Abstract
Five new natural products, including two sorbicillinoids (1–2), one indolinone alkaloid (10), one tetracyclic steroid (11), and one α-pyrone derivative (14), were identified from the endophytic Penicillium sp. NX-S-6, together with thirteen known natural products. The structures of new compounds were unambiguously elucidated by comprehensive spectroscopic analyses (NMR, MS), as well as electronic circular dichroism (ECD) calculation. Notably, quinosorbicillinol (1) was identified as a rare hybrid sorbicillinoid incorporating a quinolone moiety, representing a unique structural scaffold in this natural product class. Biological evaluation revealed that Compounds 1, 4 and 8 potently inhibited the production of nitric oxide and interleukin 6 in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. Mechanistic studies furthermore demonstrated that Compounds 4 and 8 effectively suppressed interleukin-1β secretion in LPS-induced immortalized mouse bone marrow-derived macrophages (iBMDMs) by blocking NLRP3 inflammasome activation. This inhibition was attributed to their ability to disrupt the assembly of the NLRP3-caspase-1 complex, a key event in the pathogenesis of inflammatory disorders. These findings not only expand the structural diversity of endophyte-derived natural products but also highlight their potential as lead compounds for developing anti-inflammatory therapeutics targeting the NLRP3 pathway.
1. Introduction
Marine natural products denote organic metabolites derived from marine organisms, encompassing sponges, algae, bryozoans, cnidarians, tunicates, echinoderms, microorganisms, mangroves and other intertidal flora [1,2]. These metabolites include diverse organic classes such as polyketides, peptides, terpenes, sterols, alkaloids, etc. [1,3,4,5]. Research in this field involves not only the isolation, purification and structure elucidation, but also the bioactivity profiling [3,6] and biosynthetic pathway dissection [7,8]. Sorbicillinoids, a distinct family of polyketides characterized by a sorbyl side chain, are predominantly isolated from marine fungi [9,10,11,12]. Their structural uniqueness lies in the diversity of monomeric, dimeric, trimeric architectures, and even hybrid scaffolds incorporating heterocyclic moieties (e.g., quiniline, indole) [9,13,14]. Since the first sorbicillinoid was reported in 1948 [15], over 170 sorbicillinoid congeners have been identified, most of which exhibit significant biological activities—including anti-inflammatory, anticancer, antimicrobial and antioxidant properties [9,10,13,16,17]. These attributes position them as promising lead compounds for pharmaceutical and agrochemical development, particularly due to their selective targeting of disease-relevant molecular pathways [9,10].
In the pursuit of novel bioactive natural products from microorganisms inhabiting niche ecological niches [18,19,20], the endophytic Penicillium sp. NX-S-6 was identified as a promising isolate based on its demonstrated ability to biosynthesize unique aminosorbicillinol derivatives [20]. To further expand the structural diversity of the sorbicillinoid natural product class, the “one strain many compounds” (OSMAC) approach was systematically employed by using solid rice, potato dextrose and corn meal. This strategy involves the manipulation of cultivation parameters, including nutrient composition, temperature, and aeration, to induce the production of cryptic secondary metabolites that are not expressed under standard fermentation conditions. Upon comprehensive analysis of high-resolution liquid chromatography–mass spectrometry (HR-LC-MS) data, a molecular ion with a protonated adduct [M + H]+ at m/z 407.2 was detected. The ultraviolet-visible (UV-Vis) spectral characteristics of this ion exhibited a distinctive absorption profile reminiscent of sorbicillinoids; however, the observed molecular mass was lower than that typically associated with dimeric sorbicillinoid congeners, suggesting the presence of a hitherto unreported structural architecture.
To facilitate structural elucidation, a large-scale fermentation (30 L) was conducted using solid rice medium, a proven substrate for enhancing the production of diverse secondary metabolites in filamentous fungi due to its rich nutrient composition and favorable physical properties. Following fermentation, the crude extract was subjected to a multi-step purification process. The purified compound was subsequently characterized using a suite of advanced spectroscopic techniques, including one- and two-dimensional nuclear magnetic resonance (1D/2D NMR) spectroscopy, high-resolution electrospray ionization mass spectrometry (HR-ESI-MS), and electronic circular dichroism (ECD) analysis. These studies revealed the compound to be a hybrid sorbicillinoid incorporating an anthranilic acid moiety, featuring a novel 6/6/5/6/6 fused pentacyclic carbon framework (1). In addition to this novel metabolite, four additional new natural products and thirteen known compounds were successfully isolated and identified (Figure 1). This report details the fermentation protocols, isolation procedures, structural elucidation strategies, and anti-inflammatory bioactivity evaluations of these natural products.
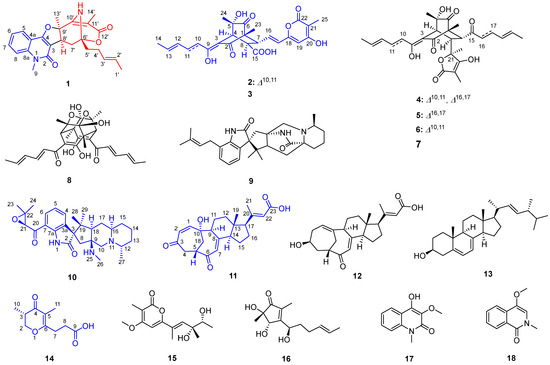
Figure 1.
Structures of Compounds 1–15 from Penicillium sp. NX-S-6.
2. Results and Discussion
Preliminary LC-MS analyses of crude extract prepared from Penicillium sp. NX-S-6 culture revealed a set of potentially new secondary metabolites. Scale-up fermentation (30 L) of this strain, followed by extraction and chromatographic purification, yielded five new compounds: quinosorbicillinol (1, yield: 0.04 mg/L), bisorbicillpyrone B (2, yield: 0.14 mg/L), citrinadin E (10, yield: 0.32 mg/L), norcyclocitrinoic acid C (11, yield: 0.12 mg/L), stapyrone I (14, yield: 0.12 mg/L).
