Multifaceted Marine Peptides and Their Therapeutic Potential
Abstract
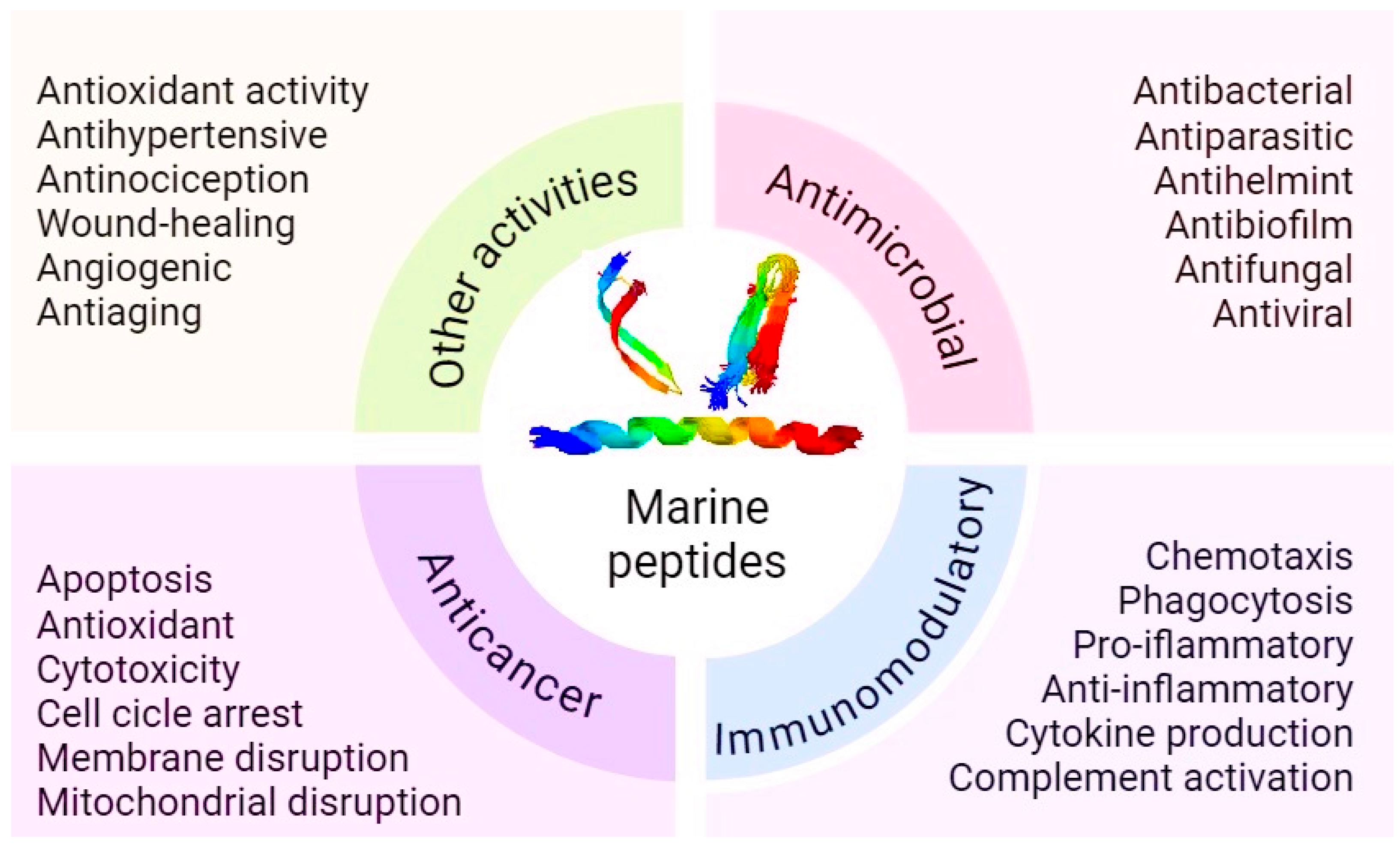
1. Introduction
2. Antimicrobial Activity
2.1. Antibacterial
2.2. Antibiofilm
2.3. Antifungal
2.4. Antiviral
2.5. Antiparasitic
3. Immunomodulatory Activities
3.1. Anti-Inflammatory
3.2. Modulation the Complement System
3.3. Chemotactic
3.4. Opsonizing
4. Anticancer Activity
5. Other Activities
5.1. Antihypertensive
5.2. Antinociceptive
5.3. Wound Healing
5.4. Antiaging
5.5. Anti-Photoaging and Skin Protection
5.6. Antiplatelet and Antithrombotic
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mah, C.L. New Genera, Species and Occurrences of Deep-Sea Asteroidea (Valvatacea, Forcipulatacea, Echinodermata) collected from the North Pacific Ocean by the CAPSTONE Expedition. Zootaxa 2022, 5164, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Opresko, D.M.; Wagner, D. New species of black corals (Cnidaria:Anthozoa: Antipatharia) from deep-sea seamounts and ridges in the North Pacific. Zootaxa 2020, 4868, zootaxa.4868.4.5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Chan, T.Y.; Sha, Z.L. A new deep-sea species of the genus Urocaridella (Crustacea: Decapoda: Caridea: Palaemonidea) from Yap Seamount in the Western Pacific. Zootaxa 2015, 4012, 191–197. [Google Scholar] [CrossRef]
- Bouchet, P.; Kantor, Y.I.; Sysoev, A.; Puillandre, N. A new operational classification of the conoidea (Gastropoda). J. Molluscan Stud. 2011, 77, 273–308. [Google Scholar] [CrossRef]
- Costello, M.J.; Chaudhary, C. Marine Biodiversity, Biogeography, Deep-Sea Gradients, and Conservation. Curr. Biol. 2017, 27, R511–R527. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, G. Marine biodiversity characteristics. Comptes Rendus Biol. 2011, 334, 435–440. [Google Scholar] [CrossRef]
- Falanga, A.; Lombardi, L.; Franci, G.; Vitiello, M.; Iovene, M.R.; Morelli, G.; Galdiero, M.; Galdiero, S. Marine Antimicrobial Peptides: Nature Provides Templates for the Design of Novel Compounds against Pathogenic Bacteria. Int. J. Mol. Sci. 2016, 17, 785. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V. Structure, Function, and Therapeutic Potential of Marine Bioactive Peptides. Mar. Drugs 2019, 17, 505. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V. Marine Peptides: Structure, Bioactivities, and a New Hope for Therapeutic Application. Mar. Drugs 2021, 19, 407. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Gigani, O.B.; Gudima, G.O.; Kataeva, A.M.; Kolesnikova, N.V. Dual Effect of Low-Molecular-Weight Bioregulators of Bacterial Origin in Experimental Model of Asthma. Life 2022, 12, 192. [Google Scholar] [CrossRef]
- Guryanova, S.V. Regulation of Immune Homeostasis via Muramyl Peptides-Low Molecular Weight Bioregulators of Bacterial Origin. Microorganisms 2022, 10, 1526. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Kataeva, A. Inflammation Regulation by Bacterial Molecular Patterns. Biomedicines 2023, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Sigmatulin, I.A.; Gigani, O.O.; Lipkina, S.A. Mechanisms of regulation allergic and autoimmune reactions by bacterial origin bioregulators. RUDN J. Med. 2023, 27, 470–482. [Google Scholar] [CrossRef]
- Guryanova, S.V. Bacteria and Allergic Diseases. Int. J. Mol. Sci. 2024, 25, 10298. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V. Influence of muramyl peptides on the production of chemokines, growth factors, pro-inflammatory and anti-inflammatory cytokines. RUDN J. Med. 2024, 28, 365–376. [Google Scholar]
- Chi, C.-F.; Wang, B. Marine Bioactive Peptides—Structure, Function and Application. Mar. Drugs 2023, 21, 275. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Shi, Q. Aquatic genomics and transcriptomics for evolutionary biology. Front. Genet. Sec. Evol. Popul. Genet. 2023, 14, 1183637. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Bioactive Peptide of Marine Origin for the Prevention and Treatment of Non-Communicable Diseases. Mar. Drugs 2017, 15, 67. [Google Scholar] [CrossRef]
- Zhang, Q.-T.; Liu, Z.-D.; Wang, Z.; Wang, T.; Wang, N.; Wang, N.; Zhang, B.; Zhao, Y.-F. Recent Advances in Small Peptides of Marine Origin in Cancer Therapy. Mar. Drugs 2021, 19, 115. [Google Scholar] [CrossRef]
- Sukmarini, L. Antiviral Peptides (AVPs) of Marine Origin as Propitious Therapeutic Drug Candidates for the Treatment of Human Viruses. Molecules 2022, 27, 2619. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Balandin, S.V.; Aleshina, G.M.; Tagaev, A.A.; Leonova, Y.F.; Krasnodembsky, E.G.; Men’shenin, A.V.; Kokryakov, V.N. Aurelin, a novel antimicrobial peptide from jellyfish Aurelia aurita with structural features of defensins and channel-blocking toxins. Biochem. Biophys. Res. Commun. 2006, 348, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Ovchinnikova, T.V. Innate Immunity Mechanisms in Marine Multicellular Organisms. Mar. Drugs 2022, 20, 549. [Google Scholar] [CrossRef]
- Sridhar, K.; Inbaraj, B.S.; Chen, B.H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021, 147, 110468. [Google Scholar] [CrossRef]
- Tripoteau, L.; Bedoux, G.; Gagnon, J.; Bourgougnon, N. In Vitro Antiviral Activities of Enzymatic Hydrolysates Extracted from Byproducts of the Atlantic Holothurian Cucumaria frondosa. Process Biochem. 2015, 50, 867–875. [Google Scholar] [CrossRef]
- Guerard, F.; Sumaya-Martinez, M.T.; Laroque, D.; Chabeaud, A.; Dufossé, L. Optimization of free radical scavenging activity by response surface methodology in the hydrolysis of shrimp processing discards. Process Biochem. 2007, 42, 1486–1491. [Google Scholar] [CrossRef]
- Panteleev, P.V.; Balandin, S.V.; Ivanov, V.T.; Ovchinnikova, T.V. A therapeutic potential of animal β-hairpin antimicrobial peptides. Curr. Med. Chem. 2017, 24, 1724–1746. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Panteleev, P.V.; Balandin, S.V.; Gizatullina, A.K.; Altukhov, D.A.; Finkina, E.I.; Kokryakov, V.N.; Arseniev, A.S.; Ovchinnikova, T.V. Recombinant expression and solution structure of antimicrobial peptide aurelin from jellifish Aurelia aurita. Biochem. Biophys. Res. Commun. 2012, 429, 63–69. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, Z.; Zhang, X. In silico evaluation of marine fish proteins as nutritional supplements for COVID-19 patients. Food Funct. 2020, 11, 5565–5572. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Shi, Q. Omics-based identification, characterization, and validation of marine bioactive peptides. Front. Mar. Sci. 2023, 10, 1323847. [Google Scholar] [CrossRef]
