Neoxanthin: A Promising Medicinal and Nutritional Carotenoid
Abstract
1. Introduction
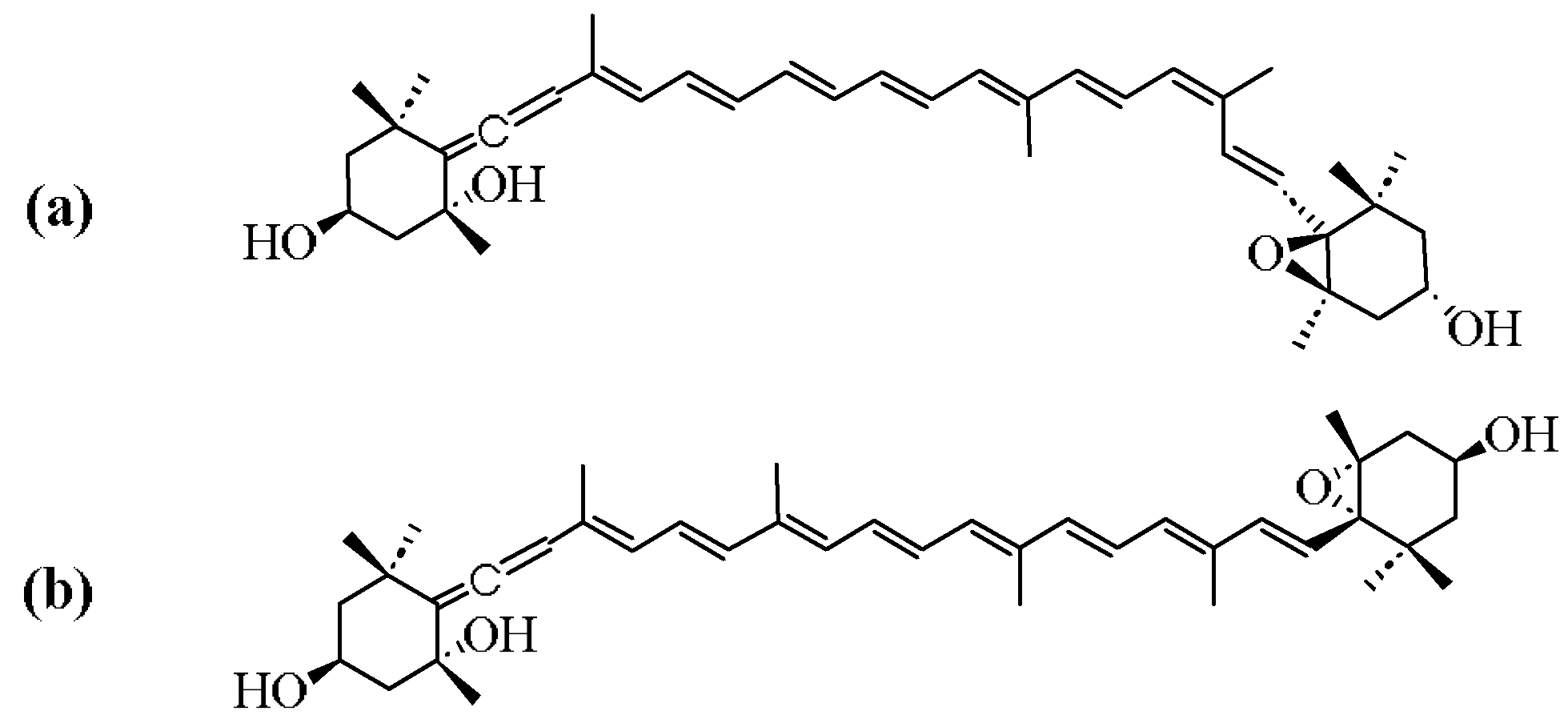
2. Structure of Neoxanthin
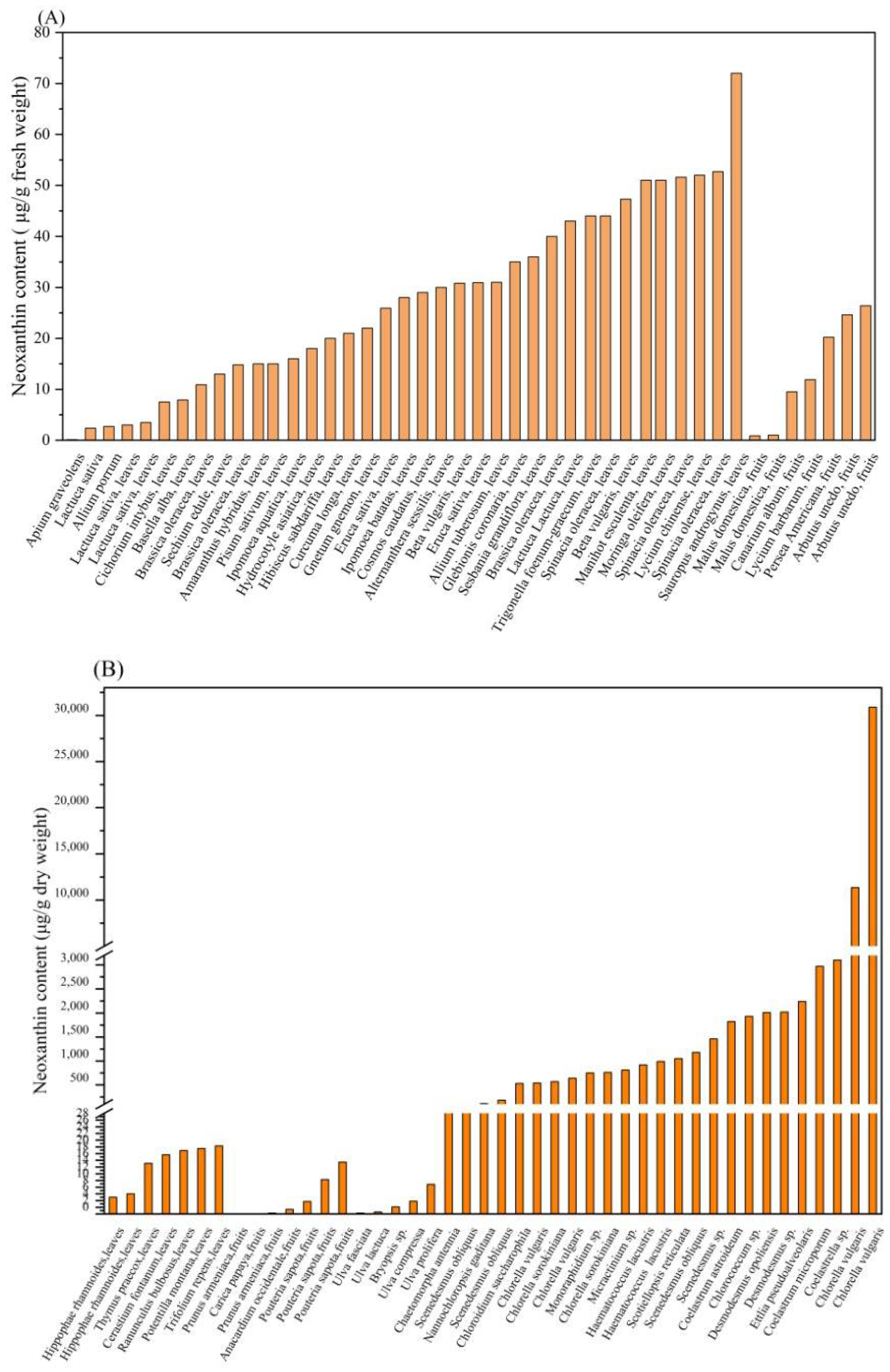
3. Neoxanthin Source
| High Plant | Content of Neoxanthin | Reference |
|---|---|---|
| Allium porrum | 2.7 ± 0.9 a | [26] |
| Allium tuberosum | 31 ± 2 a | [26] |
| Alternanthera sessilis | 30 ± 3 a | [26] |
| Amaranthus hybridus | 15 ± 5 a | [26] |
| Apium graveolens | 0.11 ± 0.09 a | [26] |
| Basella alba | 7.9 ± 0.7 a | [26] |
| Beta vulgaris | 30.8 a | [27] |
| Beta vulgaris | 47.3 a | [27] |
| Brassica oleracea | 40 ± 10 a | [26] |
| Brassica oleracea | 14.8 a | [28] |
| Brassica oleracea | 10.9 a | [28] |
| Cerastium fontanum | 17.6 ± 0.860 b | [29] |
| Cichorium intybus | 7.5 a | [28] |
| Cosmos caudatus | 29 ± 7 a | [26] |
| Curcuma longa | 21 ± 1 a | [26] |
| Eruca sativa | 30.9 a | [27] |
| Eruca sativa | 25.9 a | [27] |
| Glebionis coronaria | 35 ± 6 a | [26] |
| Gnetum gnemon | 22 ± 2 a | [26] |
| Hibiscus sabdariffa | 20 ± 2 a | [26] |
| Hippophae rhamnoides | 5.0 ± 0.0 b | [30] |
| Hippophae rhamnoides | 6.0 ± 0.0 b | [30] |
| Hydrocotyle asiatica | 18 ± 1 a | [26] |
| Ipomoea aquatica | 16 ± 1 a | [26] |
| Ipomoea batatas | 28 ± 3 a | [26] |
| Lactuca sativa var. capitata | 3 ± 1 a | [26] |
| Lactuca sativa var. Longifolia | 43 ± 1 a | [26] |
| Lactuca sativa var. Longifolia | 2.36 a | [27] |
| Lactuca sativa var. Longifolia | 3.49 a | [27] |
| Lycium chinense | 52 ± 8 a | [26] |
| Manihot esculenta | 51 ± 5 a | [26] |
| Moringa oleifera | 51 ± 12 a | [26] |
| Pisum sativum | 15 ± 2 a | [26] |
| Potentilla montana | 19.5 ± 0.787 b | [29] |
| Ranunculus bulbosus | 18.9 ± 0.179 b | [29] |
| Sauropus androgynus | 72 ± 6 a | [26] |
| Sesbania grandiflora | 36 ± 4 a | [26] |
| Sechium edule | 13 ± 6 a | [26] |
| Spinacia oleracea | 44 ± 9 a | [26] |
| Spinacia oleracea | 52.7 a | [27] |
| Spinacia oleracea | 51.6 a | [27] |
| Thymus praecox | 15.1 ± 0.0803 b | [29] |
| Trifolium repens | 20.3 ± 0.556 b | [29] |
| Trigonella foenum-graecum | 44 ± 7 a | [26] |
| High Plant | Content of Neoxanthin | Reference |
|---|---|---|
| Arbutus unedo | 26.4 ± 1.8 a | [31] |
| Arbutus unedo | 24.6 ± 1.9 a | [31] |
| Anacardium occidentale | 1.36 b | [32] |
| Canarium album | 9.5 a | [33] |
| Carica papaya | 0.007 b | [34] |
| Lycium barbarum | 11.9 ± 0.0 a | [35] |
| Malus domestica | 0.99 ± 0.05 a | [36] |
| Malus domestica | 0.87 ± 0.05 a | [36] |
| Persea Americana | 20.2 ± 2.80 a | [37] |
| Pouteriasapota | 15.45 ± 1.32 b | [38] |
| Pouteriasapota | 10.24 ± 2.63 b | [38] |
| Pouteriasapota | 3.70 ± 0.99 b | [38] |
| Prunus armeniaca | 0.005 b | [39] |
| Prunus armeniaca | 0.257 b | [39] |
| Microalgal Species | Neoxanthin Content | Reference |
|---|---|---|
| Bryopsis sp. | 2.11 ± 0.10 a | [12] |
| Chaetomorpha antennia | 33.35 ± 0.23 a | [12] |
| Chloroidium saccharophilum (formerly Chlorella saccharophila) | 530 ± 40 a | [10] |
| Chlorella sorokiniana | 570 ± 20 a | [10] |
| Chlorella sorokiniana | 760 ± 120 a | [10] |
| Chlorella vulgaris | 540 ± 80 a | [10] |
| Chlorella vulgaris | 640 ± 50 a | [10] |
| Chlorella vulgaris | 11,350 ± 17 a | [11] |
| Chlorella vulgaris | 30,880 ± 426 a | [11] |
| Chlorococcum sp. | 1930 ± 130 a | [10] |
| Coelastrella sp. | 3100 ± 220 a | [10] |
| Coelastrum astroideum | 1820 ± 80 a | [10] |
| Coelastrum microporum | 2970 ± 170 a | [10] |
| Desmodesmus opoliensis | 2010 ± 160 a | [10] |
| Desmodesmus sp. | 990 ± 70 a | [10] |
| Desmodesmus sp. | 2020 ± 150 a | [10] |
| Ettlia pseudoalveolaris | 2240 ± 90 a | [10] |
| Haematococcus lacustris (formerly Haematococcus pluvialis) | 920 ± 20 a | [10] |
| Micractinium sp. | 810 ± 50 a | [10] |
| Monoraphidium sp. | 750 ± 70 a | [10] |
| Nannochloropsis gaditana | 110 ± 20 a | [41] |
| Scenedesmus obliquus | 1180 ± 30 a | [10] |
| Scenedesmus obliquus | 55.72 ± 1.72 a | [42] |
| Scenedesmus obliquus | 180.33 ± 11.23 a | [42] |
| Scenedesmus sp. | 1460 ± 100 a | [10] |
| Scotiellopsis reticulata | 1050 ± 110 a | [10] |
| Ulva compressa | 3.81 ± 0.08 a | [12] |
| Ulva fasciata | 0.26 ± 0.00 a | [12] |
| Ulva lactuca | 0.61 ± 0.07 a | [12] |
| Ulva prolifera | 8.84 ± 0.12 a | [12] |
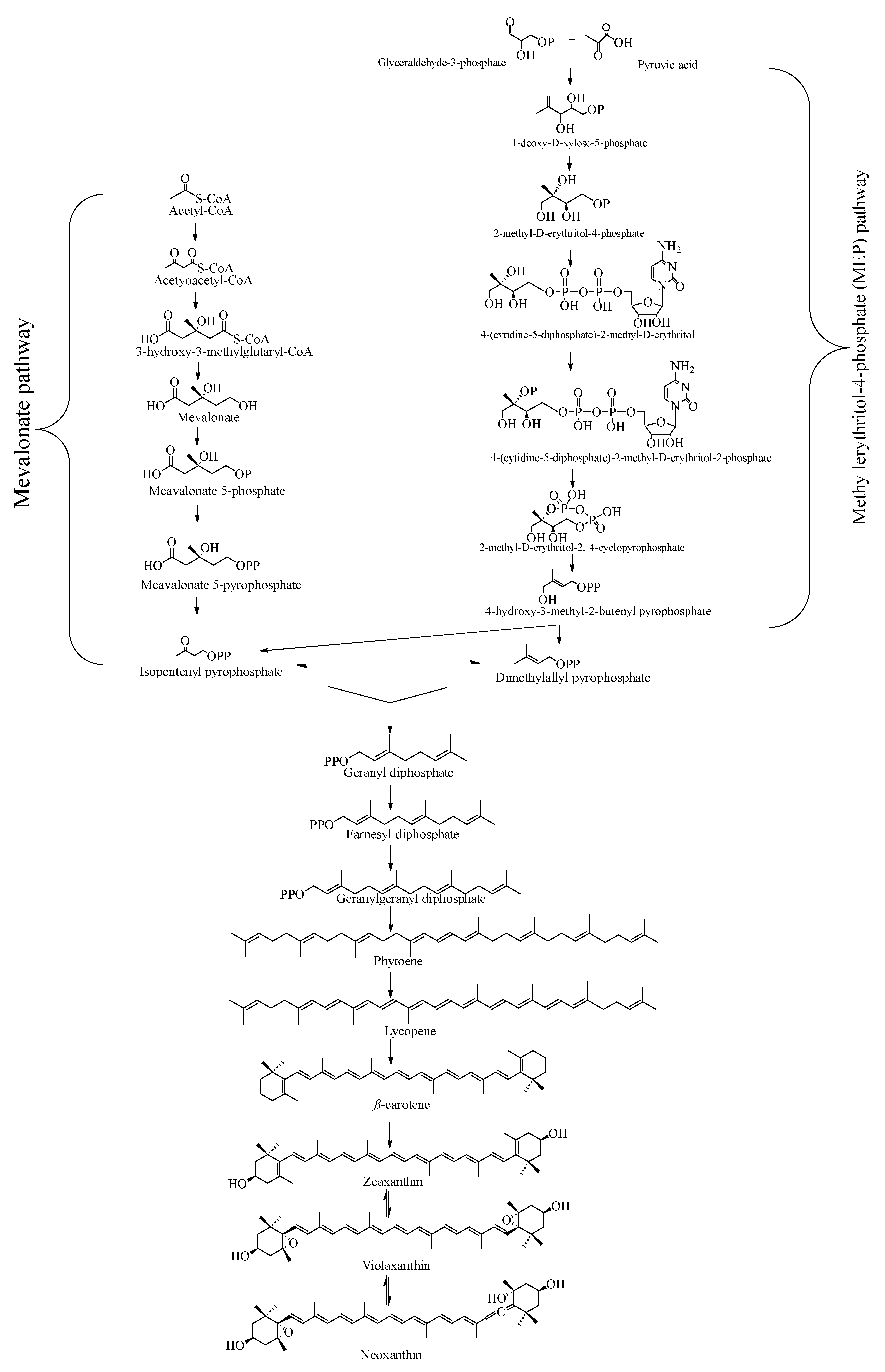
4. Biosynthetic Pathway of Neoxanthin
5. Extraction of Neoxanthin
5.1. Organic Solvents Extraction
5.2. Ionic Liquids Extraction
5.3. Supercritical Liquid Extraction
5.4. Ultrasound-Assisted Extraction
