Exploring the Catalytic Mechanisms of a Newly Identified Salt-Activated Alginate Lyase from Pseudoalteromonas carrageenovora ASY5
Abstract
1. Introduction
2. Results
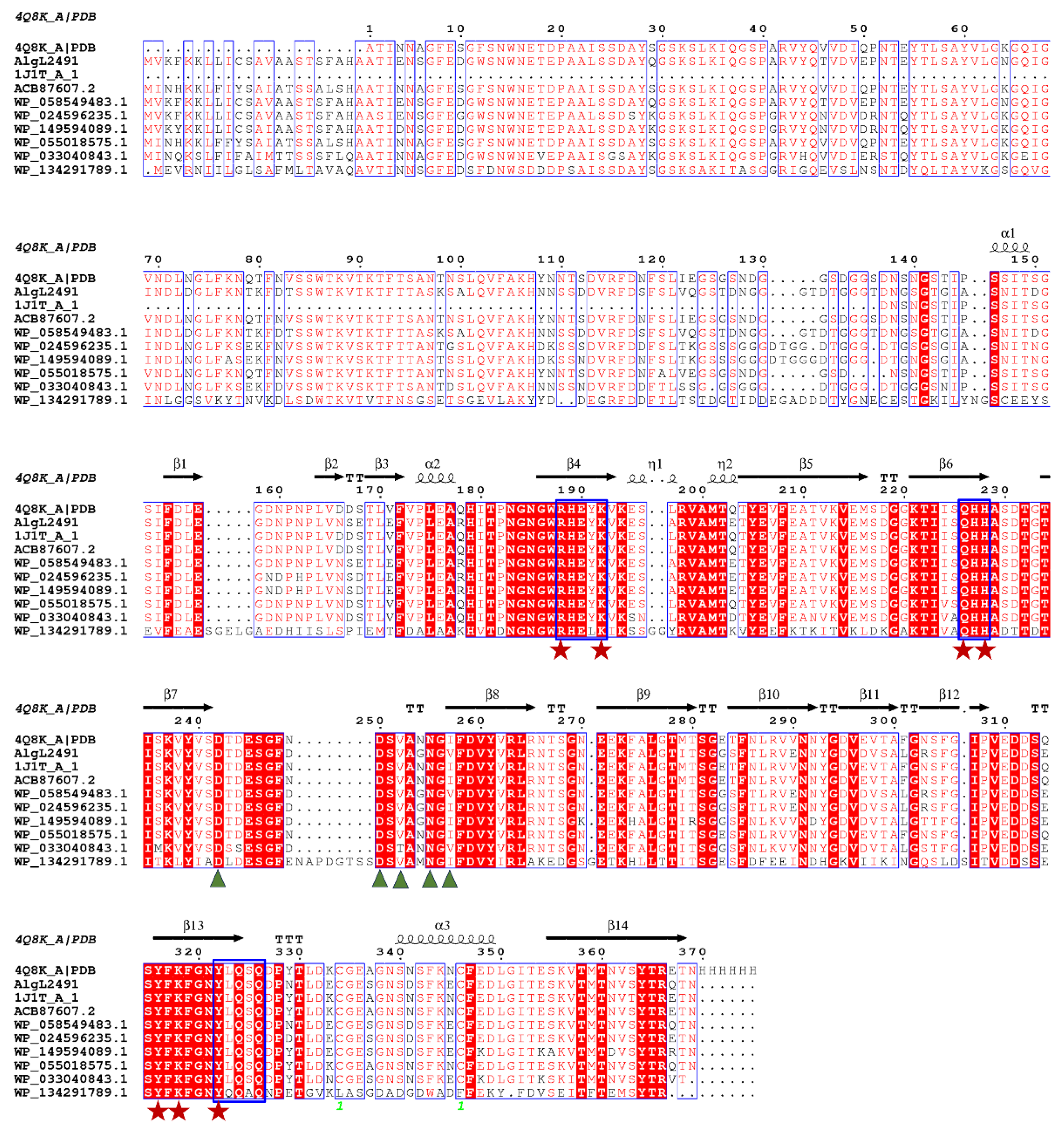
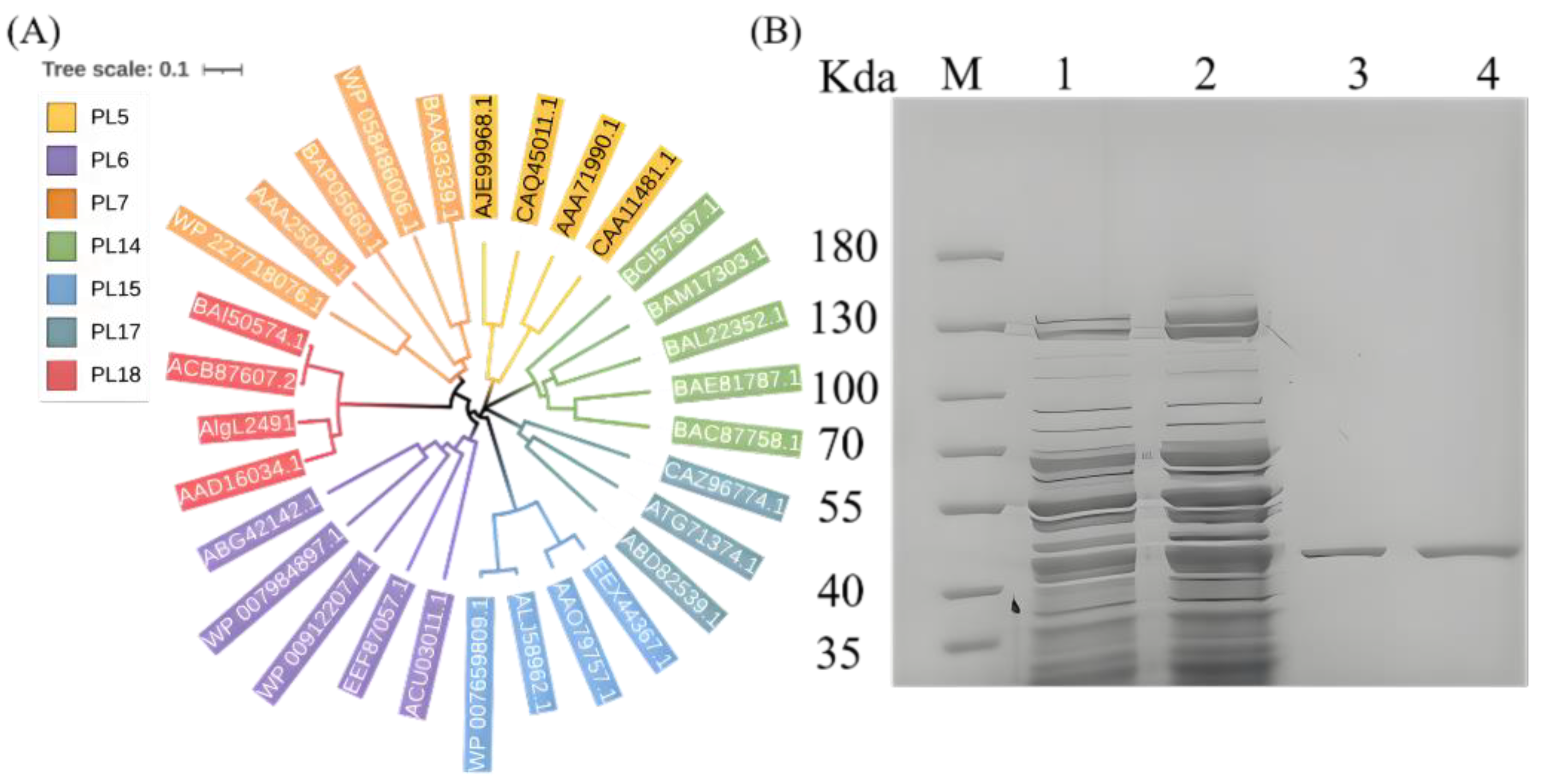
2.1. AlgL2491 Sequence Information
2.2. Expression and Purification of Recombinant Enzyme AlgL2491
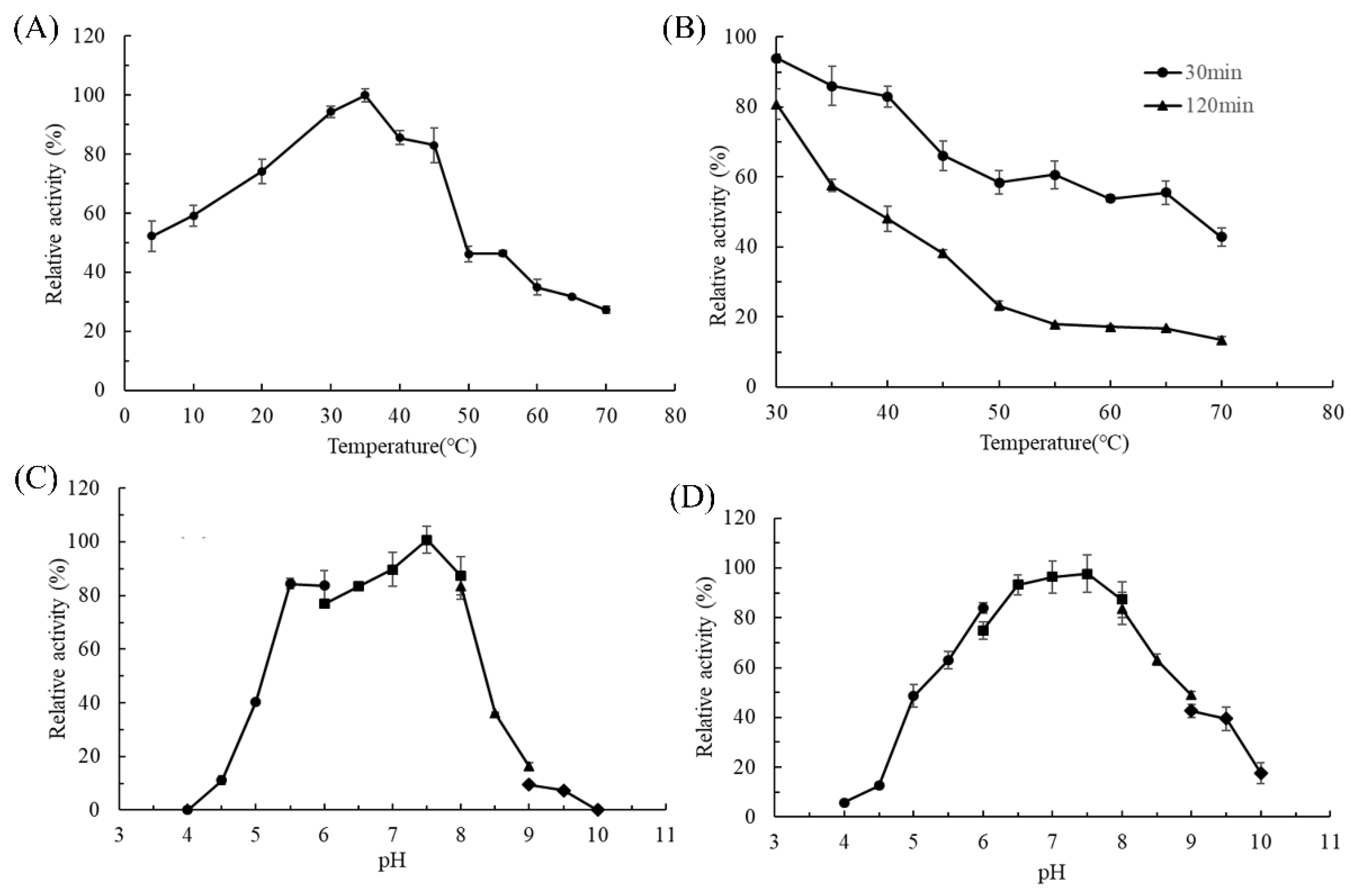
2.3. Biochemical Characterization of AlgL2491
2.4. Salt Stabilization of AlgL2491
2.5. Substrate Specificity and Mode of Enzyme Action of AlgL2491
2.6. Antioxidant Function of the Hydrolysates of AlgL2491
3. Materials and Methods
3.1. Strains and Plasmids
3.2. Construction of Recombinant E. coli
3.3. Expression and Purification of AlgL2491
3.4. Phylogenetic Analyses of the AlgL2491 Sequence
3.5. Measurement of AlgL2491 Activity
3.6. Effects of Temperature and pH on AlgL2491 Activity and Stability
3.7. Effects of Salts on the Activity and Kinetic Parameters of AlgL2491
3.8. Substrate Specificity of AlgL2491
3.9. ESI-MS Analysis of the Degradation Products of AlgL2491
3.10. Antioxidant Function of the Alginate Degradation Products of AlgL2491
3.10.1. Scavenging Activity of Hydroxyl Radical
3.10.2. Scavenging Activity of 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)
3.10.3. Scavenging Activity of 2,2′-Azinobis-(3-Ethylbenzthiazoline-6-Sulphonate) (ABTS)
3.10.4. Ferric Reducing Power
3.11. Protein Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Technavio Plus. Seaweed Market Analysis APAC, Europe, North America, South America, Middle East and Africa—China, Indonesia, Japan, US, South Korea—Size and Forecast 2024–2028. Available online: https://www.technavio.com/report/seaweed-market-industry-analysis (accessed on 8 June 2025).
- Jiang, J.; Hu, Z.; Wang, Y.; Jiang, Z.; Yan, Q.; Yang, S. Directed Evolution of an Alginate Lyase from Flammeovirga sp. for Seaweed Fertilizer Production from the Brown Seaweed Laminaria japonica. J. Agric. Food Chem. 2025, 73, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Moon, J.H.; Woo, S.; Lee, C.W.; Jung, G.Y.; Lim, H.G. Recent Advances in Alginate Lyase Engineering for Efficient Conversion of Alginate to Value-Added Products. Microb. Biotechnol. 