A Review on Marine Microbial Docosahexaenoic Acid Production Through Circular Economy, Fermentation Engineering, and Antioxidant Technology
Abstract
1. Introduction
2. Sources of DHA
2.1. Traditional Sources of DHA
2.2. New Sources of DHA
- High productivity in fermentation systems: Under controlled fermentation conditions, selected heterotrophic strains exhibit rapid growth and can accumulate oil at levels as high as 50% of their dry cell weight.
- Closed-tank bioprocessing: Fermentation occurs in sterile bioreactors with defined and uncontaminated culture media. This controlled environment produces DHA oil with fewer impurities, superior quality, and enhanced safety.
- Efficient extraction processes: Microbial fermentation DHA production integrates seamlessly with downstream processing. Following fermentation, the oil can be efficiently extracted and refined without the geographical and seasonal constraints associated with fish-derived DHA, ensuring consistent production quality.
- Utilization of agricultural by-products: These microorganisms can be cultured using low-cost agricultural by-products and waste materials, such as waste molasses and glycerol, as fermentation substrates. This approach not only reduces production costs but also valorizes waste, contributing to environmental sustainability by mitigating pollution from waste disposal.
2.3. DHA-Producing Strains
3. Marine Microbial DHA Fermentation
3.1. DHA Synthesis Pathway
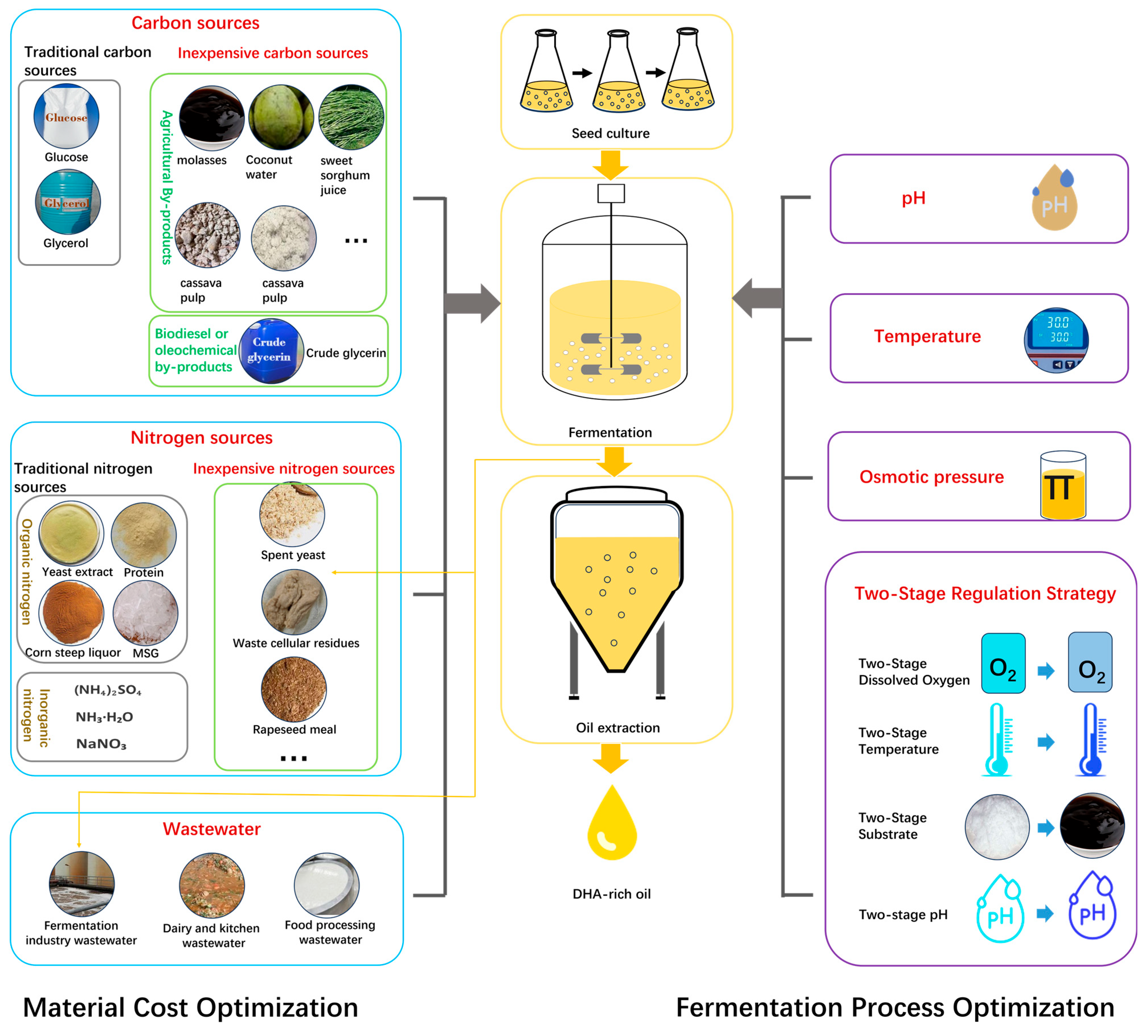
3.2. Materials for Microbial DHA Fermentation
3.2.1. Traditional Carbon Sources
3.2.2. Traditional Nitrogen Sources
3.2.3. Non-Traditional Low-Cost Materials
Cheap Carbon Sources
Cheap Nitrogen Sources
Wastewater
3.3. pH Control in DHA Fermentation Processes
3.4. Osmotic Control in DHA Fermentation Processes
3.5. Two-Stage Regulation Strategy for DHA Fermentation Processes
3.5.1. Two-Stage Dissolved Oxygen Regulation
3.5.2. Two-Stage Temperature Regulation
3.5.3. Two-Stage Substrate Regulation
4. Post-Treatment of Marine Microbial DHA Oils
4.1. Extraction of Oils
4.1.1. Cell Lysis
- Chemical lysis: This method uses strong acids or bases to break down the cell wall by dissolving glycoproteins, cellulose, and other structural components, thereby releasing the oil. While chemical lysis is relatively simple and does not require extensive sample pretreatment, the hot acid method is ineffective for extracting oils from cell membranes. Moreover, the use of acids and alkalis is highly corrosive and poses significant safety risks, making it unsuitable for large-scale production.
- Mechanical lysis: This technique utilizes high shear force from a high-pressure homogenizer to rupture cell walls and membrane components. Unlike chemical methods, mechanical lysis does not introduce chemicals, minimizing the risk of damaging the oil components and improving extraction efficiency. However, this method is energy-intensive and involves complex operational procedures, leading to higher labor and energy costs.
- Enzymatic lysis: This method uses specific enzymes tailored to the composition of the cell wall. The enzymes interact with the structural components of the cell wall and membrane, breaking them down and releasing the intracellular contents. Due to the complexity of the cell wall structures, a combination of enzymes is often required to achieve optimal lysis. With advancements in biotechnological enzymes and the increasing demand for efficient production methods, enzymatic lysis has become the predominant technique for disrupting DHA-producing microbial cells [120,121,122].
