High Inter- and Intraspecific Variability in Amphidinol Content and Toxicity of Amphidinium Strains
Abstract
1. Introduction
2. Results and Discussion
2.1. Search for Amphidinols
2.2. New Amphidinols
2.2.1. Variant ARC-1
2.2.2. Variant ARC-2
2.2.3. Variant ARC-3
2.2.4. Variant ARC-4
2.2.5. Variant ARC-5
2.2.6. Variant ARC-6
2.2.7. Variant ARC-7
2.2.8. Variant ARC-8
2.3. Diversity, Toxicity, and Amphidinol Profile of Strains
3. Materials and Methods
3.1. Culturing and Phylogenetic Analysis of Amphidinium Strains
3.2. Amphidinol Extraction
3.3. Analysis via Ultra-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry
3.3.1. Selected Reaction Monitoring (SRM) Mode
3.3.2. Neutral Loss (NL) Measurement Mode
3.3.3. Full-Scan (FS) Measurement Mode
3.3.4. Collision-Induced Dissociation (CID) Measurement Mode
3.3.5. Selection of Neutral Loss Scans
3.4. Brine Shrimp Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HAB | Harmful Algal Blooms |
| AM | Amphidinols |
| LP | Luteophanols |
| LS | Lingshuiols |
| SP | Symbiopolyols |
| KAR | Karatungiols |
| CAR | Carteraols |
| LC-MS/MS | Liquid chromatography tandem mass spectrometry |
| UHPLC-MS/MS | Ultra-high performance liquid chromatography tandem mass spectrometry |
| SRM | Selected reaction monitoring |
| NMR | Nuclear magnetic resonance spectroscopy |
| MS | Mass spectometry |
| NL | Neutral loss |
| NL | Neutral mode |
| FS | Full scan |
| CID | Collision induced dissociation |
| ESI | Electrospray ionization |
| ARC | Algal Resources Collection |
References
- Dodge, J.D. Marine Dinoflagellates of the British Isles; Informa UK Limited: London, UK, 1982; p. 303. [Google Scholar] [CrossRef]
- Larsen, J.; Patterson, D.J. Some flagellates (Protista) from tropical marine sediments. J. Nat. Hist. 1990, 24, 801–937. [Google Scholar] [CrossRef]
- Murray, S.; Patterson, D.J. The benthic dinoflagellate genus Amphidinium in south-eastern Australian waters, including three new species. Eur. J. Phycol. 2002, 37, 279–298. [Google Scholar] [CrossRef]