Quinosorbicillinol (1) was isolated as a yellow powder. The molecular formula was decided as C24H26N2O4 from HRESIMS peak at m/z 405.1819 [M − H]− (calcd for C24H25N2O4, 405.1814), indicating 13 degrees of unsaturation. The 1H NMR spectrum of 1 (Table 1) showed signals attributable to four aromatic protons [δH 7.23 (t), 7.34 (d), 7.59 (t), 7.84 (d)], two olefinic protons [δH 5.58 (m), 5.65 (m)], one methine group [δH 3.84 (brs)], one N-methyl group [δH 3.70 (s)], three methylene groups [δH 2.08 (1H, m), 2.17 (2H, t), 2.41 (2H, m), 2.62 (1H, dd)], and three methyl groups [δH 1.51 (s), 1.73 (d), 1.75 (s)]. The 13C NMR spectrum (Table 1) showed total of 24 carbon signals, which can be assigned to two carbonyls (δC 162.3, 173.5), six sp2 quaternary carbons (δC 101.2, 105.3, 114.9, 139.6, 155.9, 165.6), six sp2 methines (δC 114.5, 121.8, 122.7, 127.0, 129.9, 131.3), one N-methyl (δC 29.9), one methine (δC 34.7), three methylene (δC 26.9, 31.1, 39.4), three methyl (δC 6.5, 18.1, 26.0) and two oxygenated quaternary carbons (δC 82.0, 89.2), as revealed by HSQC spectrum. Further detailed analysis of 2D NMR data allowed the construction of two substructures, as shown in Figure 2. The 1H-1H COSY spectra revealed the existence of an ortho-substituted phenyl ring according to the main spin system from H-5 to H-8, which shown at 7.84 (d, J = 7.9 Hz, H-5), 7.23 (t, J = 7.6 Hz, H-6), 7.59 (t, J = 7.7 Hz, H-7) and 7.34 (d, J = 8.5 Hz, H-8). The key HMBC correlations from N-methyl (CH3-9) to C-2 and C-8a, and from H-5 to C-4, and C-8a, supported the construction of the quinolone structure (subunit A in Figure 2), which turned out to be 4-hydroxy-3-methoxy-1-methyl-2(1H)-quinolone (17) [21] with different C-3 substitution. The 1H-1H COSY correlations from H-1′ to H-5′ indicated the characteristic sorbyl side chain of sorbicillinoid, while the hexatomic ring varied greatly. Firstly, an α,β-unsaturated ketone can be readily recognized from the 13C NMR of C-10′ (δC 165.6), C-11′ (δC 101.2) and C-12′ (δC 173.5). And a methyl was located at C-11′ through the HMBC correlations from H3-11′ to C-10′, C-11′and C-12′. Another methyl at δH 1.75 (s) showed HMBC correlations to C-8′ (δC 34.7), C-9′ (δC 82.0) and C-10′, indicating the C-9′ with a methyl substitution. The 1H-1H COSY cross peaks of H2-7′ (δH 2.08 and 2.62)/H-8′ (δH 3.84), together with the HMBC correlations from H-8′ to C-6′ (δC 89.2) and C-10′, and from H-5′ (δH 2.17) to C-7′ (δC 31.1), and from H2-7′ to C-12′ (δC 173.5), revealed the existence of a 10-membered macrocyclic lactone. Moreover, an amino proton at δH 4.94 displayed strong HMBC correlation peaks C-7′ and C-9′, which helped to construct an amino bridge between C-6′ and C-9′. The quinolone and variational sorbicillinoid were hybridized through a dihydrofuran ring, elucidated through key HMBC correlations from H2-7′ to C-2, and from H-8′ to C-2 and C-4 (Figure 2). Thus, the planar structure of 1 was elucidated as shown in Figure 1.

Table 1.
1H (500 MHz) and 13C (125 MHz) NMR data for quinosorbicillinol (1) in CDCl3.
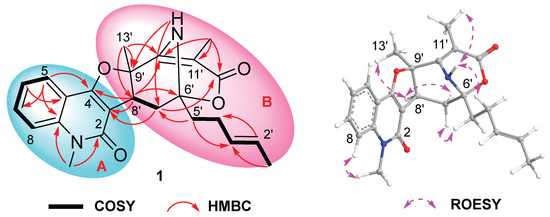
Figure 2.
1H−1H COSY, key HMBC and ROESY correlations of Compound 1.
Compound 1′s relative configuration was established by the ROESY spectrum. ROESY correlations of H-8′/H-13′ and H-7′α/H-8′ indicated they were co-facial and arbitrarily fixed in α-orientation. The NOE cross peaks of H-4′/N-H and N-H/H3-11′ indicated the nitrogen bridge was β-oriented (Figure 2). Several attempts to crystallize 1 did not produce material suitable for X-ray experiments. Alternatively, electronic circular dichroism (ECD) analysis was used for absolute configuration determination [22,23]. Specifically, time-dependent density functional theory (TDDFT) was used to calculate the ECD spectra of the two possible enantiomers of 1. And the theoretical ECD spectra were then compared to the actual ECD spectrum. The better match of the 6′R, 8′S, 9′R isomer in the ECD spectrum (Figure 3) helped to decide the absolute configuration of 1 as 6′R, 8′S, 9′R. Based on the above analysis, Compound 1 was identified as a new hybrid sorbicillinoid with a quinolone moiety, and it was named quinosorbicillinol.
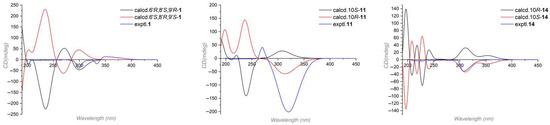
Figure 3.
The experimental and calculated ECD spectra of Compounds 1, 11 and 14.
From a biosynthetic perspective, quinosorbicillinol was constructed from two distinct precursors—a sorbicillinoid and a quinolone—derived from separate pathways. Although both precursor types are widely distributed in fungi [9,24], no examples of their biosynthetic pathway hybridization have been reported to date. The discovery of quinosorbicillinol has highlighted nature’s astonishing ability to synthesize unexpected natural products. Here, we proposed a hypothetical biosynthetic pathway for quinosorbicillinol (Scheme S1). Initially, the sorbicillinoid precursor was generated by iterative polyketide synthases. An aminotransferase then replaced the C-10 ketone group with an amino group. Nucleophilic attack by this amino group on the C-6 ketone led to the formation of the first nitrogen-containing six-membered heterocyclic ring. Subsequently, the highly nucleophilic C-3 position of 4-hydroxy-N-methyl-2-quinolone attacked the C-8 ketone group of the polyketide chain to form a C-C bond. A furan ring was then constructed through dehydration and cyclization. Finally, the product was released as an ester, and a putative oxidation step yielded the final compound, quinosorbicillinol (1).