4.1.2. Oils Extraction
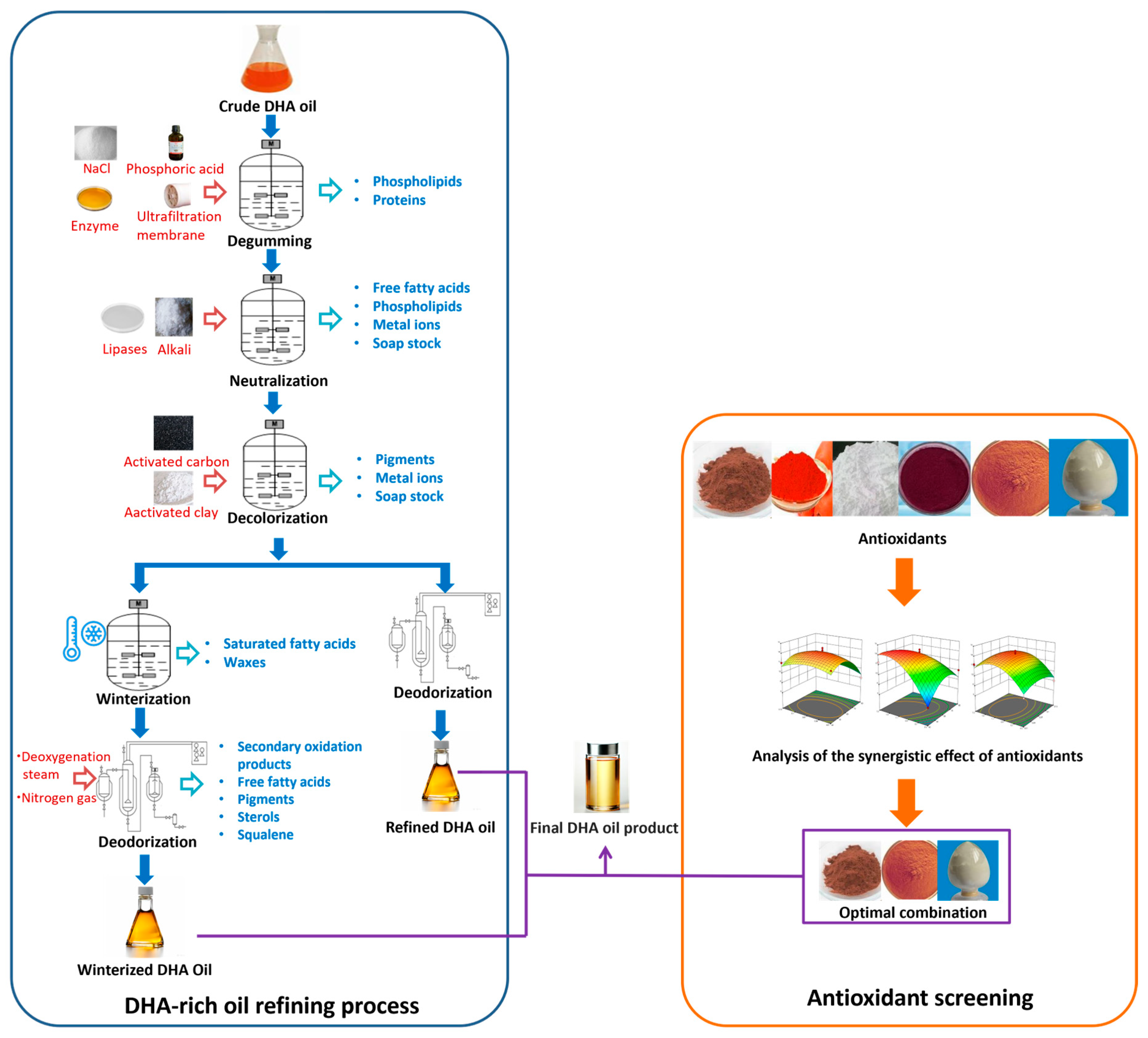
4.2. Oils Refining
- Degumming: This step removes phospholipids and other gum-forming agents that can affect the oil’s clarity and quality.
- Neutralization: Free fatty acids are removed, which helps reduce acidity and improve the oil’s stability.
- Decolorization: Pigments and certain other contaminants are removed, improving the oil’s color and purity.
- Deodorization: Volatile compounds responsible for off-flavors and undesirable odors are eliminated to improve the oil’s sensory characteristics.
4.2.1. Degumming
4.2.2. Neutralization
4.2.3. Decolorization
4.2.4. Deodorization
5. Antioxidant Technology of Marine Microbial DHA Oils
5.1. Oxidation Processes in PUFA-Rich Oils
5.1.1. Autoxidation of Oils
5.1.2. Photo-Oxidation of Oils
5.2. Antioxidants for Marine Microbial DHA Oils
5.3. Types of Antioxidants
5.3.1. Synthetic Antioxidants
5.3.2. Natural Antioxidants
- Tocopherol: A fat-soluble compound, tocopherol enhances DHA oil stability [149], though its efficacy is concentration-dependent. Structural isomers (α-, γ-, δ-tocopherols) exhibit varying activities; while tocopherol scavenges singlet oxygen and donates hydrogen to lipid radicals, excessive concentrations (>740 mg/kg) can paradoxically promote oxidation (e.g., by generating free radicals during decomposition) [150]).
- Rosemary extract: Derived from Rosmarinus officinalis, rosemary extract contains bioactive compounds (e.g., rosmarinic acid, carnosic acid, carnosol) and exists as water- or fat-soluble fractions. It is widely used in food preservation for its strong antioxidant properties [151,152], where a 50 mg/kg dose effectively controls oxidation rates [153], and it preserves the sensory quality of sardine oil during cold storage [154].
- Ascorbic acid: A water-soluble antioxidant, ascorbic acid neutralizes reactive oxygen species (ROS) via redox reactions [155] but exhibits limited solubility in oils. Esterification with fatty acids (e.g., ascorbyl palmitate) improves lipid compatibility while retaining antioxidant activity, making it suitable for DHA oil systems.
- Tea polyphenols: Extracted from tea leaves, tea polyphenols (e.g., catechins) are water-soluble and exhibit strong antioxidant activity. O’Sullivan et al. showed that 0.04% (w/w) tea polyphenols inhibit thermal degradation in frying oil [156]. Recent studies have further validated the antioxidant properties of tea polyphenols and demonstrated their successful application in protecting DHA algae oil [12,157,158].
5.4. Synergistic Effects of Antioxidants
5.4.1. Vitamins and Polyphenols
5.4.2. Vitamins and Carotenoids
5.4.3. Application of Synergistic Antioxidants in DHA Oils
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n-3 and n-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef] [PubMed]
- Goff, Z.D.; Nissen, S.E. N-3 polyunsaturated fatty acids for cardiovascular risk. Curr. Opin. Cardiol. 2022, 37, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Bellou, S.; Triantaphyllidou, I.-E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Yang, W.; Mohamed, H.; Zhang, Y.; Song, Y. Microbes: A Hidden Treasure of Polyunsaturated Fatty Acids. Front. Nutr. 2022, 9, 827837. [Google Scholar] [CrossRef]
- Yan, C.X.; Zhang, Y.; Yang, W.Q.; Ma, W.; Sun, X.M.; Huang, H. Universal and unique strategies for the production of polyunsaturated fatty acids in industrial oleaginous microorganisms. Biotechnol. Adv. 2024, 70, 108298. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Dietary DHA and health: Cognitive function ageing. Nutr. Res. Rev. 2016, 29, 281–294. [Google Scholar] [CrossRef]
- Díaz, M.; Mesa-Herrera, F.; Marín, R. DHA and Its Elaborated Modulation of Antioxidant Defenses of the Brain: Implications in Aging and AD Neurodegeneration. Antioxidants 2021, 10, 907. [Google Scholar] [CrossRef]
- Li, J.; Pora, B.L.R.; Dong, K.; Hasjim, J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Sci. Nutr. 2021, 9, 5229–5243. [Google Scholar] [CrossRef]
- Santos, H.O.; Price, J.C.; Bueno, A.A. Beyond Fish Oil Supplementation: The Effects of Alternative Plant Sources of Omega-3 Polyunsaturated Fatty Acids upon Lipid Indexes and Cardiometabolic Biomarkers—An Overview. Nutrients 2020, 12, 3159. [Google Scholar] [CrossRef]
- Xu, X.; Huang, C.; Xu, Z.; Xu, H.; Wang, Z.; Yu, X. The strategies to reduce cost and improve productivity in DHA production by Aurantiochytrium sp.: From biochemical to genetic respects. Appl. Microbiol. Biotechnol. 2020, 104, 9433–9447. [Google Scholar] [CrossRef]
- Jesionowska, M.; Ovadia, J.; Hockemeyer, K.; Clews, A.C.; Xu, Y. EPA and DHA in microalgae: Health benefits, biosynthesis, and metabolic engineering advances. J. Am. Oil Chem. Soc. 2023, 100, 831–842. [Google Scholar] [CrossRef]
- Kumari, A.; Pabbi, S.; Tyagi, A. Recent advances in enhancing the production of long chain omega-3 fatty acids in microalgae. Crit. Rev. Food Sci. Nutr. 2024, 64, 10564–10582. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Lu, T.; Liu, X.Y.; Zhao, M.T.; Yin, F.W.; Rakariyatham, K.; Zhou, D.Y. Improving the oxidative stability and lengthening the shelf life of DHA algae oil with composite antioxidants. Food Chem. 2020, 313, 126139. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, L.; Wang, D.; Sun, Y.; Huang, J.; Shahidi, F. Stability and stabilization of omega-3 oils: A review. Trends Food Sci. Technol. 2021, 118, 17–35. [Google Scholar] [CrossRef]
- Yin, F.; Sun, X.; Zheng, W.; Luo, X.; Peng, C.; Jia, Q.; Fu, Y. Improving the quality of microalgae DHA-rich oil in the deodorization process using deoxygenated steam. J. Food Process. Preserv. 2020, 44, e14602. [Google Scholar] [CrossRef]
- Singer, P.; Richter, V.; Singer, K.; Loehlein, I. Analyses and Declarations of Omega-3 Fatty Acids in Canned Seafood May Help to Quantify Their Dietary Intake. Nutrients 2021, 13, 2970. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Fan, C.; Wu, W.; Zhang, W.; Wang, Y. Health Benefits, Food Applications, and Sustainability of Microalgae-Derived N-3 PUFA. Foods 2022, 11, 1883. [Google Scholar] [CrossRef]
- Jiang, M.; Hu, Z.; Huang, Y.; Chen, X.D.; Wu, P. Impact of wall materials and DHA sources on the release, digestion and absorption of DHA microcapsules: Advancements, challenges and future directions. Food Res. Int. 2024, 191, 114646. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Makhutova, O.N. Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins Other Lipid Mediat. 2013, 107, 117–126. [Google Scholar] [CrossRef]
- Lenihan-Geels, G.; Bishop, K.S.; Ferguson, L.R. Alternative sources of omega-3 fats: Can we find a sustainable substitute for fish? Nutrients 2013, 5, 1301–1315. [Google Scholar] [CrossRef]
- Magoni, C.; Bertacchi, S.; Giustra, C.M.; Guzzetti, L.; Cozza, R.; Ferrari, M.; Torelli, A.; Marieschi, M.; Porro, D.; Branduardi, P.; et al. Could microalgae be a strategic choice for responding to the demand for omega-3 fatty acids? A European perspective. Trends Food Sci. Technol. 2022, 121, 142–155. [Google Scholar] [CrossRef]
