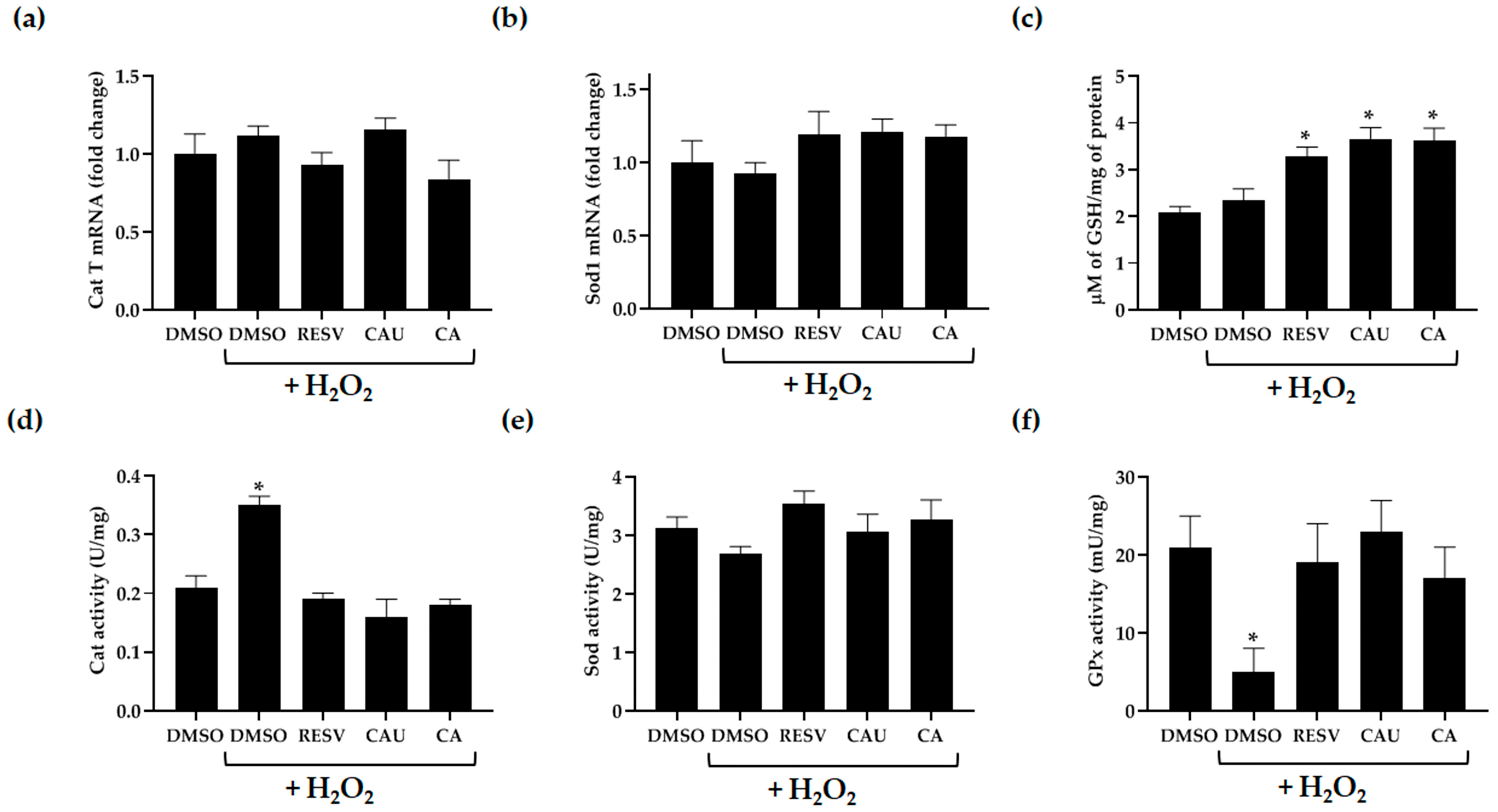
2.4. Effect of Marine-Derived Compounds on the Enzymatic and Non-Enzymatic Defense Systems
3. Discussion
4. Materials and Methods
4.1. Purification and Alkaline Hydrolysis of CAU
4.2. Yeast Strains and Growth Conditions
4.3. Evaluation of S. cerevisiae Cells’ Sensitivity to Marine-Derived Compounds
4.4. Cellular Assays for the Evaluation of Antioxidant Activity
4.5. Estimation of Intracellular Oxidation
4.6. Preparation of Yeast Cell–Free Extracts
4.7. Measurement of Oxidative Damage
4.8. Determination of Cat, Sod, and GPx Activity
4.9. Quantification of Intracellular GSH Levels
4.10. Gene Expression Analysis by Quantitative Real-Time PCR (qRT-PCR)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BSA | Bovine Serum Albumin |
| CA | Caulerpinic Acid |
| Cat | Catalase |
| CAU | Caulerpin |
| CFU | Colony-Forming Units |
| DCF | 2′,7′-dichlorofluorescein |
| ddH2O | double-distilled water |
| DMSO | Dimethyl sulfoxide |
| DTNB | 5,5′-dithiobis-(2-nitrobenzoic acid) |
| GPx | Glutathione Peroxidase |
| GSH | Glutathione |
| H2DCF-DA | 2′,7′-dichlorofluorescein diacetate |
| HRP | Horseradish peroxidase |
| MDA | Malondialdehyde |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| OD | Optical Density |
| PMSF | Phenylmethylsulfonyl fluoride |
| qRT-PCR | Quantitative Real-Time PCR |
| RESV | Resveratrol |
| ROS | Reactive Oxygen Species |
| SC | Synthetic Complete |
| SD | Standard Deviation |
| Sod | Superoxide dismutase |
| TBA | Thiobarbituric acid |
| TBARS | Thiobarbituric acid-reactive substances |
| TLC | Thin-Layer Chromatography |
| XO | Xanthine Oxidase |
References
- Alam Bhuiyan, M.K.; Qureshi, S. Proximate Chemical Composition of Sea Grapes Caulerpa Racemosa (J. Agardh, 1873) Collected from a Sub-Tropical Coast. Virol. Mycol. 2016, 5, 158. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kim, E.-A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; de Silva, E.D.; et al. FTIR Characterization and Antioxidant Activity of Water Soluble Crude Polysaccharides of Sri Lankan Marine Algae. Algae 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant Activity of Three Edible Seaweeds from Two Areas in South East Asia. LWT—Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- De Souza, É.T.; Pereira de