Marine Jellyfish Collagen and Other Bioactive Natural Compounds from the Sea, with Significant Potential for Wound Healing and Repair Materials
Abstract
1. Introduction
2. Results
2.1. Chemical Characteristics for Ingredients
Proximate Composition Data of Collagen Peptide Extract and Hydroalcoholic Algal Extract
2.2. Jellyfish R. pulmo Physico—Date for the Collagen Structure
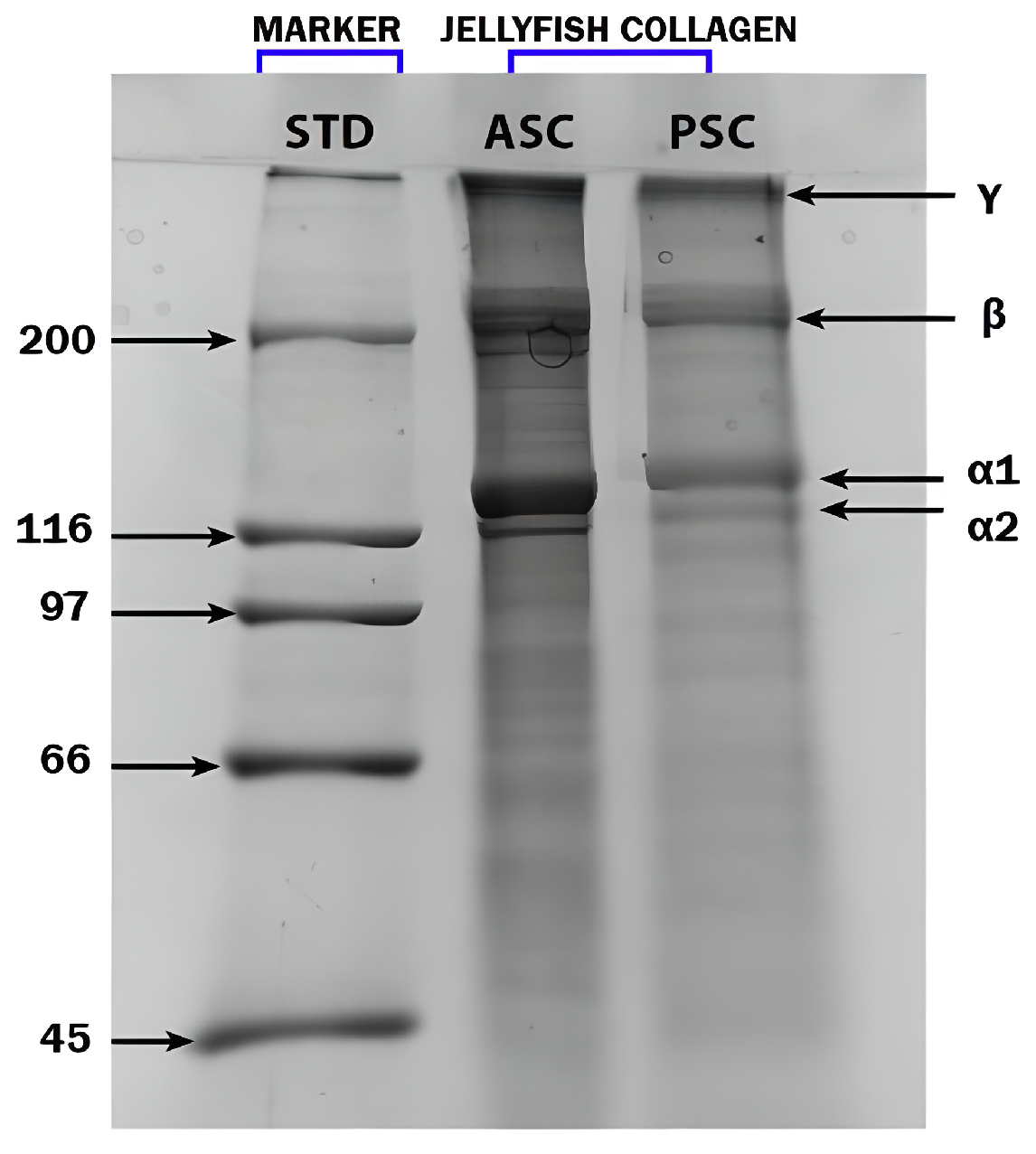
2.2.1. SDS-PAGE Analysis
2.2.2. Circular Dichroism Spectral Analyses
2.2.3. FT-IR Analysis
2.2.4. Amino Acid Composition
2.3. Polyphenols Content
2.3.1. Total Phenols Content and Total Flavonoid Content
2.3.2. Individual Phenolic Acids
2.4. Physico-Chemical Characteristics for the New Wound-Healing Preparations
2.4.1. Organoleptic Characteristics of the New Preparations Obtained
2.4.2. Rheological Study of JPC-ALG Composite Hydrogels
2.4.3. Microscopic Study of JPC-ALG Composites Intended for Wound Healing
2.5. Antioxidant Activity
2.5.1. DPPH Test
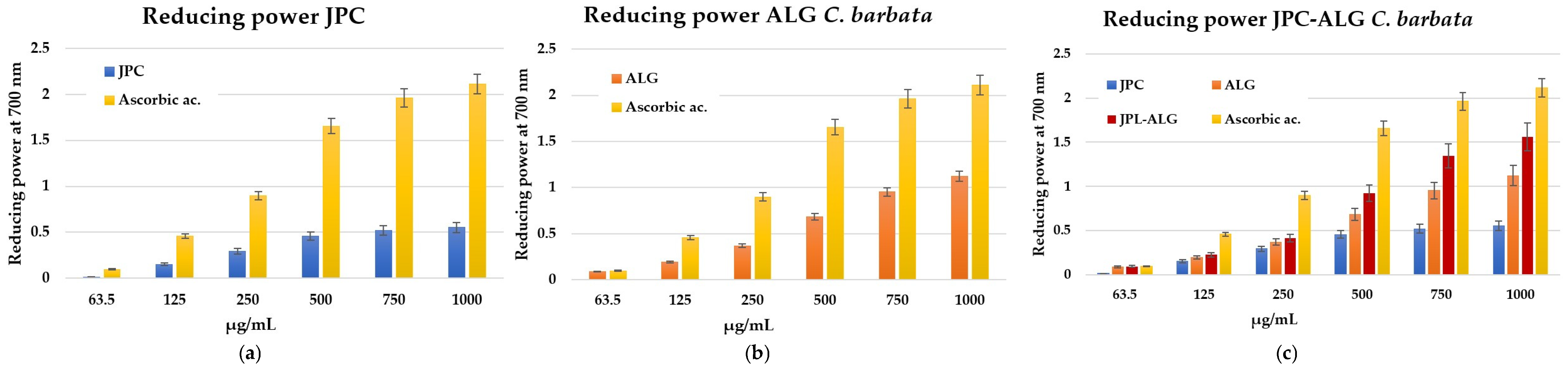
2.5.2. Antioxidant Activity by Reducing Power
2.6. Antimicrobial Activity
2.7. Biological Evaluation of New Composite with JPC-ALG for Wound-Healing Application
2.7.1. Wound Healing by the Fibroblast Scratch Test
2.7.2. Wound Healing by Keratocyte Scratch Assay
3. Discussions
4. Materials and Methods
4.1. Chemical Reagents
4.2. Obtaining Extracts from Marine Resources
4.2.1. Obtaining Collagen Extracts R. pulmo
4.2.2. Obtaining Collagen Peptides
4.2.3. Extraction of C. barbata Extracts
4.3. Preparation of New Composite Preparations Based on Collagen Peptides from R. pulmo and Brown Alga C. barbata (JPC-ALG)
4.4. Determination of the Biochemical Compositions of Biocompounds from R. pulmo and C. barbata
4.4.1. The Biochemical Composition of Collagen Extracts from Rhizostoma pulmo
4.4.2. Biochemical Composition for Brown Algae C. barbata
4.5. Jellyfish R. pulmo Physico-Chemical Data for the Collagen Structure
4.5.1. SDS-PAGE Analysis
4.5.2. Circular Dichroism Spectral Analysis
4.5.3. FT-IR Spectroscopy Analysis of Collagen Peptides
4.5.4. Amino Acid Analysis of R. pulmo Collagen
4.6. Evaluation of Polyphenol Content
4.6.1. Evaluation of Total Polyphenol Content (TPC) in Marine Resources
4.6.2. Evaluation of Total Flavonoid Compound (TFC) Content
4.6.3. Individual Phenol Content
4.7. Physico-Chemical Characteristics for JPC-ALG Preparations
4.7.1. Organoleptic Characteristics
4.7.2. Rheological Characteristics of Preparations
4.7.3. Optical Microscopy Studies for the JPC-ALG Composite
4.8. Antioxidant Activity Was Assessed by DPPH Test and Reducing Power Assay
4.8.1. DPPH Test
4.8.2. Reducing Power Assay
4.9. Antimicrobial Activity
4.10. Biological Investigation
4.10.1. Cell Viability
4.10.2. Scratch Test on BALB/3T3 Cells and HaCat Cells
4.11. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JPC | Jellyfish Collagen Peptides |
| ALG | Alga (brown algae C. barbata) |
| JPC-ALG | Composite Jellyfish Peptides-Brown Algae |
| SDS-PAGE | Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| ASC | Acid Solubil Collagen process |
| PSC | Pepsin Soluble Collagen |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| MIC | Minimal inhibitory concentration |
| BALB/3T3 | A fibroblast cell line |
| HaCaT | Keratinocyte cell line |
| ECM | Extracellular Matrix |
| ROS | Reactive Oxygen Species |
| TGF-α | Transforming Growth Factor-Alfa |
| TGF-β | Transforming Growth Factor-Beta |
| FGF | Fibroblast Growth Factor |
| PDGF | Platelet-Derived Growth Factor |
| VEGF | Vascular Endothelial Growth Factor |
References
- Gu, X.; Li, Z.; Su, J. Air pollution and skin diseases: A comprehensive evaluation of the associated mechanism. Ecotoxicol. Environ. Saf. 2024, 278, 116429. [Google Scholar] [CrossRef] [PubMed]
- Childs, D.R.; Murthy, A.S. Overview of wound healing and management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar] [CrossRef]