5.5. Pressurized Liquid Extraction
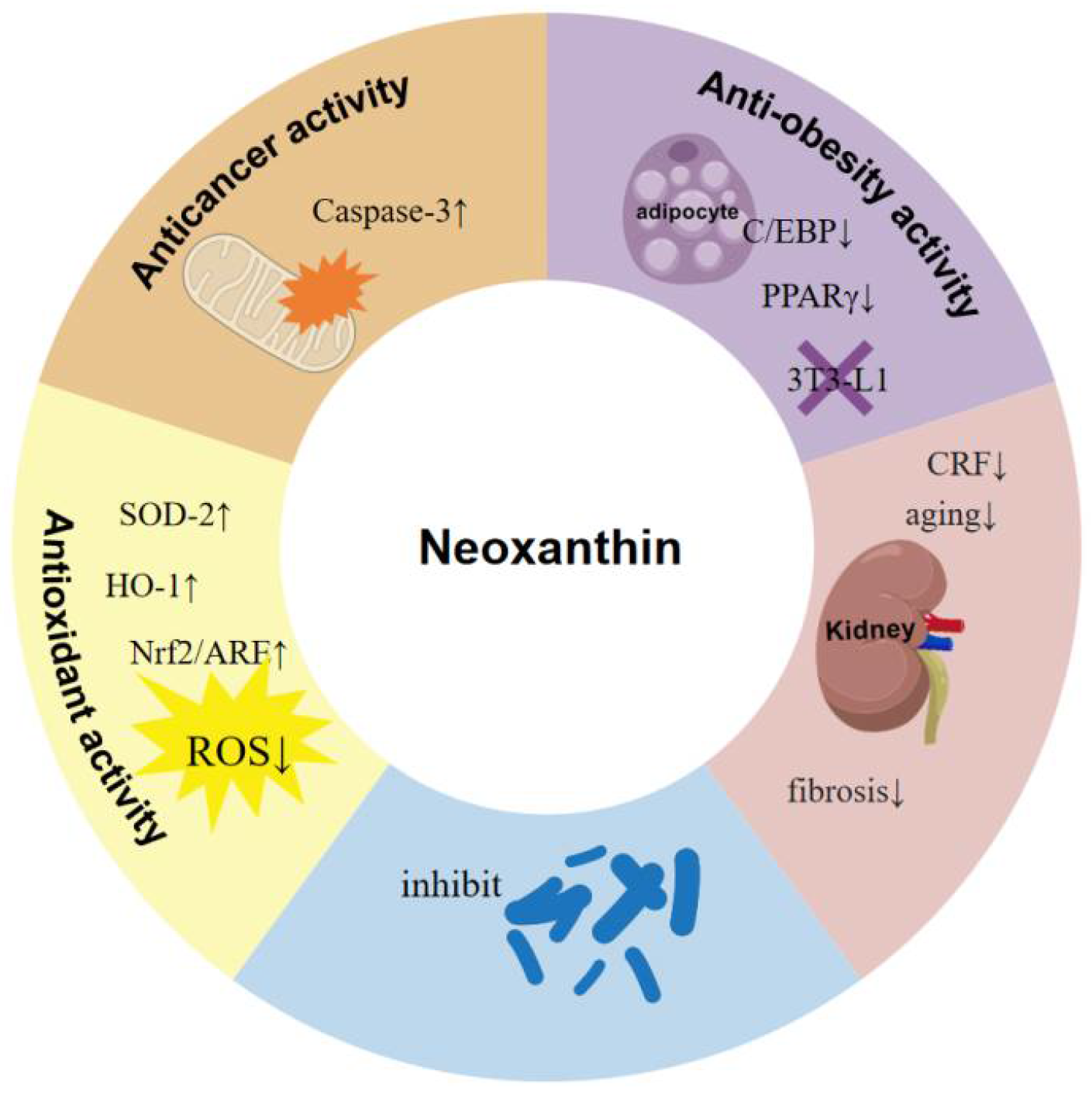
6. Biological Activities of Neoxanthin
6.1. Anti-Cancer Activity
6.2. Anti-Oxidant Activity
6.3. Anti-Obesity Activity
6.4. Anti-Inflammatory Activity
6.5. Anti-Bacterial Activity
7. Current Challenges and Opportunities
7.1. The Prospect of Microalgae as a Neoxanthin Source
7.2. Extraction and Purification of Neoxanthin
7.3. Biological Activity of Neoxanthin
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Britton, G. Carotenoid research: History and new perspectives for chemistry in biological systems. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2020, 1865, 158699. [Google Scholar] [CrossRef]
- Sourkes, T.L. The discovery and early history of carotene. Hist. Chem 2009, 34, 33. [Google Scholar] [CrossRef]
- Steenbock, H.; Coward, K.H. Fat-soluble vitamins. J. Biol. Chem. 1927, 72, 765–779. [Google Scholar] [CrossRef]
- Uray, I.P.; Dmitrovsky, E.; Brown, P.H. Retinoids and rexinoids in cancer prevention: From laboratory to clinic. Semin. Oncol. 2016, 43, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Kolašinac, S.M.; Dajić Stevanović, Z.; Kilibarda, S.N.; Kostić, A.Ž. Carotenoids: New applications of “old” pigments. Phyton 2021, 90, 1041–1062. [Google Scholar] [CrossRef]
- Choi, S.S.; Seo, Y.B.; Nam, S.-W.; Kim, G.-D. Enhanced production of astaxanthin by co-culture of Paracoccus haeundaensis and lactic acid bacteria. Front. Mar. Sci. 2021, 7, 597553. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef]
- Giossi, C.; Cartaxana, P.; Cruz, S. Photoprotective role of neoxanthin in plants and algae. Molecules 2020, 25, 4617. [Google Scholar] [CrossRef] [PubMed]
- León-Vaz, A.; León, R.; Vigara, J.; Funk, C. Exploring nordic microalgae as a potential novel source of antioxidant and bioactive compounds. New Biotechnol. 2023, 73, 1–8. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Avalo, B.; Cifuentes, A.; Reglero, G.; García-Blairsy Reina, G.; Señoráns, F.J.; Ibáñez, E. Comprehensive characterization of the functional activities of pressurized liquid and ultrasound-assisted extracts from Chlorella vulgaris. LWT—Food Sci. Technol. 2012, 46, 245–253. [Google Scholar] [CrossRef]
- Bhat, I.; Haripriya, G.; Jogi, N.; Mamatha, B.S. Carotenoid composition of locally found seaweeds of Dakshina Kannada district in India. Algal Res. 2021, 53, 102154. [Google Scholar] [CrossRef]
- Parida, S.; Jena, M.; Behera, A.K.; Mandal, A.K.; Nayak, R.; Patra, S. A novel phytocolorant, neoxanthin, as a potent chemopreventive: Current progress and future prospects. Curr. Med. Chem. 2024, 31, 5149–5164. [Google Scholar] [CrossRef]
- Cezare-Gomes, E.A.; Mejia-da-Silva, L.D.C.; Pérez-Mora, L.S.; Matsudo, M.C.; Ferreira-Camargo, L.S.; Singh, A.K.; De Carvalho, J.C.M. Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotechnol. 2019, 188, 602–634. [Google Scholar] [CrossRef]
- Heller, J.; Szarkowska, L.; Michalek, H. Ubiquinone (coenzyme Q) in insects. Nature 1960, 188, 491. [Google Scholar] [CrossRef]
- Bungard, R.A.; Ruban, A.V.; Hibberd, J.M.; Press, M.C.; Horton, P.; Scholes, J.D. Unusual carotenoid composition and a new type of xanthophyll cycle in plants. Proc. Natl. Acad. Sci. USA 1999, 96, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tu, W.; Liu, C.; Rao, Y.; Gao, Z.; Yang, C. 9-cis neoxanthin in light harvesting complexes of photosystem II regulates the binding of violaxanthin and xanthophyll cycle. Plant Physiol. 2017, 174, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.D.; Horgan, R. Carotenoids and abscisic acid (ABA) biosynthesis in higher plants. Physiol. Plant. 1991, 82, 320–326. [Google Scholar] [CrossRef]
- Frank, H.A.; Bautista, J.A.; Josue, J.; Pendon, Z.; Hiller, R.G.; Sharples, F.P.; Gosztola, D.; Wasielewski, M.R. Effect of the solvent environment on the spectroscopic properties and dynamics of the lowest excited states of carotenoids. J. Phys. Chem. B 2000, 104, 4569–4577. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the sea: Algae as source of bioactive carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Gandul-Rojas, B. Thermal degradation kinetics of neoxanthin, violaxanthin, and antheraxanthin in virgin olive oils. J. Agric. Food Chem. 2012, 60, 5180–5191. [Google Scholar] [CrossRef]
- Takaichi, S.; Mirauro, M. Distribution and geometric isomerism of neoxanthin in oxygenic phototrophs: 9’-cis, a sole molecular form. Plant Cell Physiol. 1998, 39, 968–977. [Google Scholar] [CrossRef]
