Phycobilins Versatile Pigments with Wide-Ranging Applications: Exploring Their Uses, Biological Activities, Extraction Methods and Future Perspectives
Abstract
1. Introduction
2. Selection Methods
- “phycobiliproteins” OR “phycobilins” to retrieve results that included either term, broadening the search scope.
- “phycobiliproteins OR phycobilins” AND “biological activity” to find articles discussing the bioactivity of these compounds.
- “phycobilins” AND (“extraction methods” OR “purification techniques”) to locate information on technological approaches.
- “phycobiliproteins” NOT “chlorophyll” to exclude unrelated pigment studies.
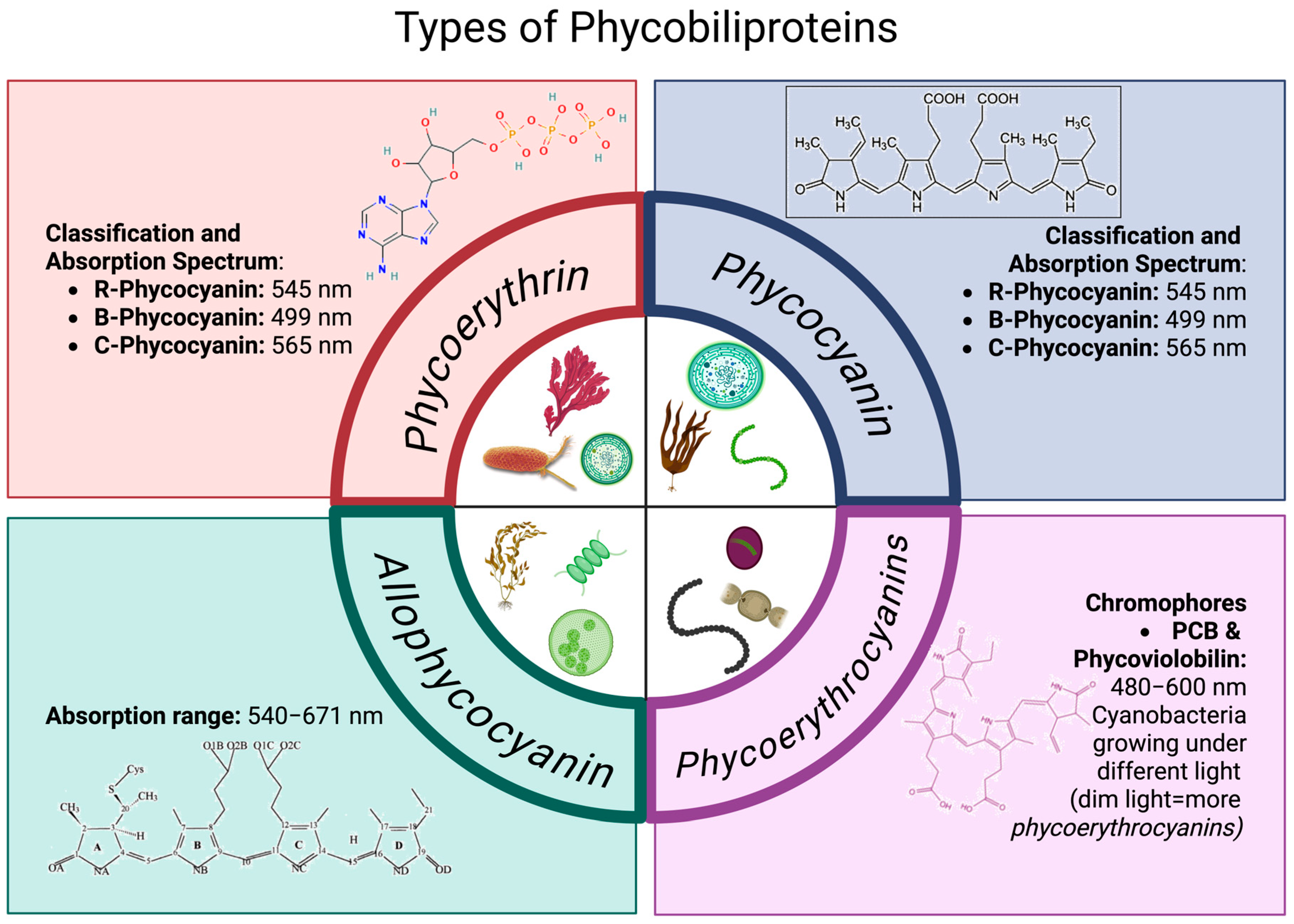
3. Types of Phycobiliproteins
3.1. Phycoerythrin
- R-Phycocyanin (R-PE), with absorbance peaks at 499 and 565 nm and a shoulder at 545 nm.
- B-Phycocyanin (B-PE), with absorbance peaks at 545 and 565 nm and a shoulder at 499 nm.
- C-Phycocyanin (C-PE), with an absorbance peak at 565 nm.
3.2. Phycoerythrocyanins
3.3. Phycocyanin
- R-PC-I: These are the most abundant and are found in red algae. They were the first phycocyanins to be spectroscopically characterized. This protein contains three chromophores of phycocyanobilin (PCB) in its structure: one attached to cysteine 84 of the α chain, and the other two to cysteine 84 and 155 of the β chain. The absorption spectrum of this R-PC shows two peaks of maximum absorption: the first, at less than 555 nm, is attributed to the PEB molecule, and the second, higher peak, associated with PCB molecules, is at 646 nm.
- R-PC-II: This was the first phycocyanin from cyanobacteria in which a PEB molecule was reported. This type of phycocyanin has a PEB molecule attached to cysteine 84 of the α subunit, while the β subunit contains a PCB molecule attached to cysteine 84 and a PEB molecule at cysteine 155. Its absorption spectrum shows three peaks at 533, 545, and 615 nm. Its fluorescence emission is at 646 nm.
- R-PC-III: This phycocyanin contains two molecules of PCB and one of PEB. Unlike the previous R-PCs, the PCB molecules are attached at residues 84 and 153 of the β subunit, while the PEB molecule is attached at residue 84 of the α subunit. Its absorption spectrum shows two peaks of maximum absorption, one at 560 nm and another with maximum intensity at 603 nm. The maximum fluorescence emission of this phycocyanin is at 648 nm.
- R-PC-IV: This phycocyanin differs from the other three types by having a PUB molecule attached at the 84 positions of the α subunit and two PCB molecules at residues 84 and 155 of the β subunit. Its absorption spectrum shows two maximum peaks, one at 490 nm and another at 592 nm. The maximum emission of this protein is at 644 nm.
- R-PC-V: This protein is characterized by having three different types of PUB chromophores at the 84 positions of the α subunit, one PCB molecule at residue 82, and one PEB molecule at residue 153 of the β subunit. Its absorption spectrum shows three maximum peaks: one at 495 nm, one at 540 nm, and one at 590 nm. The fluorescence emission is at 640 nm.
3.4. Allophycocyanin
3.5. Habitat Influence on Phycobiliprotein (PBPs) Production
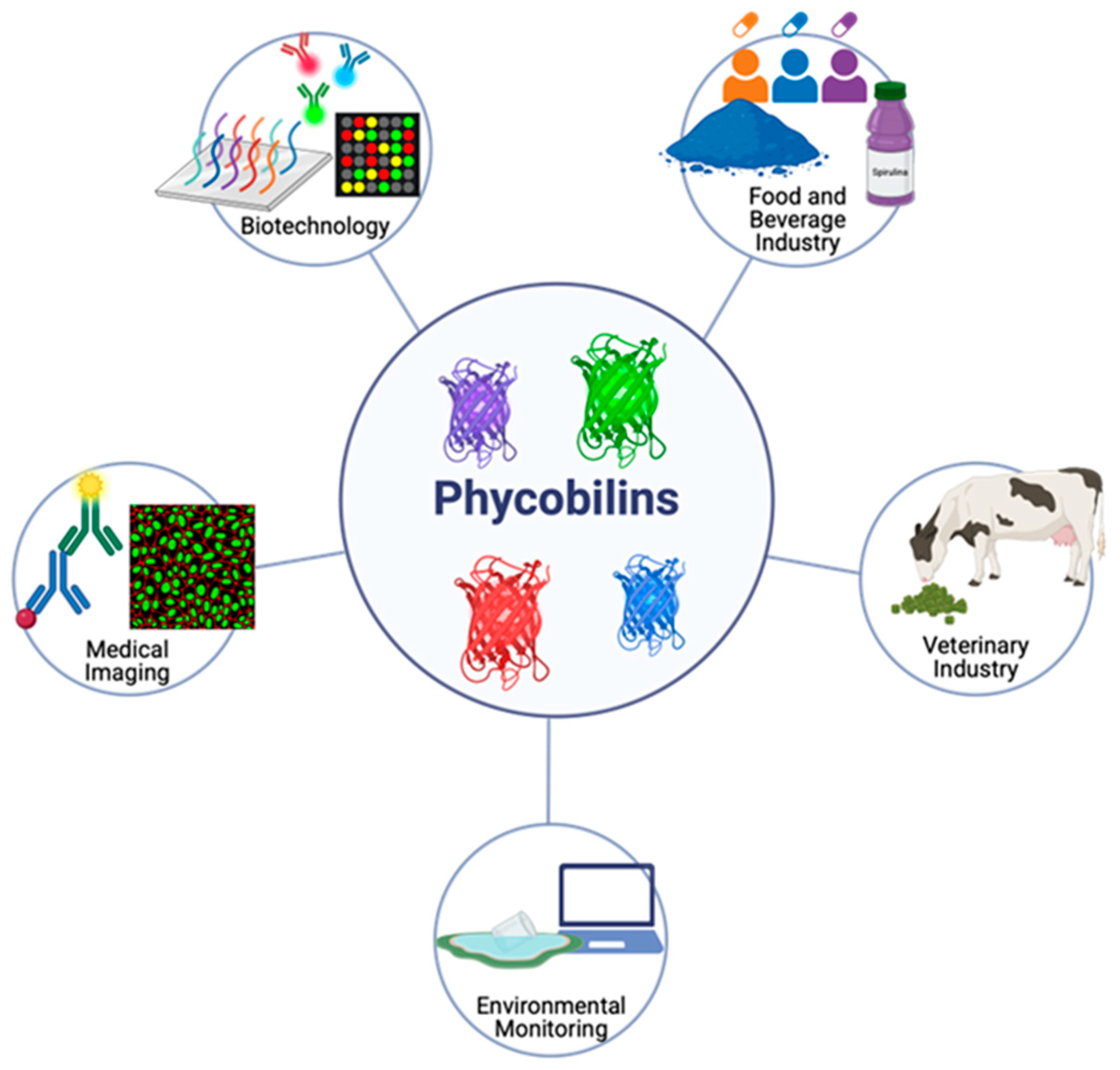
4. Industrial Applications
4.1. Biotechnology
4.2. Food and Beverage Industry
4.3. Medical Imaging
4.4. Environmental Monitoring
4.5. Veterinary Industry
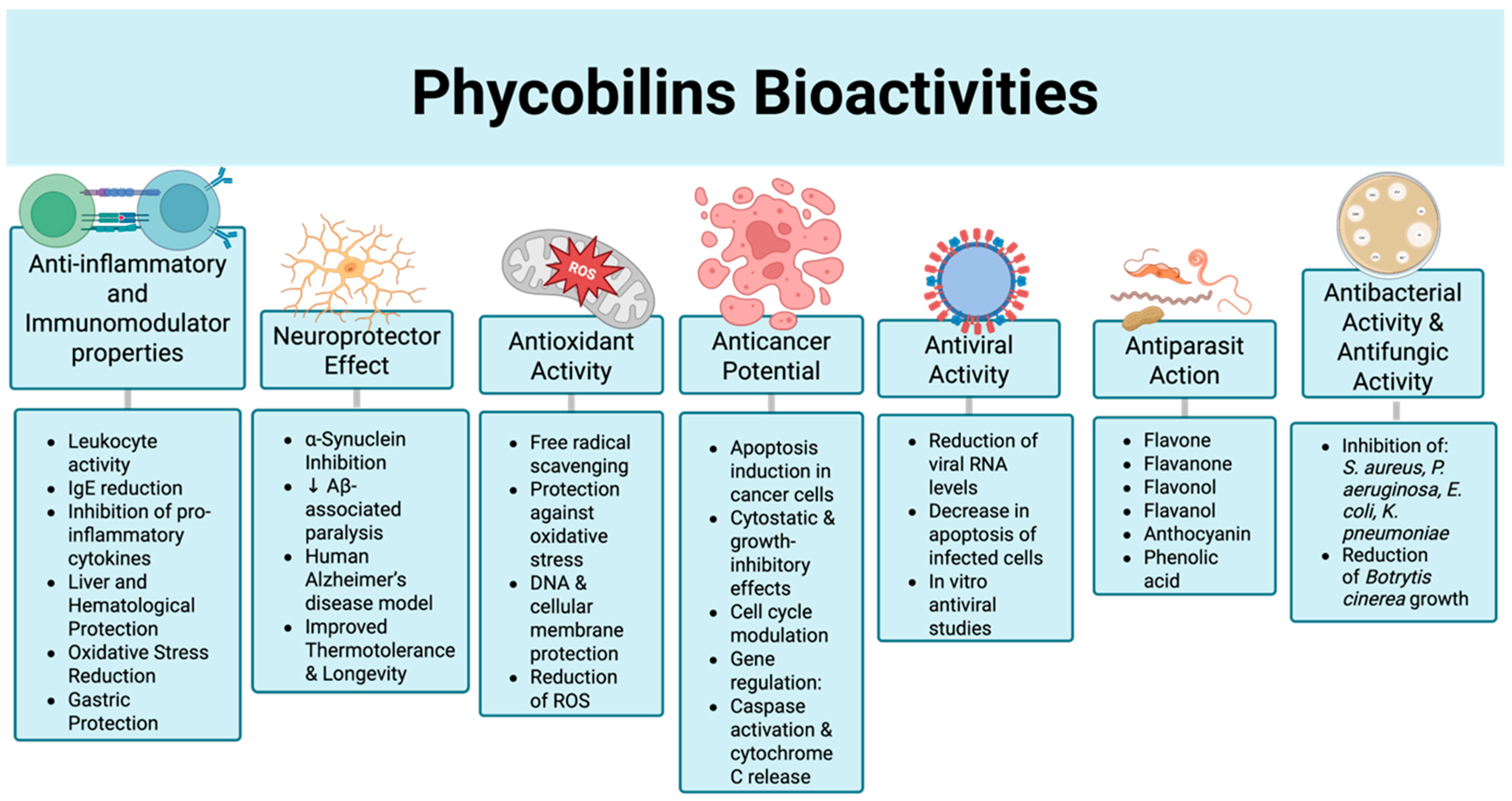
5. Bioactivities
5.1. Therapeutic Agents
5.2. Functional Properties of PBPs
5.2.1. Anti-Inflammatory and Immunomodulator Properties
| Organism | Assay Results | Reference |
|---|---|---|
| Phycocyanin (PC) | ||
| Spirulina platensis | Hepatoprotective effect against carbon tetrachloride (CCl4)-induced toxicity in Wistar rats. A dose-dependent reduction in Alanine Transaminase (ALT) and Aspartate Transaminase (AST) levels was observed at 100, 150, and 200 mg/kg over 28 days. Histological analysis revealed a recovery comparable to the negative control. | [68] |
| S. platensis | The administration of C-PC to Wistar rats at doses of 250 and 500 mg/kg over 28 days did not induce toxicity or cause changes in organ weights or in biochemical or hematological profiles; it did not result in damage to the heart, lungs, liver, stomach, kidneys, uterus or testes. | [69] |
| Phormidium versicolor NCC466 | Treatment with 50 mg/kg of C-PC reduced ALT, AST, and bilirubin levels in a cadmium (Cd)-induced liver injury model in Wistar rats. | [70] |
| N/D | It reduced serum malondialdehyde (MDA) levels and gastric pro-inflammatory markers, including TNF-α, IL-1β, IL-6, ICAM-1, and MPO (myeloperoxidase). | [64] |
| Spirulina sp. | Administration of C-PC to C57BL/6 mice significantly reduced plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Histological analysis confirmed protection against radiation-induced hepatotoxicity. The treatment increased the relative mRNA expression of superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX) while reducing ROS levels in the liver. Furthermore, H2AX expression (a marker of DNA damage) was markedly lower compared to the irradiated group. Also, can inhibit the formation of Aβ40/42 fibrils. | [57,71] |
| S. platensis | C-PC exhibited antidiabetic activity with IC50 values of 231.45 ± 0.47 μg/mL for α-amylase and 198.11 ± 0.25 μg/mL for β-glucosidase. These values were compared to the standard control, acarbose, which showed IC50 values of 151.96 ± 0.57 μg/mL and 141.33 ± 0.34 μg/mL, respectively. Additionally, C-PC demonstrated dose-dependent anti-inflammatory activity, inhibiting proteinase activity by 5, 11, 17, 29, and 36% at concentrations of 100, 200, 300, 400, and 500 μg/mL, respectively. | [72] |
