Mannan-Containing Polymers from Hadal Bacterium Psychrobacter pulmonis: Preparation, Structural Analysis, Immunological Activity and Antitumor Effects
Abstract
1. Introduction
2. Result
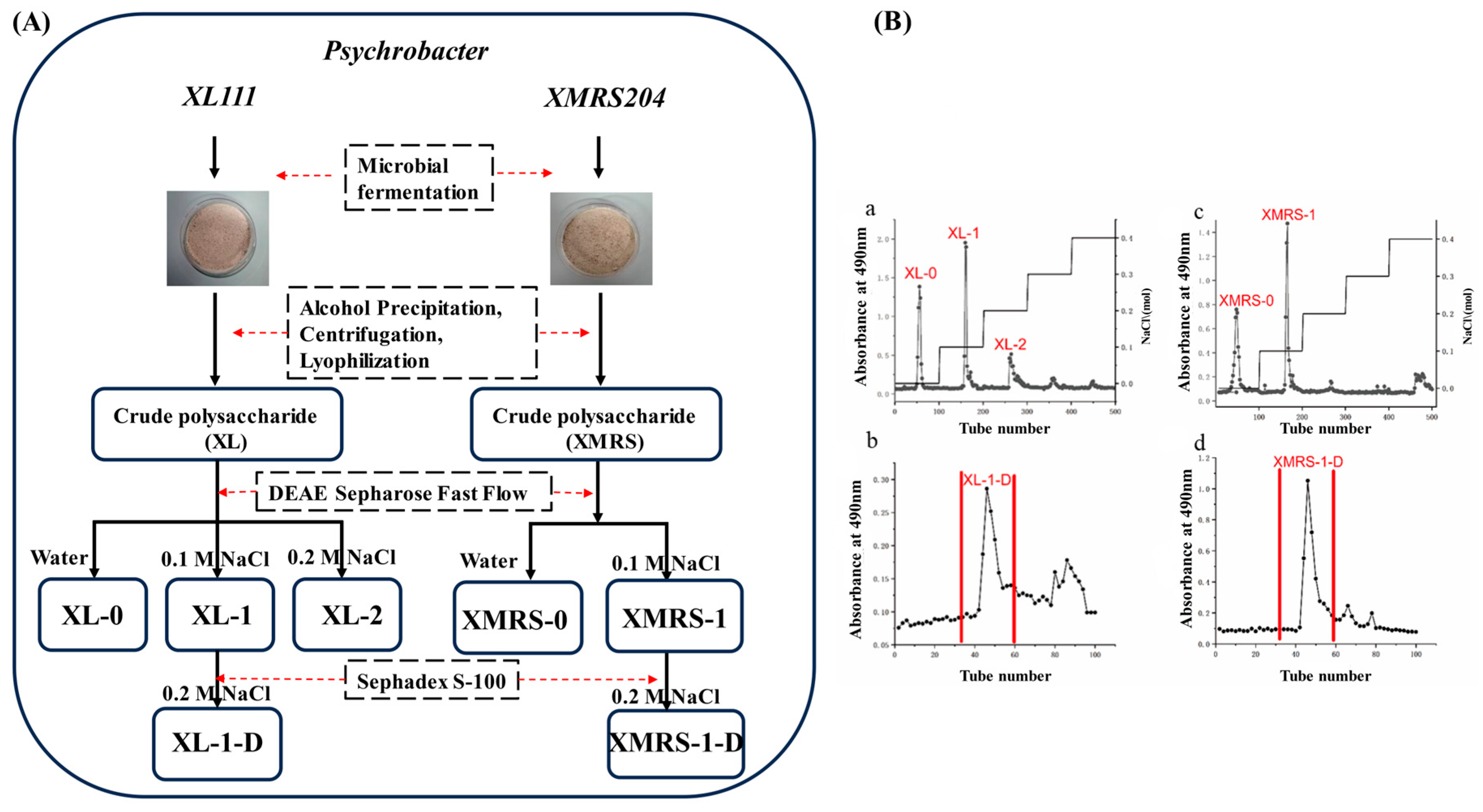
2.1. Extraction, Separation and Purification of XL-1, XL-1-D, XMRS-1, and XMRS-1-D
2.2. Structural Characteristics of XL-1, XL-1-D, XMRS-1, and XMRS-1-D
2.2.1. Infrared Spectroscopic Characterization of XL-1, XL-1-D, XMRS-1, and XMRS-1-D
2.2.2. Morphological Characterization of XL-1, XL-1-D, XMRS-1, and XMRS-1-D
2.2.3. Amino Acid Composition of XL-1-D and XMRS-1-D
2.2.4. NMR Analysis of XL-1-D and XMRS-1-D
2.3. Immunomodulatory Effects of XL-1, XL-1-D, XMRS-1, and XMRS-1-D
2.3.1. Effect of XL-1, XL-1-D, XMRS-1, and XMRS-1-D on the Viability and Proliferation of RAW264.7 Cells
2.3.2. Effect of XL-1, XL-1-D, XMRS-1, and XMRS-1-D on Macrophage Phagocytic Function
2.3.3. Effect of These Fractions on Nitric Oxide (NO) Production by Macrophages
2.3.4. Effect of XL-1, XL-1-D, XMRS-1, and XMRS-1-D on Reactive Oxygen Species (ROS) Production by Macrophages
2.3.5. XL-1, XL-1-D, XMRS-1, and XMRS-1-D Promoted the Expression of IL-6 and TNF-α
2.3.6. Effect of XL-1, XL-1-D, XMRS-1, and XMRS-1-D on LPS-Induced mRNA Expression of iNOS, TNF-α, and IL-6 in RAW264.7 Cells
2.4. Anti-Tumor Effects In Vitro
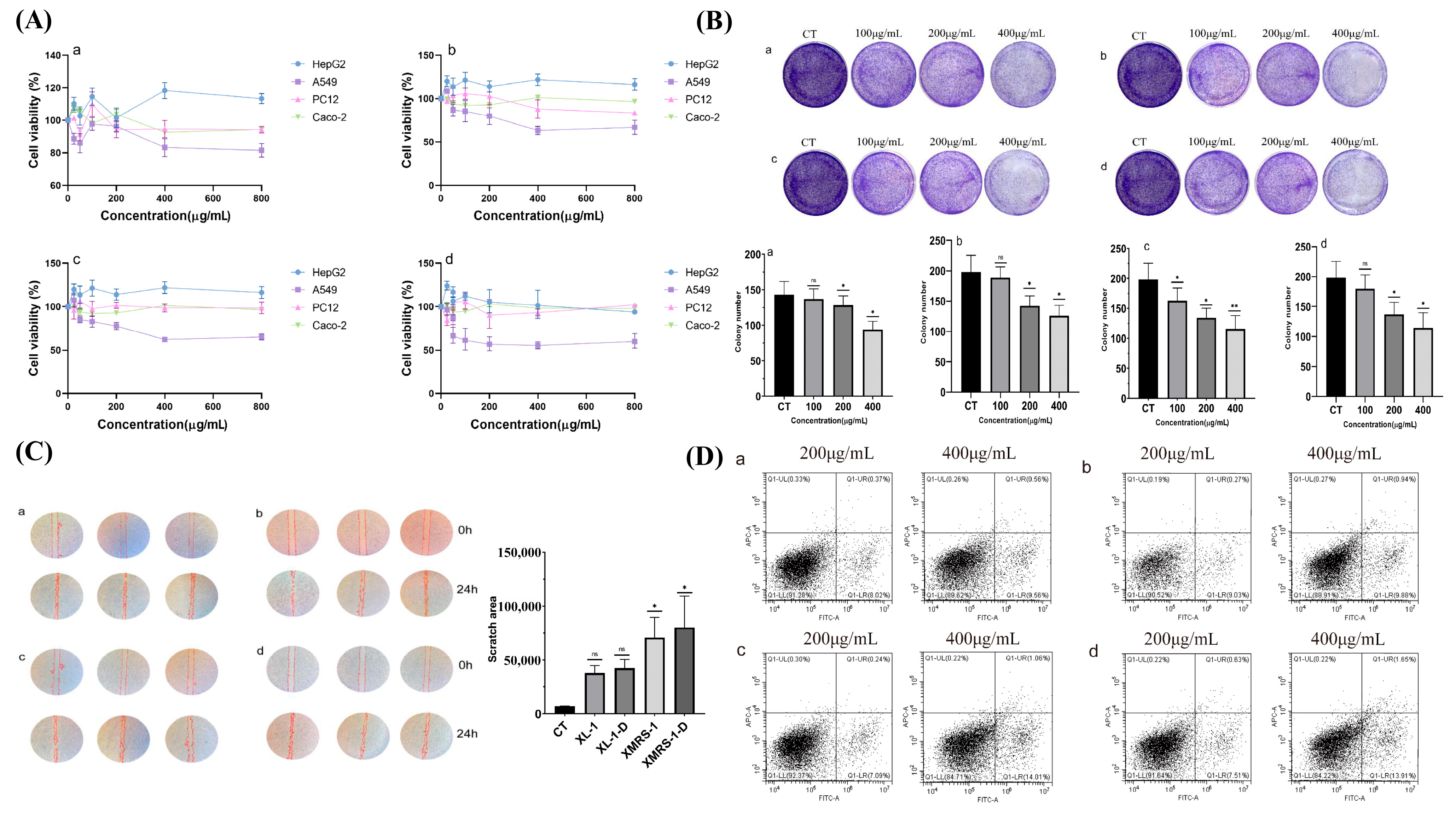
2.4.1. XL-1, XL-1-D, XMRS-1, and XMRS-1-D Inhibited A549 Cells Proliferation
2.4.2. XL-1, XL-1-D, XMRS-1, and XMRS-1-D Inhibited the Colony Formation Ability of A549 Cells
2.4.3. XL-1, XL-1-D, XMRS-1, and XMRS-1-D Inhibited the Migration Ability of A549 Cells
2.4.4. XL-1, XL-1-D, XMRS-1, and XMRS-1-D Induced Apoptosis in A549 Cells
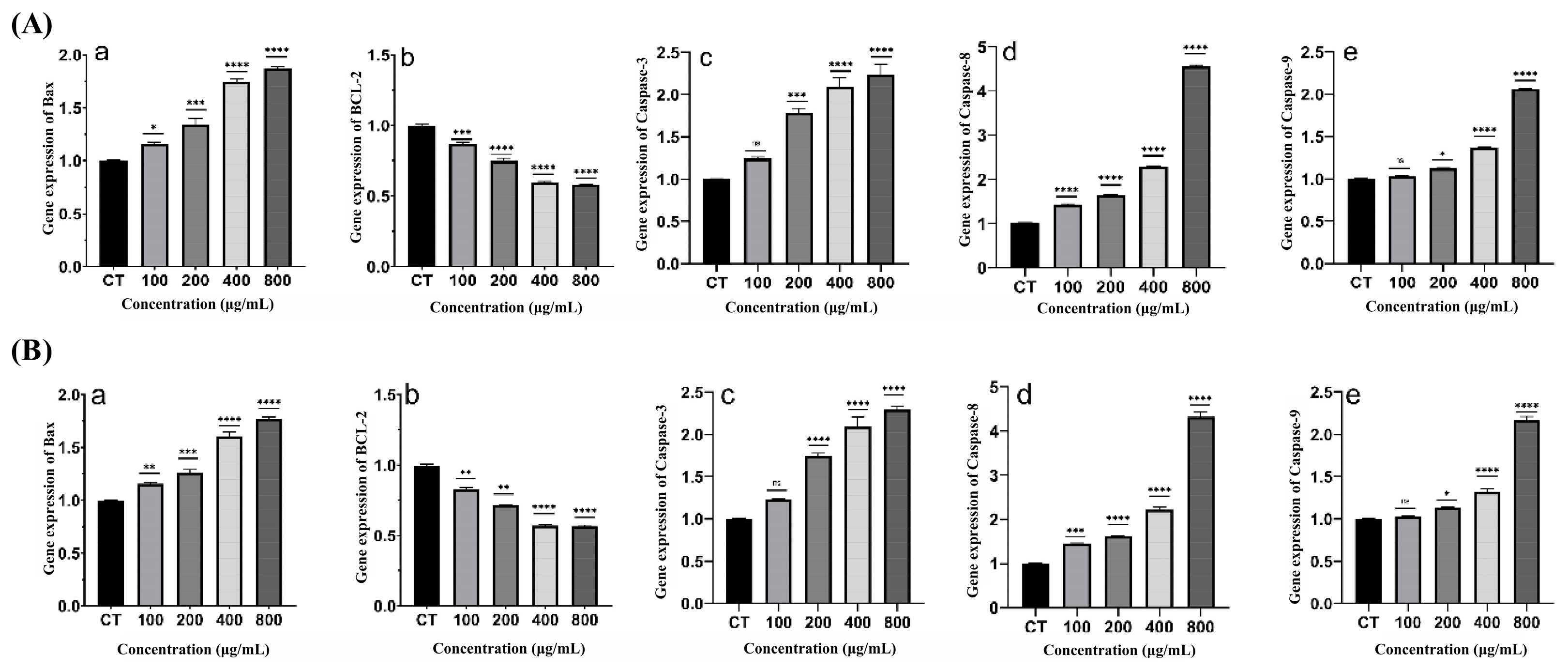
2.4.5. Effect of XMRS-1 and XMRS-1-D on Apoptosis-Related Gene Expression in A549 Cells
3. Discussions
4. Materials and Methods
4.1. Strains and Experimental Consumables
4.2. Strain Isolation and Purification
4.2.1. Colony Characteristics
4.2.2. Large-Scale Cultivation and Crude Fractions Extraction
4.2.3. Preparation of Extracellular Fractions from Marine Microorganisms
4.3. Analysis of the Physicochemical Properties of the Four Fractions
4.3.1. Determination of Molecular Weight
4.3.2. Determination of Monosaccharide Composition
4.3.3. Fourier Transform Infrared Spectroscopy
4.3.4. Morphological Characterization of Fractions
4.3.5. Amino Acid Composition of Purified Fractions
4.3.6. NMR Analysis
4.4. Immunomodulatory Effects of Fractions
4.4.1. Effect of XL-1, XL-1-D, XMRS-1, and XMRS-1-D on the Viability and Proliferation of RAW264.7 Cells
4.4.2. Effects on Macrophage Phagocytic Function
4.4.3. Effect on Nitric Oxide (NO) Production by Macrophages
4.4.4. Effect on Reactive Oxygen Species (ROS) Production by Macrophages