- Panteleev, P.V.; Safronova, V.N.; Duan, S.; Komlev, A.S.; Bolosov, I.A.; Kruglikov, R.N.; Kombarova, T.I.; Korobova, O.V.; Pereskokova, E.S.; Borzilov, A.I.; et al. Novel BRICHOS-related antimicrobial peptides from the marine worm Heteromastus filiformis: Transcriptome mining, synthesis, biological activities, and therapeutic potential. Mar. Drugs 2023, 21, 639. [Google Scholar] [CrossRef]
- Wang, H.; Liao, Z.; Yang, Z.; Xiao, W.; Yang, Z.; He, J.; Zhang, X.; Yan, X.; Tang, C. Histone derived antimicrobial peptides identified from Mytilus coruscus serum by peptidomics. Fish Shellfish Immunol. 2024, 149, 109546. [Google Scholar] [CrossRef] [PubMed]
- Fidor, A.; Konkel, R.; Mazur-Marzec, H. Bioactive peptides produced by cyanobacteria of the genus Nostoc. Mar. Drugs 2019, 17, 561. [Google Scholar] [CrossRef]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, R.; Ouyang, Z.; He, T.; Zhang, W.; Chen, X. Identification of a new antifungal peptide W1 from a marine Bacillus amyloliquefaciens reveals its potential in controlling fungal plant diseases. Front. Microbiol. 2022, 13, 922454. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wei, Y.; Chen, Y.; Jiang, S.; Xu, F.; Zhang, C.; Wang, H.; Shao, X. Epinecidin-1, a marine antifungal peptide, inhibits Botrytis cinerea and delays gray mold in postharvest peaches. Food Chem. 2023, 403, 134419. [Google Scholar] [CrossRef]
- Solov’eva, T.F.; Bakholdina, S.I.; Naberezhnykh, G.A. Host defense proteins and peptides with lipopolysaccharide-binding activity from marine invertebrates and their therapeutic potential in Gram-negative sepsis. Mar. Drugs 2023, 21, 581. [Google Scholar] [CrossRef]
- Ovchinnikova, T.; Aleshina, G.M.; Balandin, S.V.; Krasnosdembskaya, A.D.; Markelov, M.; Frolova, E.I.; Leonova, Y.F.; Tagaev, A.A.; Krasnodembsky, E.G.; Kokryakov, V.N. Purification and primary structure of two isoforms of arenicin, a novel antimicrobial peptide from marine polychaeta Arenicola marina. FEBS Lett. 2004, 577, 209–214. [Google Scholar] [CrossRef]
- Ovchinnikova, T.V.; Shenkarev, Z.O.; Balandin, S.V.; Nadezhdin, K.D.; Paramonov, A.S.; Kokryakov, V.N.; Arseniev, A.S. Molecular insight into mechanism of antimicrobial action of the beta-hairpin peptide arenicin: Specific oligomerization in detergent micelles. Biopolymers 2008, 5, 455–464. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Balandin, S.V.; Trunov, K.I.; Paramonov, A.S.; Sukhanov, S.V.; Barsukov, L.I.; Arseniev, A.S.; Ovchinnikova, T.V. Molecular mechanism of action of β-hairpin antimicrobial peptide arenicin: Oligomeric structure in dodecylphosphocholine micelles and pore formation in planar lipid bilayers. Biochemistry 2011, 50, 6255–6265. [Google Scholar] [CrossRef]
- Sychev, S.V.; Sukhanov, S.V.; Panteleev, P.V.; Shenkarev, Z.O.; Ovchinnikova, T.V. Marine antimicrobial peptide arenicin adopts a monomeric twisted β-hairpin structure and forms low conductivity pores in zwitterionic lipid bilayers. Pept. Sci. 2017, 110, e23093. [Google Scholar] [CrossRef]
- Panteleev, P.V.; Bolosov, I.A.; Ovchinnikova, T.V. Bioengineering and functional characterization of arenicin shortened analogs with enhanced antibacterial activity and cell selectivity. J. Pept. Sci. 2015, 22, 82–91. [Google Scholar] [CrossRef]
- Barton, J.C.; Acton, R.T. Hepcidin, iron, and bacterial infection. Vitam. Horm. 2019, 110, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; An, Z.; Zhang, W.; Chen, F.; Wang, K.-J. The antimicrobial peptide LJ-hep2 from Lateolabrax japonicus exerting activities against multiple pathogenic bacteria and immune protection in vivo. Mar. Drugs 2022, 20, 651. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.; Maisetta, G.; Batoni, G.; Tavanti, A. Insights into the antimicrobial properties of hepcidins: Advantages and drawbacks as potential therapeutic agents. Molecules 2015, 20, 6319–6341. [Google Scholar] [CrossRef]
- Chen, R.-C.; Lan, C.-Y. Human antimicrobial peptide hepcidin 25-induced apoptosis in Candida albicans. Microorganisms 2020, 8, 585. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-ferroportin interaction controls systemic iron homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Ginzburg, Y.Z. Hepcidin-ferroportin axis in health and disease. Vitam. Horm. 2019, 110, 17–45. [Google Scholar] [CrossRef]
- Safronova, V.N.; Panteleev, P.V.; Sukhanov, S.V.; Toropygin, I.Y.; Bolosov, I.A.; Ovchinnikova, T.V. Mechanism of action and therapeutic potential of the beta-hairpin antimicrobial peptide capitellacin from the marine polychaeta Capitella teleta. Mar. Drugs 2022, 20, 167. [Google Scholar] [CrossRef]
- Safronova, V.N.; Bolosov, I.A.; Kruglikov, R.N.; Korobova, O.V.; Pereskokova, E.S.; Borzilov, A.I.; Panteleev, P.V.; Ovchinnikova, T.V. Novel β-hairpin peptide from marine polychaeta with a high efficacy against Gram-negative pathogens. Mar. Drugs 2022, 20, 517. [Google Scholar] [CrossRef]
- Panteleev, P.V.; Tsarev, A.V.; Safronova, V.N.; Reznikova, O.V.; Bolosov, I.A.; Sychev, S.V.; Shenkarev, Z.O.; Ovchinnikova, T.V. Structure elucidation and functional studies of a novel β-hairpin antimicrobial peptide from the marine polychaeta Capitella teleta. Mar. Drugs 2020, 18, 620. [Google Scholar] [CrossRef]
- Tincu, J.A.; Menzel, L.P.; Azimov, R.; Sands, J.; Hong, T.; Waring, A.J.; Taylor, S.W.; Lehrer, R.I. Plicatamide, an antimicrobial octapeptide from Styela plicata hemocytes. J. Biol. Chem. 2003, 278, 13546–13553. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, K.N.; Lee, Y.S.; Nam, M.H.; Lee, I.H. Halocidin: A new antimicrobial peptide from hemocytes of the solitary tunicate, Halocynthia aurantium. FEBS Lett. 2002, 521, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Franchi, N.; Ballarin, L.; Cima, F. Botryllin, a novel antimicrobial peptide from the colonial ascidian Botryllus schlosseri. Mar. Drugs 2023, 21, 74. [Google Scholar] [CrossRef]
- Panteleev, P.V.; Balandin, S.V.; Ovchinnikova, T.V. Effect of arenicins and other beta-hairpin antimicrobial peptides on Pseudomonas aeruginosa PAI1 biofilms. Pharm. Chem. J. 2017, 50, 715–720. [Google Scholar] [CrossRef]
- Rivardo, F.; Turner, R.J.; Allegrone, G.; Ceri, H.; Martinotti, M.G. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl. Microbiol. Biotechnol. 2009, 83, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Larroze, S.; Mayer, C.; Teixeira, T.; Wengier, R.; Benayahu, Y.; Otero, A. Evaluation of the anti-fouling efficacy of Bacillus licheniformis extracts under environmental and natural conditions. Front. Mar. Sci. 2021, 8, 711108. [Google Scholar] [CrossRef]
