Abstract
Antarctic krill (Euphausia superba) is a nutrient-rich marine resource. Although several terrestrial proteases have been used to prepare Antarctic krill peptides (AKPs), there has been no report on the preparation of AKPs using a marine protease. Here, marine bacterial protease A69 was used to prepare AKPs with multi-bioactivities. Through optimizing hydrolysis parameters, we established a process for AKPs preparation by hydrolyzing Antarctic krill powder with A69. In the prepared AKPs, peptides less than 3000 Da and 1000 Da accounted for 99.23% and 88.37%, respectively. The scavenging ratios of the AKPs to ABTS+, DPPH· and ·OH reached 93.23 ± 0.09%, 99.90 ± 0.15%, and 93.90 ± 0.47%, respectively. The AKPs also had high angiotensin-converting enzyme (ACE)-inhibitory activity, with an IC50 of 0.22 ± 0.04 mg/mL. At 40 mg/mL, the AKPs inhibited α-glucosidase and dipeptidyl peptidase IV (DPP-IV) activities by 7.18% and 13.62%, respectively, and displayed antibacterial activity to Escherichia coli. Moreover, 14 antioxidant peptides, 24 ACE-inhibitory peptides, 2 α-glucosidase-inhibitory peptides, and 10 DPP-Ⅳ-inhibitory peptides were identified from the AKPs. These results demonstrate that the prepared AKPs contain diverse bioactive peptides and have multi-bioactivities. This study indicates that marine bacterial protease A69 has promising application potential in preparing AKPs with multi-bioactivities.
1. Introduction
Active peptides, consisting of 2 to 50 amino acid residues, have a variety of biological activities, and have been widely used as bioactive ingredients in the fields of food [1,2], medicine [3], healthcare products [1,4] and others. Active peptides have been shown to have a variety of biological functions, such as antioxidant [5,6,7], anti-inflammatory [8,9], antibacterial [10,11,12], immune regulation [13,14,15], lowering blood pressure [16,17] and lowering uric acid [18], etc. Active peptides are now commonly prepared by enzymatic hydrolysis technology. Many proteases from terrestrial animals, plants and microorganisms have been used in the preparation of active peptides, including pepsin, trypsin, papain, alkaline protease, neutral protease and others [19,20]. In contrast, marine-derived proteases used in the preparation of active peptides are still limited.
Marine environments harbor more than 80% of global biodiversity, and serve as a vast reservoir of novel enzymes and bioactive compounds [21,22]. Marine-derived bioactive compounds have been shown to have anti-inflammatory [23], antimicrobial [24,25], anticancer [26,27] and other activities, some of which have been developed into FDA-approved drugs [25,27]. Antarctic krill (Euphausia superba) is a small crustacean widely distributed in Antarctic waters. As a key species in the Antarctic marine ecosystem, Antarctic krill is not only an important part of the Antarctic food chain and provides a major food source for a wide variety of marine life [28], but also an important source of high-value bioactive substances. Antarctic krill has been used as raw material for the production of astaxanthin [29,30], chitin [31,32], krill oil [33,34,35], krill peptides [34,36], and feed [35,37,38], etc. In addition, Antarctic krill has attracted more and more attention in the food [36,39,40,41], healthcare [34,42] and pharmaceutical fields [42,43,44] due to its rich nutritional composition and bioactive characteristics. Although some terrestrial proteases, including neutral protease, alkaline protease, Corolase PP, trypsin, pepsin, papain, flavor protease, thermoase PC10F, protamex and animal proteolytic enzyme, have been used in the preparation of Antarctic krill peptides (AKPs), which have antioxidant, anti-hypertensive, anti-diabetic and/or antibacterial activities [45,46,47,48,49,50,51,52,53], there has been no report on the preparation of Antarctic krill peptides using marine-derived proteases.
Protease A69 is a MEROPS M4 family metalloprotease derived from the marine bacterium Anoxybacillus caldiproteolyticus 1A02591 [54]. In previous studies, protease A69 was heterologously expressed in Escherichia coli and Bacillus subtilis, and recombinant protease A69 has an apparent molecular weight of approximately 34 kDa, an optimal temperature of 60 °C, and an optimal pH of 7.0 [54,55]. Recombinant protease A69 has been used to prepare bovine collagen peptides [54], soy protein peptides [55], and peanut peptides [56]. During protein hydrolysis, protease A69 preferentially hydrolyzes peptide bonds with hydrophobic residues at the P1’ site, such as Leu, Phe, Ile, Val, and Ala, and the prepared peptides exhibit bioactivities. The prepared bovine collagen peptides have moisturizing ability and antioxidant activity, the prepared soybean peptides have angiotensin-converting enzyme (ACE)-inhibitory activity, and the prepared peanut peptides have antioxidant activity and ACE-inhibitory activity. These studies showed that the marine-derived protease A69 has good potential in preparing bioactive peptides.
The aim of this study was to evaluate the potential of marine bacterial protease A69 in the preparation of AKPs with biological activities. With Antarctic krill powder, a by-product from krill oil production, as the material, on the basis of optimizing hydrolysis parameters, a process of preparing AKPs by hydrolyzing Antarctic krill powder with protease A69 was established. The characteristics and biological activities of the prepared AKPs were further analyzed. The AKPs have high proportion of low molecular weight peptides, contain 20 amino acids, and have antioxidant, ACE-inhibitory, α-glucosidase-inhibitory, dipeptidyl peptidase IV (DPP-IV)-inhibitory and antibacterial activities. The results show that the marine bacterial protease A69 has great application potential in the preparation of AKPs with good nutritional function, and antioxidant, anti-hypertensive, anti-diabetic and antibacterial activities.
2. Results and Discussion
2.1. Optimization of the Hydrolysis Parameters of Protease A69 on Antarctic Krill Powder
Protease A69 was previously shown to achieve the highest activity at 60 °C and pH 7.0 [55]. To determine the optimal conditions for A69 to hydrolyze Antarctic krill powder for AKPs production, two additional enzymatic hydrolytic parameters, enzyme/substrate (E/S) ratio and hydrolysis time, were optimized at 60 °C and pH 7.0 by single-factor experiments. As shown in Figure 1A, when Antarctic krill powder was hydrolyzed with A69 at the E/S ratio range from 500 U/g to 6000 U/g, the hydrolysate yield increased rapidly with the E/S ratio from 500 U/g to 5000 U/g, but only showed a slight increase from 5000 U/g to 6000 U/g. In addition, the content of oligopeptides with a molecular weight < 500 Da in the hydrolysates from 5000 U/g and 6000 U/g hydrolysis was almost the same, reaching the highest (Table 1). Based on these results, the optimal E/S ratio for A69 to hydrolyze Antarctic krill powder for AKPs production was determined to be 5000 U/g. As shown in Figure 1B, the hydrolysate yield increased with the hydrolysis time and reached the peak at 5 h. The content of oligopeptides with a molecular weight < 1000 Da in the hydrolysate from 6 h hydrolysis was the highest, and the contents of those < 500 Da were similar in the hydrolysates from 5 h and 6 h hydrolysis (Table 2). Based on these results, the optimal hydrolysis time for A69 to hydrolyze Antarctic krill powder for AKPs production was determined to be 6 h.

Figure 1.
Optimization of the parameters for protease A69 to hydrolyze Antarctic krill powder. (A) The effect of the E/S ratio on the hydrolysate yield. Antarctic krill powder was hydrolyzed at 60 °C and pH 7.0 for 6 h by A69 under different E/S ratios. (B) The effect of hydrolysis time on the hydrolysate yield. Antarctic krill powder was hydrolyzed at the E/S ratio of 5000 U/g, 60 °C, and pH 7.0 for different time durations. The graphs show data from triplicate experiments (mean ± SD).

Table 1.
Molecular weight distribution of the AKPs prepared from Antarctic krill powder hydrolysis by A69 at 60 °C and pH 7.0 for 6 h under different E/S ratios.

Table 2.
Molecular weight distribution of the AKPs prepared from Antarctic krill powder hydrolysis by A69 at the E/S ratio of 5000 U/g, 60 °C, and pH 7.0 for different hydrolysis time durations.