4.4.5. Effect on IL-6 and TNF-α Expression by Macrophages
4.4.6. LPS Induces mRNA Expression of iNOS, TNF-α, and IL-6 in RAW264.7 Cells
4.5. Anti-Tumor Effects
4.5.1. Assessment of Cytotoxicity Against Various Tumor Cell Lines
4.5.2. Effect on the Colony Formation Ability of A549 Cells
4.5.3. Effect on the Migration Ability of A549 Cells
4.5.4. Effect on Apoptosis in A549 Cells
4.5.5. Effect on Apoptosis-Related Gene Expression in A549 Cells
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gan, L.; Huang, X.; He, Z.; He, T. Exopolysaccharide Production by Salt-Tolerant Bacteria: Recent Advances, Current Challenges, and Future Prospects. Int. J. Biol. Macromol. 2024, 264, 130731. [Google Scholar] [CrossRef]
- Pandey, S.; Kannaujiya, V.K. Bacterial Extracellular Biopolymers: Eco-Diversification, Biosynthesis, Technological Development and Commercial Applications. Int. J. Biol. Macromol. 2024, 279, 135261. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Microbial Exopolysaccharides: Structure, Diversity, Applications, and Future Frontiers in Sustainable Functional Materials. Polysaccharides 2024, 5, 241–287. [Google Scholar] [CrossRef]
- Nguyen, H.-T.; Pham, T.-T.; Nguyen, P.-T.; Le-Buanec, H.; Rabetafika, H.N.; Razafindralambo, H.L. Advances in Microbial Exopolysaccharides: Present and Future Applications. Biomolecules 2024, 14, 1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, T. Types, Synthesis Pathways, Purification, Characterization, and Agroecological Physiological Functions of Microbial Exopolysaccharides: A Review. Int. J. Biol. Macromol. 2024, 281, 136317. [Google Scholar] [CrossRef]
- Ahuja, V.; Chauhan, S.; Dasgupta, D.; Wadhwa, P.; Raj, T.; Yang, Y.-H.; Bhatia, S.K. Microbial Exopolysaccharide Composites with Inorganic Materials and Their Biomedical Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100482. [Google Scholar] [CrossRef]
- Salimi, F.; Farrokh, P. Recent Advances in the Biological Activities of Microbial Exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 213. [Google Scholar] [CrossRef]
- Waoo, A.A.; Singh, S.; Pandey, A.; Kant, G.; Choure, K.; Amesho, K.T.T.; Srivastava, S. Microbial Exopolysaccharides in the Biomedical and Pharmaceutical Industries. Heliyon 2023, 9, e18613. [Google Scholar] [CrossRef]
- Abbasi, A.; Rahbar Saadat, T.; Rahbar Saadat, Y. Microbial Exopolysaccharides–β-Glucans–as Promising Postbiotic Candidates in Vaccine Adjuvants. Int. J. Biol. Macromol. 2022, 223, 346–361. [Google Scholar] [CrossRef]
- Prasad, S.; Purohit, S.R. Microbial Exopolysaccharide: Sources, Stress Conditions, Properties and Application in Food and Environment: A Comprehensive Review. Int. J. Biol. Macromol. 2023, 242, 124925. [Google Scholar] [CrossRef]