The molecular formula C25H26O9 of Bisorbicillpyrone B (2) was determined by HRESIMS at m/z 469.1497 [M − H]− (calcd for C25H25O9, 469.1499), indicating 13 degrees of unsaturation. The 13C NMR data (Table 2) showed the presence of 25 carbon signals, which can be sorted into four methyls, three sp2 methines, twelve olefinic carbons, two quaternary carbons, two carboxyl and two ketones, with the aid of the HSQC experiment. The existence of a sorbyl side chain from H-10 to H3-14 in 1H NMR and 1H-1H COSY spectra indicated that 2 was a sorbicillinoid congener (Figure 2). Its NMR data closely resembled those of bisorbicillpyrone A (3) [25], with structural divergence about an extra double bond. Specifically, the key 1H-1H COSY correlations from H-10 to H3-14, along with related key HMBC correlations, helped to assign the Δ10,11 at 2 (Figure 4). The relative configuration of 2 was established by ROESY correlations. Compared to bisorbicillpyrone A (3) [25], the similar NOE correlations of H-4/H-8 and H-8/H-16 indicated they were α-oriented. On the other hand, the key ROESY cross-peaks of H-7/H3-23 and H3-23/H3-24 revealed that they were in β-orientation. The double bonds of Δ10,11 and Δ16,17 were both in E-geometry format from the coupling constant of H-11 (δH 7.34, dd, J = 14.9, 15.0) and H-17 (δH 6.18, d, J = 15.3). The Δ12,13 was also in E-geometry based on the ROESY correlation of H-2/H3-14 (Figure 5). Thus, thorough analyses of 1D and 2D NMR spectra cumulatively established the structure of Compound 2, as depicted in Figure 1, and it was subsequently named bisorbicillpyrone B.

Table 2.
1H (500 MHz) and 13C (125 MHz) NMR data for Compounds 2, 10 and 11.
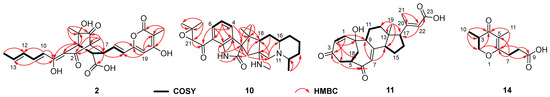
Figure 4.
1H−1H COSY and key HMBC correlations of Compounds 2, 10, 11 and 14.
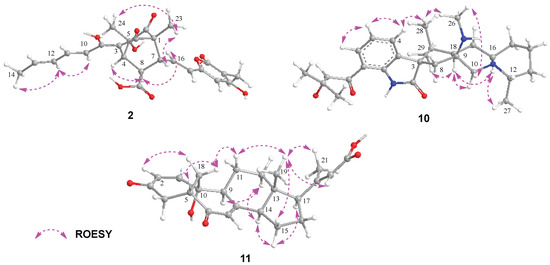
Figure 5.
Key ROESY correlations of Compounds 2, 10 and 11.
Citrinadin E (10) was isolated as a pale yellow oil; the formula C28H39N3O3 was determined by HRESIMS at m/z 466.3070 [M + H]+ (calcd for C28H40N3O3, 466.3069), indicating 11 degrees of unsaturation. Its 1H and 13C NMR data (Table 2) closely resembled those of citrinadin B [26,27]. The obvious difference was the absence of an oxygenated quaternary carbon (δC 82.4) in 10, and the HRESIMS data revealed that 10 lacked one oxygen atom compared to citrinadin B, which indicated the deficiency of a hydroxyl group at C-18. This was confirmed by the key HMBC correlations from H-28 and H-29 to C-3, C-18 and C-19 (Figure 4). Thus, the planar structure of 10 was established, as shown in Figure 1. Recently, the stereochemical structure of citrinadin B has been revised by total synthesis [27]. The relative configuration at the stereocenters of 10 was indirectly established through the analyses of ROESY correlations (Figure 4) and their comparison with those of citrinadin B [27]. The key ROESY correlations of H-16/H-18, H-16/H3-27, H-10α/H-18, and H-18/H3-29 revealed that they were on the same side of the molecule as citrinadin B and analysis of the ROESY spectrum. On the other hand, H-4, Me-26 and Me-28 were on the opposite side (Figure 5). Furthermore, the highly consistent 1H and 13C NMR data at C-7 side chain indicated the configuration at C-21 could be assigned as 21S*. Therefore, all the results were consistent with the chemical structure of 10, as displayed in Figure 1.
Compound 11 was isolated as a colorless powder. HRESIMS analysis of 11 revealed the molecular formula C23H28O5, requiring 10 degrees of unsaturation. Analyzing its 1H and 13C NMR data (Table 2) revealed the structural similarity with norcyclocitrinoic acid A [28], the nearly isolated steroid featuring an unusual 7/7/6/5-tetracyclic scaffold. The main difference was an extra keto carbonyl carbon and an oxygenated quaternary carbon observed in 11. By analysis of its 2D NMR, Compound 11 was demonstrated to have a ketone carbonyl group at C-3 instead of a hydroxyl group in norcyclocitrinoic acid A. Moreover, the double bond was shifted from positions Δ1,10 to Δ1,2, and C-10 was substituted with a hydroxyl. This was confirmed by the 1H-1H COSY correlation of H-1/H-2, and HMBC correlations from H-1 to C-3 and from H-2 to C-10. Based on the ROESY spectrum and their comparable NMR chemical shifts, the relative configuration of 11 was determined to be similar as that of norcyclocitrinoic acid A with ROESY correlations of H-9/H-11α, H-11α/H-14, H-14/H-17, H-14/H-15α, H-15α/H-17, H-15β/H3-19, H-11β/H3-19, H3-19/H3-21, H-11β/Hb-18, and Hb-18/H-5 (Figure 5). According to the ECD calculation result, the C-10’s absolute configuration of 11 was determined as 10R with an experimental ECD curve more consistent with the calculated curve (Figure 3). As a result, 11 was elucidated as a new steroid with a 7/7/6/5-tetracyclic system and given the designation norcyclocitrinoic acid C.
Stapyrone I (14) was obtained as a pale yellow oil. Based on the HRESIMS ion peak at m/z 197.0818 [M − H]−, 14 had a molecular formula of C10H14O4 with 4 degrees of unsaturation. Comparison of the NMR data (Table 3) disclosed high similarity with stapyrone G [29], with the principal differences being the replacement of the methyl formate group (δC 172.9) in stapyrone G with a carboxylic acid in 14 (δC 177.2), indicated by diagnostic HMBC correlations of H-7/C-9 and H-8/C-9. The absolute configuration of 14 was determined as 10S based on the ECD calculations (Figure 3).