Protease A69 was previously used to prepare active peptides from bovine bone collagen, soy protein, and peanut protein. The optimal E/S ratio and hydrolysis time for recombinant A69 from E. coli to prepare collagen peptides were determined to be 25 U (collagenolytic activity)/g, and 2 h, respectively [54]; those for recombinant A69 from B. subtilis to prepare soy protein peptides were 4000 U (caseinolytic activity)/g, and 3 h [55]; and those for recombinant A69 from B. subtilis to prepare peanut protein peptides were 3000 U (caseinolytic activity)/g, and 4 h [56]. In this study, the optimal E/S ratio and hydrolysis time for recombinant A69 from B. subtilis to prepare AKPs from Antarctic krill powder were determined to be 5000 U (caseinolytic activity)/g, and 6 h. These differences may be attributed to the discrepancy in the sequences and structures of these protein sources.
In addition, it is worth mentioning that, in this study, the freeze-dried supernatant derived from Antarctic krill powder hydrolysis was used to determine the hydrolysate yield in hydrolysis parameter optimization. In addition to peptides, the supernatant may contain soluble non-peptide components, such as inorganic salts, free amino acids, and others from Antarctic krill powder during hydrolysis.
2.2. Preparation and Characterization of AKPs
Based on the optimal hydrolysis parameters determined above, we set up a process to prepare AKPs using protease A69, and a flow chart of this process was shown in Figure 2. The AKPs prepared with this process were milky white powder (Figure 3) and had good water solubility when the concentration reached 30% (w/v) (Figure 4). The enzymatic hydrolysis efficiency of the Antarctic krill powder was 35.99 ± 0.03% based on its weight before and after enzymatic hydrolysis, and 60.83 ± 0.02% based on its protein content before and after enzymatic hydrolysis. The molecular weight distribution of peptides in the AKPs prepared with the established process were further analyzed. The result showed that peptides with a molecular weight < 500 Da in the AKPs accounted for 52.37% and the content of those < 1000 Da was 88.37% (Figure 5 and Table 3), indicating that the prepared AKPs were rich in peptides composed of less than 10 amino acid residues. It has been reported that bioactive peptides usually contain 2–20 amino acid residues [1]. Thus, the prepared AKPs may contain a variety of bioactive peptides with various biological activities.
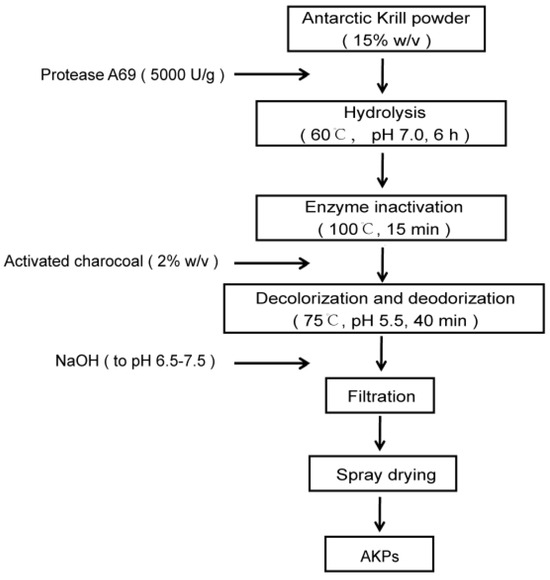
Figure 2.
A flow chart of AKPs preparation with protease A69.

Figure 3.
Antarctic krill powder (A) and the AKPs powder (B). The AKPs powder was prepared using the process shown in Figure 2.
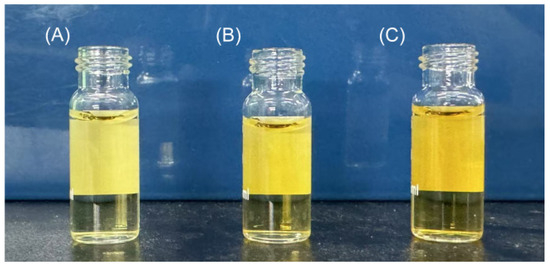
Figure 4.
Solubility of the prepared AKPs powder. (A) The AKPs solution of 10% (w/v) concentration. (B) The AKPs solution of 20% (w/v) concentration. (C) The AKPs solution of 30% (w/v) concentration.

Figure 5.
Molecular weight distribution of the prepared AKPs analyzed by HPLC gel filtration.

Table 3.
Proportions of peptides with different molecular weights in the prepared AKPs based on HPLC analysis.
2.3. Amino Acid Composition of the Prepared AKPs
To evaluate the nutritional value of the prepared AKPs, the free and total amino acid composition of the AKPs was detected. The content of free amino acids in the prepared AKPs was less than 5%, and that of total amino acids was more than 63% (Table 4). A total of 17 amino acids were detected after acid hydrolysis. However, because Asn and Gln were, respectively, converted to Asp and Glu, and Trp was degraded during acid hydrolysis, the actual total amino acid composition of the AKPs was likely comprised of 20 amino acids. Among the total amino acids in the AKPs, the detected contents of acidic amino acids Glu and Asp were quite high, accounting for 9.80 ± 0.72% and 6.58 ± 0.54%, which, however, should be the total contents of Glu + Gln and Asp + Asn, respectively. The contents of Pro and Lys were also high, reaching 8.11 ± 0.50% and 5.31 ± 0.49%, respectively. In addition, eight human essential amino acids were identified in the AKPs, totally accounting for 22.74 ± 0.27%. Considering that Trp was destroyed during acid hydrolysis, the AKPs actually contain all nine human essential amino acids. These results show that the prepared AKPs have high nutritional value.

Table 4.
The amino acid composition and content of the AKPs prepared using protease A69 a.
2.4. The Antioxidant Activity of the Prepared AKPs
The in vitro antioxidant activity of the prepared AKPs was evaluated by determining its scavenging ratios to free radicals ABTS+, DPPH·, ·OH and O2−·. Ascorbic acid (Vc), hyaluronic acid (HA), and L-reducing glutathione were used as three positive controls in determining the ABTS+ scavenging ratio, while Vc and HA were used as two positive controls in determining the scavenging ratios of DPPH·, ·OH and O2−·. As shown in Figure 6, within the investigated range of the AKPs concentration, the scavenging ratios of all free radicals rose with the increase in the AKPs concentration, demonstrating a dose-effect scavenging ability of the AKPs to the radicals. The scavenging ratio to ABTS+ of the AKPs at 10 mg/mL reached 93.23 ± 0.09%, approximately the same as those of Vc and L-reducing glutathione, and much higher than that of HA (Figure 6A). The scavenging ratios of the AKPs to DPPH· and ·OH were also high, reaching 99.90 ± 0.15% (at 30 mg/mL), and 93.90 ± 0.47% (at 40 mg/mL), respectively, basically equivalent to those of Vc, and much higher than those of HA (Figure 6B,C). By contrast, the scavenging ratio of the AKPs to O2−·was much lower, reaching 47.70 ± 1.38% at 30.00 mg/mL (Figure 6D). The EC50 values of the AKPs were 0.93 ± 0.02 mg/mL for ABTS+, 9.04 ± 0.63 mg/mL for DPPH· and 5.10 ± 0.18 mg/mL for ·OH. Altogether, these results indicated that the AKPs possess strong scavenging ability to a variety of free radicals, showing good antioxidant activity.
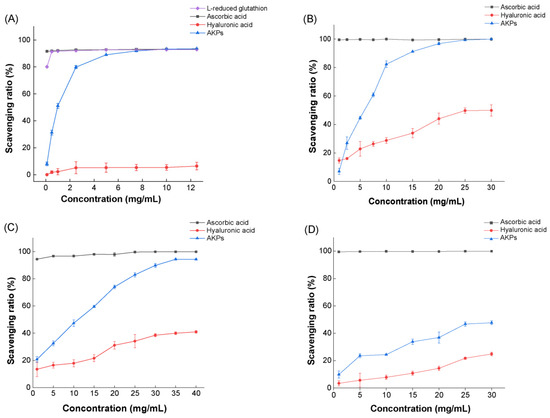
Figure 6.
Antioxidant activity of the prepared AKPs. (A) ABTS+ scavenging capacity of the AKPs. (B) •OH scavenging capacity of the AKPs. (C) DPPH• scavenging capacity of the AKPs. (D) O2−• scavenging capacity of the AKPs. The graphs show data from triplicate experiments (mean ± SD).