- Benhadda, F.; Zykwinska, A.; Colliec-Jouault, S.; Sinquin, C.; Thollas, B.; Courtois, A.; Fuzzati, N.; Toribio, A.; Delbarre-Ladrat, C. Marine Versus Non-Marine Bacterial Exopolysaccharides and Their Skincare Applications. Mar. Drugs 2023, 21, 582. [Google Scholar] [CrossRef]
- Ibrahim, H.A.H.; Abou Elhassayeb, H.E.; El-Sayed, W.M.M. Potential Functions and Applications of Diverse Microbial Exopolysaccharides in Marine Environments. J. Genet. Eng. Biotechnol. 2022, 20, 151. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Fujii, T.; Mayor, D.J.; Solan, M.; Priede, I.G. Hadal Trenches: The Ecology of the Deepest Places on Earth. Trends Ecol. Evol. 2010, 25, 190–197. [Google Scholar] [CrossRef]
- Taira, K.; Yanagimoto, D.; Kitagawa, S. Deep CTD Casts in the Challenger Deep, Mariana Trench. J. Oceanogr. 2005, 61, 447–454. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, W.; Liu, Y.; Cai, M.; Luo, Z.; Li, M. Metagenomics Reveals Microbial Diversity and Metabolic Potentials of Seawater and Surface Sediment from a Hadal Biosphere at the Yap Trench. Front. Microbiol. 2018, 9, 2402. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Li, Y.; Deng, L.; Fang, J.; Yu, X. Insight into the Adaptation Mechanisms of High Hydrostatic Pressure in Physiology and Metabolism of Hadal Fungi from the Deepest Ocean Sediment. Msystems 2023, 9, e01085-23. [Google Scholar] [CrossRef]
- Rappaport, H.B.; Oliverio, A.M. Extreme Environments Offer an Unprecedented Opportunity to Understand Microbial Eukaryotic Ecology, Evolution, and Genome Biology. Nat. Commun. 2023, 14, 4959. [Google Scholar] [CrossRef]
- Ganesh Kumar, A.; Manisha, D.; Nivedha Rajan, N.; Sujitha, K.; Magesh Peter, D.; Kirubagaran, R.; Dharani, G. Biodegradation of Phenanthrene by Piezotolerant Bacillus subtilis EB1 and Genomic Insights for Bioremediation. Mar. Pollut. Bull. 2023, 194, 115151. [Google Scholar] [CrossRef] [PubMed]
- Radchenkova, N.; Rusinova-Videva, S. Extremophiles as a “Green Source” of New Exopolysaccharides with Ecological Importance and Multifunctional Applications. Polym.-Plast. Technol. Mater. 2024, 63, 247–269. [Google Scholar] [CrossRef]
- Radchenkova, N.; Yaşar Yıldız, S. Advanced Optimization of Bioprocess Parameters for Exopolysaccharides Synthesis in Extremophiles. Processes 2025, 13, 822. [Google Scholar] [CrossRef]
- López-Ortega, M.A.; Chavarría-Hernández, N.; del Rocio Lopez-Cuellar, M.; Rodríguez-Hernández, A.I. A Review of Extracellular Polysaccharides from Extreme Niches: An Emerging Natural Source for the Biotechnology. From the Adverse to Diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, M.; Clack, B.; Edwards, J.; Tylingo, R.; Samaszko, J.; Madaj, J. Structure and Properties of the Exopolysaccharides Produced by Pseudomonas mutabilis T6 and P. mutabilis ATCC 31014. Carbohydr. Res. 2012, 348, 84–90. [Google Scholar] [CrossRef]