- Durán-Riveroll, L.M.; Juárez, O.E.; Okolodkov, Y.B.; Mejía-Camacho, A.L.; Ramírez-Corona, F.; Casanova-Gracia, D.; Osorio-Ramírez, M.d.C.; Cervantes-Urieta, V.A.; Cembella, A.D. Morphological and molecular characterization of the benthic dinoflagellate Amphidinium from coastal waters of Mexico. Phycology 2023, 3, 305–324. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Murray, S.A.; Chomérat, N.; Horiguchi, T. Marine Benthic Dinoflagellates-Unveiling Their Worldwide Biodiversity. In Kleine Senckenberg-Reihe 54; Schweizerbart’sche Verlagsbuchhandlung: Sydney, Australia, 2014; Available online: http://hdl.handle.net/10453/36179 (accessed on 18 August 2025).
- Murray, S.A.; Kohli, G.S.; Farrell, H.; Spiers, Z.B.; Place, A.R.; Dorantes-Aranda, J.J.; Ruszczyk, J. A fish kill associated with a bloom of Amphidinium carterae in a coastal lagoon in Sydney, Australia. Harmful Algae 2015, 49, 19–28. [Google Scholar] [CrossRef]
- Baig, H.; Saifullah, S.; Dar, A. Occurrence and toxicity of Amphidinium carterae Hulburt in the North Arabian Sea. Harmful Algae 2006, 5, 133–140. [Google Scholar] [CrossRef]
- Pagliara, P.; Caroppo, C. Toxicity assessment of Amphidinium carterae, Coolia cfr. monotis and Ostreopsis cfr. ovata (Dinophyta) isolated from the northern Ionian Sea (Mediterranean Sea). Toxicon 2012, 60, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.; Smith, K.; Harwood, T.; Bedford, C. Novel and toxin-producing epiphytic dinoflagellates isolated from sub-tropical Raoul Island, Kermadec Islands group. N. Z. J. Mar. Freshw. Res. 2014, 48, 594–599. [Google Scholar] [CrossRef]
- Cousseau, A.; Siano, R.; Probert, I.; Bach, S.; Mehiri, M. Marine dinoflagellates as a source of new bioactive structures. Stud. Nat. Prod. Chem. 2020, 65, 125–171. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T.; Fujita, T.; Naoki, H. Amphidinol, a polyhydroxy-polyene antifungal agent with an unprecedented structure, from a marine dinoflagellate, Amphidinium klebsii. J. Am. Chem. Soc. 1991, 113, 9859–9861. [Google Scholar] [CrossRef]
- Wellkamp, M.; García-Camacho, F.; Durán-Riveroll, L.M.; Tebben, J.; Tillmann, U.; Krock, B. LC-MS/MS method development for the discovery and identification of amphidinols produced by Amphidinium. Mar. Drugs 2020, 18, 497. [Google Scholar] [CrossRef]