| S. platensis | The administration of C-PC at a dose of 300 mg/kg in albino rats demonstrated a protective effect against arsenic-induced toxicity. | [63] |
| Mycrocystis aeruginosa | C-PC (50 mg/kg) exhibits a therapeutic renal effect against potassium dichromate (PD)-induced kidney injury in Wistar rats. This is achieved through improved renal function, downregulation of oxidative stress and the TLR4/TNF-α/HSP70 inflammatory pathway, as well as modulation of IGF-1. | [73] |
| N/D | C-PC was determined to be non-toxic at a concentration of 2000 mg/kg in Balb/c mice. Serum concentrations of SOD and CAT remained comparable to the vitamin E control at a dose of 200 mg/kg. SOD and CAT levels exhibited a dose-dependent increase at 500 and 1000 mg/kg of C-PC. | [62] |
| N/D | C-PC exhibits neuroprotective effects against chemotherapy-induced cognitive impairment (CICI), commonly known as “chemo brain”. Treatment with 50 mg/kg significantly improved the behavioral deficits of mice treated with doxorubicin (DOX). Additionally, it suppressed DOX-induced neuroinflammation and oxidative stress, mitigated mitochondrial abnormalities, rescued dendritic spine loss, and increased synaptic density in the hippocampus of DOX-treated mice. | [74] |
| N/D | C-PC is not cytotoxic to macrophages (RAW 264.7). It promotes proliferation with a relative survival of 138% at 200 μg/mL. It reduces nitric oxide (NO) levels by 39% and 41.16% at concentrations of 50 and 200 μg/mL, respectively. It exhibits inhibitory effects on TNF-α by 74.32% and 100% at concentrations of 50 and 200 μg/mL. It also inhibits IL-6 by 30.44% and 75.76% at 50 and 200 μg/mL, respectively. At a dose of 30 μg/mL, phycocyanin shows higher inhibition of Collagen I (an indicator of idiopathic pulmonary fibrosis) and promotes cell recovery in the A549 cell line. | [75] |
| P. versicolor NCC466 | The nephroprotective activity against cadmium (Cd) toxicity induced by 35 μg of Cd in HEK 293 cells was evaluated. Cells treated with 25 μg of C-PC increased cell viability by 90%. Antioxidant enzymes catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) in the kidneys of rats treated with C-PC significantly counteracted the prooxidant effect of Cd exposure. | [76] |
| Plectonema sp. | The administration of C-PC in diabetic Wistar rats for 45 days decreased triglyceride (TG) levels, blood glucose, glycated hemoglobin (HbA1c), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), liver and kidney function indices, and increased body weight in diabetic rats. | [67] |
| S. platensis | In a colorectal cancer-associated colitis model induced by azoxymethane (AOM) and dextran sulfate sodium (DSS) in C57BL/6J mice, C-PC improved inflammation induced by AOM/DSS. Treatment with C-PC significantly reduced the number of colorectal tumors and inhibited the proliferation of epithelial cells in CAC mice. | [77] |
| N/D | C-PC significantly alleviates pathological damage in an acute lung injury (ALI) model in C57BL/6 mice induced by seawater (25%) and lipopolysaccharide (5 mg/kg). | [66] |
| Phycoerythrin (PE) | ||
| Colaconema formosanum | Non-toxic to NIH-3T3 embryonic fibroblasts cells, showing viability of 92.1 ± 1.03, 90.0 ± 1.11, 89.2 ± 1.41, and 82.4 ± 1.21% in a dose-dependent manner at 1, 2, 5, and 10 μg/mL, respectively. Exhibited anti-allergic activity by arresting β-hexosaminidase release (82.4% at 20 μg/mL). Promoted type I procollagen synthesis, with 10 μg/mL generating 130.7 ± 4.2% compared to the TGF-β1 control (121.3 ± 4.6%). | [78] |
| Lyngbya sp. A09DM | Reduction in Aβ deposition on C. elegans mutant strain CL4176. | [79] |
| Allophycocyanin (APC) | ||
| Phormidium sp. A09DM | In wild-type C. elegans, treatment with 100 μg/mL of APC extended the lifespan from 16 ± 0.2 days (control) to 20 ± 0.1 days. | [58] |
5.2.2. Antioxidant Activity
| Organism | Assay Results | Reference |
|---|---|---|
| Phycocyanin (PC) | ||
| Arthrospira platensis IFRPD 1182 | The C-PC obtained through lyophilization, and dehydration showed a DPPH activity of 58% and 58.6%, respectively, at a concentration of 0.06 g/mL. | [90] |
| S. platensis | The SC50 activity for DPPH was 104 μg/mL. | [68] |
| S. platensis | The DPPH activity showed a dose-dependent response with 25%, 57%, 76.2%, 97.2%, and 99% inhibition at concentrations of 10, 25, 50, 75, and 100 μg/mL, respectively. | [69] |
| P. versicolor NCC466 | The C-PC showed a superoxide radical (O2−) and hydroxyl radical (OH) scavenging capacity of 87.42% and 88.75%, a nitric oxide (NO) radical antioxidant activity of 84.87%, and an Iron (II) chelation activity of 78.56%. | [70] |
| S. platensis | An SC50-DPPH activity of 18.59, 34.23, and 47.26 mg/mL was reported for a formulation of C-PC and maltodextrin at concentrations of 50, 100, and 150 g/L, respectively, with an SC50 control of 45.21 mg/mL for non-formulated C-PC. | [91] |
| Arthrospira sp. | The gelatin-C-PC formulation showed a dose-dependent Iron (II) chelation activity of 30%, 85%, and 92% for concentrations of 1, 2.5, and 5 mg/mL (C-PC), and a DPPH activity of 46.73%, 60%, 77%, and 78.9% for 0.5, 1, 2.5, and 5 mg/mL, respectively. | [92] |
| Nostoc sp. R76DM | In vitro, 100 μg/mL showed DPPH antioxidant activity of 63.15%, FRAP 0.22%, and reducing power of 0.40%. In vivo, it had a protective effect against oxidative stress of 84.04% in the Caenorhabditis elegans model. | [93] |
| Oscillatoria minima | At a concentration of 1 mg/mL, a DPPH activity of 44% and an ABTS activity of 95% were obtained, which was higher than the positive control Butilhidroxianisol (BHA at 90%). | [94] |