Several studies have shown that Antarctic krill hydrolysates (AKHs) prepared using terrestrial enzymes have antioxidant activity. Zhang et al. reported that the ·OH and DPPH· scavenging ratios of the AKH prepared using alcalase were 65.99 ± 1.22% and 55.32 ± 1.08% at 5 mg/mL, respectively, significantly higher than those prepared using trypsin, Neutrase, pepsin or papain [51]. Lan et al. reported that the high Fischer ratio oligopeptides, prepared from the sequential hydrolysis of Antarctic krill powder with alcalase and flavorzyme, showed scavenging activity against four free radicals, with EC50 values of 0.91 mg/mL for ABTS+, 0.83 mg/mL for O2-·, 4.90 mg/mL for DPPH·, and 4.62 mg/mL for ·OH [47]. Our results in this study showed that the AKPs prepared using marine bacterial protease A69 had a comparable EC50 value for ABTS+ (0.93 ± 0.02 mg/mL), and higher EC50 values for DPPH· (9.04 ± 0.63 mg/mL) and ·OH (5.10 ± 0.18 mg/mL).
2.5. The ACE-Inhibitory Activity of the Prepared AKPs
ACE inhibitors have been used to treat hypertension by blocking the activity of ACE in the renin–angiotensin–aldosterone system of humans [57]. Many peptides with ACE-inhibitory activity have been reported, such as tuna muscle peptide [16], broccoli peptide [58], walnut peptide [59], etc. In order to ascertain the ACE-inhibitory activity of the AKPs prepared with protease A69, the ACE-inhibitory rates of the AKPs of different concentrations were measured. As illustrated in Figure 7, the ACE-inhibitory rate increased promptly with the AKPs concentration and reached 94.12 ± 0.45% at 2.5 mg/mL. The IC50 value of the AKPs for ACE was determined to be 0.22 ± 0.04 mg/mL. Thus, the AKPs demonstrated remarkable ACE-inhibitory activity and likely contain ACE-inhibitory peptides.
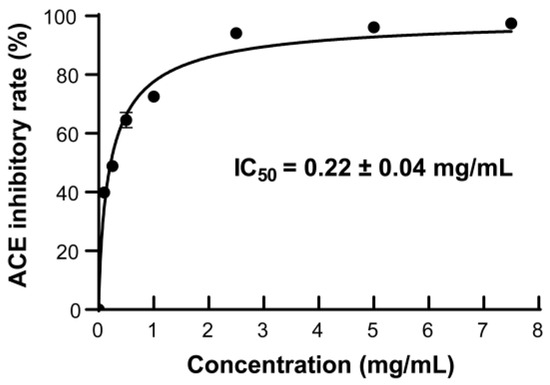
Figure 7.
The ACE-inhibitory activity of the AKPs. The graph shows data from triplicate experiments (mean ± SD).
It has been reported that AKHs prepared with commercial enzymes have ACE-inhibitory activity. The ACE-inhibitory rate of the AKH prepared with trypsin was 38.82 ± 0.71% when the concentration was 1 mg/mL [45]. The IC50 of the AKH prepared by hydrolysis of peeled Antarctic krill tail meat using thermoase PC10F was 1.944 mg/mL [48]. Ji et al. reported that the ACE-inhibitory rate of the AKH prepared with corolase PP was approximately 48% at 10 mg/mL, which was higher than those prepared with alcalase, flavourzyme, and papain [53]. The IC50 value (0.22 ± 0.04 mg/mL) of the AKPs prepared with marine bacterial protease A69 in this study is much lower than those of the reported AKHs prepared with terrestrial commercial enzymes, suggesting that the AKPs have higher ACE-inhibitory activity.
2.6. The Antibacterial Activity of the Prepared AKPs
To detect the antibacterial activity of the AKPs, the antibacterial effects of the AKPs on E. coli and S. aureus were studied by observing the inhibition zone formation on solid agar plates. As shown in Figure 8, the AKPs at 40 mg/mL formed a clear inhibition zone on the plate containing E. coli cells after 12 h incubation, and the diameter of the zone increased with incubation time, reaching 2.99 ± 0.08 mm at 48 h, in contrast to that of the positive control kanamycin (1 mg/mL) that retained approximately the same size (2.38 ± 0.20 mm) from 12 to 48 h (Figure 8, Table 5). On the plate containing S. aureus cells, the positive control kanamycin (1 mg/mL) formed a clear inhibition zone, but the AKPs did not (Figure 8). This result showed that the AKPs had antibacterial activity against the Gram-negative bacterium E. coli, but not against the Gram-positive bacterium S. aureus.
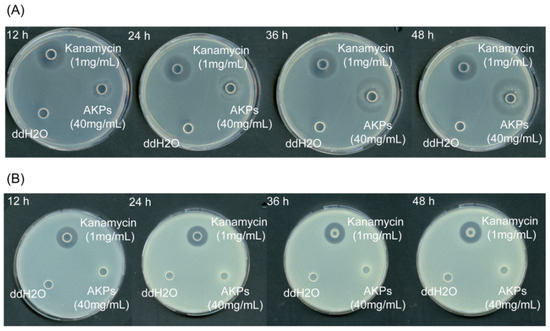
Figure 8.
Antibacterial effects of the AKPs on E. coli (A) and S. aureus (B). Plates in the figure are representatives from triplicate experiments.

Table 5.
Inhibition zone diameters of the AKPs and kanamycin against E. coli and S. aureus a.
Zhao et al. reported that the AKH prepared with protamex showed antibacterial activity against S. aureus [49]. In contrast, our study showed that the AKPs prepared with protease A69 exhibited antibacterial activity against E. coli, but no antibacterial activity against S. aureus. This difference suggests that proteases protamex and A69 likely have distinct hydrolytic sites on Antarctic krill proteins and generate bioactive peptides with differences in amino acid composition and sequence, structural characteristics and antimicrobial activity.
2.7. The Inhibitory Activities of the AKPs to α-Amylase, α-Glucosidase and DPP-IV
To evaluate the hypoglycemic potential of the AKPs, its inhibitory effects on α-amylase, α-glucosidase and DPP-Ⅳ activities were measured. When the concentration of AKPs was 40 mg/mL, its inhibitory rates on α-glucosidase and DPP-Ⅳ activities were 7.18% and 13.62%, respectively, and it had no inhibitory effect on α-amylase activity.
There has been no report on the α-amylase-inhibitory activity of AKH, and only one report on the α-glucosidase-inhibitory activity of AKH. Zheng et al. reported that the α-glucosidase-inhibitory ratio of the AKH prepared with neutral protease was 43.82%, which was higher than that of AKH prepared with trypsin, alcalase, protamex, papain or flavoenzyme [46]. In contrast, there are more reports on the DPP-Ⅳ-inhibitory activity of AKHs prepared with different enzymes. Ji et al. reported that the AKH prepared with an animal proteolytic enzyme showed DPP-Ⅳ-inhibitory activity with an IC50 value of 1.6272 mg/mL [50]. They also prepared AKHs using corolase PP, alcalase, flavourzyme, and papain, and found that the AKH prepared with corolase PP had the highest inhibition rate against DPP-IV, approximately 40% at a concentration of 10 mg/mL [53]. Lang et al. reported that the AKHs prepared with compound protease, neutral protease, alkaline protease, flavor protease, and animal hydrolase all had inhibitory activity against DPP-IV at a concentration of 100 mg/mL, and that preparation with compound protease displayed the highest value (66.81 ± 2.50%) [52]. Similar to these reports, our results in this study showed that the AKPs prepared with marine bacterial protease A69 had inhibition effects on α-glucosidase and DPP-IV activities, but not on α-amylase activity.
2.8. Identification of Bioactive Peptides from the Prepared AKPs
The peptide sequences in the prepared AKPs were determined by LC-MS/MS, and a total of 5657 peptide sequences from the AKPs were detected. By searching the AODB database, fourteen antioxidant peptides were identified from the detected peptide sequences in the prepared AKPs, including three dipeptides, five tripeptides, two tetrapeptides, one pentapeptide, one hexapeptide, and two heptapeptides (Table 6). Similarly, twenty-four ACE-inhibitory peptides were identified by searching the AHTPDB database, including eight dipeptides, eight tripeptides, six tetrapeptides, and two pentapeptides (Table 7). By searching the BIOPEP-UWM database, two α-glucosidase-inhibitory peptides (one pentapeptide and one heptapeptide) (Table 8) and ten DPP-Ⅳ-inhibitory peptides (seven dipeptides and three tetrapeptides) (Table 9), but no α-amylase-inhibitory peptide, were identified. In addition, no antimicrobial peptide was identified by searching the APD database. These data demonstrated that the AKPs prepared with protease A69 contain diverse bioactive peptides, consistent with its multiple bioactivities determined above.

Table 6.
The antioxidant peptides identified from the prepared AKPs.

Table 7.
The ACE-inhibitory peptides identified from the prepared AKPs.