Table 3.
1 H (500 MHz) and 13C (125 MHz) NMR data for stapyrone I (14) in CDCl3.
Thirteen known compounds with different structure styles were also isolated and identified as bisorbicillpyrone A (3) [25], trichotetronine (4) [30], dihydrotrichotetronine (5), 10,11-dihydrobislongiquinolide (6) [31], 10,11,16,17-tetrahydrobislongiquinolide (7) [31], trichodimerol (8) [32,33], chrysogenamide A (9) [34], norcyclocitrinoic acid A (12) [28], ergosterol (13) [20], stapyrone B (15) [29], 5-hydroxycyclopenicillone (16) [35], 4-hydroxy-3-methoxy-1-methyl-2(1H)-quinolone (17) [21], and 4-methoxy-2-methylisoquinolin-1(2H)-one (18) [36].
The anti-inflammatory activities of all yield natural products were evaluated by using routine protocols [37]. All tested compounds showed no cytotoxicity to macrophages at concentrations of 50 μM. Notably, quinosorbicillinol (1), trichotetronine (4) and trichodimerol (8) showed considerable inhibitory efficacy on both the production of nitric oxide and IL-6 in LPS-stimulated RAW264.7 cells. Meanwhile, bisorbicillpyrone B (2), 10,11-dihydrobislongiquinolide (6), chrysogenamide A (9) and norcyclocitrinoic acid C (11) showed weak inhibition on NO release (Table 4).

Table 4.
Anti-inflammatory effects (IC50 ± SD) of active compounds.
To investigate the anti-inflammatory signaling pathway of sorbicillinoid dimers, the two active trichotetronine (4) and trichodimerol (8) were selected for further investigation by an in vitro inflammatory model using LPS-induced immortalized mouse bone marrow-derived macrophages (iBMDMs). The expression levels of inflammation-related markers were assessed using quantitative real-time PCR (qRT-PCR) and Western blot analysis techniques. The results of qRT-PCR (Figure 6A) showed that, compared with the model group, Compounds 4 and 8 dose-dependently reduced the mRNA transcription levels of the inflammation-related factors interleukin-1β (Il-1β) and nitric oxide synthase 2 (Nos2). Next, we examined their effects on the NLRP3 inflammasome pathway associated with IL-1β. The results of Western blot analysis (Figure 6B) showed that both Compounds 4 and 8 dose-dependently reduced the expression of NLRP3 and inhibited the release of downstream IL-1β. Notably, Compound 8 exhibited a more pronounced reduction in the expression of these two proteins compared to Compound 4. The combined experimental results indicate that Compounds 4 and 8 exhibit significant anti-inflammatory activity and can exert their anti-inflammatory effects by modulating NLRP3.
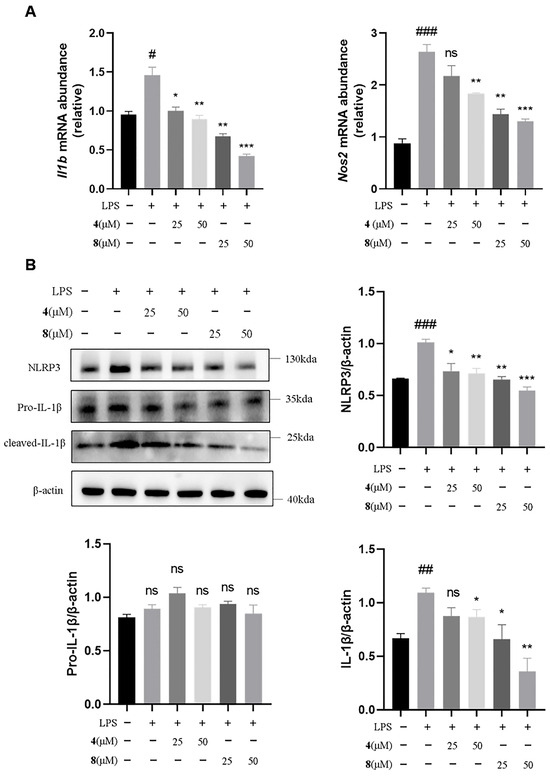
Figure 6.
Compounds 4 and 8 inhibited the NLRP3 inflammasome activation. (A) qRT–PCR detection of Il-1β and Nos2 mRNA levels (n = 3). (B) Western blot detection of NLRP3, cleaved caspase 1, pro-caspase 1 and pro-IL-1β expression level in the lysates of iBMDMs (n = 3). Resulting data were from at least three biological replicates and are presented as mean ± standard error of the mean (SEM) (A,B); # p < 0.05, ## p < 0.01, ### p < 0.001, vs. normal group; * p < 0.05, ** p < 0.01, *** p < 0.001, vs. LPS group; ns: no significance. statistical analysis was performed using one-way ANOVA.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were measured in MeOH using the MCP 5100 digital polarimeter (Anton Paar, Graz, Austria). The CD spectrum was obtained on a J-810 spectropolarimeter at room temperature (Jasco, Easton, PA, USA). Nuclear magnetic resonance spectra were measured on an Avance AV500 NMR spectrometer (Bruker, Karlsruhe, Germany). High-resolution electrospray ionization mass spectrometry (HRESIMS) data was collected on a Zeno TOF 7600 mass spectrometer (AB SCIEX, Framingham, MA, USA). HPLC analyses were conducted on an Agilent 1260 system with a PDA detector (Agilent Technologies, Santa Clara, CA, USA). Preparative HPLC separation was conducted on a Waters 1525EF LC system (Waters, Milford, CT, USA). Sephadex LH-20 (25–100 μm) was purchased from GE Healthcare (GE Healthcare, Uppsala, Sweden). Silica gel (200−300 mesh and 300−400 mesh) and precoated silica gel GF254 plates were purchased from Qingdao Marine Chemical Co., Ltd. (Qingdao, China). High-glucose Dulbecco’s modified Eagle medium (DMEM) was purchased from Gibco (Shanghai, China). Fetal bovine serum (FBS) was obtained from VIVA Cell (Shanghai, China). Phosphate-buffered saline (PBS) and dimethyl sulfoxide (DMSO) were purchased from KeyGEN Bio TECH (Shanghai, China). Lipopolysaccharide (LPS) was obtained from Sigma (Shanghai, China). Nitric oxide assay kit was obtained from Beyotime (Shanghai, China). Mouse IL-6 uncoated ELISA Kit was obtained from Invitrogen (Vienna, Austria).
