Multiblock Metabolomics Responses of the Diatom Phaeodactylum tricornutum Under Benthic and Planktonic Culture Conditions
Abstract
1. Introduction
2. Results
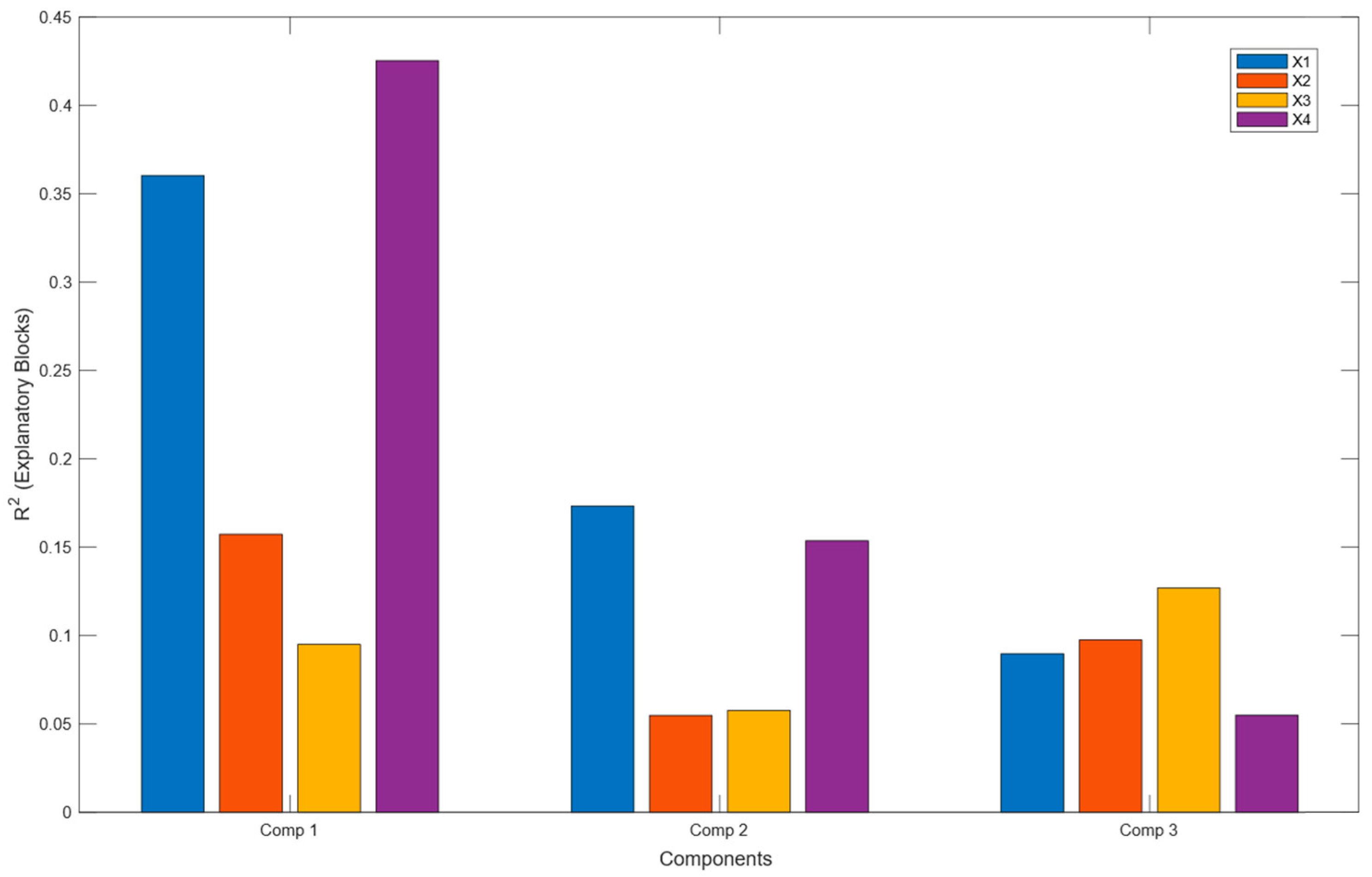
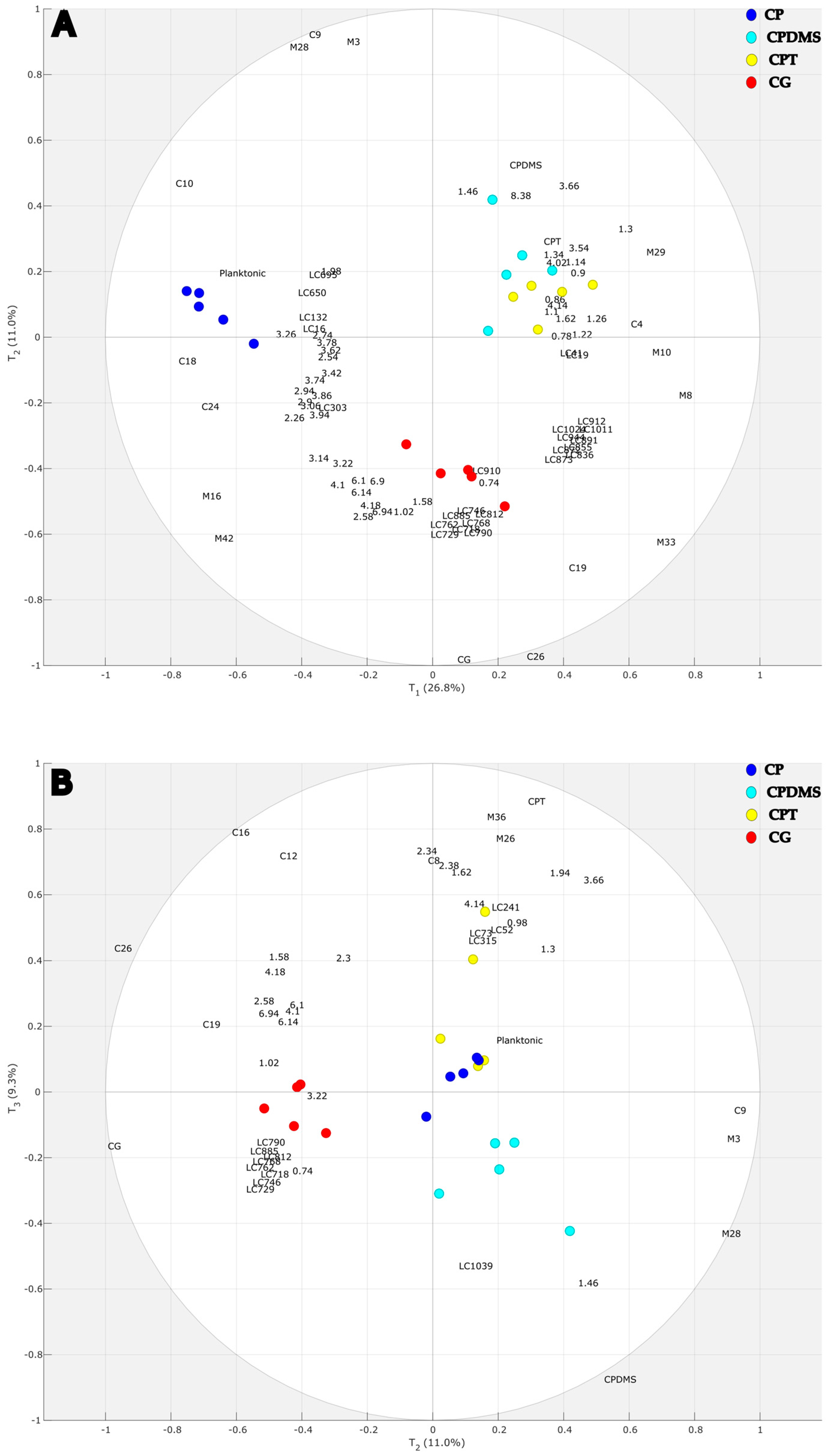
2.1. Multivariate Statistical Analyses of LC-HRMS2, GC-MS(CHCl3), GC-MS(MeOH), and 1H NMR Data
2.2. Discriminant Metabolites from X1 Matrix (LC-HRMS2) in the Multiblock Model
2.3. Analyses of Matrices X2 GC-MS(CHCl3) and X3 GC-MS(MeOH) in the Multiblock Model
2.4. Discriminant Metabolites from the X4 Matrix 1H-NMR in the Multiblock Model
3. Discussion
4. Materials and Methods
4.1. Microalgae Culture
4.2. Metabolomics
4.2.1. Analytical Methods and Data Processing
Mass Spectrometry and Metabolite Annotations
GC–MS Analyses and Metabolite Annotations
NMR Analyses and Spectral Attributions
4.2.2. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serôdio, J.; Lavaud, J. Diatoms and Their Ecological Importance. In Life Below Water; Leal Filho, W., Azul, A.M., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Encyclopedia of the UN Sustainable Development Goals; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–9. ISBN 978-3-319-71064-8. [Google Scholar]
- Nieri, P.; Carpi, S.; Esposito, R.; Costantini, M.; Zupo, V. Bioactive Molecules from Marine Diatoms and Their Value for the Nutraceutical Industry. Nutrients 2023, 15, 464. [Google Scholar] [CrossRef]
- Robinette, S.L.; Brüschweiler, R.; Schroeder, F.C.; Edison, A.S. NMR in Metabolomics and Natural Products Research: Two Sides of the Same Coin. Acc. Chem. Res. 2012, 45, 288–297. [Google Scholar] [CrossRef]
- Alipanah, L.; Rohloff, J.; Winge, P.; Bones, A.M.; Brembu, T. Whole-Cell Response to Nitrogen Deprivation in the Diatom Phaeodactylum tricornutum. J. Exp. Bot. 2015, 66, 6281–6296. [Google Scholar] [CrossRef]
- Scarsini, M.; Thiriet-Rupert, S.; Veidl, B.; Mondeguer, F.; Hu, H.; Marchand, J.; Schoefs, B. The Transition Toward Nitrogen Deprivation in Diatoms Requires Chloroplast Stand-By and Deep Metabolic Reshuffling. Front. Plant Sci. 2022, 12, 760516. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.-Y.; Yang, Z.-K.; Zheng, J.-W.; Xie, Y.; Li, D.-W.; Murugan, S.B.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Examination of Metabolic Responses to Phosphorus Limitation via Proteomic Analyses in the Marine Diatom Phaeodactylum tricornutum. Sci. Rep. 2015, 5, 10373. [Google Scholar] [CrossRef] [PubMed]
- Remmers, I.M.; D’Adamo, S.; Martens, D.E.; De Vos, R.C.H.; Mumm, R.; America, A.H.P.; Cordewener, J.H.G.; Bakker, L.V.; Peters, S.A.; Wijffels, R.H.; et al. Orchestration of Transcriptome, Proteome and Metabolome in the Diatom Phaeodactylum tricornutum during Nitrogen Limitation. Algal Res. 2018, 35, 33–49. [Google Scholar] [CrossRef]
- Russo, M.T.; Rogato, A.; Jaubert, M.; Karas, B.J.; Falciatore, A. Phaeodactylum tricornutum: An Established Model Species for Diatom Molecular Research and an Emerging Chassis for Algal Synthetic Biology. J. Phycol. 2023, 59, 1114–1122. [Google Scholar] [CrossRef]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum: A Diatom Cell Factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The Phaeodactylum Genome Reveals the Evolutionary History of Diatom Genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef]
- Filloramo, G.V.; Curtis, B.A.; Blanche, E.; Archibald, J.M. Re-Examination of Two Diatom Reference Genomes Using Long-Read Sequencing. BMC Genom. 2021, 22, 379. [Google Scholar] [CrossRef]