- Muras, A.; López-Pérez, M.; Mayer, C.; Parga, A.; Amaro-Blanco, J.; Otero, A. High prevalence of quorum-sensing and quorum-quenching activity among cultivable bacteria and metagenomic sequences in the Mediterranean Sea. Genes 2018, 9, 100. [Google Scholar] [CrossRef]
- Soler, A.; Arregui, L.; Arroyo, M.; Mendoza, J.A.; Muras, A.; Álvarez, C. Quorum sensing versus quorum quenching bacterial isolates obtained from MBR plants treating leachates from municipal solid waste. Int. J. Environ. Res. Public Health 2018, 15, 1019. [Google Scholar] [CrossRef]
- Fusetani, N. Antifungal substances from marine invertebrates. Ann. N. Y. Acad. Sci. 1988, 544, 113–127. [Google Scholar] [CrossRef]
- Li, H.Y.; Matsunaga, S.; Fusetani, N. Antifungal metabolites from marine sponges. Curr. Org. Chem. 1998, 2, 649–682. [Google Scholar] [CrossRef]
- Nakamura, T.; Furunaka, H.; Miyata, T.; Tokunaga, F.; Muta, T.; Iwanaga, S. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J. Biol. Chem. 1988, 263, 16709–16713. [Google Scholar] [CrossRef]
- Fusetani, N. Antifungal peptides in marine invertebrates. Invertebr. Surviv. J. 2010, 7, 53–66. [Google Scholar]
- Pettit, R.K.; Pettit, G.R.; Hazen, K.C. Specific activities of dolastatin 10 and peptide derivatives against Cryptococcus neoformans. Antimicrob. Agents Chemother. 1998, 42, 2961–2965. [Google Scholar] [CrossRef]
- Luesch, H.; Moore, R.E.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H. Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J. Nat. Prod. 2001, 64, 907–910. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Y.; Hua, H.; Li, D.; Tang, C. Marine antitumor peptide dolastatin 10: Biological activity, structural modification and synthetic chemistry. Mar. Drugs 2021, 19, 363. [Google Scholar] [CrossRef] [PubMed]
- Shilabin, A.G.; Kasanah, N.; Wedge, D.E.; Hamann, M.T. Lysosome and HER3 (ErbB3) selective anticancer agent kahalalide F: Semisynthetic modifications and antifungal lead-exploration studies. J. Med. Chem. 2007, 50, 4340–4350. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.M.; Swaileh, Z.; Ammar, M.; Jaghama, W.; Yousef, M.; Karaman, R.A.; Bufo, S.; Scrano, L. Antifungal and antibacterial activities of isolated marine compounds. Toxins 2023, 15, 93. [Google Scholar] [CrossRef]
- Neuhof, T.; Schmieder, P.; Preussel, K.; Dieckmann, R.; Pham, H.; Bartl, F.; von Döhren, H. Hassallidin A, a glycosylated lipopeptide with antifungal activity from the cyanobacterium Hassallia sp. J. Nat. Prod. 2005, 68, 695–700. [Google Scholar] [CrossRef]
- Watson, A.; Agius, J.; Ackerly, D.; Beddoe, T.; Helbig, K. The Role of anti-viral effector molecules in mollusc hemolymph. Biomolecules 2022, 12, 345. [Google Scholar] [CrossRef]
- Santhiravel, S.; Dave, D.; Shahidi, F. Bioactives from marine resources as natural health products: A review. Pharmacol. Rev. 2025, 77, 100006. [Google Scholar] [CrossRef]
- Yang, L.; Niu, S.; Gao, J.; Zuo, H.; Yuan, J.; Weng, S.; He, J.; Xu, X. A single WAP domain (SWD)-containing protein with antiviral activity from pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 73, 167–174. [Google Scholar] [CrossRef]
- Novoa, B.; Romero, A.; Álvarez, Á.L.; Moreira, R.; Pereiro, P.; Costa, M.M.; Dios, S.; Estepa, A.; Parra, F.; Figueras, A. Antiviral activity of myticin C peptide from mussel: An ancient defense against herpesviruses. J. Virol. 2016, 90, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Zannella, C.; Mosca, F.; Mariani, F.; Franci, G.; Folliero, V.; Galdiero, M.; Tiscar, P.G.; Galdiero, M. Microbial diseases of bivalve mollusks: Infections, immunology and antimicrobial defense. Mar. Drugs 2017, 15, 182. [Google Scholar] [CrossRef]
- Costa, M.M.; Prado-Alvarez, M.; Gestal, C.; Li, H.; Roch, P.; Novoa, B.; Figueras, A. Functional and molecular immune response of Mediterranean mussel (Mytilus galloprovincialis) haemocytes against pathogen-associated molecular patterns and bacteria. Fish Shellfish Immunol. 2009, 26, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.M.; Dios, S.; Alonso-Gutierrez, J.; Romero, A.; Novoa, B.; Figueras, A. Evidence of high individual diversity on myticin C in mussel (Mytilus galloprovincialis). Dev. Comp. Immunol. 2009, 33, 162–170. [Google Scholar] [CrossRef]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; O’Keefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [CrossRef]
- Tietjen, I.; Williams, D.E.; Read, S.; Kuang, X.T.; Mwimanzi, P.; Wilhelm, E.; Markle, T.; Kinloch, N.N.; Naphen, C.N.; Tenney, K.; et al. Inhibition of NF-κB-dependent HIV-1 replication by the marine natural product bengamide A. Antivir. Res. 2018, 152, 94–103. [Google Scholar] [CrossRef] [PubMed]
- White, K.N.; Tenney, K.; Crews, P. The bengamides: A mini-review of natural sources, analogues, biological properties, biosynthetic origins, and future prospects. J. Nat. Prod. 2017, 80, 740–755. [Google Scholar] [CrossRef]
- Porras-Alcalá, C.; Moya-Utrera, F.; García-Castro, M.; Sánchez-Ruiz, A.; López-Romero, J.M.; Pino-González, M.S.; Díaz-Morilla, A.; Kitamura, S.; Wolan, D.W.; Prados, J.; et al. The development of the bengamides as new antibiotics against drug-resistant bacteria. Mar. Drugs 2022, 20, 373. [Google Scholar] [CrossRef]
- Shin, H.J.; Rashid, M.A.; Cartner, L.K.; Bokesch, H.R.; Wilson, J.A.; McMahon, J.B.; Gustafson, K.R. Stellettapeptins A and B, HIV-inhibitory cyclic depsipeptides from the marine sponge Stelletta sp. Tetrahedron Lett. 2015, 56, 4215–4219. [Google Scholar] [CrossRef]
- Elyakova, L.A.; Vaskovsky, B.V.; Khoroshilova, N.I.; Vantseva, S.I.; Agapkina, Y.Y. Isolation and structure of a novel peptide inhibitor of HIV-1 integrase from marine polychaetes. Russ. J. Bioorg. Chem. 2011, 37, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Festa, M.; Sansone, C.; Brunet, C.; Crocetta, F.; Di Paola, L.; Lombardo, M.; Bruno, A.; Noonan, D.M.; Albini, A. Cardiovascular Active Peptides of Marine Origin with ACE Inhibitory Activities: Potential Role as Anti-Hypertensive Drugs and in Prevention of SARSCoV-2 Infection. Int. J. Mol. Sci. 2020, 21, 8364. [Google Scholar] [CrossRef]