3.2. Fermentation, Extraction, Isolation, and Purification
Isolation of Penicillium sp. NX-S-6 and its phylogenetic characterization were described previously [20]. Penicillium sp. NX-S-6 was cultivated on three 250 mL Erlenmeyer flasks with 50 mL of potato dextrose broth (200 g potato infusion and 20 g dextrose in 1 L water) for 3 days at 28 °C as seed cultures. The seed cultures, each 3 mL, were moved to thirty-three 1000 mL Erlenmeyer flasks containing solid rice medium (each contained 80 g rice and 120 mL deionized water). The flasks were incubated under static conditions at room temperature for 25 days. After the fermentation, the cultures were extracted with ethyl acetate (EtOAc) three times, and the EtOAc portion was evaporated under reduced pressure to obtain a crude extract (49.7 g). The crude extract was dispersed in water and extracted with petroleum ether (PE) and EtOAc successively, to afford EtOAc extract (11.6 g). The EtOAc extract was subjected to a silica gel chromatographic column eluted with a gradient PE/EtOAc system to produce 14 fractions (Fr.1−14). Fr. 6 was recrystallized to yield Compound 13 (36.2 mg). Fr. 11 (2.5 g) was purified by a silica gel column with a gradient DCM/MeOH system to obtain subfractions Fr.11A−11J. Fr. 11C (0.6 g) was then purified by a Sephadex LH-20 column (MeOH) to obtain subfractions Fr.11C1−11C7. Fr.11C2 (325 mg) was further purified by a preparative HPLC (50% MeCN) to obtain Compounds 4 (21.7 mg), 5 (33.4 mg), 6 (24.5 mg) and 7 (40.2 mg). Fr.11C4 (93 mg) was further purified by a preparative HPLC eluted with 15~50% MeCN to obtain Compounds 8 (3.3 mg) and 14 (3.7 mg). Fr.11C5 (68 mg) was further purified by a preparative HPLC (20% MeCN) to obtain Compound 17 (3.8 mg). Fr.11E (381 mg) was purified by a Sephadex LH-20 column (MeOH) and then subjected to a preparative HPLC (35% MeCN) to obtain Compound 12 (3.9 mg). Fr.11F (396 mg) was purified by a Sephadex LH-20 column (MeOH) followed by a preparative HPLC eluted with 20% MeCN to obtain Compounds 11 (3.6 mg) and 16 (2.3 mg). Fr. 11H and 11I were combined (408 mg) and purified by a Sephadex LH-20 column (MeOH) and then subjected to a preparative HPLC (30% MeCN) to obtain Compounds 2 (4.1 mg) and 3 (2.6 mg). Fr. 12 (902 mg) was purified by Sephadex LH-20 column (100% MeOH) to obtain subfractions Fr.12A−12E. Fr.12C (540 mg) was purified by silica gel chromatographic column eluted with DCM/MeOH (200:1, 120:1 and 90:1) to obtain subfractions Fr.12C1−12C10. Fr.12C3 (45 mg) was further purified by preparative HPLC (45% MeCN) to obtain Compound 1 (1.3 mg). Fr.12C5 (130 mg) was further purified by preparative HPLC (18% MeCN) to obtain Compound 15 (2.3 mg). Fraction 13 (3.8 g) was isolated by a silica gel chromatographic column with a gradient DCM/MeOH system to obtain subfractions Fr.13A−13N. Fr.13G (322 mg) was purified by a Sephadex LH-20 column (MeOH) followed by a preparative HPLC (10% MeCN) to obtain Compound 18 (4.0 mg). Fr.13J (741 mg) was purified by a Sephadex LH-20 column (100% MeOH) to obtain subfractions Fr.13J1−13J6. Fr.13J2 (112 mg) was further purified by preparative HPLC (18% MeCN) to obtain Compound 10 (9.7 mg). Fr.13J3 (85 mg) was further purified by preparative HPLC (15% MeCN) to obtain Compound 9 (7.8 mg).
Quinosorbicillinol (1): yellow powder; [α −227.20 (c = 0.1, MeOH); 13C NMR and 1H NMR data, see Table 1; HRESIMS m/z 405.1819 [M − H]− (calcd. for C22H25N2O4, 405.1814).
Bisorbicillpyrone B (2): yellow powder; [α +163.7 (c = 0.2, MeOH); 13C NMR and 1H NMR data, see Table 2; HRESIMS m/z 469.1497 [M − H]− (calcd. for C25H25O9, 469.1499).
Citrinadin E (10): Yellow powder; [α +66.5 (c = 0.5, MeOH); 13C NMR and 1H NMR data, see Table 2; HRESIMS m/z 466.3069 [M + Na]+ (calcd. for C28H40N3O3, 466.3070).
Norcyclocitrinoic acid C (11): Colorless powder; [α +188.9 (c = 0.1, MeOH); 13C NMR and 1H NMR data, see Table 2; HRESIMS m/z 383.1859 [M − H]− (calcd. for C23H27O5, 383.1858), 767.3785 [2M − H]− (calcd. for C46H55O10, 767.3795).
Stapyrone I (14): Colorless oil; [α +10.1 (c = 0.2, MeOH); 13C NMR and 1H NMR data, see Table 3; HRESIMS m/z 197.0818 [M − H]− (calcd. for C10H13O4, 197.0814).
3.3. Quantum Chemistry Calculation
The theoretical calculations of Compounds 1, 11 and 14 were performed using Gaussian 09 [38]. The possible conformations were initially obtained from the program Spartan’14 and then optimized at B3LYP/6-31G* level in the gas phase. Room-temperature equilibrium populations were calculated according to the Boltzmann distribution law. The ECD calculations were performed using time dependent density functional theory (TDDFT) [39] at wB97xd/6-311G** level in methanol with the PCM model. The ECD spectra of compounds were obtained by weighing the Boltzmann distribution rate of each geometric conformation, and the sigma/gamma value for processing the calculated ECD was 0.3 eV [40]. All calculated curves were shifted +20 nm to better simulate experimental spectra.
3.4. Anti-Inflammatory Activity
A Griess method was used to screen the isolated compounds for anti-inflammatory activity as previously reported [37,41].
