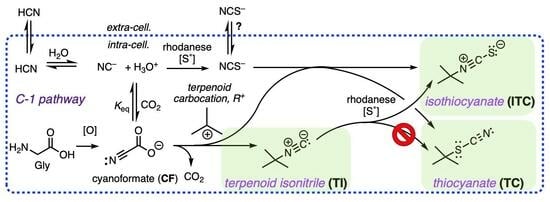
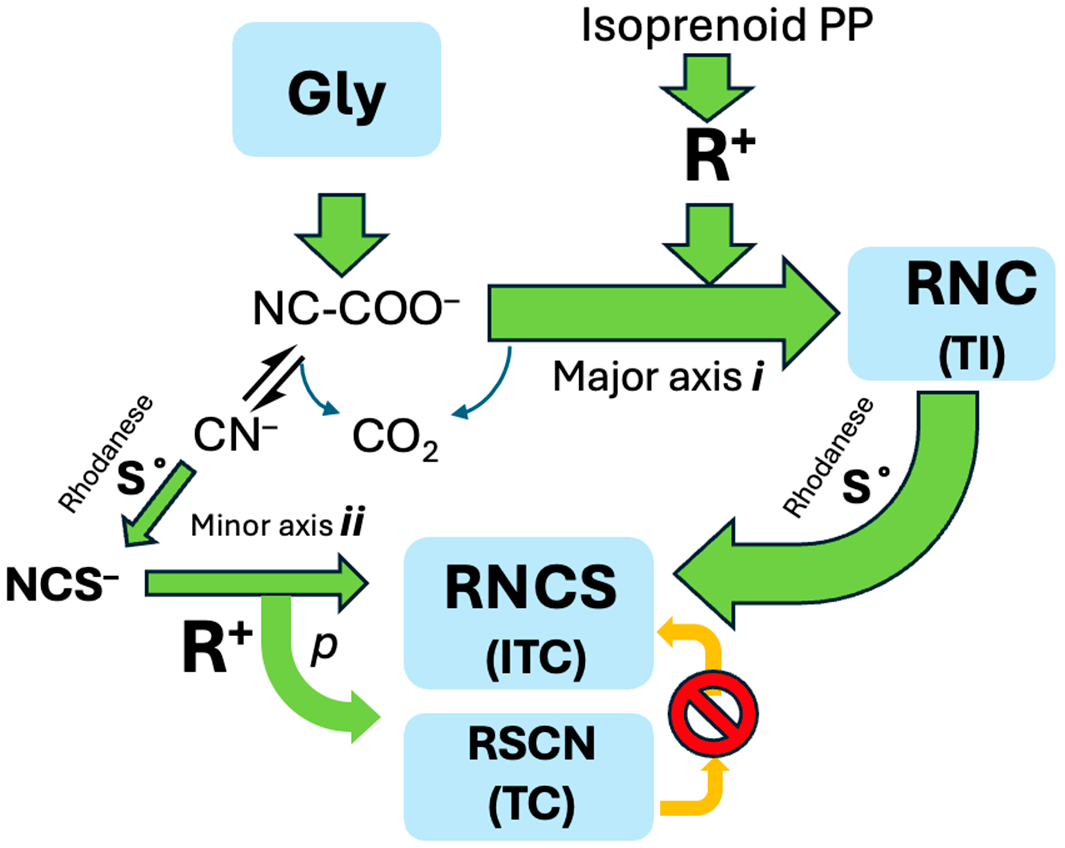
The Enigma of Sponge-Derived Terpenoid Isothiocyanate–Thiocyanate Pairs: A Biosynthetic Proposal
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry and Bonding in TCs and ITCs
2.2. Dissociation–Reassociation
2.3. Adventitious Capture of NCS−
2.4. Alkyl TC from SN Reaction with Kinetically Competent NCS−
2.5. A Simple Proposal—Thiocyanates Can Arise Adventitiously
3. Materials and Methods
General Experimental Procedures
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emsermann, J.; Kauhl, U.; Opatz, T. Marine Isonitriles and Their Related Compounds. Mar. Drugs 2016, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Garson, M.J.; Simpson, J.S. Marine isocyanides and related natural products—Structure, biosynthesis and ecology. Nat. Prod. Rep. 2004, 21, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Chena, T.-Y.; Chen, J.; Tang, Y.; Zhou, J.; Guo, Y.; Chang, W.-C. Current Understanding Toward Isonitrile Group Biosynthesis and Mechanism. Chin. J. Chem. 2021, 39, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T.; Wells, R.J.; Oberhänsli, W.E.; Hawes, G.B. A New Diisocyanide of Novel Ring Structure from a Sponge. J. Am. Chem. Soc. 1976, 98, 4010–4012. [Google Scholar] [CrossRef]
- Chang, W.J.; Patra, A.; Roll, D.M.; Scheuer, P.J.; Matsumoto, G.K.; Clardy, J. Kalihinol-A, a highly functionalized diisocyano diterpenoid antibiotic from a sponge. J. Am. Chem. Soc. 1984, 106, 4644–4646. [Google Scholar] [CrossRef]
- Patra, A.; Chang, C.W.J.; Scheuer, P.J.; Van Duyne, G.D.; Matsumoto, G.K.; Clardy, J. An Unprecedented Triisocyano Diterpenoid Antibiotic from a Sponge. J. Am. Chem. Soc. 1984, 106, 7981–7983. [Google Scholar] [CrossRef]
- Chahine, Z.; Abel, S.; Hollin, T.; Barnes, G.L.; Chung, J.H.; Daub, M.E.; Renard, I.; Choi, J.Y.; Vydyam, P.; Pal, A.; et al. A kalihinol analog disrupts apicoplast function and vesicular trafficking in P. falciparum malaria. Science 2024, 385, 7966–7978. [Google Scholar] [CrossRef]
- Cafieri, F.; Fattorusso, E.; Magno, S.; Santacroce, C.; Sica, D. Isolation and Structure of Axisonitrile-1 and Axisothiocyanate-1. Two Unusual Sesquiterpenoids from the Marine Sponge Axinella cannabina. Tetrahedron 1973, 29, 4259–4262. [Google Scholar] [CrossRef]
- Molinski, T.F. The Paradox of Antimalarial Terpenoid Isonitrile Biosynthesis Explained. Proposal of Cyanoformate as an NC Delivery Vector. J. Nat. Prod. 2025, 88, 205–210. [Google Scholar] [CrossRef]
