Chartarlactams U-X: Novel Phenylspirodrimanes from a Marine Derived Fungus Stachybotrys sp. SZU-W23 with Anti-Inflammatory Activity Mediated by the NF-κB/ROS Signaling Pathways
Abstract
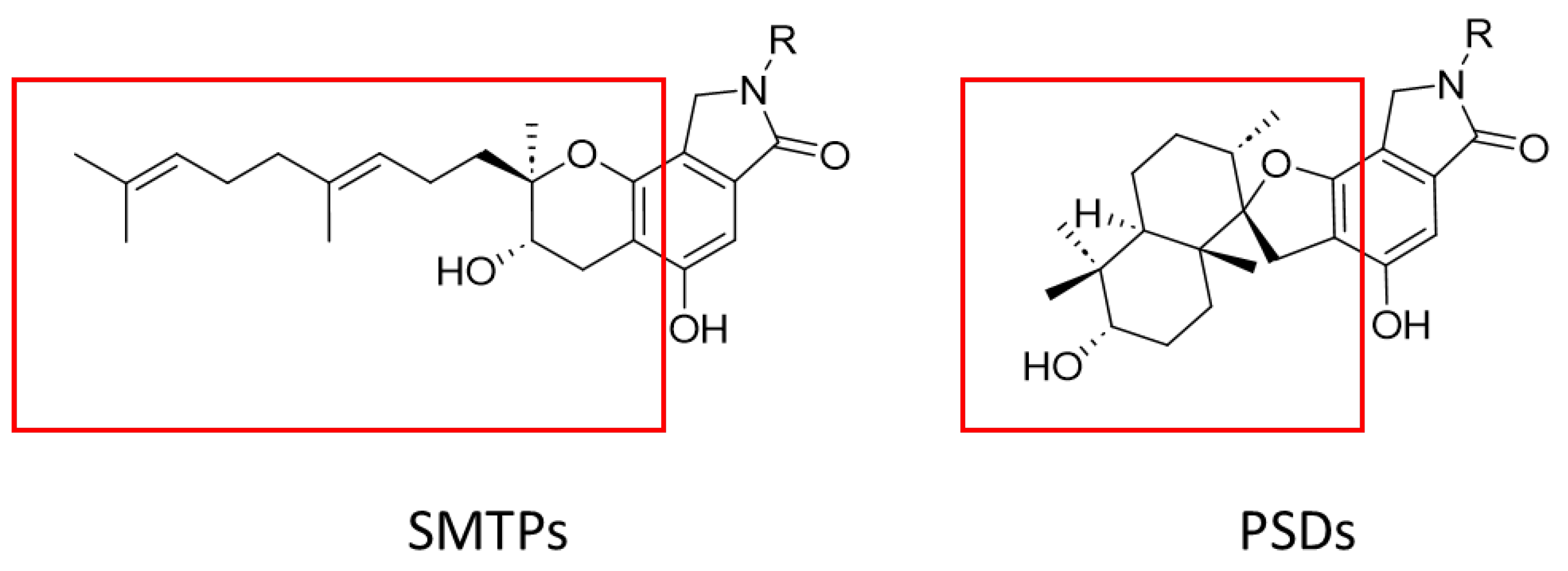
1. Introduction
2. Results
2.1. Identification of PSDs
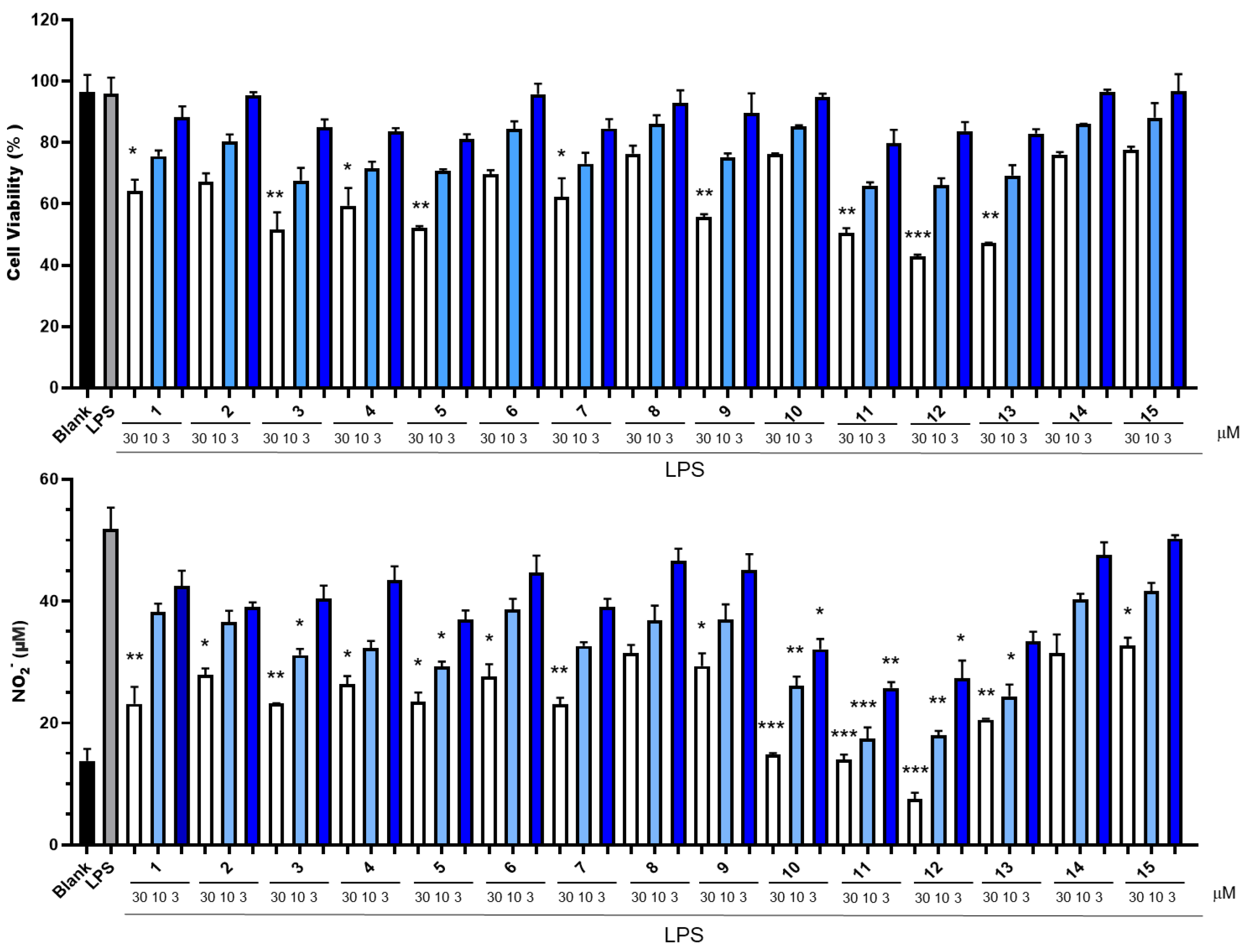
2.2. Determination of Cytotoxicity and Inhibition of NO Production
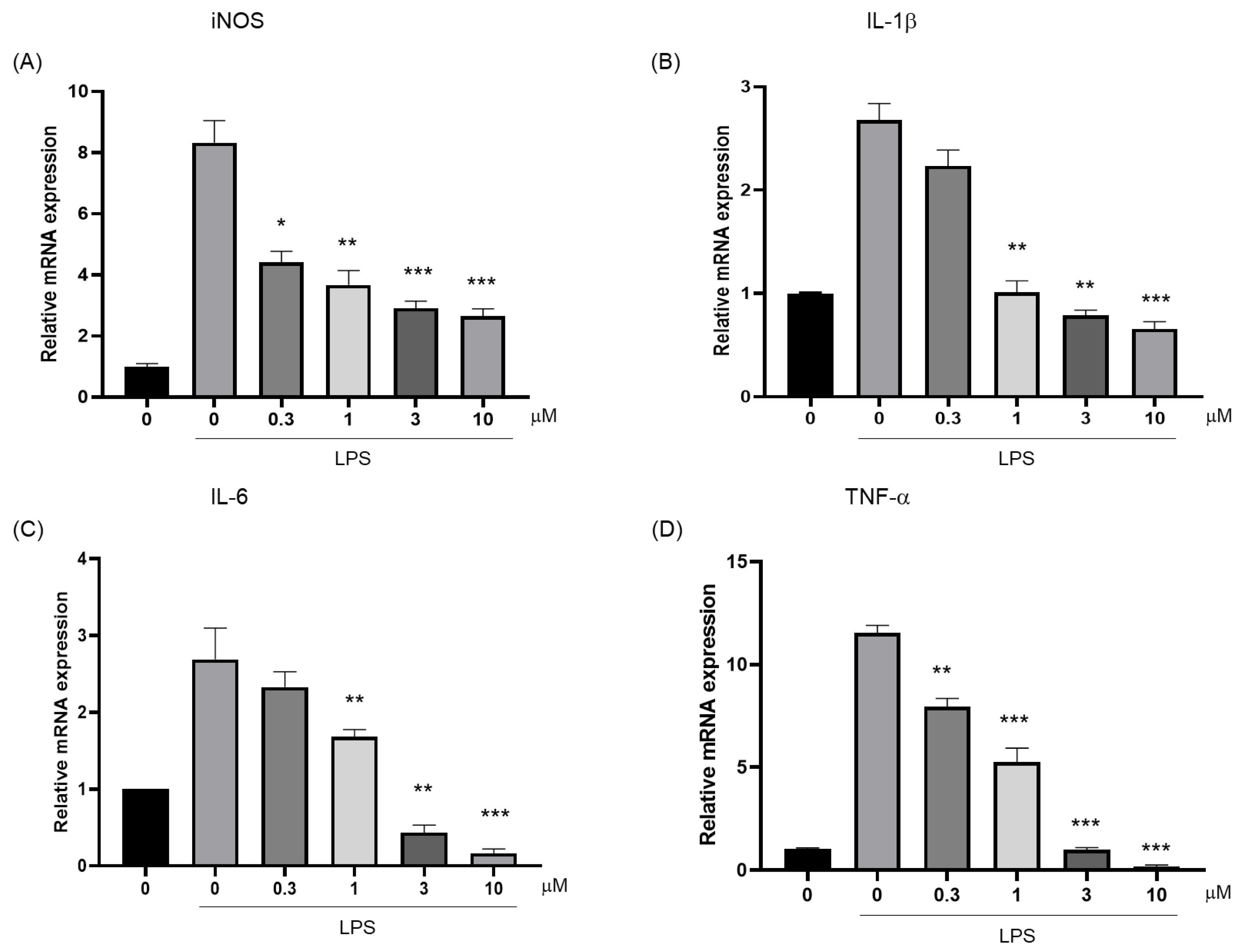
2.3. Determination of the Ability of Compound 10 to Inhibit Inflammatory Cytokines
2.4. Determination of the Inhibitory Effect of Compound 10 on the Binding Activity of Recombinant p65
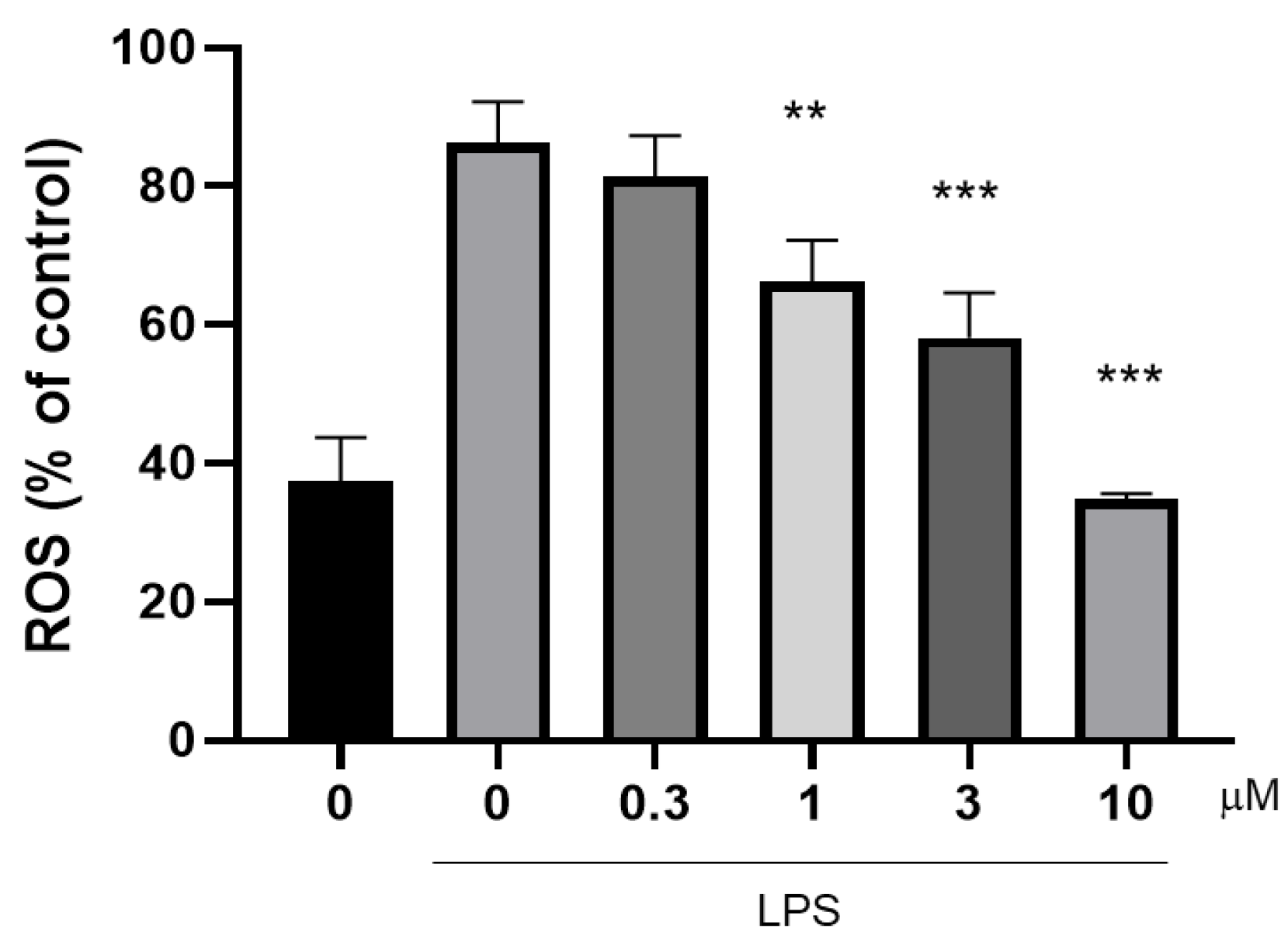
2.5. Determination of the Ability of Compound 10 to Inhibit ROS
3. Discussion
4. Materials and Methods
4.1. Fungal Strain
4.2. General Experimental Procedures
4.3. Fermentation, Extraction, and Isolation
4.4. Characterization of Novel Compounds
4.5. Cell Culture
4.6. CCK8 Assay
4.7. Measurement of NO Production
4.8. RNA Isolation and Real-Time PCR
4.9. Measurement of Binding Activity of p65
4.10. Measurement of Reactive Oxygen Species (ROS) Production
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakai, K.; Watanabe, K.; Masuda, K.; Tsuji, M.; Hasumi, K.; Endo, A. Isolation, Characterization and Biological Activities of Novel Triprenyl Phenols as Pancreatic Cholesterol Esterase Inhibitors Produced by Stachybotrys sp. F-1839. J. Antibiot. 1994, 48, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Haruki, K.; Keiko, H.; Naoko, N.; Ritsuko, N.; Keiji, H. A new series of the SMTP plasminogen modulators with a phenylamine-based side chain. J. Antibiot. 2012, 65, 361–367. [Google Scholar]