Cell culture. Murine macrophage cell line RAW 264.7 and immortalized bone marrow-derived macrophages (iBMDM) were obtained from Procell Life Science and Technology (Wuhan, China). Cells were cultured with DMEM supplemented with 10% FBS. All cell culture was incubated in a humidified chamber at 37 °C with 5% CO2.
Cell viability assay. Cell viability was tested by the CCK-8 kit. RAW264.7 cells were seeded into a 96-well plate at a density of 5 × 103 per well and subsequently maintained in a humidified atmosphere containing 5% CO2 at 37 °C. After 12 h, the medium was replaced with 50 μM of isolated compounds and dexamethasone (positive control) for 24 h. The 10 μL fresh CCK-8 solution was added to each well and incubated at 37 °C for 2 h. The absorbance was recorded at 450 nm wavelength using a microplate Reader (M200 Pro Nanoquant, TECAN, Männedorf, Switzerland).
Nitric oxide and IL-6 inhibitory activity. RAW 264.7 cells were cultured in 24-well flat-bottomed plates for 24 h at a density of 2 × 105/mL. The medium was replaced with various concentrations (2.5, 5.0, 10, 25 and 50 μM) of isolated compounds and dexamethasone (positive control) and LPS with a final concentration of 1 μg/mL was added to stimulate RAW264.7 cells for 24 h. After incubation, the cell supernatant was collected and centrifuged at 5000 rpm for 10 min. The NO and IL-6 concentrations in the supernatant were determined using the protocol of nitric oxide assay kit (Beyotime, Shanghai, China) and Mouse IL-6 uncoated ELISA kit (Invitrogen, Vienna, Austria), respectively. The absorbance was measured by a Tecan infinite M nano plate reader (Männedorf, Switzerland) at 540 nm and 450 nm. The inhibition rate was determined by the following equation:
ODM stands for the absorbance of the model
ODX stands for the absorbance of the sample
ODC stands for the absorbance of the control
qRT–PCR. Total mRNA was extracted using the FreeZol kit (Vazyme Biotech Co., Ltd., Nanjing, China) according to the manufacturer’s instructions. The total mRNA was reverse-transcribed into cDNA using HiScript®II QRT SuperMix for qPCR (+qDNA wiper) reagent (Vazyme Biotech Co., Ltd., Nanjing, China). The real-time quantitative PCR (qRT–PCR) reaction system was prepared with ChamQ SYBR qPCR Master Mix (Without ROX) reagent (Vazyme Biotech Co., Ltd., Nanjing, China) and performed on a real-time fluorescent quantitative PCR instrument (Lighe Cycler 96, Roche, Basel, Switzerland); the mRNA expression levels were normalized using GAPDH as an internal reference [13].
Western Blot. After completing the cell modeling, the cells were collected and lysed in RIPA lysis buffer (Beyotime, Shanghai, China) containing phenylmethylsulfonyl fluoride (PMSF, Beyotime, Shanghai, China) at 4 °C for 30 min. The lysate was centrifuged at 12,000× g for 15 min at 4 °C. The supernatant was collected, mixed with SDS-PAGE protein loading buffer (5×) (Beyotime, Shanghai, China), and heated in a metal bath at 100 °C for 10 min to prepare the protein samples. The protein samples were separated by SDS-PAGE and transferred to PVDF membranes, which were then blotted with specific primary and secondary antibodies [13].
4. Conclusions
In summary, aiming to discover novel natural products from microorganisms in unique ecological environments, we selected the endophytic Penicillium sp. NX-S-6, which was originally isolated from a plant growing in an extreme alpine region. This strain was fermented using a 30 L solid rice medium, a widely adopted method for enhancing the production of diverse secondary metabolites due to its ability to mimic the natural habitat of endophytic fungi and promote metabolic diversity. After the fermentation process, the extracts were subjected to a series of separation techniques, including column chromatography on silica gel, Sephadex LH-20, and semi-preparative high-performance liquid chromatography (HPLC). Through these meticulous isolation steps, five new secondary metabolites were successfully obtained, including a hybrid sorbicillinoid (1) featuring a novel 6/6/5/6/6 fused pentacyclic carbon skeleton. This unique structure represents a significant discovery, as it expands the structural diversity of sorbicillinoids, a class of natural products known for their broad biological activities.
Bioactivity assays demonstrated that Compounds 1, 4, and 8 exhibited potent anti-inflammatory activity by inhibiting the release of nitric oxide (NO) and interleukin-6 (IL-6) in LPS-stimulated RAW264.7 macrophages. These results were further validated by dose–response experiments, which revealed that the inhibitory effects of these compounds were concentration-dependent. Furthermore, Compounds 4 and 8 suppressed IL-1β secretion by inhibiting the activation of the NLRP3 inflammasome, a key player in the inflammatory response. Mechanistic studies showed that these compounds regulated the phosphorylation levels of multiple signaling proteins involved in the NLRP3 pathway, indicating a multi-target mode of action.
This study not only enriches the library of natural products from endophytic fungi but also provides experimental evidence for the further development of anti-inflammatory drugs. The discovery of these bioactive compounds with novel structures highlights the potential of microorganisms from extreme environments as a valuable source for drug discovery, suggesting that exploring understudied ecological niches could lead to the identification of more promising lead compounds in the future.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md23070280/s1, Figures S1–S35: 1D, 2D NMR, and HRESIMS spectra of compounds 1, 2, 10, 11 and 14, Tables S1–S7: Conformational analysis of the optimized isomers and the coordinates of the optimized conformers of compounds 1, 11 and 14, Figure S36: Uncropped images of gel/blot. Scheme S1: Proposed biosynthetic pathway of quinosorbicillinol (1).