- Xu, Y.; Li, N.; Jiao, W.-H.; Wang, R.-P.; Peng, Y.; Qi, S.-H.; Song, S.-J.; Chen, W.-S.; Lin, H.-W. Antifouling and cytotoxic constituents from the South China Sea sponge Acanthella cavernosa. Tetrahedron 2012, 68, 2876–2883. [Google Scholar] [CrossRef]
- Wratten, S.J.; Faulkner, D.J. Carbonimidic dichlorides from the marine sponge Pseudaxinyssa pitys. J. Am. Chem. Soc. 1977, 99, 7367–7368. [Google Scholar] [CrossRef] [PubMed]
- Brust, A.; Garson, M.J. Dereplication of complex natural product mixtures by 2D NMR: Isolation of a new carbonimidic dichloride of biosynthetic interest from the tropical marine sponge Stylotella aurantium. ACGC Chem. Res. Commun. 2004, 17, 33–37. [Google Scholar]
- Hagadone, M.R.; Burreson, B.J.; Scheuer, P.J.; Finer, J.S.; Clardy, J. Defense Allomones of the Nudibranch Phyllidia varicosa Lamarck 1801. Helv. Chim. Acta 1979, 62, 2484–2494. [Google Scholar] [CrossRef]
- Marcus, A.H.; Molinski, T.F.; Fahy, E.; Faulkner, D.J.; Xu, C.; Clardy, J. 5-Isothiocyanatopupukeanane from a sponge of the genus Axinyssa. J. Org. Chem. 1989, 54, 5184–5186. [Google Scholar] [CrossRef]
- Karuso, P.; Poiner, A.; Scheuer, P.J. Isocyanoneopupukeanane, a new tricyclic sesquiterpene from a sponge. J. Org. Chem. 1989, 54, 2095–2097. [Google Scholar] [CrossRef]
- Wratten, S.J.; Faulkner, D.J.; Hirotsu, K.; Clardy, J. Diterpenoid Isocyanides from the Marine Sponge Hymeniacidon amphilecta. Tetrahedron Lett. 1978, 19, 4345–4348. [Google Scholar] [CrossRef]
- Molinski, T.F.; Faulkner, D.J.; Van Duyne, G.D.; Clardy, J. Three New Diterpene Isonitriles from a Palauan Sponge of the Genus Halichondria. J. Org. Chem. 1987, 52, 3334–3337. [Google Scholar] [CrossRef]
- Avile, E.; Rodriguez, A.D.; Vincente, J. Two Rare-Class Tricyclic Diterpenes with Antitubercular Activity from the Caribbean Sponge Svenzea flava. Application of Vibrational Circular Dichroism Spectroscopy for Determining Absolute Configuration. J. Org. Chem. 2013, 78, 11294–11301. [Google Scholar] [CrossRef]
- He, H.Y.; Faulkner, D.J.; Shumsky, J.S.; Hong, K.; Clardy, J. A sesquiterpene thiocyanate and three sesquiterpene isothiocyanates from the sponge Trachyopsis aplysinoides. J. Org. Chem. 1989, 54, 2511–2514. [Google Scholar] [CrossRef]
- Fusetani, N.; Wolstenholme, H.J.; Matsunaga, S. Co-occurrence of 9-isocyanopupukeanane and its C-9 epimer in the nudibranch Phyllidia bourguini. Tetrahedron Lett. 1990, 31, 5623–5624. [Google Scholar] [CrossRef]
- Pham, A.T.; Ichiba, T.; Yoshida, W.Y.; Scheuer, P.J.; Uchida, T.; Tanaka, J.; Higa, T. Two marine sesquiterpene thiocyanates. Tetrahedron Lett. 1991, 32, 4843–4846. [Google Scholar] [CrossRef]