- Anderson, K.; Hamm, R.L. Factors that impair wound healing. J. Am Coll. Clin. Wound Spec. 2012, 4, 84–91. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Reza Farahpour, M. Medicinal plants in wound healing. In Wound Healing—Current Perspectives; Dogan, K.H., Ed.; IntechOpen: London, UK, 2019; pp. 33–47. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound.Care. 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Öztürk, F.; Ermertcan, A.T. Wound healing: A new approach to the topical wound care. Cutan. Ocul. Toxicol. 2011, 30, 92–99. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv Wound Care. 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Shilo, S.; Roth, S.; Amzel, T.; Harel-Adar, T.; Tamir, E.; Grynspan, F.; Shoseyov, O. Cutaneous wound healing after treatment with plant-derived human recombinant collagen flowable gel. Tissue Eng. 2013, 19, 1519–1526. [Google Scholar] [CrossRef]
- Kiritsi, D.; Nyström, A. The role of TGFβ in wound healing pathologies. Mech. Ageing Dev. 2017, 172, 51–58. [Google Scholar] [CrossRef]
- Velnar, T.V.; Ailey, T.B. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound healing in the 21-st century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Beldon, P. Basic science of wound healing. Surgery 2010, 28, 409–412. [Google Scholar] [CrossRef]
- Hochstein, A.O.; Bhatia, A. Collagen: Its role in wound healing. Podiatry Manag. 2014, 103e106–109e110. [Google Scholar]
- Felician, F.F.; Yu, R.-H.; Li, M.-Z.; Li, C.-J.; Chen, H.-Q.; Jiang, Y.; Tang, T.; Qi, W.-Y.; Xu, H.-M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-Based Biomaterials for Wound Healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional biomaterials for treatment of chronic wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef]
- Sirbu, R.; Mustafa, A.; Aneta Tomescu, S.; Stanciu, G.; Emin Cadar, E. Rheological and Microbiological Study on Bio-Composites with Marine Chitosan Polymers from Black Sea Stone Crabs used in Medical Therapy of Tissue Regeneration. Mat. Plast. 2019, 56, 148–155. [Google Scholar] [CrossRef]
- Cadar, E.; Negreanu-Pirjol, T.; Pascale, C.; Sirbu, R.; Prasacu, I.; Negreanu-Pirjol, B.S.; Tomescu, C.L.; Ionescu, A.M. Natural Bio-Compounds from Ganoderma lucidum and Their Beneficial Biological Actions for Anticancer Application: A Review. Antioxidants 2023, 12, 1907. [Google Scholar] [CrossRef]
- Fan, J.; Zhuang, Y.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients 2013, 5, 223–233. [Google Scholar] [CrossRef]
- Barzideh, Z.; Latiff, A.A.; Gan, C.-Y.; Abedin, Z.; Alias, A.K. ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.). Food Technol. Biotechnol. 2014, 52, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Filiss, I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem. Biophys. Res. Commun. 2015, 462, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ennaas, N.; Hammami, R.; Gomaa, A.; Bédard, F.; Biron, É.; Subirade, M.; Beaulieu, L.; Fliss, I. Collagencin, an antibacterial peptide from fish collagen: Activity, structure and interaction dynamics with membrane. Biochem. Biophys. Res. Commun. 2016, 473, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Cadar, E.; Pesterau, A.-M.; Prasacu, I.; Ionescu, A.-M.; Pascale, C.; Dragan, A.-M.L.; Sirbu, R.; Tomescu, C.L. Marine Antioxidants from Marine Collagen and Collagen Peptides with Nutraceuticals Applications: A Review. Antioxidants 2024, 13, 919. [Google Scholar] [CrossRef]
- Asserin, J.; Lati, E.; Shioya, T. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301. [Google Scholar] [CrossRef]
- Banerjee, P.; Suguna, L.; Shanthi, C. Wound healing activity of a collagen-derived cryptic peptide. Amino Acids. 2015, 47, 317–328. [Google Scholar] [CrossRef]
- Widdowson, P.J.; Picton, J.A.; Vinc, V.; Wright, C.J.; Mearns-Spragg, A. In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1524–1533. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs. 2011, 9, 967–983. [Google Scholar] [CrossRef]
- Yu, H.H.; Li, R.F.; Liu, S. Amino acid composition and nutritional quality of gonad from jellyfish Rhopilema esculentum. Biomed. Prev. Nutr. 2014, 4, 399–402. [Google Scholar] [CrossRef]
- Khong, N.M.; Yusoff, F.M.; Jamilah, B. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef]
