Dolabellane Diterpenoids from Soft Coral Clavularia viridis with Anti-Inflammatory Activities
Abstract
1. Introduction
2. Results
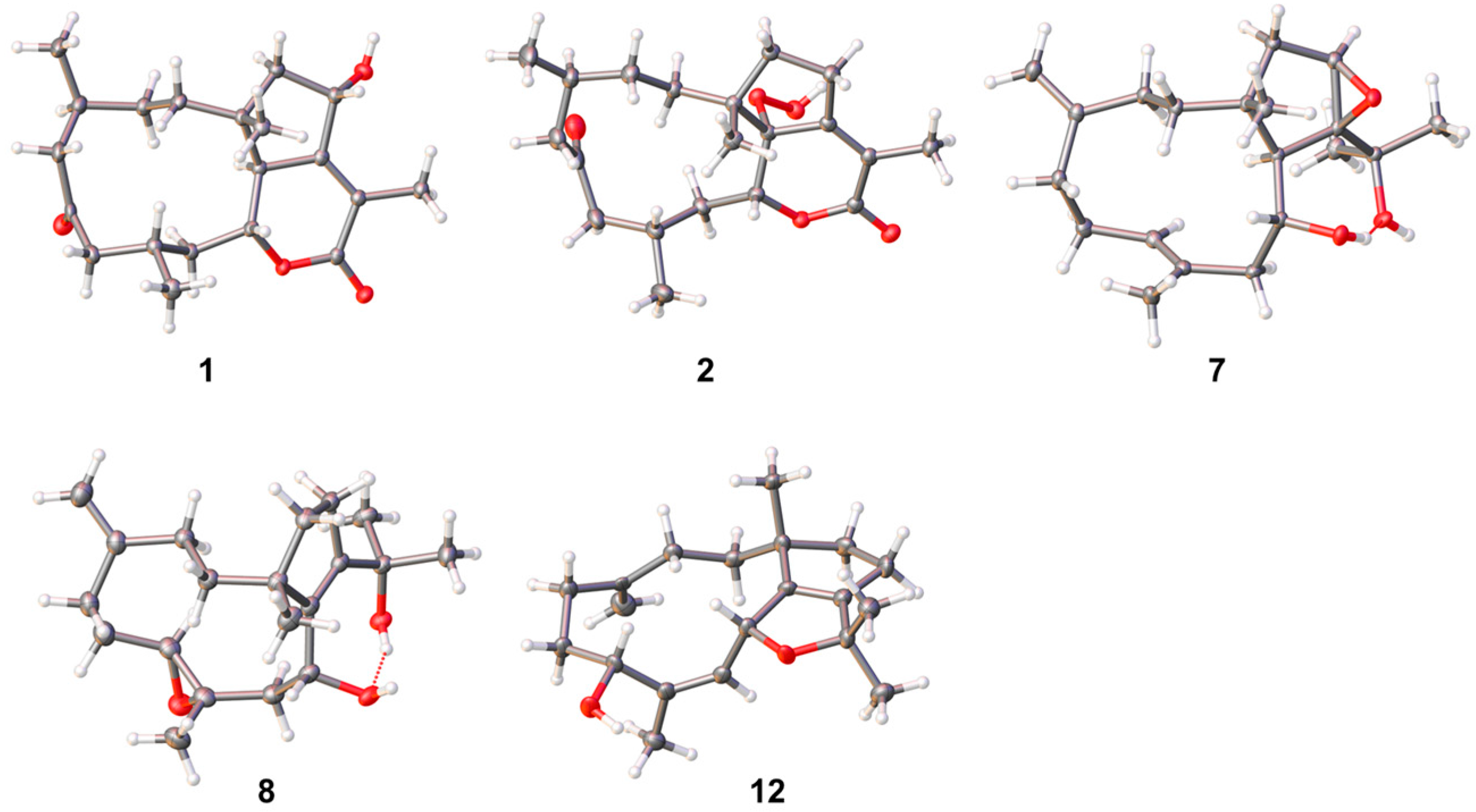
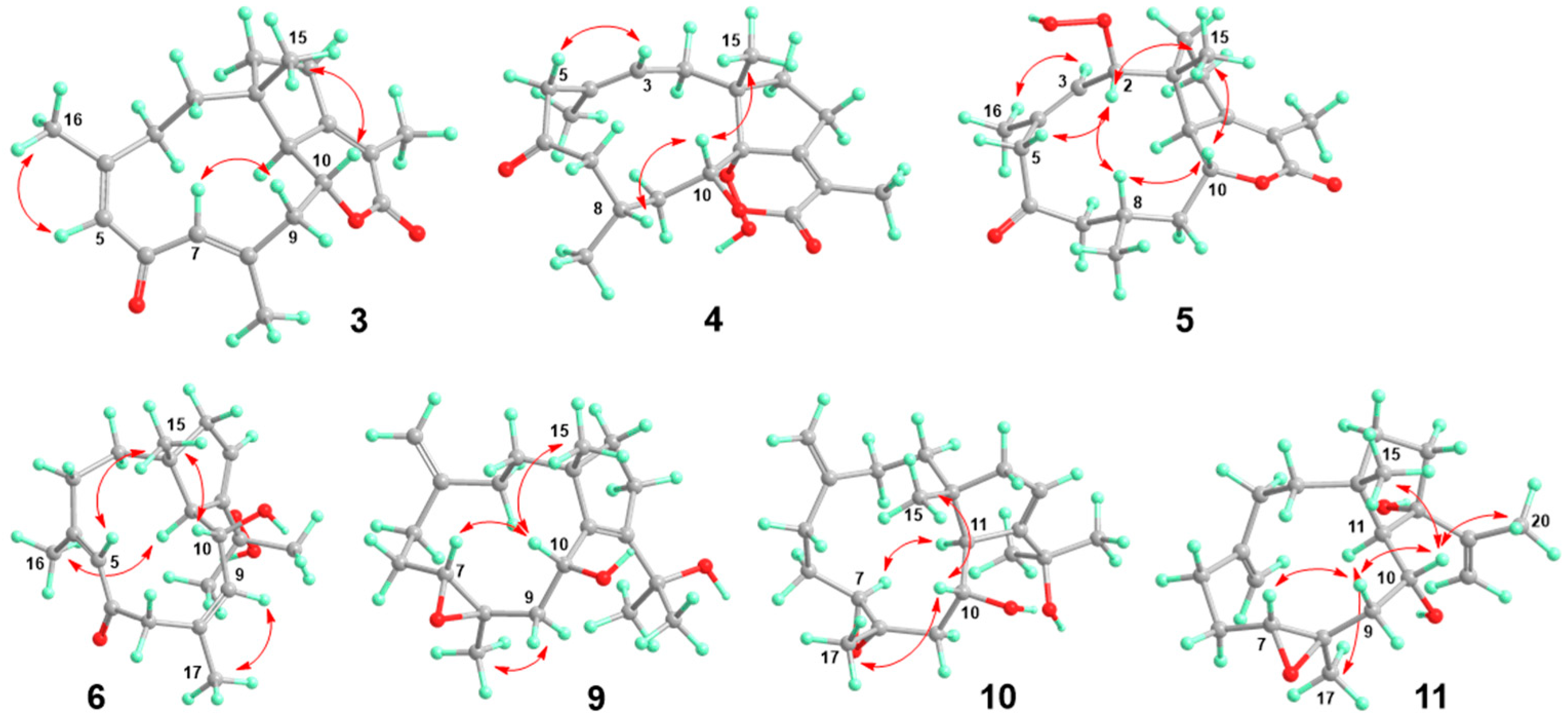
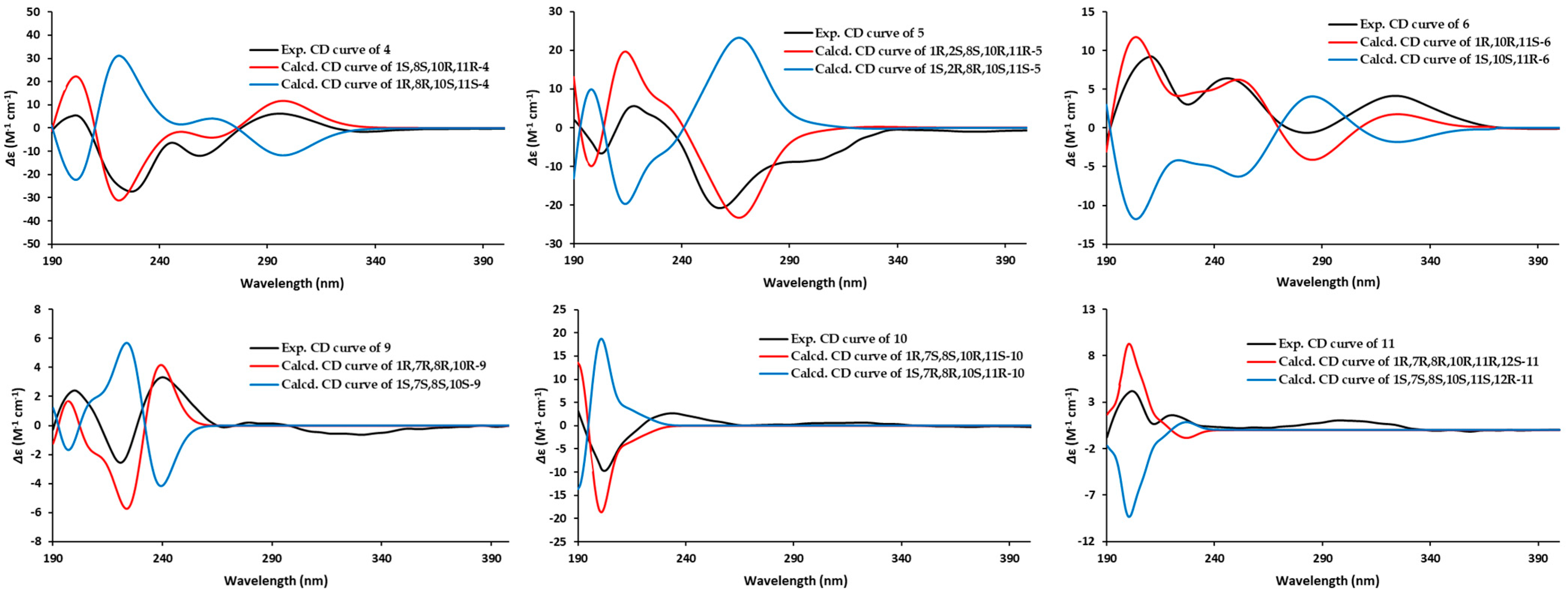
2.1. Structural Elucidation
2.2. Biological Activity Assessment
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. X-Ray Crystallography Data
3.5. ECD Calculation
3.6. Cell Culture
3.7. CCK-8 Assay
3.8. Inhibitory Activity Against NO Production
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Q.; Yu, S.; Zhou, M.; Wang, P.; Li, W.; Jin, X.; Pan, Y.; Sheng, Y.; Li, H.; Qin, L.; et al. Diterpenoids of Marine Organisms: Isolation, Structures, and Bioactivities. Mar. Drugs 2025, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, D.; Chen, X.; Zhao, F. Marine-Derived Diterpenes from 2019 to 2024: Structures, Biological Activities, Synthesis and Potential Applications. Mar. Drugs 2025, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, I.; de Rond, T.; Chen, P.Y.