2025, 18, e70150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Hu, F.; Yuan, H.; Sun, Y.; Yao, Z. Biochemical Characterization and Degradation Pattern of a Unique pH-Stable PolyM-Specific Alginate Lyase from Newly Isolated Serratia Marcescens NJ-07. Mar. Drugs 2018, 16, 129. [Google Scholar] [CrossRef]
- Dong, S.; Li, Y.; Zhu, K.; Wang, C.; Zhai, S. Advances in Structure Designing and Function Tailoring Strategy toward Alginate-Based Hydrogels for Efficient Water Remediation: A Review. Int. J. Biol. Macromol. 2025, 304, 140801. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cai, Y.; Zhong, H.; Chen, R.; Yi, Y.; Ye, Y.; Li, L. Expression and Characterization of an Efficient Alginate Lyase from Psychromonas sp. SP041 through Metagenomics Analysis of Rotten Kelp. Genes 2024, 15, 598. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Zhou, Y.; Gao, Y. Molecular Engineering of Alginate Lyases and the Potential Agricultural Applications of Their Enzymatic Products. J. Agric. Food Chem. 2025, 73, 5666–5684. [Google Scholar] [CrossRef]
- Carrasqueira, J.; Bernardino, S.; Bernardino, R.; Afonso, C. Marine-Derived Polysaccharides and Their Potential Health Benefits in Nutraceutical Applications. Mar. Drugs 2025, 23, 60. [Google Scholar] [CrossRef]
- Bi, D.; Yang, X.; Lu, J.; Xu, X. Preparation and Potential Applications of Alginate Oligosaccharides. Crit. Rev. Food Sci. Nutr. 2023, 63, 10130–10147. [Google Scholar] [CrossRef]
- Kim, H.H.; Vaidya, B.; Cho, S.-Y.; Kwon, J.; Kim, D. Anti-Hyperglycemic Potential of Alginate Oligosaccharide in a High Glucose-Induced Zebrafish Model. J. Funct. Foods 2022, 94, 105098. [Google Scholar] [CrossRef]
- Wang, Y.; Han, F.; Hu, B.; Li, J.; Yu, W. In Vivo Prebiotic Properties of Alginate Oligosaccharides Prepared through Enzymatic Hydrolysis of Alginate. Nutr. Res. 2006, 26, 597–603. [Google Scholar] [CrossRef]
