The Efficacy of Daily Salmon Oil for Adult Type 2 Asthma: An Exploratory Randomized Double-Blind Trial
Abstract
1. Introduction
2. Results
3. Discussion
- Standardize participant selection to include individuals at the same stage of treatment initiation.
- Evaluate alternative formulations, such as liquids, to enhance tolerability and adherence.
- Monitor and address GI side effects more proactively during the trial.
- Consider patient feedback on formulation acceptability during trial design.
4. Materials and Methods
4.1. Recruitment of Participants
4.2. Intervention
4.3. Assessment
4.4. Primary Outcome: The Occurrence of Exacerbations
4.5. Secondary Outcome: Asthma Control and Safety Profile
4.6. Sample Size and Power Calculation
4.7. Statistical Analysis
4.8. Informed Consent and Registration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT | Asthma Control Test |
| ACQ | Asthma Control Questionnaire |
| ACQ5 | Asthma Control Questionnaire-5 questions |
| AEs | Adverse events |
| ATS | The American Thoracic Society |
| BMI | Body mass index |
| CEs | Composite events |
| CompEx | Composite endpoint |
| COS | Composite outcome score |
| CTU | Clinical trial unit |
| DAC | Data Access Committee |
| DHA | Docosahexaenoic acid |
| DPA | Docosapentaenoic acid |
| EPA | Eicosapentaenoic acid |
| ERS | European Respiratory Society |
| FeNO | Fractional exhaled nitric oxide |
| FEV1 | Forced expiratory volume in one second |
| GDPR | General Data Protection Regulation |
| GI | Gastrointestinal |
| GINA | Global Initiative for Asthma |
| GP | General practitioner |
| ICH-GCP | International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use-Good Clinical Practice Guidelines |
| ICS | Inhaled corticosteroid |
| IgE | Immunoglobulin E |
| IL | Interleukin |
| LABA | Long-acting beta-2 agonist |
| LAMA | Long-acting muscarinic antagonist |
| LTRA | Leukotriene receptor antagonist |
| MCT | Medium-chain triglycerides |
| n-3 PUFA | Omega-3 polyunsaturated fatty acids |
| NOMA | Norwegian Medical Products Agency |
| OCS | Oral corticosteroid |
| PEF | Peak expiratory flow |
| PUFA | Polyunsaturated fatty acids |
| PROM | Patient reported outcome measures |
| REK Central | Regional Committees for Medical and Health Research Ethics, Central Norway |
| SABAs | Short-acting beta-agonists |
| SAEs | Serious adverse events |
| SAP | Statistical analysis plan |
| SD | Standard deviations |
| SOPs | Standard operating procedures |
References
- Tian, Y.; Sun, J.; Jiao, D.; Zhang, W. The potential role of n-3 fatty acids and their lipid mediators on asthmatic airway inflammation. Front. Immunol. 2024, 15, 1488570. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.L.; Bacharier, L.B.; Bateman, E.; Boulet, L.P.; Brightling, C.; Buhl, R.; Brusselle, G.; Cruz, A.A.; Drazen, J.M.; Duijts, L.; et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. Npj Prim. Care Respir. Med. 2023, 33, 7. [Google Scholar] [CrossRef]
- Hansen, S.; Bülow, A.v.; Sandin, P.; Ernstsson, O.; Janson, C.; Lehtimäki, L.; Kankaanranta, H.; Ulrik, C.; Aarli, B.B.; Wahl, H.F.; et al. Prevalence and management of severe asthma in the Nordic countries: Findings from the NORDSTAR cohort. ERJ Open Res. 2023, 9, 00687–02022. [Google Scholar] [CrossRef]
- GINA. Global Strategy for Asthma Management and Prevention. Global Iniative for Asthma. Available online: https://ginasthma.org/2024-report/ (accessed on 10 October 2024).
