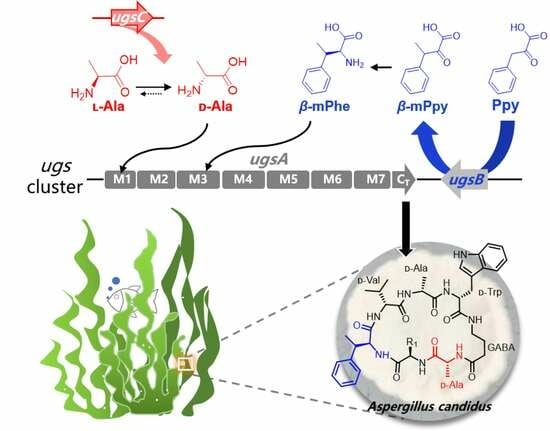
The Isolation, Structural Characterization, and Biosynthetic Pathway of Unguisin from the Marine-Derived Fungus Aspergillus candidus
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation of Unguisins from Aspergillus candidus MEFC1001
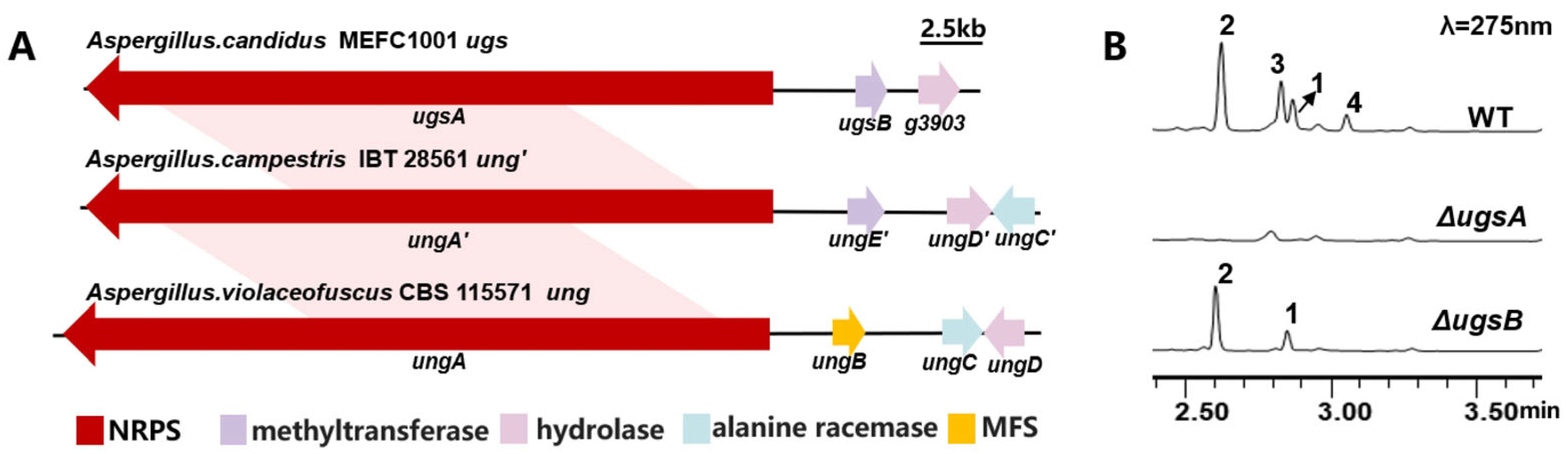
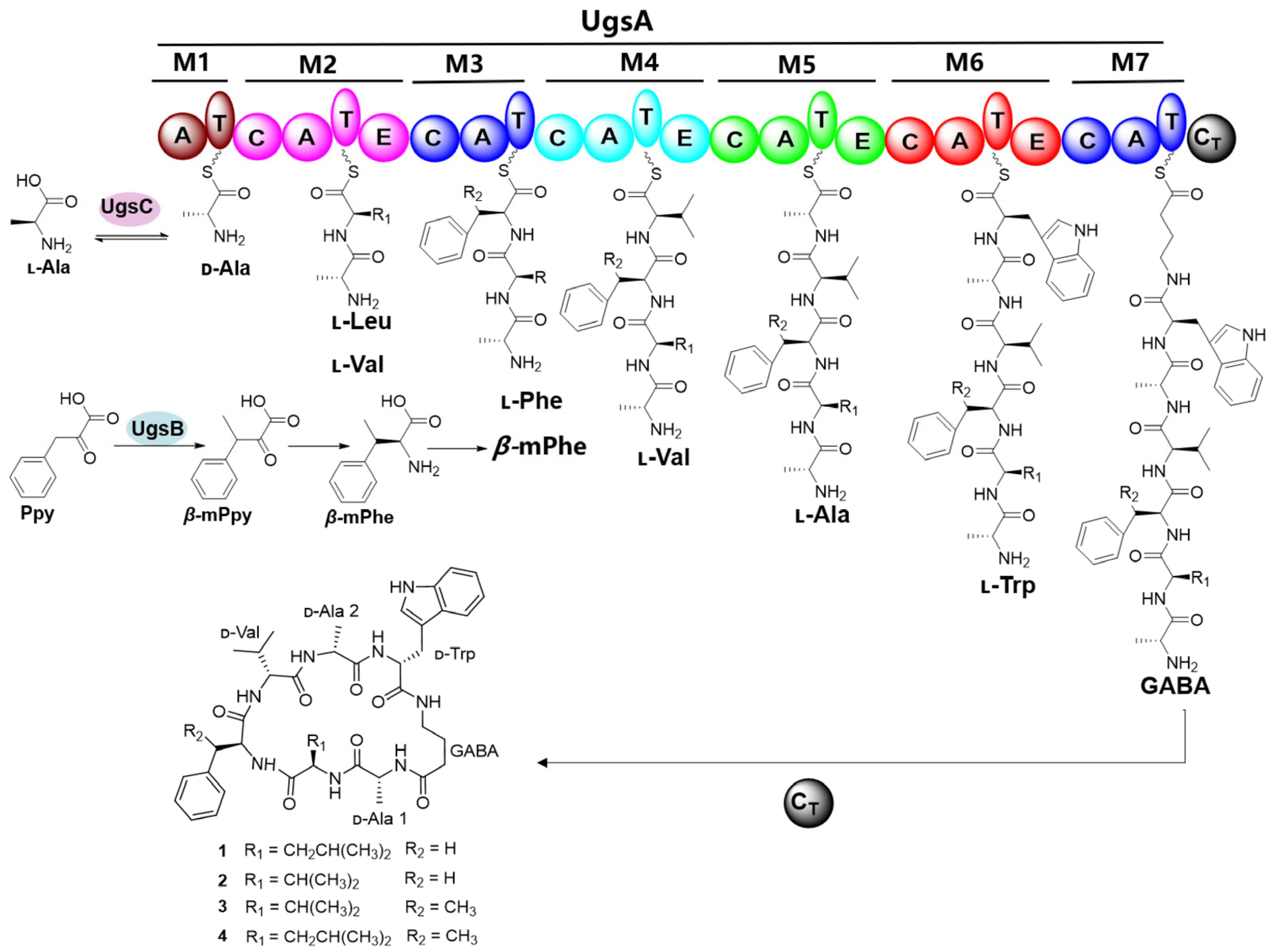
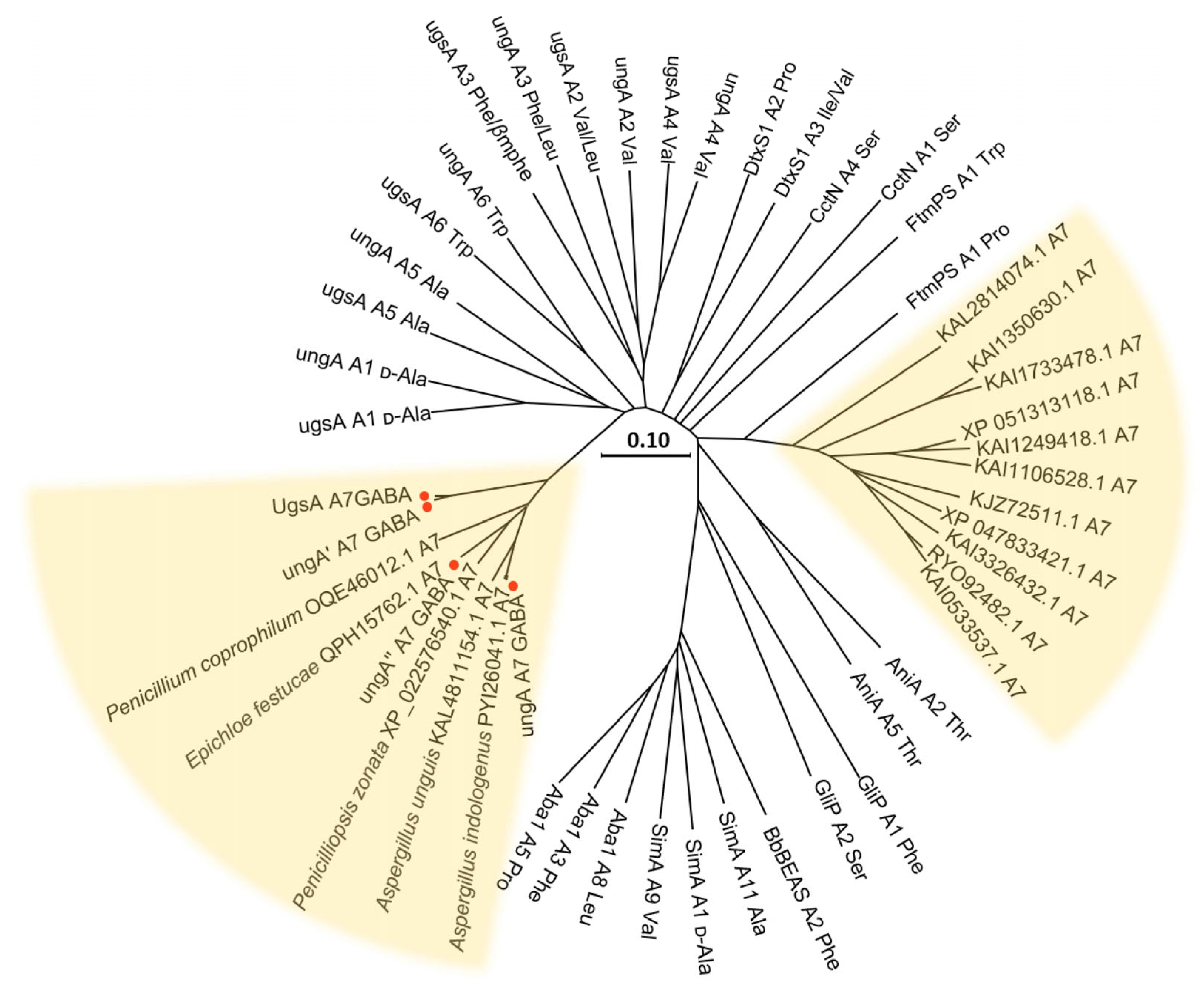
2.2. Identification of the Biosynthetic Gene Cluster of Unguisins
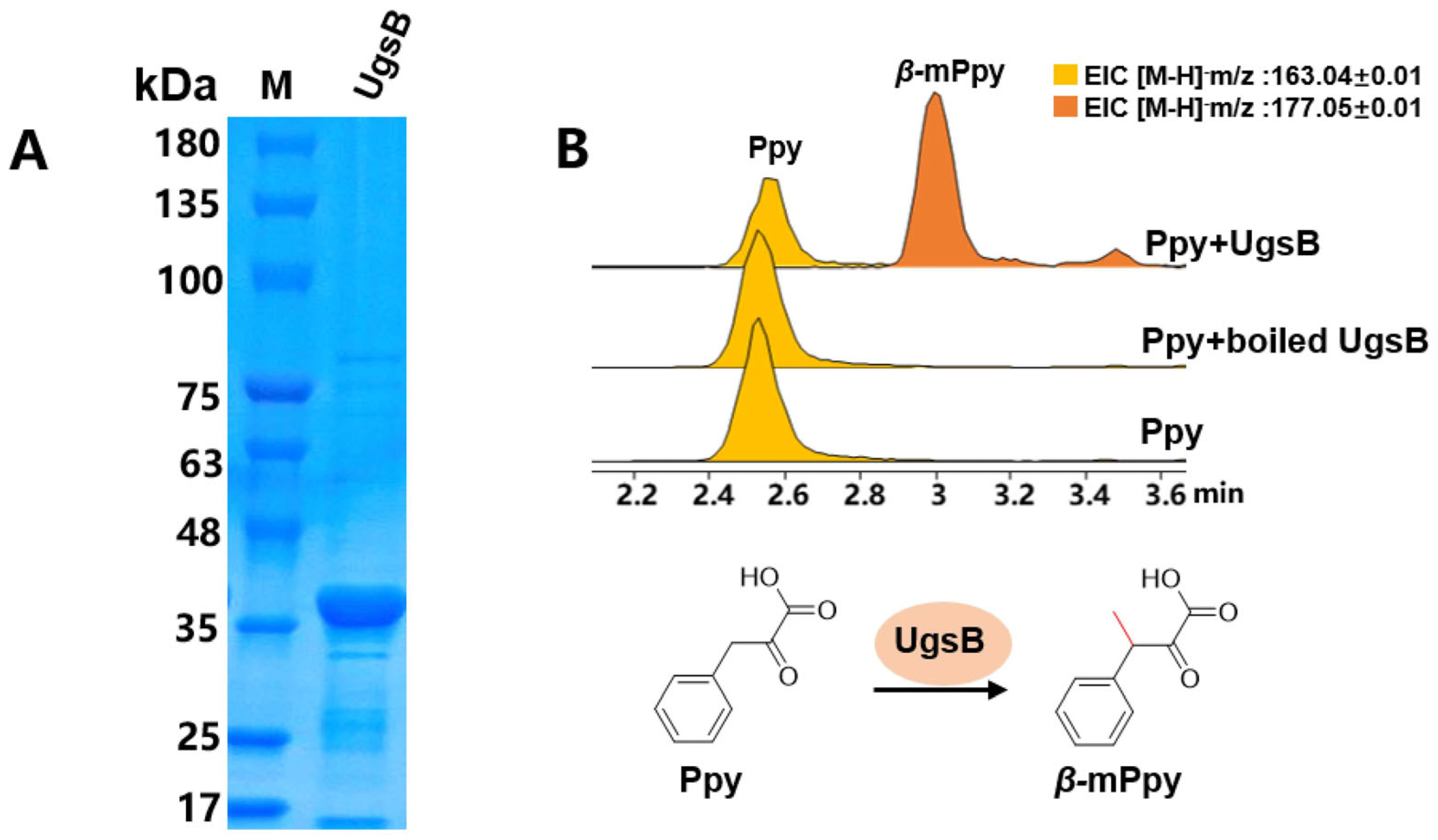
2.3. The β-Carbon Methylation at the Phe Residue Is a Pre-Modification
2.4. The Extra-Clustered Alanine Racemase UgsC Mediates d-Ala Starter Unit Provision
2.5. Proposed Biosynthetic Pathway of Unguisins and Its Biosynthetic Potential in Other Fungi
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Strains, Media, and Growth Conditions
3.3. Isolation and Analysis of Unguisins
3.4. The LC-MS/MS Analysis of 1
3.5. Advanced Marfey’s Method to Determine the Absolute Configurations of Unguisin K (1)
3.6. Gene Deletion Experiments
3.7. Phylogenetic Analysis
3.8. UgsC and UgsB Purification and Enzymatic Reaction Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawant, A.M.; Vamkudoth, K.R. Biosynthetic process and strain improvement approaches for industrial penicillin production. Biotechnol. Lett. 2022, 44, 179–192. [Google Scholar] [CrossRef]