- Cherry, P.; Yadav, S.; Strain, C.R.; Allsopp, P.J.; McSorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from Seaweeds: An Ocean of Opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, M.; Song, X.; Xue, C.; Chang, Y. Strictly G-Specific Alginate Lyase Aly7Sa for Efficient Preparation of Unsaturated Guluronate Oligosaccharides. J. Agric. Food Chem. 2025, 73, 7376–7382. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Q.; Xu, W.; Wang, X.; Zheng, Q.; Liang, X.; Dong, X.; Li, F.; Peng, L. A Bifunctional Endolytic Alginate Lyase with Two Different Lyase Catalytic Domains from Vibrio sp. H204. Front. Microbiol. 2024, 15, 1509599. [Google Scholar] [CrossRef]
- Barzkar, N.; Sheng, R.; Sohail, M.; Jahromi, S.T.; Babich, O.; Sukhikh, S.; Nahavandi, R. Alginate Lyases from Marine Bacteria: An Enzyme Ocean for Sustainable Future. Molecules 2022, 27, 3375. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, J.; Yao, Z.; Zhu, B. Recent Advances in the Production, Properties and Applications of Alginate Oligosaccharides—A Mini Review. World J. Microbiol. Biotechnol. 2023, 39, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, J.; Chen, G.; Zheng, L.; Mei, X.; Xue, C.; Chang, Y. Discovery and Characterization af a Novel Poly-Mannuronate Preferred Alginate Lyase: The First Member of a New Polysaccharide Lyase Family. Carbohydr. Polym. 2024, 343, 122474. [Google Scholar] [CrossRef]
- Grobler, C.E.; Mabate, B.; Prins, A.; Le Roes-Hill, M.; Pletschke, B.I. Expression, purification, and characterisation of recombinant alginate lyase (Flammeovirga Al2) for the bioconversion of alginate into alginate oligosaccharides. Molecules 2024, 29, 5578. [Google Scholar] [CrossRef]
- Zhu, B.; Ni, F.; Sun, Y.; Ning, L.; Yao, Z. Elucidation of Degrading Pattern and Substrate Recognition of a Novel Bifunctional Alginate Lyase from Flammeovirga sp. NJ-04 and Its Use for Preparation Alginate Oligosaccharides. Biotechnol. Biofuels 2019, 12, 13. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Lu, C.; Zhou, X.; Chen, L.; Qiu, C.; Xie, Z.; Xu, X.; Jin, Z.; Long, J. Significantly Improving the Thermal Stability of Alginate Lyase AlyC3 from Psychromonas sp. C-3 by Computational Redesign. Food Biosci. 2024, 59, 103973. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Gao, F.; Xu, X.; Chen, G.; Li, Y.; Wang, L. Low-Cost and Efficient Strategy for Brown Algal Hydrolysis: Combination of Alginate Lyase and Cellulase. Bioresour. Technol. 2024, 397, 130481. [Google Scholar] [CrossRef]