- Carvalho, D.N.; Reis, R.L.; Silva, T.H. Marine Origin Materials on Biomaterials and Advanced Therapies to Cartilage Tissue Engineering and Regenerative Medicine. Biomater. Sci. 2021, 9, 6718–6736. [Google Scholar] [CrossRef]
- Wang, C.-L.; Huang, T.-H.; Liang, T.-W.; Fang, C.-Y.; Wang, S.-L. Production and Characterization of Exopolysaccharides and Antioxidant from Paenibacillus Sp. TKU023. New Biotechnol. 2011, 28, 559–565. [Google Scholar] [CrossRef]
- Decho, A.W.; Gutierrez, T. Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems. Front. Microbiol. 2017, 8, 922. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, C.; Kraithong, S.; Gong, H.; Han, Z.; Zheng, X.; Liao, X.; Mok, S.W.-F.; Huang, R.; Zhang, X. Insights into a Novel Exopolysaccharide from Mariana Trench-Derived Aspergillus versicolor SCAU214: Structure and Immune Activity. Int. J. Biol. Macromol. 2025, 308, 142660. [Google Scholar] [CrossRef]
- Ghareeb, A.; Fouda, A.; Kishk, R.M.; El Kazzaz, W.M. Unlocking the Therapeutic Potential of Bioactive Exopolysaccharide Produced by Marine Actinobacterium Streptomyces vinaceusdrappus AMG31: A Novel Approach to Drug Development. Int. J. Biol. Macromol. 2024, 276, 133861. [Google Scholar] [CrossRef]
- Yang, W.; Liu, L.; Zhao, X.; Chen, Y.; Zhang, J.; Zhang, L. Characterizations of the Structure and Properties of Exopolysaccharide from Leuconostoc citreum SFL-2-8 and Its Potential for Use in Food Coating Preservation. Int. J. Biol. Macromol. 2025, 308, 142150. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Ricciardelli, A.; Parrilli, E.; Tutino, M.L.; Corsaro, M.M. Cell-Wall Associated Polysaccharide from the Psychrotolerant Bacterium Psychrobacter arcticus 273-4: Isolation, Purification and Structural Elucidation. Extremophiles 2020, 24, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Radchenkova, N.; Vassilev, S.; Martinov, M.; Kuncheva, M.; Panchev, I.; Vlaev, S.; Kambourova, M. Optimization of the Aeration and Agitation Speed of Aeribacillus palidus 418 Exopolysaccharide Production and the Emulsifying Properties of the Product. Process Biochem. 2014, 49, 576–582. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial Exopolysaccharides from Extreme Marine Habitats: Production, Characterization and Biological Activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef]
- Qi, M.; Zheng, C.; Wu, W.; Yu, G.; Wang, P. Exopolysaccharides from Marine Microbes: Source, Structure and Application. Mar. Drugs 2022, 20, 512. [Google Scholar] [CrossRef]
- Juni, E.; Heym, G.A. Psychrobacter immobilis Gen. Nov., Sp. Nov.: Genospecies Composed of Gram-Negative, Aerobic, Oxidase-Positive Coccobacilli. Int. J. Syst. Evol. Microbiol. 1986, 36, 388–391. [Google Scholar] [CrossRef]