- Veerasamy, V.; Neethirajan, V.; Singarayar, M.S.; Balasundaram, D.; Dharmar, P.; Thilagar, S. Microalgal biomass and lipid synergy for omega fatty acid enrichment: A sustainable source for food supplements & nutraceuticals. Algal Res. Biomass Biofuels Bioprod. 2024, 80, 103514. [Google Scholar] [CrossRef]
- Zhang, H.; Xiangying, Z.; Jiaxiang, Z.; Liping, L.; Liu, J. Overview of Docosahexaenoic Acid (DHA) Biosynthesis by Aurantiochytrium: Biosynthetic Pathway, High-Yield Mechanism, and Metabolic Engineering Strategies. Food Rev. Int. 2025, 41, 883–921. [Google Scholar] [CrossRef]
- Sharma, T.; Nisha, D.; Preeti, M.K.; Kumar, M.R.; Sudheer, P.; Kant, S.R.; Manish, K.; Abhishek, G.; Nayak, M. Microalgae as an emerging alternative raw material of docosahexaenoic acid and eicosapentaenoic acid—A review. Crit. Rev. Food Sci. Nutr. 2025, 31, 1–20. [Google Scholar] [CrossRef]
- Barclay, W.; Meager, K.; Abril, J. Heterotrophic production of long chain omega-3 fatty acids utilizing algae and algae-like microorganisms. J. Appl. Phycol. 1994, 6, 123–129. [Google Scholar] [CrossRef]
- Sun, X.M.; Xu, Y.S.; Huang, H. Thraustochytrid Cell Factories for Producing Lipid Compounds. Trends Biotechnol. 2021, 39, 648–650. [Google Scholar] [CrossRef]
- Bajpai, P.; Bajpai, P.K.; Ward, O.P. Production of docosahexaenoic acid by Thraustochytrium aureum. Appl. Microbiol. Biotechnol. 1991, 35, 706–710. [Google Scholar] [CrossRef]
- Barclay, W.R. Process for the Heterotrophic Production of Microbial Products with High Concentrations of Omega-3 Highly Unsaturated Fatty Acids. U.S. Patent 5,130,242, 14 July 1992. [Google Scholar]
- Yokoyama, R.; Honda, D. Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomic characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): Emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov. Mycoscience 2007, 48, 199–211. [Google Scholar] [CrossRef]
- Lee Chang, K.J.; Nichols, C.M.; Blackburn, S.I.; Dunstan, G.A.; Koutoulis, A.; Nichols, P.D. Comparison of Thraustochytrids Aurantiochytrium sp., Schizochytrium sp., Thraustochytrium sp., and Ulkenia sp. for production of biodiesel, long-chain omega-3 oils, and exopolysaccharide. Mar. Biotechnol. 2014, 16, 396–411. [Google Scholar] [CrossRef]
- Yin, F.W.; Zhan, C.T.; Huang, J.; Sun, X.L.; Yin, L.F.; Zheng, W.L.; Luo, X.; Zhang, Y.Y.; Fu, Y.Q. Efficient Co-production of Docosahexaenoic Acid Oil and Carotenoids in Aurantiochytrium sp. Using a Light Intensity Gradient Strategy. Appl. Biochem. Biotechnol. 2023, 195, 623–638. [Google Scholar] [CrossRef]
- Han, X.; Li, Z.; Wen, Y.; Chen, Z. Overproduction of docosahexaenoic acid in Schizochytrium sp. through genetic engineering of oxidative stress defense pathways. Biotechnol. Biofuels 2021, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Blasio, M.; Balzano, S. Fatty Acids Derivatives From Eukaryotic Microalgae, Pathways and Potential Applications. Front. Microbiol. 2021, 12, 718933. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.G.; Roessler, P.; Facciotti, D.; Levering, C.; Dittrich, F.; Lassner, M.; Valentine, R.; Lardizabal, K.; Domergue, F.; Yamada, A.; et al. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 2001, 293, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, D.; Wang, D.; Ning, K.; Jia, J.; Wei, L.; Jing, X.; Huang, S.; Chen, J.; Li, Y. Choreography of transcriptomes and lipidomes of Nannochloropsis reveals the mechanisms of oil synthesis in microalgae. Plant Cell 2014, 26, 1645–1665. [Google Scholar] [CrossRef]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef]
- Diao, J.; Li, X.; Pei, G.; Liu, L.; Chen, L. Comparative metabolomic analysis of Crypthecodinium cohnii in response to different dissolved oxygen levels during docosahexaenoic acid fermentation. Biochem. Biophys. Res. Commun. 2018, 499, 941–947. [Google Scholar] [CrossRef]
- Ding, J.; Fu, Z.; Zhu, Y.; He, J.; Ma, L.; Bu, D. Enhancing docosahexaenoic acid production of Schizochytrium sp. by optimizing fermentation using central composite design. BMC Biotechnol. 2022, 22, 39. [Google Scholar] [CrossRef]
- Hu, F.; Clevenger, A.L.; Zheng, P.; Huang, Q.; Wang, Z. Low-temperature effects on docosahexaenoic acid biosynthesis in Schizochytrium sp. TIO01 and its proposed underlying mechanism. Biotechnol. Biofuels 2020, 13, 172. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Chang, G.; Li, X.; Chang, M.; Liu, Y.; Jin, Q.; Wang, X. A strategy for the highly efficient production of docosahexaenoic acid by Aurantiochytrium limacinum SR21 using glucose and glycerol as the mixed carbon sources. Bioresour. Technol. 2015, 177, 51–57. [Google Scholar] [CrossRef]
- Sahin, D.; Tas, E.; Altindag, U.H. Enhancement of docosahexaenoic acid (DHA) production from Schizochytrium sp. S31 using different growth medium conditions. AMB Express 2018, 8, 7. [Google Scholar] [CrossRef]
- Yin, F.-W.; Zhang, Y.T.; Jiang, J.-Y.; Guo, D.-S.; Gao, S.; Gao, Z. Efficient docosahexaenoic acid production by Schizochytrium sp. via a two-phase pH control strategy using ammonia and citric acid as pH regulators. Process Biochem. 2019, 77, 1–7. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Z.; Yang, W.; Huang, P.; Gu, Y.; Sun, X.; Huang, H. Enhanced docosahexaenoic acid production from cane molasses by engineered and adaptively evolved Schizochytrium sp. Bioresour. Technol. 2023, 376, 128833. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Luo, Z.; Gu, S.; Wu, Q.; Chang, M.; Wang, X. Fatty acid shifts and metabolic activity changes of Schizochytrium sp. S31 cultured on glycerol. Bioresour. Technol. 2013, 142, 255–260. [Google Scholar] [CrossRef] [PubMed]
- de Swaaf, M.E.; Pronk, J.T.; Sijtsma, L. Fed-batch cultivation of the docosahexaenoic-acid-producing marine alga Crypthecodinium cohnii on ethanol. Appl. Microbiol. Biotechnol. 2003, 61, 40–43. [Google Scholar] [CrossRef]
- Sun, L.; Ren, L.; Zhuang, X.; Ji, X.; Yan, J.; Huang, H. Differential effects of nutrient limitations on biochemical constituents and docosahexaenoic acid production of Schizochytrium sp. Bioresour. Technol. 2014, 159, 199–206. [Google Scholar] [CrossRef]
- Li, M.; Liao, X.; Zhang, D.; Du, G.; Chen, J. Yeast extract promotes cell growth and induces production of polyvinyl alcohol-degrading enzymes. Enzyme Res. 2011, 2011, 179819. [Google Scholar] [CrossRef]
- Ling, X.; Guo, J.; Liu, X.; Zhang, X.; Wang, N.; Lu, Y.; Ng, I.S. Impact of carbon and nitrogen feeding strategy on high production of biomass and docosahexaenoic acid (DHA) by Schizochytrium sp. LU310. Bioresour. Technol. 2015, 184, 139–147. [Google Scholar] [CrossRef]
- Ren, L.J.; Sun, L.N.; Zhuang, X.Y.; Qu, L.; Ji, X.J.; Huang, H. Regulation of docosahexaenoic acid production by Schizochytrium sp.: Effect of nitrogen addition. Bioprocess Biosyst. Eng. 2014, 37, 865–872. [Google Scholar] [CrossRef]