- Lehmann, S.; Stylianou, E.; Tøndell, A.; Tollåli, T.; Gallefoss, F.; Jørstad, S.Ø. Praktisk Veileder for Alvorlig Astma hos Voksne. Available online: https://www.legeforeningen.no/fag/Forskning-kvalitetsarbeid-og-innovasjon/ferdige-prosjekter/praktisk-veileder-for-alvorlig-astma-hos-voksne/ (accessed on 5 June 2021).
- Reihman, A.E.; Holguin, F.; Sharma, S. Management of Severe Asthma Beyond the Guidelines. Curr. Allergy Asthma Rep. 2020, 20, 47. [Google Scholar] [CrossRef]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive summary and rationale for key changes. Eur. Respir. J. 2022, 59, 2102730. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Kytikova, O.; Novgorodtseva, T.; Denisenko, Y.; Antonyuk, M.; Gvozdenko, T. Pro-Resolving Lipid Mediators in the Pathophysiology of Asthma. Medicina 2019, 55, 284. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Wu, Y.; Yu, G.; Wang, H. Role of specialized pro-resolving lipid mediators in pulmonary inflammation diseases: Mechanisms and development. Respir. Res. 2021, 22, 204. [Google Scholar] [CrossRef]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Oliver, P.J.; Arutla, S.; Yenigalla, A.; Hund, T.J.; Parinandi, N.L. Lipid Nutrition in Asthma. Cell Biochem. Biophys. 2021, 79, 669–694. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.E.; Mougey, E.B.; Hossain, M.J.; Livingston, F.; Balagopal, P.B.; Langdon, S.; Lima, J.J. Fish Oil Supplementation in Overweight/Obese Patients with Uncontrolled Asthma. A Randomized Trial. Ann. Am. Thorac. Soc. 2019, 16, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Framroze, B.; Heggdal, H. An in vitro study to explore the modulation of eosinophil effector function in human allergic peripheral blood eosinophils using enzymatically extracted salmonid oil. Funct. Foods Health Dis. 2020, 10, 357–367. [Google Scholar] [CrossRef]