- Welter, D.K.; Ruaud, A.; Henseler, Z.M.; De Jong, H.N.; van Coeverden de Groot, P.; Michaux, J.; Gormezano, L.; Waters, J.L.; Youngblut, N.D.; Ley, R.E. Free-Living, Psychrotrophic Bacteria of the Genus Psychrobacter Are Descendants of Pathobionts. Msystems 2021, 6, 10.1128/msystems.258-21. [Google Scholar] [CrossRef] [PubMed]
- Kokoulin, M.S.; Kuzmich, A.S.; Romanenko, L.A.; Chikalovets, I.V.; Chernikov, O.V. Structure and In Vitro Bioactivity Against Cancer Cells of the Capsular Polysaccharide from the Marine Bacterium Psychrobacter marincola. Mar. Drugs 2020, 18, 268. [Google Scholar] [CrossRef] [PubMed]
- Kondakova, A.N.; Novototskaya-Vlasova, K.A.; Shashkov, A.S.; Drutskaya, M.S.; Senchenkova, S.N.; Shcherbakova, V.A.; Gilichinsky, D.A.; Nedospasov, S.A.; Knirel, Y.A. Structure of an Acidic Polysaccharide Isolated from Psychrobacter maritimus 3pS Containing a Bacillosamine Derivative. Carbohydr. Res. 2012, 359, 7–10. [Google Scholar] [CrossRef]
- Casillo, A.; Fabozzi, A.; Russo Krauss, I.; Parrilli, E.; Biggs, C.I.; Gibson, M.I.; Lanzetta, R.; Appavou, M.-S.; Radulescu, A.; Tutino, M.L.; et al. Physicochemical Approach to Understanding the Structure, Conformation, and Activity of Mannan Polysaccharides. Biomacromolecules 2021, 22, 1445–1457. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Z.; Zhao, Z.; Mu, Y.; Xu, J.; Dai, S.; Cui, Y.; Ying, M.; Hu, X.; Huang, L. Isolation, Structural Characterization and Multiple Activity of a Novel Exopolysaccharide Produced by Gelidibacter Sp. PG–2. Int. J. Biol. Macromol. 2025, 305, 141127. [Google Scholar] [CrossRef]
- Vinogradov, E.; Petersen, B.; Bock, K. Structural Analysis of the Intact Polysaccharide Mannan from Saccharomyces cerevisiae Yeast Using 1H and 13C NMR Spectroscopy at 750 MHz. Carbohydr. Res. 1998, 307, 177–183. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A Review of Natural Polysaccharides for Drug Delivery Applications: Special Focus on Cellulose, Starch and Glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; El-Bery, H.M.; Metwally, A.A.; Elshazly, M.; Hathout, R.M. Synthesis of CdS-Modified Chitosan Quantum Dots for the Drug Delivery of Sesamol. Carbohydr. Polym. 2019, 214, 90–99. [Google Scholar] [CrossRef]
- Pan, L.; Cai, C.; Liu, C.; Liu, D.; Li, G.; Linhardt, R.J.; Yu, G. Recent Progress and Advanced Technology in Carbohydrate-Based Drug Development. Curr. Opin. Biotechnol. 2021, 69, 191–198. [Google Scholar] [CrossRef]
- Wang, W.; Tan, J.; Nima, L.; Sang, Y.; Cai, X.; Xue, H. Polysaccharides from Fungi: A Review on Their Extraction, Purification, Structural Features, and Biological Activities. Food Chem. X 2022, 15, 100414. [Google Scholar] [CrossRef]
- Zhao, J.-H.; Wang, Y.-W.; Yang, J.; Tong, Z.-J.; Wu, J.-Z.; Wang, Y.-B.; Wang, Q.-X.; Li, Q.-Q.; Yu, Y.-C.; Leng, X.-J.; et al. Natural Products as Potential Lead Compounds to Develop New Antiviral Drugs over the Past Decade. Eur. J. Med. Chem. 2023, 260, 115726. [Google Scholar] [CrossRef]