- Satasiya, G.; Kumar, M.A.; Ray, S. Biofouling Dynamics and Antifouling Innovations: Transitioning from Traditional Biocides to Nanotechnological Interventions. Environ. Res. 2025, 269, 120943. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Chen, S.; Liu, X.; Yu, Y.; Li, T.; Wan, Y.; Tang, N.; Liu, Y.; Gu, Y. Bioinspired Marine Antifouling Coatings: Antifouling Mechanisms, Design Strategies and Application Feasibility Studies. Eur. Polym. J. 2023, 190, 111997. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, Y.; Guo, Y.; Zhang, S.; Xiong, G.; Wang, K.; Zhang, Z. Anti-Marine Biofouling Adhesion Performance and Mechanism of PDMS Fouling-Release Coating Containing PS-PEG hydrogel. Mar. Pollut. Bull. 2023, 194, 115345. [Google Scholar] [CrossRef]
- Laviale, M.; Beaussart, A.; Allen, J.; Quilès, F.; El-Kirat-Chatel, S. Probing the Adhesion of the Common Freshwater Diatom Nitzschia palea at Nanoscale. ACS Appl. Mater. Interfaces 2019, 11, 48574–48582. [Google Scholar] [CrossRef]
- Lachnit, M.; Buhmann, M.T.; Klemm, J.; Kröger, N.; Poulsen, N. Identification of Proteins in the Adhesive Trails of the Diatom Amphora coffeaeformis. Phil. Trans. R. Soc. B 2019, 374, 20190196. [Google Scholar] [CrossRef]
- Molino, P.J.; Wetherbee, R. The Biology of Biofouling Diatoms and Their Role in the Development of Microbial Slimes. Biofouling 2008, 24, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Landoulsi, J.; Cooksey, K.E.; Dupres, V. Review—Interactions between Diatoms and Stainless Steel: Focus on Biofouling and Biocorrosion. Biofouling 2011, 27, 1105–1124. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, J.; Schuhmann, W.; Rosenhahn, A. Control of Marine Bacteria and Diatom Biofouling by Constant and Alternating Potentials. Langmuir 2021, 37, 7464–7472. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.; Melo, T.; Lopes, D.; Couto, D.; Marques, F.; Domingues, M.R. Applications of Lipidomics in Marine Organisms: Progress, Challenges and Future Perspectives. Mol. Omics 2022, 18, 357–386. [Google Scholar] [CrossRef]
- Nuzzo, G.; Gallo, C.; D’Ippolito, G.; Cutignano, A.; Sardo, A.; Fontana, A. Composition and Quantitation of Microalgal Lipids by ERETIC 1H NMR Method. Mar. Drugs 2013, 11, 3742–3753. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.; Tsiafoulis, C.; Gerothanassis, I. High Resolution NMR Spectroscopy as a Structural and Analytical Tool for Unsaturated Lipids in Solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef]
- Murison, V.; Hérault, J.; Côme, M.; Guinio, S.; Lebon, A.; Chamot, C.; Bénard, M.; Galas, L.; Schoefs, B.; Marchand, J.; et al. Comparison of Two Phaeodactylum tricornutum Ecotypes under Nitrogen Starvation and Resupply Reveals Distinct Lipid Accumulation Strategies but a Common Degradation Process. Front. Plant Sci. 2023, 14, 1257500. [Google Scholar] [CrossRef]
- Lupette, J.; Tardif, M.; Brugière, S.; Couté, Y.; Salvaing, J.; Maréchal, E. Quantitative Proteomic Analyses Reveal the Impact of Nitrogen Starvation on the Proteome of the Model Diatom Phaeodactylum tricornutum. Proteomics 2022, 22, 2200155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Gu, W.; Huang, A.; Wu, S.; Liu, C.; Huan, L.; Gao, S.; Xie, X.; Wang, G. Effect of Iron on the Growth of Phaeodactylum tricornutum via Photosynthesis. J. Phycol. 2018, 54, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Chuberre, C.; Chan, P.; Walet-Balieu, M.-L.; Thiébert, F.; Burel, C.; Hardouin, J.; Gügi, B.; Bardor, M. Comparative Proteomic Analysis of the Diatom Phaeodactylum tricornutum Reveals New Insights Into Intra- and Extra-Cellular Protein Contents of Its Oval, Fusiform, and Triradiate Morphotypes. Front. Plant Sci. 2022, 13, 673113. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.; Gao, Z.; Cui, Y.; Yao, Q.; Qin, J.; Liu, X.; Lin, S. Transcriptomics and Physiological Analyses Unveil the Distinct Mechanisms of ATP and Glucose-6-Phosphate Utilization in Phaeodactylum tricornutum. Front. Mar. Sci. 2023, 10, 1163189. [Google Scholar] [CrossRef]