- Wang, X.-H.; Sun, X.-H.; Chen, X.-L.; Li, P.-Y.; Qin, Q.-L.; Zhang, Y.-Q.; Xu, F. Synergy of the Two Alginate Lyase Domains of a Novel Alginate Lyase from Vibrio sp. NC2 in Alginate Degradation. Appl. Environ. Microbiol. 2022, 88, e01559-22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liang, S.; Jiang, W.; Wang, L.; Wang, Y.; Wang, H.; Wang, L.; Cong, Y.; Lu, Y.; Yang, G. Multi-Functional Alginate Lyase AlgVR7 from Vibrio rumoiensis: Structural Insights and Catalytic Mechanisms. Mar. Drugs 2025, 23, 124. [Google Scholar] [CrossRef]
- Xu, F.; Chen, X.-L.; Sun, X.-H.; Dong, F.; Li, C.-Y.; Li, P.-Y.; Ding, H.; Chen, Y.; Zhang, Y.-Z.; Wang, P. Structural and Molecular Basis for the Substrate Positioning Mechanism of a New PL7 Subfamily Alginate Lyase from the Arctic. J. Biol. Chem. 2020, 295, 16380–16392. [Google Scholar] [CrossRef]
- Rivas-Fernández, J.P.; Vuillemin, M.; Pilgaard, B.; Klau, L.J.; Fredslund, F.; Lund-Hanssen, C.; Welner, D.H.; Meyer, A.S.; Morth, J.P.; Meilleur, F.; et al. Unraveling the Molecular Mechanism of Polysaccharide Lyases for Efficient Alginate Degradation. Nat. Commun. 2025, 16, 2670. [Google Scholar] [CrossRef]
- Li, J.; Sun, M.; Liu, G.; Zhou, J.; Chang, Y.; Xue, C. Characterization and Elucidation of a Novel M-Specific Alginate Lyase Aly7Aq with Strict Recognition at Subsites ±2. Int. J. Biol. Macromol. 2024, 277, 133972. [Google Scholar] [CrossRef]
- Kitamikado, M.; Tseng, C.H.; Yamaguchi, K.; Nakamura, T. Two Types of Bacterial Alginate Lyases. Appl. Environ. Microbiol. 1992, 58, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- DasSarma, S.L.; Capes, M.D.; DasSarma, P.; DasSarma, S. HaloWeb: The Haloarchaeal Genomes Database. Saline Syst. 2010, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Tadeo, X.; López-Méndez, B.; Trigueros, T.; Laín, A.; Castaño, D.; Millet, O. Structural Basis for the Aminoacid Composition of Proteins from Halophilic archea. PLoS Biol. 2009, 7, e1000257. [Google Scholar] [CrossRef]