Author Contributions
H.P.: Investigation, Formal analysis, Software, Writing-original draft. J.S.: Investigation, Formal analysis, Validation. R.Z.: Investigation, Validation. Y.Q.: Formal analysis, Software. Y.H. (Yu Hong): Investigation. F.Z.: Methodology. C.W.: Investigation. Y.H. (Yang Hu): Supervision, Resources, Writing—review and editing. X.W.: Supervision, Funding acquisition, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was financially supported by the National Key R&D Program of China (2023YFC2308200, Ministry of Science and Technology of the People’s Republic of China), the National Natural Science Foundation of China (81803391), Jiangsu Provincial Innovation and Entrepreneurship Talent Plan (202010528, Jiangsu Government, China).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that all relevant data supporting the results of this study are available within the article and its Supplementary Materials file, or from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2025, 42, 257–297. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiao, Y.; Li, Q.; Su, M.; Guo, Y.; Jin, X. Recent advances in natural products derived from marine Echinoderms and endophytic microbes: Chemical insights and therapeutic potential. Mar. Drugs 2025, 23, 33. [Google Scholar] [CrossRef]
- Chhetri, B.K.; Tedbury, P.R.; Sweeney-Jones, A.M.; Mani, L.; Soapi, K.; Manfredi, C.; Sorscher, E.; Sarafianos, S.G.; Kubanek, J. Marine natural products as leads against SARS-CoV-2 infection. J. Nat. Prod. 2022, 85, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wilson, B.A.P.; Li, N.; Shah, R.; Dalilian, M.; Wang, D.; Smith, E.A.; Wamiru, A.; Goncharova, E.I.; Zhang, P.; et al. Discovery and synthesis of a naturally derived protein kinase inhibitor that selectively inhibits distinct classes of serine/threonine kinases. J. Nat. Prod. 2023, 86, 2283–2293. [Google Scholar] [CrossRef]
- Xue, D.; Zou, H.; Qiu, Y.; Lv, W.; Madden, M.D.; Xu, M.; Lian, X.; Pulliam, C.; Older, E.A.; Hou, L.; et al. Discovery of acylsulfenic acid-featuring natural product sulfenicin and characterization of its biosynthesis. Nat. Chem. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Li, Y.; Li, G.; Feng, J.; Li, S.; Liu, N. Advances in research on marine natural products for modulating the inflammatory microenvironment. Phytother. Res. 2025, 39, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Teufel, R.; Muller, M. Giant polyketide synthases biosynthesize a marine polyether biotoxin. Angew. Chem. Int. Ed. Engl. 2025, 64, e202419620. [Google Scholar] [CrossRef]
- Kahlert, L.; Bassiony, E.F.; Cox, R.J.; Skellam, E.J. Diels-Alder reactions during the biosynthesis of sorbicillinoids. Angew. Chem. Int. Ed. Engl. 2020, 59, 5816–5822. [Google Scholar] [CrossRef]
- Milzarek, T.M.; Gulder, T.A.M. The fungal natural product class of the sorbicillinoids: Structures, bioactivities, biosynthesis, and synthesis. Nat. Prod. Rep. 2025, 42, 482–500. [Google Scholar] [CrossRef]
- Meng, J.; Wang, X.; Xu, D.; Fu, X.; Zhang, X.; Lai, D.; Zhou, L.; Zhang, G. Sorbicillinoids from fungi and their bioactivities. Molecules 2016, 21, 715. [Google Scholar] [CrossRef]
- Zhang, P.; Deng, Y.; Lin, X.; Chen, B.; Li, J.; Liu, H.; Chen, S.; Liu, L. Anti-inflammatory mono- and dimeric sorbicillinoids from the marine-derived fungus Trichoderma reesei 4670. J. Nat. Prod. 2019, 82, 947–957. [Google Scholar] [CrossRef]
- Chen, S.; Guo, H.; Wu, Z.; Wu, Q.; Jiang, M.; Li, H.; Liu, L. Targeted discovery of sorbicillinoid pigments with anti-inflammatory activity from the sponge-derived fungus Stagonospora sp. SYSU-MS7888 using the PMG strategy. J. Agric. Food Chem. 2022, 70, 15116–15125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, J.; Jiang, Y.; Sun, S.; Wang, R.; Sun, J.; Ma, C.; Chen, Y.; Wang, W.; Hou, X.; et al. Sorbremnoids A and B: NLRP3 inflammasome inhibitors discovered from spatially restricted crosstalk of biosynthetic pathways. J. Am. Chem. Soc. 2024, 146, 18172–18183. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Ren, X.; Chen, W.; Yang, B.; Zhou, X.; Tian, X.; Guo, P.; Wang, J.; Liu, Y. Sorbicillalanines A and B, two [6,5,6] hybrid-sorbicillinoid alkaloids from Deep-Sea-Derived Penicillium sp. co-cultured with sponge-derived Setosphaeria sp. Org. Lett. 2025, 27, 6042–6046. [Google Scholar] [CrossRef]
- Cram, D.J. Mold metabolites. II. The structure of sorbicillin, a pigment produced by the mold Penicillium notatum. J. Am. Chem. Soc. 1948, 70, 4240–4243. [Google Scholar] [CrossRef]
- Xia, Y.P.; Xie, Y.; Rao, L.; Yin, G.P. Bioactive sorbicillinoid derivatives from an endophytic fungus Trichoderma citrinoviride. Front. Microbiol. 2025, 16, 1485032. [Google Scholar] [CrossRef]
- Pang, X.; Wang, P.; Liao, S.; Zhou, X.; Lin, X.; Yang, B.; Tian, X.; Wang, J.; Liu, Y. Three unusual hybrid sorbicillinoids with anti-inflammatory activities from the deep-sea derived fungus Penicillium sp. SCSIO06868. Phytochemistry 2022, 202, 113311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, A.; Zhang, N.; Li, S.; Yuan, T.; Ding, N.; Zhang, S.; Bao, S.; Wang, C.; Zhang, Y.; et al. Insecticidal endostemonines A-J produced by endophytic Streptomyces from Stemona sessilifolia. J. Agric. Food Chem. 2020, 68, 1588–1595. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, A.; Liu, J.; Bao, S.; Peng, R.; Hu, Y.; Yuan, T.; Hou, S.; Xie, T.; Zhang, Q.; et al. Chartspiroton, a tetracyclic spiro-naphthoquinone derivative from a medicinal plant endophytic Streptomyces. Org. Lett. 2020, 22, 3739–3743. [Google Scholar] [CrossRef]
