New Polyketide and Butenolide Derivatives from the Mangrove Fungus Aspergillus spelaeus SCSIO 41433
Abstract
1. Introduction
2. Results
2.1. Structural Elucidation
2.2. Biological Activity Assay
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Source and Strain Identification
3.3. Fungal Cultivation and Fermentation
3.4. Extraction and Separation
3.5. Physicochemical Data of New Compounds
3.6. ECD Calculation
3.7. PDE4 Inhibition Rate Assay
3.8. Cell Culture
3.9. Antitumor Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, S.; Pang, X.; Cai, J.; Zhang, X.; Liu, Y.; Zhu, Y.; Zhou, X. Natural Products from Mangrove Sediments-Derived Microbes: Structural Diversity, Bioactivities, Biosynthesis, and Total Synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef] [PubMed]
- Feller, I.C.; Lovelock, C.E.; Berger, U.; McKee, K.L.; Joye, S.B.; Ball, M.C. Biocomplexity in Mangrove Ecosystems. Annu. Rev. Mar. Sci. 2010, 2, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Aghasafari, P.; George, U.; Pidaparti, R. A Review of Inflammatory Mechanism in Airway Diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Torphy, T.J. Phosphodiesterase Isozymes: Molecular targets for novel antiasthma agents. Am. J. Respir. Crit. Care Med. 1998, 157, 351–370. [Google Scholar] [CrossRef]
- Guo, Z.; Abulaizi, A.; Huang, L.; Xiong, Z.; Zhang, S.; Liu, T.; Wang, R. Discovery of P-Terphenyl Metabolites as Potential Phosphodiesterase PDE4D Inhibitors from the Coral-Associated Fungus Aspergillus sp. ITBBc1. Mar. Drugs 2022, 20, 679. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, Q.; Qi, X.; Zhang, F.; Yang, J.; Chen, C.; Zhang, K.; Chen, Z.; Luo, H.-B.; Liu, Y.; et al. Discovery of Oxidized P-Terphenyls as Phosphodiesterase 4 Inhibitors from Marine-Derived Fungi. J. Nat. Prod. 2024, 87, 1808–1816. [Google Scholar] [CrossRef]