- Ibrahim, S.R.M.; Choudhry, H.; Asseri, A.H.; Elfaky, M.A.; Mohamed, S.G.A.; Mohamed, G.A. Stachybotrys chartarum—A Hidden Treasure: Secondary Metabolites, Bioactivities, and Biotechnological Relevance. J. Fungi 2022, 8, 504. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Guo, X.; Ji, W.; Lin, W. Chartarlactams Q−T, Dimeric Phenylspirodrimanes with Antibacterial and Antiviral Activities. Chem. Biodivers. 2020, 17, 1612–1643. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Shen, Y.; Tan, Z.; Zhang, M.; Chen, R.; Zhao, J.; Zhang, D.; Yu, L.; Dai, J. Stachybotrysams A–E, prenylated isoindolinone derivatives with anti-HIV activity from the fungus Stachybotrys chartarum. Phytochem. Lett. 2017, 20, 289–294. [Google Scholar] [CrossRef]
- Vázquez, M.J.; Vega, A.; Rivera, S.A.; Jiménez, A.M.D.; Díez, E.; Hueso, R.J.A. Novel sesquiterpenoids as tyrosine kinase inhibitors produced by Stachybotrys chortarum. Tetrahedron 2004, 60, 2379–2385. [Google Scholar] [CrossRef]
- Dayras, M.; Sfecci, E.; Bovio, E.; Rastoin, O.; Dufies, M.; Fontaine, V.F.; Taffin, G.E.; Lacour, T.; Pages, G.; Varese, G.C.; et al. New Phenylspirodrimanes from the Sponge-Associated Fungus Stachybotrys chartarum MUT 3308. Mar. Drugs 2023, 21, 135. [Google Scholar] [CrossRef]