Table 8.
The α-glucosidase-inhibitory identified from the prepared AKPs.

Table 9.
The DPP-Ⅳ-inhibitory peptides identified from the prepared AKPs.
In the AODB database, no antioxidant peptides of Antarctic krill origin were deposited. The 14 antioxidant peptides we identified from the prepared AKPs show homolog sequences to those previously reported from other animals and plants, marking their first identification from Antarctic krill. In the AHTPDB database, there are four ACE-inhibitory peptides from Antarctic krill currently documented, including VW [48,85], LKY [48,85], ITRY [85], and VFER [85]. In this study, we identified 24 ACE-inhibitory peptides from the prepared AKPs that differ from known Antarctic krill-derived sequences in the database, representing their first discovery in Antarctic krill. In the APD database, no antimicrobial peptides from Antarctic krill were recorded, and none were identified from the prepared AKPs through searching this database. However, the prepared AKPs exhibited in vitro antibacterial activity against E. coli (Figure 8), suggesting that there are antimicrobial peptides in the AKPs that could not be identified through database search, which thus need future identification. By searching the BIOPEP-UWM database, no α-amylase-inhibitory peptides were identified from the prepared AKPs, consistent with the lack of in vitro α-amylase-inhibitory activity of the AKPs. However, α-glucosidase-inhibitory peptides and DPP-Ⅳ-inhibitory peptides were identified from the AKPs, consistent with the inhibitory effects of the AKPs on α-glucosidase DPP-Ⅳ activities detected above. Notably, the inhibitory effect of the AKPs on α-glucosidase activity was quite low, probably due to the small number and/or low abundance of α-glucosidase-inhibitory peptides in the AKPs.
3. Materials and Methods
3.1. Experimental Materials
Antarctic krill powder was kindly provided by Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. Aprotinin, cytochrome C, salicylic acid, pyrogallol, 2’-Azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS), potassium persulfate, L-reduced glutathione, ACE, hippuric acid, Hip-His-Leu (HHL), Starch, DPP-Ⅳ and Gly-Pro-para-nitroaniline hydrochloride (Gly-Pro-PNA) were purchased from Sigma (St Louis, MO, USA). α-amylase, α-glucosidase and 4-nitrophenyl-α-D-glucopyranoside (PNPG) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). Bacitracin and H2O2 were purchased from Aladdin (Shanghai, China). Tetrapeptide GGYR and tripeptide GGG were synthesized by Qiangyao Co., Ltd. (Shanghai, China). Vc was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). HA was purchased from Shandong Freda Bioeng Co., Ltd. (Jinan, China). DPPH• was purchased from Tokyo Chemical Industry (Tokyo, Japan). Other chemicals were of analytical grade and commercially available.
3.2. Production and Activity Assay of Protease A69
The protease A69 was produced using recombinant B. subtilis in a 15-L fermentor as previously reported [55]. The fermentation medium was composed of 10% maltodextrin, 3.5% soybean meal, 1.3% citrate sodium, 5.5% K2HPO4, 1.8% MgCl2, and 1.0% CaCl2. The supernatant after centrifugation of the fermentation culture was concentrated to approximately 40,000 U/mL, which was the enzyme solution of protease A69 to be used in Antarctic krill powder hydrolysis. The enzyme solution of protease A69 was stored at 4 °C before use, and exhibited no obvious activity loss in 1-month storage at 4 °C, and retained more than 90% activity in 6-month storage at 4 °C.
The activity of protease A69 was determined by the Folin–Ciocalteu method as previously reported [54]. Briefly, the reaction mixture containing 100 μL enzyme solution and 100 μL of 2% (w/v) casein was incubated at 60 °C for 10 min, and then the reaction was stopped by adding 200 μL trichloroacetic acid (0.4 M) into the mixture. After centrifugation of the mixture, 100 μL of the supernatant was mixed with 500 μL of sodium carbonate solution (0.4 M) and 100 μL of the Folin–phenol reagent, which was incubated at 40 °C for 20 min. Then, the OD660 of the mixture was measured. One unit (1 U) was defined as the amount of enzyme that released 1 μg of tyrosine from casein at 60 °C and pH 7.0 per minute.
3.3. Optimization of the Hydrolytic Parameters of Protease A69 Towards Antarctic Krill Powder
Because the optimal temperature and pH for the activity of A69 were previously determined to be 60 °C and 7.0, respectively [54], all the hydrolytic reactions for hydrolysis parameter optimization were performed at 60 °C and pH 7.0. The hydrolysis parameters of protease A69 towards Antarctic krill powder, including hydrolysis time and enzyme/substrate (E/S) ratio, were optimized based on the methods previously reported [54], with some modifications. Briefly, 5 g Antarctic krill powder in 30 mL ddH2O was hydrolyzed by protease A69 at 60 °C and pH 7.0 with a constant stir (180 rpm), and the pH of the mixture was maintained at 7.0 with a 10 M NaOH solution for all optimization reaction. The optimal E/S ratio was determined by hydrolyzing the Antarctic krill powder for 6 h with different E/S ratios (500 U/g, 1000 U/g, 2000 U/g, 3000 U/g, 4000 U/g, 5000 U/g, 6000 U/g). The changes in the E/S ratios were achieved by adding different amounts of enzyme solution of protease A69 to a fixed substrate. The optimal hydrolysis time was determined by hydrolyzing the Antarctic krill powder under the determined optimal E/S ratio (5000 U/g) for different times (1–6 h). After hydrolysis, the mixtures were incubated at 100 °C for 15 min to terminate the reaction, and then centrifuged (4 °C, 16,970× g, 30 min). The supernatant and precipitate were, respectively, freeze-dried, and weighed. The powder from freeze-dried supernatant was prepared as a 5 mg/mL solution with ddH2O, which was subjected to peptide molecular weight distribution analysis by HPLC (Shimadzu, Kyoto, Japan) gel filtration on a TSK gel G2000 SWXL column (7.8 × 300 mm; Tosoh, Tokyo, Japan), as previously reported [54]. The column was eluted with 45% acetonitrile containing 0.1% (v/v) trifluoroacetic acid at a flow rate of 0.5 mL/min, and peptide signals were monitored at 220 nm. The weight of the freeze-dried precipitate was used to calculate the hydrolysate yield using the formula: Hydrolysate yield (%) = (Wa − Wb)/Wa × 100, where Wa and Wb were the weight of Antarctic krill powder before hydrolysis and that of the freeze-dried precipitate after hydrolysis, respectively.
3.4. Preparation of AKPs with Protease A69
According to the determined optimal hydrolytic parameters, 150 g Antarctic krill powder in 1 L ddH2O was hydrolyzed with protease A69 (5000 U/g) at 60 °C and pH 7.0 for 6 h with constant stir (180 rpm). During hydrolysis, the pH of the mixture was maintained at 7.0 with 10 M NaOH solution. After hydrolysis, the AKPs powder was prepared from the mixture through activated carbon treatment, centrifugation, filtration and centrifugal spray drying, as previously described [56].
3.5. Characterization of the Prepared AKPs
The prepared AKPs were dissolved in ddH2O to prepare 10% (w/v), 20% (w/v), and 30% (w/v) AKPs solutions to investigate the water solubility of the AKPs. Peptide molecular weight distribution of the prepared AKPs was analyzed by HPLC with a 5 mg/mL solution using the method mentioned above. The free and total amino acid composition of the prepared AKPs was determined on an automatic amino acid analyzer, HITACHI 835 (HITACHI, Tokyo, Japan), as previously described [54].
3.6. Determination of Protein Content
The protein content of the Antarctic krill powder samples before and after A69 hydrolysis was determined using the colorimetric method according to the National Standard of the People’s Republic of China for determination of protein in foods (GB 5009.168-2016) [105], as previously described [56].
3.7. Bioactivity Assays of the Prepared AKPs
The in vitro antioxidant activity of the prepared AKPs was evaluated by determining the scavenging rates of ABTS+, DPPH·, ·OH and O2−·. The scavenging rate of ABTS+ was determined according to the National Standard of the People’s Republic of China for the Determination of antioxidant activity of peptides (GB/T 39100-2020) [106]. The clearance rates of DPPH·, ·OH and O2−· were determined according to the previously reported methods [54]. The ACE-inhibitory activity of the prepared AKPs was determined with the previously reported method [55]. The α-amylase-inhibitory activity [107], α-glucosidase-inhibitory activity [108] and the DPP-IV-inhibitory activity [109] of the prepared AKPs were determined according to the methods previously reported.