- Reynolds, D.; Huesemann, M.; Edmundson, S.; Sims, A.; Hurst, B.; Cady, S.; Beirne, N.; Freeman, J.; Berger, A.; Gao, S. Viral Inhibitors Derived from Macroalgae, Microalgae, and Cyanobacteria: A Review of Antiviral Potential throughout Pathogenesis. Algal Res. 2021, 57, 102331. [Google Scholar] [CrossRef]
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin Has Potent Preclinical Efficacy against SARS-CoV-2 by Targeting the Host Protein EEF1A. Science 2021, 371, 926–931. [Google Scholar] [CrossRef]
- Sahin, S.; Calapoğlu, F.; Ozmen, I. Didemnins Inhibit COVID-19 Main Protease (MPRO). Biointerface Res. Appl. Chem. 2021, 11, 8204–8209. [Google Scholar] [CrossRef]
- Gentile, D.; Patamia, V.; Scala, A.; Sciortino, M.T.; Piperno, A.; Rescifina, A. Putative Inhibitors of SARS-CoV-2 Main Protease from a Library of Marine Natural Products: A Virtual Screening and Molecular Modeling Study. Mar. Drugs 2020, 18, 225. [Google Scholar] [CrossRef]
- Ashhurst, A.S.; Tang, A.H.; Fajtová, P.; Yoon, M.C.; Aggarwal, A.; Bedding, M.J.; Stoye, A.; Beretta, L.; Pwee, D.; Drelich, A.; et al. Potent Anti-SARS-CoV-2 Activity by the Natural Product Gallinamide A and Analogues via Inhibition of Cathepsin L. J. Med. Chem. 2022, 65, 2956–2970. [Google Scholar] [CrossRef]
- Ribeiro, R.; Costa, L.; Pinto, E.; Sousa, E.; Fernandes, C. Therapeutic Potential of Marine-Derived Cyclic Peptides as Antiparasitic Agents. Mar. Drugs 2023, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Makioka, A.; Kawazu, S.I.; Kano, S.; Kawai, S.; Akaki, M.; Aikawa, M.; Ohtomo, H. Effect of jasplakinolide on the growth, invasion, and actin cytoskeleton of Plasmodium falciparum. Parasitol. Res. 2002, 88, 844–848. [Google Scholar] [CrossRef]
- Scott, V.R.; Boehme, R.; Matthews, T.R. New class of antifungal agents: Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis species. Antimicrob. Agents Chemother. 1988, 32, 1154–1157. [Google Scholar] [CrossRef]
- Bubb, M.R.; Senderowicz, A.M.J.; Sausville, E.A.; Duncan, K.L.K.; Korn, E.D. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 1994, 269, 14869–14871. [Google Scholar] [CrossRef] [PubMed]
- Bubb, M.R.; Spector, I.; Beyer, B.B.; Fosen, K.M. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J. Biol. Chem. 2000, 275, 5163–5170. [Google Scholar] [CrossRef]
- Makioka, A.; Kumagai, M.; Ohtomo, H.; Kobayashi, S.; Takeuchi, T. Effect of jasplakinolide on the growth, encystation, and actin cytoskeleton of Entamoeba histolytica and Entamoeba invadens. J. Parasitol. 2001, 87, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Poupel, O.; Tardieux, I. Toxoplasma gondii motility and host cell invasiveness are drastically impaired by jasplakinolide, a cyclic peptide stabilizing F-actin. Microbes Infect. 1999, 1, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Makioka, A.; Kumagai, M.; Ohtomo, H.; Kobayashi, S.; Takeuchi, T. Growth inhibition and actin aggregate formation of Entamoeba histolytica by jasplakinolide. Arch. Med. Res. 2000, 31, S145–S146. [Google Scholar] [CrossRef] [PubMed]
- Bürstner, N.; Roggo, S.; Ostermann, N.; Blank, J.; Delmas, C.; Freuler, F.; Gerhartz, B.; Hinniger, A.; Hoepfner, D.; Liechty, B.; et al. Gift from Nature: Cyclomarin A Kills Mycobacteria and Malaria Parasites by Distinct Modes of Action. ChemBioChem 2015, 16, 2433–2436. [Google Scholar] [CrossRef]
- Intaraudom, C.; Rachtawee, P.; Suvannakad, R.; Pittayakhajonwut, P. Antimalarial and antituberculosis substances from Streptomyces sp. BCC26924. Tetrahedron 2011, 67, 7593–7597. [Google Scholar] [CrossRef]
- Linington, R.G.; Edwards, D.J.; Shuman, C.F.; McPhail, K.L.; Matainaho, T.; Gerwick, W.H. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2008, 71, 22–27. [Google Scholar] [CrossRef]
- Faisal, M.R.; Kawaroe, M.; Satrija, F. Potensi Antelmintika Ekstrak Bakteri Simbion Spons Laut Terhadap Trichostrongylidae (Nematoda) Parasit Domba. J. Ilmu Pertan. Indones. 2017, 21, 41–47. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef]
- Oh, D.C.; Strangman, W.K.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Thalassospiramides A and B, immunosuppressive peptides from the marine bacterium Thalassospira sp. Org. Lett. 2007, 9, 1525–1528. [Google Scholar] [CrossRef]
- Um, S.; Pyee, Y.; Kim, E.H.; Lee, S.K.; Shin, J.; Oh, D.C. Thalassospiramide G, a new gamma-amino-acid-bearing peptide from the marine bacterium Thalassospira sp. Mar. Drugs 2013, 11, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Kim, I.H.; Nam, T.J. Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- García-Beltrán, J.M.; Arizcun, M.; Chaves-Pozo, E. Antimicrobial Peptides from Photosynthetic Marine Organisms with Potential Application in Aquaculture. Mar. Drugs 2023, 21, 290. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Jiang, X.-X.; Zhao, Q.-L.; Ye, H.-W.; Lin, Y.; Huang, J.; Tang, Y.-P. Immunoenhancing Effects of Cyclina sinensis Pentadecapeptide through Modulation of Signaling Pathways in Mice with Cyclophosphamide-Induced Immunosuppression. Mar. Drugs 2022, 20, 560. [Google Scholar] [CrossRef]
- Yao, L.; Yang, P.; Luo, W.; Li, S.; Wu, Y.; Cai, N.; Bi, D.; Li, H.; Han, Q.; Xu, X. Macrophage-stimulating activity of European eel (Anguilla anguilla) peptides in RAW264.7 cells mediated via NF-κB and MAPK signaling pathways. Food Funct. 2020, 11, 10968–10978. [Google Scholar] [CrossRef]
- Hayes, M.; Naik, A.; Mora, L.; Iñarra, B.; Ibarruri, J.; Bald, C.; Cariou, T.; Reid, D.; Gallagher, M.; Dragøy, R.; et al. Generation, Characterisation and Identification of Bioactive Peptides from Mesopelagic Fish Protein Hydrolysates Using In Silico and In Vitro Approaches. Mar. Drugs 2024, 22, 297. [Google Scholar] [CrossRef]
- Vijayanand, M.; Issac, P.K.; Velayutham, M.; Shaik, M.R.; Hussain, S.A.; Shaik, B.; Guru, A. Protective Effects of KC14 Peptide from Cyprinus carpio on Copper Sulfate-Induced Toxicity in Zebrafish Larvae: Insights into Anti-inflammatory Cytokine and Glutathione Modulations. Int. J. Pept. Res. Ther. 2025, 31, 49. [Google Scholar] [CrossRef]
- Khatun, M.S.; Hasan, M.M.; Kurata, H. PreAIP: Computational Prediction of Anti-inflammatory Peptides by Integrating Multiple Complementary Features. Front. Genet. 2019, 10, 00129. [Google Scholar] [CrossRef]
- Umnyakova, E.S.; Gorbunov, N.P.; Zhakhov, A.V.; Krenev, I.A.; Ovchinnikova, T.V.; Kokryakov, V.N.; Berlov, M.N. Modulation of Human Complement System by Antimicrobial Peptide Arenicin-1 from Arenicola marina. Mar. Drugs 2018, 16, 480. [Google Scholar] [CrossRef]