-T.; Moore, B.S. Ancient plant-like terpene biosynthesis in corals. Nat. Chem. Biol. 2022, 18, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Scesa, P.D.; Lin, Z.; Schmidt, E.W. Ancient defensive terpene biosynthetic gene clusters in the soft corals. Nat. Chem. Biol. 2022, 18, 659–663. [Google Scholar] [CrossRef]
- Grayson, N.E.; Scesa, P.D.; Moore, M.L.; Ledoux, J.-B.; Gomez-Garrido, J.; Alioto, T.; Michael, T.P.; Burkhardt, I.; Schmidt, E.W.; Moore, B.S. A widespread metabolic gene cluster family in metazoans. Nat. Chem. Biol. 2025. [Google Scholar] [CrossRef]
- Du, Y.-Q.; Gao, Y.; Zang, Y.; Li, J.; Li, X.-W.; Guo, Y.-W. Extending the record of dolabellane-type diterpenoids from the soft coral Clavularia viridis: Structures and stereochemistry. Phytochemistry 2023, 210, 113671. [Google Scholar] [CrossRef]
- Iguchi, K.; Fukaya, T.; Yasumoto, A.; Watanabe, K. New Marine Sesquiterpenoids and Diterpenoids from the Okinawan Soft Coral Clavularia koellikeri. J. Nat. Prod. 2004, 67, 577–583. [Google Scholar] [CrossRef]