The antibacterial effects of the prepared AKPs on E. coli and S. aureus were determined using the Oxford Cup method [110] with some modification. Briefly, 100 μL of the AKPs water solution (40 mg/mL) was added in the cup standing on the plates containing LB solid medium and E. coli or S. aureus cells. Kanamycin (1 mg/mL, 100 μL) was used as the positive control and sterile water (100 μL) as the negative control. The plates were incubated at 37 °C. The clear inhibition zones were observed, and their diameters were measured.
3.8. Identification of Bioactive Peptides from the Prepared AKPs
The prepared AKPs were subjected to mass spectrometry using a Q Exactive™ Hybrid Quadrupole-Orbitrap™ Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) in Beijing biotech-pack Scientific Co., Ltd. (Beijing, China). The raw MS files were analyzed and searched against protein databases based on the species in the sample using Byonic. The parameters were set as follows: the protein modifications were carbamidomethylation (C) (fixed), oxidation (M) (variable), and acetyl (N-term) (variable), the enzyme specificity was set to non-specific, the maximum missed cleavages were set to three, the precursor ion mass tolerance was set to 20 ppm, and MS/MS tolerance was 0.02 Da. Only peptides identified with high confidence were chosen for downstream protein identification analysis. In the determined peptide sequences of the prepared AKPs, peptide sequences with bioactivity deposited in databases were searched, including peptide sequences with ACE-inhibitory activity deposited in the AHTPDB database (https://webs.iiitd.edu.in/raghava/ahtpdb/, accessed on 23 March 2025), peptide sequences with antioxidant activity deposited in the AODB database (https://aodb.idruglab.cn/, accessed on 17 March 2025), peptide sequences with antimicrobial activity deposited in the APD database (https://aps.unmc.edu, accessed on 13 March 2025), peptide sequences with α-amylase-inhibitory activity, α-glucosidase-inhibitory activity, or DPP-Ⅳ-inhibitory activity deposited in BIOPEP-UWM (http://www.uwm.edu.pl/biochemia/index.php/pl/biopep, accessed on 23 March 2025).
4. Conclusions
In this study, Antarctic krill powder derived from krill oil production was hydrolyzed by marine bacterial metalloprotease A69 to prepare AKPs with multi-bioactivities. Through optimization of hydrolysis parameters, we established a process for AKPs preparation. The prepared AKPs exhibited milky white powder with good water solubility, containing high content of small peptides with molecular weights below 1000 Da (>85%), and 20 amino acids, including 9 human essential ones. Biochemical analyses showed that the prepared AKPs displayed multiple bioactivities, including antioxidant activity, ACE-inhibitory activity, antibacterial activity, α-glucosidase-inhibitory activity and DPP-IV-inhibitory activity, and the functioning concentrations of the AKPs were mostly at the mg/mL level, which are likely higher than the effective physiological concentrations. Further studies are still needed to evaluate the bioactivities of the AKPs at the cellular and animal levels. In addition, based on searching known bioactive peptide sequences deposited in databases, 14 antioxidant peptides, 24 ACE-inhibitory peptides, 2 α-glucosidase-inhibitory peptides, and 10 DPP-Ⅳ-inhibitory peptides were identified from the AKPs. However, it is likely that there are bioactive peptides with unknown sequences in the AKPs, which await further purification, identification and functional evaluation. This study represents the first report on the preparation of AKPs with more than two bioactivities and diverse bioactive peptides. The results indicate that marine bacterial metalloprotease A69 has promising potential in preparing AKPs with good nutritional value and multi-bioactivities, which may have application prospects in functional food, medicine and other industries. This study also offers a possible method for the high value-added utilization of Antarctic krill powder.
Author Contributions
Conceptualization, J.L., X.-L.C. and Y.-Q.Z.; investigation, R.L., W.-J.C., W.-X.Z. and X.-J.Y.; methodology, R.L., W.-X.Z. and W.-J.C.; data curation, Q.-L.Q., X.-Y.Z. and X.-Y.S.; writing—original draft preparation, R.L.; writing—review and editing, J.L., X.-L.C. and Y.-Q.Z.; supervision, J.L., X.-L.C. and Y.-Q.Z.; project administration, X.-L.C. and Y.-Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (grant 2022YFC2807503), the National Science Foundation of China (grants U2006205 and 32330001), and the SKLMT Frontiers and Challenges Project (grant SKLMTFCP-2023-06).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original data presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Haiyan Sui from the State Key Laboratory of Microbial Technology of Shandong University for her help and guidance in the amino acid analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Sreelekshmi, P.J.; Devika, V.; Aiswarya, L.S.; Jeevan, S.R.; Ramanunni, K.; Nair, P.B.; Sadanandan, S. Recent Advances in Bioactive Peptides as Functional Food for Health Promotions and Medicinal Applications. Protein Pept. Lett. 2023, 30, 626–639. [Google Scholar] [CrossRef]
- Agyei, D.; Ahmed, I.; Akram, Z.; Iqbal, H.M.N.; Danquah, M.K. Protein and Peptide Biopharmaceuticals: An Overview. Protein Pept. Lett. 2017, 24, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Acquah, C.; Agyei, D.; Obeng, E.M.; Pan, S.; Tan, K.X.; Danquah, M.K. Aptamers: An emerging class of bioaffinity ligands in bioactive peptide applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 1195–1206. [Google Scholar] [CrossRef]
- Ng, W.-J.; Wong, F.-C.; Abd Manan, F.; Chow, Y.-L.; Ooi, A.-L.; Ong, M.-K.; Zhang, X.; Chai, T.-T. Antioxidant Peptides and Protein Hydrolysates from Tilapia: Cellular and In Vivo Evidences for Human Health Benefits. Foods 2024, 13, 2945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lian, H.; Yang, L.; Tong, L.; Wu, Y.; Jin, S.; Guo, D. Preparation and characterization of novel antioxidant peptides from protein hydrolysate of Ophiocordyceps gracilis. Process Biochem. 2024, 146, 571–586. [Google Scholar] [CrossRef]
- Li, L.; Yang, Y.; Ma, C.-m.; Wang, B.; Bian, X.; Zhang, G.; Liu, X.-f.; Zhang, N. Structure, antioxidant activity, and neuroprotective effect of black soybean (Glycine max (L.) merr.) protein hydrolysates. Food Chem. 2025, 463, 141390. [Google Scholar] [CrossRef]
- Abril, A.G.; Pazos, M.; Villa, T.G.; Calo-Mata, P.; Barros-Velazquez, J.; Carrera, M. Proteomics Characterization of Food-Derived Bioactive Peptides with Anti-Allergic and Anti-Inflammatory Properties. Nutrients 2022, 14, 4400. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem. 2019, 43, e12531. [Google Scholar] [CrossRef]
- Chakka, A.K.; Elias, M.; Jini, R.; Sakhare, P.Z.; Bhaskar, N. In-vitro antioxidant and antibacterial properties of fermentatively and enzymatically prepared chicken liver protein hydrolysates. J. Food Sci. Technol.-Mysore 2015, 52, 8059–8067. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, B.; Li, B.; Wang, C.; Luo, Y. Preparation and identification of peptides and their zinc complexes with antimicrobial activities from silver carp (Hypophthalmichthys molitrix) protein hydrolysates. Food Res. Int. 2014, 64, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, S.; Xu, J.; Zeng, M.; Song, H.; Zhao, Y. Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin. Food Control 2008, 19, 231–235. [Google Scholar] [CrossRef]