- Xi, Y.; Fan, F.; Zhang, X. Microbial L-malic acid production: History, current progress, and perspectives. Green Carbon 2023, 1, 118–132. [Google Scholar] [CrossRef]
- Yang, H.; Luo, X.W.; Shang, Z.; Li, K.L.; Cai, J.; Chen, Y.Y.; Xin, L.C.; Ju, J.H. Metabolic blockade-based genome mining of Malbranchea circinata SDU050: Discovery of diverse secondary metabolites. Mar. Drugs 2025, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Huang, X.; Du, Z.; Guo, J.; Wang, M.; Xu, G.; Geng, C.; Zhang, Y.; Wang, Q.; Lu, X. Integration of biological synthesis & chemical catalysis: Bio-based plasticizer trans-aconitates. Green Carbon 2023, 1, 20–32. [Google Scholar]
- Mogi, T.; Kita, K. Gramicidin S and polymyxins: The revival of cationic cyclic peptide antibiotics. Cell Mol. Life Sci. 2009, 66, 3821–3826. [Google Scholar] [CrossRef]
- You, S.P.; McIntyre, G.; Passioura, T. The coming of age of cyclic peptide drugs: An update on discovery technologies. Expert Opin. Drug Discov. 2024, 19, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Giuliano Garisto Donzelli, B.; Krasnoff, S.B.; Sun-Moon, Y.; Churchill, A.C.L.; Gibson, D.M. Genetic basis of destruxin production in the entomopathogen Metarhizium robertsii. Curr. Genet. 2012, 58, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine cyclic peptides: Antimicrobial activity and synthetic strategies. Mar. Drugs 2022, 20, 397. [Google Scholar] [CrossRef]