| S. platensis LEB-52 | It exhibited an ABTS antioxidant activity of 161.66 ± 4.64 μmol TE.g−1 and an oxygen radical absorbance capacity (ORAC) of 1211.41 ± 73.65 μmol TE.g−1. | [95] |
| A. platensis | The purified extract showed a DPPH activity of 98% at 25 μg/mL and an Iron (II) chelation activity of 100% at 5 μg/mL. | [96] |
| Galdieria sulphuraria | The ABTS antioxidant activity of the phycocyanins obtained from the algae G. sulphuraria and S. platensis at 1 g/mL was 72.97% and 75.55%, respectively. | [97] |
| N/D | The IC50 of DPPH activity was 158.3 μg/mL (control 112.9 μg/mL), ferric reducing antioxidant power (FRAP) was 152.7 μg/mL (control 91.47 μg/mL), hydroxyl radical scavenging was 88.67 μg/mL (control 57.78 μg/mL), hydrogen peroxide scavenging was 110.9 μg/mL (control 44.63 μg/mL), and total antioxidant capacity (TAC) was 164.78 μg/mL (control 26.76 μg/mL). Ascorbic acid was used as the control. | [98] |
| S. platensis MK343101 | The antioxidant activity against peroxide radicals was 97.7% at 100 μg/mL. | [99] |
| Pseudanabaena sp. ABRG5-3, Limnothrix sp. SK1-2-1 y A. platensis NIES-39 | The C-PC obtained from the three strains showed an ABTS antioxidant activity above 80% at a concentration of 1 mg/mL. | [100] |
| Porphyra sp. | DPPH antioxidant activity showed a dose-dependent effect, with 23.87 ± 1.12% at 5 mg/mL and 59.46 ± 1.23% at 10 mg/mL. Iron (II) chelation activity was 67.10 ± 0.45%, 73.61 ± 0.31%, 86.18 ± 0.30%, and 90.31 ± 0.11% at concentrations of 31.25, 62.50, 125, and 250 μg/mL, respectively. | [101] |
| S. platensis | It exhibited a DPPH antioxidant activity of 94.284% at 300 mg/mL. | [63] |
| S. platensis | An antioxidant activity of 87% was reported for DPPH. | [102] |
| Plectonema sp. | The DPPH antioxidant activity was 58.75%, nitric oxide (NO) radical scavenging activity was 58.4%, and peroxide radical (O2−2) scavenging capacity was 61.5%. These data were obtained at a concentration of 1000 μg/mL. | [103] |
| A. platensis | The ORAC was 12,141 ± 1928 and 32,680 ± 3295 TE/100 g for commercial and isolated C-PC, respectively. | [104] |
| A. platensis | An IC50 value of 629.94 μg/mL for DPPH was obtained. It showed an anti-inflammatory activity of 74.49% and an anti-arthritis activity of 76.98%. | [6] |
| Caquena (CAQ-15) | PC (CAQ-15) showed ABTS and FRAP activities of 312 ± 15 and 1.55 ± 0.10 μmol TE/100 mg, respectively. PC (LLA-10) exhibited ABTS and FRAP activities of 205 ± 41 and 2.50 ± 0.15 μmol TE/100 mg, respectively. | [105] |
| Phycoerythrin (PE) | ||
| Palmaria sp. | An antioxidant activity of 21.3% for DPPH and 90.4% for ABTS was obtained at concentrations of 5 mg/mL and 1 mg/L of PE, respectively. | [106] |
| Spyridia filamentosa | It showed IC50 antioxidant activity in various assays: DPPH of 125.5 μg/mL, nitric oxide of 87.85 μg/mL, hydroxyl radical of 34.56 μg/mL, superoxide radical (O2) of 18.58 μg/mL, and ABTS of 3.13 μg/mL. | [107] |
| Kappaphycus alvarezii | An IC50 DPPH activity of 31.02 μg/mL was reported. | [108] |
| Nostoc sp. | PE exhibited ABTS and FRAP antioxidant activities of 198 ± 45 and 0.92 ± 0.15 μmol TE/100 mg, respectively. | [105] |
| Nostoc sp. A5 | The DPPH antioxidant activity showed an IC50 of 0.038 mg/mL, with ascorbic acid as the control at 0.032 mg/mL. The ABTS activity had an IC50 of 0.02 mg/mL, with butylhydroxytoluene (BHT) as the control at 0.019 mg/mL. The superoxide radical antioxidant activity had an IC50 of 0.057 mg/mL (PE) and 0.042 mg/mL (ascorbic acid). A dose-dependent inhibition of nitric oxide was observed, with inhibition percentages of 79, 84, 91, and 98% for 10, 25, 50, 100, 150, and 250 μL, respectively. | [109] |
| Porphyra sp. | The DPPH antioxidant activity showed a dose-dependent effect, with 30.17 ± 0.95% and 63.06 ± 1.08% inhibition at 5 and 10 mg/mL, respectively. The iron (II) chelation activity was 60.59 ± 0.74%, 71.61 ± 0.52%, 87.63 ± 0.23%, and 91.21 ± 0.11% at 31.25, 62.50, 125, and 250 μg/mL, respectively. | [101] |
| Nostoc sp. | Both strains exhibited antioxidant activity with an IC50 of 0.03 mg/mL for DPPH and 0.04 mg/mL for ABTS. | [110] |
| Gracilaria corticata | Effective activity in antioxidant capacity (264.90 ± 10.20 μg/mL), DPPH antioxidant activity (22.91 ± 1.90% at 0.15 mg/mL), and iron (II) chelation effect (26.06 ± 1.60% at 0.15 mg/mL). | [111] |
| Allophycocyanin (APC) | ||
| Corallina officinalis | An IC50 DPPH activity of 893.39 μg/mL was obtained. It exhibited an anti-inflammatory activity of 74.81% and an anti-arthritis activity of 78.25%. | [6] |
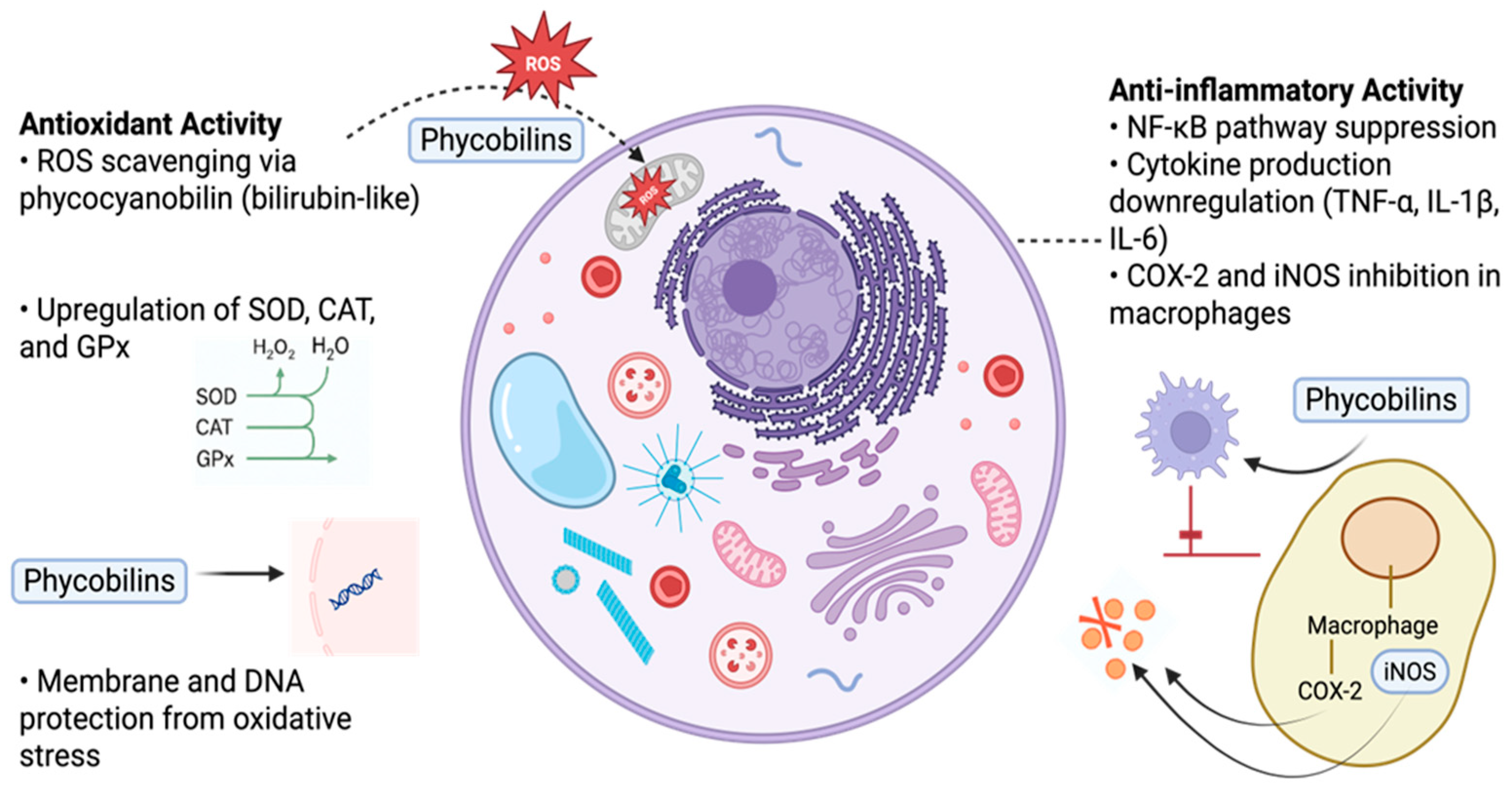
5.2.3. Mechanisms of Antioxidant and Anti-Inflammatory Activity of PBPs
5.3. Anticancer Potential
5.4. Antibacterial Activity & Antifungic Activity
5.5. Antiviral Activity
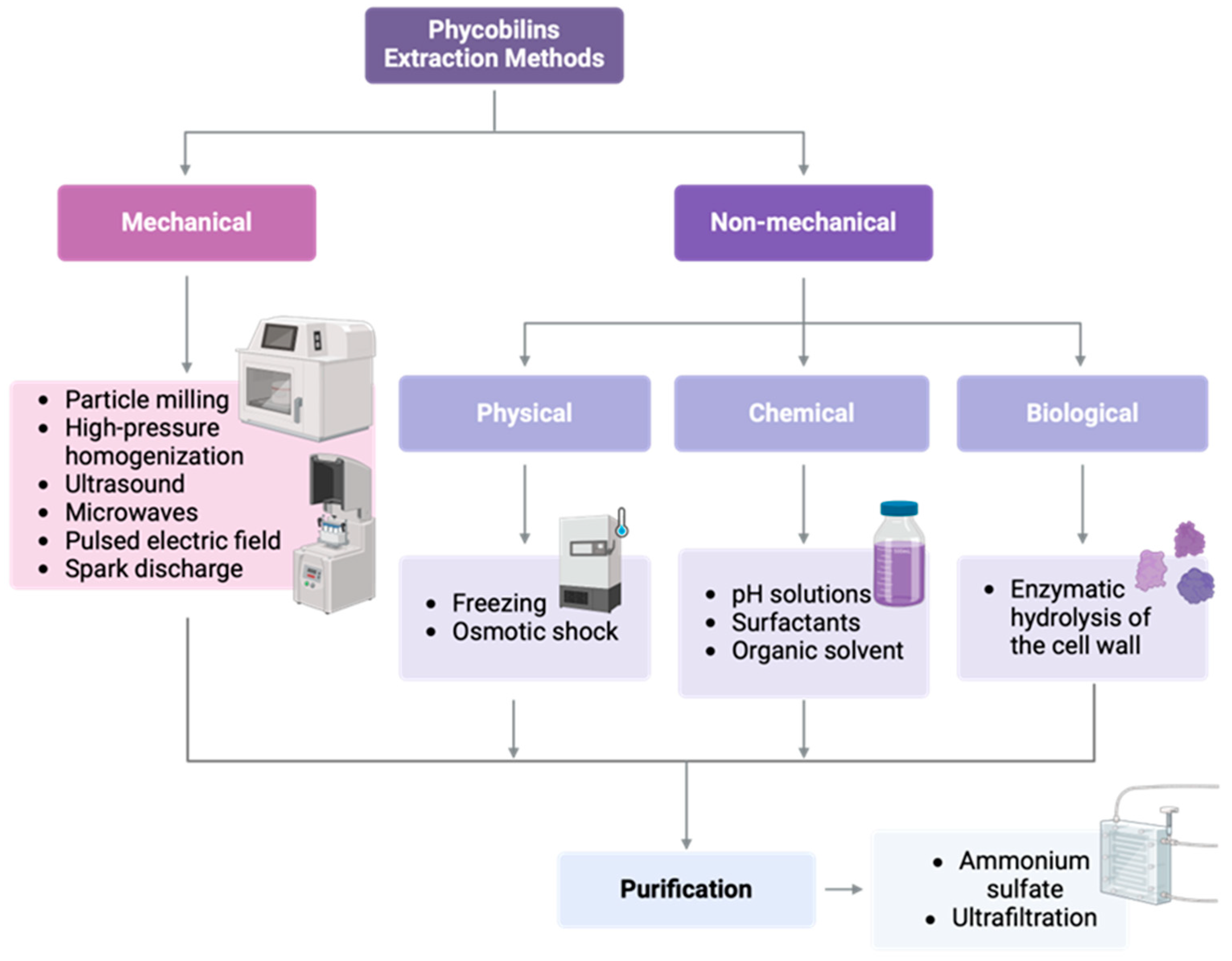
6. Extraction and Purification Methods
6.1. Extraction Techniques of Phycobiliproteins
6.2. Purification Approaches for Phycobiliproteins
6.3. Safety Considerations: Presence of Cyanotoxins in Cyanobacterial Biomass
7. Commercial Products from Phycobiliproteins and Economic Aspects
- Microalgae used in bioremediation for treating wastewater, soil, and capturing CO2 from the atmosphere.
- Production of biofuels and biofertilizers, using both whole biomass and extracts thereof.
- Utilization as ingredients in human and animal nutrition.
- Extraction of high-value products or compounds from microalgae, such as carotenoids, polyunsaturated fatty acids, and phycobiliproteins.