- Hasumi, K.; Suzuki, E. Impact of SMTP Targeting Plasminogen and Soluble Epoxide Hydrolase on Thrombolysis, Inflammation, and Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 954. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Liu, D.; Proksch, P.; Guo, P.; Lin, W. Chartarlactams A–P, Phenylspirodrimanes from the Sponge-Associated Fungus Stachybotrys chartarum with Antihyperlipidemic Activities. J. Nat. Prod. 2014, 77, 138–147. [Google Scholar] [CrossRef]
- Moritoyo, T.; Nishimura, N.; Hasegawa, K.; Ishii, S.; Kirihara, K.; Takata, M.; Svensson, A.K.; Umeda, K.Y.; Kawarasaki, S.; Ihara, R.; et al. A first-in-human study of the anti-inflammatory profibrinolytic TMS-007, an SMTP family triprenyl phenol. Br. J. Clin. Pharmacol. 2023, 89, 1809–1819. [Google Scholar] [CrossRef]
- Iwama, R.; Sasano, Y.; Hiramatsu, T.; Otake, S.; Suzuki, E.; Hasumi, K. Amine-Regulated pri-SMTP Oxidation in SMTP Biosynthesis in Stachybotrys: Possible Implication in Nitrogen Acquisition. J. Fungi 2022, 8, 975. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, L.; Zhu, T.; Ba, M.; Li, G.; Gu, Q.; Guo, Y.; Li, D. Phenylspirodrimanes with Anti-HIV Activity from the Sponge-Derived Fungus Stachybotrys chartarum MXH-X73. J. Nat. Prod. 2013, 76, 2298–2306. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, J.; Tan, Z.; Liu, J.; Zhao, J.; Chen, R.; Xie, K.; Zhang, D.; Li, Y.; Yu, L. Stachybotrysins A–G, Phenylspirodrimane Derivatives from the Fungus Stachybotrys chartarum. J. Nat. Prod. 2017, 80, 1819–1826. [Google Scholar] [CrossRef]