- Zhuang, Y.L.; Sun, L.P.; Zhao, X. Investigation of gelatin polypeptides of jellyfish (Rhopilema esculentum) for their antioxidant activity in vitro. Food Technol. Biotechnol. 2010, 48, 222–228. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, Z.; Li, C. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum kishinouye for use in hemostatic applications. PLoS ONE 2017, 12, 0169731. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Tilvi, S.; Khandeparker, R.; Sreepada, R.A.; Thakur, N.; Gauthankar, M. Jellyfish Rhizostoma pulmo collected off Goa Coast (India) as a rich source of tryptophan containing collagen and its enhanced antioxidant potential. J. Food Sci. Technol. 2023, 60, 2825–2834. [Google Scholar] [CrossRef]
- D’Ambra, I.; Malej, A. Scyphomedusae of the Mediterranean: State of the Art and Future Perspectives. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 81–94. [Google Scholar] [CrossRef]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A. Barrel Jellyfish (Rhizostoma pulmo) as Source of Antioxidant Peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef]
- Cadar, E.; Pesterau, A.-M.; Sirbu, R.; Negreanu-Pirjol, B.S.; Tomescu, C.L. Jellyfishes—Significant Marine Resources with Potential in the Wound-Healing Process: A Review. Mar. Drugs 2023, 21, 201. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Hwang, J.; Ko, J.Y.; Jeon, Y.J.; Ryu, B. In Vitro and In Vivo Antioxidant Activities of Polysaccharides Isolated from Celluclast-Assisted Extract of an Edible Brown Seaweed, Sargassum fulvellum. Antioxidants 2019, 8, 493. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Jeon, Y.J. The Potential of Sulfated Polysaccharides Isolated from the Brown Seaweed Ecklonia maxima in Cosmetics: Antioxidant, Anti-melanogenesis, and Photoprotective Activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.S.; Jeon, Y.J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef]
- Yalçın, S.; Karakaş, Ö.; Okudan, E.Ş.; Başkan, K.S.; Çekiç, S.D.; Apak, R. HPLC detection and antioxidant capacity determination of brown, red and green algal pigments in seaweed extracts. J. Chromatogr. Sci. 2021, 59, 325–337. [Google Scholar] [CrossRef]
- Cadar, E.; Negreanu-Pirjol, T.; Sirbu, R.; Dragan, A.-M.L.; Negreanu-Pirjol, B.-S.; Axente, E.R.; Ionescu, A.-M. Biocompounds from Green Algae of Romanian Black Sea Coast as Potential Nutraceuticals. Processes 2023, 11, 1750. [Google Scholar] [CrossRef]
- Cadar, E.; Popescu, A.; Dragan, A.-M.-L.; Pesterau, A.-M.; Pascale, C.; Anuta, V.; Prasacu, I.; Velescu, B.S.; Tomescu, C.L.; Bogdan-Andreescu, C.F.; et al. Bioactive Compounds of Marine Algae and Their Potential Health and Nutraceutical Applications: A Review. Mar. Drugs 2025, 23, 152. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, N.M.; Lee, H.G.; Nagahawatta, D.P.; Jayawardhana, H.; Song, K.M.; Choi, Y.S.; Jeon, Y.J.; Kang, M.C. Fucoidan from Sargassum autumnale Inhibits Potential Inflammatory Responses via NF-kappaB and MAPK Pathway Suppression in Lipopolysaccharide-Induced RAW264.7 Macrophages. Mar. Drugs 2023, 21, 374. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, A.M.K.; Kirindage, K.; Fernando, I.P.S.; Kim, K.N.; Oh, J.Y.; Ahn, G. The Anti-Inflammatory Effect of Low MolecularWeight Fucoidan from Sargassum siliquastrum in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages via Inhibiting NFkappaB/MAPK Signaling Pathways. Mar. Drugs 2023, 21, 347. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean. Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef]
- D’Ambra, I.; Merquiol, L. Jellyfish from Fisheries By-Catches as a Sustainable Source of High-Value Compounds with Biotechnological Applications. Mar. Drugs 2022, 20, 266. [Google Scholar] [CrossRef]
- Pesterau, A.-M.; Sirbu, R.; Cadar, E. Extraction and Method Validation for Collagen Obtained from the Jellyfish Rhizostoma Pulmo Found on the Romanian Black Sea Coast. Eur. J. Nat. Sci. Med. 2024, 7, 1–20. Available online: https://revistia.com/files/articles/ejnm_v7_24/Pesterau.pdf (accessed on 16 May 2025). [CrossRef]
- Cadar, E.; Axinte, E.R.; Amzoiu, M.; Jurja, S.; Cherim, M. Preliminary Study on the Marine Algae from the Romanian Black Sea coast. J. Sci. Arts 2019, 4, 989–1000. Available online: http://www.josa.ro/docs/josa_2019_4/b_06_Cadar_989-1000_12p.pdf (accessed on 16 May 2025).