- Iwashima, M.; Matsumoto, Y.; Takenaka, Y.; Iguchi, K.; Yamori, T. New Marine Diterpenoids from the Okinawan Soft Coral Clavularia koellikeri. J. Nat. Prod. 2002, 65, 1441–1446. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Chia, M.-C.; Wang, S.-K.; Chen, H.-J.; El-Gamal, A.A.H.; Dai, C.-F. Cytotoxic Dolabellane Diterpenes from the Formosan Soft Coral Clavularia inflata. J. Nat. Prod. 2001, 64, 1028–1031. [Google Scholar] [CrossRef]
- Geng, J.; Liu, W.; Gao, J.; Jiang, C.; Fan, T.; Sun, Y.; Qin, Z.-H.; Xu, Q.; Guo, W.; Gao, J. Andrographolide alleviates Parkinsonism in MPTP-PD mice via targeting mitochondrial fission mediated by dynamin-related protein 1. Br. J. Pharmacol. 2019, 176, 4574–4591. [Google Scholar] [CrossRef]
- González, Y.; Torres-Mendoza, D.; Jones, G.E.; Fernandez, P.L. Marine Diterpenoids as Potential Anti-Inflammatory Agents. Mediat. Inflamm. 2015, 2015, 263543. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.-C.; Niu, G.-H.; Lo, C.-F.; Wang, J.-Y.; Chi, Y.-H.; Huang, W.-C.; Tung, C.-W.; Sung, P.-J.; Tsou, L.K.; Zhang, M.M. Marine diterpenoid targets STING palmitoylation in mammalian cells. Commun. Chem. 2023, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, X.; Guo, X.; Cheng, W.; Qi, X.; Huang, J.; Lin, W. Briarane-type diterpenoids suppress osteoclastogenisis by regulation of Nrf2 and MAPK/NF-kB signaling pathway. Bioorg. Chem. 2021, 112, 104976. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, X.; Meng, J.; Wu, J.; Cheng, W.; Huang, J.; Lin, W. Briarane-type diterpenoids, the inhibitors of osteoclast formation by interrupting Keap1-Nrf2 interaction and activating Nrf2 pathway. Eur. J. Med. Chem. 2023, 246, 114948. [Google Scholar] [CrossRef]
- Qi, X.; Meng, J.; Li, C.; Cheng, W.; Fan, A.; Huang, J.; Lin, W. Praelolide alleviates collagen-induced arthritis through increasing catalase activity and activating Nrf2 pathway. Phytomedicine 2024, 135, 156040. [Google Scholar] [CrossRef]
- Dong, X.; Wu, J.; Jia, H.; Cen, S.; Cheng, W.; Lin, W. Targeted Isolation of Dolabellane Diterpenoids from the Soft Coral Clavularia viridis Using Molecular Networking. ACS Omega 2023, 8, 21254–21264. [Google Scholar] [CrossRef]
- Su, J.; Zhong, Y.; Zeng, L. Four Novel Diterpenoids: Clavirolides B, C, D, and E from the Chinese Soft Coral Clavularia viridis. J. Nat. Prod. 1991, 54, 380–385. [Google Scholar] [CrossRef]
- Gao, Y.; Du, Y.-Q.; Zang, Y.; Liu, H.-C.; Wan, H.-Y.; Li, J.; Li, X.-W.; Guo, Y.-W. Dolabellane Diterpenoids from the Xisha Soft Coral Clavularia viridis. ACS Omega 2022, 7, 3052–3059. [Google Scholar] [CrossRef]
- Han, X.; Luo, X.; Xue, L.; van Ofwegen, L.; Zhang, W.; Liu, K.; Zhang, Y.; Tang, X.; Li, P.; Li, G. Dolabellane Diterpenes and Elemane Alkaloids from the Soft Coral Clavularia inflata Collected in the South China Sea. J. Nat. Prod. 2022, 85, 276–283. [Google Scholar] [CrossRef]
- Su, J.; Zhong, Y.; Shi, K.; Cheng, Q.; Snyder, J.K.; Hu, S.; Huang, Y. Clavudiol A and clavirolide A, two marine dolabellane diterpenes from the soft coral Clavularia viridis. J. Org. Chem. 1991, 56, 2337–2344. [Google Scholar] [CrossRef]
- Cheng, W.; Ji, M.; Li, X.; Ren, J.; Yin, F.; van Ofwegen, L.; Yu, S.; Chen, X.; Lin, W. Fragilolides A-Q, norditerpenoid and briarane diterpenoids from the gorgonian coral Junceella fragilis. Tetrahedron 2017, 73, 2518–2528. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
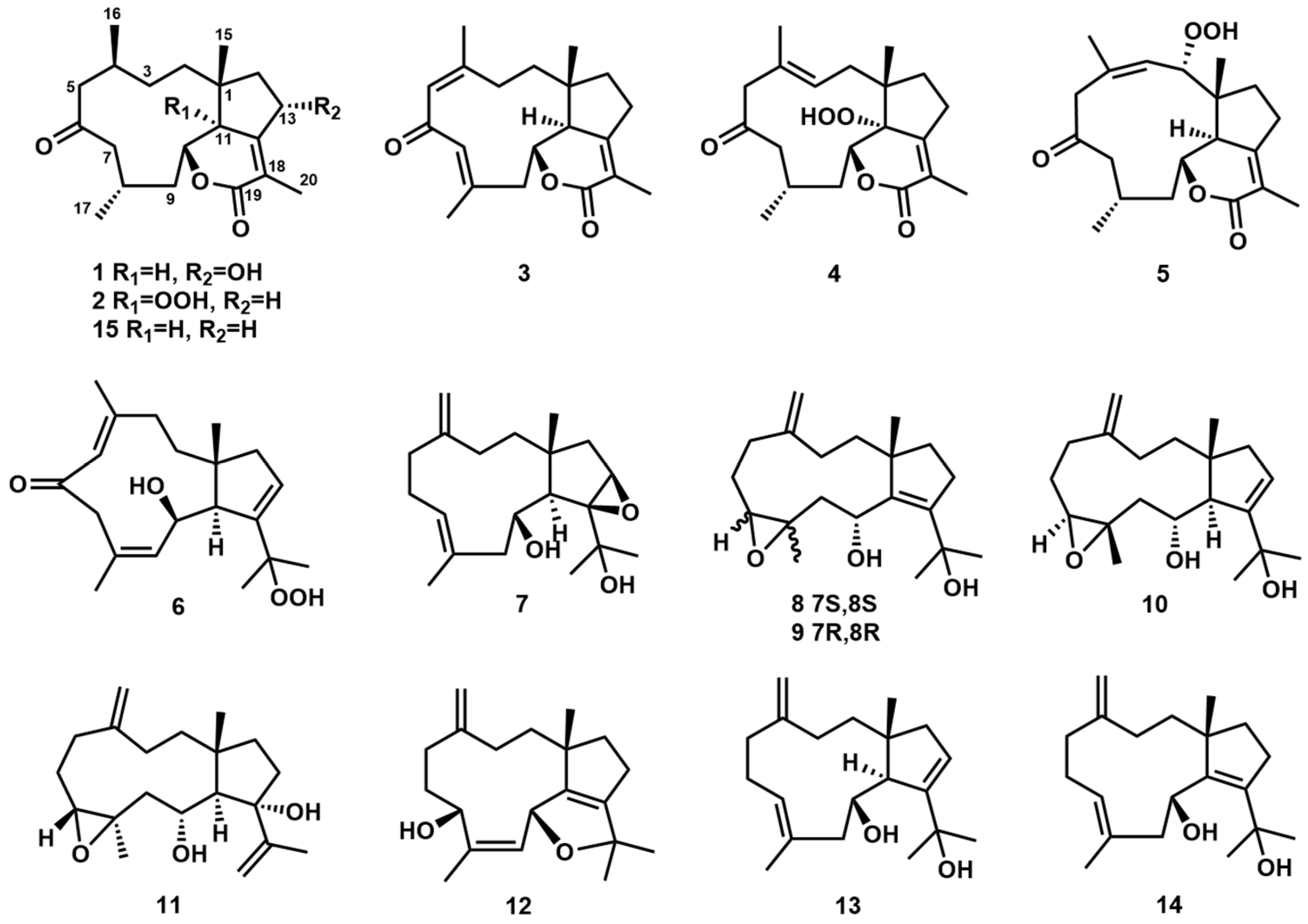
 ) and 1H-1H COSY (
) and 1H-1H COSY ( ) correlations of 1, 6, 7, and 12.