- Desmiaty, Y.; Sandhiutami, N.M.D.; Mulatsari, E.; Maziyah, F.A.; Rahmadhani, K.; Algifari, H.O.Z.; Jantuna, F.A. Antioxidant and anti-inflammatory activity through inhibition of NF-κB and sEH of some citrus peel and phytoconstituent characteristics. Saudi Pharm. J. 2024, 32, 101959–101969. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, W.; Sun, K.; Gu, X.; Zeng, X.; Zhang, H.; Zhong, T.; Shao, Z.; Zhang, Y. Two new phenylspirodrimanes from the deep-sea derived fungus Stachybotrys sp. MCCC 3A00409. Nat. Prod. Res. 2018, 33, 386–392. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Hu, Z.; Wang, L. Novel Sesquiterpene and Diterpene Aminoglycosides from the Deep-Sea-Sediment Fungus Trichoderma sp. SCSIOW21. Mar. Drugs 2022, 21, 7. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Li, X.; Hu, Z.; Wang, L. Novel Harziane Diterpenes from Deep-Sea Sediment Fungus Trichoderma sp. SCSIOW21 and Their Potential Anti-Inflammatory Effects. Mar. Drugs 2021, 19, 689. [Google Scholar] [CrossRef]
- Lu, X.; He, J.; Wu, Y.; Du, N.; Li, X.; Ju, J.; Hu, Z.; Umezawa, K.; Wang, L. Isolation and Characterization of New Anti-Inflammatory and Antioxidant Components from Deep Marine-Derived Fungus Myrothecium SP. Bzo-l062. Mar. Drugs 2020, 18, 597. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Lin, Y.; Du, S.; Liu, Z.; Ju, J.; Suzuki, H.; Sawada, M.; Umezawa, K. Inhibition of cellular inflammatory mediator production and amelioration of learning deficit in flies by deep sea Aspergillus-derived cyclopenin. J. Antibiot. 2020, 73, 622–629. [Google Scholar] [CrossRef]