- Krenev, I.A.; Umnyakova, E.S.; Eliseev, I.E.; Dubrovskii, Y.A.; Gorbunov, N.P.; Pozolotin, V.A.; Komlev, A.S.; Panteleev, P.V.; Balandin, S.V.; Ovchinnikova, T.V.; et al. Antimicrobial Peptide Arenicin-1 Derivative Ar-1-(C/A) as Complement System Modulator. Mar. Drugs 2020, 18, 631. [Google Scholar] [CrossRef]
- Berlov, M.N.; Umnyakova, E.S.; Leonova, T.S.; Milman, B.L.; Krasnodembskaya, A.D.; Ovchinnikova, T.V.; Kokryakov, V.N. Interaction of Arenicin-1 with C1q Protein. Russ. J. Bioorg. Chem. 2015, 41, 597–601. [Google Scholar] [CrossRef]
- Krenev, I.A.; Panteleev, P.V.; Umnyakova, E.S.; Gorbunov, N.P.; Kostevich, V.A.; Balandin, S.V.; Ovchinnikova, T.V.; Aleshina, G.M.; Berlov, M.N. In Vitro Modulation of Complement Activation by Therapeutically Prospective Analogues of the Marine Polychaeta Arenicin Peptides. Mar. Drugs 2022, 20, 612. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Mao, W.; Yi, Y.; Lu, Y.; Liu, F.; Deng, L. The effects of four paralogous piscidin antimicrobial peptides on the chemotaxis, macrophage respiratory burst, phagocytosis and expression of immune-related genes in orange-spotted grouper (Epinephelus coicodes). Dev. Comp. Immunol. 2024, 154, 105144. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, G.; Capillo, G.; Fernandes, J.M.O.; Kiron, V.; Lauriano, E.R.; Alesci, A.; Lo Cascio, P.; Guerrera, M.C.; Kuciel, M.; Zuwala, K.; et al. Expression of the Antimicrobial Peptide Piscidin 1 and Neuropeptides in Fish Gill and Skin: A Potential Participation in Neuro-Immune Interaction. Mar. Drugs 2022, 20, 145. [Google Scholar] [CrossRef]
- Arockiaraj, J.; Gnanam, A.J.; Muthukrishnan, D.; Gudimella, R.; Milton, J.; Singh, A. Crustin, a Wap Domain Containing Antimicrobial Peptide from Freshwater Prawn Macrobrachium rosenbergii: Immune Characterization. Fish Shellfish Immunol. 2013, 34, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Pisuttharachai, D.; Fagutao, F.F.; Yasuike, M.; Aono, H.; Yano, Y.; Murakami, K. Characterization of Crustin Antimicrobial Proteins from Japanese Spiny Lobster Panulirus japonicus. Dev. Comp. Immunol. 2009, 33, 1049–1054. [Google Scholar] [CrossRef]
- Barreto, C.; Coelho, J.D.R.; Yuan, J.; Xiang, J.; Perazzolo, L.M.; Rosa, R.D. Specific Molecular Signatures for Type II Crustins in Penaeid Shrimp Uncovered by the Identification of Crustin-Like Antimicrobial Peptides in Litopenaeus vannamei. Mar. Drugs 2018, 16, 31. [Google Scholar] [CrossRef]
- Supungul, P.; Tang, S.; Maneeruttanarungroj, C.; Rimphanitchayakit, V.; Hirono, I.; Aoki, T. Cloning, Expression and Antimicrobial Activity of CrustinPm1, a Major Isoform of Crustin, From the Black Tiger Shrimp Penaeus monodon. Dev. Comp. Immunol. 2008, 32, 61–70. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, R.; Chen, F.; Zhu, X.; Wang, K.J. A New Crustin Gene Homolog SpCrus8 Identified in Scylla paramamosain Exerting In Vivo Protection Through Opsonization and Immunomodulation. Front. Immunol. 2022, 13, 946227. [Google Scholar] [CrossRef]
- Jiang, H.S.; Sun, C.; Wang, T.; Zhao, X.F.; Wang, J.X. A Single Whey Acidic Protein Domain Containing Protein (Swd) Inhibits Bacteria Invasion and Dissemination in Shrimp Marsupenaeus japonicus. Fish Shellfish Immunol. 2013, 35, 310–318. [Google Scholar] [CrossRef]
- Liu, N.; Lan, J.F.; Sun, J.J.; Jia, W.M.; Zhao, X.F.; Wang, J.X. A Novel Crustin from Marsupenaeus japonicus Promotes Hemocyte Phagocytosis. Dev. Comp. Immunol. 2015, 49, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Shockey, J.E.; O’Leary, N.A.; de la Vega, E.; Browdy, C.L.; Baatz, J.E.; Gross, P.S. The role of crustins in Litopenaeus vannamei in response to infection with shrimp pathogens: An in vivo approach. Dev. Comp. Immunol. 2009, 33, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs 2017, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.C.; Wilke, D.V.; Branco, P.C.; Bauermeister, A.; Rezende-Teixeira, P.; Gaudêncio, S.P.; Costa-Lotufo, L.V. Enriching cancer pharmacology with drugs of marine origin. Br. J. Pharmacol. 2020, 177, 3–27. [Google Scholar] [CrossRef]
- Beckwith, M.; Urba, W.J.; Longo, D.L. Growth inhibition of human lymphoma cell lines by the marine products, dolastatins 10 and 15. J. Natl. Cancer Inst. 1993, 85, 483–488. [Google Scholar] [CrossRef]
- Ahmed, S.; Aschner, M.; Alsharif, K.F.; Allahyani, M.; Huang, G.; Wan, C.; Khan, H. Marine peptides in lymphoma: Surgery at molecular level for therapeutic understanding. Naunyn Schmiedebergs Arch. Pharmacol. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Rosati, R.; Pettit, G.R.; Kalemkerian, G.P. Dolastatin 15 induces apoptosis and BCL-2 phosphorylation in small cell lung cancer cell lines. Anticancer Res. 1998, 18, 1021–1026. [Google Scholar] [PubMed]
- Gong, F.; Chen, M.-F.; Zhang, Y.-Y.; Li, C.-Y.; Zhou, C.-X.; Hong, P.-Z.; Sun, S.-L.; Qian, Z.-J. A Novel Peptide from Abalone (Haliotis discus hannai) to Suppress Metastasis and Vasculogenic Mimicry of Tumor Cells and Enhance Anti-Tumor Effect In Vitro. Mar. Drugs 2019, 17, 244. [Google Scholar] [CrossRef]
- Anderson, H.J.; Coleman, J.E.; Andersen, R.J.; Roberge, M. Cytotoxic peptides hemiasterlin, hemiasterlin A and hemiasterlin B induce mitotic arrest and abnormal spindle formation. Cancer Chemother. Pharmacol. 1997, 39, 223–226. [Google Scholar] [CrossRef]
- Lu, S.; Lin, J.; Jin, J.; Zhang, L.; Guan, Y.; Chen, H.; Wu, Y.; Zhang, W.; Luan, X. Tachyplesin I and its derivatives: A pharmaco-chemical perspective on their antimicrobial and antitumor potential. Expert Opin. Drug Discov. 2022, 17, 1407–1423. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, X.; Zhang, J.; Chen, J.; Wang, Y.; Wei, T.; Ma, J.; Li, Y.; Mo, T.; He, Z.; et al. Tachyplesin induces apoptosis in non-small cell lung cancer cells and enhances the chemosensitivity of A549/DDP cells to cisplatin by activating Fas and necroptosis pathway. Chem. Biol. Drug Des. 2021, 97, 809–820. [Google Scholar] [CrossRef]
- Kuzmin, D.V.; Emel’yanova, A.A.; Kalashnikova, M.B.; Panteleev, P.V.; Ovchinnikova, T.V. In Vitro Study of Antitumor Effect of Antimicrobial Peptide Tachyplesin I in Combination with Cisplatin. Bull. Exp. Biol. Med. 2018, 165, 220–224. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Balandin, S.V.; Belogurova-Ovchinnikova, O.Y.; Ovchinnikova, T.V. Marine Invertebrate Antimicrobial Peptides and Their Potential as Novel Peptide Antibiotics. Mar. Drugs 2023, 21, 503. [Google Scholar] [CrossRef] [PubMed]