- Arias Cardona, H.R.; Cerqueira da Silva, B.; de Lima, F.O.; Andrade Leite, F.H.; de Souza, B.C.; Brandão, H.N.; David, J.M.; Alves, C.Q.; Kijjoa, A. Hydroxytakakiamide and Other Constituents from a Marine Sponge-Associated Fungus Aspergillus Fischeri MMERU23, and Antinociceptive Activity of Ergosterol Acetate, Acetylaszonalenin and Helvolic Acid. Mar. Drugs 2024, 22, 97. [Google Scholar] [CrossRef]
- Feng, D.; Tan, L.; Qiu, L.; Ju, F.; Kuang, Q.-X.; Chen, J.-F.; Li, X.-N.; Gu, Y.-C.; Guo, D.-L.; Deng, Y. Three New Polyketides Produced by Penicillium Crustosum, a Mycoparasitic Fungus from Ophiocordyceps sinensis. Phytochem. Lett. 2020, 36, 150–155. [Google Scholar] [CrossRef]
- Hühner, E.; Öqvist, K.; Li, S.-M. Design of α-Keto Carboxylic Acid Dimers by Domain Recombination of Nonribosomal Peptide Synthetase (NRPS)-Like Enzymes. Org. Lett. 2019, 21, 498–502. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, W.; Lin, X.; Xiao, J.; Liu, Y.; Zhou, X. Butenolides from the Coral-Derived Fungus Aspergillius terreus SCSIO41404. Mar. Drugs 2022, 20, 212. [Google Scholar] [CrossRef] [PubMed]
- Ui, H.; Shiomi, K.; Yamaguchi, Y.; Masuma, R.; Nagamitsu, T.; Takano, D.; Sunazuka, T.; Namikoshi, M.; Omura, S. Nafuredin, a Novel Inhibitor of NADH-Fumarate Reductase, Produced by Aspergillus Niger FT-0554. J. Antibiot. 2001, 54, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Y.; Guo, Y.; Song, Z.; Li, J.; Feng, W. Isolation and identification of chemical components from the aerial parts of Achyranthes bidentata. J. Int. Pharm. Res. 2020, 47, 450–455. [Google Scholar]