- Currie, C.; Framroze, B.; Singh, D.; Sharma, D.; Bjerknes, C.; Hermansen, E. Pharmacological evaluation of the effects of enzymatically liberated fish oil on eosinophilic inflammation in animal models. Biotechnol. Appl. Biochem. 2023, 70, 157–163. [Google Scholar] [CrossRef]
- Aaron, S.D.; Boulet, L.P.; Reddel, H.K.; Gershon, A.S. Underdiagnosis and Overdiagnosis of Asthma. Am. J. Respir. Crit. Care Med. 2018, 198, 1012–1020. [Google Scholar] [CrossRef]
- Fuhlbrigge, A.L.; Bengtsson, T.; Peterson, S.; Jauhiainen, A.; Eriksson, G.; Da Silva, C.A.; Johnson, A.; Sethi, T.; Locantore, N.; Tal-Singer, R.; et al. A novel endpoint for exacerbations in asthma to accelerate clinical development: A post-hoc analysis of randomised controlled trials. Lancet Respir. Med. 2017, 5, 577–590. [Google Scholar] [CrossRef]
- Barua, U.K.; Karmaker, P.; Saha, A.K.; Mostofa, M.M.; Ghosh, D.K.; Biswas, K.K. The effects of omega-3 fatty acids supplementation in bronchial asthma. J. Biosci. Med. 2023, 11, 208–219. [Google Scholar] [CrossRef]
- Farjadian, S.; Moghtaderi, M.; Kalani, M.; Gholami, T.; Hosseini Teshnizi, S. Effects of omega-3 fatty acids on serum levels of T-helper cytokines in children with asthma. Cytokine 2016, 85, 61–66. [Google Scholar] [CrossRef]
- Lorensia, A.; Suryadinata, R.V.; Ratnasari, R. Pilot Study of Lung Function Improvement in Peak Expiratory Flow (PEF) Value Using Fish Oil Containing Omega-3 Therapy in Asthma. Glob. Med. Health Commun. 2020, 8, 211–218. [Google Scholar] [CrossRef]