- Dittmann, J.; Wenger, R.M.; Kleinkauf, H.; Lawen, A. Mechanism of cyclosporine A biosynthesis—Evidence for synthesis via a single linear undecapeptide precursor. J. Biol. Chem. 1994, 269, 2841–2846. [Google Scholar] [CrossRef]
- Emri, T.; Majoros, L.; Tóth, V.; Pócsi, I. Echinocandins: Production and applications. Appl. Microbiol. Biot. 2013, 97, 3267–3284. [Google Scholar] [CrossRef]
- Akone, S.H.; Daletos, G.; Lin, W.; Proksch, P. Unguisin F, a new cyclic peptide from the endophytic fungus Mucor irregularis. Z. Naturforschung C J. Biosci. 2016, 71, 15–19. [Google Scholar] [CrossRef]
- Li, W.; Jiao, F.W.; Wang, J.Q.; Shi, J.; Wang, T.T.; Khan, S.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Unguisin G, a new kynurenine-containing cyclic heptapeptide from the sponge-associated fungus Aspergillus candidus NF2412. Tetrahedron Lett. 2020, 61, 152322. [Google Scholar] [CrossRef]
- Liu, S.S.; Shen, Y.M. A new cyclic peptide from the marine fungal strain Aspergillus sp. AF119. Chem. Nat. Compd. 2011, 47, 786–788. [Google Scholar] [CrossRef]
- Malmstrom, J. Unguisins A and B: New cyclic peptides from the marine-derived fungus Emericella unguis. J. Nat. Prod. 1999, 62, 787–789. [Google Scholar] [CrossRef]