- Rastogi, A.; Maheswari, U.; Dorrell, R.G.; Vieira, F.R.J.; Maumus, F.; Kustka, A.; McCarthy, J.; Allen, A.E.; Kersey, P.; Bowler, C.; et al. Integrative Analysis of Large Scale Transcriptome Data Draws a Comprehensive Landscape of Phaeodactylum tricornutum Genome and Evolutionary Origin of Diatoms. Sci. Rep. 2018, 8, 4834. [Google Scholar] [CrossRef] [PubMed]
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Santos, M.; Freitas, M.; Mata, T.; Martins, A.; Caetano, N. Potential of Phaeodactylum tricornutum for Biodiesel Production under Natural Conditions in Chile. Energies 2017, 11, 54. [Google Scholar] [CrossRef]
- Prestegard, S.; Erga, S.; Steinrücken, P.; Mjøs, S.; Knutsen, G.; Rohloff, J. Specific Metabolites in a Phaeodactylum tricornutum Strain Isolated from Western Norwegian Fjord Water. Mar. Drugs 2015, 14, 9. [Google Scholar] [CrossRef]
- Coffey, N.R.; Dewey, C.; Manning, K.; Corilo, Y.; Kew, W.; Babcock-Adams, L.; McKenna, A.M.; Stuart, R.K.; Boiteau, R.M. Annotation of DOM Metabolomes with an Ultrahigh Resolution Mass Spectrometry Molecular Formula Library. Org. Geochem. 2024, 197, 104880. [Google Scholar] [CrossRef]
- Popko, J.; Herrfurth, C.; Feussner, K.; Ischebeck, T.; Iven, T.; Haslam, R.; Hamilton, M.; Sayanova, O.; Napier, J.; Khozin-Goldberg, I.; et al. Metabolome Analysis Reveals Betaine Lipids as Major Source for Triglyceride Formation, and the Accumulation of Sedoheptulose during Nitrogen-Starvation of Phaeodactylum tricornutum. PLoS ONE 2016, 11, e0164673. [Google Scholar] [CrossRef]
- Olofsson, M.; Ferrer-González, F.X.; Uchimiya, M.; Schreier, J.E.; Holderman, N.R.; Smith, C.B.; Edison, A.S.; Moran, M.A. Growth-Stage-Related Shifts in Diatom Endometabolome Composition Set the Stage for Bacterial Heterotrophy. ISME Commun. 2022, 2, 28. [Google Scholar] [CrossRef]
- Alothman, A.; Emwas, A.-H.; Singh, U.; Jaremko, M.; Agusti, S. Metabolomics-Based Analysis of the Diatom Cheatoceros tenuissimus Combining NMR and GC–MS Techniques. MethodsX 2024, 12, 102695. [Google Scholar] [CrossRef]
- Huang, B.; Marchand, J.; Thiriet-Rupert, S.; Carrier, G.; Saint-Jean, B.; Lukomska, E.; Moreau, B.; Morant-Manceau, A.; Bougaran, G.; Mimouni, V. Betaine Lipid and Neutral Lipid Production under Nitrogen or Phosphorus Limitation in the Marine Microalga Tisochrysis lutea (Haptophyta). Algal Res. 2019, 40, 101506. [Google Scholar] [CrossRef]
- Hoffmann, D.Y.; Shachar-Hill, Y. Do Betaine Lipids Replace Phosphatidylcholine as Fatty Acid Editing Hubs in Microalgae? Front. Plant Sci. 2023, 14, 1077347. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Cuiné, S.; Légeret, B.; Wijffels, R.H.; Hulatt, C.J.; Li-Beisson, Y.; Kiron, V. Phosphorus Starvation Induces the Synthesis of Novel Lipid Class Diacylglyceryl Glucuronide and Diacylglyceryl- N,N,N -trimethylhomoserine in Two Species of Cold-adapted Microalgae Raphidonema (Chlorophyta). Plant J. 2025, 121, e17227. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Yamada, M. Biosynthesis of Polyunsaturated Fatty Acids in the Marine Diatom, Phaeodactylum tricornutum. Phytochemistry 1994, 35, 1177–1181. [Google Scholar] [CrossRef]
- Gaubert-Boussarie, J.; Prado, S.; Hubas, C. An Untargeted Metabolomic Approach for Microphytobenthic Biofilms in Intertidal Mudflats. Front. Mar. Sci. 2020, 7, 250. [Google Scholar] [CrossRef]
- Dong, Y.; Li, S.; Zhao, D.; Liu, J.; Ma, S.; Geng, J.; Lu, C.; Liu, Y. IolR, a Negative Regulator of the Myo-Inositol Metabolic Pathway, Inhibits Cell Autoaggregation and Biofilm Formation by Downregulating RpmA in Aeromonas Hydrophila. NPJ Biofilms Microbiomes 2020, 6, 22. [Google Scholar] [CrossRef]