- Paradiso, F.; Fitzgerald, J.; Yao, S.; Barry, F.; Taraballi, F.; Gonzalez, D.; Conlan, R.S.; Francis, L. Marine Collagen Substrates for 2D and 3D Ovarian cancer Cell Systems. Front. Bioeng. Biotechnol. 2019, 7, 343. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Mhadhebi, A.; Robert, J.; Bouraoui, A. Antioxidant, Anti-inflammatory and Antiproliferative Effects of Aqueous Extracts of Three Mediterranean Brown Seaweeds of the Genus Cystoseira. Iran. J. Pharm. Res. 2014, 13, 207–220. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3985253/ (accessed on 28 March 2025).
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic Content of Brown Algae (Pheophyceae) Species: Extraction, Identification, and Quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Manev, Z.; Iliev, A.; Vachkova, V. Chemical characterization of brown seaweed–Cystoseira barbata. Bulg. J. Agric. Sci. 2013, 19, 12–15. Available online: https://www.researchgate.net/publication/258432496 (accessed on 1 May 2025).
- Kosanić, M.; Ranković, B.; Stanojković., T. Biological potential of marine macroalgae of the genus Cystoseira. Acta Biol. Hung. 2015, 66, 374–384. Available online: https://link.springer.com/article/10.1556/018.66.2015.4.2 (accessed on 29 March 2025). [CrossRef] [PubMed]
- Cadar, E. Research and Development of Semi-Solid Pharmaceutical Systems Based on Marine Resources. Ph.D. Thesis, IOSUD Carol Davila UMF, Bucharest, Romania, 2018. [Google Scholar]
- Dragan, A.M.L.; Sirbu, R.; Cadar, E. Brown Seaweeds from Black Sea Coast as an Important Source of Bioactive Compounds of Interest for Human Health. Eur. J. Nat. Sci. Med. 2023, 6, 101–113. Available online: https://revistia.com/files/articles/ejnm_v6_i1_23/Dragan.pdf (accessed on 12 April 2025).
- Cadar, E.; Cadar, E.M.; Erimia, C.-L.; Tomescu, A. New Formulation with Marine Algae from Black Sea. EJMNS 2019, 2, 69–75. Available online: https://revistia.com/files/articles/ejmn_v2_i2_19/Cadar.pdf (accessed on 25 March 2025). [CrossRef]
- Cherim, M.; Sirbu, R.; Tomescu, A.; Popa, M.F.; Cadar, E. Comparative Studies on the Physico-chemical Characteristics of Bio-materials with Collagen from Calf and Fish Skins from Black Sea. Mater. Plast. 2019, 56, 179–185. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Sirbu, R.; Stanciu, G.; Tomescu, A.; Ionescu, A.M.; Cadar, E. Evaluation of Antioxidant and Antimicrobial Activity in Relation to Total Phenolic Content of Green Algae from Black Sea, Evaluation of Antioxidant and Antimicrobial Activity in Relation to Total Phenolic Content of Green Algae from Black Sea. Rev. Chim. 2019, 70, 1197–1203. [Google Scholar] [CrossRef]
- ISO-10993-1; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. International Organization for Standardisation: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/68936.html (accessed on 12 May 2025).
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–411. [Google Scholar] [CrossRef]
- Migone, C.; Scacciati, N.; Grassiri, B.; De Leo, M.; Braca, A.; Puppi, D.; Zambito, Y.; Piras, A.M. Jellyfish Polysaccharides for Wound Healing Applications. Int. J. Mol. Sci. 2022, 23, 11491. [Google Scholar] [CrossRef]
- Li, Q.M.; Wang, J.F.; Zha, X.Q.; Pan, L.H.; Zhang, H.L.; Luo, J.P. Structural characterization and immunomodulatory activity of a new polysaccharide from jellyfish. Carbohydr. Polym. 2017, 159, 188–194. [Google Scholar] [CrossRef]
- Morgner, B.; Husmark, J.; Arvidsson, A.; Wiegand, C. Effect of a DACC-coated dressing on keratinocytes and fibroblasts in wound healing using an in vitro scratch model. J. Mater. Sci. Mater. Med. 2022, 33, 22. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.J.; Martin, P. Wound repair at a glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Gonzalez, A.C.D.O.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The Role of the Extracellular Matrix Components in Cutaneous Wound Healing. Biomed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 2: Role of growth factors in normal and pathological wound healing: Therapeutic potential and methods of delivery. Adv. Ski. Wound Care 2012, 25, 349–370. [Google Scholar] [CrossRef]
- Barroso, A.F.N. Technological Advances in Cutaneous Wound Repair, Ed, Univ. del Lisboa. 2019; pp. 15–20. Available online: https://repositorio.ul.pt/bitstream/10451/43417/1/MICF_Andreia_Barroso.pdf (accessed on 1 April 2025).