- He, H.; Salvá, J.; Caiíalos, R.F.; Faulkner, D.J. Sesquiterpene Thiocyanates and Isothiocyanates from Axinyssa aplysinoides. J. Org. Chem. 1992, 57, 3191–3194. [Google Scholar] [CrossRef]
- Blaževića, I.; Montaut, S.; Burčul, F.; Olsend, C.E.; Burowe, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Winstein, S.; Trifan, D.S. The Structure of the Bicyclo[1,2]2-Heptyl (Norbornyl) Carbonium Ion. J. Am. Chem. Soc. 1949, 71, 2953. [Google Scholar] [CrossRef]
- Fusetani, N.; Wolstenholme, H.J.; Shinoda, K.; Asai, N.; Matsunaga, S.; Onuki, H.; Hirota, H. Two sesquiterpene isocyanides and a sesquiterpene thiocyanate from the marine sponge Acanthella cf. cavernosa and the Nudibranch Phyllidia ocellata. Tetrahedron Lett. 1992, 33, 6823–6826. [Google Scholar] [CrossRef]
- Yasman, Y.; Edrada, R.A.; Wray, V.; Proksch, P. New 9-Thiocyanatopupukeanane Sesquiterpenes from the Nudibranch Phyllidia varicosa and Its Sponge-Prey Axinyssa aculeata. J. Nat. Prod. 2003, 66, 1512–1514. [Google Scholar] [CrossRef]
- Simpson, J.S.; Garson, M.J.; Hooper, J.N.A.; Cline, E.I.; Angerhofer, C.K. Terpene Metabolites from the Tropical Marine Sponge Axinyssa sp. nov. Aust. J. Chem. 1997, 50, 1123–1128. [Google Scholar] [CrossRef]
- Emerson, D.W. The Preparation and Isomerization of Allyl Thiocyanate. J. Chem. Ed. 1971, 48, 81–82. [Google Scholar] [CrossRef]
- Carlson, A.S.; Topczewski, J.J. Allylic azides: Synthesis, reactivity, and the Winstein rearrangement. Org. Biomol. Chem. 2019, 17, 4406–4429. [Google Scholar] [CrossRef]
- Gagneux, A.; Winstein, S.; Young, W.G. Rearrangement of allylic azides. J. Am. Chem. Soc. 1960, 82, 5956–5957. [Google Scholar] [CrossRef]
- Knowles, C.J.; Bunch, A.W. Microbial Cyanide Metabolism. Adv. Microb. Physiol. 1986, 27, 73–111. [Google Scholar] [PubMed]
- Harris, R.E.; Bunch, A.W.; Knowles, C.J. Microbial cyanide and nitrile metabolism. Sci. Prog. 1987, 71, 293–304. [Google Scholar]
- Blumer, C.; Haas, D. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch. Microbiol. 2000, 173, 170–177. [Google Scholar] [CrossRef]
- Hering, C.; von Langermann, J.; Shulz, A. The Elusive Cyanoformate: An Unusual Cyanide Shuttle. Angew. Chem. Int. Ed. 2014, 53, 8282–8284. [Google Scholar] [CrossRef]
- Dose, B.; Niehs, S.P.; Scherlach, K.; Shahda, S.; Flórez, L.; Kaltenpoth, M.; Hertweck, C. Biosynthesis of Sinapigladioside, an Antifungal Isothiocyanate from Burkholderia Symbionts. ChemBioChem 2021, 22, 1920–1924. [Google Scholar] [CrossRef]
- Mlotek, M.D.; Bose, B.; Hertweck, C. Bacterial Isothiocyanate Biosynthesis by Rhodanese-Catalyzed Sulfur Transfer onto Isonitriles. ChemBioChem 2024, 25, e202300732. [Google Scholar] [CrossRef]