- Pierre, G.; Graber, M.; Rafiliposon, B.A.; Dupuy, C.; Orvain, F.; De Crignis, M.; Maugard, T. Biochemical Composition and Changes of Extracellular Polysaccharides (ECPS) Produced during Microphytobenthic Biofilm Development (Marennes-Oléron, France). Microb. Ecol. 2012, 63, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Nikitashina, V.; Stettin, D.; Pohnert, G. Metabolic Adaptation of Diatoms to Hypersalinity. Phytochemistry 2022, 201, 113267. [Google Scholar] [CrossRef]
- Chiovitti, A.; Molino, P.; Crawford, S.A.; Teng, R.; Spurck, T.; Wetherbee, R. The Glucans Extracted with Warm Water from Diatoms Are Mainly Derived from Intracellular Chrysolaminaran and Not Extracellular Polysaccharides. Eur. J. Phycol. 2004, 39, 117–128. [Google Scholar] [CrossRef]
- Hubas, C.; Monti, D.; Mortillaro, J.-M.; Augagneur, S.; Carbon, A.; Duran, R.; Karama, S.; Meziane, T.; Pardon, P.; Risser, T.; et al. Chlordecone-Contaminated Epilithic Biofilms Show Increased Adsorption Capacities. Sci. Total Environ. 2022, 825, 153942. [Google Scholar] [CrossRef]
- Torstensson, A.; Young, J.N.; Carlson, L.T.; Ingalls, A.E.; Deming, J.W. Use of Exogenous Glycine Betaine and Its Precursor Choline as Osmoprotectants in Antarctic Sea-ice Diatoms 1. J. Phycol. 2019, 55, 663–675. [Google Scholar] [CrossRef]
- Iglesias, M.J.; Soengas, R.; Probert, I.; Guilloud, E.; Gourvil, P.; Mehiri, M.; López, Y.; Cepas, V.; Gutiérrez-del-Río, I.; Redondo-Blanco, S.; et al. NMR Characterization and Evaluation of Antibacterial and Antiobiofilm Activity of Organic Extracts from Stationary Phase Batch Cultures of Five Marine Microalgae (Dunaliella sp., D. salina, Chaetoceros calcitrans, C. gracilis and Tisochrysis lutea). Phytochemistry 2019, 164, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Nader, C.; Cella, H.; Lopes, R.G.; Oliveira, C.Y.B.; D’Alessadro, E.B.; Filho, N.R.A.; Derner, R.B. Effect of different cultivation conditions on the production of volatile organic compounds by the microalgae Arthrospira platensis and Chlorella sp. J. Appl. Phycol. 2024, 34, 203–217. [Google Scholar] [CrossRef]
- Stonik, V.; Stonik, I. Low-Molecular-Weight Metabolites from Diatoms: Structures, Biological Roles and Biosynthesis. Mar. Drugs 2015, 13, 3672–3709. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A Review of Biomedical Activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Santana-Casiano, J.M.; González-Dávila, M.; González, A.G.; Rico, M.; López, A.; Martel, A. Characterization of Phenolic Exudates from Phaeodactylum tricornutum and Their Effects on the Chemistry of Fe(II)–Fe(III). Mar. Chem. 2014, 158, 10–16. [Google Scholar] [CrossRef]
- Uzlasir, T.; Selli, S.; Kelebek, H. Effect of Salt Stress on the Phenolic Compounds, Antioxidant Capacity, Microbial Load, and In Vitro Bioaccessibility of Two Microalgae Species (Phaeodactylum tricornutum and Spirulina platensis). Foods 2023, 12, 3185. [Google Scholar] [CrossRef]
- Van Tol, H.M.; Armbrust, E.V. Genome-Scale Metabolic Model of the Diatom Thalassiosira pseudonana Highlights the Importance of Nitrogen and Sulfur Metabolism in Redox Balance. PLoS ONE 2021, 16, e0241960. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Hu, F.; Yu, C.; Li, C.; Huang, T.; Hu, H. Amino Acid Catabolism During Nitrogen Limitation in Phaeodactylum tricornutum. Front. Plant Sci. 2020, 11, 589026. [Google Scholar] [CrossRef] [PubMed]
- Cointet, E.; Séverin, E.; Couzinet-Mossion, A.; Méléder, V.; Gonçalves, O.; Wielgosz-Collin, G. Assessment of the Lipid Production Potential of Six Benthic Diatom Species Grown in Airlift Photobioreactors. J. Appl. Phycol. 2021, 33, 2093–2103. [Google Scholar] [CrossRef]
- Lippold, F.; Vom Dorp, K.; Abraham, M.; Hölzl, G.; Wewer, V.; Yilmaz, J.L.; Lager, I.; Montandon, C.; Besagni, C.; Kessler, F.; et al. Fatty Acid Phytyl Ester Synthesis in Chloroplasts of Arabidopsis. Plant Cell 2012, 24, 2001–2014. [Google Scholar] [CrossRef]