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Okur, N.U.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Liang, R.; Zhang, M.; Zhao, Z.; Li, Y. Oral administration of marine collagen peptides prepared from chum salmon (Oncorhynchus keta) improves wound healing Following Cesarean Section in Rats. Food Nutr Res. 2015, 59, 26411. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef]
- Okamura, Y.; Inagaki, Y.; Nakao, S.; Kawaguchi, A.T.; Yanagawa, T.; Sumiyoshi, H.; Nakano, Y.; Endo, H. A novel composite biomaterial made of jellyfish and porcine collagens accelerates dermal wound healing by enhancing reepithelization and granulation tissue formation in mice. Adv. Wound Care 2020, 9, 295–311. [Google Scholar] [CrossRef]
- Sumiyoshi, H.; Okamura, Y.; Kawaguchi, A.T.; Kubota, T.; Endo, H.; Yanagawa, T.; Yasuda, J.; Matsuki, Y.; Nakao, S.; Inagaki, Y. External administration of moon jellyfish collagen solution accelerates physiological wound healing and improves delayed wound closure in diabetic model mice. Regen. Ther. 2021, 18, 223–230. [Google Scholar] [CrossRef]
- Pesterau, A.M.; Sirbu, R.; Cadar, E. Method for Obtaining and Physico-Chemical Characterization of Collagenic Extract of Rhizostoma Pulmo from the Black Sea. Eur. J. Nat. Sci. Med. 2022, 5, 48–57. [Google Scholar] [CrossRef]
- Pesterau, A.M.; Sirbu, R.; Cadar, E. Biomedical Applications Based on Marine Collagen Obtained from the Jellyfish Species Rhizostoma Pulmo Extracted from the Black Sea. Eur. J. Nat. Sci. Med. 2023, 6, 89–110. [Google Scholar] [CrossRef]
- Pesterau, A.M.; Sirbu, R.; Cadar, E. Extraction, Identification and Characterization by Sds-Page Method of Collagen Extracted from Rhizostoma Pulmo, a Jellyfish Found in the Black Sea Basin. Eur. J. Nat. Sci. Med. 2024, 7, 54–63. Available online: https://revistia.com/files/articles/ejnm_v7_24/Pesterau2.pdf (accessed on 7 April 2025). [CrossRef]
- Gopinath, A.; Reddy, S.M.M.; Madhan, B. Effect of aqueous ethanol on the triple helical structure of collagen. Eur. Biophys. J. 2014, 43, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.A.; Aroso, I.M.; Silva, T.H.; Mano, J.F.; Duarte, A.R.C.; Reis, R.L. Water and Carbon Dioxide: Green Solvents for the Extraction of Collagen/Gelatin from Marine Sponges. ACS Sustain. Chem. Eng. 2015, 3, 254–260. [Google Scholar] [CrossRef]
- Smith, I.P.; Domingos, M.; Richardson, S.M.; Bella, J. Characterization of the Biophysical Properties and Cell Adhesion Interactions of Marine Invertebrate Collagen from Rhizostoma pulmo. Mar. Drugs 2023, 21, 59. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef]
- Yuan, D.; Li, C.; Huang, Q.; Fu, X.; Dong, H. Current advances in the anti-inflammatory effects and mechanisms of natural polysaccharides. Crit. Rev. Food Sci. Nutr. 2022, 12, 5890–5910. [Google Scholar] [CrossRef]
- Riccio, G.; Martinez, K.A.; Martín, J.; Reyes, F.; D’Ambra, I.; Lauritano, C. Jellyfish as an Alternative Source of Bioactive Antiproliferative Compounds. Mar. Drugs 2022, 20, 350. [Google Scholar] [CrossRef]
- Nudelman, R.; Alhmoud, H.; Delalat, B.; Fleicher, S.; Fine, E.; Guliakhmedova, T.; Elnathan, R.; Nyska, A.; Voelcker, N.H.; Gozin, M. Jellyfish-based smart wound dressing devices containing in situ synthesized antibacterial nanoparticles. Adv. Funct. Mater. 2019, 29, 1902783. [Google Scholar] [CrossRef]
- Wang, X.; Huang, C.; Yang, F.; Wang, K.; Cha, S.-H.; Mao, X.; Jeon, Y.-J.; Wang, L. Fucoidan isolated from the edible seaweed Sargassum fusiforme suppresses skin damage stimulated by airborne particulate matter. Algal Res. 2024, 77, 103339. [Google Scholar] [CrossRef]
- Sirbu, R.; Zaharia, T.; Maximov, V.; Bechir, A.M.; Maris, M.; Negreanu-Pirjol, B.S.; Artenie Maris, D.; Negreanu-Pirjol, T.; Leca, M.; Cadar, E.; et al. Clean bio-technologies for obtaining new pharmaceutical formulations based on collagen gels and marine algae extracts for medical applications. JEPE 2010, 11, 654–665. Available online: https://www.researchgate.net/publication/288687642_Clean_bio-technologies_for_obtaining_new_pharmaceutical_formulations_based_on_collagen_gels_and_marine_algae_extracts_for_medical_applications (accessed on 22 April 2025).