- Uras, I.S.; Korinek, M.; Albohy, A.; Abdulrazik, B.S.; Lin, W.; Ebada, S.S.; Konuklugil, B. Anti-Inflammatory, Antiallergic and COVID-19 Main Protease (Mpro) Inhibitory Activities of Butenolides from a Marine-Derived Fungus Aspergillus Costaricaensis. ChemistrySelect 2022, 7, e202200130. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, J.; Ma, H.; Cheng, L.; Zhang, G. Study on secondary metabolites of endophytic Chaetomium sp. Chin. Tradit. Herb. Drugs 2017, 48, 1298–1301. [Google Scholar]
- Tanahashi, T.; Takenaka, Y.; Nagakura, N.; Hamada, N. 2,3-Dialkylchromones from Mycobiont Cultures of the Lichen Graphis Scripta. Heterocycles 2000, 53, 1589. [Google Scholar] [CrossRef]
- Smetanina, O.F.; Yurchenko, A.N.; Ivanets, E.V.; Gerasimenko, A.V.; Trinh, P.T.H.; Ly, B.M.; Nhut, N.D.; Van, T.T.T.; Yurchenko, E.A.; Afiyatullov, S.S. Aromatic Metabolites of Marine Fungus Penicillium sp. KMM 4672 Associated with a Brown Alga Padina sp. Chem. Nat. Compd. 2017, 53, 600–602. [Google Scholar] [CrossRef]
- Garo, E.; Starks, C.M.; Jensen, P.R.; Fenical, W.; Lobkovsky, E.; Clardy, J. Trichodermamides A and B, Cytotoxic Modified Dipeptides from the Marine-Derived Fungus Trichoderma Virens. J. Nat. Prod. 2003, 66, 423–426. [Google Scholar] [CrossRef]
- Capon, R.J.; Ratnayake, R.; Stewart, M.; Lacey, E.; Tennant, S.; Gill, J.H. Aspergillazines A–E: Novel Heterocyclic Dipeptides from an Australian Strain of Aspergillus Unilateralis. Org. Biomol. Chem. 2005, 3, 123–129. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, Z.; Feng, H.; Gan, Q.; Che, Q.; Zhu, T.; Gu, Q.; Han, B.; Li, D. Trichodermamides D–F, Heterocyclic Dipeptides with a Highly Functionalized 1,2-Oxazadecaline Core Isolated from the Endophytic Fungus Penicillium Janthinellum HDN13-309. RSC Adv. 2017, 7, 48019–48024. [Google Scholar] [CrossRef]