8. Conclusions
9. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Allophycocyanin |
| PBP | Phycobiliprotein |
| PBs | Phycobilins |
| PBSs | Phycobilisomes |
| PC | Phycocyanin |
| PE | Phycoerythrin |
| PEC | Phycoerythrocyanin |
| PEF | Pulsed electrical field |
| IC50 | Half Maximal Inhibitory Concentration |
| SC50 | Half Scavenging Concentration |
| g/mL | Grams per milliliter |
| N/D | Not described. |
| mm | Millimeter |
| mg/mL | Milligram per milliliter |
| UAE | Ultrasonic Assisted Extraction |
| μg/mL | Microgram per milliliter |
| SD | Standard deviation |
References
- Garza-Valverde, E.; Cortez-Guardiola, S.A.; Guzmán-Rodríguez, M.F.; Vidales-Contreras, J.A.; García-Gómez, C. Optimización de Métodos de Extracción de R-Ficoeritrina a Partir de Porphyridium Cruentum. Investig. Y Desarro. En Cienc. Y Tecnol. De Aliment. 2023, 8, 73–83. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from Cyanobacteria: Chemistry and Biotechnological Applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Grossman, A.R.; Schaefer, M.R.; Chiang, G.G.; Collier, J.L. The Phycobilisome, a Light-Harvesting Complex Responsive to Environmental Conditions. Microbiol. Rev. 1993, 57, 725–749. [Google Scholar] [CrossRef] [PubMed]
- Dagnino-Leone, J.; Figueroa, C.P.; Castañeda, M.L.; Youlton, A.D.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pavón Pérez, J.; Agurto-Muñoz, C. Phycobiliproteins: Structural Aspects, Functional Characteristics, and Biotechnological Perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1527. [Google Scholar] [CrossRef]
- Tounsi, L.; Ben Hlima, H.; Hentati, F.; Hentati, O.; Derbel, H.; Michaud, P.; Abdelkafi, S. Microalgae: A Promising Source of Bioactive Phycobiliproteins. Mar. Drugs 2023, 21, 440. [Google Scholar] [CrossRef]
- Ismail, M.M.; El-Fakharany, E.M.; Hegazy, G.E. Purification and Fractionation of Phycobiliproteins from Arthrospira platensis and Corallina officinalis with Evaluating Their Biological Activities. Sci. Rep. 2023, 13, 14270. [Google Scholar] [CrossRef]
- Aguirre Cavazos, D.; Orozco Flores, A.; Ek Ramos, M.; Elizondo Luevano, J. FICOBILINAS: USOS, APLICACIONES Y PERSPECTIVAS. Rev. Planta 2023, 19, 13–20. [Google Scholar]
- Glazer, A.N. Phycobiliproteins—A Family of Valuable, Widely Used Fluorophores. J. Appl. Phycol. 1994, 6, 105–112. [Google Scholar] [CrossRef]
- Chen, H.; Deng, J.; Li, L.; Liu, Z.; Sun, S.; Xiong, P. Recent Progress of Natural and Recombinant Phycobiliproteins as Fluorescent Probes. Mar. Drugs 2023, 21, 572. [Google Scholar] [CrossRef]
- Kovaleski, G.; Kholany, M.; Dias, L.M.S.; Correia, S.F.H.; Ferreira, R.A.S.; Coutinho, J.A.P.; Ventura, S.P.M. Extraction and Purification of Phycobiliproteins from Algae and Their Applications. Front. Chem. 2022, 10, 1065355. [Google Scholar] [CrossRef]
- De, F.; Manuel, J.; Sierra, S.; Emilia, D.; Salmerón, O. Estudio de la Estabilidad de la B-Ficoeritrina Procedente del Alga Roja Porphyridium cruentum en Función de la Temperatura, Para su Utilización Como Colorante Natural. Bachelor’s Thesis, Universidad de Almeria, Almeria, Spain, 2014. [Google Scholar]
- Jacob-Lopes, E.; Queiroz, M.I.; Queiroz, L.; Editors, Z. Pigments from Microalgae Handbook; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Ortiz Bernal, N.V. Obtención de un Extracto Proteínico Rico en B-Ficoeritrina a Partir del Cultivo de la Microalga Roja Porphyridium cruentum. Master’s Thesis, Universidad Autonoma de Sinaloa, Culiacan, Mexico, 2020. [Google Scholar]
- Alfaro-Alfaro, Á.E.; Alpízar-Cambronero, V.; Duarte-Rodríguez, A.I.; Feng-Feng, J.; Rosales-Leiva, C.; Mora-Román, J.J. C-Ficocianinas: Modulación Del Sistema Inmune y Su Posible Aplicación Como Terapia Contra El Cáncer. Rev. Tecnol. En Marcha 2020, 33, 125–139. [Google Scholar] [CrossRef]
- Pandey, V.D.; Pandey, A.; Sharma, V. Biotechnological Applications of Cyanobacterial Phycobiliproteins. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 89–97. [Google Scholar]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P.K. Purification and Characterization of C-Phycocyanin from Cyanobacterial Species of Marine and Freshwater Habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef]
- Glazer, A.N. Adaptive Variations in Phycobilisome Structure. In Advances in Molecular and Cell Biology; Elsevier: Amsterdam, The Netherlands, 1994; Volume: 10, pp. 119–149. [Google Scholar]
- Michalak, I.; Tiwari, R.; Dhawan, M.; Alagawany, M.; Farag, M.R.; Sharun, K.; Emran, T.B.; Dhama, K. Antioxidant Effects of Seaweeds and Their Active Compounds on Animal Health and Production—A Review. Vet. Q. 2022, 42, 48–67. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Kumar, D.; Singh, V.; Sinha, R.P. Advances in Phycobiliproteins Research: Innovations and Commercialization. Innovations and Commercialization. In Natural Bioactive Compounds: Technological Advancements; Academic Press: Cambridge, MA, USA, 2021; pp. 57–81. [Google Scholar] [CrossRef]
- Shukla, P. (Ed.) Frontier Discoveries and Innovations in Interdisciplinary Microbiology; Springer: New Delhi, India, 2016; ISBN 978-81-322-2609-3. [Google Scholar]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a Commodity: Trends in Applied Research, Patents and Commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Pendyala, B.; Patras, A.; Dash, C. Phycobilins as Potent Food Bioactive Broad-Spectrum Inhibitors Against Proteases of SARS-CoV-2 and Other Coronaviruses: A Preliminary Study. Front. Microbiol. 2021, 12, 645713. [Google Scholar] [CrossRef]
- Morya, S.; Kumar Chattu, V.; Khalid, W.; Zubair Khalid, M.; Siddeeg, A. Potential Protein Phycocyanin: An Overview on Its Properties, Extraction, and Utilization. Int. J. Food Prop. 2023, 26, 3160–3176. [Google Scholar] [CrossRef]
- Mysliwa-Kurdziel, B.; Solymosi, K. Phycobilins and Phycobiliproteins Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2017, 17, 1173–1193. [Google Scholar] [CrossRef]
- Campos Assumpção de Amarante, M.; Cavalcante Braga, A.R.; Sala, L.; Juliano Kalil, S. Colour Stability and Antioxidant Activity of C-Phycocyanin-Added Ice Creams after in Vitro Digestion. Food Res. Int. 2020, 137, 109602. [Google Scholar] [CrossRef]
- Zhang, Z.; Cho, S.; Dadmohammadi, Y.; Li, Y.; Abbaspourrad, A. Improvement of the Storage Stability of C-Phycocyanin in Beverages by High-Pressure Processing. Food Hydrocoll. 2021, 110, 106055. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Jagatheesan, A.; Perumal, K.; Arunkumar, K. Methods of Phycobiliprotein Extraction from Gracilaria crassa and Its Applications in Food Colourants. Algal Res. 2015, 8, 115–120. [Google Scholar] [CrossRef]
- Díaz Domínguez, G.; Marsán Suárez, V.; del Valle Pérez, L.O. Principales Propiedades Inmunomoduladoras y Antinflamatorias de La Ficobiliproteína C-Ficocianina. Rev. Cuba. De Hematol. Inmunol. Y Hemoter. 2016, 32, 447–454. [Google Scholar]
- Chen, H.; Qi, H.; Xiong, P. Phycobiliproteins—A Family of Algae-Derived Biliproteins: Productions, Characterization and Pharmaceutical Potentials. Mar. Drugs 2022, 20, 450. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Guedes, A.C. Pigments from Microalgae. In Handbook of Microalgae-Based Processes and Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 465–492. [Google Scholar]
- Pérez-González, R.; Sòria-Perpinyà, X.; Soria, J.M.; Delegido, J.; Urrego, P.; Sendra, M.D.; Ruíz-Verdú, A.; Vicente, E.; Moreno, J. Phycocyanin Monitoring in Some Spanish Water Bodies with Sentinel-2 Imagery. Water 2021, 13, 2866. [Google Scholar] [CrossRef]
- Zamyadi, A.; McQuaid, N.; Prévost, M.; Dorner, S. Monitoring of Potentially Toxic Cyanobacteria Using an Online Multi-Probe in Drinking Water Sources. J. Environ. Monit. 2012, 14, 579–588. [Google Scholar] [CrossRef]
- Qi, H.; Liu, Y.; Qi, X.; Liang, H.; Chen, H.; Jiang, P.; Wang, D. Dietary Recombinant Phycoerythrin Modulates the Gut Microbiota of H22 Tumor-Bearing Mice. Mar. Drugs 2019, 17, 665. [Google Scholar] [CrossRef]
- El-Abd, N.M.; Hamouds, R.A.; Saddiq, A.A.; Al-Shaikh, T.M.; Khusaifan, T.J.; Abou-El-Souod, G. Effect of Dietary Arthrospira platensis Phycocyanin on Broiler Chicken Growth Performance, Physiological Status, Fatty and Amino Acid Profiles. Vet. World 2024, 17, 1098–1107. [Google Scholar] [CrossRef]
- Kang, H.K.; Salim, H.M.; Akter, N.; Kim, D.W.; Kim, J.H.; Bang, H.T.; Kim, M.J.; Na, J.C.; Hwangbo, J.; Choi, H.C.; et al. Effect of Various Forms of Dietary Chlorella Supplementation on Growth Performance, Immune Characteristics, and Intestinal Microflora Population of Broiler Chickens. J. Appl. Poult. Res. 2013, 22, 100–108. [Google Scholar] [CrossRef]
- Hamprakorn, K.; Maneewan, B.; Jantasin, W.; Lani, M.N.; Moonmanee, T.; Panatuk, J. Effect of Extracted Phycocyanin By-Products as a Synbiotic Supplement on the Production Performance and Intestinal Morphology of Broilers. Vet. World 2025, 18, 52–59. [Google Scholar] [CrossRef]
- Omar, A.E.; Al-Khalaifah, H.S.; Osman, A.; Gouda, A.; Shalaby, S.I.; Roushdy, E.M.; Abdo, S.A.; Ali, S.A.; Hassan, A.M.; Amer, S.A. Modulating the Growth, Antioxidant Activity, and Immunoexpression of Proinflammatory Cytokines and Apoptotic Proteins in Broiler Chickens by Adding Dietary Spirulina platensis Phycocyanin. Antioxidants 2022, 11, 991. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Gao, Y.; Liu, H.-R.; Wang, T.; Feng, M.-L.; Xue, F.-R.; Ding, K.; Yang, Q.; Jiang, Z.-Y.; Sun, D.; et al. C-Phycocyanin Improves the Quality of Goat Oocytes after in Vitro Maturation and Vitrification. Theriogenology 2024, 222, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.M. Chlorella and Spirulina Microalgae as Sources of Functional Foods, Nutraceuticals, and Food Supplements; an Overview. MOJ Food Process. Technol. 2018, 6, 144. [Google Scholar] [CrossRef]
- Costa, D.F.A.; Quigley, S.P.; Isherwood, P.; McLennan, S.R.; Poppi, D.P. Supplementation of Cattle Fed Tropical Grasses with Microalgae Increases Microbial Protein Production and Average Daily Gain1. J. Anim. Sci. 2016, 94, 2047–2058. [Google Scholar] [CrossRef]
- Amir, A.; Panjaitan, T.S.S.; Adinata, Y.; Krishna, N.H.; Zubir; Tambunan, R.D.; Al Zahra, W.; Prabowo, S. Microalgae Application in Ruminants Feeds on the Production and Quality of Meat and Milk: A Review. IOP Conf. Ser. Earth Environ. Sci. 2024, 1360, 012011. [Google Scholar] [CrossRef]