- Marggraf, M.B.; Panteleev, P.V.; Emelianova, A.A.; Sorokin, M.I.; Bolosov, I.A.; Buzdin, A.A.; Kuzmin, D.V.; Ovchinnikova, T.V. Cytotoxic potential of the novel horseshoe crab peptide polyphemusin III. Mar. Drugs 2018, 16, 466. [Google Scholar] [CrossRef] [PubMed]
- Kuentzel, S.L.; Li, L.H. Didemnins: Antiviral and antitumor depsipeptides from a caribbean tunicate. Science 1981, 212, 933–935. [Google Scholar] [CrossRef]
- Rinehart, K.L., Jr.; Gloer, J.B.; Wilson, G.R.; Hughes, R.G., Jr.; Li, L.H.; Renis, H.E.; McGovren, J.P. Antiviral and antitumor compounds from tunicates. Fed. Proc. 1983, 42, 87–90. [Google Scholar] [PubMed]
- SirDeshpande, B.V.; Toogood, P.L. Mechanism of protein synthesis inhibition by didemnin B in vitro. Biochemistry 1995, 34, 9177–9184. [Google Scholar] [CrossRef]
- Ahuja, D.; Vera, M.D.; SirDeshpande, B.V.; Morimoto, H.; Williams, P.G.; Joullié, M.M.; Toogood, P.L. Inhibition of protein synthesis by didemnin B: How EF-1alpha mediates inhibition of translocation. Biochemistry 2000, 39, 4339–4346. [Google Scholar] [CrossRef]
- Lobo, C.; García-Pozo, S.G.; Núñez de Castro, I.; Alonso, F.J. Effect of dehydrodidemnin B on human colon carcinoma cell lines. Anticancer Res. 1997, 17, 333–336. [Google Scholar] [PubMed]
- Dmitriev, S.E.; Vladimirov, D.O.; Lashkevich, K.A. A Quick Guide to Small-Molecule Inhibitors of Eukaryotic Protein Synthesis. Biochemistry 2020, 85, 1389–1421. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.-C.; Kuo, H.-M.; Yu, C.-L.; Selvam, P.; Su, I.-L.; Tseng, C.-C.; Yuan, C.-H.; Wen, Z.-H. Marine-derived antimicrobial peptide piscidin-1 triggers extrinsic and intrinsic apoptosis in oral squamous cell carcinoma through reactive oxygen species production and inhibits angiogenesis. Free Radic. Biol. Med. 2024, 220, 28–42. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Liu, Z.; Pe, X.; Han, X.; Li, Y. Antioxidant effect of a marine oligopeptide preparation from chum salmon (Oncorhynchus keta) by enzymatic hydrolysis in radiation injured mice. Mar. Drugs 2011, 9, 2304–2315. [Google Scholar] [CrossRef]
- Suetsuna, K.; Nakano, T. Identification of an Antihypertensive Peptide from Peptic Digest of Wakame (Undaria pinnatifida). J. Nutr. Biochem. 2000, 11, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, Y.; San, S. Efficacy of a Novel ACE-Inhibitory Peptide from Sargassum maclurei in Hypertension and Reduction of Intracellular Endothelin-1. Nutrients 2020, 12, 653. [Google Scholar] [CrossRef]
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef]
- Kawasaki, T.; Seki, E.; Osajima, K.; Yoshida, M.; Asada, K.; Matsui, T.; Osajima, Y. Antihypertensive effect of valyl-tyrosine, a short chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects. J. Hum. Hypertens. 2000, 14, 519–523. [Google Scholar] [CrossRef]
- Yokoyama, K.; Chiba, H.; Yoshikawa, M. Peptide inhibitors for angiotensin i-converting enzyme from thermolysin digest of dried bonitot. Biosci. Biotechnol. Biochem. 1992, 56, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Yoshikawa, M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology 1999, 44, 123–127. [Google Scholar] [CrossRef]
- Aissaoui, N.; Abidi, F.; Hardouin, J.; Abdelkafi, Z.; Marrakchi, N.; Jouenne, T.; Marzouki, M.N. Two novel peptides with angiotensin I converting enzyme inhibitory and antioxidative activities from Scorpaena notata muscle protein hydrolysate. Biotechnol. Appl. Biochem. 2017, 64, 201–210. [Google Scholar] [CrossRef]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xiang, B.; Dou, B.; Hu, J.; Zhang, L.; Kang, X.; Lyu, M.; Wang, S. Novel Angiotensin-Converting Enzyme-Inhibitory Peptides Obtained from Trichiurus lepturus: Preparation, Identification and Potential Antihypertensive Mechanism. Biomolecules 2024, 14, 581. [Google Scholar] [CrossRef] [PubMed]
- Layer, R.T.; McIntosh, J.M. Conotoxins: Therapeutic Potential and Application. Mar. Drugs 2006, 4, 119–142. [Google Scholar] [CrossRef] [PubMed Central]
- Smith, M.T.; Cabot, P.J.; Ross, F.B.; Robertson, A.D.; Lewis, R.J. The novel N-type calcium channel blocker, AM336, produces potent dose-dependent antinociception after intrathecal dosing in rats and inhibits substance P release in rat spinal cord slices. Pain 2002, 96, 119–127. [Google Scholar] [CrossRef]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef]
- Herzig, V.; Cristofori-Armstrong, B.; Israel, M.R.; Nixon, S.A.; Vetter, I.; King, G.F. Animal toxins—Nature’s evolutionary-refined toolkit for basic research and drug discovery. Biochem. Pharmacol. 2020, 181, 114096. [Google Scholar] [CrossRef]
- Vetter, I.; Lewis, R.J. Therapeutic potential of cone snail venom peptides (conopeptides). Curr. Top. Med. Chem. 2012, 12, 1546–1552. [Google Scholar] [CrossRef]
- Robinson, S.D.; Vetter, I. Pharmacology and therapeutic potential of venom peptides. Biochem. Pharmacol. 2020, 181, 114207. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Sarandy, M.M.; de Oliveira, L.L.; da Matta, S.L.P.; Novaes, R.D.; Gonçalves, R.V. Positive Effect of Peptides Obtained from Nile Tilapia (Oreochromis niloticus) on Inflammation Regulation and Wound Healing. Cosmetics 2024, 11, 133. [Google Scholar] [CrossRef]
- Peng, X.; Xu, J.; Tian, Y.; Liu, W.; Peng, B. Marine fish peptides (collagen peptides) compound intake promotes wound healing in rats after cesarean section. Food Nutr. Res. 2020, 64, 4247. [Google Scholar] [CrossRef]
- Sitje, T.S.; Cornelius, E.G.; Sørensen, J.A. Clinical innovation: Fish-derived wound product for cutaneous wounds. Wounds Int. 2018, 9, 44–50. [Google Scholar]
- Rey-Campos, M.; Moreira, R.; Romero, A.; Medina-Gali, R.M.; Novoa, B.; Gasset, M.; Figueras, A. Transcriptomic Analysis Reveals the Wound Healing Activity of Mussel Myticin, C. Biomolecules 2020, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Shin, Y.P.; Shin, S.H.; Park, S.; Kim, M.H.; Lee, I.H. Therapeutic efficacy of halocidin-derived peptide HG1 in a mouse model of surgical wound infection with methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 1296–1299. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Zhang, B.; Zhao, Y.; Wang, N. Potential Active Marine Peptides as Anti-Aging Drugs or Drug Candidates. Mar. Drugs 2023, 21, 144. [Google Scholar] [CrossRef]
- Piskovatska, V.; Strilbytska, O.; Koliada, A.; Vaiserman, A.; Lushchak, O. Health benefits of anti-aging drugs. Biochem. Cell Biol. Ageing Part II Clin. Sci. 2019, 91, 339–392. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Zheng, L.; Zhao, M.; Fan, J.; Liu, S. Preparation of sea cucumber (Stichopus variegates) peptide fraction with desired organoleptic property and its anti-aging activity in fruit flies and D-galactose-induced aging mice. J. Funct. Foods 2020, 69, 103954. [Google Scholar] [CrossRef]