- Castro-Ferreira, C.; Gomes-Dias, J.S.; Ferreira-Santos, P.; Pereira, R.N.; Vicente, A.A.; Rocha, C.M.R. Phaeodactylum tricornutum Extracts as Structuring Agents for Food Applications: Physicochemical and Functional Properties. Food Hydrocoll. 2022, 124, 107276. [Google Scholar] [CrossRef]
- Castaldi, A.; Truong, B.N.; Vu, Q.T.; Le, T.H.M.; Marie, A.; Le Pennec, G.; Rouvier, F.; Brunel, J.-M.; Longeon, A.; Pham, V.C.; et al. Computational Methods Reveal a Series of Cyclic and Linear Lichenysins and Surfactins from the Vietnamese Marine Sediment-Derived Streptomyces Strain G222. Molecules 2024, 29, 1458. [Google Scholar] [CrossRef]
- Rutz, A.; Dounoue-Kubo, M.; Ollivier, S.; Bisson, J.; Bagheri, M.; Saesong, T.; Ebrahimi, S.N.; Ingkaninan, K.; Wolfender, J.-L.; Allard, P.-M. Taxonomically Informed Scoring Enhances Confidence in Natural Products Annotation. Front. Plant Sci. 2019, 10, 1329. [Google Scholar] [CrossRef]
- Allard, P.-M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.-L. Integration of Molecular Networking and In-Silico MS/MS Fragmentation for Natural Products Dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef]
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS Initiative for Open Knowledge Management in Natural Products Research. eLife 2022, 11, e70780. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Van Santen, J.A.; Jacob, G.; Singh, A.L.; Aniebok, V.; Balunas, M.J.; Bunsko, D.; Neto, F.C.; Castaño-Espriu, L.; Chang, C.; Clark, T.N.; et al. The Natural Products Atlas: An Open Access Knowledge Base for Microbial Natural Products Discovery. ACS Cent. Sci. 2019, 5, 1824–1833. [Google Scholar] [CrossRef]
- Ono, K.; Demchak, B.; Ideker, T. Cytoscape Tools for the Web Age: D3.Js and Cytoscape.Js Exporters. F1000Research 2014, 3, 143. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Beniddir, M.A.; Le Moyec, L.; Triba, M.N.; Longeon, A.; Deville, A.; Blond, A.; Pham, V.C.; De Voogd, N.J.; Bourguet-Kondracki, M.-L. Metabolomics with Multi-Block Modelling of Mass Spectrometry and Nuclear Magnetic Resonance in Order to Discriminate Haplosclerida Marine Sponges. Anal. Bioanal. Chem. 2022, 414, 5929–5942. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Hoch, J.C.; Baskaran, K.; Burr, H.; Chin, J.; Eghbalnia, H.R.; Fujiwara, T.; Gryk, M.R.; Iwata, T.; Kojima, C.; Kurisu, G.; et al. Biological Magnetic Resonance Data Bank. Nucleic Acids Res. 2023, 51, D368–D376. [Google Scholar] [CrossRef]
- Azizan, A.; Ahamad Bustamam, M.S.; Maulidiani, M.; Shaari, K.; Ismail, I.S.; Nagao, N.; Abas, F. Metabolite Profiling of the Microalgal Diatom Chaetoceros calcitrans and Correlation with Antioxidant and Nitric Oxide Inhibitory Activities via 1H NMR-Based Metabolomics. Mar. Drugs 2018, 16, 154. [Google Scholar] [CrossRef]
- Pantami, H.A.; Shaari, K.; Bustamam, M.S.A.; Ismail, I.S. Metabolite Profiling of Different Solvent Extracts of the Microalgae Chlorella vulgaris Via 1H NMR-Based Metabolomics. Curr. Metabolomics Syst. Biol. 2021, 8, 61–74. [Google Scholar] [CrossRef]
- Bisht, B.; Kumar, V.; Gururani, P.; Tomar, M.S.; Nanda, M.; Vlaskin, M.S.; Kumar, S.; Kurbatova, A. The Potential of Nuclear Magnetic Resonance (NMR) in Metabolomics and Lipidomics of Microalgae—A Review. Arch. Biochem. Biophys. 2021, 710, 108987. [Google Scholar] [CrossRef]
- Caprara, C.D.S.C.; Mathias, T.K.; Santos, M.D.F.C.; D’Oca, M.G.M.; D’Oca, C.D.R.M.; Roselet, F.; Abreu, P.C.; Ramos, D.F. Application of 1H HR-MAS NMR-Based Metabolite Fingerprinting of Marine Microalgae. Metabolites 2023, 13, 202. [Google Scholar] [CrossRef]
- Noonan, M.J.; Tinnesand, H.V.; Buesching, C.D. Normalizing Gas-Chromatography–Mass Spectrometry Data: Method Choice Can Alter Biological Inference. BioEssays 2018, 40, 1700210. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, J.A.; Kourti, T.; MacGregor, J.F. Analysis of Multiblock and Hierarchical PCA and PLS Models. J. Chemom. 1998, 12, 301–321. [Google Scholar] [CrossRef]
