OSMAC-Driven Discovery of Six New Alkaloids from the Cold-Seep-Derived Fungus Talaromyces amestolkiae HDN21-0307
Abstract
1. Introduction
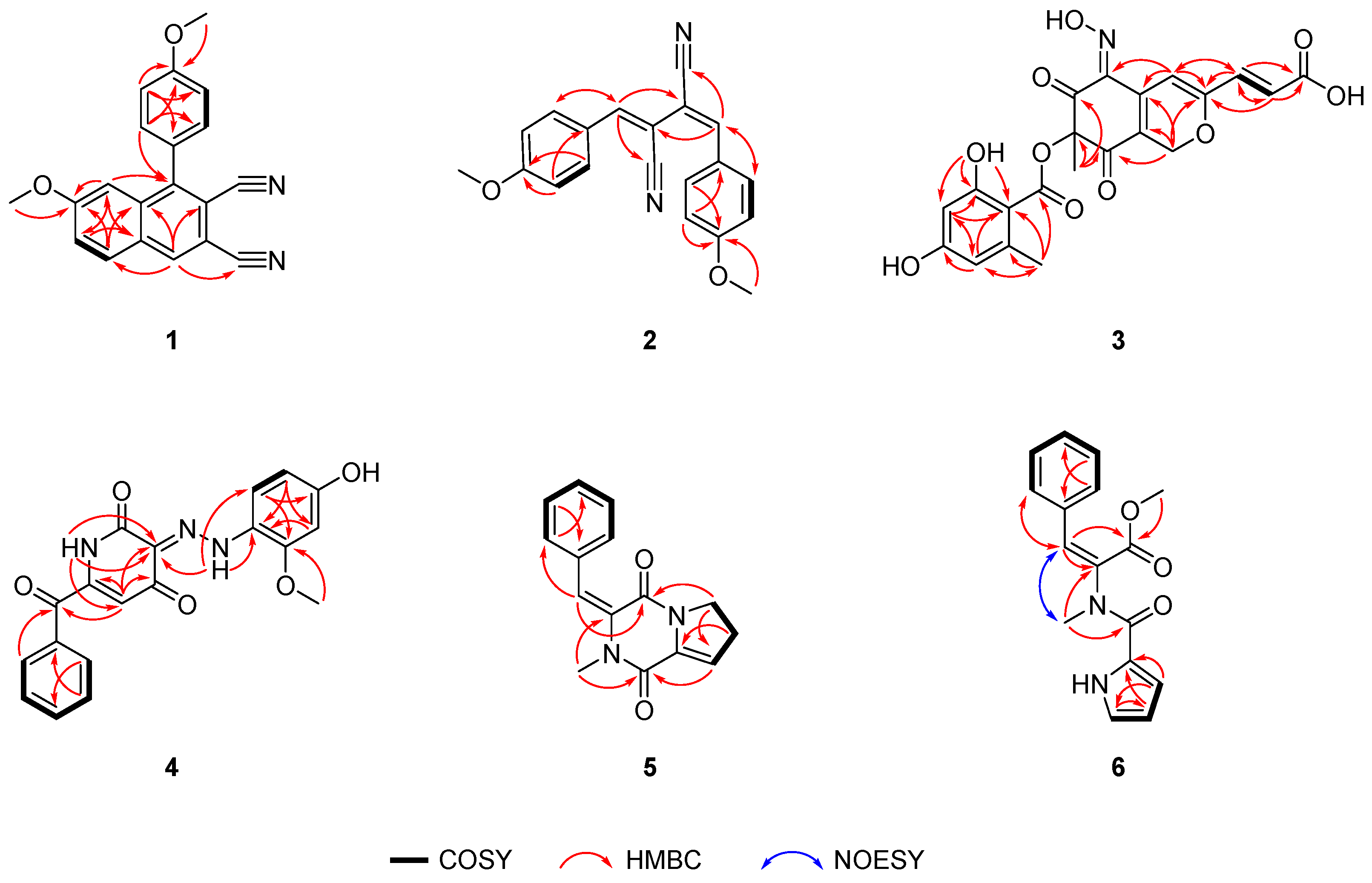
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material and Fermentation
3.3. OSMAC Research, Fermentation, and Extraction
3.4. Separation and Purification of Compounds
3.5. Computation Section
3.6. Assay of Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Meng, W.; Cao, C.; Wang, J.; Shan, W.; Wang, Q. Antibacterial and Antifungal Compounds from Marine Fungi. Mar. Drugs 2015, 13, 3479–3513. [Google Scholar] [CrossRef]
- Yin, Q.; Liu, X.; Zhang, Z.; Lei, H.; Wu, B. Chemistry and Bioactivities of Alkaloids Isolated from Marine Fungi (Covering 2016–2022). Fitoterapia 2023, 164, 105377. [Google Scholar] [CrossRef] [PubMed]
- Cong, M.; Zhang, Y.; Feng, X.; Pang, X.; Liu, Y.; Zhang, X.; Yang, Z.; Wang, J. Anti-Inflammatory Alkaloids from the Cold-Seep-Derived Fungus Talaromyces helicus SCSIO41311. 3 Biotech 2022, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.-R.; Gong, L.-Q.; Jin, M.-Y.; Wang, R.; Liu, R.; Gao, J.; Liu, M.-D.; Huang, L.; Wang, G.-Z.; Wang, D.; et al. Research Advances in the Structures and Biological Activities of Secondary Metabolites from Talaromyces. Front. Microbiol. 2022, 13, 984801. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Tan, H.; Guo, R.; Ma, X.; Wen, H. Research progress on the structure and activity of secondary metabolites of Talaromyces sp. Chin. J. Antibiot. 2024, 49, 961–985. [Google Scholar]
- Wu, J.; Wang, W.; Yang, Y.; Shah, M.; Peng, J.; Zhou, L.; Zhang, G.; Che, Q.; Li, J.; Zhu, T.; et al. Phenylhydrazone Alkaloids from the Deep-Sea Cold Seep Derived Fungus Talaromyces amestolkiae HDN21-0307. J. Nat. Prod. 2024, 87, 1407–1415. [Google Scholar] [CrossRef]
- Paoli, L.; Ruscheweyh, H.-J.; Forneris, C.C.; Hubrich, F.; Kautsar, S.; Bhushan, A.; Lotti, A.; Clayssen, Q.; Salazar, G.; Milanese, A.; et al. Biosynthetic Potential of the Global Ocean Microbiome. Nature 2022, 607, 111–118. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big Effects from Small Changes: Possible Ways to Explore Nature’s Chemical Diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Jin, J.; Fang, M.; Yin, K.; Jiang, M.; Wang, Y. Research progress of secondary metabolites of marine fungi by OSMAC strategy. Chin. J. Antibiot. 2023, 48, 481–491. [Google Scholar]