- Waters, A.L.; Oh, J.; Place, A.R.; Hamann, M.T. Stereochemical studies of the karlotoxin class using NMR spectroscopy and DP4 chemical-shift analysis: Insights into their mechanism of action. Angew. Chem. 2015, 127, 15931–15936. [Google Scholar] [CrossRef]
- Barone, M.E.; Murphy, E.; Parkes, R.; Fleming, G.T.; Campanile, F.; Thomas, O.P.; Touzet, N. Antibacterial activity and amphidinol profiling of the marine dinoflagellate Amphidinium carterae (Subclade III). Int. J. Mol. Sci. 2021, 22, 12196. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Cornelio, K.; Hanashima, S.; Malabed, R.; Murata, M.; Matsumori, N.; Zhang, H.; Hayashi, F.; Mori, S.; Kim, J.S. Structures of the largest amphidinol homologues from the dinoflagellate Amphidinium carterae and structure–activity relationships. J. Nat. Prod. 2017, 80, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N.; Houdai, T.; Matsuoka, S.; Matsumori, N.; Adachi, S.; Oishi, T.; Murata, M.; Iwashita, T.; Fujita, T. Structures of new amphidinols with truncated polyhydroxyl chain and their membrane-permeabilizing activities. Bioorg. Med. Chem. 2006, 14, 6548–6554. [Google Scholar] [CrossRef]
- Echigoya, R.; Rhodes, L.; Oshima, Y.; Satake, M. The structures of five new antifungal and hemolytic amphidinol analogs from Amphidinium carterae collected in New Zealand. Harmful Algae 2005, 4, 383–389. [Google Scholar] [CrossRef]
- Morsy, N.; Matsuoka, S.; Houdai, T.; Matsumori, N.; Adachi, S.; Murata, M.; Iwashita, T.; Fujita, T. Isolation and structure elucidation of a new amphidinol with a truncated polyhydroxyl chain from Amphidinium klebsii. Tetrahedron 2005, 61, 8606–8610. [Google Scholar] [CrossRef]
- Place, A.R.; Bowers, H.A.; Bachvaroff, T.R.; Adolf, J.E.; Deeds, J.R.; Sheng, J. Karlodinium veneficum—The little dinoflagellate with a big bite. Harmful Algae 2012, 14, 179–195. [Google Scholar] [CrossRef]
- Morsy, N.; Houdai, T.; Konoki, K.; Matsumori, N.; Oishi, T.; Murata, M. Effects of lipid constituents on membrane-permeabilizing activity of amphidinols. Bioorg. Med. Chem. 2008, 16, 3084–3090. [Google Scholar] [CrossRef]