- Yoshii, Y.; Takaichi, S.; Maoka, T.; Inouye, I. Photosynthetic pigment composition in the primitive green alga Mesostigma viride (prasinophyceae): Phylogenetic and evolutionary implications. J. Phycol. 2003, 39, 570–576. [Google Scholar] [CrossRef]
- Nurafifah, I.; Hardianto, M.A.; Erfianti, T.; Amelia, R.; Suyono, E.A. The effect of acidic ph on chlorophyll, carotenoids, and carotenoid derivatives of Euglena sp. as antioxidants. AACL Bioflux. 2023, 16, 2391–2401. [Google Scholar]
- Mc Gee, D.; Gillespie, E. The bioactivity and chemotaxonomy of microalgal carotenoids. In Biodiversity and Chemotaxonomy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 215–237. [Google Scholar]
- Wen Lee, H.; Bi, X.; Jeyakumar Henry, C. Carotenoids, tocopherols and phylloquinone content of 26 green leafy vegetables commonly consumed in Southeast Asia. Food Chem. 2022, 385, 132729. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Suzuki, S.; Ushida, Y.; Suganuma, H. Neoxanthin in young vegetable leaves prevents fat accumulation in differentiated adipocytes. Biosci. Biotechnol. Biochem. 2021, 85, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- De Sá, M.C.; Rodriguez-Amaya, D.B. Optimization of hplc quantification of carotenoids in cooked green vegetables—Comparison of analytical and calculated data. J. Food Compos. Anal. 2004, 17, 37–51. [Google Scholar] [CrossRef]
- Valdivielso, I.; Bustamante, M.Á.; Ruiz De Gordoa, J.C.; Nájera, A.I.; De Renobales, M.; Barron, L.J.R. Simultaneous analysis of carotenoids and tocopherols in botanical species using one step solid–liquid extraction followed by high performance liquid chromatography. Food Chem. 2015, 173, 709–717. [Google Scholar] [CrossRef]
- Pop, R.M.; Weesepoel, Y.; Socaciu, C.; Pintea, A.; Vincken, J.-P.; Gruppen, H. Carotenoid composition of berries and leaves from six romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Food Chem. 2014, 147, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Carotenoid composition of strawberry tree (Arbutus unedo L.) fruits. Food Chem. 2016, 199, 165–175. [Google Scholar] [CrossRef]
- De Abreu, F.P.; Dornier, M.; Dionisio, A.P.; Carail, M.; Caris-Veyrat, C.; Dhuique-Mayer, C. Cashew apple (Anacardium occidentale L.) extract from by-product of juice processing: A focus on carotenoids. Food Chem. 2013, 138, 25–31. [Google Scholar] [CrossRef]
- Criado, M.N.; Motilva, M.J.; Goñi, M.; Romero, M.P. Comparative study of the effect of the maturation process of the olive fruit on the chlorophyll and carotenoid fractions of drupes and virgin oils from Arbequina and Farga cultivars. Food Chem. 2007, 100, 748–755. [Google Scholar] [CrossRef]
- Barreto, G.P.M.; Fabi, J.P.; De Rosso, V.V.; Cordenunsi, B.R.; Lajolo, F.M.; Do Nascimento, J.R.O.; Mercadante, A.Z. Influence of ethylene on carotenoid biosynthesis during papaya postharvesting ripening. J. Food Compos. Anal. 2011, 24, 620–624. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Lu, H.; Hung, C.F.; Wu, W.B.; Lin, C.L.; Chen, B.H. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC–DAD–APCI–MS. J. Pharm. Biomed. Anal. 2008, 47, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and carotenoid pigments in the peel and flesh of commercial apple fruit varieties. Food Res. Int. 2014, 65, 272–281. [Google Scholar] [CrossRef]
- Hong, H.T.; Takagi, T.; O’Hare, T.J. An optimal saponification and extraction method to determine carotenoids in avocado. Food Chem. 2022, 387, 132923. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Ordóñez, T.; Schweiggert, R.M.; Bosy-Westphal, A.; Jiménez, V.M.; Carle, R.; Esquivel, P. Carotenoids and carotenoid esters of orange- and yellow-fleshed mamey sapote (Pouteria sapota (Jacq.) H.E. Moore & Stearn) fruit and their post-prandial absorption in humans. Food Chem. 2017, 221, 673–682. [Google Scholar]