Lira, D.; Cavalcanti de Queiroz, A.; Costa da Silva, D.J.; Bezerra de Aquino, A.; Campessato Mella, E.A.; Prates Lorenzo, V.; De Miranda, G.E.C.; De Araújo-Júnior, J.X.; De Oliveira Chaves, M.C.; et al. The Antinociceptive and Anti-Inflammatory Activities of Caulerpin, a Bisindole Alkaloid Isolated from Seaweeds of the Genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, I.H.; Bandaranayake, U.; Keerthirathna, L.R.; Manawadu, C.; Silva, R.M.; Mohamed, B.; Ali, R.; Peiris, D.C. Integration of in Vitro and In-Silico Analysis of Caulerpa Racemosa against Antioxidant, Antidiabetic, and Anticancer Activities. Sci. Rep. 2022, 12, 20848. [Google Scholar] [CrossRef] [PubMed]
- Sidrônio, M.G.S.; Freitas, M.E.G.; Magalhães, D.W.A.; Carvalho, D.C.M.; Gonçalves, V.A.B.; de Queiroz Oliveira, A.C.M.; Paulino, G.C.; Borges, G.C.; Ribeiro, R.L.; de Sousa, N.F.; et al. Host-Mediated Antimicrobial Effects and NLRP3 Inflammasome Modulation by Caulerpin and Its Derivatives in Macrophage Models of Mycobacterial Infections. Microorganisms 2025, 13, 561. [Google Scholar] [CrossRef]
- El Habitri, N.; Belkacemi, L. Antidiabetic Effect of Oral Supplementation with Caulerpa Racemosa Powder. Eur. J. Biol. Res. Res. Artic. Eur. J. Biol. Res. 2022, 12, 141–152. [Google Scholar] [CrossRef]
- Ferramosca, A.; Conte, A.; Guerra, F.; Felline, S.; Rimoli, M.G.; Mollo, E.; Zara, V.; Terlizzi, A. Metabolites from Invasive Pests Inhibit Mitochondrial Complex II: A Potential Strategy for the Treatment of Human Ovarian Carcinoma? Biochem. Biophys. Res. Commun. 2016, 473, 1133–1138. [Google Scholar] [CrossRef]
- Klein, J.; Verlaque, M. The Caulerpa Racemosa Invasion: A Critical Review. Mar. Pollut. Bull. 2008, 56, 205–225. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Ferramosca, A.; Pinto Provenzano, S.; Montagna, D.D.; Coppola, L.; Zara, V. Oxidative Stress Negatively Affects Human Sperm Mitochondrial Respiration. Urology 2013, 82, 78–83. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; El Yazbi, A.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci.-Landmark 2022, 27, 105. [Google Scholar] [CrossRef]