- Morsy, N.; Konoki, K.; Houdai, T.; Matsumori, N.; Oishi, T.; Murata, M.; Aimoto, S. Roles of integral protein in membrane permeabilization by amphidinols. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 1453–1459. [Google Scholar] [CrossRef]
- Haq, S.; Oyler, B.L.; Williams, E.; Khan, M.M.; Goodlett, D.R.; Bachvaroff, T.; Place, A.R. Investigating a multi-domain polyketide synthase in Amphidinium carterae. Mar. Drugs 2023, 21, 425. [Google Scholar] [CrossRef]
- Haq, S.; Bachvaroff, T.R.; Place, A.R. Characterization of acetyl-CoA carboxylases in the basal dinoflagellate Amphidinium carterae. Mar. Drugs 2017, 15, 149. [Google Scholar] [CrossRef]
- Bausch, A.R.; Juhl, A.R.; Donaher, N.A.; Cockshutt, A.M. Combined effects of simulated acidification and hypoxia on the harmful dinoflagellate Amphidinium carterae. Mar. Biol. 2019, 166, 80. [Google Scholar] [CrossRef]
- Lee, J.J.; Shpigel, M.; Freeman, S.; Zmora, O.; Mcleod, S.; Bowen, S.; Pearson, M.; Szostek, A. Physiological ecology and possible control strategy of a toxic marine dinoflagellate, Amphidinium sp., from the benthos of a mariculture pond. Aquaculture 2003, 217, 351–371. [Google Scholar] [CrossRef]
- Lin, Q.; Shang, L.; Wang, X.; Hu, Z.; Du, H.; Wang, H. Different dimethylsulphoniopropionate-producing ability of dinoflagellates could affect the structure of their associated bacterial community. Algal Res. 2021, 57, 102359. [Google Scholar] [CrossRef]
- Murray, S.A.; Garby, T.; Hoppenrath, M.; Neilan, B.A. Genetic diversity, morphological uniformity and polyketide production in dinoflagellates (Amphidinium, Dinoflagellata). PLoS ONE 2012, 7, e38253. [Google Scholar] [CrossRef]
- Flø Jørgensen, M.; Murray, S.; Daugbjerg, N. Amphidinium revisited. I. Redefinition of Amphidinium (Dinophyceae) based on cladistic and molecular phylogenetic analyses. J. Phycol. 2004, 40, 351–365. [Google Scholar] [CrossRef]
- Karafas, S.; Teng, S.T.; Leaw, C.P.; Alves-de-Souza, C. An evaluation of the genus Amphidinium (Dinophyceae) combining evidence from morphology, phylogenetics, and toxin production, with the introduction of six novel species. Harmful Algae 2017, 68, 128–151. [Google Scholar] [CrossRef]