- Li, S.; Hu, Z.; Yang, X.; Li, Y. Effect of Nitrogen Sources on Omega-3 Polyunsaturated Fatty Acid Biosynthesis and Gene Expression in Thraustochytriidae sp. Mar. Drugs 2020, 18, 612. [Google Scholar] [CrossRef]
- Ganuza, E.; Anderson, A.J.; Ratledge, C. High-cell-density cultivation of Schizochytrium sp. in an ammonium/pH-auxostat fed-batch system. Biotechnol. Lett. 2008, 30, 1559–1564. [Google Scholar] [CrossRef]
- Xie, D.; Jackson, E.N.; Zhu, Q. Sustainable source of omega-3 eicosapentaenoic acid from metabolically engineered Yarrowia lipolytica: From fundamental research to commercial production. Appl. Microbiol. Biotechnol. 2015, 99, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Chen, C.-W.; Tambat, V.S.; Singhania, R.R.; Chang, J.-S.; Dong, C.-D.; Patel, A.K. Bioprocess engineering to produce essential polyunsaturated fatty acids from Thraustochytrium sp. Bioresour. Technol. 2023, 383, 129209. [Google Scholar] [CrossRef] [PubMed]
- Burja, A.M.; Radianingtyas, H.; Windust, A.; Barrow, C.J. Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: Screening of strains and optimization of omega-3 production. Appl. Microbiol. Biotechnol. 2006, 72, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S.; Patel, A.K.; Chen, C.-W.; Chang, J.-S.; Michaud, P.; Dong, C.-D.; Singhania, R.R. Enhanced production of high-value polyunsaturated fatty acids (PUFAs) from potential thraustochytrid Aurantiochytrium sp. Bioresour. Technol. 2023, 370, 128536. [Google Scholar] [CrossRef]
- Trovao, M.; Pereira, H.; Costa, M.; Machado, A.; Barros, A.; Soares, M.; Carvalho, B.; Silva, J.T.; Varela, J.; Silva, J. Lab-Scale Optimization of Aurantiochytrium sp. Culture Medium for Improved Growth and DHA Production. Appl. Sci. 2020, 10, 2500. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Liu, L.; Zhu, X.; Sen, B.; Wang, G. Nitrogen Starvation Enhances the Production of Saturated and Unsaturated Fatty Acids in Aurantiochytrium sp. PKU#SW8 by Regulating Key Biosynthetic Genes. Mar. Drugs 2022, 20, 621. [Google Scholar] [CrossRef]
- Nazir, Y.; Shuib, S.; Kalil, M.S.; Song, Y.; Hamid, A.A. Optimization of Culture Conditions for Enhanced Growth, Lipid and Docosahexaenoic Acid (DHA) Production of Aurantiochytrium SW1 by Response Surface Methodology. Sci. Rep. 2018, 8, 8909. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, J.; Zhao, J.; Gao, Z.; Zhang, C.; Chen, M. Regulation of lipid accumulation in Schizochytrium sp ATCC 20888 in response to different nitrogen sources. Eur. J. Lipid Sci. Technol. 2017, 119, 1700025. [Google Scholar] [CrossRef]
- Guo, D.-S.; Ji, X.-J.; Ren, L.-J.; Yin, F.-W.; Sun, X.-M.; Huang, H.; Zhen, G. Development of a multi-stage continuous fermentation strategy for docosahexaenoic acid production by Schizochytrium sp. Bioresour. Technol. 2018, 269, 32–39. [Google Scholar] [CrossRef]
- Ju, J.-H.; Ko, D.-J.; Heo, S.-Y.; Lee, J.-J.; Kim, Y.-M.; Lee, B.-S.; Kim, M.-S.; Kim, C.-H.; Seo, J.-W.; Oh, B.-R. Regulation of lipid accumulation using nitrogen for microalgae lipid production in Schizochytrium sp. ABC101. Renew. Energy 2020, 153, 580–587. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.D.; Kim, N.; Kang, J. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Aini, U.N.; Lunprom, S.; Reungsang, A.; Salakkam, A. Docosahexaenoic acid (DHA) production by Aurantiochytrium limacinum using cassava pulp hydrolysate as an alternative low-cost carbon source. Front. Mar. Sci. 2022, 9, 985119. [Google Scholar] [CrossRef]
- Unagul, P.; Assantachai, C.; Phadungruengluij, S.; Suphantharika, M.; Tanticharoen, M.; Verduyn, C. Coconut water as a medium additive for the production of docosahexaenoic acid (C22:6 n3) by Schizochytrium mangrovei Sk-02. Bioresour. Technol. 2007, 98, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Su, C.-H.; Yu, Y.-K.; Huong, D.T.M. Sugarcane bagasse as a novel carbon source for heterotrophic cultivation of oleaginous microalga Schizochytrium sp. Ind. Crops Prod. 2018, 121, 99–105. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Xu, Y.-S.; Li, Z.-J.; Xu, L.-W.; Ma, W.; Li, Y.-F.; Guo, D.-S.; Sun, X.-M.; Huang, H. Turning waste into treasure: A new direction for low-cost production of lipid chemicals from Thraustochytrids. Biotechnol. Adv. 2024, 73, 108354. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y.; Yesuf, J.; Trushenski, J.; Blackburn, J.W. Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresour. Technol. 2010, 101, 3623–3627. [Google Scholar] [CrossRef]
- Yu, X.J.; Yu, Z.Q.; Liu, Y.L.; Sun, J.; Zheng, J.Y.; Wang, Z. Utilization of High-Fructose Corn Syrup for Biomass Production Containing High Levels of Docosahexaenoic Acid by a Newly Isolated Aurantiochytrium sp. YLH70. Appl. Biochem. Biotechnol. 2015, 177, 1229–1240. [Google Scholar] [CrossRef]
- Fan, K.W.; Chen, F.; Jones, E.B.; Vrijmoed, L.L. Eicosapentaenoic and docosahexaenoic acids production by and okara-utilizing potential of thraustochytrids. J. Ind. Microbiol. Biotechnol. 2001, 27, 199–202. [Google Scholar] [CrossRef]
- Lung, Y.-T.; Tan, C.H.; Show, P.L.; Ling, T.C.; Lan, J.C.-W.; Lam, H.L.; Chang, J.-S. Docosahexaenoic acid production from crude glycerol by Schizochytrium limacinum SR21. Clean Technol. Environ. Policy 2016, 18, 2209–2216. [Google Scholar] [CrossRef]
- Kujawska, N.; Talbierz, S.; Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Optimizing Docosahexaenoic Acid (DHA) Production by Schizochytrium sp. Grown on Waste Glycerol. Energies 2021, 14, 1685. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.; Jiang, M.; Liang, Z.; Jin, H.; Hu, X.; Wan, X.; Hu, C. Improvement of Omega-3 Docosahexaenoic Acid Production by Marine Dinoflagellate Crypthecodinium cohnii Using Rapeseed Meal Hydrolysate and Waste Molasses as Feedstock. PLoS ONE 2015, 10, e0125368. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.W.; Zhu, S.Y.; Guo, D.S.; Ren, L.J.; Ji, X.J.; Huang, H.; Gao, Z. Development of a strategy for the production of docosahexaenoic acid by Schizochytrium sp. from cane molasses and algae-residue. Bioresour. Technol. 2019, 271, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Olsen, P.M.; Kósa, G.; Klüver, M.; Kohler, A.; Shapaval, V.; Horn, S.J. Production of docosahexaenoic acid from spruce sugars using Aurantiochytrium limacinum. Bioresour. Technol. 2023, 376, 128827. [Google Scholar] [CrossRef] [PubMed]
- Didrihsone, E.; Dubencovs, K.; Grube, M.; Shvirksts, K.; Suleiko, A.; Suleiko, A.; Vanags, J. Crypthecodinium cohnii Growth and Omega Fatty Acid Production in Mediums Supplemented with Extract from Recycled Biomass. Mar. Drugs 2022, 20, 68. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.-F.; Wang, Z.-Y.; Wang, Y.; Hao, T.-B.; Balamurugan, S.; Li, D.-W.; He, Y.; Li, H.-Y.; Lin, C.S.K. A waste upcycling loop: Two-factor adaptive evolution of microalgae to increase polyunsaturated fatty acid production using food waste. J. Clean. Prod. 2022, 331, 130018. [Google Scholar] [CrossRef]