- Malmstrom, J.; Ryager, A.; Anthoni, U.; Nielsen, P.H. Unguisin C, a GABA-containing cyclic peptide from the fungus Emericella unguis. Phytochemistry 2002, 60, 869–872. [Google Scholar] [CrossRef]
- Neupane, S.; de Amorim, M.R.; Skellam, E. Discovery of unguisin J, a new cyclic peptide from Aspergillus heteromorphus CBS 117.55, and phylogeny-based bioinformatic analysis of UngA NRPS domains. Beilstein J. Org. Chem. 2024, 20, 321–330. [Google Scholar] [CrossRef]
- Hunter, L.; Chung, J.H. Total synthesis of unguisin A. J. Org. Chem. 2011, 76, 5502–5505. [Google Scholar] [CrossRef]
- Ariawan, A.D.; Webb, J.E.A.; Howe, E.N.W.; Gale, P.A.; Thordarson, P.; Hunter, L. Cyclic peptide unguisin A is an anion receptor with high affinity for phosphate and pyrophosphate. Org. Biomol. Chem. 2017, 15, 2962–2967. [Google Scholar] [CrossRef]
- Cacho, R.A.; Jiang, W.; Chooi, Y.H.; Walsh, C.T.; Tang, Y. Identification and characterization of the echinocandin B biosynthetic gene cluster from Emericella rugulosa NRRL 11440. J. Am. Chem. Soc. 2012, 134, 16781–16790. [Google Scholar] [CrossRef]
- Men, P.; Geng, C.; Zhang, X.; Zhang, W.; Xie, L.; Feng, D.D.; Du, S.Y.; Wang, M.; Huang, X.N.; Lu, X.F. Biosynthesis mechanism, genome mining and artificial construction of echinocandin O-sulfonation. Metab. Eng. 2022, 74, 160–167. [Google Scholar] [CrossRef]