- Pearson, R.G.; Sobel, H.; Songstad, J. Nucleophilic Reactivity Constants toward Methyl Iodide and trans-[Pt(py)2Cl2]. J. Am. Chem. Soc. 1968, 90, 319–326. [Google Scholar] [CrossRef]
- Walling, C. An Innocent Bystander Looks at the 2-Norbornyl Cation. Acc. Chem. Res. 1983, 16, 448–454. [Google Scholar] [CrossRef]
- Belluco, U.; Cattalini, U.; Basolo, F.; Pearson, R.G.; Turco, A. Nucleophilic Constants and Substrate Discrimination Factors for Substitution Reactions of Platinum (II) Complexes. J. Am. Chem. Soc. 1965, 87, 241–246. [Google Scholar] [CrossRef]
- Pedemonte, N.; Caci, E.; Sondo, E.; Caputo, A.; Rhoden, K.; Pfeffer, U.; Di Candia, M.; Bandettini, R.; Ravazzolo, R.; Zegarra-Moran, O.; et al. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: Role of pendrin and anion channels. J. Immunol. 2007, 178, 5144–5153. [Google Scholar] [CrossRef]
- Oshiki, M.; Fukushima, T.; Kawano, S.; Kasahara, Y.; Nakagawa, J. Thiocyanate Degradation by a Highly Enriched Culture of the Neutrophilic Halophile Thiohalobacter sp. Strain FOKN1 from Activated Sludge and Genomic Insights into Thiocyanate Metabolism. Microbes Environ. 2019, 34, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Raniga, P.; Garson, M.J. Biosynthesis of dichloroimines in the tropical marine sponge Stylotella aurantium. Tetrahedron Lett. 1997, 38, 7947–7950. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Yu, Z.-G.; Guo, Y.-W. New N-Containing Sesquiterpenes from Hainan Marine Sponge Axinyssa sp. Helv. Chim. Acta 2008, 91, 1553–1558. [Google Scholar] [CrossRef]
- Pala, G.; Mantegani, A.; Coppi, G. Terpene Compounds Mono- and Sesquiterpene Thiocyanates and Isothiocyanates. J. Med. Chem. 1969, 12, 725–726. [Google Scholar] [CrossRef]
- Karuso, P.; Scheuer, P.J. Long-chain α,ω-bisisothiocyanates from a marine sponge. Tetrahedron Lett. 1987, 28, 4633–4636. [Google Scholar] [CrossRef]
- Pawlik, J.R.; Kernan, M.K.; Molinski, T.F.; Harper, M.K.; Faulkner, D.J. Defensive Chemicals of the Spanish Dancer Hexabranchus sanguineus, and its Egg Ribbons: Macrolides Derived from a Sponge Diet. J. Exp. Mar. Biol. Ecol. 1988, 119, 99–109. [Google Scholar] [CrossRef]
- Garson, M.J.; Simpson, J.S.; Flowers, A.E.; Dumdei, E.J. Cyanide and thiocyanate-derived functionality in marine organisms—Structures, biosynthesis and ecology. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 21, Part B; pp. 329–372. [Google Scholar]
- Cimino, C.; De Rosa, S.; De Stefano, S.; Sodano, G. Marine natural products: New results from Mediterranean invertebrates. Pure Appl. Chem. 1986, 58, 375–386. [Google Scholar] [CrossRef]