- Guo, K.; Su, L.; Wang, Y.; Liu, H.; Lin, J.; Cheng, P.; Yin, X.; Liang, M.; Wang, Q.; Huang, Z. Antioxidant and anti-aging effects of a sea cucumber protein hydrolyzate and bioinformatic characterization of its composing peptides. Food Funct. 2020, 11, 5004–5016. [Google Scholar] [CrossRef]
- Lin, L.; Yang, K.; Zheng, L.; Zhao, M.; Sun, W.; Zhu, Q.; Liu, S. Anti-aging effect of sea cucumber (Cucumaria frondosa) hydrolysate on fruit flies and d-galactose-induced aging mice. J. Funct. Foods 2018, 47, 11–18. [Google Scholar] [CrossRef]
- Kuang, X.; Chen, Y.-S.; Wang, L.-F.; Li, Y.-J.; Liu, K.; Zhang, M.-X.; Li, L.-J.; Chen, C.; He, Q.; Wang, Y.; et al. Klotho upregulation contributes to the neuroprotection of ligustilide in an Alzheimer’s disease mouse model. Neurobiol. Aging 2014, 35, 169–178. [Google Scholar] [CrossRef]
- Alvariño, R.; Alonso, E.; Bornancin, L.; Bonnard, I.; Inguimbert, N.; Banaigs, B.; Botana, L.M. Biological Activities of Cyclic and Acyclic B-Type Laxaphycins in SH-SY5Y Human Neuroblastoma Cells. Mar. Drugs 2020, 18, 364. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, J.; Zhong, H.; Abdullah; Zhuang, J.; Zhang, J.; Wang, J.; Zhang, X.; Feng, F. High-degree hydrolysis sea cucumber peptides improve exercise performance and exert antifatigue effect via activating the NRF2 and AMPK signaling pathways in mice. J. Funct. Foods 2021, 86, 104677. [Google Scholar] [CrossRef]
- Han, J.; Huang, Z.; Tang, S.; Lu, C.; Wan, H.; Zhou, J.; Li, Y.; Ming, T.; Wang, Z.J.; Su, X. The novel peptides ICRD and LCGEC screened from tuna roe show antioxidative activity via Keap1/Nrf2-ARE pathway regulation and gut microbiota modulation. Food Chem. 2020, 327, 94. [Google Scholar] [CrossRef]
- Chen, J.; Liang, P.; Xiao, Z.; Chen, M.-F.; Gong, F.; Li, C.; Zhou, C.; Hong, P.; Jung, W.-K.; Qian, Z.-J. Antiphotoaging effect of boiled abalone residual peptide ATPGDEG on UVB-induced keratinocyte HaCaT cells. Food Nutr. Res. 2019, 63, 3508. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.-W.; Hu, X.-M.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments. Mar. Drugs 2022, 20, 626. [Google Scholar] [CrossRef]
- Pan, N.; Li, Z.-C.; Li, Z.-H.; Chen, S.-H.; Jiang, M.-H.; Yang, H.-Y.; Liu, Y.-S.; Hu, R.; Zeng, Y.-W.; Dai, L.-H.; et al. Antiplatelet and Antithrombotic Effects of Isaridin E Isolated from the Marine-Derived Fungus via Downregulating the PI3K/Akt Signaling Pathway. Mar. Drugs 2022, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Battistuzzi, F.U.; Feijao, A.; Hedges, S.B. A genomic timescale of prokaryote evolution: Insights into the origin of methanogenesis, phototrophy, and the colonization of land. BMC Evol. Biol. 2004, 4, 44. [Google Scholar] [CrossRef]
- Guryanova, S.V. Immunomodulation, Bioavailability and Safety of Bacteriocins. Life 2023, 13, 1521. [Google Scholar] [CrossRef]
- Parfrey, L.W.; Lahr, D.J.; Knoll, A.H.; Katz, L.A. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc. Natl. Acad. Sci. USA 2011, 108, 13624–13629. [Google Scholar] [CrossRef]
- Pundir, P.; Catalli, A.; Leggiadro, C.; Douglas, S.E.; Kulka, M. Pleurocidin, a novel antimicrobial peptide, induces human mast cell activation through the FPRL1 receptor. Mucosal Immunol. 2014, 7, 177–187. [Google Scholar] [CrossRef]
- Ebrahimdoost, M.; Mohammadi, M.; Obeidi, N.; Mohammadi, S.A.; Khamisipour, G. A Pleurocidin-Like Peptide from Poecilia Mexicana Fish Induces Selective Cytotoxicity in Leukemia Jurkat Cells Through the Apoptosis Pathway. Cell 2023, 25, 76–84. [Google Scholar] [CrossRef]
- Velayutham, M.; Arockiaraj, J. Aquatic peptides: Prospects and limitations in developing them as therapeutic products. AACL Bioflux 2022, 15, 195–211. [Google Scholar]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef] [PubMed]
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs? Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 803, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Tasiamide, a cytotoxic peptide from the marine cyanobacterium Symploca sp. J. Nat. Prod. 2002, 65, 1336–1339. [Google Scholar] [CrossRef]
- Kanaujia, K.A.; Wagh, S.; Pandey, G.; Phatale, V.; Khairnar, P.; Kolipaka, T.; Rajinikanth, P.S.; Saraf, S.A.; Srivastava, S.; Kumar, S. Harnessing marine antimicrobial peptides for novel therapeutics: A deep dive into ocean-derived bioactives. Int. J. Biol. Macromol. 2025, 307, 142158. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Li, W.; Li, Y.; Zhang, J.; Qin, S. Progress in Preparation Technology and Functional Research on Marine Bioactive Peptides. Mar. Biotechnol. 2025, 27, 42. [Google Scholar] [CrossRef]
- Qiao, K.; Huang, Q.; Sun, T.; Chen, B.; Huang, W.; Su, Y.; Lin, H.; Liu, Z. Preparation and Efficacy Evaluation of Antihyperuricemic Peptides from Marine Sources. Nutrients 2024, 16, 4301. [Google Scholar] [CrossRef]
- Hoeng, J.; Boue, S.; Fields, B.; Park, J.; Peitsch, M.C.; Schlage, W.K.; Talikka, M.; Binenbaum, I.; Bondarenko, V.; Bulgakov, O.V.; et al. Enhancement of COPD biological networks using a web-based collaboration interface. F1000 Res. 2015, 4, 32. [Google Scholar] [CrossRef]
- Guryanova, S.; Guryanova, A. sbv IMPROVER: Modern Approach to Systems Biology. Methods Mol. Biol. 2017, 1613, 21–29. [Google Scholar] [CrossRef]
- Ouyang, Y.; Yue, Y.; Wu, N.; Wang, J.; Geng, L.; Zhang, Q. Identification and anticoagulant mechanisms of novel factor XIa inhibitory peptides by virtual screening of a in silico generated deep-sea peptide database. Food Res. Int. 2024, 197 Pt 2, 115308. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.-L.; Harris, J.L.; Khanna, K.K.; Hong, J.-H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- Rahman, A.; Rehmani, R.; Pirvu, D.G.; Huang, S.M.; Puri, S.; Arcos, M. Unlocking the Therapeutic Potential of Marine Collagen: A Scientific Exploration for Delaying Skin Aging. Mar. Drugs 2024, 22, 159. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110963. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef]