- Ryu, B.G.; Kim, K.; Kim, J.; Han, J.I.; Yang, J.W. Use of organic waste from the brewery industry for high-density cultivation of the docosahexaenoic acid-rich microalga, Aurantiochytrium sp. KRS101. Bioresour. Technol. 2013, 129, 351–359. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Verardo, V.; Martín-García, B.; Di Pierro, P.; Sorrentino, A.; Baselice, M.; Oliviero, M.; Sacchi, R.; Masi, P. Formulation of New Media from Dairy and Brewery Wastes for a Sustainable Production of DHA-Rich Oil by Aurantiochytrium mangrovei. Mar. Drugs 2021, 20, 30. [Google Scholar] [CrossRef]
- Bryant, H.L.; Gogichaishvili, I.; Anderson, D.; Richardson, J.W.; Sawyer, J.; Wickersham, T.; Drewery, M.L. The value of post-extracted algae residue. Algal Res. Biomass Biofuels Bioprod. 2012, 1, 185–193. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, Z.; Yin, F.; Ji, X.; Huang, H. Lipid production of Chlorella vulgaris from lipid-extracted microalgal biomass residues through two-step enzymatic hydrolysis. Bioresour. Technol. 2012, 117, 1–6. [Google Scholar] [CrossRef]
- Yin, F.-W.; Huang, J.; Zhan, C.-T.; Sun, X.-L.; Zheng, W.-L.; Luo, X.; Zhang, Y.-Y.; Yin, L.-F.; Fu, Y.-Q. Recycling Fermentation Strategy for Waste Cellular Residues in the Production of Polyunsaturated Fatty Acids. Fermentation 2024, 10, 81. [Google Scholar] [CrossRef]
- Wang, S.K.; Wang, X.; Tian, Y.T.; Cui, Y.H. Nutrient recovery from tofu whey wastewater for the economical production of docosahexaenoic acid by Schizochytrium sp. S31. Sci. Total. Environ. 2020, 710, 136448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Yang, L.H.; He, S.J.; Du, Y.H.; Guo, D.S. Development of a green fermentation strategy with resource cycle for the docosahexaenoic acid production by Schizochytrium sp. Bioresour. Technol. 2023, 385, 129434. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.W.; Guo, D.S.; Ren, L.J.; Ji, X.J.; Huang, H. Development of a method for the valorization of fermentation wastewater and algal-residue extract in docosahexaenoic acid production by Schizochytrium sp. Bioresour. Technol. 2018, 266, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Aki, T.; Shinozaki, M.; Taguchi, M.; Kawamoto, S.; Ono, K. Utilization of Shochu distillery wastewater for production of polyunsaturated fatty acids and xanthophylls using thraustochytrid. J. Biosci. Bioeng. 2006, 102, 323–327. [Google Scholar] [CrossRef]
- Song, X.; Ma, Z.; Tan, Y.; Zhang, H.; Cui, Q. Wastewater recycling technology for fermentation in polyunsaturated fatty acid production. Bioresour. Technol. 2017, 235, 79–86. [Google Scholar] [CrossRef]
- Wang, S.K.; Tian, Y.T.; Dai, Y.R.; Wang, D.; Liu, K.C.; Cui, Y.H. Development of an alternative medium via completely replaces the medium components by mixed wastewater and crude glycerol for efficient production of docosahexaenoic acid by Schizochytrium sp. Chemosphere 2022, 291, 132868. [Google Scholar] [CrossRef]
- Lee, G.-I.; Shin, W.-S.; Jung, S.M.; Kim, W.; Lee, C.; Kwon, J.-H. Effects of soybean curd wastewater on growth and DHA production in Aurantiochytrium sp. LWT-Food Sci. Technol. 2020, 134, 110245. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, W.; Qin, Y.; Zhou, X.; Han, W.; Gao, S.; Li, X.; Naushad, M.; Jiang, G.; Liu, H. Development of an alternative low-cost culture medium for a new isolated high-production DHA strain using kitchen wastewater. Process Saf. Environ. Prot. 2024, 183, 698–707. [Google Scholar] [CrossRef]
- Humhal, T.; Kastanek, P.; Jezkova, Z.; Cadkova, A.; Kohoutkova, J.; Branyik, T. Use of saline waste water from demineralization of cheese whey for cultivation of Schizochytrium limacinum PA-968 and Japonochytrium marinum AN-4. Bioprocess Biosyst. Eng. 2017, 40, 395–402. [Google Scholar] [CrossRef]
- Song, X.; Zang, X.; Zhang, X. Production of High Docosahexaenoic Acid by Schizochytrium sp Using Low-cost Raw Materials from Food Industry. J. Oleo Sci. 2015, 64, 197–204. [Google Scholar] [CrossRef]
- Park, W.-K.; Moon, M.; Shin, S.-E.; Cho, J.M.; Suh, W.I.; Chang, Y.K.; Lee, B. Economical DHA (Docosahexaenoic acid) production from Aurantiochytrium sp KRS101 using orange peel extract and low cost nitrogen sources. Algal Res. Biomass Biofuels Bioprod. 2018, 29, 71–79. [Google Scholar] [CrossRef]
- Nazir, Y.; Halim, H.; Al-Shorgani, N.K.N.; Manikan, V.; Hamid, A.A.; Song, Y. Efficient conversion of extracts from low-cost, rejected fruits for high-valued Docosahexaenoic acid production by Aurantiochytrium sp. SW1. Algal Res. 2020, 50, 101977. [Google Scholar] [CrossRef]
- Moniz, P.; Silva, C.; Oliveira, A.C.; Reis, A.; Lopes da Silva, T. Raw Glycerol Based Medium for DHA and Lipids Production, Using the Marine Heterotrophic Microalga Crypthecodinium cohnii. Processes 2021, 9, 2005. [Google Scholar] [CrossRef]
- Lowrey, J.; Armenta, R.E.; Brooks, M.S. Recycling of lipid-extracted hydrolysate as nitrogen supplementation for production of thraustochytrid biomass. J. Ind. Microbiol. Biotechnol. 2016, 43, 1105–1115. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, J.-N.; He, A.-Y.; Wu, H.; Kong, X.-P.; Liu, J.-L.; Yin, C.-Y.; Chen, W.-F.; Chen, P. Enhanced acetone/butanol/ethanol production by Clostridium beijerinckii IB4 using pH control strategy. Process Biochem. 2014, 49, 1238–1244. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Selection method of pH conditions to establish Pseudomonas taetrolens physiological states and lactobionic acid production. Appl. Microbiol. Biotechnol. 2013, 97, 3843–3854. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Zeng, G.; Dong, H.; Chen, Y.; Huang, C.; Zhu, Y.; Xu, R.; Cheng, Y.; Hou, K.; et al. Multivariate relationships between microbial communities and environmental variables during co-composting of sewage sludge and agricultural waste in the presence of PVP-AgNPs. Bioresour. Technol. 2018, 261, 10–18. [Google Scholar] [CrossRef]
- Kautharapu, K.B.; Rathmacher, J.; Jarboe, L.R. Growth condition optimization for docosahexaenoic acid (DHA) production by Moritella marina MP-1. Appl. Microbiol. Biotechnol. 2013, 97, 2859–2866. [Google Scholar] [CrossRef][Green Version]
- Song, P.; Yuan, K.; Qin, T.; Zhang, K.; Ji, X.J.; Ren, L.; Guan, R.; Wen, J.; Huang, H. Metabolomics profiling reveals the mechanism of increased pneumocandin B(0) production by comparing mutant and parent strains. J. Ind. Microbiol. Biotechnol. 2018, 45, 767–780. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Mbifile, M.D.; Li, C.; Yang, H.; Wang, W. Improvement of docosahexaenoic acid fermentation from Schizochytrium sp. AB-610 by staged pH control based on cell morphological changes. Eng. Life Sci. 2017, 17, 981–988. [Google Scholar] [CrossRef]