- Garson, M.J. Biosynthesis of the novel diterpene isonitrile diisocyanoadociane by a marine sponge of the Amphimedon genus: Incorporation studies with sodium [14C] cyanide and sodium [2-14C] acetate. J. Chem. Soc. Chem. Commun. 1986, 35–36. [Google Scholar] [CrossRef]
- Fookes, C.J.R.; Garson, M.J.; MacLeod, J.K.; Skelton, B.W.; White, A.H. Biosynthesis of diisocyanoadociane, a novel diterpene from the marine sponge Amphimedon sp. Crystal structure of a monoamide derivative. J. Chem. Soc. Perkin Trans. 1 1988, 1003–1011. [Google Scholar] [CrossRef]
- Dumdei, E.J.; Flowers, A.E.; Garson, M.J.; Moore, C.J. The Biosynthesis of Sesquiterpene Isocyanides and Isothiocyanates in the Marine Sponge Acanthella cavernosa (Dendy); Evidence for Dietary Transfer to the Dorid Nudibranch Phyllidiela pustulosa. Comp. Biochem. Physiol. 1997, 118A, 1385–1392. [Google Scholar] [CrossRef]
- Simpson, J.S.; Garson, M.J. Advanced Precursors in Marine Biosynthetic Study: The Biosynthesis of Diisocyanoadociane in Amphimedon terpenensis. Tetrahedron Lett. 1999, 40, 3909–3912. [Google Scholar] [CrossRef]
- Seto, Y. Oxidative conversion of thiocyanate to cyanide by oxyhemoglobin during acid denaturation. Arch. Biochem. Biophys. 1995, 321, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.S.; Garson, M.J. Thiocyanate Biosynthesis in the Tropical Marine Sponge Axinyssa n. sp. Tetrahedron Lett. 1988, 39, 5819–5822. [Google Scholar] [CrossRef]
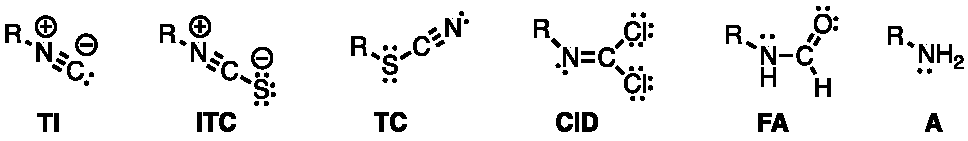




| Entry | Nu: or Nu:− | 103·k2/M−1·s−1 | Entry | Nu: or Nu:− | 103·k2/M−1·s−1 |
|---|---|---|---|---|---|
| 1 | MeOH | 1.3 × 10−7 | 7 | PhO− | 0.073 |
| 2 | NH3 | 0.041 | 8 | NCS− | 0.574 |
| 3 | N3− | 0.078 | 9 | NC− | 0.645 |
| 4 | Br− | 0.0798 | 10 | NCSe− | 9.13 |
| 5 | I− | 3.42 | 11 | PhS− | 1070 |
| 6 | (CH3)2S | 0.045 | 12 | S2O32– | 114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinski, T.F. The Enigma of Sponge-Derived Terpenoid Isothiocyanate–Thiocyanate Pairs: A Biosynthetic Proposal. Mar. Drugs 2025, 23, 220. https://doi.org/10.3390/md23050220
Molinski TF. The Enigma of Sponge-Derived Terpenoid Isothiocyanate–Thiocyanate Pairs: A Biosynthetic Proposal. Marine Drugs. 2025; 23(5):220. https://doi.org/10.3390/md23050220
Chicago/Turabian StyleMolinski, Tadeusz F. 2025. "The Enigma of Sponge-Derived Terpenoid Isothiocyanate–Thiocyanate Pairs: A Biosynthetic Proposal" Marine Drugs 23, no. 5: 220. https://doi.org/10.3390/md23050220
APA StyleMolinski, T. F. (2025). The Enigma of Sponge-Derived Terpenoid Isothiocyanate–Thiocyanate Pairs: A Biosynthetic Proposal. Marine Drugs, 23(5), 220. https://doi.org/10.3390/md23050220