- Gao, J.; Ning, C.; Wang, M.; Wei, M.; Ren, Y.; Li, W. Structural, antioxidant activity, and stability studies of jellyfish collagen peptide–calcium chelates. Food Chem. X 2024, 23, 101706. [Google Scholar] [CrossRef] [PubMed]
- Diao, P.; He, H.; Tang, J.; Xiong, L.; Li, L. Natural compounds protect the skin from airborne particulate matter by attenuating oxidative stress. Biomed. Pharmacother. 2021, 138, 111534. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Vasincu, A.; Luca, S.V.; Neophytou, C.; Wolfram, E.; Opitz, S.E.W.; Sava, D.; Bucur, L.; Cioroiui, B.I.; Miron, A.; et al. Unravelling the potential of seaweeds from the Black Sea coast of Romania as bioactive compounds sources. Part I: Cystoseira barbata (Stackhouse) C. Agardh. Food Chem. Toxicol. 2019, 134, 110820. [Google Scholar] [CrossRef]
- Yegdaneh, A.; Ghannadi, A.; Dayani, L. Chemical constituents and biological activities of two Iranian Cystoseira species. Res. Pharm. Sci. 2016, 11, 311–317. [Google Scholar] [CrossRef]
- Edwards, R.; Harding, K.G. Bacteria and wound healing. Curr. Opin. Infect. Dis. 2004, 17, 91–96. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I. Wound microbiology and associated approaches to wound management. Clin. Microb. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Ovington, L. Bacterial toxins and wound healing. Clin. Microb. Rev. 2003, 49, 8–12. Available online: https://pubmed.ncbi.nlm.nih.gov/12883157/ (accessed on 18 March 2025).
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Ozdemir, G.; Horzum, Z.; Sukatar, A.; Karabay-Yavasoglu, N.U. Antimicrobial Activities of Volatile Components and Various Extracts of Dictyopteris membranaceae and Cystoseira barbata from the Coast of Izmir, Turkey. Pharm. Biol. 2006, 44, 183–188. [Google Scholar] [CrossRef]
- Taskin, E.; Ozturk, M.; Taskin, E.; Kurt, O. Antibacterial activities of some marine algae from the Aegean Sea (Turkey). Afr. J. Biotechnol. 2007, 6, 2746–2751. [Google Scholar] [CrossRef]
- Alghazeer, R.; Whida, F.; Abduelrhman, E.; Gammoudi, F.; Azwai, S. Screening of antibacterial activity in marine green, red and brown macroalgae from the western coast of Libya. Nat. Sci. 2013, 5, 7–14. [Google Scholar] [CrossRef]
- Heijenoort, J. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology 2001, 11, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ibtissam, C.; Hassane, R.; Jose, M.L.; Francisco, D.S.J.; Antonio, G.V.J.; Hassan, B.; Mohamed, K. Screening of antibacterial activity in marine green and brown macroalgae from the coast of Morocco. Afr. J. Biotechnol. 2009, 8, 1258–1262. Available online: https://www.researchgate.net/publication/242184423_Screening_of_antibacterial_activity_in_marine_green_and_brown_macroalgae_from_the_coast_of_Morocco (accessed on 19 April 2025).
- Cadar, E.; Tomescu, A.; Negreanu-Pirjol, B.S. Studies on the identification of bioactive compounds in algae biomass in the Black Sea with major therapeutic actions. JOSA 2017, 3, 533–538. Available online: https://www.academia.edu/76714177/Studies_on_the_Identification_of_Bioactive_Compounds_in_Algae_Biomass_in_the_Black_Sea_with_Major_Therapeutic_Actions (accessed on 2 May 2025).
- Bechir, A.; Sirbu, R.; Pacurar, M.; Podariu, A.C.; Monea, M.; Bechir, E.S.; Ghergic, D.L. The Effect of Collagenic Gels with Marine Algae Extracts Mixtures in the Treatment of Recurrent Aphthous Stomatitis. Rev. Chim. 2014, 65, 362–368. Available online: http://bch.ro/pdfRC/BECHIR%20A.pdf%203%2014.pdf (accessed on 14 April 2025).
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Washington, DC, USA, 2019. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Percy, J.; Fife, F. The Biochemical Composition and Energy Content of Arctic Marine Macrozooplankton. Arctic 1981, 34, 307–313. [Google Scholar] [CrossRef]
- Zhang, H.L.; Cui, S.H.; Zha, X.Q.; Bansal, V.; Xue, L.; Li, X.L.; Hao, R.; Pan, L.H.; Luo, J.P. Jellyfish skin polysaccharides: Extraction and inhibitory activity on macrophage-derived foam cell formation. Carbohydr. Polym. 2014, 106, 393–402. [Google Scholar] [CrossRef]
- Sirbu, R.; Negreanu-Pirjol, T.; Mirea, M.; Negreanu-Pirjol, B.S. Bioactive Compounds from Three Green Algae Species along the Romanian Black Sea Coast with Therapeutically Properties. Eur. J. Med. Nat. Sci. 2019, 3, 5–15. [Google Scholar] [CrossRef]
- Rohani-Ghadikolaei, K.; Abdulalian, E.; Wing-Keon, N.G. Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J. Food Sci. Technol. 2012, 49, 774–780. [Google Scholar] [CrossRef]
- Ansari, F.A.; Shriwastav, A.; Gupta, S.K.; Rawat, I.; Guldhe, A.; Bux, F. Lipid extracted algae as a source for protein and reduced sugar: A step closer to the biorefinery. Biores. Technol. 2015, 179, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Albalasmeh, A.A.; Berhe, A.A.; Ghezzehei, T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometr. Carbohydr. Polym. 2013, 97, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Yaich, H.; Garna, H.; Bchir, B.; Besbes, S.; Paquot, M.; Richel, A.; Blecker, C.; Attia, H. Chemical composition and functional properties of dietary fibre extracted by Englyst and Prosky methods from the alga Ulva lactuca collected in Tunisia. Algal Res. 2015, 9, 65–73. [Google Scholar] [CrossRef]
- Barzideh, Z.; Latiff, A.A.; Gan, C.-Y.; Benjakul, S.; Karim, A.A. Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.). Int. J. Food Scie. Technol. 2014, 49, 1490–1499. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, M.; Milisenda, G.; Piraino, S. Mediterranean jellyfish as novel food: Effects of thermal processing on antioxidant, phenolic, and protein contents. Eur. Food Res. Technol. 2019, 245, 1611–1627. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Caprioli, R.; Leone, A.; Piraino, S. Jellyfish Bioprospecting in the Mediterranean Sea: Antioxidant and Lysozyme-Like Activities from Aurelia coerulea (Cnidaria, Scyphozoa) Extracts. Mar. Drugs 2021, 19, 619. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Antioxidant Activities, Total Phenolics and Flavonoids Content in Two Varieties of Malaysia Young Ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Lukiati, B.; Sulisetijono; Nugrahaningsih; Rahmi, M. Determination of total phenol and flavonoid levels and antioxidant activity of methanolic and ethanolic extract Zingiber officinale Rosc var. Rubrum rhizome. AIP Conf. Proc. 2020, 2231, 040003. [Google Scholar] [CrossRef]
- Goupy, P.; Hugues, M.; Boivin, P.; Amiot, M.J. Antioxidant Composition and Activity of Barley (Hordeum vulgare) and Malt Extracts and of Isolated Phenolic Compounds. J. Sci. Food Agric. 1999, 79, 1625–1634. [Google Scholar] [CrossRef]
- Brand, W.W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hsu, C.L.; Chen, W.; Weng, Y.M.; Tseng, C.Y. Chemical composition, physical properties and antioxidant activities of yam flours as affected by different drying methods. Food Chem. 2003, 83, 85–92. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction: Antioxidative Activity of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Ilkhani, M.; Rustaiyan, A.; Larijani, K.; Sartavi, K.; Tahmasebi, R.; Asayesh, G. Antibacterial effect of the brown alga Cystoseira trinodis. J. Med. Plants Res. 2011, 5, 4654–4657. Available online: https://academicjournals.org/journal/JMPR/article-full-text-pdf/0E6DD5125820 (accessed on 3 April 2025).