- Murray, S.; Flø Jørgensen, M.; Daugbjerg, N.; Rhodes, L. Amphidinium revisited. II. Resolving species boundaries in the Amphidinium operculatum species complex (dinophyceae), including the descriptions of Amphidinium trulla sp. nov. and Amphidinium gibbosum. comb. nov. J. Phycol. 2004, 40, 366–382. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, N.; Mohamed, H.F.; Liang, Y.; Pei, L.; Huang, S.; Gu, H.; Luo, Z.; Wang, N.; Mohamed, H.F. Amphidinium stirisquamtum sp. nov. (Dinophyceae), a new marine sand-dwelling dinoflagellate with a novel type of body scale. Algae 2021, 36, 241–261. [Google Scholar] [CrossRef]
- Lee, J.J.; Olea, R.; Cevasco, M.; Pochon, X.; Correia, M.; Shpigel, M.; Pawlowski, J. A marine dinoflagellate, Amphidinium eilatiensis n. sp., from the benthos of a mariculture sedimentation pond in Eilat, Israel. J. Eukaryot. Microbiol. 2003, 50, 439–448. [Google Scholar] [CrossRef]
- Murray, S.; Patterson, D.J. Amphidiniopsis korewalensis sp. nov., a new heterotrophic benthic dinoflagellate. Phycologia 2002, 41, 382–388. [Google Scholar] [CrossRef]
- Molina-Miras, A.; Morales-Amador, A.; De Vera, C.; López-Rosales, L.; Sánchez-Mirón, A.; Souto, M.; Fernández, J.; Norte, M.; García-Camacho, F.; Molina-Grima, E. A pilot-scale bioprocess to produce amphidinols from the marine microalga Amphidinium carterae: Isolation of a novel analogue. Algal Res. 2018, 31, 87–98. [Google Scholar] [CrossRef]
- Abreu, A.C.; Molina-Miras, A.; Aguilera-Sáez, L.M.; López-Rosales, L.; Cerón-García, M.d.C.; Sánchez-Mirón, A.; Olmo-García, L.; Carrasco-Pancorbo, A.; García-Camacho, F.; Molina-Grima, E. Production of amphidinols and other bioproducts of interest by the marine microalga Amphidinium carterae unraveled by nuclear magnetic resonance metabolomics approach coupled to multivariate data analysis. J. Agric. Food Chem. 2019, 67, 9667–9682. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Matsuoka, S.; Matsumori, N.; Paul, G.K.; Tachibana, K. Absolute configuration of amphidinol 3, the first complete structure determination from amphidinol homologues: Application of a new configuration analysis based on carbon− hydrogen spin-coupling constants. J. Am. Chem. Soc. 1999, 121, 870–871. [Google Scholar] [CrossRef]