- Kuang, M.; Peng, W.; Xu, L.; Zheng, Y.; Sang, Z.; Qin, S.; Zou, Z. Study on the Chemical Constituents of the Plant Rhizosphere Fungus Trichoderma velutinum. Cent. South Pharm. 2022, 20, 1975–1981. [Google Scholar]
- Liao, J.; Yuan, C.; Di, Y.; He, H.; Hu, X. A New Indole Alkaloid from the Fruits of Capparis Masaikai. Asian J. Chem. 2014, 26, 4504–4506. [Google Scholar] [CrossRef]
- Yu, Z.; Han, C.; Yu, B.; Zhao, J.; Yan, Y.; Huang, S.; Liu, C.; Xiang, W. Taxonomic Characterization, and Secondary Metabolite Analysis of Streptomyces triticiradicis sp. nov.: A Novel Actinomycete with Antifungal Activity. Microorganisms 2020, 8, 77. [Google Scholar] [CrossRef]
- Evidente, A.; Ricciardiello, G.; Andolfi, A.; Sabatini, M.A.; Ganassi, S.; Altomare, C.; Favilla, M.; Melck, D. Citrantifidiene and Citrantifidiol: Bioactive Metabolites Produced by Trichoderma Citrinoviride with Potential Antifeedant Activity toward Aphids. J. Agric. Food Chem. 2008, 56, 3569–3573. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive Metabolites from Spilanthes Acmella Murr. Molecules 2009, 14, 850–867. [Google Scholar] [CrossRef]
- Evidente, A.; Andolfi, A.; Maddau, L.; Franceschini, A.; Marras, F. Biscopyran, a Phytotoxic Hexasubstituted Pyranopyran Produced by Biscogniauxia Mediterranea, a Fungus Pathogen of Cork Oak. J. Nat. Prod. 2005, 68, 568–571. [Google Scholar] [CrossRef]
- Li, X.; Gao, J.; Chen, H.; Zhang, A.; Tang, M. Toxins from a Symbiotic Fungus, Leptographium Qinlingensis Associated with Dendroctonus Armandi and Their In Vitro Toxicities to Pinus Armandi Seedlings. Eur. J. Plant Pathol. 2012, 134, 239–247. [Google Scholar] [CrossRef]
- Wu, B.; Wu, L.; Zhang, L.; Jin, Z. Studies on the Antibacterial Chemical Constituents of Senecio cannabifolius Less. (I). J. Shenyang Pharm. Univ. 2004, 21, 341–345. [Google Scholar]