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef] [PubMed]
- Giddings, L.-A.; Newman, D.J. Extremophilic Fungi from Marine Environments: Underexplored Sources of Antitumor, Anti-Infective and Other Biologically Active Agents. Mar. Drugs 2022, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Hemmerling, F.; Piel, J. Strategies to Access Biosynthetic Novelty in Bacterial Genomes for Drug Discovery. Nat. Rev. Drug Discov. 2022, 21, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Ragozzino, C.; Casella, V.; Coppola, A.; Scarpato, S.; Buonocore, C.; Consiglio, A.; Palma Esposito, F.; Galasso, C.; Tedesco, P.; Della Sala, G.; et al. Last Decade Insights in Exploiting Marine Microorganisms as Sources of New Bioactive Natural Products. Mar. Drugs 2025, 23, 116. [Google Scholar] [CrossRef]
- Tan, L.T. Impact of Marine Chemical Ecology Research on the Discovery and Development of New Pharmaceuticals. Mar. Drugs 2023, 21, 174. [Google Scholar] [CrossRef]
- Zain ul Arifeen, M.; Ma, Y.-N.; Xue, Y.-R.; Liu, C.-H. Deep-Sea Fungi Could Be the New Arsenal for Bioactive Molecules. Mar. Drugs 2020, 18, 9. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Xue, Y.-R.; Liu, C.-H. A Brief Review of Bioactive Metabolites Derived from Deep-Sea Fungi. Mar. Drugs 2015, 13, 4594–4616. [Google Scholar] [CrossRef]
- Shang, S.; Li, X.; Wang, H.; Zhou, Y.; Pang, K.; Li, P.; Liu, X.; Zhang, M.; Li, W.; Li, Q.; et al. Targeted Therapy of Kidney Disease with Nanoparticle Drug Delivery Materials. Bioact. Mater. 2024, 37, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kashyap, S.; Mazahir, F.; Sharma, R.; Yadav, A.K. Unveiling the Potential of Molecular Imprinting Polymer-Based Composites in the Discovery of Advanced Drug Delivery Carriers. Drug Discov. Today 2024, 29, 104164. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Hou, L.; Ching, Y.C.; Ching, K.Y.; Hai, N.D.; Chuah, C.H. A Review of Recent Advances of Cellulose-Based Intelligent-Responsive Hydrogels as Vehicles for Controllable Drug Delivery System. Int. J. Biol. Macromol. 2024, 264, 130525. [Google Scholar] [CrossRef]
- Qian, Y.; Shi, C.; Cheng, C.; Liao, D.; Liu, J.; Chen, G. Ginger Polysaccharide UGP1 Suppressed Human Colon Cancer Growth via P53, Bax/Bcl-2, Caspase-3 Pathways and Immunomodulation. Food Sci. Hum. Wellness 2023, 12, 467–476. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yu, X.; Yu, T.; Zheng, X.; Chu, Q. Radix tetrastigma Inhibits the Non-Small Cell Lung Cancer via Bax/Bcl-2/Caspase-9/Caspase-3 Pathway. Nutr. Cancer 2022, 74, 320–332. [Google Scholar] [CrossRef]