- Murugesan, N.; Saxena, D.; Dileep, A.; Adrish, M.; Hanania, N.A. Update on the Role of FeNO in Asthma Management. Diagnostics 2023, 13, 1428. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.; ten Brinke, A.; Sizoo, D.; Pepels, J.J.S.; ten Have, L.; van der Wiel, E.; van Zutphen, T.; Kerstjens, H.A.M.; de Jong, K. Effect of dietary interventions on markers of type 2 inflammation in asthma: A systematic review. Respir. Med. 2024, 221, 107504. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.W.; Ghushchyan, V.H.; Marvel, J.; Barrett, Y.C.; Fuhlbrigge, A.L. Association Between Pulmonary Function and Asthma Symptoms. J. Allergy Clin. Immunol. Pract. 2019, 7, 2319–2325. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.; Pistelli, R.; Carpagnano, G.E.; Celi, A.; Puxeddu, I.; Scichilone, N.; Spanevello, A.; Canonica, G.W.; Paggiaro, P. Symptom versus exacerbation control: An evolution in GINA guidelines? Ther. Adv. Respir. Dis. 2023, 17, 17534666231159261. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Reddel, H.K.; Taylor, D.R.; Bateman, E.D.; Boulet, L.P.; Boushey, H.A.; Busse, W.W.; Casale, T.B.; Chanez, P.; Enright, P.L.; Gibson, P.G.; et al. An official American Thoracic Society/European Respiratory Society statement: Asthma control and exacerbations: Standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 2009, 180, 59–99. [Google Scholar] [CrossRef]
- Bolton, C.; Harrison, T.; Lugogo, N.; Fuhlbrigge, A.; Hirsch, I.; Bengtsson, T.; Peterson, S.; Sidaway, M.; Garcia Gil, E.; Fagerås, M.; et al. Use of CompEx in eosinophilic patients with severe, uncontrolled asthma on benralizumab. ERJ Open Res. 2024, 10, 01025–02023. [Google Scholar] [CrossRef]