- Takahashi, H.; Nozawa, K.; Kawai, K.I. Isolation and Structures of Dicyanide Derivatives, Epurpurins A to C, from Emericella purpurea. Chem. Pharm. Bull. 1996, 44, 2227–2230. [Google Scholar] [CrossRef]
- Scotti, C.; Barlow, J.W. Natural Products Containing the Nitrile Functional Group and Their Biological Activities. Nat. Prod. Commun. 2022, 17, 1–24. [Google Scholar] [CrossRef]
- Liu, M.; Li, S. Nitrile Biosynthesis in Nature: How and Why? Nat. Prod. Rep. 2024, 41, 649–671. [Google Scholar] [CrossRef] [PubMed]
- Komai, S.; Hosoe, T.; Itabashi, T.; Nozawa, K.; Okada, K.; De Campos Takaki, G.M.; Chikamori, M.; Yaguchi, T.; Fukushima, K.; Miyaji, M.; et al. A New Funicone Derivative Isolated from Talaromyces flavus IFM52668. Mycotoxins 2004, 54, 15–19. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Liao, Y.-Y.; Cheng, G.; Chen, J.; Mo, S.; Yuan, L.; Cheng, X.-D.; Qin, J.-J.; Shao, Z. Aspeterreurone A, a Cytotoxic Dihydrobenzofuran–Phenyl Acrylate Hybrid from the Deep-Sea-Derived Fungus Aspergillus terreus CC-S06-18. J. Nat. Prod. 2020, 83, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z. Azaphilone Alkaloids: Prospective Source of Natural Food Pigments. Appl. Microbiol. Biotechnol. 2022, 106, 469–484. [Google Scholar] [CrossRef]
- Jin, S.; Liebscher, J. Optically Active Precursors for Quaternary Amino Acids by Addition of N-Heteroaromatics to 3-Alkylidene-2,5-Diketopiperazines. Synlett 1999, 1999, 459–461. [Google Scholar] [CrossRef]
- Tian, H.; Ermolenko, L.; Gabant, M.; Vergne, C.; Moriou, C.; Retailleau, P.; Al-Mourabit, A. Pyrrole-Assisted and Easy Oxidation of Cyclic α-Amino Acid- Derived Diketopiperazines under Mild Conditions. Adv. Synth. Catal. 2011, 353, 1525–1533. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Chang, Y.; Zhou, L.; Hou, X.; Zhu, T.; Pfeifer, B.A.; Li, D.; He, X.; Zhang, G.; Che, Q. Microbial Dimerization and Chlorination of Isoflavones by a Takla Makan Desert-Derived Streptomyces sp. HDN154127. J. Nat. Prod. 2023, 86, 34–44. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision A.1; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An Improved Probability for the Stereochemical Assignment of Isomeric Compounds Using Quantum Chemical Calculations of NMR Shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]