) correlations of 1, 6, 7, and 12.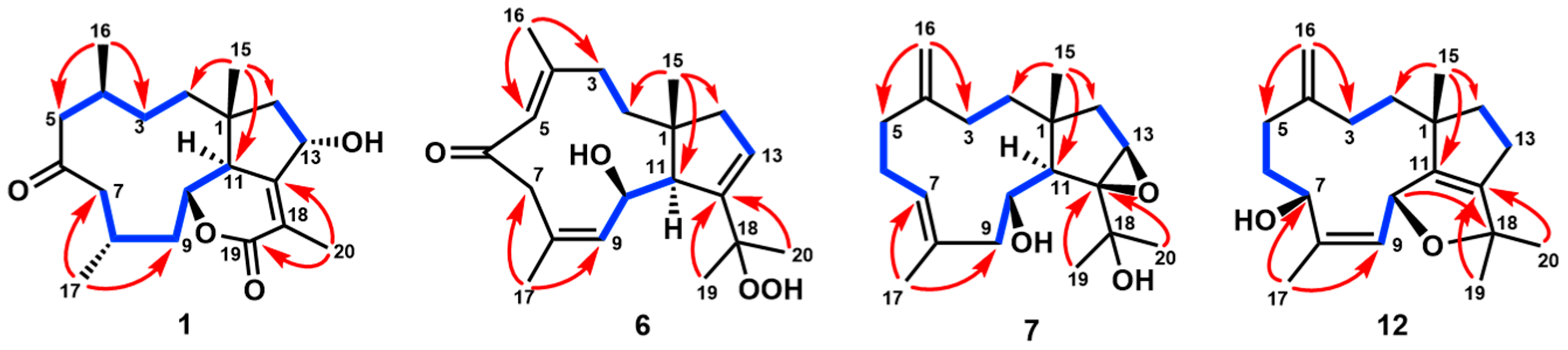
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 2 | 1.46, m | 1.47, m 1.32, m | 1.80, m 1.75, m | 2.70, dd (15.2, 11.5) 1.87, d (15.2) | 5.18, d (11.0) |
| 3 | 1.46, m 1.31, m | 1.33, m 1.00, m | 2.75, m 1.82, m | 5.56, d (11.5) | 5.47, d (11.0) |
| 4 | 2.52, m | 2.20, m | |||
| 5 | 2.72, dd (15.0, 12.0) 2.09, m | 2.37, dd (14.5, 10.8) 2.26, dd (14.5, 2.7) | 5.86, br s | 3.47, d (10.8) 2.86, d (10.8) | 4.17, d (12.2) 2.62, d (12.2) |
| 7 | 2.54, m 2.10, m | 2.35, m | 6.36, br s | 2.59, m 2.21, dd (14.1, 11.0) | 2.71, dd (13.8, 4.2) 2.14, d (13.8) |
| 8 | 2.12, m | 2.10, m | 2.32, m | 2.43, m | |
| 9 | 1.94, m 1.70, m | 2.33, m 1.69, m | 2.86, dd (14.5, 4.6) 2.57, dd (14.5, 4.6) | 2.45, ddd (15.6, 3.6, 2.4) 1.49, ddd (15.6, 10.2, 5.2) | 1.72, m 1.61, m |
| 10 | 4.18, dd (10.8, 9.3) | 4.49, t (4.4) | 4.56, dt (11.2, 4.6) | 4.46, dd (5.2, 2.4) | 4.29, dd (11.0, 9.5) |
| 11 | 2.78, d (10.8) | 2.71, d (11.2) | 2.65, d (11.0) | ||
| 13 | 4.82, m | 2.56, m | 2.52, m | 2.58, m | 2.48, m |
| 14 | 2.01, m; 1.69, m | 2.06, m 1.52, m | 1.92, m 1.43, m | 2.14, m 1.60, m | 1.81, m 1.73, m |
| 15 | 0.87, s | 0.91, s | 0.95, s | 1.09, s | 1.16, s |
| 16 | 0.99, d (6.8) | 1.01, d (6.8) | 1.92, d (1.2) | 1.77, br s | 2.07, s |
| 17 | 1.17, d (6.0) | 1.28, d (6.5) | 2.07, s | 1.17, d (6.6) | 1.12, d (6.6) |
| 19 | 1.99, s | 1.92, brs | 1.87, brs | 1.95, br s | 1.84, s |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41.9 | 49.6 | 45.1 | 51.3 | 48.1 | 45.1 | 40.9 | 52.4 | 53.1 | 45.8 | 43.3 | 45.3 |
| 2 | 32.3 | 30.9 | 36.2 | 33.5 | 86.8 | 40.1 | 38.0 | 37.8 | 37.0 | 38.5 | 38.9 | 34.3 |
| 3 | 27.0 | 32.0 | 29.2 | 126.5 | 120.9 | 36.7 | 30.7 | 31.8 | 31.4 | 31.8 | 31.4 | 34.8 |
| 4 | 29.1 | 31.3 | 153.2 | 130.1 | 140.7 | 153.2 | 151.8 | 150.3 | 150.4 | 148.8 | 149.1 | 149.5 |
| 5 | 46.2 | 50.9 | 128.9 | 54.0 | 43.1 | 129.3 | 37.3 | 33.5 | 31.3 | 32.0 | 32.1 | 30.9 |
| 6 | 211.8 | 212.5 | 196.4 | 207.2 | 206.4 | 196.8 | 29.7 | 30.9 | 27.8 | 26.4 | 26.8 | 32.1 |
| 7 | 53.5 | 52.3 | 133.1 | 53.4 | 56.1 | 46.5 | 128.7 | 65.3 | 64.1 | 62.9 | 68.1 | 67.7 |
| 8 | 26.9 | 31.3 | 142.9 | 31.3 | 26.2 | 129.3 | 131.7 | 60.2 | 60.5 | 59.4 | 60.2 | 135.8 |
| 9 | 43.2 | 36.8 | 45.1 | 37.2 | 43.0 | 132.5 | 47.0 | 51.6 | 47.6 | 48.2 | 44.4 | 132.7 |