- Waheed, D.M.; El-Diasty, M.; Gabr, E.M. Spirulina as an Animal Feed and Its Effect on Animal Health and Productivity. J. Adv. Vet. Res. 2024, 14, 342–344. [Google Scholar]
- Satyaraj, E.; Reynolds, A.; Engler, R.; Labuda, J.; Sun, P. Supplementation of Diets with Spirulina Influences Immune and Gut Function in Dogs. Front. Nutr. 2021, 8, 667072. [Google Scholar] [CrossRef]
- Krishnaveni, R.; Palanivelu, K.; Velavan, S. Effects of Probiotics and Spirulina Supplementation on Haemato-Immunological Function of Catla Catla. Int. J. Res. Fish. Aquac. 2013, 3, 176–181. [Google Scholar]
- Şahan, A.; Taşbozan, O.; Aydin, F.; Özütok, S.; Erbaş, C.; Duman, S.; Uslu, L.; Özcan, F. Determination of Some Haematological and Non-Specific Immune Parameters in Nile Tilapia (Oreochromis niloticus L., 1758) Fed with Spirulina (Spirulina platensis) Added Diets. J. Aquac. Eng. Fish. Res. 2015, 1, 133–139. [Google Scholar] [CrossRef]
- Saddiqa, A.; Faisal, Z.; Akram, N.; Afzaal, M.; Saeed, F.; Ahmed, A.; Almudaihim, A.; Touqeer, M.; Ahmed, F.; Asghar, A.; et al. Algal Pigments: Therapeutic Potential and Food Applications. Food Sci. Nutr. 2024, 12, 6956–6969. [Google Scholar] [CrossRef]
- Hirata, T.; Tanaka, M.; Ooike, M.; Tsunomura, T.; Sakaguchi, M. Antioxidant Activities of Phycocyanobilin Prepared from Spirulina platensis. J. Appl. Phycol. 2000, 12, 435–439. [Google Scholar] [CrossRef]
- Romay, C.; Armesto, J.; Remirez, D.; González, R.; Ledon, N.; García, I. Antioxidant and Anti-Inflammatory Properties of C-Phycocyanin from Blue-Green Algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Li, S.; Wang, J.; Zhao, L.; Yan, Y.; Wu, T.; Zhang, J.; Wang, C. C-Phycocyanin Suppresses the In Vitro Proliferation and Migration of Non-Small-Cell Lung Cancer Cells through Reduction of RIPK1/NF-ΚB Activity. Mar. Drugs 2019, 17, 362. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Yan, Y.; Li, S.; Zhao, L.; Zhang, C.; Liu, L.; Wang, C. The In Vitro Anti-Tumor Activity of Phycocyanin against Non-Small Cell Lung Cancer Cells. Mar. Drugs 2018, 16, 178. [Google Scholar] [CrossRef]
- Strasky, Z.; Zemankova, L.; Nemeckova, I.; Rathouska, J.; Wong, R.J.; Muchova, L.; Subhanova, I.; Vanikova, J.; Vanova, K.; Vitek, L.; et al. Spirulina Platensis and Phycocyanobilin Activate Atheroprotective Heme Oxygenase-1: A Possible Implication for Atherogenesis. Food Funct. 2013, 4, 1586. [Google Scholar] [CrossRef]
- McCarty, M. Supplementation with Phycocyanobilin, Citrulline, Taurine, and Supranutritional Doses of Folic Acid and Biotin—Potential for Preventing or Slowing the Progression of Diabetic Complications. Healthcare 2017, 5, 15. [Google Scholar] [CrossRef]
- Soni, B.; Visavadiya, N.P.; Madamwar, D. Attenuation of Diabetic Complications by C-Phycoerythrin in Rats: Antioxidant Activity of C-Phycoerythrin Including Copper-Induced Lipoprotein and Serum Oxidation. Br. J. Nutr. 2009, 102, 102–109. [Google Scholar] [CrossRef]
- Benedetti, S.; Benvenuti, F.; Scoglio, S.; Canestrari, F. Oxygen Radical Absorbance Capacity of Phycocyanin and Phycocyanobilin from the Food Supplement Aphanizomenon flos-Aquae. J. Med. Food 2010, 13, 223–227. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Marín-Prida, J.; McCarty, M.F. C-Phycocyanin-Derived Phycocyanobilin as a Potential Nutraceutical Approach for Major Neurodegenerative Disorders and COVID-19- Induced Damage to the Nervous System. Curr. Neuropharmacol. 2021, 19, 2250–2275. [Google Scholar] [CrossRef]
- Shang, M.-H.; Sun, J.-F.; Bi, Y.; Xu, X.-T.; Zang, X.-N. Fluorescence and Antioxidant Activity of Heterologous Expression of Phycocyanin and Allophycocyanin from Arthrospira platensis. Front. Nutr. 2023, 10, 1127422. [Google Scholar] [CrossRef]
- Liu, Y.; Jovcevski, B.; Pukala, T.L. C-Phycocyanin from Spirulina Inhibits α-Synuclein and Amyloid-β Fibril Formation but Not Amorphous Aggregation. J. Nat. Prod. 2019, 82, 66–73. [Google Scholar] [CrossRef]
- Chaubey, M.G.; Patel, S.N.; Rastogi, R.P.; Madamwar, D.; Singh, N.K. Cyanobacterial Pigment Protein Allophycocyanin Exhibits Longevity and Reduces Aβ-Mediated Paralysis in C. Elegans: Complicity of FOXO and NRF2 Ortholog DAF-16 and SKN-1. 3 Biotech. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nemoto-Kawamura, C.; Hirahashi, T.; Nagai, T.; Yamada, H.; Katoh, T.; Hayashi, O. Phycocyanin Enhances Secretary IgA Antibody Response and Suppresses Allergic IgE Antibody Response in Mice Immunized with Antigen-Entrapped Biodegradable Microparticles. J. Nutr. Sci. Vitaminol. 2004, 50, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Pentón-Rol, G.; Martínez-Sánchez, G.; Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Acosta-Medina, E.F.; Falcón-Cama, V.; Alonso-Ramírez, R.; Valenzuela-Silva, C.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; et al. C-Phycocyanin Ameliorates Experimental Autoimmune Encephalomyelitis and Induces Regulatory T Cells. Int. Immunopharmacol. 2011, 11, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Yang, T.-S.; Chen, M.-J.; Chang, Y.-C.; Wang, E.I.-C.; Ho, C.-L.; Lai, Y.-J.; Yu, C.-C.; Chou, J.-C.; Chao, L.K.-P.; et al. Purification and Immunomodulating Activity of C-Phycocyanin from Spirulina platensis Cultured Using Power Plant Flue Gas. Process Biochem. 2014, 49, 1337–1344. [Google Scholar] [CrossRef]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Narayan Bhatt, A.; Kumar Nishad, D.; Purkayastha, J. C-Phycocyanin-a Novel Protein from Spirulina platensis—In Vivo Toxicity, Antioxidant and Immunomodulatory Studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef]
- Al-Yasiri, T.; Alchalabi, S.M.M. Use of C-Phycocyanin to Reduce the Toxicity of Arsenic on Rats. Nat. Volatiles Essent. Oils 2021, 8, 4460–4471. [Google Scholar]
- Lian, Y.Z.; Lin, I.H.; Yang, Y.-C.; Chao, J.C.J. Gastroprotective Effect of Lycium barbarum Polysaccharides and C-Phyocyanin in Rats with Ethanol-Induced Gastric Ulcer. Int. J. Biol. Macromol. 2020, 165, 1519–1528. [Google Scholar] [CrossRef]
- Li, B.; Gao, M.; Zhang, X.; Chu, X. Molecular Immune Mechanism of C-phycocyanin from Spirulina platensis Induces Apoptosis in HeLa Cells in Vitro. Biotechnol. Appl. Biochem. 2006, 43, 155–164. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, D.; Huang, J.; Wang, Q.; Shao, L. The Therapeutic Effect and the Possible Mechanism of C-Phycocyanin in Lipopolysaccharide and Seawater-Induced Acute Lung Injury. Drug Des. Devel Ther. 2022, 16, 1025–1040. [Google Scholar] [CrossRef]
- Husain, A.; Alouffi, S.; Khanam, A.; Akasha, R.; Farooqui, A.; Ahmad, S. Therapeutic Efficacy of Natural Product ‘C-Phycocyanin’ in Alleviating Streptozotocin-Induced Diabetes via the Inhibition of Glycation Reaction in Rats. Int. J. Mol. Sci. 2022, 23, 14235. [Google Scholar] [CrossRef]
- Osman, A.; Abd-Elaziz, S.; Salama, A.; Eita, A.A.; Sitohy, M. Health Protective Actions of Phycocyanin Obtained from an Egyptian Isolate of Spirulina platensis on Albino Rats. Eurasian J. Biosci. 2019, 13, 105–112. [Google Scholar]
- Namasivayam, S.K.R.; Shivaramakrishnan, K.; Bharani, R.S.A. Potential Antioxidative Protein-Pigment Complex Spirulina platensis Mediated Food Grade Phycocyanin C-Extraction, Purification, Antioxidative Activity and Biocompatibility. Indian. J. Biochem. Biophys. 2019, 56, 230–239. [Google Scholar]
- Gammoudi, S.; Athmouni, K.; Nasri, A.; Diwani, N.; Grati, I.; Belhaj, D.; Bouaziz-Ketata, H.; Fki, L.; El Feki, A.; Ayadi, H. Optimization, Isolation, Characterization and Hepatoprotective Effect of a Novel Pigment-Protein Complex (Phycocyanin) Producing Microalga: Phormidium versicolor NCC-466 Using Response Surface Methodology. Int. J. Biol. Macromol. 2019, 137, 647–656. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Qin, S. Therapeutic Effect of Phycocyanin on Acute Liver Oxidative Damage Caused by X-Ray. Biomed. Pharmacother. 2020, 130, 110553. [Google Scholar] [CrossRef]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and Characterization of Phycocyanin from Spirulina platensis and Evaluation of Its Anticancer, Antidiabetic and Antiinflammatory Effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef]
- Salama, A.; Hussein, R.A.; Mettwally, W.S.A.; Helmy, M.S.; Ali, G.H. C-Phycocyanin Isolated from Microcystis Aeruginosa Kützing Mitigates Renal Injury Induced by Potassium Dichromate via Toll-like Receptor-4 down Regulation in Rats. Egypt. J. Chem. 2021, 64, 3439–3450. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Wang, L.; Pan, S.; Liu, Y.; Li, S.; Wang, D. C-Phycocyanin Mitigates Cognitive Impairment in Doxorubicin-Induced Chemobrain: Impact on Neuroinflammation, Oxidative Stress, and Brain Mitochondrial and Synaptic Alterations. Neurochem. Res. 2021, 46, 149–158. [Google Scholar] [CrossRef]
- Liu, R.-Z.; Li, W.-J.; Zhang, J.-J.; Liu, Z.-Y.; Li, Y.; Liu, C.; Qin, S. The Inhibitory Effect of Phycocyanin Peptide on Pulmonary Fibrosis in Vitro. Mar. Drugs 2022, 20, 696. [Google Scholar] [CrossRef]
- Guermazi, W.; Athmouni, K.; Diwani, N.; Bidhi, M.; Aloulou, A.; Ayadi, H.; Gammoudi, S. The Potential Protective Effect of C-Phycocyanin from New Extremophile Strain Phormidium Versicolor NCC-466 against Cadmium-Induced Nephrotoxicity in HEK293 Cells and Rats Kidney. Res. Sq. 2022; Preprint. [Google Scholar] [CrossRef]
- Pan, D.; Huang, B.; Gan, Y.; Gao, C.; Liu, Y.; Tang, Z. Phycocyanin Ameliorates Colitis-Associated Colorectal Cancer by Regulating the Gut Microbiota and the IL-17 Signaling Pathway. Mar. Drugs 2022, 20, 260. [Google Scholar] [CrossRef]
- Lee, P.-T.; Yeh, H.-Y.; Lung, W.-Q.-C.; Huang, J.; Chen, Y.J.; Chen, B.; Nan, F.H.; Lee, M.C. R-Phycoerythrin from Colaconema formosanum (Rhodophyta), an Anti-Allergic and Collagen Promoting Material for Cosmeceuticals. Appl. Sci. 2021, 11, 9425. [Google Scholar] [CrossRef]
- Chaubey, M.G.; Patel, S.N.; Rastogi, R.P.; Srivastava, P.L.; Singh, A.K.; Madamwar, D.; Singh, N.K. Therapeutic Potential of Cyanobacterial Pigment Protein Phycoerythrin: In Silico and in Vitro Study of BACE1 Interaction and in Vivo Aβ Reduction. Int. J. Biol. Macromol. 2019, 134, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Choi, Y.H.; Nam, T. Identification and Antioxidant Activity of Synthetic Peptides from Phycobiliproteins of Pyropia yezoensis. Int. J. Mol. Med. 2018, 42, 789–798. [Google Scholar] [CrossRef]