| Comp. | RT | m/z (Adduct) | [Adduct], Compound’s Molecular Formula | Compound’s Name | Superclass/Natural Product Class | Err. ppm | Sirius Score | T1: Increased in |
|---|---|---|---|---|---|---|---|---|
| LC16 | 9.395 | 111.0437 | [M + H]+, C6H6O2 | Catechol (a) (c) | Organic oxygen compounds/ Phenolic acids (C6-C1) | −1.3 | 100.00% | CP |
| LC19 | 3.619 | 115.0389 | [M-H2O + H]+, C5H8O4 | N.A. | Organic acids and derivatives/ Dicarboxylic acids | −0.5 | 100.00% | CG/CPT/CPDMS |
| LC41 | 3.572 | 133.0494 | [M-H2O + H]+, C5H10O5 | Ribose (a) (c) | Carboxylic acids/Saccharides | −0.7 | 100.00% | CG/CPT/CPDMS |
| LC132 | 11.582 | 181.1221 | [M + H]+, C11H16O2 | Dihydroactinidiolide (a) (b) (c) (d) | Lipids and lipid-like molecules/ Terpenoids | −2.7 | 100.00% | CP |
| LC303 | 0.943 | 249.0377 | [M + H2O + K]+, C7H12O6 | Quinic acid (a) (c) (d) | Organic oxygen compounds/Cyclitols | 1.6 | 100.00% | CP |
| LC650 | 0.967 | 395.1156 | [M + Na]+, C13H24O12 | N.A. | Organic oxygen compounds/ Disaccharides | 0.7 | 100.00% | CP |
| LC695 | 21.422 | 433.3304 | [M + H]+, C27H44O4 | N.A. | Lipids and lipid-like molecules/ Terpenoids | 3.8 | 99.78% | CP |
| LC836 | 13.488 | 560.3786 | [M + H]+, C29H53NO9 | DGTA/S 5:1;O2/14:0 (e) | Organic acids and derivatives/ Glycerolipids | 1.2 | 100.00% | CG/CPT/CPDMS |
| LC855 | 13.643 | 574.3941 | [M + H]+, C30H55NO9 | DGTA/S 6:1;O2/14:0 (e) | Organic acids and derivatives/ Glycerolipids | 0.5 | 99.90% | CG/CPT/CPDMS |
| LC872 | 13.916 | 588.4099 | [M + H]+, C31H57NO9 | DGTA/S 7:1;O2/14:0 (e) | Organic acids and derivatives/ Glycerolipids | 0.3 | 99.95% | CG/CPT/CPDMS |
| LC873 | 14.567 | 588.4100 | [M + H]+, C31H57NO9 | DGTA/S 5:1;O2/16:0 (e) | Organic acids and derivatives/ Glycerolipids | −1.4 | 99.95% | CG/CPT/CPDMS |
| LC891 | 14.106 | 602.4255 | [M + H]+, C32H59NO9 | DGTA/S 8:1;O2/14:0 (e) | Organic acids and derivatives/ Glycerolipids | −0.1 | 98.14% | CG/CPT/CPDMS |
| LC912 | 14.434 | 616.4411 | [M + H]+, C33H61NO9 | DGTA/S 9:1;O2/14:0 (e) | Organic acids and derivatives/ Glycerolipids | 0.3 | 67.18% | CG/CPT/CPDMS |
| LC944 | 15.495 | 644.4726 | [M + H]+, C35H65NO9 | DGTA/S 9:1;O2/16:0 (e) | Organic acids and derivatives/ Glycerolipids | 0.1 | 94.37% | CG/CPT/CPDMS |
| LC1011 | 18.141 | 724.5712 | [M + H]+, C42H77NO8 | DGTA/S 18:2;O1/14:0 (e) | Organic acids and derivatives/ Glycerolipids | 1.5 | 97.89% | CG/CPT/CPDMS |
| LC1024 | 19.204 | 752.6032 | [M + H]+, C44H81NO8 | DGTA/S 18:2;O1/16:0 (e) | Organic acids and derivatives/ Glycerolipids | 1.5 | 99.46% | CG/CPT/CPDMS |
| Comp. | RT | m/z (Adduct) | [Adduct], Compound’s Molecular formula | Compound’s Name | Superclass/Natural Product Class | Err. ppm | Sirius Score | T2: Increased in |
| LC718 | 7.663 | 454.1831 | [M + H]+, C18H32NO12 | DGTA/S 5:1;O2/3:1;O3 (e) | Organic acids and derivatives/ Glycerolipids | 23.50 | N.A. | CG |
| LC729 | 6.495 | 464.2121 | [M + H]+, C20H34NO11 | DGTA/S 5:1;O2/5:1;O2 (e) | Organic acids and derivatives/ Glycerolipids | −0.04 | 86.99% | CG |
| LC746 | 6.94 | 478.2278 | [M + H]+, C21H35NO11 | DGTA/S 5:1;O2/6:1,O2 (e) | Organic acids and derivatives/ Glycerolipids | 1.28 | 40.19% | CG |
| LC762 | 7.553 | 492.2435 | [M + H]+, C22H37NO11 | DGTA/S 5:1;O2/7:1;O2 (e) | Organic acids and derivatives/ Glycerolipids | −1.14 | 89.30% | CG |
| LC768 | 9.304 | 496.2300 | [M + H]+, C21H37NO12 | DGTA/S 5:1;O2/6:0,O3 (e) | Organic acids and derivatives/ Glycerolipids | 19.67 | N.A. | CG |
| LC790 | 9.823 | 510.2456 | [M + H]+, C22H39NO12 | DGTA/S 5:1;O2/7:0;O3 (e) | Organic acids and derivatives/ Glycerolipids | 14.00 | N.A. | CG |