| 10 | 78.2 | 80.8 | 79.9 | 81.1 | 77.4 | 69.5 | 68.3 | 64.1 | 65.9 | 67.1 | 69.4 | 75.5 |
| 11 | 50.8 | 88.4 | 46.2 | 88.1 | 52.7 | 56.5 | 46.6 | 143.0 | 141.7 | 55.1 | 57.1 | 150.2 |
| 12 | 159.0 | 153.0 | 161.3 | 153.4 | 159.3 | 141.0 | 73.3 | 144.7 | 145.8 | 146.7 | 86.7 | 149.1 |
| 13 | 69.6 | 26.1 | 27.5 | 25.3 | 26.9 | 133.7 | 59.9 | 33.6 | 33.4 | 127.7 | 39.3 | 23.7 |
| 14 | 47.9 | 37.0 | 35.1 | 39.2 | 31.1 | 50.9 | 36.7 | 33.8 | 32.4 | 44.9 | 36.7 | 47.1 |
| 15 | 23.9 | 23.1 | 23.1 | 23.2 | 20.3 | 22.2 | 28.6 | 28.7 | 28.1 | 24.9 | 25.4 | 21.9 |
| 16 | 19.9 | 21.5 | 25.5 | 16.7 | 25.1 | 17.7 | 111.9 | 110.7 | 111.5 | 111.3 | 112.0 | 109.9 |
| 17 | 21.7 | 22.9 | 18.2 | 20.9 | 20.1 | 26.2 | 17.5 | 17.3 | 19.8 | 17.6 | 19.6 | 17.4 |
| 18 | 123.6 | 126.3 | 120.4 | 126.7 | 120.7 | 81.0 | 71.1 | 73.0 | 73.2 | 71.1 | 151.4 | 82.6 |
| 19 | 11.9 | 12.9 | 12.8 | 13.1 | 12.7 | 24.6 | 26.3 | 31.6 | 32.0 | 31.6 | 113.3 | 28.0 |
| 20 | 166.6 | 165.2 | 165.7 | 164.3 | 165.7 | 25.2 | 28.6 | 30.1 | 29.2 | 31.2 | 22.0 | 28.1 |
| No. | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|
| 2 | 1.96, dd (14.0, 3.6) 1.37, dt (14.0, 4.3) | 1.33, m 1.09, dt (12.0, 2.0) | 1.49, m | 1.79, m | 1.61, m | 1.75, ddd (14.5, 10.0, 5.8) 1.38, ddd (14.5, 10.0, 6.4) | 1.54, m 1.41, m |
| 3 | 2.54, td (13.0, 3.6) 2.12, m | 2.13, m 1.78, t (10.8) | 2.19, m 1.78, m | 2.19, m 1.88, m | 2.28, m 1.97, m | 2.25, m | 2.10, m 1.85, m |
| 5 | 6.36, s | 2.41, m 2.11, m | 2.35, m 2.04, m | 2.36, m 2.04, m | 2.29, m | 2.48, m 2.29, m | 1.99, m |
| 6 | 2.33, m 2.13, m | 2.10, m 1.38, m | 2.21, m 1.19 m | 2.28, m 1.48, m | 2.07, m 1.49, m | 1.89, m | |
| 7 | 3.70, d (10.6) 2.63, d (10.6) | 5.44, dd (9.2, 5.6) | 2.85, dd (10.2, 2.9) | 3.04, d (10.5) | 3.14, t dd (9.4, 2.3) | 2.95, dd (10.0, 1.1) | 4.88, m |
| 9 | 6.44, d (9.8) | 2.77, dd (12.6, 11.6) 2.26, dd (12.6, 1.9) | 2.47, d (13.7) 2.33, m | 3.23, t (12.7) 1.79, m | 2.22, m 2.02, m | 2.44, dd (14.8, 7.0) 1.61, dd (14.8, 3.9) | 5.17, d (9.6) |
| 10 | 4.88, d (9.8) | 3.89, dt (11.6, 1.9) | 3.99, d (9.9) | 4.15, dd (11.0, 4.7) | 3.91, dd (11.8, 1.5) | 4.03, ddd (7.4, 7.0, 3.9) | 5.36, dd (9.6, 2.9) |
| 11 | 2.43, s | 2.77, d (1.9) | 3.00, d (1.5) | 2.53, d (7.4) | |||