- Briyal, S.; Ranjan, A.K.; Gulati, A. Oxidative Stress: A Target to Treat Alzheimer’s Disease and Stroke. Neurochem. Int. 2023, 165, 105509. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hu, Y.; Jiang, L.; Lei, L.; Fu, C.; Wu, S.; Zhang, X.; Zhu, L.; Zhang, F.; Chen, J.; et al. Decreased Oxidative Stress Response and Oxidant Detoxification of Skin during Aging. Mech. Ageing Dev. 2023, 216, 111878. [Google Scholar] [CrossRef] [PubMed]
- Zara, V.; Assalve, G.; Ferramosca, A. Insights into the Malfunctioning of the Mitochondrial Citrate Carrier: Implications for Cell Pathology. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2023, 1869, 166758. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative Stress and Its Role in Cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.; Ranasinghe, P.; Peiris, L.D.C. Antidiabetic Potential of Marine Brown Algae—A Mini Review. J. Diabetes Res. 2020, 2020, 1230218. [Google Scholar] [CrossRef]
- Mehra, R.; Bhushan, S.; Bast, F.; Singh, S. Marine Macroalga Caulerpa: Role of Its Metabolites in Modulating Cancer Signaling. Mol. Biol. Rep. 2019, 46, 3545–3555. [Google Scholar] [CrossRef]
- dos Santos, J.S.P.; Silva, D.K.C.; da Silva Oliveira, V.; Junior, S.S.S.; dos Santos Rodrigues, E.; de Souza, C.V.C.; Martinez, S.T.; Santos-Filho, O.A.; Meira, C.S.; Soares, M.B.P. The Alkaloid Caulerpin Exhibits Potent and Selective Anti-Inflammatory Activity Through Interaction with the Glucocorticoid Receptor. Mar. Drugs 2025, 23, 232. [Google Scholar] [CrossRef]
- Ur Rehman, N.; Rafiq, K.; Khan, A.; Ahsan Halim, S.; Ali, L.; Al-Saady, N.; Hilal Al-Balushi, A.; Al-Busaidi, H.K.; Al-Harrasi, A. α-Glucosidase Inhibition and Molecular Docking Studies of Natural Brominated Metabolites from Marine Macro Brown Alga Dictyopteris Hoytii. Mar. Drugs 2019, 17, 666. [Google Scholar] [CrossRef] [PubMed]
- Stabili, L.; Fraschetti, S.; Acquaviva, M.; Cavallo, R.; De Pascali, S.; Fanizzi, F.; Gerardi, C.; Narracci, M.; Rizzo, L. The Potential Exploitation of the Mediterranean Invasive Alga Caulerpa Cylindracea: Can the Invasion Be Transformed into a Gain? Mar. Drugs 2016, 14, 210. [Google Scholar] [CrossRef]
- Erol, E.; Orhan, M.D.; Avsar, T.; Akdemir, A.; Okudan, E.S.; Alim Toraman, G.O.; Topcu, G. Anti-SARS-CoV-2 and Cytotoxic Activity of Two Marine Alkaloids from Green Alga Caulerpa cylindracea Sonder in the Dardanelles. RSC Adv. 2022, 12, 29983–29990. [Google Scholar] [CrossRef]