- Lin, L.; Cheng, K.; Xie, Z.; Chen, C.; Chen, L.; Huang, Y.; Liang, Z. Purification and Characterization a Polysaccharide from Hedyotis diffusa and Its Apoptosis Inducing Activity Toward Human Lung Cancer Cell Line A549. Int. J. Biol. Macromol. 2019, 122, 64–71. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, N.; Chen, X. Structurally Modified Polysaccharides: Physicochemical Properties, Biological Activities, Structure–Activity Relationship, and Applications. J. Agric. Food Chem. 2024, 72, 3259–3276. [Google Scholar] [CrossRef]
- Han, D.; Yang, L.; Liang, Q.; Sun, H.; Sun, Y.; Yan, G.; Zhang, X.; Han, Y.; Wang, X.; Wang, X. Natural Resourced Polysaccharides: Preparation, Purification, Structural Elucidation, Structure-Activity Relationships and Regulating Intestinal Flora, a System Review. Int. J. Biol. Macromol. 2024, 280, 135956. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Fu, Y.; Jiao, H.; Wang, X.; Wang, Q.; Zhou, M.; Yong, Y.; Liu, J. A Structure-Functionality Insight into the Bioactivity of Microbial Polysaccharides Toward Biomedical Applications: A Review. Carbohydr. Polym. 2024, 335, 122078. [Google Scholar] [CrossRef]
- Cui, Y.; Xiao, Y.; Wang, Z.; Ji, P.; Zhang, C.; Li, Y.; Fang, J.; Yu, X. Microbial Community Structure and Functional Traits Involved in the Adaptation of Culturable Bacteria Within the Gut of Amphipods from the Deepest Ocean. Microbiol. Spectr. 2025, 13, e0072324. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, Deproteinization and Comparison of Bioactive Polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, Y.; Leng, F.; Wang, S.; Wang, F.; Zhuang, Y.; Liu, X.; Wang, X.; Ma, X. Establishment and Application of a Method for Rapid Determination of Total Sugar Content Based on Colorimetric Microplate. Sugar Tech 2017, 19, 424–431. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New Method for Quantitative Determination of Uronic Acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Yao, J.; Shi, Y.; Li, P.; Ding, K. An Arabinogalactan from Flowers of Panax notoginseng Inhibits Angiogenesis by BMP2/Smad/Id1 Signaling. Carbohydr. Polym. 2015, 121, 328–335. [Google Scholar] [CrossRef]
- Ji, M.; Sun, L.; Zhang, M.; Liu, Y.; Zhang, Z.; Wang, P. RN0D, a Galactoglucan from Panax notoginseng Flower Induces Cancer Cell Death via PINK1/Parkin Mitophagy. Carbohydr. Polym. 2024, 332, 121889. [Google Scholar] [CrossRef]
- Sun, L.; Ni, X.; Liu, Y.; Jiang, Y.; Wang, P.-P.; Gao, J. A Neutral Glucan Extracted from Dried Ginger (Zingiberis rhizoma): Preparation, Structure Characterization, and Immunomodulatory Activity. Planta Med. 2025. [Google Scholar] [CrossRef]





| Sample Name | Total Sugar Content | Protein Content | Uronic Acid Content | Monosaccharide Composition |
|---|---|---|---|---|
| Man:GlcN:GlcA:GalN:Gal:Glc | ||||
| XL-1 | 43.56% | 17.31% | 3.65% | 35.45:7.28:14.66:36.80:2.84:0 |