- Yang, A.; Hong, Y.; Zhou, F.; Zhang, L.; Zhu, Y.; Wang, C.; Hu, Y.; Yu, L.; Chen, L.; Wang, X. Endophytic microbes from medicinal plants in Fenghuang Mountain as a source of antibiotics. Molecules 2023, 28, 6301. [Google Scholar] [CrossRef]
- Yao, G.; Chen, X.; Zheng, H.; Liao, D.; Yu, Z.; Wang, Z.; Chen, J. Genomic and chemical investigation of bioactive secondary metabolites from a marine-derived fungus Penicillium steckii P2648. Front. Microbiol. 2021, 12, 600991. [Google Scholar] [CrossRef]
- Wang, X.; Elshahawi, S.I.; Shaaban, K.A.; Fang, L.; Ponomareva, L.V.; Zhang, Y.; Copley, G.C.; Hower, J.C.; Zhan, C.G.; Kharel, M.K.; et al. Ruthmycin, a new tetracyclic polyketide from Streptomyces sp. RM-4-15. Org. Lett. 2014, 16, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zeng, Y.; Zhang, R.; Yang, L.; Wu, F.; Gai, C.; Yuan, J.; Chang, W.; Dai, H.; Wang, X. Cembranoid diterpenes from South China Sea soft coral Sarcophyton crassocaule. Mar. Drugs 2024, 22, 536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Liu, Z.; Yang, S.; Ding, N.; Gao, X. Quinolactacin biosynthesis involves non-ribosomal-peptide-synthetase-catalyzed Dieckmann condensation to form the quinolone-gamma-lactam hybrid. Angew. Chem. Int. Ed. Engl. 2020, 59, 19108–19114. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhou, X.; Lin, X.; Yang, B.; Tian, X.; Wang, J.; Xu, S.; Liu, Y. Structurally various sorbicillinoids from the deep-sea sediment derived fungus Penicillium sp. SCSIO06871. Bioorg. Chem. 2021, 107, 104600. [Google Scholar] [CrossRef]
- Mugishima, T.; Tsuda, M.; Kasai, Y.; Ishiyama, H.; Fukushi, E.; Kawabata, J.; Watanabe, M.; Akao, K.; Kobayashi, J. Absolute stereochemistry of citrinadins A and B from marine-derived fungus. J. Org. Chem. 2005, 70, 9430–9435. [Google Scholar] [CrossRef]
- Bian, Z.; Marvin, C.C.; Pettersson, M.; Martin, S.F. Enantioselective total syntheses of citrinadins A and B. Stereochemical revision of their assigned structures. J. Am. Chem. Soc. 2014, 136, 14184–14192. [Google Scholar] [CrossRef]
- He, Z.H.; Xie, C.L.; Wu, T.; Yue, Y.T.; Wang, C.F.; Xu, L.; Xie, M.M.; Zhang, Y.; Hao, Y.J.; Xu, R.; et al. Tetracyclic steroids bearing a bicyclo [4.4.1] ring system as potent antiosteoporosis agents from the deep-sea-derived Fungus Rhizopus sp. W23. J. Nat. Prod. 2023, 86, 157–165. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, H.; Wu, Q.; Jiang, M.; Chen, J.; Chen, B.; Li, H.; Liu, L.; Chen, S. Absolute configuration of cyclopropanes and the structural revision of pyrones from marine-derived fungus Stagonospora sp. SYSU-MS7888. Bioorg. Chem. 2023, 136, 106542. [Google Scholar] [CrossRef]
- Shirota, O.; Pathak, V.; Hossain, C.F.; Sekita, S.; Takatori, K.; Satake, M. Structural elucidation of trichotetronines: Polyketides possessing a bicyclo [2.2.2]octane skeleton with a tetronic acid moiety isolated from Trichoderma sp. J. Chem. Soc. Perkin Trans. 1997, 1, 2961–2964. [Google Scholar]
- Yu, J.; Han, H.; Zhang, X.; Ma, C.; Sun, C.; Che, Q.; Gu, Q.; Zhu, T.; Zhang, G.; Li, D. Discovery of two new sorbicillinoids by overexpression of the global regulator LaeA in a marine-derived fungus Penicillium dipodomyis YJ-11. Mar. Drugs 2019, 17, 446. [Google Scholar] [CrossRef]
- Barnes-Seeman, D.; Corey, E.J. A two-step total synthesis of the natural pentacycle trichodimerol, a novel inhibitor of TNF-alpha production. Org. Lett. 1999, 1, 1503–1504. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Simonsen, K.B.; Vassilikogiannakis, G.; Baran, P.S.; Vidali, V.P.; Pitsinos, E.N.; Couladouros, E.A. Biomimetic explorations towards the bisorbicillinoids: Total synthesis of bisorbicillinol, bisorbibutenolide, and trichodimerol. Angew. Chem. Int. Ed. Engl. 1999, 38, 3555–3559. [Google Scholar] [CrossRef]
- Lin, Z.; Wen, J.; Zhu, T.; Fang, Y.; Gu, Q.; Zhu, W. Chrysogenamide A from an endophytic fungus associated with Cistanche deserticola and its neuroprotective effect on SH-SY5Y cells. J. Antibiot. 2008, 61, 81–85. [Google Scholar] [CrossRef]
- Fang, F.; Zhao, J.; Ding, L.; Huang, C.; Naman, C.B.; He, S.; Wu, B.; Zhu, P.; Luo, Q.; Gerwick, W.H.; et al. 5-hydroxycyclopenicillone, a new beta-amyloid fibrillization inhibitor from a sponge-derived fungus Trichoderma sp. HPQJ-34. Mar. Drugs 2017, 15, 260. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Z.; Ruan, J.; Xu, L.; Li, G.; Luo, L. A new isoquinolone alkaloid from Penicillium citrinum P4-5-1, an endophytic fungus isolated from mangrove plant Acanthus ilicifolius. Chin. J. Mar. Drugs 2023, 42, 31–35. [Google Scholar]
- Hu, Y.; Yang, H.; Ding, X.; Liu, J.; Wang, X.; Hu, L.; Liu, M.; Zhang, C. Anti-inflammatory octahydroindolizine alkaloid enantiomers from Dendrobium crepidatum. Bioorg. Chem. 2020, 100, 103809. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Srebro-Hooper, M.; Autschbach, J. Calculating natural optical activity of molecules from first principles. J. Annu. Rev. Phys. Chem. 2017, 68, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y. SpecDis, Version 1.64; University of Wuerzburg: Würzburg, Germany, 2015. [Google Scholar]
- Wang, J.; Li, H.; Li, Y.; Yang, A.; Bao, S.; Zhang, Y.; Du, Q.; Zheng, Z.; Wang, X. Sinoflavonoids NJ and NK, anti-inflammatory prenylated flavonoids from the fruits of Podophyllum hexandrum Royle. Nat. Prod. Res. 2024, 38, 701–705. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).