- Mert-Ozupek, N.; Calibasi-Kocal, G.; Olgun, N.; Basbinar, Y.; Cavas, L.; Ellidokuz, H. An Efficient and Quick Analytical Method for the Quantification of an Algal Alkaloid Caulerpin Showed In-Vitro Anticancer Activity against Colorectal Cancer. Mar. Drugs 2022, 20, 757. [Google Scholar] [CrossRef]
- Assalve, G.; Lunetti, P.; Zara, V.; Ferramosca, A. In Vivo Antioxidant Activity of Common Dietary Flavonoids: Insights from the Yeast Model Saccharomyces cerevisiae. Antioxidants 2024, 13, 1103. [Google Scholar] [CrossRef]
- Dani, C.; Bonatto, D.; Salvador, M.; Pereira, M.D.; Henriques, J.A.P.; Eleutherio, E. Antioxidant Protection of Resveratrol and Catechin in Saccharomyces cerevisiae. J. Agric. Food Chem. 2008, 56, 4268–4272. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, W.; Bartosz, G. Estimation of Oxidative Stress in Saccharomyces Cerevisae with Fluorescent Probes. Int. J. Biochem. Cell Biol. 1997, 29, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Bayliak, M.; Semchyshyn, H.; Lushchak, V. Effect of Hydrogen Peroxide on Antioxidant Enzyme Activities in Saccharomyces cerevisiae Is Strain-Specific. Biochemistry 2006, 71, 1013–1020. [Google Scholar] [CrossRef]
- Martins, D.; English, A.M. Catalase Activity Is Stimulated by H2O2 in Rich Culture Medium and Is Required for H2O2 Resistance and Adaptation in Yeast. Redox Biol. 2014, 2, 308–313. [Google Scholar] [CrossRef]
- Luk, E.; Yang, M.; Jensen, L.T.; Bourbonnais, Y.; Culotta, V.C. Manganese Activation of Superoxide Dismutase 2 in the Mitochondria of Saccharomyces cerevisiae. J. Biol. Chem. 2005, 280, 22715–22720. [Google Scholar] [CrossRef]
- Baron, J.A.; Chen, J.S.; Culotta, V.C. Cu/Zn Superoxide Dismutase and the Proton ATPase Pma1p of Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2015, 462, 251–256. [Google Scholar] [CrossRef]
- Macierzyńska, E.; Grzelak, A.; Bartosz, G. The Effect of Growth Medium on the Antioxidant Defense of Saccharomyces cerevisiae. Cell Mol. Biol. Lett. 2007, 12, 448–456. [Google Scholar] [CrossRef]
- SJ, S.; Veerabhadrappa, B.; Subramaniyan, S.; Dyavaiah, M. Astaxanthin Enhances the Longevity of Saccharomyces cerevisiae by Decreasing Oxidative Stress and Apoptosis. FEMS Yeast Res. 2018, 19, foy113. [Google Scholar] [CrossRef]