- Thangam, R.; Suresh, V.; Asenath Princy, W.; Rajkumar, M.; Senthilkumar, N.; Gunasekaran, P.; Rengasamy, R.; Anbazhagan, C.; Kaveri, K.; Kannan, S. C-Phycocyanin from Oscillatoria tenuis Exhibited an Antioxidant and in Vitro Antiproliferative Activity through Induction of Apoptosis and G0/G1 Cell Cycle Arrest. Food Chem. 2013, 140, 262–272. [Google Scholar] [CrossRef]
- Li, Y. The Bioactivities of Phycocyanobilin from Spirulina. J. Immunol. Res. 2022, 2022, 4008991. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Zhao, J.; Jiang, P. Fusion Proteins of Streptavidin and Allophycocyanin Alpha Subunit for Immunofluorescence Assay. Biochem. Eng. J. 2017, 125, 97–103. [Google Scholar] [CrossRef]
- Romay, C.; Ledón, N.; González, R. Further Studies on Anti-Inflammatory Activity of Phycocyanin in Some Animal Models of Inflammation. Inflamm. Res. 1998, 47, 334–338. [Google Scholar] [CrossRef]
- Liu, J.; Bai, X.; Fu, P. In Silico and in Vitro Assessment of Bioactive Peptides from Arthrospira platensis Phycobiliproteins for DPP-IV Inhibitory Activity, ACE Inhibitory Activity, and Antioxidant Activity. J. Appl. Phycol. 2022, 34, 1497–1511. [Google Scholar] [CrossRef]
- Sonani, R.R.; Singh, N.K.; Kumar, J.; Thakar, D.; Madamwar, D. Concurrent Purification and Antioxidant Activity of Phycobiliproteins from Lyngbya Sp. A09DM: An Antioxidant and Anti-Aging Potential of Phycoerythrin in Caenorhabditis elegans. Process Biochem. 2014, 49, 1757–1766. [Google Scholar] [CrossRef]
- Benedetti, S.; Benvenuti, F.; Pagliarani, S.; Francogli, S.; Scoglio, S.; Canestrari, F. Antioxidant Properties of a Novel Phycocyanin Extract from the Blue-Green Alga Aphanizomenon flos-Aquae. Life Sci. 2004, 75, 2353–2362. [Google Scholar] [CrossRef]
- Madhyastha, H.K.; Sivashankari, S.; Vatsala, T.M. C-Phycocyanin from Spirulina Fussiformis Exposed to Blue Light Demonstrates Higher Efficacy of in Vitro Antioxidant Activity. Biochem. Eng. J. 2009, 43, 221–224. [Google Scholar] [CrossRef]
- Li, W.; Su, H.N.; Pu, Y.; Chen, J.; Liu, L.N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular Structure, Production, Applications, and Prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Pan-utai, W.; Iamtham, S. Extraction, Purification and Antioxidant Activity of Phycobiliprotein from Arthrospira platensis. Process Biochem. 2019, 82, 189–198. [Google Scholar] [CrossRef]
- Agustina, S.; Aidha, N.N.; Oktarina, E. Effect of Maltodextrin Concentration on the Characteristic of Phycocyanin Powder as a Functional Food. AIP Conf. Proc. 2019, 2175, 1–9. [Google Scholar] [CrossRef]
- Chentir, I.; Kchaou, H.; Hamdi, M.; Jridi, M.; Li, S.; Doumandji, A.; Nasri, M. Biofunctional Gelatin-Based Films Incorporated with Food Grade Phycocyanin Extracted from the Saharian Cyanobacterium Arthrospira Sp. Food Hydrocoll. 2019, 89, 715–725. [Google Scholar] [CrossRef]
- Sonani, R.R.; Rastogi, R.P.; Patel, S.N.; Chaubey, M.G.; Singh, N.K.; Gupta, G.D.; Kumar, V.; Madamwar, D. Phylogenetic and Crystallographic Analysis of Nostoc Phycocyanin Having Blue-Shifted Spectral Properties. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Venugopal, V.C.; Thakur, A.; Chennabasappa, L.K.; Mishra, G.; Singh, K.; Rathee, P.; Ranjan, A. Phycocyanin Extracted from Oscillatoria Minima Shows Antimicrobial, Algicidal, and Antiradical Activities: In Silico and in Vitro Analysis. Antiinflamm. Antiallergy Agents Med. Chem. 2020, 19, 240–253. [Google Scholar] [CrossRef]
- de Amarante, M.C.A.; Braga, A.R.C.; Sala, L.; Moraes, C.C.; Kalil, S.J. Design Strategies for C-Phycocyanin Purification: Process Influence on Purity Grade. Sep. Purif. Technol. 2020, 252, 117453. [Google Scholar] [CrossRef]
- Viana Carlos, T.A.; dos Santos Pires Cavalcante, K.M.; de Cássia Evangelista de Oliveira, F.; do Ó Pessoa, C.; Sant’Ana, H.B.; Feitosa, F.X.; Rocha, M.V.P. Pressurized Extraction of Phycobiliproteins from Arthrospira platensis and Evaluation of Its Effect on Antioxidant and Anticancer Activities of These Biomolecules. J. Appl. Phycol. 2021, 33, 929–938. [Google Scholar] [CrossRef]
- Wan, M.; Zhao, H.; Guo, J.; Yan, L.; Zhang, D.; Bai, W.; Li, Y. Comparison of C-Phycocyanin from Extremophilic Galdieria sulphuraria and Spirulina platensis on Stability and Antioxidant Capacity. Algal Res. 2021, 58, 102391. [Google Scholar] [CrossRef]
- Agrawal, M.; Bansal, S.; Chopra, K. Evaluation of the in Vitro and in Vivo Antioxidant Potentials of Food Grade Phycocyanin. J. Food Sci. Technol. 2021, 58, 4382–4390. [Google Scholar] [CrossRef] [PubMed]
- Sivasankari, S.; Vinoth, M.; Ravindran, D.; Baskar, K.; Alqarawi, A.A.; Abd_Allah, E.F. Efficacy of Red Light for Enhanced Cell Disruption and Fluorescence Intensity of Phycocyanin. Bioprocess. Biosyst. Eng. 2021, 44, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Sasaki, D.; Asayama, M. Development of a Method for Phycocyanin Recovery from Filamentous Cyanobacteria and Evaluation of Its Stability and Antioxidant Capacity. BMC Biotechnol. 2021, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Chen, W.-C.; Gao, Y.-H.; Chen, G.-W.; Lin, H.-T.V.; Pan, C.-L. Enzyme-Assisted Method for Phycobiliproteins Extraction from Porphyra and Evaluation of Their Bioactivity. Processes 2021, 9, 560. [Google Scholar] [CrossRef]
- BEl-Sayed, A.E.-K.; El-Feky, A.; Mounier, M.; Reda, M. C-Phycocyanin, Anticancer Activity and Nutritional Value of Mass-Produced Spirulina platensis Egypt. J. Chem. 2022, 65, 611–625. [Google Scholar] [CrossRef]
- Husain, A.; Alouffi, S.; Khanam, A.; Akasha, R.; Khan, S.; Khan, M.; Farooqui, A.; Ahmad, S. Non-Inhibitory Effects of the Potent Antioxidant c-Phycocyanin from Plectonema Sp. on the in Vitro Glycation Reaction. Rev. Română De Med. De de Lab. 2022, 30, 199–213. [Google Scholar] [CrossRef]
- Puglisi, R.; Biazzi, E.; Gesmundo, D.; Vanni, R.; Tava, A.; Cenadelli, S. The Antioxidant Activity of a Commercial and a Fractionated Phycocyanin on Human Skin Cells in Vitro. Molecules 2022, 27, 5276. [Google Scholar] [CrossRef]
- Galetovic, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Nuñez, J.; Tapia, C.; Neira, I.; Sanzana, S.; et al. Use of Phycobiliproteins from Atacama Cyanobacteria as Food Colorants in a Dairy Beverage Prototype. Foods 2020, 9, 244. [Google Scholar] [CrossRef]
- Sato, N.; Furuta, T.; Takeda, T.; Miyabe, Y.; Ura, K.; Takagi, Y.; Yasui, H.; Kumagai, Y.; Kishimura, H. Antioxidant Activity of Proteins Extracted from Red Alga Dulse Harvested in Japan. J. Food Biochem. 2019, 43, e12709. [Google Scholar] [CrossRef]
- Brabakaran, A.; Venkatesan, S.; Jayappriyan, K.R.; Roselin, L.; Thangaraju, N. Antioxidant Properties of R-Phycoerythrin from Red Alga Spyridia filamentosa (Wulfen) Harvey Collected on the Pudumadam Coast. Adv. Sci. Eng. Med. 2020, 12, 489–498. [Google Scholar] [CrossRef]
- Uju; Dewi, N.P.S.U.K.; Santoso, J.; Setyaningsih, I.; Hardingtyas, S.D. Yopi Extraction of Phycoerythrin from Kappaphycus alvarezii Seaweed Using Ultrasonication. IOP Conf. Ser. Earth Environ. Sci. 2020, 414, 12028. [Google Scholar] [CrossRef]
- Nowruzi, B.; Fahimi, H.; Lorenzi, A.S. Recovery of Pure C-Phycoerythrin from a Limestone Drought Tolerant Cyanobacterium Nostoc Sp. and Evaluation of Its Biological Activity. An. Biol. 2020, 42, 115–128. [Google Scholar] [CrossRef]
- Nowruzi, B.; Porzani, S.J. Study of Temperature and Food-Grade Preservatives Affecting the in Vitro Stability of Phycocyanin and Phycoerythrin Extracted from Two Nostoc Strains. Acta Biol. Slov. 2022, 65, 28–47. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Dharani, G.; Paramasivam, A. Evaluation of Antimicrobial, Antioxidant and Cytotoxicity Potential of R-Phycoerythrin Extracted from Gracilaria corticata Seaweed. Curr. Res. Green. Sustain. Chem. 2023, 6, 100352. [Google Scholar] [CrossRef]
- García-Ishimine, R.; Rodríguez-Vega, J.; Mejía-Pinedo, D. Hepatoprotective, Antioxidant and Anticancer Effect of Spirulina. Rev. Haban. Cienc. Méd. 2020, 19, e2960. [Google Scholar]
- Gdara, N.B.; Belgacem, A.; Khemiri, I.; Mannai, S.; Bitri, L. Protective Effects of Phycocyanin on Ischemia/Reperfusion Liver Injuries. Biomed. Pharmacother. 2018, 102, 196–202. [Google Scholar] [CrossRef]
- Romay, C.; González, R.; Ledón, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef]
- Riedel, C.-U.; Foata, F.; Philippe, D.; Adolfsson, O.; Eikmanns, B.-J.; Blum, S. Anti-Inflammatory Effects of Bifidobacteria by Inhibition of LPS-Induced NF-KappaB Activation. World J. Gastroenterol. 2006, 12, 3729–3735. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, R.; Cao, L.; Shen, Z.; Cao, Y. The Role of MAPK Pathways in Airborne Fine Particulate Matter-Induced Upregulation of Endothelin Receptors in Rat Basilar Arteries. Toxicol. Sci. 2016, 149, 213–226. [Google Scholar] [CrossRef]
- Liu, R.; Qin, S.; Li, W. Phycocyanin: Anti-Inflammatory Effect and Mechanism. Biomed. Pharmacother. 2022, 153, 113362. [Google Scholar] [CrossRef] [PubMed]
- Peña-Medina, R.L.; Fimbres-Olivarría, D.; Enríquez-Ocaña, L.F.; Martínez-Córdova, L.R.; Del-Toro-Sánchez, C.L.; López-Elías, J.A.; González-Vega, R.I. Erythroprotective Potential of Phycobiliproteins Extracted from Porphyridium cruentum. Metabolites 2023, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Rodríguez, A.L.; Ramón-Gallegos, E. Efecto in Vitro de Las Ficobiliproteínas En El Tratamiento Del Carcinoma Cervicouterino. Rev. Del Cent. De Investig. De La Univ. La Salle 2014, 7, 5–12. [Google Scholar]
- Ge, B.; Tang, Z.; Lin, L.; Ren, Y.; Yang, Y.; Qin, S. Pilot-Scale Fermentation and Purification of the Recombinant Allophycocyanin over-Expressed in Escherichia Coli. Biotechnol. Lett. 2005, 27, 783–787. [Google Scholar] [CrossRef]
- Wen, R.; Sui, Z.; Zhang, X.; Zhang, S.; Qin, S. Expression of the Phycoerythrin Gene of Gracilaria lemaneiformis (Rhodophyta) in E. coli and Evaluation of the Bioactivity of Recombinant PE. J. Ocean. Univ. China 2007, 6, 373–377. [Google Scholar] [CrossRef]
- Noore, S.; Tiwari, B.K.; Jambrak, A.R.; Dukić, J.; Wanigasekara, J.; Curtin, J.F.; Fuentes-Grunewald, C.; O’Donnell, C. Extraction Yield and Biological Activity of Phycobiliproteins from Porphyridium purpureum Using Atmospheric Cold Plasma Discharge and Jet Systems. LWT 2023, 187, 115204. [Google Scholar] [CrossRef]