- Man, H.-W.; Schafer, P.; Wong, L.M.; Patterson, R.T.; Corral, L.G.; Raymon, H.; Blease, K.; Leisten, J.; Shirley, M.A.; Tang, Y.; et al. Discovery of (S)-N-{2-[1-(3-Ethoxy-4-Methoxyphenyl)-2-Methanesulfonylethyl]-1,3-Dioxo-2,3-Dihydro-1H-Isoindol-4-Yl}acetamide (Apremilast), a Potent and Orally Active Phosphodiesterase 4 and Tumor Necrosis Factor-α Inhibitor. J. Med. Chem. 2009, 52, 1522–1524. [Google Scholar] [CrossRef]
- Luo, X.; Lin, X.; Tao, H.; Wang, J.; Li, J.; Yang, B.; Zhou, X.; Liu, Y. Isochromophilones A–F, Cytotoxic Chloroazaphilones from the Marine Mangrove Endophytic Fungus Diaporthe sp. SCSIO 41011. J. Nat. Prod. 2018, 81, 934–941. [Google Scholar] [CrossRef]
- Chen, S.-K.; Zhao, P.; Shao, Y.-X.; Li, Z.; Zhang, C.; Liu, P.; He, X.; Luo, H.-B.; Hu, X. Moracin M from Morus Alba L. Is a Natural Phosphodiesterase-4 Inhibitor. Bioorg. Med. Chem. Lett. 2012, 22, 3261–3264. [Google Scholar] [CrossRef] [PubMed]

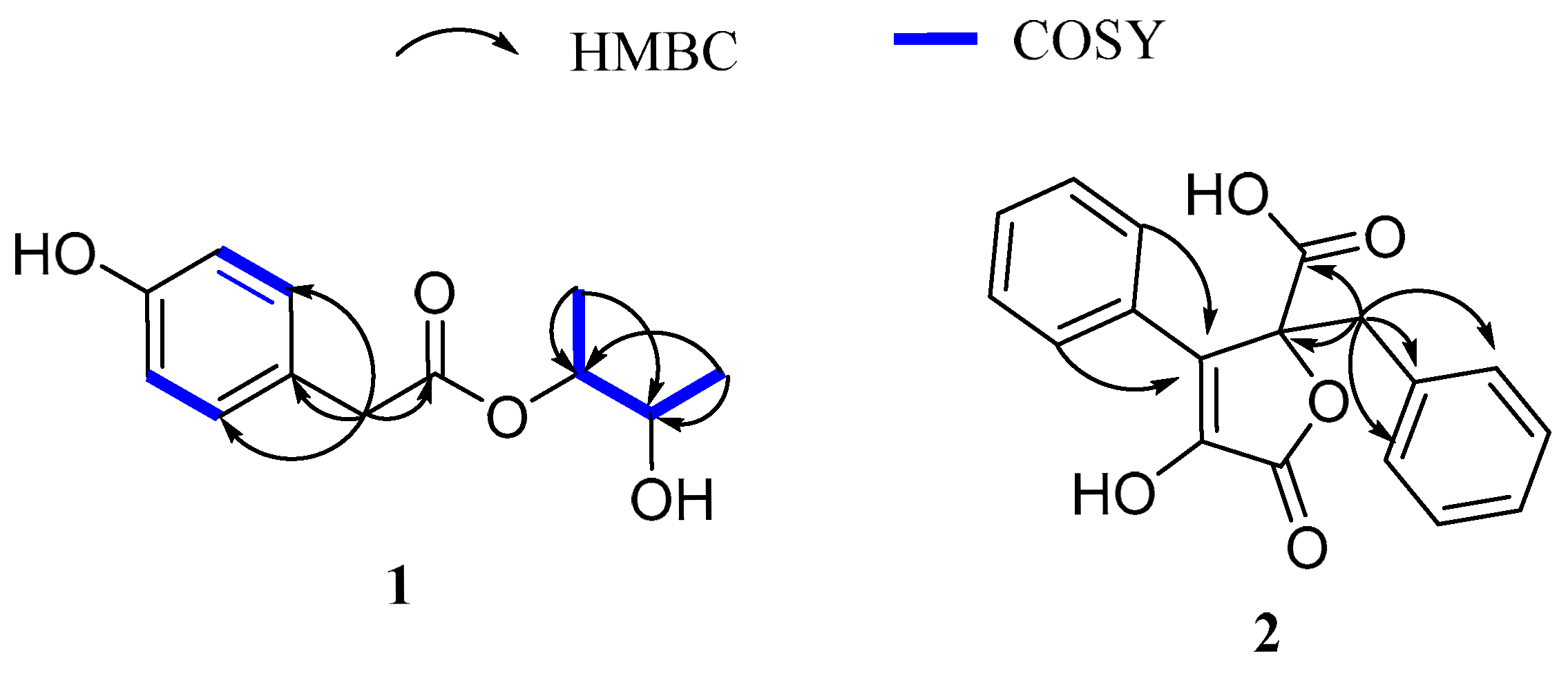
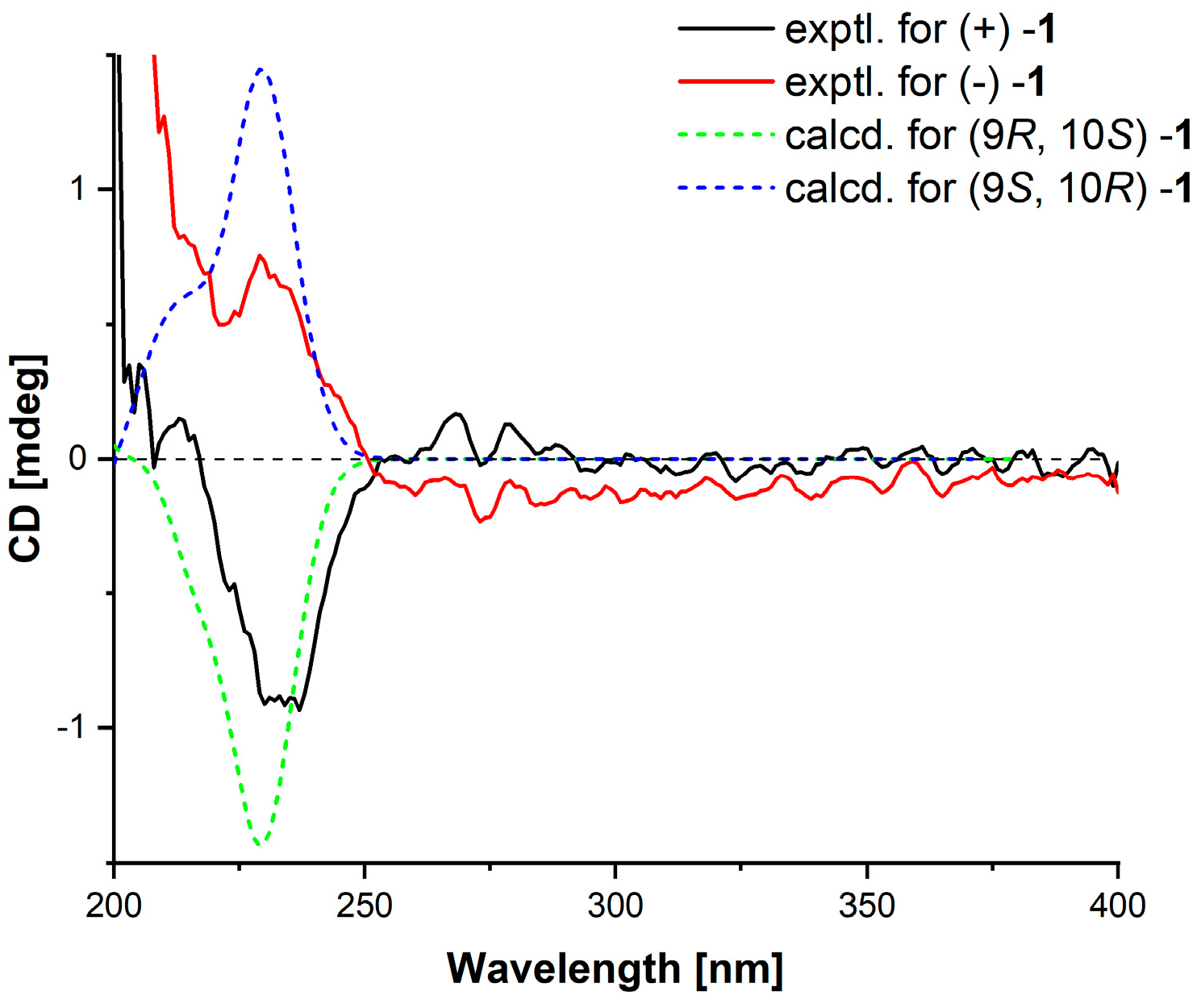
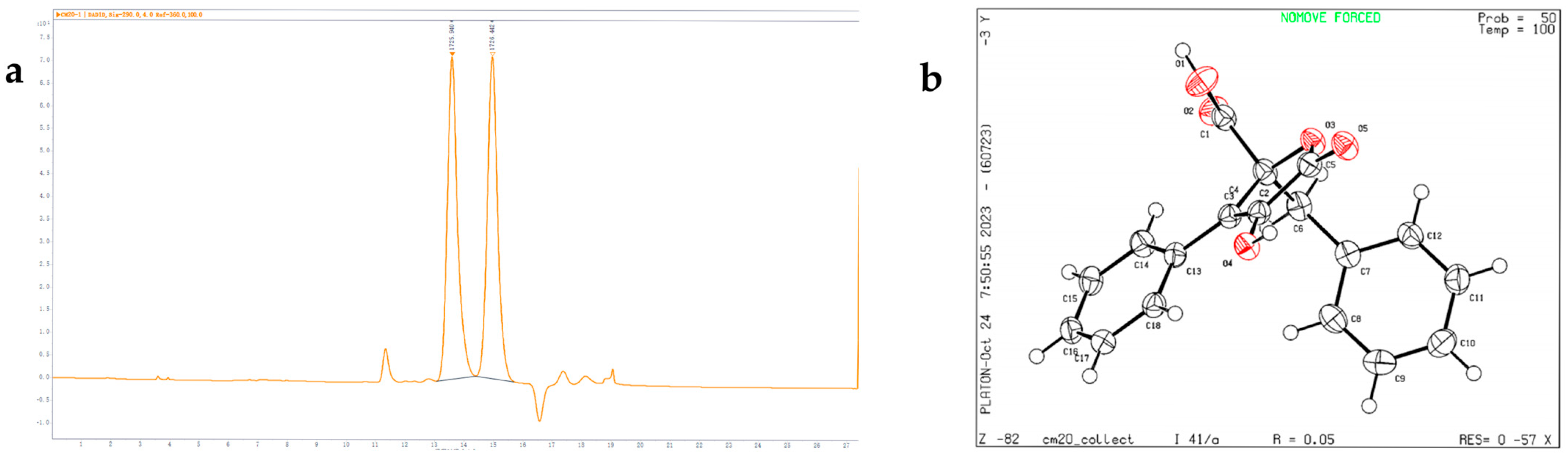
| Pos. | 1 | 2 | ||
|---|---|---|---|---|
| δC, Type | δH, (J in Hz) | δC, Type | δH, (J in Hz) | |
| 1 | 172.4, C | 172.4, C | ||
| 2 | 40.9, CH2 | 3.54, s | 87.0, C | |
| 3 | 125.6, C | 128.8, C | ||
| 4 | 130.4, CH | 7.08, d (8.1) | 141.8, C | |
| 5 | 115.7, CH | 6.72, d (8.0) | 170.1, C | |
| 6 | 155.3, C | 40.1, CH2 | 3.56, s | |
| 7 | 115.7, CH | 6.72, d (8.0) | 135.1, CH | |
| 8 | 130.4, CH | 7.08, d (8.1) | 131.5, CH | 6.83, m |
| 9 | 74.9, CH | 4.87, m | 128.8, CH | 7.09, dd (8.2, 6.5) |
| 10 | 69.7, CH | 3.85, qd (6.5, 3.1) | 129.73, CH | 7.39, m |
| 11 | 14.2, CH3 | 1.18, d (6.5) | 128.8, CH | 7.09, dd (8.2, 6.5) |
| 12 | 17.6, CH3 | 1.11, d (6.5) | 131.5, CH | 6.83, m |
| 13 | 132.0, C | |||
| 14 | 128.8, CH | 7.78, m | ||
| 15 | 129.7, CH | 7.46, t (7.8) | ||