| No. | 1 | No. | 2 | ||
|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | ||
| 1 | 148.2, C | 1 | 119.1, C | ||
| 2 | 111.7, C | 2 | 106.1, C | ||
| 3 | 108.5, C | 3 | 100.8, C | ||
| 4 | 136.2, CH | 8.50, s | 4 | 116.1, C | |
| 4a | 130.5, C | 1′ | 125.4, C | ||
| 5 | 132.2, C | 8.07, d (9.0) | 2′ | 132.5, CH | 7.41, d (8.9) |
| 6 | 123.8, CH | 7.46, dd (9.0, 2.5) | 3′ | 114.4, CH | 6.93, d (8.9) |
| 7 | 162.9, CH | 4′ | 161.9, C | ||
| 8 | 107.1, CH | 7.06, d (2.5) | 5′ | 114.4, CH | 6.93, d (8.9) |
| 8a | 136.5, C | 6′ | 132.5, CH | 7.41, d (8.9) | |
| 9 | 117.0, C | 7′ | 146.1, CH | 7.35, s | |
| 10 | 117.7, C | 8′ | 55.6, CH3 | 3.88, s | |
| 11 | 56.0, CH3 | 3.77, s | 1″ | 125.4, C | |
| 1′ | 128.9, C | 2″ | 132.1, CH | 7.87, d (8.9) | |
| 2′ | 132.3, CH | 7.42, d (8.7) | 3″ | 114.8, CH | 6.98, d (8.9) |
| 3′ | 115.5, CH | 7.18, d (8.7) | 4″ | 162.7, C | |
| 4′ | 162.2, C | 5″ | 114.8, CH | 6.98, d (8.9) | |
| 5′ | 115.5, CH | 7.18, d (8.7) | 6″ | 132.1, CH | 7.87, d (8.9) |
| 6′ | 132.3, CH | 7.42, d (8.7) | 7″ | 149.0, CH | 7.46, s |
| 7′ | 55.9, CH3 | 3.93, s | 8″ | 55.7, CH3 | 3.85, s |
| No. | 3 | No. | 4 | ||
|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | ||
| 1 | 189.0, C | 1 | |||
| 2 | 86.9, C | 2 | 160.9, C | ||
| 3 | 185.4, C | 3 | 127.9, C | ||
| 4 | 142.4, C | 4 | 178.2, C | ||
| 4a | 120.0, C | 5 | 108.6, CH | 5.91, s | |
| 5 | 104.9, CH | 6.56, s | 6 | 145.1, C | |
| 6 | 158.8, C | 7 | 180.5, C | ||
| 8 | 64.6, CH2 | 5.14, m | 8 | 134.6, C | |
| 8a | 141.3, C | 9 | 129.9, CH | 7.91, m a | |
| 9 | 137.3, CH | 7.23, d (15.5) | 10 | 129.0, CH | 7.61, m a |
| 10 | 124.4, CH | 6.42, d (15.5) | 11 | 134.4, CH | 7.76, m a |
| 11 | 166.9, CH | 12 | 128.6, CH | 7.61, m a | |
| 12 | 21.9, CH3 | 1.77, s | 13 | 129.1, CH | 7.91, m a |
| 1″ | 170.5, C | 16 | 122.6, C | ||
| 2″ | 104.3, C | 17 | 151.6, C | ||
| 3″ | 166.6, C | 18 | 99.7, CH | 6.62, d (2.3) | |
| 4″ | 101.8, CH | 6.25, d (2.4) | 19 | 159.8, C | |
| 5″ | 164.4, C | 20 | 109.3, CH | 6.57, dd (8.8, 2.1) | |
| 6″ | 113.0, CH | 6.38, d (2.1) | 21 | 117.9, CH | 7.69, d (8.3) |