- Nuzzo, G.; Cutignano, A.; Sardo, A.; Fontana, A. Antifungal amphidinol 18 and its 7-sulfate derivative from the marine dinoflagellate Amphidinium carterae. J. Nat. Prod. 2014, 77, 1524–1527. [Google Scholar] [CrossRef]
- Lincoln, R.; Strupinski, K.; Walker, J. The use of Artemia nauplii (brine shrimp larvae) to detect toxic compounds from microalgal cultures. Int. J. Pharmacogn. 1996, 34, 384–389. [Google Scholar] [CrossRef]
- Chan, W.; Shaughnessy, A.E.; van den Berg, C.P.; Garson, M.J.; Cheney, K.L. The validity of brine shrimp (Artemia sp.) toxicity assays to assess the ecological function of marine natural products. J. Chem. Ecol. 2021, 47, 834–846. [Google Scholar] [CrossRef]
- Martínez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martín, J.; Díaz, C.; Cautain, B.; de la Cruz, M. Amphidinol 22, a new cytotoxic and antifungal amphidinol from the dinoflagellate Amphidinium carterae. Mar. Drugs 2019, 17, 385. [Google Scholar] [CrossRef] [PubMed]
- Orefice, I.; Balzano, S.; Romano, G.; Sardo, A. Amphidinium spp. as a source of antimicrobial, antifungal, and anticancer compounds. Life 2023, 13, 2164. [Google Scholar] [CrossRef]
- Doi, Y.; Ishibashi, M.; Nakamichi, H.; Kosaka, T.; Ishikawa, T.; Kobayashi, J.i. Luteophanol A, a new polyhydroxyl compound from symbiotic marine dinoflagellate Amphidinium sp. J. Org. Chem. 1997, 62, 3820–3823. [Google Scholar] [CrossRef]
- Meng, Y.; Van Wagoner, R.M.; Misner, I.; Tomas, C.; Wright, J.L. Structure and biosynthesis of amphidinol 17, a hemolytic compound from Amphidinium carterae. J. Nat. Prod. 2010, 73, 409–415. [Google Scholar] [CrossRef]
- Kubota, T.; Iwai, T.; Sakai, K.; Gonoi, T.; Kobayashi, J.i. Amphidinins C–F, amphidinolide Q analogues from marine dinoflagellate Amphidinium sp. Org. Lett. 2014, 16, 5624–5627. [Google Scholar] [CrossRef]