| 13 | 6.00, m | 3.16, br s | 2.34, m 2.22, m | 2.29, m | 5.71, dd (4.4, 2.4) | 2.20, m 1.67, m | 2.31, m 2.03, m |
| 14 | 2.45, m 2.08, m | 1.78, d (15.4) 1.71, d (15.4) | 1.78, m 1.56, m | 2.43, m 1.88, m | 2.23, m 2.10, d (16.5) | 1.95, dt (12.8, 7.5) 1.47, m | 2.03, m |
| 15 | 1.48, s | 1.10, s | 1.17, s | 1.10, s | 1.17, s | 1.05, s | 1.05, s |
| 16 | 1.83, s | 4.80, brs 4.69, brs | 4.80, brs 4.78, brs | 4.71, brs 4.69, brs | 4.90, brs 4.85, brs | 4.90, brs 4.81, brs | 4.79, brs 4.74, brs |
| 17 | 1.91, d (1.4) | 1.73, s | 1.38, s | 1.34, s | 1.43, s | 1.51, s | 1.72, s |
| 19 | 1.18, s | 1.14, s | 1.38, s | 1.39, s | 1.34, s | 5.32, brs 5.13, brs | 1.24, s |
| 20 | 1.58, s | 1.47, s | 1.40, s | 1.47, s | 1.55, s | 2.03, s | 1.42,rs |
| Compound | NO Inhibitory Activity | CC50 (μM) | |
|---|---|---|---|
| % Inhibition (20 μM) | IC50 (μM) | ||
| 1 | 15.1 ± 2.4 | >50 | |
| 2 | 36.4 ± 1.7 | >50 | |
| 3 | 42.7 ± 3.5 | 27.0 ± 0.6 | >50 |
| 4 | 29.0 ± 2.8 | >50 | |
| 5 | 26.8 ± 3.6 | >50 | |
| 6 | 14.6 ± 1.2 | >50 | |
| 7 | 24.0 ± 3.8 | >50 | |
| 8 | 35.9 ± 4.2 | >50 | |
| 9 | 12.6 ± 2.6 | >50 | |
| 10 | 68.9 ± 3.6 | 18.3 ± 0.6 | >50 |
| 11 | 18.4 ± 2.8 | >50 | |
| 12 | 48.8 ± 4.2 | 20.3 ± 0.3 | >50 |
| 13 | 45.6 ± 3.5 | 20.3 ± 0.3 | >50 |
| 14 | 25.4 ± 2.4 | >50 | |
| 15 | 48.3 ± 3.6 | 23.4 ± 0.4 | >50 |
| L-NMMA a | 76.7 ± 4.2 | 17.8 ± 0.6 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, C.; Jia, H.; Zhou, K.; Wang, B.; Lin, W.; Cheng, W. Dolabellane Diterpenoids from Soft Coral Clavularia viridis with Anti-Inflammatory Activities. Mar. Drugs 2025, 23, 312. https://doi.org/10.3390/md23080312
Gu C, Jia H, Zhou K, Wang B, Lin W, Cheng W. Dolabellane Diterpenoids from Soft Coral Clavularia viridis with Anti-Inflammatory Activities. Marine Drugs. 2025; 23(8):312. https://doi.org/10.3390/md23080312
Chicago/Turabian StyleGu, Chufan, Hongli Jia, Kang Zhou, Bin Wang, Wenhan Lin, and Wei Cheng. 2025. "Dolabellane Diterpenoids from Soft Coral Clavularia viridis with Anti-Inflammatory Activities" Marine Drugs 23, no. 8: 312. https://doi.org/10.3390/md23080312
APA StyleGu, C., Jia, H., Zhou, K., Wang, B., Lin, W., & Cheng, W. (2025). Dolabellane Diterpenoids from Soft Coral Clavularia viridis with Anti-Inflammatory Activities. Marine Drugs, 23(8), 312. https://doi.org/10.3390/md23080312