- Liu, S.; An, M.; Zhao, Y.; Zhao, W.; Li, P.; Du, B. Immunomodulatory peptides from sturgeon cartilage: Isolation, identification, molecular docking and effects on RAW264.7 cells. Food Chem.-X 2024, 24, 101863. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, Y.; Zeng, Y.; Yang, X.; Yu, F.-M.; Wang, B. Immunomodulatory peptides from thick-shelled mussel (Mytilus coruscus): Isolation, identification, molecular docking and immunomodulatory effects on RAW264.7 cells. Food Biosci. 2024, 59, 103874. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Villanueva-Lazo, A.; Millan, F.; Martin-Santiago, V.; Rivero-Pino, F.; Millan-Linares, M.C. Production and identification of immunomodulatory peptides in intestine cells obtained from hemp industrial by-products. Food Res. Int. 2023, 174, 113616. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Wang, H.; Liu, J.; Hu, Y.; Tu, Z. Angiotensin converting enzyme (ACE) inhibitory peptide from the tuna (Thunnus thynnus) muscle: Screening, interaction mechanism and stability. Int. J. Biol. Macromol. 2024, 279, 135469. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef]
- Mehmood, A.; Iftikhar, A.; Chen, X. Food-derived bioactive peptides with anti-hyperuricemic activity: A comprehensive review. Food Chem. 2024, 451, 139444. [Google Scholar] [CrossRef]
- Mohammad, S.S.; Barbosa, M.; Gamallo, O.; Junior, J.B.B. The production of bioactive peptides by optimization of enzymatic hydrolysis process of protein from tilapia fish skin waste (Oreochromis niloticus, Linnaeus 1758) using alcalase 2.4.L. Curr. Bioact. Compd. 2023, 19, 1. [Google Scholar]
- Weng, Z.; Chen, Y.; Liang, T.; Lin, Y.; Cao, H.; Song, H.; Xiong, L.; Wang, F.; Shen, X.; Xiao, J. A review on processing methods and functions of wheat germ-derived bioactive peptides. Crit. Rev. Food Sci. Nutr. 2023, 63, 5577–5593. [Google Scholar] [CrossRef]
- Cai, C.; Yang, D.; Cao, Y.; Peng, Z.; Wang, Y.; Xi, J.; Yan, C.; Li, X. Anticancer potential of active alkaloids and synthetic analogs derived from marine invertebrates. Eur. J. Med. Chem. 2024, 279, 116850. [Google Scholar] [CrossRef]
- Nagarajan, P.; Sivakumar, A.S.; Govindasamy, C.; El Newehy, A.S.; Louis, L.R.P.; Sivanandham, M.; Rangarajalu, K.; Sangeetha, C.C.; Ghidan, A.Y.; Ghidan, A.Y. Molecular perspective on starfish tissue extracts: Targeting human carcinoma KB cells for anticancer therapy. J. King Saud Univ. Sci. 2024, 36, 103035. [Google Scholar] [CrossRef]
- Ghelani, H.; Khursheed, M.; Adrian, T.E.; Jan, R.K. Anti-Inflammatory Effects of Compounds from Echinoderms. Mar. Drugs 2022, 20, 693. [Google Scholar] [CrossRef] [PubMed]
- Kumla, D.; Pereira, J.A.; Dethoup, T.; Gales, L.; Freitas-Silva, J.; Costa, P.M.; Lee, M.; Silva, A.M.S.; Sekeroglu, N.; Pinto, M.M.M.; et al. Chromone Derivatives and Other Constituents from Cultures of the Marine Sponge-Associated Fungus Penicillium erubescens KUFA0220 and Their Antibacterial Activity. Mar. Drugs 2018, 16, 289. [Google Scholar] [CrossRef]
- Bharathi, D.; Lee, J. Recent Advances in Marine-Derived Compounds as Potent Antibacterial and Antifungal Agents: A Comprehensive Review. Mar. Drugs 2024, 22, 348. [Google Scholar] [CrossRef] [PubMed]
- Katanaev, V.L.; Di Falco, S.; Khotimchenko, Y. The Anticancer Drug Discovery Potential of Marine Invertebrates from Russian Pacific. Mar. Drugs 2019, 17, 474. [Google Scholar] [CrossRef]
- Tamzi, N.N.; Rahman, M.M.; Das, S. Recent Advances in Marine-Derived Bioactives Towards Cancer Therapy. Int. J. Transl. Med. 2024, 4, 740–781. [Google Scholar] [CrossRef]
- Cavan, E.L.; Belcher, A.; Atkinson, A.; Hill, S.L.; Kawaguchi, S.; McCormack, S.; Meyer, B.; Nicol, S.; Ratnarajah, L.; Schmidt, K.; et al. The importance of Antarctic krill in biogeochemical cycles. Nat. Commun. 2019, 10, 4742. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhao, J.; Xia, G.; Liu, Z.; Shi, H. Astaxanthin Esters as Functional Food: A Review of Their Nutrition, Phytochemical Structure, Biological Features, and Food Industry Prospects. J. Agric. Food Chem. 2024, 72, 13467–13475. [Google Scholar] [CrossRef]
- Cong, X.-Y.; Miao, J.-K.; Zhang, H.-Z.; Sun, W.-H.; Xing, L.-H.; Sun, L.-R.; Zu, L.; Gao, Y.; Leng, K.-L. Effects of Drying Methods on the Content, Structural Isomers, and Composition of Astaxanthin in Antarctic Krill. ACS Omega 2019, 4, 17972–17980. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, X.; Miao, J.; Leng, K. Chitin from Antarctic krill shell: Eco-preparation, detection, and characterization. Int. J. Biol. Macromol. 2020, 164, 4125–4137. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Q.; Xu, Y.; Xia, W. Recovery of Chitin from Antarctic Krill (Euphausia superba) Shell Waste by Microbial Deproteinization and Demineralization. J. Aquat. Food Prod. Technol. 2017, 26, 1210–1220. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Duo, L.; Yang, J.; Wang, X.; Zhang, G.; Zhao, J.; Zou, H.; Wang, Z.; Li, Y. Krill oil: Nutraceutical potential in skin health and disease. Front. Nutr. 2024, 11, 1388155. [Google Scholar] [CrossRef]
- Teng, X.-N.; Wang, S.-C.; Zeb, L.; Dong, Y.-S.; Xiu, Z.-L. Two-Step Enzymolysis of Antarctic Krill for Simultaneous Preparation of Value-Added Oil and Enzymolysate. Mar. Drugs 2023, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, B. Utilization of Antarctic krill for food and feed. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 2004; Volume 42, pp. 45–54. [Google Scholar]
- Ge, M.-X.; Chen, R.-P.; Zhang, L.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Novel Ca-Chelating Peptides from Protein Hydrolysate of Antarctic Krill (Euphausia superba): Preparation, Characterization, and Calcium Absorption Efficiency in Caco-2 Cell Monolayer Model. Mar. Drugs 2023, 21, 579. [Google Scholar] [CrossRef]
- Hou, H.; Wang, S.; Zhu, X.; Li, Q.; Fan, Y.; Cheng, D.; Li, B. A novel calcium-binding peptide from Antarctic krill protein hydrolysates and identification of binding sites of calcium-peptide complex. Food Chem. 2018, 243, 389–395. [Google Scholar] [CrossRef]
- Mildenberger, J.; Bruheim, I.; Solibakke, P.; Atanassova, M. Development of a protein concentrate for human consumption by direct enzymatic hydrolysis of antarctic krill (Euphausia superba). LWT-Food Sci. Technol. 2023, 173, 114254. [Google Scholar] [CrossRef]
- Chi, H.; Fu, Z.; Wang, P.; Yu, D.; Zhao, L.; Li, L.; Liu, Y.; Zheng, J. Process Optimization for Antarctic Krill (Euphausia superba) Sauce Based on Back Propagation Neural Network Combined with Genetic Algorithm. Appl. Sci. 2024, 14, 7337. [Google Scholar] [CrossRef]
- Tang, S.; Wang, J.J.; Li, Y.; Malakar, P.K.; Zhao, Y. Recent advances in the use of antarctic krill (Euphausia superba) as a sustainable source of high-quality protein: A comprehensive review. Trends Food Sci. Technol. 2024, 152, 104684. [Google Scholar] [CrossRef]
- Nicol, S.; Foster, J.; Kawaguchi, S. The fishery for Antarctic krill—Recent developments. Fish Fish. 2012, 13, 30–40. [Google Scholar] [CrossRef]
- Suzuki, Y.; Fukushima, M.; Sakuraba, K.; Sawaki, K.; Sekigawa, K. Krill Oil Improves Mild Knee Joint Pain: A Randomized Control Trial. PLoS ONE 2016, 11, e0162769. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, F.; Storsve, A.B.; Burri, L.; Ding, Y.; Banquells, M.; Riera, J.; Bjork, P.; Ferrer-Roca, V.; Domingo, J.C. Krill-Oil-Dependent Increases in HS-Omega-3 Index, Plasma Choline and Antioxidant Capacity in Well-Conditioned Power Training Athletes. Nutrients 2021, 13, 4237. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Zhang, L.; Tao, J.; Chi, C.-F.; Wang, B. Eight antihypertensive peptides from the protein hydrolysate of Antarctic krill (Euphausia superba): Isolation, identification, and activity evaluation on human umbilical vein endothelial cells (HUVECs). Food Res. Int. 2019, 121, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Wu, Y.; Dai, Q.; Yan, X.; Liu, Y.; Sun, D.; Yu, Z.; Jiang, S.; Ma, Q.; Jiang, W. Extraction, identification, and molecular mechanisms of α-glucosidase inhibitory peptides from defatted Antarctic krill (Euphausia superba) powder hydrolysates. Int. J. Biol. Macromol. 2024, 266, 131126. [Google Scholar] [CrossRef]