- Wei, X.X.; Chan, T.K.; Kong, C.T.D.; Matsuda, Y. Biosynthetic characterization, heterologous production, and genomics-guided discovery of GABA-containing fungal heptapeptides. J. Nat. Prod. 2023, 86, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, X.; Feng, D.D.; Liang, Y.J.; Wu, Z.Y.; Du, S.Y.; Zhou, Y.; Geng, C.; Men, P.; Fu, C.X.; et al. Discovery of a unique flavonoid biosynthesis mechanism in fungi by genome mining. Angew. Chem. Int. Edit. 2023, 62, e202215529. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Lyu, S.Y.; Chuang, P.H.; Hsu, N.S.; Li, Y.S.; Chan, H.C.; Huang, C.J.; Liu, Y.C.; Wu, C.J.; Yang, W.B.; et al. In vitro characterization of enzymes involved in the synthesis of nonproteinogenic residue (2,3)-β-methylphenylalanine in glycopeptide antibiotic mannopeptimycin. Chembiochem 2009, 10, 2480–2487. [Google Scholar] [CrossRef] [PubMed]
- Schafhauser, T.; Jahn, L.; Kirchner, N.; Kulik, A.; Flor, L.; Lang, A.; Caradec, T.; Fewer, D.P.; Sivonen, K.; van Berkel, W.J.H.; et al. Antitumor astins originate from the fungal endophyte Cyanodermella asteris living within the medicinal plant Aster tataricus. Proc. Natl. Acad. Sci. USA 2019, 116, 26909–26917. [Google Scholar] [CrossRef]