- Wang, L.; Umezawa, K. Cellular Signal Transductions and Their Inhibitors Derived from Deep-Sea Organisms. Mar. Drugs 2021, 19, 205. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, J.; Wang, L.; Lin, Y.; Kojima, S.; Okada, S.; Umezawa, K. Isolation of the Anti-Inflammatory Agent Myceliostatin from a Methionine-Enriched Culture of Myceliophthora thermophila ATCC 42464. J. Nat. Prod. 2023, 86, 239–245. [Google Scholar] [CrossRef]
- Lang, X.; Wen, H.; Qing, Y.; Wei, L.; Zhang, Y.; Bo, Z.; Ren, X. Three new phenylspirodrimanes from a conch-derived fungus Stachybotrys sp. NF02434. Tetrahedron Lett. 2023, 119, 154426–154429. [Google Scholar]
- Lingappan, K. NF-kB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Eshima, H.; Shahtout, J.L.; Siripoksup, P.; Pearson, M.J.; Mahmassani, Z.S.; Ferrara, P.J.; Lyons, A.W.; Maschek, J.A.; Peterlin, A.D.; Verkerke, A.R.P.; et al. Lipid hydroperoxides promote sarcopenia through carbonyl stress. Cell Biol. 2023, 12, e85289. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Jia, C.; Lang, J.; Niaz, S.I.; Li, J.; Yuan, J.; Yu, J.; Chen, S.; Liu, L. Antiviral and anti-inflammatory meroterpenoids:Stachybonoids A–F from the crinoid–derived fungus Stachybotrys cchartarum 952. RSC Adv. 2017, 7, 49910–49916. [Google Scholar] [CrossRef]
- Michael, J.M.; Zheng, L. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar]




| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| No. | δCa | δH (J in Hz) b | δCa | δH (J in Hz) b | δCa | δH (J in Hz) b | δCa | δH (J in Hz) b |
| 1 | 33.3, CH2 | 1.60, t (12.0) | 24.3, CH2 | 0.93, m c | 33.3, CH2 | 1.57, t (11.7) | 33.3, CH2 | 1.63, m |
| 1.10, dd (12.0, 4.4) | 1.79, m c | 1.14, dd (11.7, 4.4) | 1.12, dd (11.9, 4.3) | |||||
| 2 | 65.3, CH | 3.81, m c | 25.3, CH2 | 1.79, m c | 65.3, CH | 3.81, m c | 65.3, CH | 3.82, br d (12.1) |
| 1.41, m c | ||||||||
| 3 | 78.1, CH | 3.14, brs | 73.9, CH | 3.20, brs | 77.9, CH | 3.16, brs | 78.1, CH | 3.16, m c |
| 4 | 38.6, C | 37.8, C | 38.6, C | 38.6, C | ||||
| 5 | 39.2, CH | 1.92, dd (12.5, 4.0) | 39.8, CH | 2.04, dd (12.1, 3.1) | 39.2, CH | 1.97, dd (12.5, 3.1) | 39.2, CH | 1.95, dd (12.5, 3.0) |
| 6 | 20.7, CH2 | 1.46, m c | 20.9, CH2 | 1.48, m c | 20.7, CH2 | 1.49, m c | 20.7, CH2 | 1.49, m c |
| 1.36, m c | 1.42, m c | 1.42, m c | 1.38, m c | |||||
| 7 | 31.2, CH2 | 1.53, m c | 31.2, CH2 | 1.53, m c | 31.4, CH2 | 1.55, m c | 31.2, CH2 | 1.54, m c |
| 1.36, m c | 1.42, m c | 1.40, m c | 1.38, m c | |||||
| 8 | 36.6, CH | 1.78, m c | 37.0, CH | 1.77, m c | 36.7, CH | 1.84, m c | 36.6, CH | 1.80, m c |
| 9 | 98.1, C | 98.2, C | 100.4, C | 98.1, C | ||||
| 10 | 43.4, C | 42.2, C | 43.3, C | 43.4, C | ||||
| 11 | 32.3, CH2 | 3.13, d (14.2) | 32.2, CH2 | 3.11, d (16.7) | 30.9, CH2 | 3.11, d (17.2) | 32.3, CH2 | 3.15, d (16.9) |