- Jamieson, D.J. Oxidative Stress Responses of the Yeast Saccharomyces cerevisiae. Yeast 1998, 14, 1511–1527. [Google Scholar] [CrossRef]
- Đorđević, N.O.; Todorović, N.; Novaković, I.T.; Pezo, L.L.; Pejin, B.; Maraš, V.; Tešević, V.V.; Pajović, S.B. Antioxidant Activity of Selected Polyphenolics in Yeast Cells: The Case Study of Montenegrin Merlot Wine. Molecules 2018, 23, 1971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative Stress and Diabetes: Antioxidative Strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Yi, X.; Zhu, Q.-X.; Wu, X.-L.; Tan, T.-T.; Jiang, X.-J. Histone Methylation and Oxidative Stress in Cardiovascular Diseases. Oxid. Med. Cell Longev. 2022, 2022, 6023710. [Google Scholar] [CrossRef] [PubMed]
- Matulja, D.; Vranješević, F.; Kolympadi Markovic, M.; Pavelić, S.K.; Marković, D. Anticancer Activities of Marine-Derived Phenolic Compounds and Their Derivatives. Molecules 2022, 27, 1449. [Google Scholar] [CrossRef] [PubMed]
- Koleva, D.I.; Petrova, V.Y.; Kujumdzieva, A.V. Comparison of Enzymatic Antioxidant Defence Systems in Different Metabolic Types of Yeasts. Can. J. Microbiol. 2008, 54, 957–963. [Google Scholar] [CrossRef]
- Izawa, S.; Inoue, Y.; Kimura, A. Importance of Catalase in the Adaptive Response to Hydrogen Peroxide: Analysis of Acatalasaemic Saccharomyces cerevisiae. Biochem. J. 1996, 320, 61–67. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In Vivo Antioxidant Activity of Grape, Pomace and Wine from Three Red Varieties Grown in Argentina: Its Relationship to Phenolic Profile. J. Funct. Foods 2016, 20, 332–345. [Google Scholar] [CrossRef]
- Magliozzi, L.; Almada, F.; Robalo, J.; Mollo, E.; Polese, G.; Gonçalves, E.J.; Felline, S.; Terlizzi, A.; D’Aniello, B. Cryptic effects of biological invasions: Reduction of the aggressive behaviour of a native fish under the influence of an “invasive” biomolecule. PLoS ONE 2017, 12, e0185620. [Google Scholar] [CrossRef] [PubMed]
- Assalve, G.; Lunetti, P.; Zara, V.; Ferramosca, A. Ctp1 and Yhm2: Two Mitochondrial Citrate Transporters to Support Metabolic Flexibility of Saccharomyces cerevisiae. Int. J. Mol. Sci. 2024, 25, 1870. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Marin, I.K.; González-Hernández, J.C.; Regalado-Gonzalez, C.; Madrigal-Perez, L.A. Saccharomyces cerevisiae Exponential Growth Kinetics in Batch Culture to Analyze Respiratory and Fermentative Metabolism. J. Vis. Exp. 2018, 139, 58192. [Google Scholar] [CrossRef]