- Li, Y.; Sun, S.; Pu, X.; Yang, Y.; Zhu, F.; Zhang, S.; Xu, N. Evaluation of Antimicrobial Activities of Seaweed Resources from Zhejiang Coast, China. Sustainability 2018, 10, 2158. [Google Scholar] [CrossRef]
- Jeyanthi, R.L.; Dhanalakshmi, V.; Thomas, T. A comparison between the effects of three algal extracts against pathogenic bacteria. J. Chem. Pharm. Res. 2012, 4, 4859–4863. Available online: https://www.researchgate.net/publication/279535792 (accessed on 16 May 2024).
- Fabiano, A.; Migone, C.; Cerri, L.; Piras, A.M.; Mezzetta, A.; Maisetta, G.; Esin, S.; Batoni, G.; Di Stefano, R.; Zambito, Y. Combination of Two Kinds of Medicated Microparticles Based on Hyaluronic Acid or Chitosan for a Wound Healing Spray Patch. Pharmaceutics 2021, 13, 2195. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]













| Characteristics | Hydrogels Extract JPC from R. pulmo | Algal Hydroalcoholic Extract ALG C. barbata | ||
|---|---|---|---|---|
| with 10% Pepsin | References | C. barbata | References | |
| Moisture % (DW) | 15.1 ± 0.1 | - | 12.6 ± 0.33 | 12.27 ± 0.42 [49] |
| Ash 600–800 °C % (DW) | 0.55 ± 0.1 | - | 17.28 ± 0.88 | 18.63 ± 1.73 [49] |
| Proteins % (DW) | 60.48 ± 1.72 23.59 ± 1.89 B; 32 ± 1.19 OA; 17.56 ± 1.98 G | 61.8 [46] 6 W; 8.7–13.7 B; [47] 27 OA; 18 G [47] | 20.98 ± 0.65 | 18.13 ± 2.11 [49] |
| Collagen content % (DW) | 57.1 ± 0.6 | 56.3 [36] | - | - |
| Lipid % (DW) | 4.9 ± 0.81 W; 1.95 ± 0.3 G | 2.3 W; [47] 4.0 ± 0.1 W; 0.8 OA; 1.2 G; [47] | 6.28 ± 0.58 | 1.63 ± 0.54 [49] |
| Carbohydrates % (DW) | 0.59 ± 1.25 W; 0.25 ± 0.65 G | - | 60.25 ± 1.56 | 61.95 ± 1.06 [49] |
| Total dietary fiber % (DW) | - | - | 59.26 ± 1.05 | 61.075 ± 1.66 [49] |
| Insoluble fiber % (DW) | - | - | 28.22 ± 1.42 | 30.62 ± 1.26 [49] |
| Soluble fiber % (DW) | - | - | 31.04 ± 1.03 | 30.45 ± 1.33 [49] |
| Amino Acids | R. pulmo from Black Sea Coast Residues/1000 Residues | R. pulmo from Goa Coast India [34] % | R. pulmo from Mediterranean Sea [46] mg/100 g |
|---|---|---|---|
| Tissue | Whole body | Whole body | Whole body |
| Essential amino acids (EAAs) | |||
| Arginine (Arg) | 6.2 | 5.63 | 1.8 |
| Cystine (Cys) | 1.2 | - | 1.2 |
| Glutamic acid (Glu) | 15.2 | 13.46 | 13.7 |
| Glycine (Gly) | 33.4 | 29.34 | 4.8 |
| Histidine (His) | 0.6 | - | 5.0 |
| Isoleucine (Ile) | - | - | 4.9 |
| Leucine (Leu) | 8.6 | 6.35 | 8.2 |
| Lysine (Lys) | 6.3 | 4.62 | 6.2 |
| Methionine (Met) | - | - | 4.1 |
| Proline (Pro) | 3.9 | 2.97 | 3.5 |
| Hydroxiproline (Hyp) | 3.65 | 4.82 | - |
| Phenylalanine (Phe) | - | - | 8.4 |
| Threonine (Thr) | 5.25 | 3.18 | 4.5 |
| Triptophan (Trp) | 2.8 | 4.72 | - |
| Tyrosine (Tyr) | 3.90 | 1.77 | 6.8 |
| Valine (Val) | 4.9 | 2.8 | 4.4 |
| Non-essential aminoacids (NEAAs) | |||
| Alanine (Ala) | 6.9 | 10.38 | 3.5 |
| Aspartic acid (Asp) | 6.65 | 10.91 | 2.9 |
| Serine (Ser) | 1.7 | - | 6.0 |
| Type of Acid | Mean Value for Extract ALG ± SD mg/100 g f.w. | Percentage for Extract ALG% | Mean Value for JPC ± SD mg/100 g f.w. | Percentage for JPC% |
|---|---|---|---|---|
| Pyrogallol Acid | 4.2 ± 0.05 | 1.36 | - | - |
| Gallic Acid | 3.5 ± 0.03 | 1.13 | 5.84 ± 0.02 | 88.75 |
| Protocatechuic Acid | 7.12 ± 0.01 | 2.3 | - | - |
| 4-Amino-benzoic Acid | 5.2 ± 0.09 | 1.68 | - | - |
| Chlorogenic Acid | 5.3 ± 0.05 | 1.71 | - | - |
| p-Hydroxy-benzoic Acid | 26.9 ± 0.06 | 8.70 | - | - |
| Vanillic Acid | 99.5 ± 0.08 | 32.18 | - | - |
| Caffeic Acid | 21.2 ± 0.06 | 6.86 | - | - |
| Caftaric Acid | - | - | 0.24 ± 0.01 | 3.65 |