| 16 | 128.04, CH | 7.12, m | ||
| 17 | 129.7, CH | 7.46, t (7.8) | ||
| 18 | 128.8, CH | 7.78, m | ||
| Compound | Inhibition Rate (%) | Compound | Inhibition Rate (%) |
|---|---|---|---|
| 2 | 14.5 | 11 | 18.0 |
| 3 | 19.4 | 12 | 12.7 |
| 5 | 9.4 | 13 | 12.7 |
| 8 | 9.5 | 14 | 11.7 |
| 9 | 19.2 | 16 | 19.4 |
| Tested Compounds | |||||
|---|---|---|---|---|---|
| Cells (IC50 ± SD, µM) | 1–4 | 7–9 | 11 | 15–21 | cis-Platinum |
| MDA-MB-231 | / | / | 7.7 ± 0.5 | / | 44.4 ± 3.3 |
| HCT116 | / | / | 19.1 ± 1.2 | / | 38.2 ± 5.9 |
| MDA-MB-435 | >50 | >50 | 9.2 ± 0.1 | >50 | 14.7 ± 1.3 |
| SNB-19 | / | / | 3.4 ± 0.1 | / | 26.4 ± 7.6 |
| PC3 | / | / | 23.7 ± 0.5 | / | 26.5 ± 1.5 |
| A549 | / | / | 5.7 ± 0.6 | / | 33.0 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Z.; Liang, J.; Yang, C.; Cai, J.; Yang, B.; Zhou, X.; Yuan, J.; Tao, H. New Polyketide and Butenolide Derivatives from the Mangrove Fungus Aspergillus spelaeus SCSIO 41433. Mar. Drugs 2025, 23, 251. https://doi.org/10.3390/md23060251
Xiao Z, Liang J, Yang C, Cai J, Yang B, Zhou X, Yuan J, Tao H. New Polyketide and Butenolide Derivatives from the Mangrove Fungus Aspergillus spelaeus SCSIO 41433. Marine Drugs. 2025; 23(6):251. https://doi.org/10.3390/md23060251
Chicago/Turabian StyleXiao, Zimin, Jiaqi Liang, Chun Yang, Jian Cai, Bin Yang, Xuefeng Zhou, Jie Yuan, and Huaming Tao. 2025. "New Polyketide and Butenolide Derivatives from the Mangrove Fungus Aspergillus spelaeus SCSIO 41433" Marine Drugs 23, no. 6: 251. https://doi.org/10.3390/md23060251
APA StyleXiao, Z., Liang, J., Yang, C., Cai, J., Yang, B., Zhou, X., Yuan, J., & Tao, H. (2025). New Polyketide and Butenolide Derivatives from the Mangrove Fungus Aspergillus spelaeus SCSIO 41433. Marine Drugs, 23(6), 251. https://doi.org/10.3390/md23060251