- Guo, D.S.; Tong, L.L.; Ji, X.J.; Ren, L.J.; Ding, Q.Q. Development of a Strategy to Improve the Stability of Culture Environment for Docosahexaenoic Acid Fermentation by Schizochytrium sp. Appl. Biochem. Biotechnol. 2020, 192, 881–894. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hou, L.; Dong, M.; Shi, J.; Huang, X.; Ding, Y.; Cong, X.; Zhang, F.; Zhang, X.; Zang, X. Transcriptome Analysis in Haematococcus pluvialis: Astaxanthin Induction by High Light with Acetate and Fe2+. Int. J. Mol. Sci. 2018, 19, 175. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, A.N.; Aasen, I.M.; Strøm, A.R. Endogenously synthesized (–)-proto-quercitol and glycine betaine are principal compatible solutes of Schizochytrium sp. strain S8 (ATCC 20889) and three new isolates of phylogenetically related thraustochytrids. Appl. Environ. Microbiol. 2007, 73, 5848–5856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hounslow, E.; Kapoore, R.V.; Vaidyanathan, S.; Gilmour, D.J.; Wright, P.C. The Search for a Lipid Trigger: The Effect of Salt Stress on the Lipid Profile of the Model Microalgal Species Chlamydomonas reinhardtii for Biofuels Production. Curr. Biotechnol. 2016, 5, 305–313. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Q.; Li, Y.; Shi, Z.; Zhu, Y.; Du, G.; Chen, J. Enhancement of pyruvate production by osmotic-tolerant mutant of Torulopsis glabrata. Biotechnol. Bioeng. 2007, 97, 825–832. [Google Scholar] [CrossRef]
- Weete, J.D.; Kim, H.; Gandhi, S.R.; Wang, Y.; Dute, R. Lipids and ultrastructure of Thraustochytrium sp. ATCC 26185. Lipids 1997, 32, 839–845. [Google Scholar] [CrossRef]
- Hu, X.C.; Ren, L.J.; Chen, S.L.; Zhang, L.; Ji, X.J.; Huang, H. The roles of different salts and a novel osmotic pressure control strategy for improvement of DHA production by Schizochytrium sp. Bioprocess Biosyst. Eng. 2015, 38, 2129–2136. [Google Scholar] [CrossRef]
- Qu, L.; Ji, X.J.; Ren, L.J.; Nie, Z.K.; Feng, Y.; Wu, W.J.; Ouyang, P.K.; Huang, H. Enhancement of docosahexaenoic acid production by Schizochytrium sp. using a two-stage oxygen supply control strategy based on oxygen transfer coefficient. Lett. Appl. Microbiol. 2011, 52, 22–27. [Google Scholar] [CrossRef]
- Jia, L.; Li, T.; Yang, Z.; He, T.; Ding, J.; Li, T.; Huang, A. Eliminating the accumulation of reactive oxygen species through periodic hypoxic stress control for effective DHA production by Schizochytrium sp. Chem. Eng. Sci. 2023, 280, 119040. [Google Scholar] [CrossRef]
- Guo, D.-S.; Ji, X.-J.; Ren, L.-J.; Li, G.-L.; Huang, H. Improving docosahexaenoic acid production by Schizochytrium sp using a newly designed high-oxygen-supply bioreactor. AICHE J. 2017, 63, 4278–4286. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Premaratne, M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Two-stage cultivation of microalgae for production of high-value compounds and biofuels: A review. Algal Res. Biomass Biofuels Bioprod. 2021, 57, 102353. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, H.; Lai, Y.; Wu, J.; Chen, H. Improving docosahexaenoic acid productivity of Schizochytrium sp. by a two-stage AEMR/shake mixed culture mode. Bioresour. Technol. 2013, 142, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ren, L.; Guo, D.; Wu, W.; Ji, X.; Huang, H. CFD investigation of Schizochytrium sp. impeller configurations on cell growth and docosahexaenoic acid synthesis. Bioprocess Biosyst. Eng. 2016, 39, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Hoseinkhani, N.; Jalili, H.; Ansari, S.; Amrane, A. Impact of bubble size on docosahexaenoic acid production by Crypthecodinium cohnii in bubble column bioreactor. Biomass Convers. Biorefin. 2021, 11, 1137–1144. [Google Scholar] [CrossRef]
- Hu, X.; Luo, Y.; Man, Y.; Tang, X.; Bi, Z.; Ren, L. Lipidomic and transcriptomic analysis reveals the self-regulation mechanism of Schizochytrium sp. in response to temperature stresses. Algal Res. Biomass Biofuels Bioprod. 2022, 64, 102664. [Google Scholar] [CrossRef]
- Zeng, Y.; Ji, X.J.; Lian, M.; Ren, L.J.; Jin, L.J.; Ouyang, P.K.; Huang, H. Development of a temperature shift strategy for efficient docosahexaenoic acid production by a marine fungoid protist, Schizochytrium sp. HX-308. Appl. Biochem. Biotechnol. 2011, 164, 249–255. [Google Scholar] [CrossRef]
- Rosa, S.M.; Soria, M.A.; Vélez, C.G.; Galvagno, M.A. Improvement of a two-stage fermentation process for docosahexaenoic acid production by Aurantiochytrium limacinum SR21 applying statistical experimental designs and data analysis. Bioresour. Technol. 2010, 101, 2367–2374. [Google Scholar] [CrossRef]
- Jia, L.; Li, T.; Wang, R.; Ma, M.; Yang, Z. Enhancing docosahexaenoic acid production from Schizochytrium sp. by using waste Pichia pastoris as nitrogen source based on two-stage feeding control. Bioresour. Technol. 2024, 403, 130891. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Matsakas, L. An Overview of Current Pretreatment Methods Used to Improve Lipid Extraction from Oleaginous Microorganisms. Molecules 2018, 23, 1562. [Google Scholar] [CrossRef]
- Lin, Y.; Xie, X.; Yuan, B.; Fu, J.; Liu, L.; Tian, H.; Chen, T.; He, D. Optimization of Enzymatic Cell Disruption for Improving Lipid Extraction from Schizochytrium sp through Response Surface Methodology. J. Oleo Sci. 2018, 67, 215–224. [Google Scholar] [CrossRef]
- Isa, M.H.; Metin, C.; Ercan, E.; Alparslan, Y. Effect of different cell disruption methods on lipid yield of Schizochytrium sp. J. Am. Oil Chem. Soc. 2022, 99, 129–139. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Chen, G.; Zhang, J.; Wang, C.; Liu, B. Extraction and purification of eicosapentaenoic acid and docosahexaenoic acid from microalgae: A critical review. Algal Res. 2019, 43, 101619. [Google Scholar] [CrossRef]
- Dhara, O.; Rani, K.N.P.; Chakrabarti, P.P. Supercritical Carbon Dioxide Extraction of Vegetable Oils: Retrospective and Prospects. Eur. J. Lipid Sci. Technol. 2022, 124, 2200006. [Google Scholar] [CrossRef]
- Gilcher, E.B.; Lane, M.K.M.; Pontious, R.S.; Apatoff, M.B.L.; Ahrens-Viquez, M.M.; Zimmerman, J.B. Sequential Extraction and Purification of Triglycerides and Carotenoids with Supercritical Carbon Dioxide for Valorization of the Integrated Algal Biorefinery. ACS Sustain. Chem. Eng. 2025, 13, 1667–1676. [Google Scholar] [CrossRef]
- Crampon, C.; Nikitine, C.; Zaier, M.; Lepine, O.; Tanzi, C.D.; Vian, M.A.; Chemat, F.; Badens, E. Oil extraction from enriched Spirulina platensis microalgae using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 119, 289–296. [Google Scholar] [CrossRef]
- Tzima, S.; Georgiopoulou, I.; Louli, V.; Magoulas, K. Recent Advances in Supercritical CO2 Extraction of Pigments, Lipids and Bioactive Compounds from Microalgae. Molecules 2023, 28, 1410. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Du, S.; Chen, H.; Wang, Q. Microalgal polyunsaturated fatty acids: Hotspots and production techniques. Front. Bioeng. Biotechnol. 2023, 11, 1146881. [Google Scholar] [CrossRef]
- Dunford, N.T. Enzyme-aided oil and oilseed processing: Opportunities and challenges. Curr. Opin. Food Sci. 2022, 48, 100943. [Google Scholar] [CrossRef]