- Zhou, W.; Niu, Y.; Ding, X.; Zhao, S.; Li, Y.; Fan, G.; Zhang, S.; Liao, K. Analysis of carotenoid content and diversity in apricots (Prunus armeniaca L.) grown in china. Food Chem. 2020, 330, 127223. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-derived pigments for the food industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Muylaert, K.; Foubert, I. Influence of extraction solvent system on extractability of lipid components from different microalgae species. Algal Res. 2014, 3, 36–43. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Caetano, P.A.; Jacob-Lopes, E.; Zepka, L.Q.; De Rosso, V.V. Alternative green solvents associated with ultrasound-assisted extraction: A green chemistry approach for the extraction of carotenoids and chlorophylls from microalgae. Food Chem. 2024, 455, 139939. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Gómez-Villegas, P.; Gonda, M.L.; León-Vaz, A.; León, R.; Mildenberger, J.; Rebours, C.; Saravia, V.; Vero, S.; Vila, E.; et al. Microalgae, seaweeds and aquatic bacteria, archaea, and yeasts: Sources of carotenoids with potential antioxidant and anti-inflammatory health-promoting actions in the sustainability era. Mar. Drugs 2023, 21, 340. [Google Scholar] [PubMed]
- Raposo, M.; De Morais, A.; De Morais, R. Carotenoids from marine microalgae: A valuable natural source for the prevention of chronic diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef]
- Amaro, H.M.; Macedo, A.C.; Malcata, F.X. Microalgae: An alternative as sustainable source of biofuels? Energy 2012, 44, 158–166. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A promising source of valuable bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Kim, J.K.; Park, S.U. An update on biosynthesis and regulation of carotenoids in plants. South Afr. J. Bot. 2021, 140, 290–302. [Google Scholar] [CrossRef]
- Sun, T.; Li, L. Toward the ‘golden’ era: The status in uncovering the regulatory control of carotenoid accumulation in plants. Plant Sci. 2020, 290, 110331. [Google Scholar] [CrossRef] [PubMed]
- Miras-Moreno, B.; Pedreño, M.Á.; Romero, L.A. Bioactivity and bioavailability of phytoene and strategies to improve its production. Phytochem. Rev. 2019, 18, 359–376. [Google Scholar] [CrossRef]
- Dautermann, O.; Lyska, D.; Andersen-Ranberg, J.; Becker, M.; Fröhlich-Nowoisky, J.; Gartmann, H.; Krämer, L.C.; Mayr, K.; Pieper, D.; Rij, L.M.; et al. An algal enzyme required for biosynthesis of the most abundant marine carotenoids. Sci. Adv. 2020, 6, eaaw9183. [Google Scholar] [CrossRef]
- Dautermann, O.; Lohr, M. A functional zeaxanthin epoxidase from red algae shedding light on the evolution of light-harvesting carotenoids and the xanthophyll cycle in photosynthetic eukaryotes. Plant J. 2017, 92, 879–891. [Google Scholar] [CrossRef]
- Gómez-García, M.; Ochoa-Alejo, N. Biochemistry and molecular biology of carotenoid biosynthesis in chili peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef] [PubMed]
- Eghbali Babadi, F.; Boonnoun, P.; Nootong, K.; Powtongsook, S.; Goto, M.; Shotipruk, A. Identification of carotenoids and chlorophylls from green algae Chlorococcum humicola and extraction by liquefied dimethyl ether. Food Bioprod. Process. 2020, 123, 296–303. [Google Scholar] [CrossRef]
- Bianchini, C.B.; Ramos-Souza, C.; Schappo, F.B.; Farina, M.; De Rosso, V.V.; Nunes, I.L. Ionic liquid and ultrasound as a fast and innovative combination for improved extraction of Chlorella sorokiniana-derived carotenoids. Algal Res. 2024, 82, 103650. [Google Scholar] [CrossRef]
- Ramos-Souza, C.; Nass, P.; Jacob-Lopes, E.; Zepka, L.Q.; Braga, A.R.C.; De Rosso, V.V. Changing Despicable Me: Potential replacement of azo dye yellow tartrazine for pequi carotenoids employing ionic liquids as high-performance extractors. Food Res. Int. 2023, 174, 113593. [Google Scholar] [CrossRef]
- Abrahamsson, V.; Rodriguez-Meizoso, I.; Turner, C. Determination of carotenoids in microalgae using supercritical fluid extraction and chromatography. J. Chromatogr. A 2012, 1250, 63–68. [Google Scholar] [CrossRef]
- Fei, T.; Wei, Y.; Xiao, L.; Lin, X.; Wang, L. Green extraction of carotenoids from canistel (Lucuma nervosa) pulps using ultrasound-assisted fatty acid-based deep eutectic solvents: Optimization and mechanism. J. Food Compos. Anal. 2024, 134, 106510. [Google Scholar] [CrossRef]
- Song, J.; Yang, Q.; Huang, W.; Xiao, Y.; Li, D.; Liu, C. Optimization of trans lutein from pumpkin (Cucurbita moschata) peel by ultrasound-assisted extraction. Food Bioprod. Process. 2018, 107, 104–112. [Google Scholar] [CrossRef]
- Singh, A.; Ahmad, S.; Ahmad, A. Green extraction methods and environmental applications of carotenoids-a review. RSC Adv. 2015, 5, 62358–62393. [Google Scholar] [CrossRef]
- Fatima, I.; Munir, M.; Qureshi, R.; Hanif, U.; Gulzar, N.; Sheikh, A.A. Advanced methods of algal pigments extraction: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 9771–9788. [Google Scholar] [CrossRef] [PubMed]