- Kumagai, K.; Minamida, M.; Akakabe, M.; Tsuda, M.; Konishi, Y.; Tominaga, A.; Tsuda, M.; Fukushi, E.; Kawabata, J. Amphirionin-2, a novel linear polyketide with potent cytotoxic activity from a marine dinoflagellate Amphidinium species. Bioorg. Med. Chem. Lett. 2015, 25, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.A.; Suggett, D.J.; Doblin, M.A.; Kohli, G.S.; Seymour, J.R.; Fabris, M.; Ralph, P.J. Unravelling the functional genetics of dinoflagellates: A review of approaches and opportunities. Perspect. Phycol. 2016, 3, 37–52. [Google Scholar] [CrossRef]
- Verma, A.; Hoppenrath, M.; Dorantes-Aranda, J.J.; Harwood, D.T.; Murray, S.A. Molecular and phylogenetic characterization of Ostreopsis (Dinophyceae) and the description of a new species, Ostreopsis rhodesae sp. nov., from a subtropical Australian lagoon. Harmful Algae 2016, 60, 116–130. [Google Scholar] [CrossRef]
- Paul, G.K.; Matsumori, N.; Murata, M.; Tachibana, K. Isolation and chemical structure of amphidinol 2, a potent hemolytic compound from marine dinoflagellate Amphidinium klebsii. Tetrahedron Lett. 1995, 36, 6279–6282. [Google Scholar] [CrossRef]
- Waggett, R.J.; Tester, P.A.; Place, A.R. Anti-grazing properties of the toxic dinoflagellate Karlodinium veneficum during predator–prey interactions with the copepod Acartia tonsa. Mar. Ecol. Prog. Ser. 2008, 366, 31–42. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
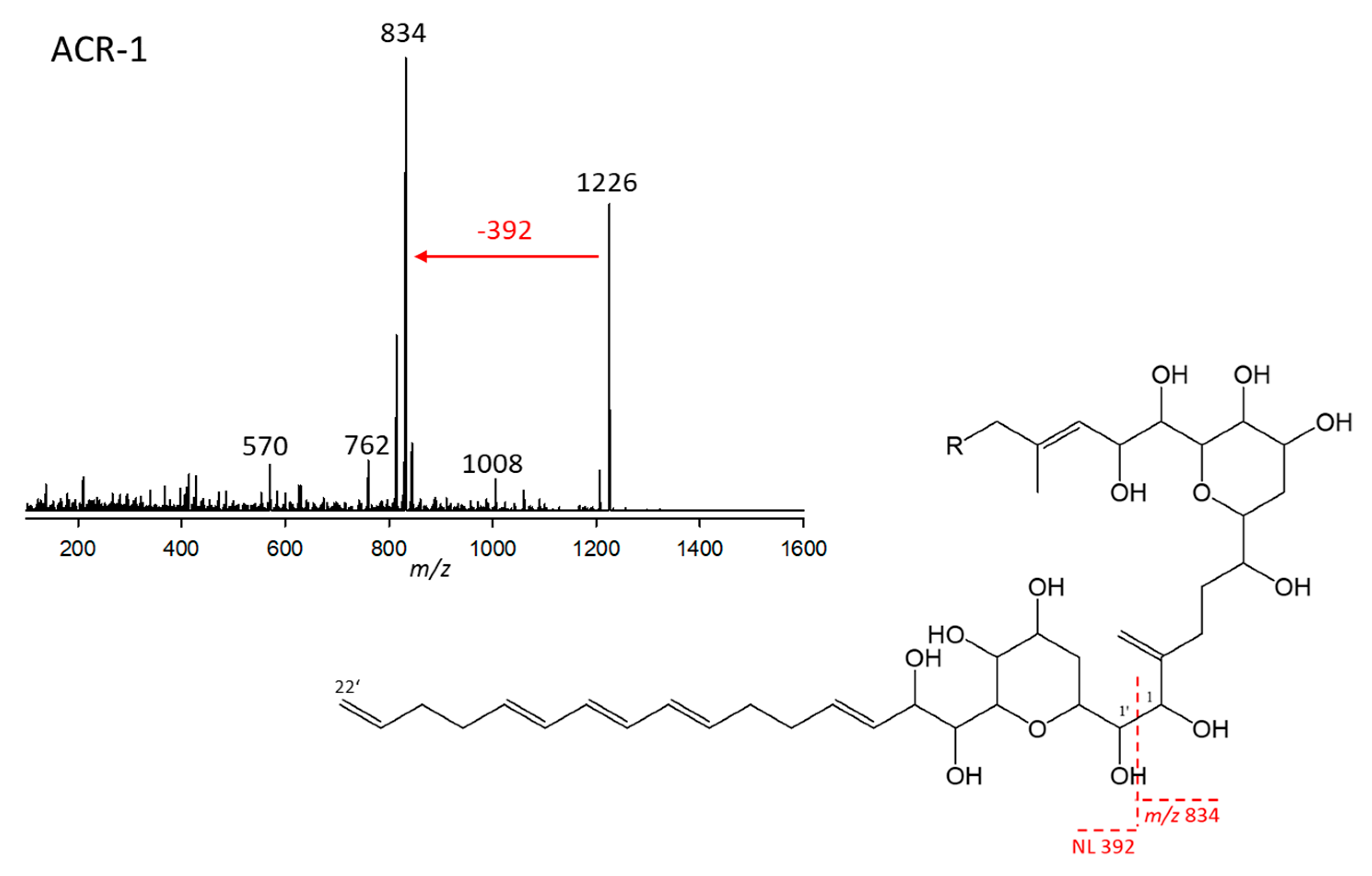
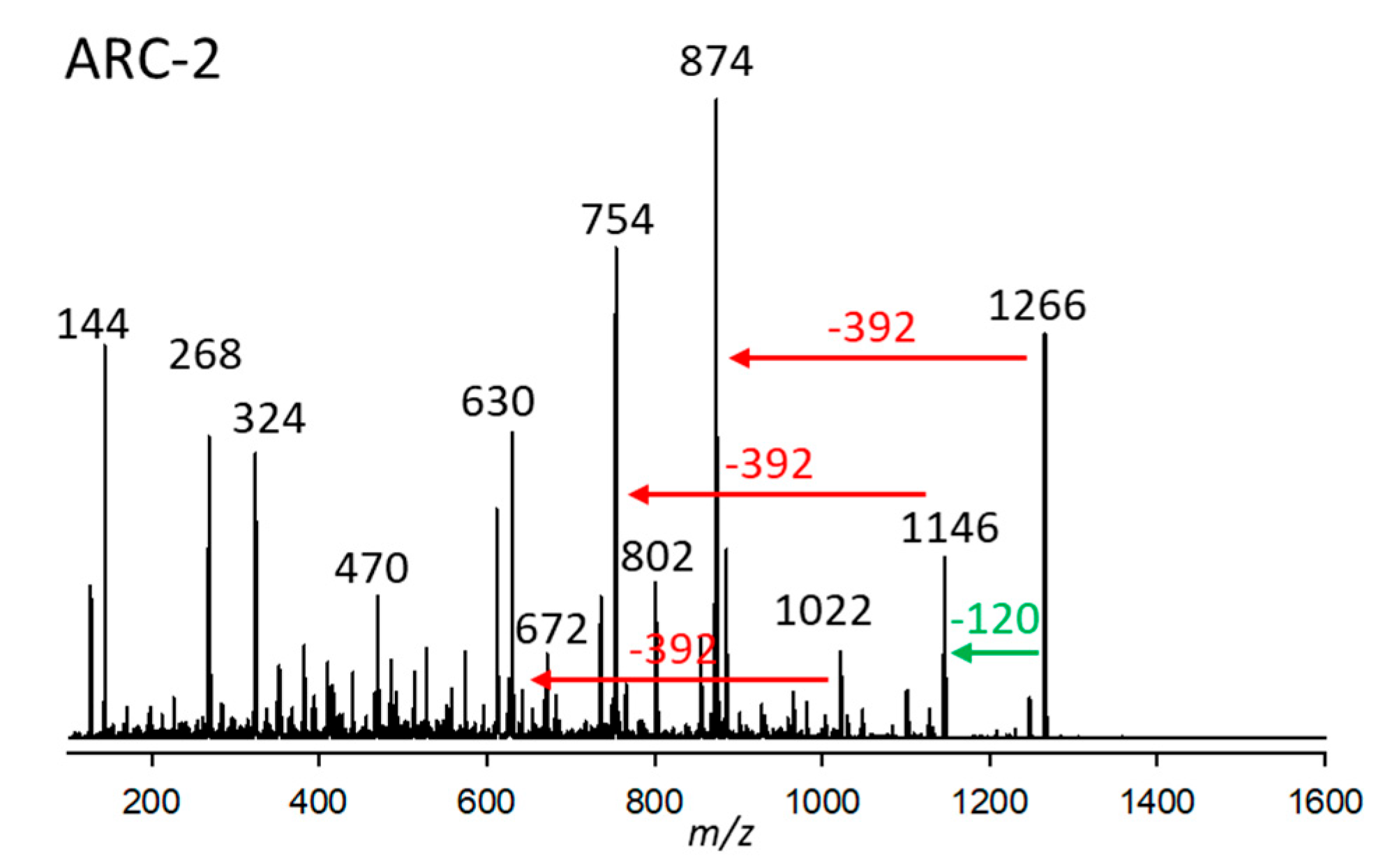
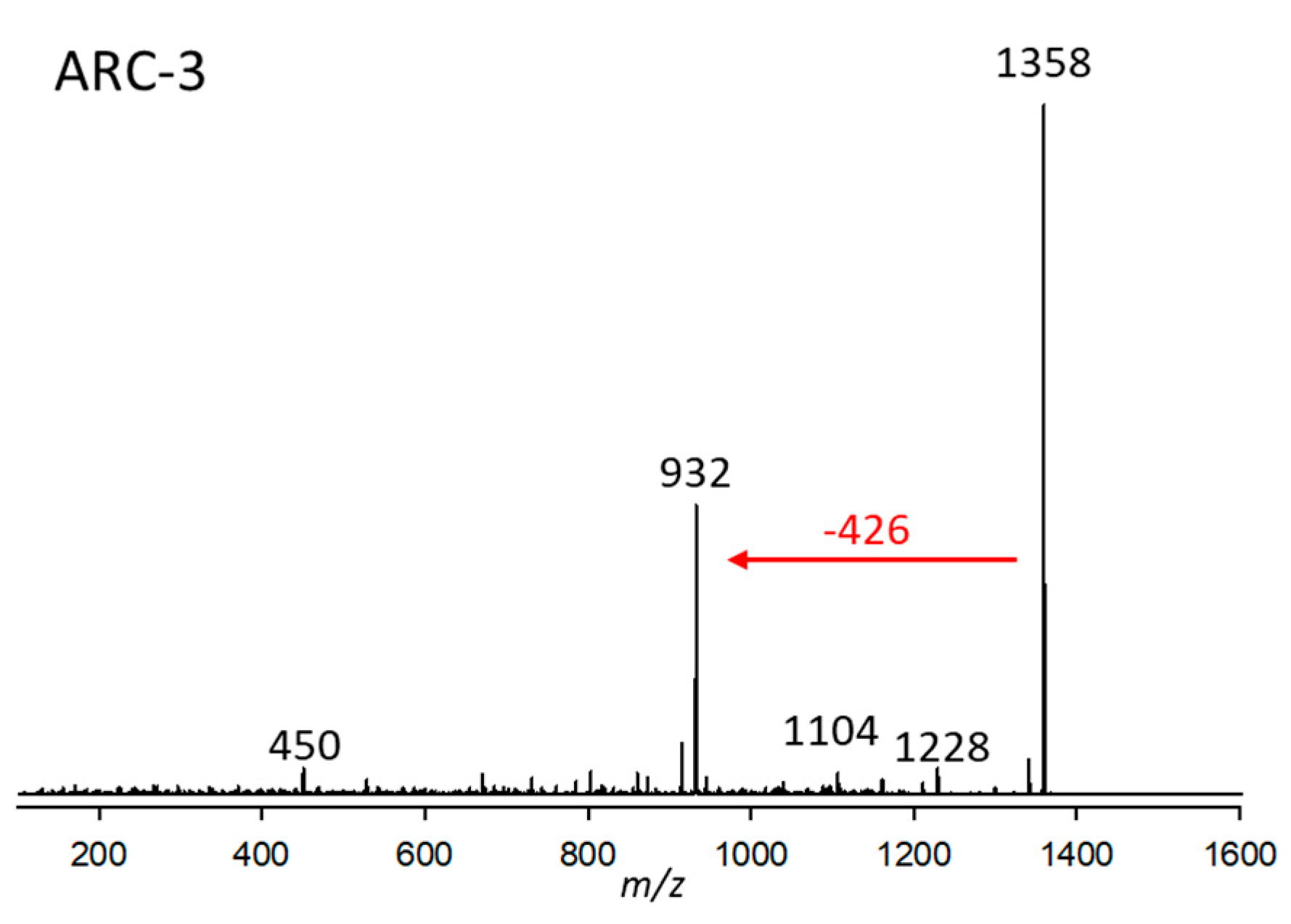
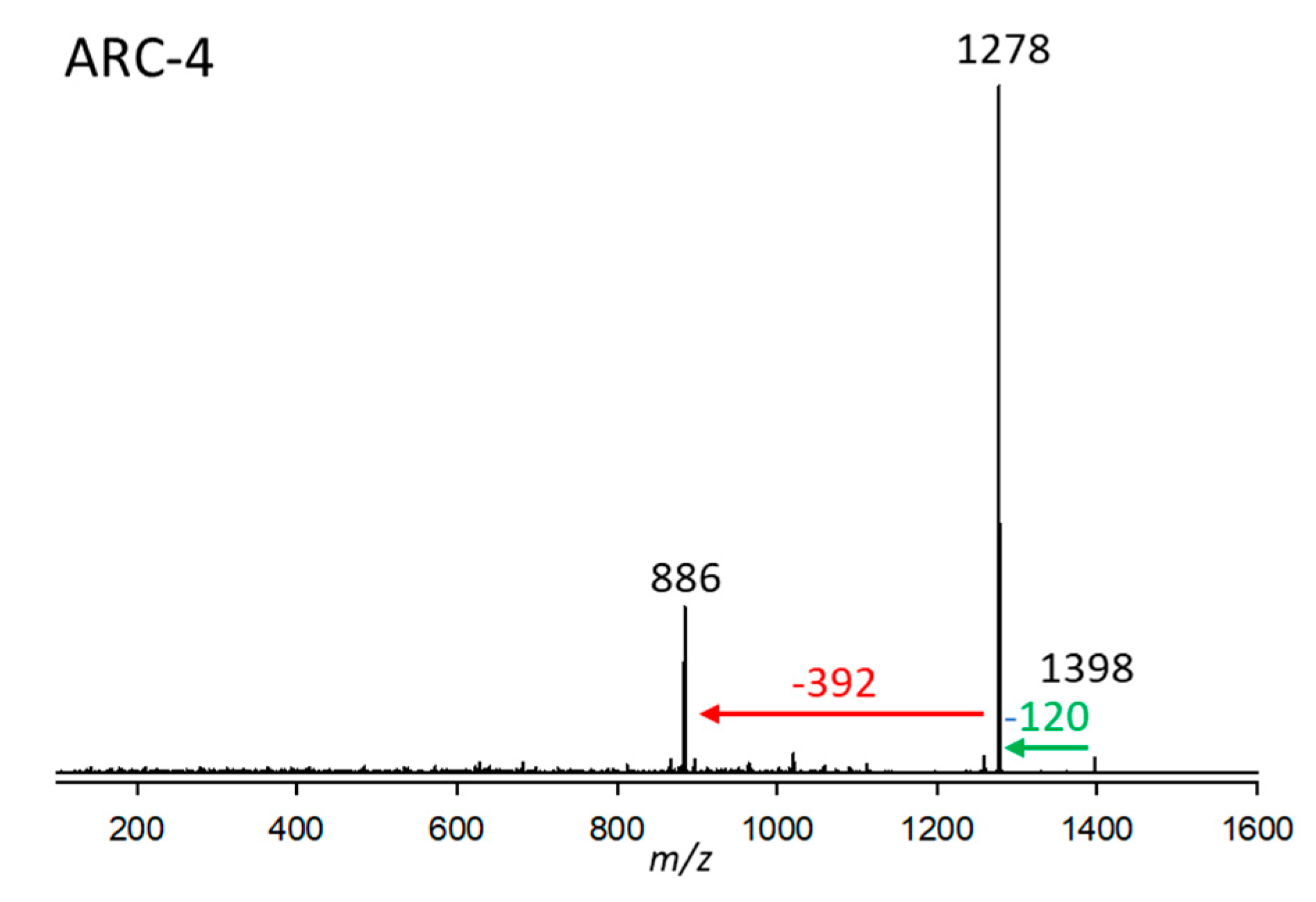
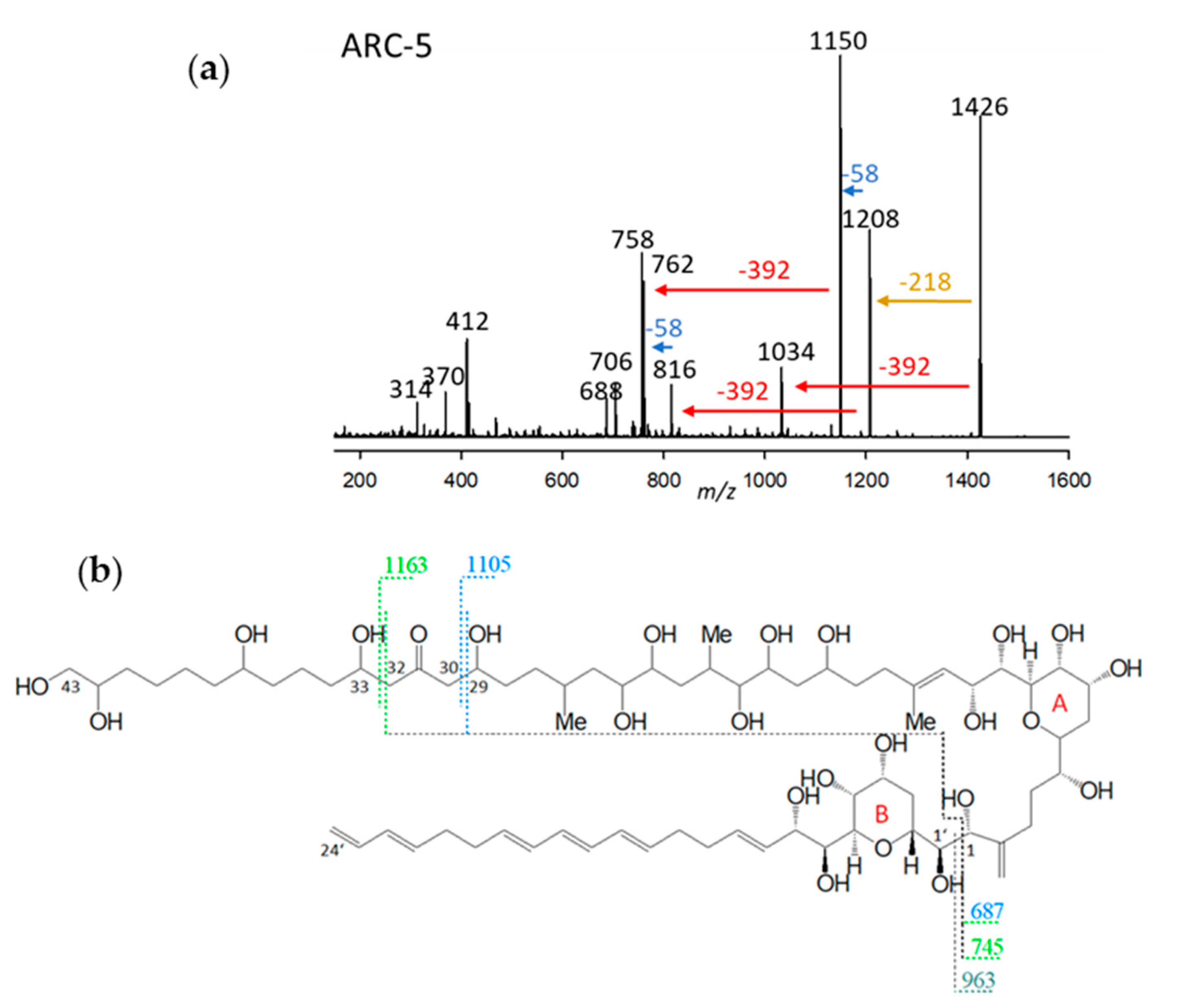
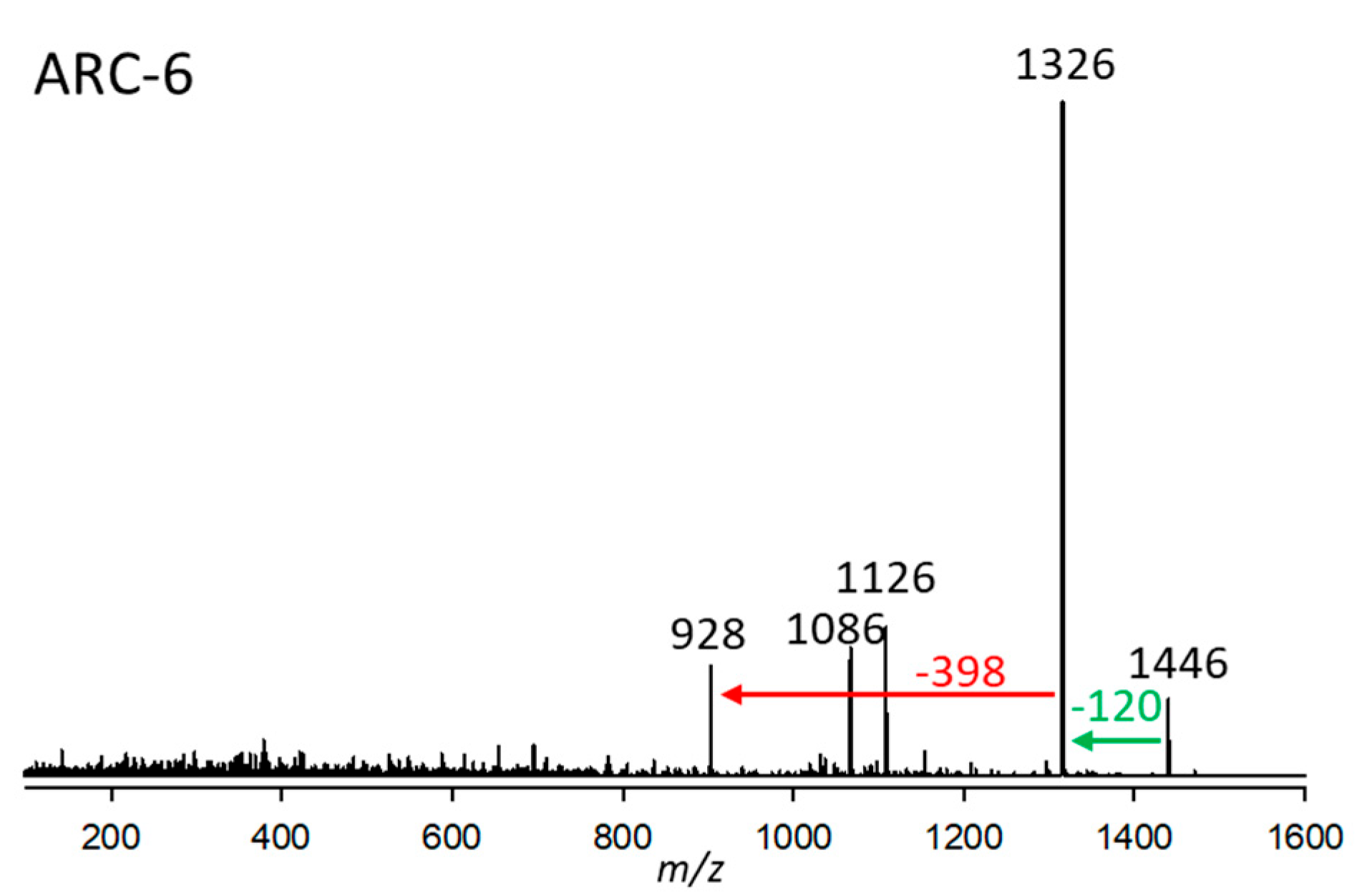
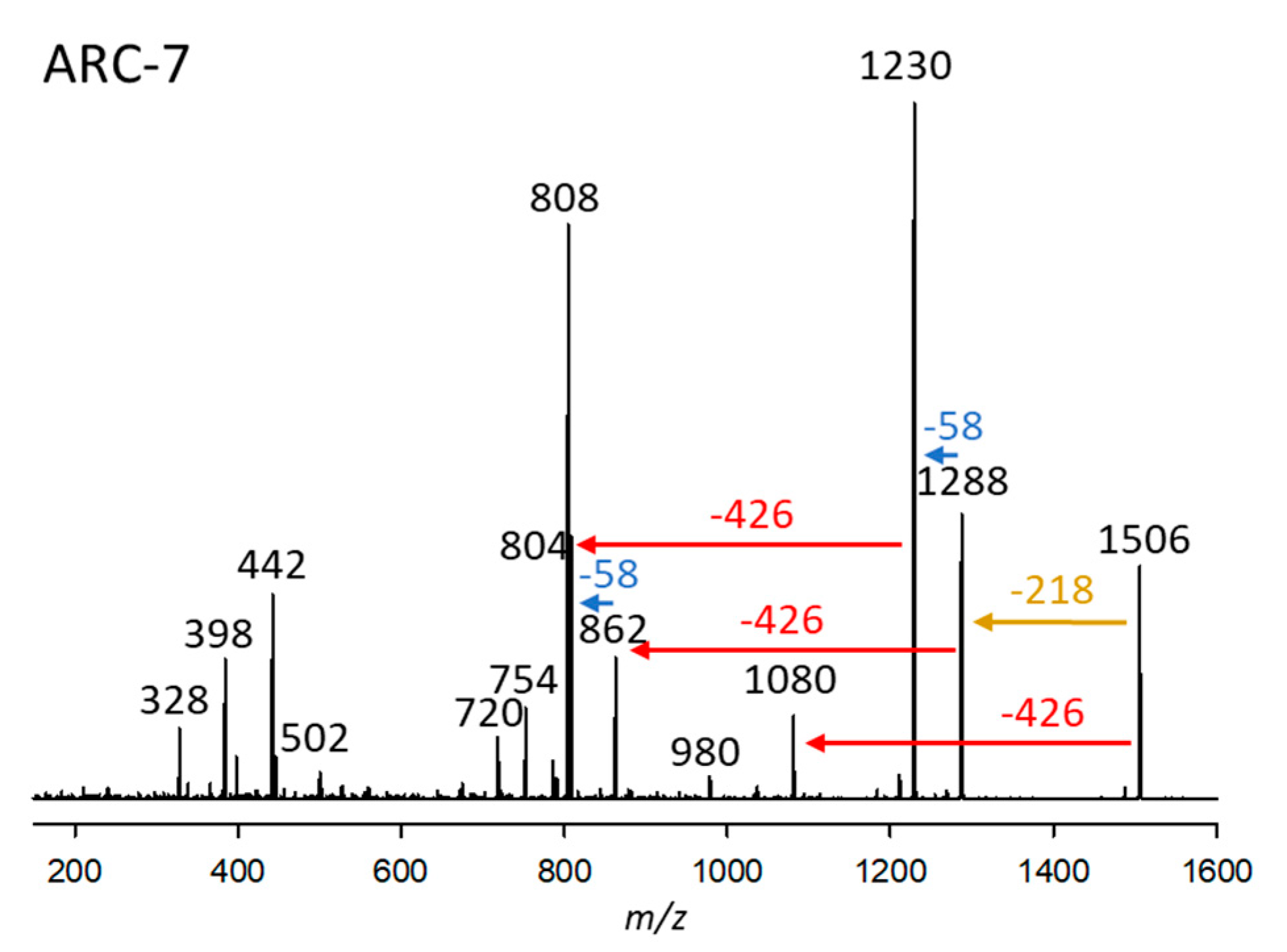
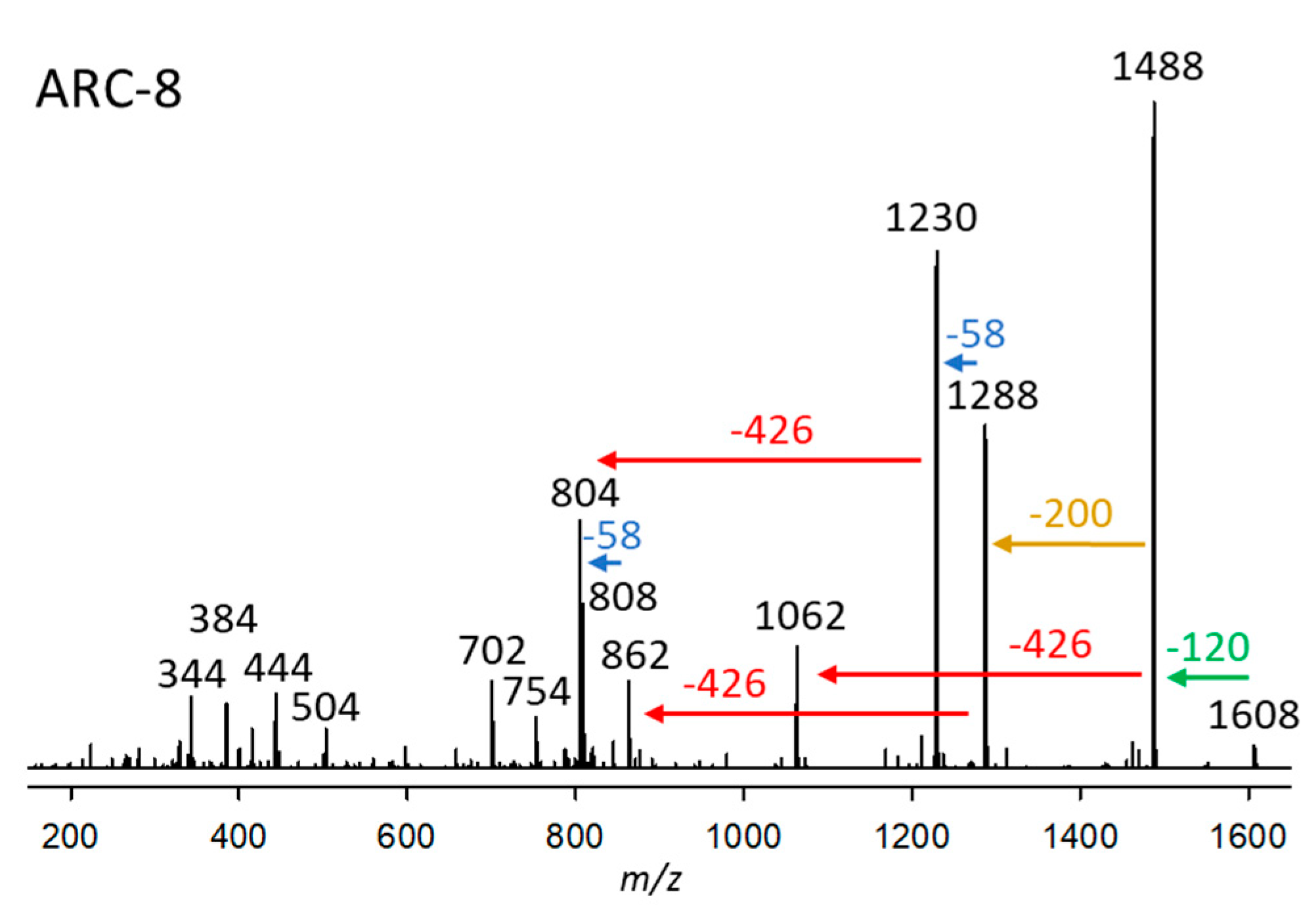
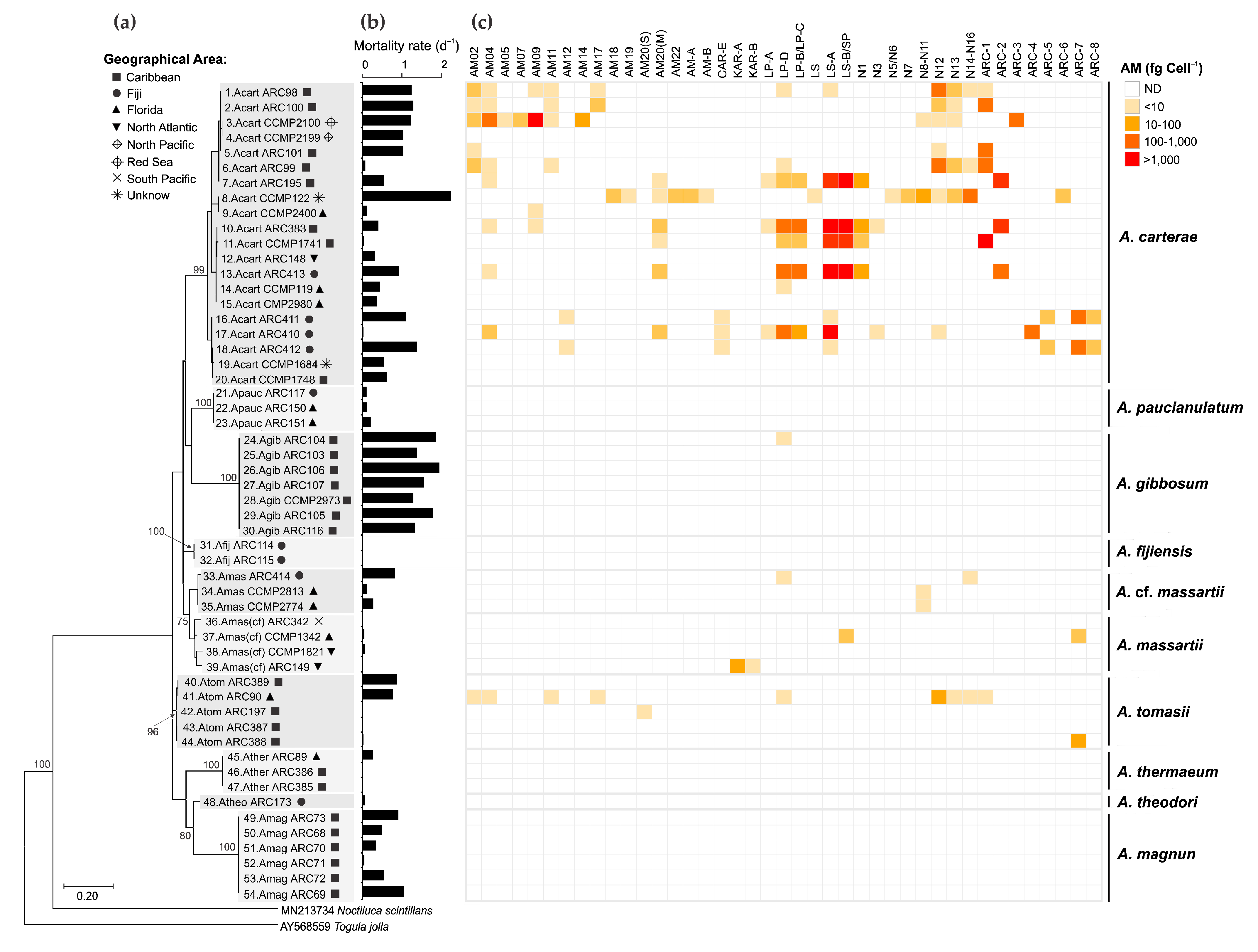
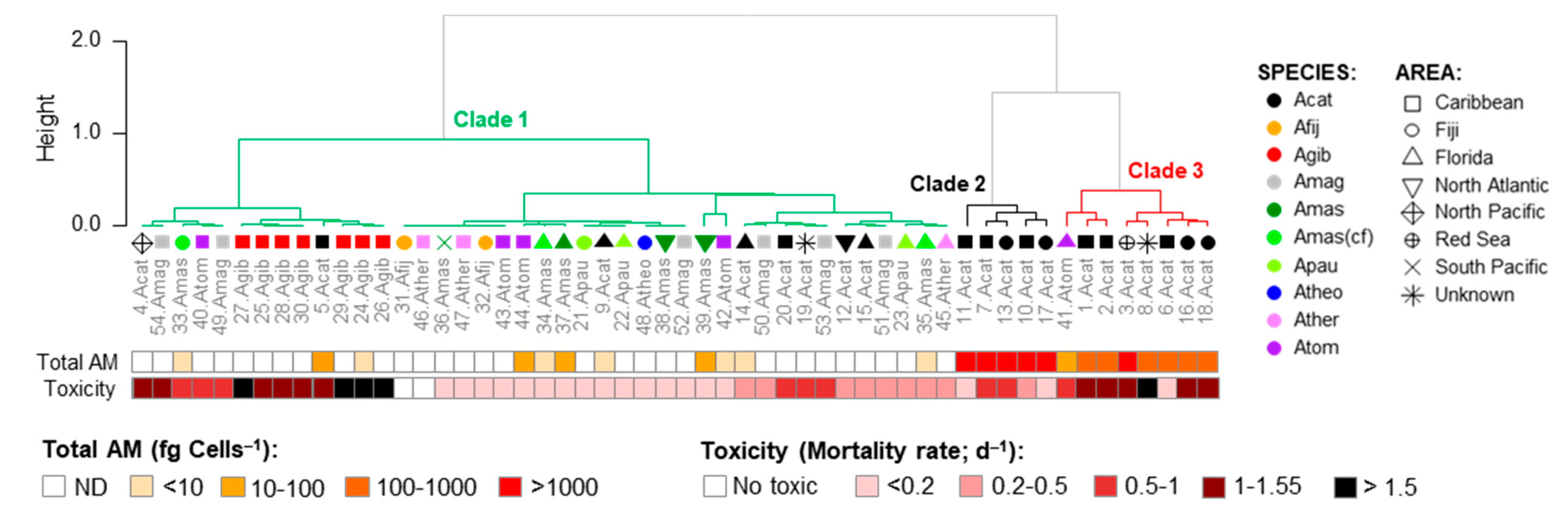
| Variant Name | Strain Code | Strain Name | tR (min) | Q1-Mass (m/z) | Detection Mode |
|---|---|---|---|---|---|
| ARC-1 | 1.Acart | ARC101 | 3.89 | 1226 | Neutral loss |
| ARC-2 | 10.Acart | ARC383 | 3.03 | 1266 | SRM |
| ARC-3 | 3.Acart | CCMP2100 | 2.90 | 1358 | Neutral loss |
| ARC-4 | 17.Acart | ARC410 | 2.93 | 1398 | SRM |
| ARC-5 | 19.Acart | ARC412 | 3.87 | 1426 | SRM |
| ARC-6 | 8.Acart | CCMP122 | 3.20 | 1446 | Neutral loss |