- McCrae, C.; Olsson, M.; Gustafson, P.; Malmgren, A.; Aurell, M.; Fagerås, M.; Da Silva, C.A.; Cavallin, A.; Paraskos, J.; Karlsson, K.; et al. INEXAS: A Phase 2 Randomized Trial of On-demand Inhaled Interferon Beta-1a in Severe Asthmatics. Clin. Exp. Allergy 2021, 51, 273–283. [Google Scholar] [CrossRef]
- Barnes, P.J.; Casale, T.B.; Dahl, R.; Pavord, I.D.; Wechsler, M.E. The Asthma Control Questionnaire as a clinical trial endpoint: Past experience and recommendations for future use. Allergy 2014, 69, 1119–1140. [Google Scholar] [CrossRef]
- Juniper, E.F.; Bousquet, J.; Abetz, L.; Bateman, E.D. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir. Med. 2006, 100, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Ng, H.K.; Tang, M.L.; Schucany, W.R. Testing the ratio of two poisson rates. Biom. J. 2008, 50, 283–298. [Google Scholar] [CrossRef] [PubMed]

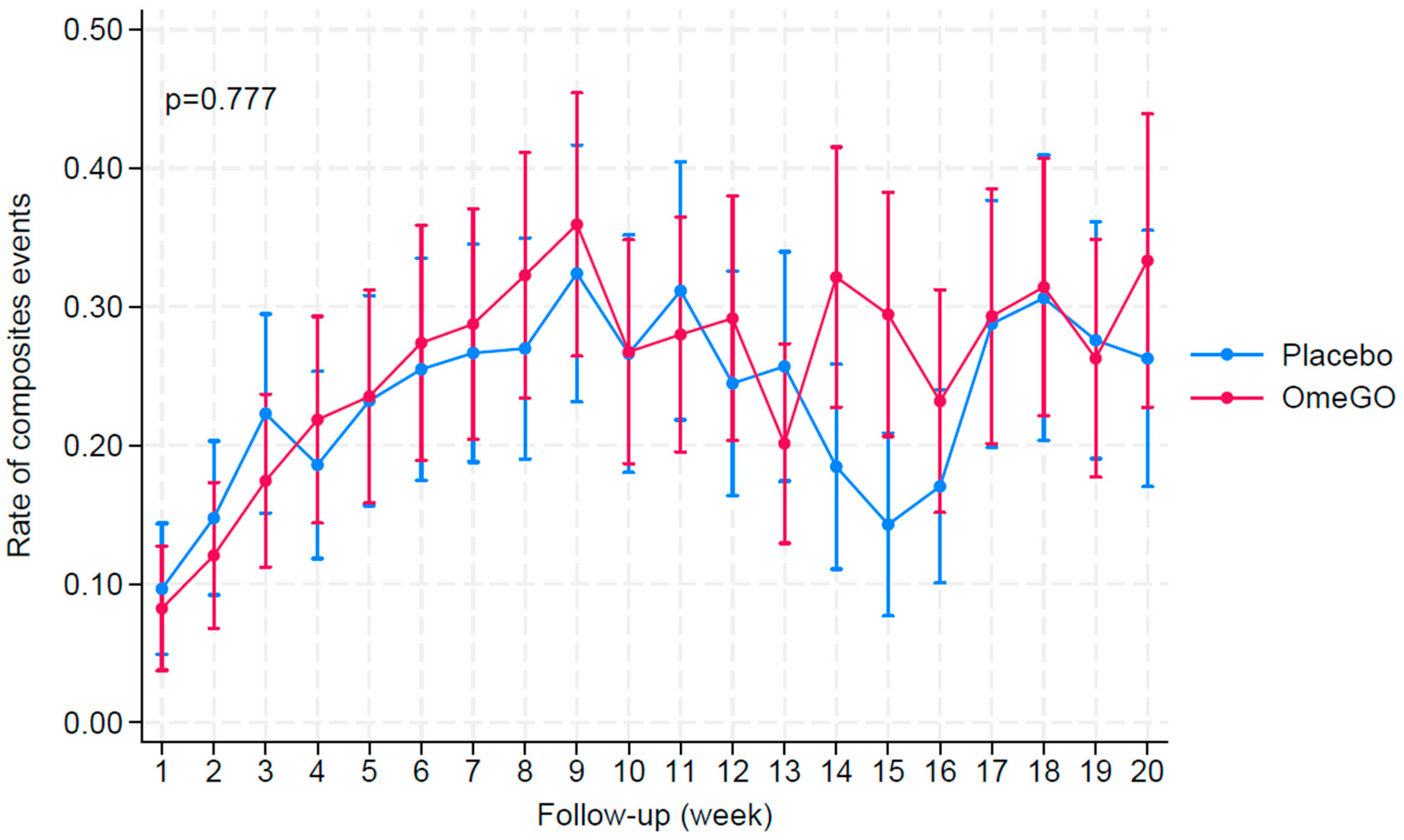
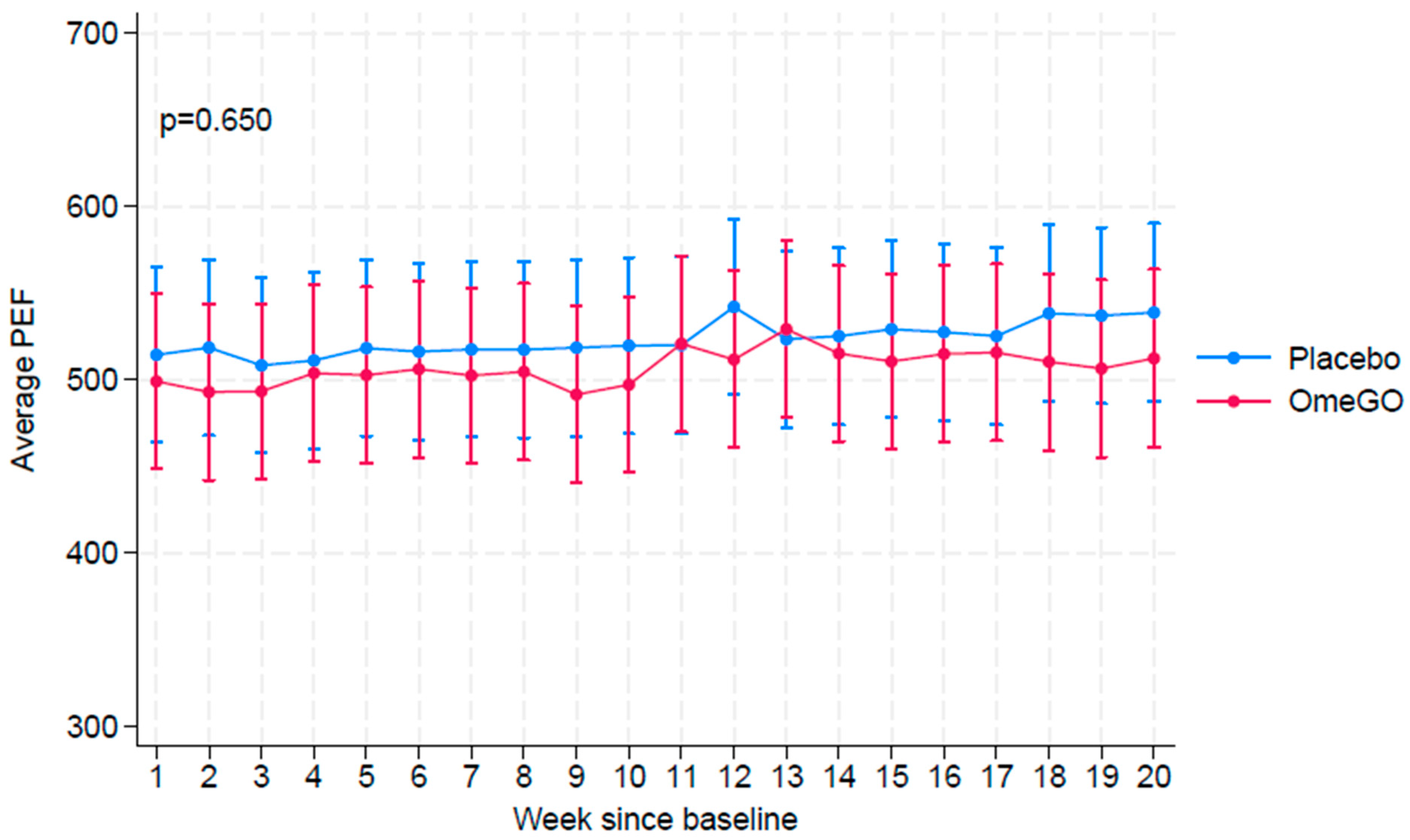
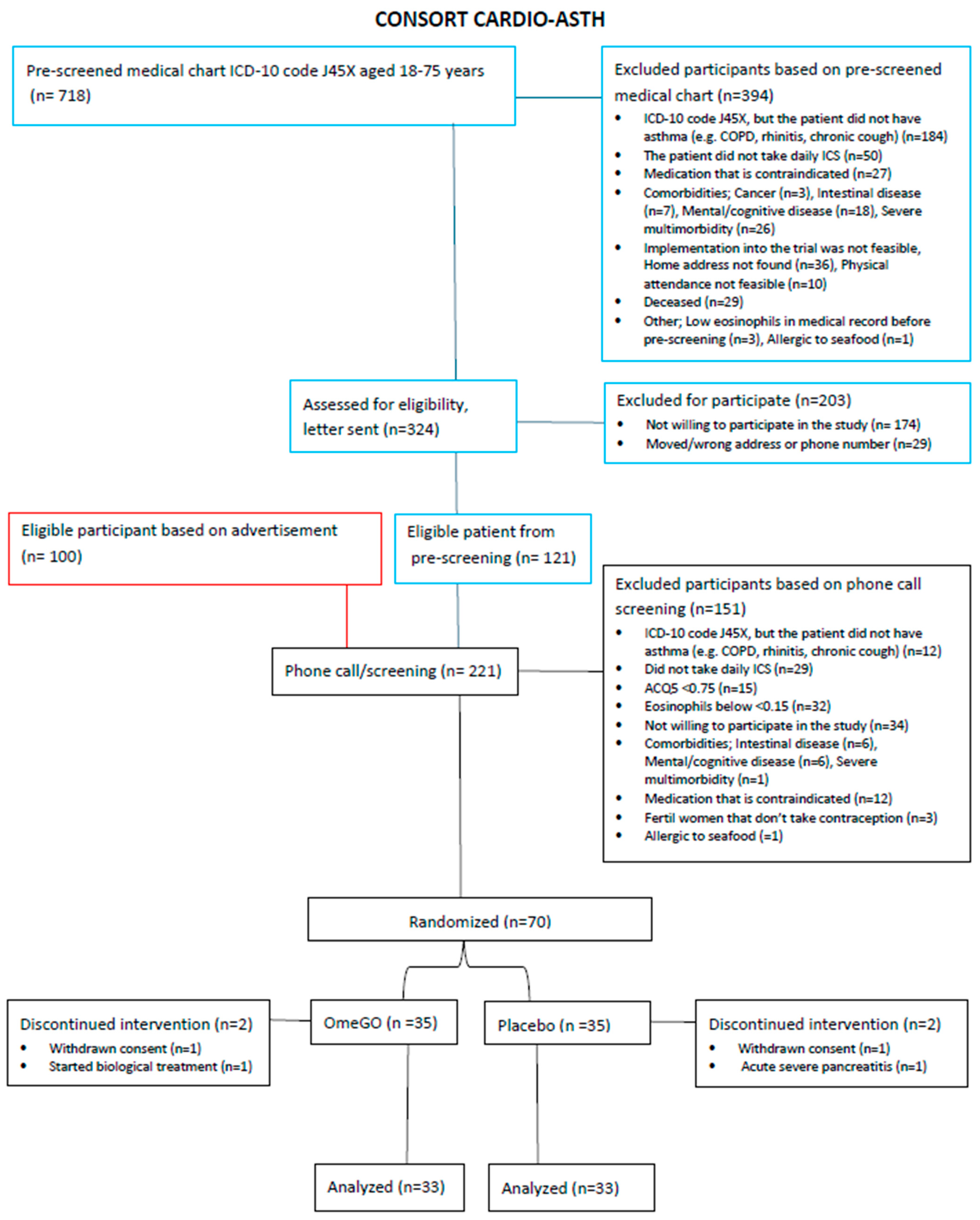
| OmeGO (N = 35) | Placebo (N = 35) | |
|---|---|---|
| Screening | ||
| Eosinophils in blood (109/L), mean (SD) | 0.44 (0.44) | 0.36 (0.30) |
| ACQ5 1, mean (SD) | 1.9 (0.8) | 1.9 (0.9) |
| Baseline | ||
| Demographic: | ||
| Age, mean (SD) | 58 (11) | 55 (11) |
| Female, n (%) | 21 (60) | 19 (54) |
| Body mass index (kg/m2), mean (SD) | 29 (6) | 29 (5) |
| Inclusion month, n (%) | ||
| February–August | 20 (57) | 22 (63) |
| September–January | 15 (43) | 13 (37) |
| Clinical characteristics: | ||
| Systolic blood pressure (mm Hg), mean (SD) | 135 (18) | 131 (12) |
| Diastolic blood pressure (mm Hg), mean (SD) | 84 (9) | 82 (7) |
| Heart rate (beats/minute), mean (SD) | 68 (9) | 73 (13) |