- Eltringham, W.; Catchpole, O.J. Relative permittivity measurements of gaseous, liquid, and supercritical dimethyl ether. J. Chem. Eng. Data 2007, 52, 363–367. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Afraz, M.T.; Yılmaz, B.B.; Adil, M.; Arshad, N.; Goksen, G.; Ali, M.; Zeng, X.-A. Recent progress in natural seaweed pigments: Green extraction, health-promoting activities, techno-functional properties and role in intelligent food packaging. J. Agric. Food Res. 2024, 15, 100991. [Google Scholar] [CrossRef]
- Li, Z.; Smith, K.H.; Stevens, G.W. The use of environmentally sustainable bio-derived solvents in solvent extraction applications—A review. Chin. J. Chem. Eng. 2016, 24, 215–220. [Google Scholar] [CrossRef]
- Adadi, P.; Barakova, N.V.; Krivoshapkina, E.F. Selected methods of extracting carotenoids, characterization, and health concerns: A review. J. Agric. Food Chem. 2018, 66, 5925–5947. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol. Biofuels 2019, 12, 240. [Google Scholar]
- Chiappe, C.; Pieraccini, D. Ionic liquids: Solvent properties and organic reactivity. J. Phys. Org. Chem. 2005, 18, 275–297. [Google Scholar] [CrossRef]
- Cheng, W.; Yu, Q.; Huang, H.; Hu, K.; Gao, J. Extraction of astaxanthin from Haematococcus pluvialis utilizing acidic deep eutectic solvents: A comparison with ionic liquids and organic solvents. Microchem. J. 2025, 210, 113005. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic liquids toxicity—Benefits and threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of ionic liquids in electrochemistry—Recent advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef] [PubMed]
- De Souza Mesquita, L.M.; Ventura, S.P.M.; Braga, A.R.C.; Pisani, L.P.; Dias, A.C.R.V.; De Rosso, V.V. Ionic liquid-high performance extractive approach to recover carotenoids from bactris gasipaes fruits. Green Chem. 2019, 21, 2380–2391. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of supercritical carbon dioxide in materials processing and synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical carbon dioxide applications in food processing. Food Eng. Rev. 2021, 13, 570–591. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Barreto, G.D.A.; Costa, A.S.; Costa, S.S.; Silva, R.P.D.; Da Silva, D.F.; Brandão, H.N.; Da Rocha, J.L.C.; Nunes, S.B.; Umsza-Guez, M.A.; et al. Determination of parameters for the supercritical extraction of antioxidant compounds from green propolis using carbon dioxide and ethanol as co-solvent. PLoS ONE 2015, 10, e0134489. [Google Scholar] [CrossRef]
- Cooper, D.A. Carotenoids in health and disease: Recent scientific evaluations, research recommendations and the consumer. J. Nutr. 2004, 134, 221S–224S. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; Panić, M.; Bagović, M.; Radošević, K.; Esteve, M.J.; Radojčić Redovniković, I. Green approach to extract bioactive compounds from orange peel employing hydrophilic and hydrophobic deep eutectic solvents. Sustain. Chem. Pharm. 2023, 31, 100942. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. mechanisms, techniques, combinations, protocols and applications. a review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, D.-P.; Lin, S.-J.; Li, Y.; Zheng, J.; Zhou, Y.; Zhang, J.-J.; Li, H.-B. Ultrasound-assisted extraction and identification of natural antioxidants from the fruit of Melastoma sanguineum Sims. Molecules 2017, 22, 306. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, G.E.; Kim, S. Optimization of accelerated solvent extraction of zeaxanthin from orange paprika using response surface methodology and an artificial neural network coupled with a genetic algorithm. Food Sci. Biotechnol. 2024, 33, 2521–2531. [Google Scholar] [CrossRef]
- Carabias-Martínez, R.; Rodríguez-Gonzalo, E.; Revilla-Ruiz, P.; Hernández-Méndez, J. Pressurized liquid extraction in the analysis of food and biological samples. J. Chromatogr. A 2005, 1089, 1–17. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Berman, J.; Zorrilla-López, U.; Farré, G.; Zhu, C.; Sandmann, G.; Twyman, R.M.; Capell, T.; Christou, P. Nutritionally important carotenoids as consumer products. Phytochem. Rev. 2015, 14, 727–743. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.-S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Buhrmann, C.; Samuel, S.M.; Varghese, E.; Ashrafizadeh, M.; Najafi, M.; Shakibaei, M.; Büsselberg, D.; et al. Carotenoids in cancer apoptosis—The road from bench to bedside and back. Cancers 2020, 12, 2425. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef] [PubMed]