- Kastritis, P.L.; Papandreou, N.C.; Hamodrakas, S.J. Haloadaptation: Insights from Comparative Modeling Studies of Halophilic Archaeal DHFRs. Int. J. Biol. Macromol. 2007, 41, 447–453. [Google Scholar] [CrossRef]
- Zorgani, M.A.; Patron, K.; Desvaux, M. New Insight in the Structural Features of Haloadaptation in α-Amylases from Halophilic Archaea Following Homology Modeling Strategy: Folded and Stable Conformation Maintained through Low Hydrophobicity and Highly Negative Charged Surface. J. Comput. Aided Mol. Des. 2014, 28, 721–734. [Google Scholar] [CrossRef]
- Warden, A.C.; Williams, M.; Peat, T.S.; Seabrook, S.A.; Newman, J.; Dojchinov, G.; Haritos, V.S. Rational Engineering of a Mesohalophilic Carbonic Anhydrase to an Extreme Halotolerant Biocatalyst. Nat. Commun. 2015, 6, 10278. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Ohmae, E.; Nakasone, K.; Katayanagi, K. Effects of Salt on the Structure, Stability, and Function of a Halophilic Dihydrofolate Reductase from a Hyperhalophilic archaeon, Haloarcula japonica Strain TR-1. Extremophiles 2015, 19, 479–493. [Google Scholar] [CrossRef]
- Binbuga, B.; Boroujerdi, A.F.B.; Young, J.K. Structure in an Extreme Environment: NMR at High Salt. Protein Sci. 2007, 16, 1783–1787. [Google Scholar] [CrossRef]
- Luk, L.Y.P.; Loveridge, E.J.; Allemann, R.K. Protein Motions and Dynamic Effects in Enzyme Catalysis. Phys. Chem. Chem. Phys. 2015, 17, 30817–30827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhuang, X.; Liu, J.; Huang, J.; Lin, L.; Tang, Y.; Zhao, S.; Li, R.; Wang, B.; Fang, B.; et al. Catalytic Cycle of Formate Dehydrogenase Captured by Single-Molecule Conductance. Nat. Catal. 2023, 6, 266–275. [Google Scholar] [CrossRef]
- Warshel, A.; Bora, R.P. Perspective: Defining and Quantifying the Role of Dynamics in Enzyme Catalysis. J. Chem. Phys. 2016, 144, 180901. [Google Scholar] [CrossRef]