- Sampaio, K.A.; Zyaykina, N.; Wozniak, B.; Tsukamoto, J.; De Greyt, W.; Stevens, C.V. Enzymatic degumming: Degumming efficiency versus yield increase. Eur. J. Lipid Sci. Technol. 2015, 117, 81–86. [Google Scholar] [CrossRef]
- Lamas, D.L. Effect of enzymatic degumming process on the physicochemical and nutritional properties of fish byproducts oil. Appl. Food Res. 2022, 2, 100170. [Google Scholar] [CrossRef]
- Kim, I.-C.; Kim, J.-H.; Lee, K.-H.; Tak, T.-M. Phospholipids separation (degumming) from crude vegetable oil by polyimide ultrafiltration membrane. J. Membr. Sci. 2002, 205, 113–123. [Google Scholar] [CrossRef]
- de Moura, J.M.; Gonçalves, L.A.; Petrus, J.C.C.; Viotto, L.A. Degumming of vegetable oil by microporous membrane. J. Food Eng. 2005, 70, 473–478. [Google Scholar] [CrossRef]
- Yi, M.; You, Y.; Zhang, Y.; Wu, G.; Karrar, E.; Zhang, L.; Zhang, H.; Jin, Q.; Wang, X. Highly valuable fish oil: Formation process, enrichment, subsequent utilization, and storage of eicosapentaenoic acid ethyl esters. Molecules 2023, 28, 672. [Google Scholar] [CrossRef]
- Byreddy, A.R. Thraustochytrids as an alternative source of omega-3 fatty acids, carotenoids and enzymes. Lipid Technol. 2016, 28, 68–70. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, Y.; Tang, J.; Wang, Y.; Xiao, Z.; Chenfeng, J.; Liang, D. Method of Producing Oil Containing Polyunsaturated Fatty Acids by Using Schizochytrium sp. Chinese Patent Application No. 201910418891.5, 9 August 2019. [Google Scholar]
- Singh, D.; Rani, R.; Sharma, A.K.; Gupta, R.P.; Ramakumar, S.S.V.; Mathur, A.S. Extraction, separation and purification of fatty acid ethyl esters for biodiesel and DHA from Thraustochytrid biomass. Biotechnol. J. 2024, 19, 2300350. [Google Scholar] [CrossRef]
- Sanchez-Machado, D.I.; Lopez-Cervantes, J.; Nunez-Gastelum, J.A.; Servin de la Mora-Lopez, G.; Lopez-Hernandez, J.; Paseiro-Losada, P. Effect of the refining process on Moringa oleifera seed oil quality. Food Chem. 2015, 187, 53–57. [Google Scholar] [CrossRef]
- Mjøs, S.A.; Solvang, M. Geometrical isomerisation of eicosapentaenoic and docosahexaenoic acid at high temperatures. Eur. J. Lipid Sci. Technol. 2006, 108, 589–597. [Google Scholar] [CrossRef]
- O’brien, R.D. Fats and Oils: Formulating and Processing for Applications; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Zuo, W.; Hu, X.; Yang, Y.; Jiang, L.; Ren, L.; Huang, H. Development of an Improved Method to Determine Saturated Aliphatic Aldehydes in Docosahexaenoic Acid-Rich Oil: A Supplement to p-Anisidine Value. Eur. J. Lipid Sci. Technol. 2017, 119, 1700243. [Google Scholar] [CrossRef]
- Yu, D.; Dong, T.; Zhang, L.; Zhou, X.; Wang, L.; Yang, F.; Liu, T. Effects of different deodorization methods on the oxidation of sterol components in rice bran oil. Food Chem. 2023, 404, 134568. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Medina-Meza, I.G.; Barnaba, C. Kinetics of Cholesterol Oxidation in Model Systems and Foods: Current Status. Food Eng. Rev. 2013, 5, 171–184. [Google Scholar] [CrossRef]
- Johnson, D.R.; Decker, E.A. The Role of Oxygen in Lipid Oxidation Reactions: A Review. Annu. Rev. Food Sci. Technol. 2015, 6, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev. 2010, 39, 4067–4079. [Google Scholar] [CrossRef]
- Amarachukwu Uzombah, T. The Implications of Replacing Synthetic Antioxidants with Natural Ones in the Food Systems. In Natural Food Additives; Prieto, M.Á., Otero, P., Carpena Rodriguez, M., Eds.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Sahari, M.A.; Moghimi, H.R.; Hadian, Z.; Barzegar, M.; Mohammadi, A. Physicochemical properties and antioxidant activity of α-tocopherol loaded nanoliposome’s containing DHA and EPA. Food Chem. 2017, 215, 157–164. [Google Scholar] [CrossRef]
- Jacobsen, C.; Hartvigsen, K.; Lund, P.; Thomsen, M.K.; Skibsted, L.H.; Hølmer, G.; Adler-Nissen, J.; Meyer, A.S. Oxidation in fish oil-enriched mayonnaise: 4. Effect of tocopherol concentration on oxidative deterioration. Eur. Food Res. Technol. 2001, 212, 308–318. [Google Scholar] [CrossRef]
- Yang, J.; Goksen, G.; Zhang, W. Rosemary essential oil: Chemical and biological properties, with emphasis on its delivery systems for food preservation. Food Control 2023, 154, 110003. [Google Scholar] [CrossRef]
- Qiu, K.; Wang, S.; Duan, F.; Sang, Z.; Wei, S.; Liu, H.; Tan, H. Rosemary: Unrevealing an old aromatic crop as a new source of promising functional food additive—A review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13273. [Google Scholar] [CrossRef]
- Guo, M.; Yang, L.; Li, X.; Tang, H.; Li, X.; Xue, Y.; Duan, Z. Antioxidant Efficacy of Rosemary Extract in Improving the Oxidative Stability of Rapeseed Oil during Storage. Foods 2023, 12, 3583. [Google Scholar] [CrossRef]
- Ozogul, Y.; Ayas, D.; Yazgan, H.; Ozogul, F.; Boga, E.K.; Ozyurt, G. The capability of rosemary extract in preventing oxidation of fish lipid. Int. J. Food Sci. Technol. 2010, 45, 1717–1723. [Google Scholar] [CrossRef]
- Ren, L.-J.; Sun, X.-M.; Ji, X.-J.; Chen, S.-L.; Guo, D.-S.; Huang, H. Enhancement of docosahexaenoic acid synthesis by manipulation of antioxidant capacity and prevention of oxidative damage in Schizochytrium sp. Bioresour. Technol. 2017, 223, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-X.; Yu, J.; Chen, N.; Zeng, W.-C. Effects and mechanism of tea polyphenols on the quality of oil during frying process. J. Food Sci. 2020, 85, 3786–3796. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sun, X.; Zheng, W.; Luo, X.; Zhang, Y.; Yin, L.; Jia, Q.; Fu, Y. Screening of highly effective mixed natural antioxidants to improve the oxidative stability of microalgal DHA-rich oil. RSC Adv. 2021, 11, 4991–4999. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.-C.; Yin, F.-W.; Zhong, X.; Liu, H.-L.; Song, L.; Zhao, G.-H.; Wang, Y.-F.; Zhou, D.-Y. Effects of different antioxidants and their combinations on the oxidative stability of DHA algae oil and walnut oil. Food Sci. Nutr. 2022, 10, 2804–2812. [Google Scholar] [CrossRef]
- Huang, P.-W.; Yan, C.-X.; Sun, X.-M.; Huang, H. Economical downstream processing of microbial polyunsaturated fatty acids. Trends Biotechnol. 2023, 41, 857–859. [Google Scholar] [CrossRef]
- Dai, F.; Chen, W.-F.; Zhou, B. Antioxidant synergism of green tea polyphenols with α-tocopherol and l-ascorbic acid in SDS micelles. Biochimie 2008, 90, 1499–1505. [Google Scholar] [CrossRef]
- Pedrielli, P.; Skibsted, L.H. Antioxidant Synergy and Regeneration Effect of Quercetin, (−)-Epicatechin, and (+)-Catechin on α-Tocopherol in Homogeneous Solutions of Peroxidating Methyl Linoleate. J. Agric. Food Chem. 2002, 50, 7138–7144. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef]