- Gagez, A.-L.; Thiery, V.; Pasquet, V.; Cadoret, J.-P.; Picot, L. Epoxycarotenoids and cancer. Review. Curr. Bioact. Compd. 2012, 8, 109–141. [Google Scholar] [CrossRef]
- Terasaki, M.; Asai, A.; Zhang, H.; Nagao, A. A highly polar xanthophyll of 9′-cis neoxanthin induces apoptosis in HCT116 human colon cancer cells through mitochondrial dysfunction. Mol. Cell. Biochem. 2007, 300, 227. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Asai, A.; Nagao, A. Neoxanthin and fucoxanthin induce apoptosis in pc-3 human prostate cancer cells. Cancer Lett. 2005, 220, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Racha, S.; Wongrattanakamon, P.; Raiwa, A.; Jiranusornkul, S. Discovery of novel potent small natural molecules able to enhance attenuation of the pathobiology of gastric cancer-associated Helicobacter pylori by molecular modeling. Int. J. Pept. Res. Ther. 2019, 25, 881–896. [Google Scholar] [CrossRef]
- Saini, R.K.; Moon, S.H.; Gansukh, E.; Keum, Y.-S. An efficient one-step scheme for the purification of major xanthophyll carotenoids from lettuce, and assessment of their comparative anticancer potential. Food Chem. 2018, 266, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Murata-Kamiya, N.; Kikuchi, K.; Hayashi, T.; Higashi, H.; Hatakeyama, M. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host Microbe 2010, 7, 399–411. [Google Scholar] [CrossRef]
- Gan, Y.; Li, X.; Han, S.; Liang, Q.; Ma, X.; Rong, P.; Wang, W.; Li, W. The cGAS/STING pathway: A novel target for cancer therapy. Front. Immunol. 2022, 12, 795401. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Miyashita, K.; Nagao, A.; Kushiro, M.; Zhang, H.; Sugawara, T. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Mareel, M.; Leroy, A. Clinical, cellular, and molecular aspects of cancer invasion. Physiol. Rev. 2003, 83, 337–376. [Google Scholar] [CrossRef]
- Martin, L. Fucoxanthin and its metabolite fucoxanthinol in cancer prevention and treatment. Mar. Drugs 2015, 13, 4784–4798. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar]
- Pérez-Gálvez, A.; Sánchez-García, A.; Garrido-Fernández, J.; Ríos, J.J. MS tools for a systematic approach in survey for carotenoids and their common metabolites. Arch. Biochem. Biophys. 2018, 650, 85–92. [Google Scholar] [CrossRef]
- Srivastava, R. Physicochemical, antioxidant properties of carotenoids and its optoelectronic and interaction studies with chlorophyll pigments. Sci. Rep. 2021, 11, 18365. [Google Scholar] [CrossRef]
- Sahin, S.; Aybastıer, Ö.; Dawbaa, S.; Karkar, B.; Cakmak, T. Study of the ability of lutein and neoxanthin as standards and in the extract of Chlamydomonas reinhardtii to prevent oxidatively induced dna base damage using ultrasensitive GC–MS/MS analysis. Chromatographia 2020, 83, 919–926. [Google Scholar] [CrossRef]
- Udayawara Rudresh, D.; Maradagi, T.; Stephen, N.M.; Niraikulam, A.; Nambi Ramudu, K.; Ponesakki, G. Neoxanthin prevents H2O2-induced cytotoxicity in HepG2 cells by activating endogenous anti-oxidant signals and suppressing apoptosis signals. Mol. Biol. Rep. 2021, 48, 6923–6934. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Udomsak, W.; Kucinska, M.; Pospieszna, J.; Dams-Kozlowska, H.; Chatuphonprasert, W.; Murias, M. Antioxidant enzymes in cancer cells: Their role in photodynamic therapy resistance and potential as targets for improved treatment outcomes. Int. J. Mol. Sci. 2024, 25, 3164. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, U.T.; Yusof, Z.N.B. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef]
- Shivarudrappa, A.H.; Ponesakki, G. Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells. J. Cell Commun. Signal. 2020, 14, 207–221. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Slater, A.F.G.; Stefan, C.; Nobel, I.; Van Den Dobbelsteen, D.J.; Orrenius, S. Signalling mechanisms and oxidative stress in apoptosis. Toxicol. Lett. 1995, 82, 149–153. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Rahman, T.; Hosen, I.; Islam, M.M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 03, 997–1019. [Google Scholar] [CrossRef]
- Williams, E.P.; Mesidor, M.; Winters, K.; Dubbert, P.M.; Wyatt, S.B. Overweight and obesity: Prevalence, consequences, and causes of a growing public health problem. Curr. Obes. Rep. 2015, 4, 363–370. [Google Scholar] [CrossRef]