| Feluric Acid | 54.5 ± 0.01 | 17.62 | - | - |
| Benzoic Acid | 65.7 ± 0.06 | 21.25 | - | - |
| Ellagic Acid | 5.6 ± 0.02 | 1.81 | - | - |
| Salicylic Acid | 10.5 ± 0.03 | 3.4 | - | - |
| Syringic Acid | - | - | 0.50 ± 0.009 | 7.60 |
| Appearance | Color | Appearance |
|---|---|---|
| Collagen peptides from jellyfish R pulmo | white | powder |
| Lamellar film from jellyfish R. pulmo | white | showing porosity |
| Collagen peptide hydrogel from R. pulmo with hydroalcoholic extract of C. barbata 5% | yellowish white | gelatins viscous |
| R. pulmo collagen peptide hydrogel with hydroalcoholic extract of C. barbata 10% | white yellow dark | gelatins viscous |
| R. pulmo-collagenic peptide films with hydroalcoholic extract of C. barbata 5% | yellowish white | porous composite material |
| Collagen peptide hydrogel of R. pulmo with hydroalcoholic extract of C. barbata 10% | white yellow dark | viscous composite material |
| R. pulmo collagen peptide films with hydroalcoholic extract of C. barbata 15% | brownish white | porous composite material |
| Type of Bacteria | MIC (µg/mL) | ||
|---|---|---|---|
| JPC R. pulmo | ALG C. barbata | JPC-ALG | |
| Escherichia coli | 75 ± 0.3 | 75 ± 0.2 | 75 ± 0.3 |
| Pseudomonas aeruginosa | 50 ± 0.6 | 50 ± 0.5 | 50 ± 0.4 |
| Proteus mirabilis | 25 ± 0.5 | 25 ± 0.4 | 25 ± 0.5 |
| Klebsiella pneumonia | 50 ± 0.3 | 75 ± 0.1 | 75 ± 0.2 |
| Staphylococcus aureus | 75 ± 0.4 | >100 ± 0.1 | >100 ± 0.1 |
| Streptococcus epidermidis | 50 ± 0.5 | 50 ± 0.4 | 50 ± 0.4 |
| Viscosity ɳ (cP) Depending on Shear Speed D (s−1) | Shear Speed D (s−1) in Correlation with the Selected Rotation Speed ω (rpm) | Shear Speed D (s−1) Depending on Shear Stress τ (Pa) | Shear Stress τ (Pa) Depending on Viscosity ɳ (cP) and SHEAR Speed D (s−1) |
|---|---|---|---|
| ɳ = f(D) (6) | D = ω * R (7) | D = f(τ) (8) | τ = ɳ * D (9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesterau, A.-M.; Popescu, A.; Sirbu, R.; Cadar, E.; Busuricu, F.; Dragan, A.-M.L.; Pascale, C.; Ionescu, A.-M.; Bogdan-Andreescu, C.F.; Radu, M.-D.; et al. Marine Jellyfish Collagen and Other Bioactive Natural Compounds from the Sea, with Significant Potential for Wound Healing and Repair Materials. Mar. Drugs 2025, 23, 252. https://doi.org/10.3390/md23060252
Pesterau A-M, Popescu A, Sirbu R, Cadar E, Busuricu F, Dragan A-ML, Pascale C, Ionescu A-M, Bogdan-Andreescu CF, Radu M-D, et al. Marine Jellyfish Collagen and Other Bioactive Natural Compounds from the Sea, with Significant Potential for Wound Healing and Repair Materials. Marine Drugs. 2025; 23(6):252. https://doi.org/10.3390/md23060252
Chicago/Turabian StylePesterau, Ana-Maria, Antoanela Popescu, Rodica Sirbu, Emin Cadar, Florica Busuricu, Ana-Maria Laura Dragan, Carolina Pascale, Ana-Maria Ionescu, Claudia Florina Bogdan-Andreescu, Marius-Daniel Radu, and et al. 2025. "Marine Jellyfish Collagen and Other Bioactive Natural Compounds from the Sea, with Significant Potential for Wound Healing and Repair Materials" Marine Drugs 23, no. 6: 252. https://doi.org/10.3390/md23060252
APA StylePesterau, A.-M., Popescu, A., Sirbu, R., Cadar, E., Busuricu, F., Dragan, A.-M. L., Pascale, C., Ionescu, A.-M., Bogdan-Andreescu, C. F., Radu, M.-D., & Tomescu, C. L. (2025). Marine Jellyfish Collagen and Other Bioactive Natural Compounds from the Sea, with Significant Potential for Wound Healing and Repair Materials. Marine Drugs, 23(6), 252. https://doi.org/10.3390/md23060252