| LC812 | 8.925 | 534.2902 | [M + H]+, C25H43NO11 | DGTA/S 6:1;O2/9:1;O2 (e) | Organic acids and derivatives/ Glycerolipids | 1.50 | 79.50% | CG |
| LC885 | 11.867 | 600.3733 | [M + H], C31H53NO10 | DGTA/S 5:1;O2/16:2;O (e) | Organic acids and derivatives/ Glycerolipids | 0.10 | 94.66% | CG |
| Comp. | RT | m/z (Adduct) | [Adduct], Compound’s Molecular formula | Compound’sName | Superclass/Natural Product Class | Err. ppm | Sirius Score | T3: Increased in |
| LC52 | 2.648 | 141.1385 | [M + H]+, C8H16N2 | N.A. | N.A. | 0.9 | 100.00% | CPT |
| LC73 | 8.993 | 149.0706 | [M + H]+, C8H8N2O | N.A. | Organic oxygen compounds/ Alkaloids | 2.3 | 100.00% | CPT |
| LC241 | 12.962 | 225.1957 | [M + H]+, C13H24N2O | N.A. | Organic oxygen compounds/ Alkaloids | −0.6 | 94.37% | CPT |
| LC315 | 14.774 | 253.1908 | [M + H]+, C14H24N2O2 | N.A. | Organic oxygen compounds/ Alkaloids | 1.8 | 97.89% | CPT |
| LC1039 | 22.596 | 791.6748 | [M + H2O + H]+, C49H88O6 | TG 14:0/16:0/16:2 (e) | Lipids and lipid-liked molecules/ Fatty acids | 2.1 | 92.21% | CPDMS |
| Comp. | Molecular Name | Pathway | Raw Formula | Match NIST | CAS Number | Exp. RI | Est. RI | Litt. RI | T1: Increased in |
|---|---|---|---|---|---|---|---|---|---|
| C4 | Tetradecanoic acid (C14:0) | Fatty acids | C14H28O2 | 956 | 544-63-8 | 1731 | - | 1725 | CG/CPT/CPDMS |
| C10 | (7Z,10Z)-Hexadecadienoic acid (C16:2n-6) | Fatty acids | C16H28O2 | 844 | 28290-73-5 | 1894 | 1894 | - | CP |
| C18 | Phytol | Terpenoids | C20H40O | 723 | 150-86-7 | 2071 | - | 2114 | CP |
| C24 | 9,10,12-Trihydroxyoctadecanoic acid (C18:0;O3) | Fatty acids | C18H36O5 | 651 * | 25027-95-6 | 2282 | - | - | CP |
| Comp. | Molecular Name | Pathway | Raw Formula | Match NIST | CAS Number | Exp. RI | Est. RI | Litt. RI | T2: Increased in |
| C9 | 2,6,10,15-Tetramethylheptadecane | Alkane | C21H44 | 730 | 54833-48-6 | 1889 | - | 1889 | CP/CPT/CPDMS |
| C19 | (6Z,9Z,12Z,15Z)-Octadecatetraenoic acid (C18:4n-3) | Fatty acids | C18H28O2 | 925 | 20290-75-9 | 2094 | - | 2088 | CG |
| C26 | 4,7,10,13,16,19-Docosahexaenoic acid (C22:6n-3) | Fatty acids | C22H32O2 | 819 | 2091-24-9 | 2456 | - | 2471 | CG |
| Comp. | Molecular Name | Pathway | Raw Formula | Match NIST | CAS Number | Exp. RI | Est. RI | Litt. RI | T3: Increased in |
| C8 | 2-Methyloctadecane | Alkane | C19H40 | 810 | 1560-88-9 | 1856 | - | 1863 | CPT |
| C12 | (9Z)-Hexadecenoic acid (C16:1n-7) | Fatty acids | C16H30O2 | 960 | 10030-73-6 | 1913 | - | 1904 | CPT |
| C16 | Isophytol, acetate | Terpenoids | C22H42O2 | 718 | 58425-36-8 | 2043 | - | 2064 | CPT |
| Comp. | Molecular Name | Pathway | Raw Formula | Match NIST | CAS Number | Exp. RI | Est. RI | Litt. RI | T1: Increased in |
|---|---|---|---|---|---|---|---|---|---|
| M8 | D-Ribofuranose (isomer 2) | Carbohydrates | C5H10O5 | 836 | 613-83-2 | 1629 | - | 1641 | CG/CPT/CPDMS |
| M10 | D-Ribose | Carbohydrates | C5H10O5 | 868 | 10257-32-6 | 1677 | 1651 | - | CG/CPT/CPDMS |
| M16 | Unknown sugar | Carbohydrates | N.A. | N.A. | N.A. | 1784 | - | - | CP |
| M29 | Myo-Inositol | Carbohydrates | C6H12O6 | 762 | 551-72-4 | 2068 | 2194 | - | CG/CPT/CPDMS |
| M33 | (9Z)-Octadecenoic acid (C18:1n-9) | Fatty acids | C18H34O2 | 780 | 112-80-1 | 2166 | - | 2141 | CG/CPT/CPDMS |
| M42 | D-Cellobiose | Carbohydrates | C12H22O11 | 711 | 528-50-7 | - | - | 2762 | CP |
| Comp. | Molecular Name | Pathway | Raw Formula | Match NIST | CAS Number | Exp. RI | Est. RI | Litt. RI | T2: Increased in |
| M3 | Glycerol | Polyol | C3H8O3 | 894 | 56-81-5 | 1221 | 1066 | 1247 | CP/CPT/CPDMS |
| M28 | N-Acetyl-D-Glucosamine (isomer 1) | Carbohydrates | C8H15NO6 | 637 | 7512-17-6 | 2061 | - | 2068 | CP/CPT/CPDMS |
| Comp. | Molecular Name | Pathway | Raw Formula | Match NIST | CAS Number | Exp. RI | Est. RI | Litt. RI | T3: Increased in |