- Silva-Núñez, A.; Donoso-Quezada, J.; González-Valdez, J. Enhanced Antiproliferative Activity of Phycoerythrin through Microencapsulation. J. Appl. Phycol. 2024, 36, 205–215. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Cheng, N.; Lin, L.; Zhang, C. Inhibitory Effect of Phycocyanin from Spirulina platensis on the Growth of Human Leukemia K562 Cells. J. Appl. Phycol. 2000, 12, 125–130. [Google Scholar] [CrossRef]
- Subhashini, J.; Mahipal, S.V.K.; Reddy, M.C.; Mallikarjuna Reddy, M.; Rachamallu, A.; Reddanna, P. Molecular Mechanisms in C-Phycocyanin Induced Apoptosis in Human Chronic Myeloid Leukemia Cell Line-K562. Biochem. Pharmacol. 2004, 68, 453–462. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hussein, M.H.; El-Sawah, A.A. Bio-Fabrication of Silver Nanoparticles by Phycocyanin, Characterization, in Vitro Anticancer Activity against Breast Cancer Cell Line and in Vivo Cytotxicity. Sci. Rep. 2017, 7, 10844. [Google Scholar] [CrossRef]
- Gupta, N.K.; Gupta, K.P. Effects of C-Phycocyanin on the Representative Genes of Tumor Development in Mouse Skin Exposed to 12-O-Tetradecanoyl-Phorbol-13-Acetate. Environ. Toxicol. Pharmacol. 2012, 34, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Gantar, M.; Dhandayuthapani, S.; Rathinavelu, A. Phycocyanin Induces Apoptosis and Enhances the Effect of Topotecan on Prostate Cell Line LNCaP. J. Med. Food 2012, 15, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Rashed, S.A.; Hammad, S.F.; Eldakak, M.M.; Khalil, I.A.; Osman, A. Assessment of the Anticancer Potentials of the Free and Metal-Organic Framework (UiO-66)—Delivered Phycocyanobilin. J. Pharm. Sci. 2023, 112, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Maleki, H.; Soleimanpour, H.; Norouzy, A.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Development of a Novel Method for the Purification of C-Phycocyanin Pigment from a Local Cyanobacterial Strain Limnothrix Sp. NS01 and Evaluation of Its Anticancer Properties. Sci. Rep. 2019, 9, 9474. [Google Scholar] [CrossRef]
- Hao, S.; Li, S.; Wang, J.; Yan, Y.; Ai, X.; Zhang, J.; Ren, Y.; Wu, T.; Liu, L.; Wang, C. Phycocyanin Exerts Anti-Proliferative Effects through down-Regulating TIRAP/NF-ΚB Activity in Human Non-Small Cell Lung Cancer Cells. Cells 2019, 8, 588. [Google Scholar] [CrossRef]
- Kefayat, A.; Ghahremani, F.; Safavi, A.; Hajiaghababa, A.; Moshtaghian, J. C-Phycocyanin: A Natural Product with Radiosensitizing Property for Enhancement of Colon Cancer Radiation Therapy Efficacy through Inhibition of COX-2 Expression. Sci. Rep. 2019, 9, 19161. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Do, J.-M.; Kim, K.H.; Stoica, A.R.; Jo, S.-W.; Kim, U.-K.; Yoon, H.-S. C-Phycocyanin from Limnothrix Species KNUA002 Alleviates Cisplatin-Induced Ototoxicity by Blocking the Mitochondrial Apoptotic Pathway in Auditory Cells. Mar. Drugs 2019, 17, 235. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, L.; Wang, J.; Zhang, L.; Zhou, D.; Qu, J.; Wang, H.; Yin, M.; Hong, J.; Zhao, W. C-Phycocyanin Ameliorates Mitochondrial Fission and Fusion Dynamics in Ischemic Cardiomyocyte Damage. Front. Pharmacol. 2019, 10, 733. [Google Scholar] [CrossRef]
- Ferraro, G.; Imbimbo, P.; Marseglia, A.; Illiano, A.; Fontanarosa, C.; Amoresano, A.; Olivieri, G.; Pollio, A.; Monti, D.M.; Merlino, A. A Thermophilic C-Phycocyanin with Unprecedented Biophysical and Biochemical Properties. Int. J. Biol. Macromol. 2020, 150, 38–51. [Google Scholar] [CrossRef]
- Kaur, P.; Dhandayuthapani, S.; Venkatesan, T.; Gantor, M.; Rathinavelu, A. Molecular Mechanism of C-Phycocyanin Induced Apoptosis in LNCaP Cells. Bioorg Med. Chem. 2020, 28, 115272. [Google Scholar] [CrossRef]
- Ma, P.; Huang, R.; Jiang, J.; Ding, Y.; Li, T.; Ou, Y. Potential Use of C-Phycocyanin in Non-Alcoholic Fatty Liver Disease. Biochem. Biophys. Res. Commun. 2020, 526, 906–912. [Google Scholar] [CrossRef] [PubMed]
- El-Mohsnawy, E.; Abu-Khudir, R. A Highly Purified C-Phycocyanin from Thermophilic Cyanobacterium Thermosynechococcus elongatus and Its Cytotoxic Activity Assessment Using an in Vitro Cell-Based Approach. J. Taibah Univ. Sci. 2020, 14, 1218–1225. [Google Scholar] [CrossRef]
- Hao, S.; Li, Q.; Liu, Y.; Li, F.; Yang, Q.; Wang, J.; Wang, C. Insulin Receptor Substrate 1 Is Involved in the Phycocyanin-Mediated Antineoplastic Function of Non-Small Cell Lung Cancer Cells. Molecules 2021, 26, 4711. [Google Scholar] [CrossRef] [PubMed]
- Kefayat, A.; Yuosefzadeh, M.; Goli, P.; Babayi, A.; Rezaei, A.; Esmaeil, N. Phycocyanin c a Natural Product with Impressive Therapeutic Efficacy for Inhibition of Breast Tumors’ Growth and Metastasis in Vivo. Middle East. J. Cancer 2021, 12, 261–268. [Google Scholar] [CrossRef]
- Hussein, N.A.; Ebied, S.A.; Saleh, M.M. Evaluation of the Anticancer Effect of Violacein, Phycocyanin and Phycocyanobilin on Apoptotic Genes Expression and Glycan Profiles in Breast Cancer Cells. Int. J. Cancer Biomed. Res. 2021, 5, 81–97. [Google Scholar] [CrossRef]
- Rashed, S.A.; Osman, A.; Hammad, S.F.; Eldakak, M.M.; Khalil, I.A. Phycocyanobilin: A Potential Anticancer Therapy—A Tale of a Natural Chromophore. Int. J. Pharma Med. Biol. Sci. 2022, 11, 30–35. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chiang, Y.-F.; Huang, C.-Y.; Shieh, T.-M.; Kao, C.; Chang, F.-K.; Huang, T.-C.; Ali, M.; Chang, H.-Y.; Hong, Y.-H.; et al. Spirulina Phycocyanin Extract and Its Active Components Suppress Epithelial-Mesenchymal Transition Process in Endometrial Cancer via Targeting TGF-Beta1/SMAD4 Signaling Pathway. Biomed. Pharmacother. 2022, 152, 113219. [Google Scholar] [CrossRef]
- Righini, H.; Francioso, O.; Di Foggia, M.; Quintana, A.M.; Roberti, R. Preliminary Study on the Activity of Phycobiliproteins against Botrytis cinerea. Mar. Drugs 2020, 18, 600. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Chiniforush, N.; Bahador, A. Antimicrobial Action of Photoactivated C-Phycocyanin against Enterococcus faecalis Biofilms: Attenuation of Quorum-Sensing System. Photodiagnosis Photodyn. Ther. 2019, 28, 286–291. [Google Scholar] [CrossRef]
- Nowruzi, B.; Anvar, S.A.A.; Ahari, H. Extraction, Purification and Evaluation of Antimicrobial and Antioxidant Properties of Phycoerythrin from Terrestrial Cyanobacterium Nostoc Sp. FA1. J. Microb. World 2020, 13, 138–153. [Google Scholar]
- Sabat, S.; Bej, S.; Swain, S.; Bishoyi, A.K.; Sahoo, C.R.; Sabat, G.; Padhy, R.N. Phycochemistry and pharmacological significance of filamentous cyanobacterium Spirulina sp. Bioresour. Bioprocess 2025, 12, 27. [Google Scholar] [CrossRef]
- Singh, U.; Gandhi, H.A.; Nikita; Bhattacharya, J.; Tandon, R.; Tiwari, G.L.; Tandon, R. Cyanometabolites: Molecules with Immense Antiviral Potential. Arch. Microbiol. 2023, 205, 164. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.; Tsai, K.; Li, Y.; Chueh, C.; Chan, E. Inhibition of Enterovirus 71-induced Apoptosis by Allophycocyanin Isolated from a Blue-green Alga Spirulina platensis. J. Med. Virol. 2003, 70, 119–125. [Google Scholar] [CrossRef]
- Jadaun, P.; Seniya, C.; Pal, S.K.; Kumar, S.; Kumar, P.; Nema, V.; Kulkarni, S.S.; Mukherjee, A. Elucidation of Antiviral and Antioxidant Potential of C-Phycocyanin against HIV-1 Infection through in Silico and in Vitro Approaches. Antioxidants 2022, 11, 1942. [Google Scholar] [CrossRef]
- Ismail, G.A.; El-Sheekh, M.M.; Samy, R.M.; Gheda, S.F. Antimicrobial, Antioxidant, and Antiviral Activities of Biosynthesized Silver Nanoparticles by Phycobiliprotein Crude Extract of the Cyanobacteria Spirulina platensis and Nostoc linckia. Bionanoscience 2021, 11, 355–370. [Google Scholar] [CrossRef]
- Munawaroh, H.S.H.; Gumilar, G.G.; Pratiwi, R.N.; Khoiriah, S.F.; Ningrum, A.; Martha, L.; Chew, K.W.; Show, P.-L. In Silico Antiviral Properties of Spirulina platensis Phycobiliprotein and Phycobilin as Natural Inhibitor for SARS-CoV-2. Algal Res. 2024, 79, 103468. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary Chromatic Adaptation in a Filamentous Blue-Green Alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Kumar, D.; Dhar, D.W.; Pabbi, S.; Kumar, N.; Walia, S. Extraction and Purification of C-Phycocyanin from Spirulina platensis (CCC540). Indian J. Plant Physiol. 2014, 19, 184–188. [Google Scholar] [CrossRef]
- Patil, G.; Chethana, S.; Sridevi, A.S.; Raghavarao, K.S.M.S. Method to Obtain C-Phycocyanin of High Purity. J. Chromatogr. A 2006, 1127, 76–81. [Google Scholar] [CrossRef]
- Eriksen, N.T. Production of Phycocyanin—A Pigment with Applications in Biology, Biotechnology, Foods and Medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef]
- Hemlata; Fatma, T. Screening of Cyanobacteria for Phycobiliproteins and Effect of Different Environmental Stress on Its Yield. Bull. Environ. Contam. Toxicol. 2009, 83, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, S.; Xiong, H.; Zhao, Q. Effect of Different Extraction Methods on Physicochemical Characteristics and Antioxidant Activity of C-Phycocyanin from Dry Biomass of Arthrospira platensis. Foods 2022, 11, 1296. [Google Scholar] [CrossRef]
- Zhao, L.; Peng, Y.L.; Gao, J.M.; Cai, W.M. Bioprocess Intensification: An Aqueous Two-Phase Process for the Purification of C-Phycocyanin from Dry Spirulina platensis. Eur. Food Res. Technol. 2014, 238, 451–457. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Loha, V.; Tia, S.; Bunnag, B. Stepwise Extraction of High-Value Chemicals from Arthrospira (Spirulina) and an Economic Feasibility Study. Biotechnol. Rep. 2018, 20, e00280. [Google Scholar] [CrossRef]
- Wang, F.; Yu, X.; Cui, Y.; Xu, L.; Huo, S.; Ding, Z.; Hu, Q.; Xie, W.; Xiao, H.; Zhang, D. Efficient Extraction of Phycobiliproteins from Dry Biomass of Spirulina platensis Using Sodium Chloride as Extraction Enhancer. Food Chem. 2023, 406, 135005. [Google Scholar] [CrossRef]
- Sánchez-Laso, J.; Espada, J.J.; Rodríguez, R.; Vicente, G.; Bautista, L.F. Novel Biorefinery Approach for Phycocyanin Extraction and Purification and Biocrude Production from Arthrospira platensis. Ind. Eng. Chem. Res. 2023, 62, 5190–5198. [Google Scholar] [CrossRef]
- Rodrigues, R.D.P.; Silva, A.S.e.; Carlos, T.A.V.; Bastos, A.K.P.; de Santiago-Aguiar, R.S.; Rocha, M.V.P. Application of Protic Ionic Liquids in the Microwave-Assisted Extraction of Phycobiliproteins from Arthrospira platensis with Antioxidant Activity. Sep. Purif. Technol. 2020, 252, 117448. [Google Scholar] [CrossRef]