| 7″ | 145.0, C | 22 | 56.3, CH3 | 3.92, s | |
| 8″ | 24.3, CH3 | 2.62, s | 1 | -NH | 11.15, s |
| 3″ | -OH | 10.68, s | 15 | -NH | 17.30, s |
| 19 | -OH | 10.30, s | |||
| No. | 5 | No. | 6 | ||
|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | ||
| 1 | 1 | 167.1, C | |||
| 2 | 156.8, C | 2 | 134.1, C | ||
| 3 | 133.5, C | 3 | |||
| 4 | 120.3, CH | 6.26, t (3.1) | 4 | 164.8, C | |
| 5 | 28.3, CH2 | 2.84, td (9.1, 3.1) | 5 | 126.1, C | |
| 6 | 46.0, CH2 | 4.13, t (9.1) | 6 | 114.7, CH | 6.53, dd (3.8, 1.1) |
| 7 | 7 | 110.5, CH | 6.03, dd (3.8, 2.6) | ||
| 8 | 156.79, C | 8 | 123.6, CH | 6.87, dd (2.4, 1.2) | |
| 9 | 131.7, C | 9 | |||
| 10 | 56.3, CH3 | 2.91, s | 10 | 35.9, CH3 | 3.06, s |
| 11 | 119.6, CH | 7.30, s | 11 | 137.9, CH | 7.63, s |
| 12 | 12 | 53.2, CH3 | 3.68, s | ||
| 1′ | 134.7, C | 1′ | 134.0, C | ||
| 2′ | 129.4, CH | 7.22, t (7.5) | 2′ | 131.2, CH | 7.61, ma |
| 3′ | 128.3, CH | 7.33, ma | 3′ | 130.2, CH | 7.40, m a |
| 4′ | 128.2, CH | 7.33, ma | 4′ | 131.9, CH | 7.40, m a |
| 5′ | 128.3, CH | 7.33, ma | 5′ | 130.2, CH | 7.40, m a |
| 6′ | 128.3, CH | 7.22, t (7.5) | 6′ | 131.2, CH | 7.61, m a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Wu, J.; Zhou, L.; Wang, Z.; Che, Q.; Chen, L.; Wang, W.; Zhu, T.; Li, D. OSMAC-Driven Discovery of Six New Alkaloids from the Cold-Seep-Derived Fungus Talaromyces amestolkiae HDN21-0307. Mar. Drugs 2025, 23, 337. https://doi.org/10.3390/md23090337
Huang X, Wu J, Zhou L, Wang Z, Che Q, Chen L, Wang W, Zhu T, Li D. OSMAC-Driven Discovery of Six New Alkaloids from the Cold-Seep-Derived Fungus Talaromyces amestolkiae HDN21-0307. Marine Drugs. 2025; 23(9):337. https://doi.org/10.3390/md23090337
Chicago/Turabian StyleHuang, Xinsheng, Jiajin Wu, Luning Zhou, Zhengjie Wang, Qian Che, Liangzhen Chen, Wenxue Wang, Tianjiao Zhu, and Dehai Li. 2025. "OSMAC-Driven Discovery of Six New Alkaloids from the Cold-Seep-Derived Fungus Talaromyces amestolkiae HDN21-0307" Marine Drugs 23, no. 9: 337. https://doi.org/10.3390/md23090337
APA StyleHuang, X., Wu, J., Zhou, L., Wang, Z., Che, Q., Chen, L., Wang, W., Zhu, T., & Li, D. (2025). OSMAC-Driven Discovery of Six New Alkaloids from the Cold-Seep-Derived Fungus Talaromyces amestolkiae HDN21-0307. Marine Drugs, 23(9), 337. https://doi.org/10.3390/md23090337