| 2.76, d (14.2) | 2.72, d (16.7) | 2.76, d (17.2) | 2.78, d (16.9) | |||||
| 12 | 15.9, CH3 | 0.64, d (6.4) | 16.0, CH3 | 0.60, d (6.5) | 15.8, CH3 | 0.64, d (6.5) | 15.9, CH3 | 0.66, d (6.5) |
| 13 | 29.4, CH3 | 0.92, s | 29.1, CH3 | 0.90, s | 29.4, CH3 | 0.96, s | 29.4, CH3 | 0.94, s |
| 14 | 22.5, CH3 | 0.81, s | 22.8, CH3 | 0.81, s | 22.5, CH3 | 0.84, s | 22.5, CH3 | 0.83, s |
| 15 | 17.2, CH3 | 0.97, s | 16.3, CH3 | 0.95, s | 17.3, CH3 | 0.99, s | 17.2, CH3 | 0.99, s |
| 1′ | 116.8, C | 116.5, C | 119.8, C | 116.7, C | ||||
| 2′ | 154.2, C | 153.9, C | 159.7, C | 154.2, C | ||||
| 3′ | 101.3, CH | 6.55, s | 101.1, CH | 6.46, s | 104.0, CH | 6.67, s | 101.3, CH | 6.57, s |
| 4′ | 134.6, C | 134.7, C | 135.6, C | 134.6, C | ||||
| 5′ | 112.4, C | 112.8, C | 103.1, C | 112.4, C | ||||
| 6′ | 156.2, C | 156.2, C | 158.7, C | 156.3, C | ||||
| 7′ | 167.7, C | 168.2, C | 169.5, C | 167.9, C | ||||
| 8′ | 47.4, CH2 | 4.26, d (16.8) | 44.6, CH2 | 4.51, d (16.4) | 167.6, C | 47.0, CH2 | 4.30, d (16.8) | |
| 4.20, d (16.8) | 4.11, d (16.4) | 4.19, d (16.8) | ||||||
| 1″ | 44.1, CH2 | 3.61, t (7.0) | 40.7, CH2 | 4.15, brs | 41.7, CH2 | 3.47, t (7.2) | ||
| 2.40, brs | ||||||||
| 2″ | 33.6, CH2 | 2.79, m c | 29.5, CH2 | 2.63, brs | 27.7, CH2 | 1.62, m c | ||
| 1.25, brs | ||||||||
| 3″ | 129.5, C | 56.9, CH | 4.85, brs | 22.4, CH2 | 1.46, m c | |||
| 4″ | 129.9, CH | 7.04, d (7.0) | 36.3, CH2 | 3.42, brs | 33.8, CH2 | 2.25, t (7.4) | ||
| 3.06, m | ||||||||
| 5″ | 115.6, CH | 6.67, d (7.0) | 140.1, C | 175.0, C | ||||