| Cardiovascular disease 2, n (%) | 10 (29) | 8 (23) |
| Eosinophils in blood (109/L), mean (SD) | 0.36 (0.27) | * 0.32 (0.27) |
| Pulmonary assessment: | ||
| FEV1 in % of predicted value 3, mean (SD) | 87 (17) | 82 (15) |
| FEV1 (L/s) Below LLN 3, n (%) | 9 (26) | 13 (37) |
| FEV1 (L/s) Above LLN 3, n (%) | 26 (74) | 22 (63) |
| FeNO (ppb) 4, mean (SD) | 57 (42) | 68 (65) |
| Asthma therapy 5: | ||
| ICS, n (%) | 2 (6) | 4 (11) |
| ICS + LABA, n (%) | 27 (77) | 28 (80) |
| ICS + LAMA, n (%) | 1 (3) | 0 (0) |
| ICS + LABA + LAMA, n (%) | 5 (14) | 3 (9) |
| LTRA, n (%) | ||
| Yes | 9 (26) | 6 (17) |
| No | 26 (74) | 29 (83) |
| Events | OmeGO (N = 33) ** | Placebo (N = 33) ** | p-Value * |
|---|---|---|---|
| Adverse Event (AE) | |||
| Post-traumatic stress disorder (PTSD) | 1 (a) | 0.314 | |
| Increased waist measurement/gained weight | 1 (b) | 0.314 | |
| Change in pre-existing medical conditions, increased asthma symptoms | 1 (b) | 0.314 | |
| Nausea | 1 (c) | 0.314 | |
| Dyspepsia (obstipation and bloating) | 1 (b) | 0.314 | |
| Stomach pain | 2 (c) | 0.151 | |
| Diarrhea | 2 (a) | 1 (a) | 0.555 |
| Serious Adverse Event (SAE) *** | |||
| Urine tract infection | 1 (a) | 0.314 | |
| Asthma exacerbation | 1 (b) | 0.314 | |
| Complication post cardio atrial fibrillation | 1 (a) | 0.314 | |
| Idiopathic pancreatitis | 1 (b) | 0.314 |
| OmeGO (N = 33) | Placebo (N = 33) | |||||
|---|---|---|---|---|---|---|
| Number of days with two consecutive days of potential COS calculation | Days * (2965) | Days * (2920) | ||||
| Number of events (%) | N *** | Number of events (%) | N *** | RR (95% CI) | p-Value ** | |
| Total days with COS **** | 746 (25) | 24 | 679 (23) | 27 | 1.08 (0.58–2.03) | 0.806 |