| XL-1-D | 66.82% | 10.02% | 5.14% | 89.41:4.36:0:0:0:6.22 |
| XMRS-1 | 37.54% | 13.99% | 5.27% | 56.42:1.97:0:0:0:41.61 |
| XMRS-1-D | 49.80% | 12.30% | 5.60% | 93.26:1.96:0:0:1.04:3.74 |
| Sample Name | Amino Acid Species | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp | Thr | Ser | Glu | Gly | Ala | Val | Ile | Leu | Tyr | Phe | Lys | His | Arg | Pro | |
| XL-1-D | 82.35 ± 2.20 | 23.17 ± 0.61 | 42.86 ± 1.16 | 104.09 ± 2.78 | 389.13 ± 10.20 | 123.62 ± 3.34 | 18.54 ± 4.50 | 7.16 ± 0.23 | 14.41 ± 0.42 | 1.90 ± 0.07 | 5.55 ± 0.16 | 23.13 ± 0.62 | 2.16 ± 0.06 | 24.06 ± 0.65 | 137.88 ± 3.65 |
| XMRS-1-D | 88.36 ± 0.96 | 25.04 ± 0.27 | 42.19 ± 0.46 | 106.15 ± 1.16 | 383.70 ± 1.09 | 124.58 ± 1.39 | 1.89 ± 0.02 | 10.13 ± 0.11 | 22.95 ± 0.25 | 3.47 ± 0.04 | 8.26 ± 0.09 | 25.65 ± 0.28 | 3.28 ± 0.03 | 17.44 ± 0.19 | 136.88 ± 3.65 |
| Upstream Primer | Downstream Primer | |
|---|---|---|
| GAPDH | AATGGATTTGGACGCATTGGT | TTTGCACTGGTACGTGTTGAT |
| iNOS | CTCTTCGACGACCCAGAAAAC | CAAGGCCATGAAGTGAGGCTT |
| TNF-α | CAGGTTCTCTTCAAGGGACAAGGC | TGACGGCAGAGAGGAGGTTGAC |
| IL-6 | CTTCTTGGGACTGATGCTGGTGAC | TCTGTTGGGAGTGGTATCCTCTGTG |
| Upstream Primer | Downstream Primer | |
|---|---|---|
| GAPDH | TGTGGGCATCAATGGATTTGG | ACACCATGTATTCCGGGTCAAT |
| Bax | CCCGAGAGGTCTTTTTCCGAG | CCAGCCCATGATGGTTCTGAT |
| Caspase-3 | CATGGAAGCGAATCAATGGACT | CTGTACCAGACCGAGATGTCA |
| Caspase-8 | TTTCTGCCTACAGGGTCATGC | GCTGCTTCTCTCTTTGCTGAA |
| Caspase-9 | CTCAGACCAGAGATTCGCAAAC | GCATTTCCCCTCAAACTCTCAA |
| BCL-2 | GGTGGGGTCATGTGTGTGG | CGGTTCAGGTACTCAGTCATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, M.; Yan, S.; Cui, Y.; Huang, Y.; Liu, Y.; Wu, W.; Yu, X.; Wang, P. Mannan-Containing Polymers from Hadal Bacterium Psychrobacter pulmonis: Preparation, Structural Analysis, Immunological Activity and Antitumor Effects. Mar. Drugs 2025, 23, 326. https://doi.org/10.3390/md23080326
Qi M, Yan S, Cui Y, Huang Y, Liu Y, Wu W, Yu X, Wang P. Mannan-Containing Polymers from Hadal Bacterium Psychrobacter pulmonis: Preparation, Structural Analysis, Immunological Activity and Antitumor Effects. Marine Drugs. 2025; 23(8):326. https://doi.org/10.3390/md23080326
Chicago/Turabian StyleQi, Mingxing, Shuqiang Yan, Yukun Cui, Yanan Huang, Yang Liu, Wenhui Wu, Xi Yu, and Peipei Wang. 2025. "Mannan-Containing Polymers from Hadal Bacterium Psychrobacter pulmonis: Preparation, Structural Analysis, Immunological Activity and Antitumor Effects" Marine Drugs 23, no. 8: 326. https://doi.org/10.3390/md23080326
APA StyleQi, M., Yan, S., Cui, Y., Huang, Y., Liu, Y., Wu, W., Yu, X., & Wang, P. (2025). Mannan-Containing Polymers from Hadal Bacterium Psychrobacter pulmonis: Preparation, Structural Analysis, Immunological Activity and Antitumor Effects. Marine Drugs, 23(8), 326. https://doi.org/10.3390/md23080326