| 6″ | 156.2, C | 128.6, CH | 7.23, m c | |||||
| 7″ | 128.9, CH | 7.20, m c | ||||||
| 8″ | 126.3, CH | 7.10, t (7.1) | ||||||
| 2′-OH | 0.75, s | 9.84, s | ||||||
| 2-OH | 4.18, brs | |||||||
| 3-OH | 3.99, d (3.3) | |||||||
| NH | 8.39, brs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Lu, L.; Zhang, P.; Wang, L. Chartarlactams U-X: Novel Phenylspirodrimanes from a Marine Derived Fungus Stachybotrys sp. SZU-W23 with Anti-Inflammatory Activity Mediated by the NF-κB/ROS Signaling Pathways. Mar. Drugs 2025, 23, 216. https://doi.org/10.3390/md23050216
Wu Y, Lu L, Zhang P, Wang L. Chartarlactams U-X: Novel Phenylspirodrimanes from a Marine Derived Fungus Stachybotrys sp. SZU-W23 with Anti-Inflammatory Activity Mediated by the NF-κB/ROS Signaling Pathways. Marine Drugs. 2025; 23(5):216. https://doi.org/10.3390/md23050216
Chicago/Turabian StyleWu, Yanhua, Lanyi Lu, Peng Zhang, and Liyan Wang. 2025. "Chartarlactams U-X: Novel Phenylspirodrimanes from a Marine Derived Fungus Stachybotrys sp. SZU-W23 with Anti-Inflammatory Activity Mediated by the NF-κB/ROS Signaling Pathways" Marine Drugs 23, no. 5: 216. https://doi.org/10.3390/md23050216
APA StyleWu, Y., Lu, L., Zhang, P., & Wang, L. (2025). Chartarlactams U-X: Novel Phenylspirodrimanes from a Marine Derived Fungus Stachybotrys sp. SZU-W23 with Anti-Inflammatory Activity Mediated by the NF-κB/ROS Signaling Pathways. Marine Drugs, 23(5), 216. https://doi.org/10.3390/md23050216