| ARC-7 | 16.Acart | ARC411 | 4.35 | 1506 | Full scan |
| ARC-8 | 16.Acart | ARC411 | 3.26 | 1608 | Full scan |
| Chromatographic parameters | |
|---|---|
| Eluent composition | A: 500 mL ultrapure water + 252.5 µL NH4OH (25%) B: 450 mL acetonitrile + 50 mL ultrapure water + 252.5 µL NH4OH (25%) |
| Eluent gradient | From 80% Eluent A to 10% Eluent A |
| Total duration (min) | 5 |
| Flow rate (mL min−1) | 0.2 |
| Injection volume (µL) | 0.5 |
| Collision energy (eV) | 75 85 for AMs over 1500 m/z during product ion scans |
| Scanning time (sec) | 0.133 |
| Scanning rate (points per peak) | 12 |
| Spectrometric parameters | |
| Ion source | |
| Capillary voltage (kV) Cone voltage (V) Desolvation temperature (°C) | 3.00 40 600 |
| Gas flow | |
| Desolvation gas flow (L h−1) Cone gas flow (L h−1) Nebulizer gas flow (bar) | 1000 150 7.0 |
| Further settings | |
| Autosampler temperature (°C) Column temperature (°C) Electrospray Full-scan mass range (m/z) | 10 40 ES+ 1000–1800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-de-Souza, C.; Weber, J.; Schmitt, M.; York, R.; Karafas, S.; Tomas, C.; Krock, B. High Inter- and Intraspecific Variability in Amphidinol Content and Toxicity of Amphidinium Strains. Mar. Drugs 2025, 23, 332. https://doi.org/10.3390/md23090332
Alves-de-Souza C, Weber J, Schmitt M, York R, Karafas S, Tomas C, Krock B. High Inter- and Intraspecific Variability in Amphidinol Content and Toxicity of Amphidinium Strains. Marine Drugs. 2025; 23(9):332. https://doi.org/10.3390/md23090332
Chicago/Turabian StyleAlves-de-Souza, Catharina, Jannik Weber, Mathew Schmitt, Robert York, Sarah Karafas, Carmelo Tomas, and Bernd Krock. 2025. "High Inter- and Intraspecific Variability in Amphidinol Content and Toxicity of Amphidinium Strains" Marine Drugs 23, no. 9: 332. https://doi.org/10.3390/md23090332
APA StyleAlves-de-Souza, C., Weber, J., Schmitt, M., York, R., Karafas, S., Tomas, C., & Krock, B. (2025). High Inter- and Intraspecific Variability in Amphidinol Content and Toxicity of Amphidinium Strains. Marine Drugs, 23(9), 332. https://doi.org/10.3390/md23090332