| M26 | D-Glucuronic acid | Carbohydrates | C6H10O7 | 795 | 528-16-5 | 2024 | - | 2012 | CPT |
| M36 | D-Glucose | Carbohydrates | C6H12O6 | 841 | 2280-44-6 | 2193 | 2173 | - | CPT |
| Discriminant Chemical Shift on Axis 1 (δ in ppm) and Multiplicity | Attribution | Annotation Confidence | T1: Increased in |
|---|---|---|---|
| 0.90, t | Fatty acids CH3 | 4 | CG/CPT/CPDMS |
| 1.12, t | N.A. | 0 | CG/CPT/CPDMS |
| 1.30, bs | Fatty acids CH2 | 4 | CG/CPT/CPDMS |
| 1.60, t | Fatty acids CH2 β-ester | 4 | CG/CPT/CPDMS |
| 2.35, t | Fatty acids CH2 α-ester | 4 | CG/CPT/CPDMS |
| 2.90, dd | 2,3-dihydroxypropane-1-sulfonate (DHPS) | 4 | CP |
| 2.95, s | Dimethylsulfoniopropionate (DMSP) | 4 | CP |
| 3.04, dd | 2,3-dihydroxypropane-1-sulfonate (DHPS) | 4 | CP |
| 3.21, s | Choline | 4 | CP |
| 3.40, t | Dimethylsulfoniopropionate (DMSP) | 4 | CP |
| 3.44, | Carbohydrates (glucose/galactose) | 2 | CP |
| 3.54, tt | Glycerophospholipids/Glycerolipids | 2 | CG/CPT/CPDMS |
| 3.62, dd | 2,3-dihydroxypropane-1-sulfonate (DHPS) | 4 | CP |
| 3.65, bs | Glycerol | 4 | CG/CPT/CPDMS |
| 3.75, d | Carbohydrates (glucose/galactose) | 2 | CP |
| 3.82, m | Glycerophospholipids/Glycerolipids | 2 | CG/CPT/CPDMS |
| 3.85, bs | Carbohydrates (glucose/galactose) | 2 | CP |
| 3.94, m | Carbohydrates (glucose/galactose) | 2 | CP |
| 4.01, dd | Glycerophospholipids/Glycerolipids | 2 | CG/CPT/CPDMS |
| 4.10, dd | Glycerophospholipids/Glycerolipids | 2 | CG/CPT/CPDMS |
| 8.40, s | Formate | 4 | CG/CPT/CPDMS |
| Discriminant Chemical Shift on Axis 2 (δ in ppm) and Multiplicity | Attribution | Annotation Confidence | T2: Increased in |
| 0.74, t | Sterols | 3 | CG |
| 1.01, d | Isoleucine | 4 | CG |
| 1.03, d | Valine | 4 | CG |
| 1.45, d | Lactate | 4 | CPT/CPDMS/CP |
| 1.60, t | Fatty acids CH2 β ester | 3 | CG |
| 2.32, m | Proline | 4 | CG |
| 2.60, t | Fatty acids CH2 α ester | 3 | CG |
| 2.82, s | N.A. | 0 | CG |
| 3.21, s | Choline | 4 | CG |
| 3.65, bs | Glycerol | 4 | CPT/CPDMS/CP |
| 4.12, dd | Sulphoquinovosyldiacylglycerols (SQDGs) | 4 | CG |
| 4.19, dd | Sulphoquinovosyldiacylglycerols (SQDGs) | 4 | CG |
| 6.10, dd | N.A. | 0 | CG |
| 6.92, dd | N.A. | 0 | CG |
| Discriminant Chemical Shift on Axis 3 (δ in ppm) and Multiplicity | Attribution | Annotation Confidence | T3: Increased in |
| 0.90, t | Fatty acids CH3 | 3 | CPT |
| 1.30, bs | Fatty acids CH2 | 3 | CPT |
| 1.60, t | Fatty acids CH2 β ester | 3 | CPT |
| 2.35, t | Fatty acids CH2 α ester | 3 | CPT |
| 4.10, dd | Glycerophospholipids/Glycerolipids | 1 | CPT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castaldi, A.; Triba, M.N.; Le Moyec, L.; Hubas, C.; Le Pennec, G.; Bourguet-Kondracki, M.-L. Multiblock Metabolomics Responses of the Diatom Phaeodactylum tricornutum Under Benthic and Planktonic Culture Conditions. Mar. Drugs 2025, 23, 314. https://doi.org/10.3390/md23080314
Castaldi A, Triba MN, Le Moyec L, Hubas C, Le Pennec G, Bourguet-Kondracki M-L. Multiblock Metabolomics Responses of the Diatom Phaeodactylum tricornutum Under Benthic and Planktonic Culture Conditions. Marine Drugs. 2025; 23(8):314. https://doi.org/10.3390/md23080314
Chicago/Turabian StyleCastaldi, Andrea, Mohamed Nawfal Triba, Laurence Le Moyec, Cédric Hubas, Gaël Le Pennec, and Marie-Lise Bourguet-Kondracki. 2025. "Multiblock Metabolomics Responses of the Diatom Phaeodactylum tricornutum Under Benthic and Planktonic Culture Conditions" Marine Drugs 23, no. 8: 314. https://doi.org/10.3390/md23080314
APA StyleCastaldi, A., Triba, M. N., Le Moyec, L., Hubas, C., Le Pennec, G., & Bourguet-Kondracki, M.-L. (2025). Multiblock Metabolomics Responses of the Diatom Phaeodactylum tricornutum Under Benthic and Planktonic Culture Conditions. Marine Drugs, 23(8), 314. https://doi.org/10.3390/md23080314