| Sub-scores | ||||||
| Days with daytime symptoms and use of SABA | 488 (17) | 486 (17) | 0.99 (0.46–2.12) | 0.977 | ||
| Days with coughing | 272 (9) | 310 (11) | 0.86 (0.34–2.18) | 0.757 | ||
| Days with wheezing | 688 (23) | 496 (17) | 1.37 (0.61–3.07) | 0.451 | ||
| Days with chest tightness | 334 (11) | 359 (12) | 0.92 (0.33–2.57) | 0.868 | ||
| Days with breathlessness | 363 (12) | 457 (16) | 0.78 (0.29–2.09) | 0.624 | ||
| Days with use of SABA | 219 (7) | 326 (11) | 0.66 (0.32–1.39) | 0.274 | ||
| Nighttime awakening with use of rescue medication | 49 (1.7) | 5 (0.2) | 9.65 (2.11–44.17) | 0.003 | ||
| Increased dose rate of ≥4 puffs/day of rescue medication | 11 (0.4) | 12 (0.4) | 0.90 (0.11–7.53) | 0.925 | ||
| Number of events with ≥20% reduction in peak expiratory flow (PEF) | 284 (10) | 281 (10) | 1.00 (0.28–3.57) | 0.994 |
| OmeGO (N = 33) | Placebo (N = 33) | ||||||
|---|---|---|---|---|---|---|---|
| Well Controlled | Partly Controlled | Un Controlled | Well Controlled | Partly Controlled | Un Controlled | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | p-Value * | |
| Month 1 | 3 (9) | 12 (36) | 18 (55) | 0 (0) | 15 (45) | 18 (55) | 0.189 |
| Month 2 | 3 (9) | 11 (33) | 19 (58) | 1 (3) | 17 (52) | 15 (45) | 0.252 |
| Month 3 | 3 (9) | 12 (36) | 18 (55) | 5 (15) | 14 (42) | 14 (43) | 0.562 |
| Month 4 | 3 (9) | 17 (52) | 13 (39) | 4 (12) | 15 (46) | 14 (42) | 0.859 |
| Month 5 | 2 (6) | 16 (49) | 15 (45) | 4 (12) | 19 (58) | 10 (30) | 0.382 |
| OmeGO (N = 33) (95% CI) | Placebo (N = 33) (95% CI) | p-Value * | |
|---|---|---|---|
| ACQ5-score 1 mean | |||
| Baseline | 1.6 (1.3–1.9) | 1.4 (1.2–1.7) | |
| 20 weeks | 1.5 (1.2–1.9) | 1.1 (0.8–1.3) | 0.211 |