- Lan, C.; Zhao, Y.-Q.; Li, X.-R.; Wang, B. High Fischer ratio oligopeptides determination from Antartic krill: Preparation, peptides profiles, and in vitro antioxidant activity. J. Food Biochem. 2019, 43, e12827. [Google Scholar] [CrossRef]
- Hatanaka, A.; Miyahara, H.; Suzuki, K.I.; Sato, S. Isolation and Identification of Antihypertensive Peptides from Antarctic Krill Tail Meat Hydrolysate. J. Food Sci. 2009, 74, H116–H120. [Google Scholar] [CrossRef]
- Zhao, L.; Yin, B.; Liu, Q.; Cao, R. Purification of antimicrobial peptide from Antarctic Krill (Euphausia superba) and its function mechanism. J. Ocean Univ. China 2013, 12, 484–490. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, C.; Ji, H. Purification, identification and molecular mechanism of two dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from Antarctic krill (Euphausia superba) protein hydrolysate. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2017, 1064, 56–61. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhao, G.-X.; Suo, S.-K.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Purification, Identification, Activity Evaluation, and Stability of Antioxidant Peptides from Alcalase Hydrolysate of Antarctic Krill (Euphausia superba) Proteins. Mar. Drugs 2021, 19, 347. [Google Scholar] [CrossRef]
- Lang, M.; Song, Y.; Li, Y.; Xiang, X.; Ni, L.; Miao, J. Purification, identification, and molecular mechanism of DPP-IV inhibitory peptides from defatted Antarctic krill powder. J. Food Biochem. 2021, 45, e13872. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhang, C.; Ji, H. Two Novel Bioactive Peptides from Antarctic Krill with Dual Angiotensin Converting Enzyme and Dipeptidyl Peptidase IV Inhibitory Activities. J. Food Sci. 2017, 82, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-H.; Zhang, X.-Y.; Wang, Z.; Zhang, X.; Liu, S.-C.; Song, X.-Y.; Zhang, Y.-Z.; Ding, J.-M.; Chen, X.-L.; Xu, F. Potential of Thermolysin-like Protease A69 in Preparation of Bovine Collagen Peptides with Moisture-Retention Ability and Antioxidative Activity. Mar. Drugs 2021, 19, 676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.-X.; Wang, Y.; Cheng, J.-H.; Bao, K.; He, J.; Chen, X.-L. Production of marine bacterial metalloprotease A69 and evaluation of its potential in preparing soybean peptides with angiotensin-converting enzyme-inhibitory activity. J. Sci. Food Agric. 2023, 103, 7153–7163. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.-J.; Liu, R.; Zhao, W.-X.; Li, J.; Wang, Y.; Yuan, X.-J.; Wang, H.-L.; Zhang, Y.-Z.; Chen, X.-L.; Zhang, Y.-Q. Potential of Marine Bacterial Metalloprotease A69 in the Preparation of Peanut Peptides with Angiotensin-Converting Enzyme (ACE)-Inhibitory and Antioxidant Properties. Mar. Drugs 2024, 22, 305. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Shu, G.; Yuan, J.; Zhang, J.; Qin, S.; Li, J. Enhanced antihypertensive potential of fermented pomegranate juice: The contribution of phenolic compounds biotransformation and the resultant angiotensin-I-converting enzyme inhibition mechanism. Food Chem. 2023, 404, 134745. [Google Scholar] [CrossRef]
- Li, Y.; Pan, D.; Zhang, W.; Xie, X.; Dang, Y.; Gao, X. Identification and molecular mechanism of novel ACE inhibitory peptides from broccoli protein. Food Biosci. 2024, 61, 104678. [Google Scholar] [CrossRef]
- Liu, M.; Du, M.; Zhang, Y.; Xu, W.; Wang, C.; Wang, K.; Zhang, L. Purification and identification of an ACE inhibitory peptide from walnut protein. J. Agric. Food Chem. 2013, 61, 4097–4100. [Google Scholar] [CrossRef]
- De Gobba, C.; Tompa, G.; Otte, J. Bioactive peptides from caseins released by cold active proteolytic enzymes from Arsukibacterium ikkense. Food Chem. 2014, 165, 205–215. [Google Scholar] [CrossRef]
- Chen, H.M.; Muramoto, K.; Yamauchi, F.; Nokihara, K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem. 1996, 44, 2619–2623. [Google Scholar] [CrossRef]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-oxidative capacity of enzymatically released peptides from soybean protein isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Dávalos, A.; Miguel, M.; Bartolomé, B.; López-Fandiño, R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1939–1944. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.-M.; Li, L.-Y.; Chi, C.-F.; Wang, B. Twelve Antioxidant Peptides From Protein Hydrolysate of Skipjack Tuna (Katsuwonus pelamis) Roe Prepared by Flavourzyme: Purification, Sequence Identification, and Activity Evaluation. Front. Nutr. 2022, 8, 813780. [Google Scholar] [CrossRef]
- Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Anwar, F.; Abu Bakar, F.; Philip, R.; Saari, N. Identification and characterization of papain-generated antioxidant peptides from palm kernel cake proteins. Food Res. Int. 2014, 62, 726–734. [Google Scholar] [CrossRef]
- Saito, K.; Jin, D.H.; Ogawa, T.; Muramoto, K.; Hatakeyama, E.; Yasuhara, T.; Nokihara, K. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J. Agric. Food Chem. 2003, 51, 3668–3674. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, W.; Santhanam, R.K.; Wang, C.; Gao, X.; Chen, Y.; Wang, C.; Xu, L.; Chen, H. Bioactive peptide with antioxidant and anticancer activities from black soybean Glycine max (L.) Merr. byproduct: Isolation, identification and molecular docking study. Eur. Food Res. Technol. 2019, 245, 677–689. [Google Scholar] [CrossRef]
- Feng, L.; Peng, F.; Wang, X.; Li, M.; Lei, H.; Xu, H. Identification and characterization of antioxidative peptides derived from simulated in vitro gastrointestinal digestion of walnut meal proteins. Food Res. Int. 2019, 116, 518–526. [Google Scholar] [CrossRef]
- del Mar Contreras, M.; Sanchez, D.; Angeles Sevilla, M.; Recio, I.; Amigo, L. Resistance of casein-derived bioactive peptides to simulated gastrointestinal digestion. Int. Dairy J. 2013, 32, 71–78. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, W.; Li, J.; Wang, L.; Wu, H.; Wang, X.; Shi, L. Rapid identification of bioactive peptides with antioxidant activity from the enzymatic hydrolysate of Mactra veneriformis by UHPLC-Q-TOF mass spectrometry. Food Chem. 2015, 167, 484–489. [Google Scholar] [CrossRef]
- Zielinska, E.; Baraniak, B.; Karas, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar] [CrossRef]
- Huang, Y.; Ruan, G.; Qin, Z.; Li, H.; Zheng, Y. Antioxidant activity measurement and potential antioxidant peptides exploration from hydrolysates of novel continuous microwave-assisted enzymolysis of the Scomberomorus niphonius protein. Food Chem. 2017, 223, 89–95. [Google Scholar] [CrossRef] [PubMed]
- van Platerink, C.J.; Janssen, H.-G.M.; Haverkamp, J. Application of at-line two-dimensional liquid chromatography-mass spectrometry for identification of small hydrophilic angiotensin I-inhibiting peptides in milk hydrolysates. Anal. Bioanal. Chem. 2008, 391, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Aristoy, M.-C.; Angel Sentandreu, M.; Vignolo, G.; Toldra, F. Peptides with angiotensin I converting enzyme (ACE) inhibitory activity generated from porcine skeletal muscle proteins by the action of meat-borne Lactobacillus. J. Proteom. 2013, 89, 183–190. [Google Scholar] [CrossRef]