- James, W.P.T. The epidemiology of obesity: The size of the problem. J. Intern. Med. 2008, 263, 336–352. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.; D’Orazio, N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Mounien, L.; Tourniaire, F.; Landrier, J.-F. Anti-obesity effect of carotenoids: Direct impact on adipose tissue and adipose tissue-driven indirect effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef]
- Okada, T.; Nakai, M.; Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Suppressive effect of neoxanthin on the differentiation of 3T3-L1 adipose cells. J. Oleo Sci. 2008, 57, 345–351. [Google Scholar] [CrossRef]
- Hu, X.; Tao, N.; Wang, X.; Xiao, J.; Wang, M. Marine-derived bioactive compounds with anti-obesity effect: A review. J. Funct. Foods 2016, 21, 372–387. [Google Scholar] [CrossRef]
- Mikami, K.; Hosokawa, M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int. J. Mol. Sci. 2013, 14, 13763–13781. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. Evid. Based Complement. Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Y.; Yin, W.; Zhang, L.; Li, G.; Ma, J.; Xu, L.; Xiong, Y.; Liu, L.; Zhang, W.; et al. Neoxanthin alleviates the chronic renal failure-induced aging and fibrosis by regulating inflammatory process. Int. Immunopharmacol. 2023, 114, 109429. [Google Scholar] [CrossRef]
- Molnár, P.; Deli, J.; Tanaka, T.; Kann, Y.; Tani, S.; Gyémánt, N.; Molnár, J.; Kawase, M. Carotenoids with anti-Helicobacter pylori activity from golden delicious apple. Phytother. Res. 2010, 24, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Yonekura, L.; Nagao, A. Low bioavailability of dietary epoxyxanthophylls in humans. Br. J. Nutr. 2008, 100, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Terasaki, M.; Nagao, A. An epoxide–furanoid rearrangement of spinach neoxanthin occurs in the gastrointestinal tract of mice and in vitro: Formation and cytostatic activity of neochrome stereoisomers. J. Nutr. 2004, 134, 2237–2243. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Cui, Y.; Sun, F.; Zhang, H.; Fan, J.; Ge, B.; Cao, Y.; Wang, X.; Zhu, X.; Wei, Z.; et al. Metabolic engineering and synthetic biology strategies for producing high-value natural pigments in microalgae. Biotechnol. Adv. 2023, 68, 108236. [Google Scholar] [CrossRef]
- Ahmad, S.; Iqbal, K.; Kothari, R.; Singh, H.M.; Sari, A.; Tyagi, V.V. A critical overview of upstream cultivation and downstream processing of algae-based biofuels: Opportunity, technological barriers and future perspective. J. Biotechnol. 2022, 351, 74–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef]
- Chen, F.; Li, H.B.; Wong, R.N.S.; Ji, B.; Jiang, Y. Isolation and purification of the bioactive carotenoid zeaxanthin from the microalga Microcystis aeruginosa by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1064, 183–186. [Google Scholar] [CrossRef]
- Li, H.B.; Fan, K.W.; Chen, F. Isolation and purification of canthaxanthin from the microalga Chlorella zofingiensis by high-speed counter-current chromatography. J. Sep. Sci. 2006, 29, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.T.; Chen, P.Y.; Wu, J.J.; Lee, T.M.; Hsu, S.L.; Chang, C.M.J.; Young, C.C.; Shieh, C.-J. Purification of algal anti-tyrosinase zeaxanthin from Nannochloropsis oculata using supercritical anti-solvent precipitation. J. Supercrit. Fluids 2011, 55, 955–962. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Yang, M.; Xuan, Z.; Wang, Q.; Yan, S.; Zhou, D.; Naman, C.B.; Zhang, J.; He, S.; Yan, X.; Cui, W. Fucoxanthin has potential for therapeutic efficacy in neurodegenerative disorders by acting on multiple targets. Nutr. Neurosci. 2022, 25, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
| Extraction Methods | Plant Species | Extraction Solvents | Extraction Conditions | Neoxanthin Yield | Reference |
|---|---|---|---|---|---|
| Organic solvents extraction | Chlorococcum humicola | Dimethyl ether | 45:1 Solid/liquid ratio (w/w), 41 °C, for 20 min | 2.55 mg/g | [53] |
| Caryocar Brasiliense | Acetone | 3 mL/g Solid/liquid ratio, for 300 s, three times extraction | 0.70 μg/g | [55] | |
| Scenedesmus obliquus | Ethanol | 10 mL/g Solid/liquid ratio, for 3 min, three times extraction | 180.33 μg/g | [42] | |
| Brazil Pouteria | Acetone | 40–60 °C | 193 μg/g | [55] | |
| Ionic liquids extraction | Caryocar Brasiliense | 1:3 (1-hexyl-3-methylimidazolium chlorid): ethanol (v/v) | 3 mL/g Solid/liquid ratio, for 300 s, three times extraction | 1.88 μg/g | [55] |
| Chlorella sorokiniana | 1:4 (1-hexyl-3-methylimidazolium chloride): ethanol (v/v) | 10 mL/g Solid/liquid ratio, for 7.5 min, two times extraction | 0.03 mg/g | [54] | |