| FEV1% of predicted value 2, mean | |||
| Baseline | 87 (81–93) | 82 (76–87) | |
| 20 weeks | 87 (81–93) | 82 (76–88) | 0.661 |
| FeNO ppb 3, mean | |||
| Baseline | 57 (42–72) | 69 (46–93) | |
| 20 weeks | 61 (49–74) | 66 (45–87 | 0.329 |
| Concomitant medication 4, n (%) | |||
| Increased | 5 (15.2%) | 4 (12.1%) | |
| Reduced | 3 (9.1%) | 5 (15.2%) | 0.729 |
| Oral corticosteroids (OCS) 5, n (%) | 4 (12%) | 2 (6%) | 0.392 |
| Compliance of intervention capsules taken 6, mean | 96 (94–98) | 92 (88–96) | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mølsæter, K.; Roth, K.; Myklebust, T.Å.; Hermansen, E.; Singh, D.; Currie, C.; Hoff, D.A.L. The Efficacy of Daily Salmon Oil for Adult Type 2 Asthma: An Exploratory Randomized Double-Blind Trial. Mar. Drugs 2025, 23, 328. https://doi.org/10.3390/md23080328
Mølsæter K, Roth K, Myklebust TÅ, Hermansen E, Singh D, Currie C, Hoff DAL. The Efficacy of Daily Salmon Oil for Adult Type 2 Asthma: An Exploratory Randomized Double-Blind Trial. Marine Drugs. 2025; 23(8):328. https://doi.org/10.3390/md23080328
Chicago/Turabian StyleMølsæter, Katarina, Kjetil Roth, Tor Åge Myklebust, Erland Hermansen, Dave Singh, Crawford Currie, and Dag Arne Lihaug Hoff. 2025. "The Efficacy of Daily Salmon Oil for Adult Type 2 Asthma: An Exploratory Randomized Double-Blind Trial" Marine Drugs 23, no. 8: 328. https://doi.org/10.3390/md23080328
APA StyleMølsæter, K., Roth, K., Myklebust, T. Å., Hermansen, E., Singh, D., Currie, C., & Hoff, D. A. L. (2025). The Efficacy of Daily Salmon Oil for Adult Type 2 Asthma: An Exploratory Randomized Double-Blind Trial. Marine Drugs, 23(8), 328. https://doi.org/10.3390/md23080328