- Zeng, J.; An, D.; Jiao, C.; Xiao, Q.; Weng, H.; Yang, Q.; Xiao, A. Cloning, Expression, and Characterization of a New pH− and Heat-stable Alginate Lyase from Pseudoalteromonas carrageenovora ASY5. J. Food Biochem. 2019, 43, e12886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Shao, Y.; Jiao, C.; Yang, Q.-M.; Weng, H.-F.; Xiao, A.-F. Characterization and Application of an Alginate Lyase, Aly1281 from Marine Bacterium Pseudoalteromonas carrageenovora ASY5. Mar. Drugs 2020, 18, 95. [Google Scholar] [CrossRef]
- He, Z.; Meng, S.; Xu, Y.; Zhong, M.; Han, X.; Xie, Q.; Ding, M.; Li, J.; Hu, Z. Direct Influence of the Conserved Motif in PL7 Family Alginate Lyases on Enzyme Cold Adaptability. J. Agric. Food Chem. 2025, 73, 4320–4330. [Google Scholar] [CrossRef]
- Dong, S.; Wei, T.-D.; Chen, X.-L.; Li, C.-Y.; Wang, P.; Xie, B.-B.; Qin, Q.-L.; Zhang, X.-Y.; Pang, X.-H.; Zhou, B.-C.; et al. Molecular Insight into the Role of the N-Terminal Extension in the Maturation, Substrate Recognition, and Catalysis of a Bacterial Alginate Lyase from Polysaccharide Lyase Family 18. J. Biol. Chem. 2014, 289, 29558–29569. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, Z.; Li, K.; Wang, W.; Jia, X.; Li, T.; Yin, H. Characterization of Two New Alginate Lyases from Pseudomonas mendocina E03. Int. J. Biol. Macromol. 2025, 285, 138304. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, Y.; Wang, X.; Zhu, X.; Chen, L.; Liu, W.; Lyu, Q.; Ran, L.; Cheng, H.; Zhang, X.-H. Characterization of Multiple Alginate Lyases in a Highly Efficient Alginate-Degrading Vibrio Strain and Its Degradation Strategy. Appl. Environ. Microbiol. 2022, 88, e01389-22. [Google Scholar] [CrossRef]
- Li, J.; Liu, G.; Song, X.; Zhang, Y.; Zhou, J.; Chang, Y.; Xue, C. Elucidation of a Specific Alginate Lyase Aly7Rm: The Products Demonstrated the Strict Recognition of G Residue at Subsites ±2. Food Biosci. 2024, 62, 105196. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Li, Y.; Wang, L. Identification and Characterization of a Novel Alginate Lyase VSAly7C with Potential Application for Alginate Di- and Tri-Saccharide Preparation. J. Agric. Food Chem. 2025, 73, 11855–11865. [Google Scholar] [CrossRef]