- Cavazos, A.; de Mejia, E.G. Identification of Bioactive Peptides from Cereal Storage Proteins and Their Potential Role in Prevention of Chronic Diseases. Compr. Rev. Food Sci. Food Saf. 2013, 12, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Iwaniak, A.; Dziuba, B. Motifs with potential physiological activity in food proteins–BIOPEP database. Acta Sci. Pol. Technol. Aliment. 2009, 8, 59–85. [Google Scholar]
- Tian, F.; Zhou, P.; Lv, F.; Song, R.; Li, Z. Three-dimensional holograph vector of atomic interaction field (3D-HoVAIF): A novel rotation-translation invariant 3D structure descriptor and its applications to peptides. J. Pept. Sci. 2007, 13, 549–566. [Google Scholar] [CrossRef]
- Wu, J.P.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship study of di- and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef]
- Pripp, A.H.; Ardo, Y. Modelling relationship between angiotensin-(I)-converting enzyme inhibition and the bitter taste of peptides. Food Chem. 2007, 102, 880–888. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, C.; Ren, Y.; Wang, C.; Tian, F. What are the ideal properties for functional food peptides with antihypertensive effect? A computational peptidology approach. Food Chem. 2013, 141, 2967–2973. [Google Scholar] [CrossRef]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar] [CrossRef]
- Cu, Y.; Majumder, K.; Wu, J. QSAR-aided in silico approach in evaluation of food proteins as precursors of ACE inhibitory peptides. Food Res. Int. 2011, 44, 2465–2474. [Google Scholar] [CrossRef]
- Pihlanto, A.; Mäkinen, S. Antihypertensive properties of plant protein derived peptides. In Bioactive Food Peptides in Health and Disease; IntechOpen: London, UK, 2013; pp. 144–182. [Google Scholar]
- Garcia, M.C.; Puchalska, P.; Esteve, C.; Marina, M.L. Vegetable foods: A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Marina Alegre, M.L.; Garcia Lopez, M.C. Isolation and Characterization of Peptides with Antihypertensive Activity in Foodstuffs. Crit. Rev. Food Sci. Nutr. 2015, 55, 521–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Liu, Y.-L.; Ning, J.-H.; Yu, J.; Li, X.-H.; Wang, F.-X. Is the structural diversity of tripeptides sufficient for developing functional food additives with satisfactory multiple bioactivities? J. Mol. Struct. 2013, 1040, 164–170. [Google Scholar] [CrossRef]
- Matsufuji, H.; Matsui, T.; Seki, E.; Osajima, K.; Nakashima, M.; Osajima, Y. Angiotensin I-converting enzyme inhibitory peptides in an alkaline protease hydrolyzate derived from sardine muscle. Biosci. Biotechnol. Biochem. 1994, 58, 2244–2245. [Google Scholar] [CrossRef]
- Silva-Sanchez, C.; de la Rosa, A.P.B.; Leon-Galvan, M.F.; de Lumen, B.O.; de Leon-Rodriguez, A.; de Mejia, E.G. Bioactive peptides in amaranth (Amaranthus hypochondriacus) seed. J. Agric. Food Chem. 2008, 56, 1233–1240. [Google Scholar] [CrossRef]
- Yano, S.; Suzuki, K.; Funatsu, G. Isolation from alpha-zein of thermolysin peptides with angiotensin I-converting enzyme inhibitory activity. Biosci. Biotechnol. Biochem. 1996, 60, 661–663. [Google Scholar] [CrossRef]
- He, H.-L.; Liu, D.; Ma, C.-B. Review on the Angiotensin-I-Converting Enzyme (ACE) Inhibitor Peptides from Marine Proteins. Appl. Biochem. Biotechnol. 2013, 169, 738–749. [Google Scholar] [CrossRef]
- He, R.; Malomo, S.A.; Girgih, A.T.; Ju, X.; Auko, R.E. Glycinyl-Histidinyl-Serine (GHS), a Novel Rapeseed Protein-Derived Peptide Has Blood Pressure-Lowering Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2013, 61, 8396–8402. [Google Scholar] [CrossRef]
- Majumder, K.; Wu, J. Angiotensin I Converting Enzyme Inhibitory Peptides from Simulated in Vitro Gastrointestinal Digestion of Cooked Eggs. J. Agric. Food Chem. 2009, 57, 471–477. [Google Scholar] [CrossRef]
- Meisel, H.; Schlimme, E. Milk proteins: Precursors of bioactive peptides. Trends Food Sci. Technol. 1990, 1, 41–43. [Google Scholar] [CrossRef]
- Tan, J.; Tian, F.; Lv, Y.; Liu, W.; Zhong, L.; Liu, Y.; Yang, L. Integration of QSAR modelling and QM/MM analysis to investigate functional food peptides with antihypertensive activity. Mol. Simul. 2013, 39, 1000–1006. [Google Scholar] [CrossRef]
- Miyoshi, S.; Ishikawa, H.; Kaneko, T.; Fukui, F.; Tanaka, H.; Maruyama, S. Structures and activity of angiotensin-converting enzyme inhibitors in an alpha-zein hydrolysate. Agric. Biol. Chem. 1991, 55, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Stromeck, A.; Loponen, J.; Lopes-Lutz, D.; Schieber, A.; Gaenzle, M.G. LC-MS/MS Quantification of Bioactive Angiotensin I-Converting Enzyme Inhibitory Peptides in Rye Malt Sourdoughs. J. Agric. Food Chem. 2011, 59, 11983–11989. [Google Scholar] [CrossRef]
- Martinez-Maqueda, D.; Miralles, B.; Recio, I.; Hernandez-Ledesma, B. Antihypertensive peptides from food proteins: A review. Food Funct. 2012, 3, 350–361. [Google Scholar] [CrossRef]
- Loponen, J. Angiotensin converting enzyme inhibitory peptides in Finnish cereals: A database survey. Agric. Food Sci. 2004, 13, 39–45. [Google Scholar] [CrossRef]
- Terashima, M.; Oe, M.; Ogura, K.; Matsumura, S. Inhibition Strength of Short Peptides Derived from an ACE Inhibitory Peptide. J. Agric. Food Chem. 2011, 59, 11234–11237. [Google Scholar] [CrossRef]
- Kohmura, M.; Nio, N.; Kubo, K.; Minoshima, Y.; Munekata, E.; Ariyoshi, Y. Inhibition of Angiotensin-converting Enzyme by Synthetic Peptides of Human β-Casein. Agric. Biol. Chem. 1989, 53, 2107–2114. [Google Scholar] [CrossRef]
- Phelan, M.; Kerins, D. The potential role of milk-derived peptides in cardiovascular disease. Food Funct. 2011, 2, 153–167. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. Bioactive Peptides of Buffalo, Camel, Goat, Sheep, Mare, and Yak Milks and Milk Products. Food Rev. Int. 2013, 29, 1–23. [Google Scholar] [CrossRef]
- Tavares, T.G.; Malcata, F.X. Whey proteins as source of bioactive peptides against hypertension. In Bioactive Food Peptides in Health and Disease; IntechOpen: London, UK, 2017. [Google Scholar]
- Gómez-Ruiz, J.A.; Ramos, M.; Recio, I. Angiotensin-converting enzyme-inhibitory peptides in Manchego cheeses manufactured with different starter cultures. Int. Dairy J. 2002, 12, 697–706. [Google Scholar] [CrossRef]
- GB/T5009.5-2003; Determination of Protein in Foods. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2003.
- GB/T 39100-2020; Determination of Antioxidant Activity for Polypeptides—DPPH and ABTS Methods. State Administration for Market Regulation, Standardization Administration of China: Beijing, China, 2020.
- Zhou, H.; Safdar, B.; Li, H.; Yang, L.; Ying, Z.; Liu, X. Identification of a novel α-amylase inhibitory activity peptide from quinoa protein hydrolysate. Food Chem. 2023, 403, 134434. [Google Scholar] [CrossRef] [PubMed]
- Daou, M.; Elnaker, N.A.; Ochsenkuhn, M.A.; Amin, S.A.; Yousef, A.F.; Yousef, L.F. In vitro α-glucosidase inhibitory activity of Tamarix nilotica shoot extracts and fractions. PLoS ONE 2022, 17, e0264969. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, L.; Zhang, Y.; Sheng, N.-J.; Wang, Z.-K.; Wu, T.-Z.; Wang, X.-Z.; Wu, H. Rapid Identification of Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Peptides from Ruditapes philippinarum Hydrolysate. Molecules 2017, 22, 1714. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Zhang, S.; Sun, M.-L.; Su, H.-N.; Li, H.-Y.; Zhang, Y.-Z.; Chen, X.-L.; Cao, H.-Y.; Song, X.-Y. Antibacterial activity of peptaibols from Trichoderma longibrachiatum SMF2 against gram-negative Xanthomonas oryzae pv. oryzae, the causal agent of bacterial leaf blight on rice. Front. Microbiol. 2022, 13, 1034779. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).