- Bauer, M.; Glowacka, M.; Kamysz, W.; Kleczkowska, P. Marine Peptides: Potential Basic Structures for the Development of Hybrid Compounds as Multitarget Therapeutics for the Treatment of Multifactorial Diseases. Int. J. Mol. Sci. 2024, 25, 12601. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; He, D.; Zhao, X.; Miao, L.; Cao, Z. Structure, function, and therapeutic potential of defensins from marine animals. Fish Shellfish Immunol. 2025, 163, 110365. [Google Scholar] [CrossRef]
- Yang, G.; Lin, M.; Kaliaperumal, K.; Lu, Y.; Qi, X.; Jiang, X.; Xu, X.; Gao, C.; Liu, Y.; Luo, X. Recent Advances in Anti-Inflammatory Compounds from Marine Microorganisms. Mar. Drugs 2024, 22, 424. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Y.; Lin, X.; Yuan, Z.; Liu, M.; Cao, S.; Zhang, F.; Linhardt, R.J. Recent Progress of Marine Polypeptides as Anticancer Agents. Recent Pat. Anti-Cancer Drug Discov. 2018, 13, 445–454. [Google Scholar] [CrossRef]
- Baindara, P.; Dinata, R.; Mandal, S.M. Marine Bacteriocins: An Evolutionary Gold Mine to Payoff Antibiotic Resistance. Mar. Drugs 2024, 22, 388. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Ma, Q.Y.; Gao, X.Z.; Wang, X.; Zhang, B.L. Research progress in anti-inflammatory bioactive substances derived from marine microorganisms, sponges, algae, and corals. Mar. Drugs 2021, 19, 572. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, J.T.; Waddad, A.Y.; Albericio, F.; de la Torre, B.G. Antimicrobial Peptide Synergies for Fighting Infectious Diseases. Adv. Sci. 2023, 10, e2300472. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Moscoso, C.A.; Merlano, J.A.R.; Olivera Gálvez, A.; Volcan Almeida, D. Antimicrobial peptides (AMPs) from microalgae as an alternative to conventional antibiotics in aquaculture. Prep. Biochem. Biotechnol. 2025, 55, 26–35. [Google Scholar] [CrossRef]

| Taxa | Source Organism | Peptide | Activity | References |
|---|---|---|---|---|
| Microorganisms | Marine bacteria Thalassospira sp. | Thalassospiramides A and D | Immunomodulation: suppress LPS-induced NO production, inhibit IL-5 expression in TH-2-mediated inflammatory diseases | [100,101,102] |
| Cyanobacterium Schizothrix | Gallinamide A 1 | Immunomodulation: inhibits SARS-CoV-2 | [87] | |
| Cyanobacteria Symploca sp. | Symplocamide | Antiparasitic activity: inhibits P. falciparum, T. cruzi and L. donovani | [98] | |
| Sponge-derived bacterium Bacillus licheniformis | Lipopeptide | Antibiofilm activity against Staphylococcus aureus, Escherichia coli, and Bugula neritina | [55] | |
| Bacterium Streptomyces sp. | Cyclomarins | Antiparasitic activity against multidrug-resistant P. falciparum | [96,97] | |
| Bearded seal-derived cyanobacteria | Dolastatins | Antifungal activity against Cryptococcus neoformans; antiproliferative activity (inhibition of tubulin polymerization); antitumor activity | [63,64,65] | |
| Cyanobacteria Hassallia sp. | Hassallidin A | Antifungal activity against A. fumigatus and C. albicans | [67,68] | |
| Algae and plants | Brown algae Undaria pinnatifida | AIYK, YKYY, KFYG, and YNKL | Antihypertensive activity (inhibition of ACE) | [144] |
| Brown algae Sargassum maclurei | RWDISQPY | Antihypertensive activity; endothelin-1 suppressing activity | [145] | |
| Sea slug Elysia rufescens, green algae Bryopsis pennata | Kahalalide F | Antifungal activity against C. albicans, C. neoformans, and Aspergillus fumigatus | [66] | |
| Blue-green alga Nostoc ellipsosporum | Cyanovirin-N | Antiviral activity: inhibits HIV by binding to glycoproteins of the viral envelope | [76] | |
| Marine algae Pyropia yezoensis | PPY1 | Anti-inflammatory acivity: inhibits LPS-stimulated NO release, reduces the release of proiflammatory cytokines in LPS-stimulated macrophages | [99,103,104] | |
| Invertebrates | Mussel Mytilus galloprovincialis | Myticin C | Antiviral activity against herpesviruses | [72,75] |
| Jellyfish Aurelia aurita | Aurelin | Antimicrobial activity | [21,27] | |
| Polychaeta Nicomache minor | Nicomicin | Antimicrobial activity | [30] | |
| Polychaeta Capitella teleta | Capitellacin | Antimicrobial and antibiofilm activities | [48,49,50] | |
| Polychaeta Arenicola marina | Arenicin | Antimicrobial activity; influence on complement system | [37,38,39,40,41,54,110,111,112,113] | |
| Polychaeta Abarenicola pacifica | Abarenicin | Antimicrobial and antibiofilm activities | [48,49,50] | |
| Polychaeta Urechis unicinctus | UuBRI-2 | Antimicrobial and antibiofilm activities | [48,49,50] | |
| Porifera sponge Discodermia kiiensis | Discodermin A | Antifungal activity against Candida albicans | [59,60] | |
| Horseshoe crab Tachypleus tridentatus, | Tachyplesin | Antifungal activity against Candida albicans | [61,62] | |
| Subphylum Crustacea (crabs, shrimps, and lobsters) | Crustins | Opsonization of bacteria | [115,116,117,118,119,120,121,122] | |
| Vertebrates | Fish Japanese sea bass Lateolabrax japonicus | Hepcidine | Antimicrobial activity | [42,43,44,45,46,47] |
| Orange-spotted grouper Epinephelus coioides | Epinecidin-1 | Antifungal activity against Botrytis cinerea and gray mold on peach fruits | [31,32] | |
| Orange-spotted grouper Epinephelus coioides | Piscidin ecPis2S | Chemotactic and phagocytic activities, the respiratory burst of macrophages, mRNA expression of chemokine receptors, Toll-like receptors, T-cell receptors, and proinflammatory cytokines | [114,115] | |
| Hybrid striped bass Morone saxatilis | Piscidin-1 | Anticancer activity: inhibits angiogenesis and induces apoptosis in oral squamous cell carcinoma through reactive oxygen species production | [142] | |
| Fish Maurolicus muelleri and Benthosema glaciale | QCPLHRPWAL | Anti-inflammatory and analgesic activities | [107] | |
| Carp Cyprinus carpio | KC14 | Anti-inflammatory and anti-oxidant activities | [108] | |
| Chum salmon Oncorhynchus keta | MOP | Antioxidant activity | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guryanova, S.V.; Ovchinnikova, T.V. Multifaceted Marine Peptides and Their Therapeutic Potential. Mar. Drugs 2025, 23, 288. https://doi.org/10.3390/md23070288
Guryanova SV, Ovchinnikova TV. Multifaceted Marine Peptides and Their Therapeutic Potential. Marine Drugs. 2025; 23(7):288. https://doi.org/10.3390/md23070288
Chicago/Turabian StyleGuryanova, Svetlana V., and Tatiana V. Ovchinnikova. 2025. "Multifaceted Marine Peptides and Their Therapeutic Potential" Marine Drugs 23, no. 7: 288. https://doi.org/10.3390/md23070288
APA StyleGuryanova, S. V., & Ovchinnikova, T. V. (2025). Multifaceted Marine Peptides and Their Therapeutic Potential. Marine Drugs, 23(7), 288. https://doi.org/10.3390/md23070288