- Chen, Y.; Ci, F.; Jiang, H.; Meng, D.; Hamouda, H.I.; Liu, C.; Quan, Y.; Chen, S.; Bai, X.; Zhang, Z.; et al. Catalytic Properties Characterization and Degradation Mode Elucidation of a polyG-Specific Alginate Lyase OUC-FaAly7. Carbohydr. Polym. 2024, 333, 121929. [Google Scholar] [CrossRef]
- Doshi, A.; Pascoe, S.; Coglan, L.; Rainey, T.J. Economic and Policy Issues in the Production of Algae-Based Biofuels: A Review. Renew. Sustain. Energy Rev. 2016, 64, 329–337. [Google Scholar] [CrossRef]
- Zhu, B.; Sun, Y.; Ni, F.; Ning, L.; Yao, Z. Characterization of a New Endo-Type Alginate Lyase from Vibrio sp. NJU-03. Int. J. Biol. Macromol. 2018, 108, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Tan, H.; Qin, Y.; Xu, Q.; Du, Y.; Yin, H. Characterization of a New Endo-Type Alginate Lyase from Vibrio sp. W13. Int. J. Biol. Macromol. 2015, 75, 330–337. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Bao, M.; Wu, Y.; Yu, W.; Han, F. Family 13 Carbohydrate-Binding Module of Alginate Lyase from Agarivorans sp. L11 Enhances Its Catalytic Efficiency and Thermostability, and Alters Its Substrate Preference and Product Distribution. FEMS Microbiol. Lett. 2015, 362, fnv054. [Google Scholar] [CrossRef]
- Gao, T.; Li, Y.; Wang, X.; Ren, F. Alginate Oligosaccharide-Mediated Butyrate-HIF-1α Axis Improves Skin Aging in Mice. J. Pharm. Anal. 2024, 14, 100911. [Google Scholar] [CrossRef]
- Kawada, A.; Hiura, N.; Shiraiwa, M.; Tajima, S.; Hiruma, M.; Hara, K.; Ishibashi, A.; Takahara, H. Stimulation of Human Keratinocyte Growth by Alginate Oligosaccharides, a Possible Co-factor for Epidermal Growth Factor in Cell Culture. FEBS Lett. 1997, 408, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Meng, Y.; Yan, B.; Zhou, Q.; Wang, X. The Biochemical Pathways of Apoptotic, Necroptotic, Pyroptotic, and Ferroptotic Cell Death. Mol. Cell 2024, 84, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Q.; Zhang, J.; Zhou, X.; Lyu, F.; Zhao, P.; Ding, Y. Preparation, Composition Analysis and Antioxidant Activities of Konjac Oligo-Glucomannan. Carbohydr. Polym. 2015, 130, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Alashi, A.M.; Blanchard, C.L.; Mailer, R.J.; Agboola, S.O.; Mawson, A.J.; He, R.; Girgih, A.; Aluko, R.E. Antioxidant Properties of Australian Canola Meal Protein Hydrolysates. Food Chem. 2014, 146, 500–506. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Venkatesalu, V. Chemical Constituents, in Vitro Antioxidant and Antimicrobial Activities of Essential Oil from the Fruit Pulp of Wood Apple. Ind. Crops Prod. 2013, 46, 66–72. [Google Scholar] [CrossRef]
- Gülçin, İ.; Bursal, E.; Şehitoğlu, M.H.; Bilsel, M.; Gören, A.C. Polyphenol Contents and Antioxidant Activity of Lyophilized Aqueous Extract of Propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef]
- Available online: http://zhanglab.ccmb.med.umich.edu/I-TASSER (accessed on 30 May 2025).




| Substrate | Relative Activity (%) |
|---|---|
| alginate | 100.00 ± 5.41 |
| polyM | 83.86 ± 0.41 |
| polyG | 90.42 ±1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, X.; Jiao, C.; Guo, Z.; Xiao, Q.; Chen, J.; Chen, F.; Yang, Q.; Ru, Y.; Weng, H.; Wang, S.; et al. Exploring the Catalytic Mechanisms of a Newly Identified Salt-Activated Alginate Lyase from Pseudoalteromonas carrageenovora ASY5. Mar. Drugs 2025, 23, 254. https://doi.org/10.3390/md23060254
Zhuang X, Jiao C, Guo Z, Xiao Q, Chen J, Chen F, Yang Q, Ru Y, Weng H, Wang S, et al. Exploring the Catalytic Mechanisms of a Newly Identified Salt-Activated Alginate Lyase from Pseudoalteromonas carrageenovora ASY5. Marine Drugs. 2025; 23(6):254. https://doi.org/10.3390/md23060254
Chicago/Turabian StyleZhuang, Xiaoyan, Chao Jiao, Zewang Guo, Qiong Xiao, Jun Chen, Fuquan Chen, Qiuming Yang, Yi Ru, Huifen Weng, Siyuan Wang, and et al. 2025. "Exploring the Catalytic Mechanisms of a Newly Identified Salt-Activated Alginate Lyase from Pseudoalteromonas carrageenovora ASY5" Marine Drugs 23, no. 6: 254. https://doi.org/10.3390/md23060254
APA StyleZhuang, X., Jiao, C., Guo, Z., Xiao, Q., Chen, J., Chen, F., Yang, Q., Ru, Y., Weng, H., Wang, S., Xiao, A., & Zhang, Y. (2025). Exploring the Catalytic Mechanisms of a Newly Identified Salt-Activated Alginate Lyase from Pseudoalteromonas carrageenovora ASY5. Marine Drugs, 23(6), 254. https://doi.org/10.3390/md23060254