- Magistrati, M.; Gilea, A.I.; Gerra, M.C.; Baruffini, E.; Dallabona, C. Drug Drop Test: How to Quickly Identify Potential Therapeutic Compounds for Mitochondrial Diseases Using Yeast Saccharomyces cerevisiae. Int. J. Mol. Sci. 2023, 24, 10696. [Google Scholar] [CrossRef]
- De Blasi, G.; Lunetti, P.; Zara, V.; Ferramosca, A. Mitochondrial Citrate Transporters Ctp1-Yhm2 and Respiratory Chain: A Coordinated Functional Connection in Saccharomyces cerevisiae Metabolism. Int. J. Biol. Macromol. 2024, 270, 132364. [Google Scholar] [CrossRef]
- Thomas, P.B.; Kaluç, N.; Aybastıer, Ö. SLX5 Deletion Confers Tolerance to Oxidative Stress in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2022, 369, fnac077. [Google Scholar] [CrossRef] [PubMed]
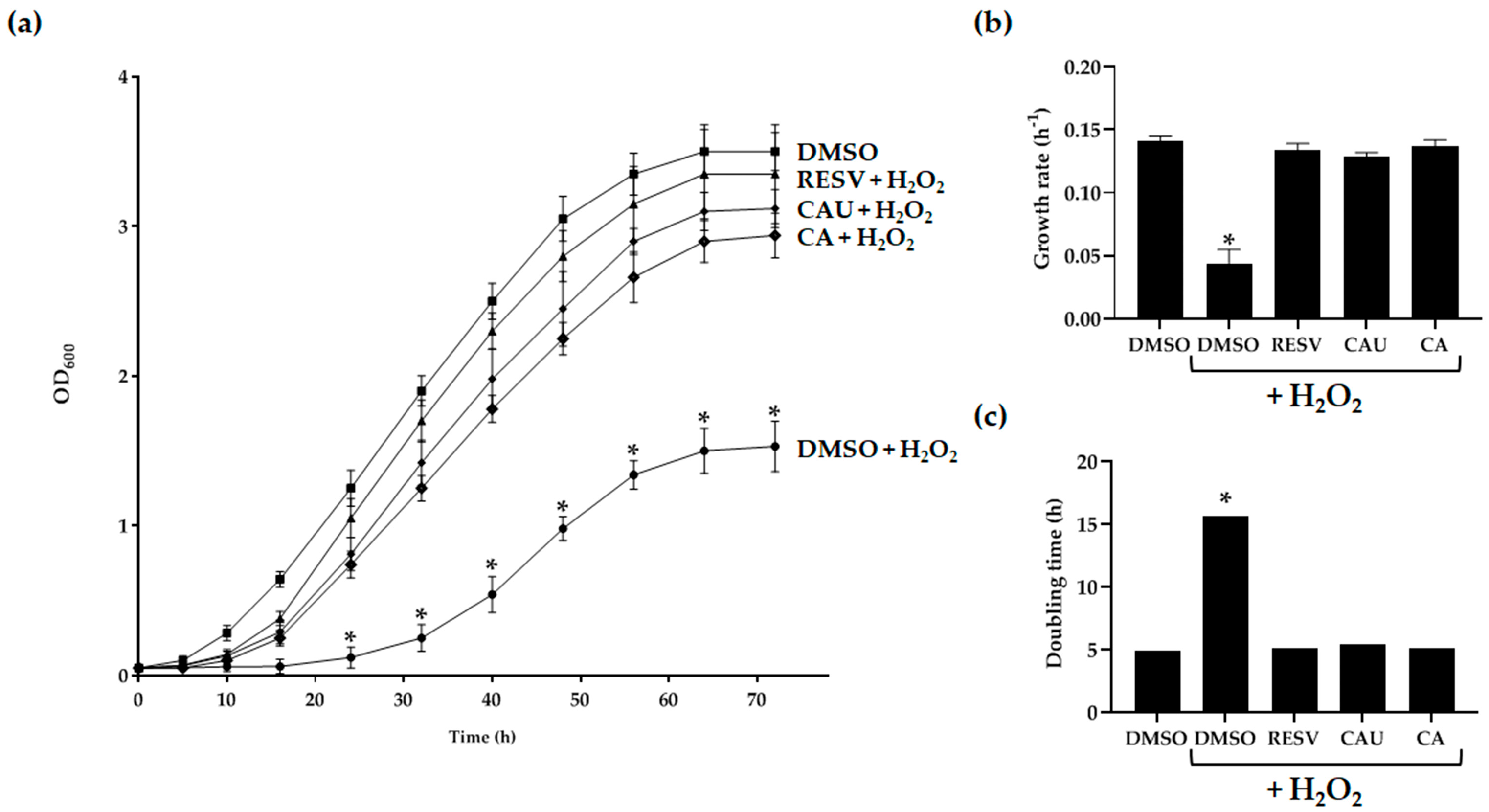


| Growth Rate (h−1) | Doubling Time (h) | ||
|---|---|---|---|
| −H2O2 | DMSO | 0.141 ± 0.004 | 4.91 |
| +H2O2 | DMSO | 0.044 ± 0.011 | 15.68 |
| RESV | 0.134 ± 0.005 | 5.16 | |
| CAU | 0.129 ± 0.003 | 5.38 | |
| CA | 0.137 ± 0.005 | 5.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assalve, G.; Lunetti, P.; Fai, A.; Terlizzi, A.; Zara, V.; Ferramosca, A. Marine Algal Metabolites as Cellular Antioxidants: A Study of Caulerpin and Caulerpinic Acid in Saccharomyces cerevisiae. Mar. Drugs 2025, 23, 338. https://doi.org/10.3390/md23090338
Assalve G, Lunetti P, Fai A, Terlizzi A, Zara V, Ferramosca A. Marine Algal Metabolites as Cellular Antioxidants: A Study of Caulerpin and Caulerpinic Acid in Saccharomyces cerevisiae. Marine Drugs. 2025; 23(9):338. https://doi.org/10.3390/md23090338
Chicago/Turabian StyleAssalve, Graziana, Paola Lunetti, Annalisa Fai, Antonio Terlizzi, Vincenzo Zara, and Alessandra Ferramosca. 2025. "Marine Algal Metabolites as Cellular Antioxidants: A Study of Caulerpin and Caulerpinic Acid in Saccharomyces cerevisiae" Marine Drugs 23, no. 9: 338. https://doi.org/10.3390/md23090338
APA StyleAssalve, G., Lunetti, P., Fai, A., Terlizzi, A., Zara, V., & Ferramosca, A. (2025). Marine Algal Metabolites as Cellular Antioxidants: A Study of Caulerpin and Caulerpinic Acid in Saccharomyces cerevisiae. Marine Drugs, 23(9), 338. https://doi.org/10.3390/md23090338