- Enko, J.; Gliszczynska-Swiglo, A. Influence of the interactions between tea (Camellia sinensis) extracts and ascorbic acid on their antioxidant activity: Analysis with interaction indexes and isobolograms. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 1234–1242. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Gil-Chavez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; Gonzalez-Aguilar, G.A. Antioxidant Interactions between Major Phenolic Compounds Found in ‘Ataulfo’ Mango Pulp: Chlorogenic, Gallic, Protocatechuic and Vanillic Acids. Molecules 2012, 17, 12657–12664. [Google Scholar] [CrossRef]
- Murugesan, N.; Damodaran, C.; Krishnamoorthy, S.; Raja, M. In-vitro evaluation of synergism in antioxidant efficiency of Quercetin and Resveratrol. Chem. Biol. Lett. 2023, 10, 534. [Google Scholar]
- Chang, M.; Zhang, T.; Guo, X.; Liu, Y.; Liu, R.; Jin, Q.; Wang, X. Optimization of cultivation conditions for efficient production of carotenoid-rich DHA oil by Schizochytrium sp. S31. Process Biochem. 2020, 94, 190–197. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.; Zhang, T.; Gong, M.; Liu, R.; Chang, M.; Wang, X. Interactions between liposoluble antioxidants: A critical review. Food Res. Int. 2022, 155, 111104. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef]
- Capitani, C.D.; Carvalho, A.C.L.; Botelho, P.B.; Carrapeiro, M.M.; Castro, I.A. Synergism on antioxidant activity between natural compounds optimized by response surface methodology. Eur. J. Lipid Sci. Technol. 2009, 111, 1100–1110. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, C.; Lu, T.; Ding, X.-Y.; Zhao, M.-T.; Zhang, M.; Liu, H.-L.; Song, L.; Zhou, D.-Y. Effects of gallic acid alkyl esters and their combinations with other antioxidants on oxidative stability of DHA algae oil. Food Res. Int. 2021, 143, 110280. [Google Scholar] [CrossRef]
| Strains | Carbon Sources | Nitrogen Sources | Fermentation Conditions | DHA Content (g/L) | DHA Productivity (mg/L/h) | Ref. |
|---|---|---|---|---|---|---|
| Thraustochytrium sp. | Glucose | Yeast extract, peptone | Batch fermentation (shake flasks, 26 °C, 150 rpm, 120 h) | 1.34 | 11.17 | [53] |
| Thraustochytrium sp. ONC-T18 | Glucose | Yeast extract, MSG | Batch fermentation (5 L bioreactor, 25 °C, 120 rpm, 168 h) | 4.6 | 38.33 | [54] |
| Aurantiochytrium sp. | Glucose | Yeast extract, peptone | Batch fermentation (shake flasks, 26 °C, 150 rpm, 120 h) | 1.34 | 11.17 | [55] |
| Aurantiochytrium sp. AF0043 | Glucose, glycerol | MSG, Corn steep powder | Fed-batch fermentation (shake flasks, 28 °C, 150 rpm, 120 h) | 2.75 | 22.92 | [56] |
| Aurantiochytrium sp. PKU#SW8 | Glucose | MSG | Batch fermentation (shake flasks, 28 °C, 170 rpm, 96 h) | 3.64 | 37.92 | [57] |
| Aurantiochytrium SW1 | Fructose | MSG | Batch fermentation (shake flasks, 30 °C, 250 rpm, 120 h) | 4.75 | 39.58 | [58] |
| Aurantiochytrium limacinum SR21 | Glucose, glycerol | Yeast extract, MSG | Fed-batch fermentation (5 L bioreactor, 25 °C, 300–400 rpm, 96 h) | 32.36 | 337.1 | [40] |
| Schizochytrium sp. I-F-9 | Glucose, glycerol | Peptone, MSG | Fed-batch fermentation (shake flasks, 28 °C, 200 rpm, 120 h) | 8.33 | 69.41 | [38] |
| Schizochytrium sp. ATCC 20888 | Glucose | Yeast extract, MSG | Batch fermentation (shake flasks, 25 °C, 200 rpm, 96 h) | 6.95 | 72.4 | [59] |
| Schizochytrium sp. HX-308 | Glucose | Yeast extract, MSG | Three stage continuous fermentation (50 L bioreactor, 30 °C, 300 rpm, 147 h) | 23.0 | 156.46 | [60] |
| Schizochytrium sp. ABC101 | Glucose | Yeast extract, corn steep liquor | Fed-batch fermentation (5 L bioreactor, 28 °C, 200 rpm, 84 h) | 16.7 | 183.3 | [61] |
| Strains | Fermented Raw Materials | Biomass (g/L) | Lipid (g/L) | DHA (g/L) | Ref. |
|---|---|---|---|---|---|
| Schizochytrium limacinum SR21 | Crude glycerol | 7.89 | 4.94 | 1.84 | [70] |
| Schizochytrium limacinum SR21 | Sorghum straw sweat | 9.38 | 6.90 | 2.35 | [67] |
| Schizochytrium limacinum PA-968 | Saline wastewater | 28.40 | 9.82 | 3.1 | [90] |
| Schizochytrium mangrovei Sk-02 | Coconut wastewater | 28.6 | 14.13 | 5.5 | [64] |
| Schizochytrium sp. HX-308 | Algal residues and cane molasses | 78.26 | 35.54 | 15.22 | [73] |
| Schizochytrium limacinum OUC88 | Soybean meal hydrolysate | 81.84 | 44.68 | 19.2 | [91] |
| Aurantiochytrium sp. KRS101 | Orange peel extract | 5.5 | 2.85 | 0.78 | [92] |
| Aurantiochytrium sp. KRS101 | Spent yeast | 31.8 | 12.12 | 10.4 | [77] |
| Aurantiochytrium sp. SW1 | Waste fruit extract | 41.5 | 25.6 | 12.67 | [93] |
| Aurantiochytrium sp. TZ209 | Waste cellular residues | 70.12 | 40.55 | 17.78 | [81] |
| Aurantiochytrium sp. YLH70 | Corn syrup | 78.5 | 51 | 20.1 | [68] |
| Crypthecodinium cohnii ATCC 30772 | Crude glycerol | 5.34 | 1.31 | 1.34 | [94] |
| Thraustochytrium sp. (T18) | Lipid-extracted hydrolysate | 14.86 | 6.43 | 2.07 | [95] |
| Oil Refining Processes | Major Impurity Components |
|---|---|
| Degumming | Phospholipids, proteins |
| Neutralization | Free fatty acids, phospholipids, metal ions, soap stock |
| Decolorization | Pigment, metal ions, and soap stock |
| Deodorization | Secondary oxidation products, free fatty acids, pigments, sterols, and squalene |
| Winterization | Saturated fatty acids |
| Antioxidants | Examples | Sources |
|---|---|---|
| Tocopherol | α-, β-, γ-, δ-tocopherol | Seeds, grains, nuts, vegetable oils, etc. |
| Trienyltocopherol | α-, β-, γ-, δ- triene tocopherols | Palm oil, rice bran oil |
| Ascorbic acid | Vitamin C, ascorbate derivatives | Fruits, vegetables, etc. |
| Carotenoids | β-carotene, lycopene, lutein, astaxanthin | Carrots, tomatoes, microalgae, etc. |
| Phenols | Flavonoids, phenolic acids, tannins, lignans | Fruits, vegetables, grains, etc. |
| Peptides | Glutathione, metallothioneins, antioxidant peptides | Animal liver, eggs, milk, etc. |
| Enzymes | Superoxide dismutase, catalase, glutathione peroxidase | Plant and animal tissues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.; Sun, X.; Luo, X.; Zheng, W.; Yin, L.; Zhang, Y.; Fu, Y. A Review on Marine Microbial Docosahexaenoic Acid Production Through Circular Economy, Fermentation Engineering, and Antioxidant Technology. Mar. Drugs 2025, 23, 256. https://doi.org/10.3390/md23060256
Yin F, Sun X, Luo X, Zheng W, Yin L, Zhang Y, Fu Y. A Review on Marine Microbial Docosahexaenoic Acid Production Through Circular Economy, Fermentation Engineering, and Antioxidant Technology. Marine Drugs. 2025; 23(6):256. https://doi.org/10.3390/md23060256
Chicago/Turabian StyleYin, Fengwei, Xiaolong Sun, Xi Luo, Weilong Zheng, Longfei Yin, Yingying Zhang, and Yongqian Fu. 2025. "A Review on Marine Microbial Docosahexaenoic Acid Production Through Circular Economy, Fermentation Engineering, and Antioxidant Technology" Marine Drugs 23, no. 6: 256. https://doi.org/10.3390/md23060256
APA StyleYin, F., Sun, X., Luo, X., Zheng, W., Yin, L., Zhang, Y., & Fu, Y. (2025). A Review on Marine Microbial Docosahexaenoic Acid Production Through Circular Economy, Fermentation Engineering, and Antioxidant Technology. Marine Drugs, 23(6), 256. https://doi.org/10.3390/md23060256