| Scenedesmus obliquus | 1:3 (1-butyl-3-methylimidazolium tetrafluoroborate): ethanol (v/v) | 10 mL/g Solid/liquid ratio, for 3 min, three times extraction | 48.71 μg/g | [42] | |
| Scenedesmus obliquus | 1:3 (1-butyl-3-methylimidazolium tetrafluoroborate): ethanol (v/v) | 10 mL/g Solid/liquid ratio, for 3 min, three times extraction | 122.66 μg/g | [42] | |
| Supercritical liquid extraction | Scenedesmus sp. | 9:1 CO2: Ethanol (v/v) | Pressure 300 bar, 60 °C, and CO2 flow rate 2 mL/min, for 60 min | 670.8 μg/g | [56] |
| Ultrasound-assisted extraction | Cucurbita moschata | 2:1 Ethanol: Petroleum (v/v) | 31 mL/g solid/liquid ratio, 203 W, for 30 min | 36.69 μg/g | [58] |
| Chlorella vulgaris | Ethanol | 30 mL/g Solid/liquid ratio, amplitude wave of 20%, 25 °C, for 25 min | 9.83 mg/g | [11] | |
| Pressurized liquid extraction | Chlorella vulgaris | Ethanol | Pressure 1500 psi, 50 °C, for 20 min | 11.35 mg/g | [11] |
| No. | Type of Cancer | Cell Line | Target | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| 1 | Colon cancer | HCT116 | mitochondrial function | Caspase-independent apoptotic pathway via loss of mitochondrial transmembrane potential, involving apoptosis-inducing factor (AIF), cytochrome-C, and endonuclease G (EndoG) | [93] |
| 2 | Prostate cancer | PC-3 | Caspase-3 | Caspase-3-dependent apoptosis | [94] |
| 3 | Gastric cancer | molecular docking | Cytotoxin-associated gene A (Cag-A) | Inhibits the binding of Cag-A of Helicobacter pylori to phosphatidylserine on host cell membranes | [95] |
| 4 | Lung cancer | A549 | Caspase-3 | Induce apoptosis through caspase-3 activation; increase ROS clearance and repair activity with IC50 (mg/L) = 7.5 ± 0.6 μM | [96] |
| 5 | Cervical cancer | HeLa | Caspase-3 | Modulation of the activity of various transcription factors and responsive elements; Inhibition of the clonal expansion of initiated cells through enhanced gap junctional communication; Immunomodulatory effects by enhancing tumor immunity with IC50 (mg/L) = 3.8 ± 0.2 μM | [96] |
| No. | Mechanism of Action | Experimental Data Results | Reference |
|---|---|---|---|
| 1 | Activating endogenous anti-oxidant signaling pathways | At 4 h exposure to 0.5 mM H2O2, the cell viability in the neoxanthin pretreatment groups (0.05 μM and 0.1 μM) is significantly greater compared to the group subjected solely to H2O2 treatment | [108] |
| 2 | Directly removes reactive oxygen species (ROS) produced within cells | Treatment of HepG2 cells with H2O2 resulted in a 38% increase in reactive oxygen species (ROS) levels compared to the control group. Pretreatment with neoxanthin at concentrations of 0.05 μM and 0.1 μM resulted in a 12% and 24% reduction in ROS production | [108] |
| 3 | Upregulates the expression of intracellular anti-oxidant enzymes (HO-1 and SOD-2) | H2O2 treatment significantly diminishes the expression of HO-1 and SOD-2, neoxanthin increases the expression of HO-1 by 17% and 22%, and SOD-2 by 21% and 35% at concentrations of 0.05 μM and 0.1 μM | [108] |
| 4 | Regulates transcription factor (Nrf2/ARE) expression | Treatment with H2O2 significantly suppresses Nrf2 expression, whereas pretreatment with neoxanthin at 0.1 μM markedly restores Nrf2 expression by up to 42% | [108] |
| 5 | Inhibits the apoptotic signaling pathway | Treatment with H2O2 leading to a significant increase (60%) in the expression of the pro-apoptotic protein Bax and a concurrent decrease (50%) in the expression of the anti-apoptotic protein Bcl-2. Treatment with neoxanthin at 0.1 μM demonstrates the most pronounced reversal effect, restoring approximately 51% and 44% of the expression levels of Bax and Bcl-2 | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhuang, G.; Zhang, J. Neoxanthin: A Promising Medicinal and Nutritional Carotenoid. Mar. Drugs 2025, 23, 317. https://doi.org/10.3390/md23080317
Zhao J, Zhuang G, Zhang J. Neoxanthin: A Promising Medicinal and Nutritional Carotenoid. Marine Drugs. 2025; 23(8):317. https://doi.org/10.3390/md23080317
Chicago/Turabian StyleZhao, Jiarong, Gengjie Zhuang, and Jinrong Zhang. 2025. "Neoxanthin: A Promising Medicinal and Nutritional Carotenoid" Marine Drugs 23, no. 8: 317. https://doi.org/10.3390/md23080317
APA StyleZhao, J., Zhuang, G., & Zhang, J. (2025). Neoxanthin: A Promising Medicinal and Nutritional Carotenoid. Marine Drugs, 23(8), 317. https://doi.org/10.3390/md23080317