- Wang, B.; Kang, Q.J.; Lu, Y.Z.; Bai, L.Q.; Wang, C.S. Unveiling the biosynthetic puzzle of destruxins in Metarhizium species. Proc. Natl. Acad. Sci. USA 2012, 109, 1287–1292. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, W.; Zhang, W.; Zhang, X.; Du, S.; Zheng, C.; Huang, X.; Lu, X. The Isolation, Structural Characterization, and Biosynthetic Pathway of Unguisin from the Marine-Derived Fungus Aspergillus candidus. Mar. Drugs 2025, 23, 219. https://doi.org/10.3390/md23050219
Diao W, Zhang W, Zhang X, Du S, Zheng C, Huang X, Lu X. The Isolation, Structural Characterization, and Biosynthetic Pathway of Unguisin from the Marine-Derived Fungus Aspergillus candidus. Marine Drugs. 2025; 23(5):219. https://doi.org/10.3390/md23050219
Chicago/Turabian StyleDiao, Wenjiao, Wei Zhang, Xiaoxi Zhang, Siyu Du, Caijuan Zheng, Xuenian Huang, and Xuefeng Lu. 2025. "The Isolation, Structural Characterization, and Biosynthetic Pathway of Unguisin from the Marine-Derived Fungus Aspergillus candidus" Marine Drugs 23, no. 5: 219. https://doi.org/10.3390/md23050219
APA StyleDiao, W., Zhang, W., Zhang, X., Du, S., Zheng, C., Huang, X., & Lu, X. (2025). The Isolation, Structural Characterization, and Biosynthetic Pathway of Unguisin from the Marine-Derived Fungus Aspergillus candidus. Marine Drugs, 23(5), 219. https://doi.org/10.3390/md23050219