- Käferböck, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable Extraction of Valuable Components from Spirulina Assisted by Pulsed Electric Fields Technology. Algal Res. 2020, 48, 10194. [Google Scholar] [CrossRef]
- Sommer, M.C.; Balazinski, M.; Rataj, R.; Wenske, S.; Kolb, J.F.; Zocher, K. Assessment of Phycocyanin Extraction from Cyanidium caldarium by Spark Discharges, Compared to Freeze-thaw Cycles, Sonication and Pulsed Electric Fields. Microorganisms 2021, 9. [Google Scholar] [CrossRef]
- Puzorjov, A.; Mert Unal, S.; Wear, M.A.; McCormick, A.J. Pilot Scale Production, Extraction and Purification of a Thermostable Phycocyanin from Synechocystis Sp. PCC 6803. Bioresour. Technol. 2022, 345, 126459. [Google Scholar] [CrossRef]
- Hidane, T.; Demura, M.; Morisada, S.; Ohto, K.; Kawakita, H. Mathematical Analysis of Cake Layer Formation in an Ultrafiltration Membrane of a Phycobiliprotein-Containing Solution Extracted from Nostoc Commune. Biochem. Eng. J. 2022, 179, 108333. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and Purification of High-Value Metabolites from Microalgae: Essential Lipids, Astaxanthin and Phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Hernández, J.E.; Romero-Castillo, K.D.; Parra-Arroyo, L.; Aguilar-Aguila-Isaías, M.A.; García-Reyes, I.E.; Ahmed, I.; Parra-Saldivar, R.; Bilal, M.; Iqbal, H.M.N. Mexican Microalgae Biodiversity and State-of-the-Art Extraction Strategies to Meet Sustainable Circular Economy Challenges: High-Value Compounds and Their Applied Perspectives. Mar. Drugs 2019, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Centella, M.H.; Arévalo-Gallegos, A.; Parra-Saldivar, R.; Iqbal, H.M.N. Marine-Derived Bioactive Compounds for Value-Added Applications in Bio- and Non-Bio Sectors. J. Clean. Prod. 2017, 168, 1559–1565. [Google Scholar] [CrossRef]
- Rito-Palomares, M.; Nuñez, L.; Amador, D. Practical Application of Aqueous Two-phase Systems for the Development of a Prototype Process for C-phycocyanin Recovery from Spirulina maxima. J. Chem. Technol. Biotechnol. 2001, 76, 1273–1280. [Google Scholar] [CrossRef]
- Sada-Borrego, C.; García-Gómez, C.; Guajardo-Barbosa, C.; Márquez-Reyes, J.; Nápoles-Armenta, J.; Beltrán-Rocha, J. Optimización Para La Extracción de Ficocianina de La Cianobacteria Spirulina maxima. Investig. Y Desarro. En Cienc. Y Tecnol. De Aliment. 2023, 8, 834–840. [Google Scholar] [CrossRef]
- Ruiz-Hernández, Y.A.; Garza-Valverde, E.; Márquez-Reyes, J.R.; García-Gómez, C. Extracción de Ficocianina Para Uso Como Colorante Natural: Optimización Por Metodología de Superficie de Respuesta. Investig. Y Desarro. En Cienc. Y Tecnología de Aliment. 2023, 8, 84–91. [Google Scholar] [CrossRef]
- Singh, P.; Mohanty, S.S.; Mohanty, K. Comprehensive Assessment of Microalgal-Based Treatment Processes for Dairy Wastewater. Front. Bioeng. Biotechnol. 2024, 12, 1425933. [Google Scholar] [CrossRef]
- Cai, C.; Li, C.; Wu, S.; Wang, Q.; Guo, Z.; He, P. Large Scale Preparation of Phycobiliproteins from Porphyra yezoensis Using Co-Precipitation with Ammonium Sulfate. Nat. Sci. 2012, 04, 536–543. [Google Scholar] [CrossRef]
- Patil, G.; Raghavarao, K.S.M.S. Aqueous Two Phase Extraction for Purification of C-Phycocyanin. Biochem. Eng. J. 2007, 34, 156–164. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Loha, V.; Tia, S.; Bunnag, B. Separation and Purification of Phycocyanin from Spirulina Sp. Using a Membrane Process. Bioresour. Technol. 2011, 102, 7159–7164. [Google Scholar] [CrossRef]
- Glazer, A.N. Light Harvesting by Phycobilisomes. Annu. Rev. Biophys. Biophys. Chem. 1985, 14, 47–77. [Google Scholar] [CrossRef] [PubMed]
- Dev, A.; Mohanbhai, S.J.; Kushwaha, A.C.; Sood, A.; Sardoiwala, M.N.; Choudhury, S.R.; Karmakar, S. κ-Carrageenan-C-Phycocyanin Based Smart Injectable Hydrogels for Accelerated Wound Recovery and Real-Time Monitoring. Acta Biomater. 2020, 109, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial Toxins: Risk Management for Health Protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Chorus, I. Accumulation of Cyanobacterial Toxins in Freshwater “Seafood” and Its Consequences for Public Health: A Review. Environ. Pollut. 2007, 150, 177–192. [Google Scholar] [CrossRef]
- Briand, J.-F.; Jacquet, S.; Bernard, C.; Humbert, J.-F. Health Hazards for Terrestrial Vertebrates from Toxic Cyanobacteria in Surface Water Ecosystems. Vet. Res. 2003, 34, 361–377. [Google Scholar] [CrossRef]
- Janssen, E.M.-L. Cyanobacterial Peptides beyond Microcystins—A Review on Co-Occurrence, Toxicity, and Challenges for Risk Assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.; Beach, D.; Labidi, Z.; Djabri, A.; Benayache, N.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The Diversity of Cyanobacterial Toxins on Structural Characterization, Distribution and Identification: A Systematic Review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef]
- Hosokawa, S.; Kawano, S. Worldwide Research Trends on Microalgae and Recent Work in Cytologia. Cytologia 2020, 85, 179–187. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Liang, Y.; Deng, L.; Feng, Z.; Ouyang, Q.; Wu, X.; Quan, W.; Zhu, Y.; Ye, H.; Wu, K.; Luo, H. A Chitosan-Based Flocculation Method for Efficient Recovery of High-Purity B-Phycoerythrin from a Low Concentration of Phycobilin in Wastewater. Molecules 2023, 28, 3600. [Google Scholar] [CrossRef] [PubMed]

| Activities | Action Mechanism of PBPs | Refererence |
|---|---|---|
| Antioxidant | Trapping ROS | [47,48] |
| Anticancer activity | Deregulates the expression of proinflammatory genes and components. | [49,50] |
| Multiple sclerosis | Has a protective effect on axonal structure loss | [51] |
| Diabetes | Decreases and/or suppresses NADPH oxidase activity | [52,53] |
| Atherosclerosis | Regulates atheroprotective activity, through molecular markers related to oxidative stress. | [54] |
| Organism | Assay Results | Reference |
|---|---|---|
| Phycocyanin (PC) | ||
| S. platensis | PBPs have shown dose-dependent antiproliferative activity against the MCF7 breast cancer cell line, with inhibition rates of 58, 60, and 64% at 400, 800, and 1600 μg/mL concentrations, respectively. | [70] |
| Limnothrix sp. NS01 | The IC50 values obtained were 5.92, 5.66, and 4.52 μg/mL at 24, 48, and 72 h, respectively, against the MCF7 breast cancer cell line. Flow cytometry analysis (Annexin V/PI) determined that cell death occurred via apoptosis, confirmed by the reduced expression of Bcl-2, Stat 3, and Cyclin D1. | [131] |
| S. platensis | Dose-dependent activity was observed at concentrations of 2.5, 5, 7.5, and 10 μM against the NSCLC cell lines H358, H1650, and LETP-a2. At 7.5 μM, a G1 cell cycle arrest was induced, and migration was reduced from 69.72% to 44.65% in all three cell lines. Apoptosis was confirmed via flow cytometry (Annexin V/PI). The treatment downregulated the transcription levels of RIPK1/NFκB, exerting antiproliferative and antimigratory effects. | [49] |
| S. platensis | A dose-dependent activity was observed against the NSCLC cell lines H1975, H1650, and LTEP-a2, showing enhanced efficacy at 2, 4, 6, and 8 μM. A 59.21% cell cycle arrest was induced in the G1 phase compared to the control (42.23%). Early apoptosis was observed at 7.15%, while late apoptosis was 19.6% at 6 μM. C-PC reduced Bcl-2 expression, increased Bax expression, and downregulated the transcription of TIRAP/NFκB. | [132] |
| N/D | A concentration of 200 μg/mL of C-PC significantly improved the efficacy of radiotherapy in all cancer cell lines: CT-26 (mouse colon cancer), DLD-1 (human colon cancer), HT-29 (human colon cancer), and CRL-1831 (normal human colon cells). The treatment of normal colonic cells with C-PC did not show a significant impact (p > 0.05) on the radiation effects on these cells. Administration of C-PC at a concentration of 300 μg/mL reduced the expression of COX-2 mRNA by 58% and protein levels by 68%. (This enzyme is involved in radio resistance in tumor cells, causing inhibition of apoptosis, resistance, and proliferation.) The administration of 50 mg/kg to Balb/c mice for 30 days did not alter the body weight of the mice and showed no signs of toxicity or tissue damage (lungs, liver, kidneys, brain, and spleen) upon histopathological examination. | [133] |
| Limnothrix sp. KNUA002 | C-PC showed no toxicity against HEI-OC1 cells at a concentration of 5 μg/mL. Treatment with PC at 1 and 2 μg/mL improved cell viability by 62% compared to 40% in control cells exposed to cisplatin. A 7% reduction in cell cycle arrest at the sub-G0/G1 phase was observed in cells pretreated with 1 μg/mL of PC. Additionally, treatment with PC at 30 μM induced the expression of the anti-apoptotic protein Bcl-2, while the expression of the pro-apoptotic protein Bax was lower in the phycocyanin treatment compared to cisplatin treatment. | [134] |
| N/D | C-PC treatment (10, 20, 40, and 80 μg/mL) protected H9c2 cells from intrinsic apoptosis induced by OGD/R (oxygen–glucose deprivation/reoxygenation) by modulating cytochrome c, apoptotic protease activating factor-1, and suppressing the phosphorylation of extracellular signal-regulated kinase and c-Jun N-terminal kinase. These results suggest that C-PC protects cardiomyocytes from ischemic damage by affecting mitochondrial fission and fusion dynamics, reducing apoptosis, and thus showing potential as a prophylactic or therapeutic agent for ischemic heart disease. | [135] |
| Galdieria phlegrea (009) | The IC50 values reported for various cell lines are as follows: against A431 epidermoid carcinoma cells, 9.8 ± 0.07 µM; against HaCat keratinocytes, >10 µM; against SV40-transformed mouse SVT2 cells, 3.7 ± 0.14 µM; and against Balb/c 3T3 mouse fibroblasts, >10 µM. | [136] |
| S. platensis | The IC50 value of 387.12 ± 0.34 μg/mL was observed against the Hep-G2 liver cancer cell line, with no activity detected against the Vero cell line. | [72] |
| Limnothrix sp. 37-2-1 | The compound exhibits anticancer activity with a reduction of >65% at concentrations of 500 μg/mL. It promotes the upregulation of pro-apoptotic proteins such as BAX and Apaf-1, along with the activation of caspases 8, 9, and 3. Furthermore, a decrease in the expression of anti-apoptotic proteins such as Bcl-2, Mcl-1, and surviving was demonstrated. | [137] |
| S. platensis | Phycocyanin reduced lipid accumulation in L02 steatosis cells and in the liver of mice with non-alcoholic steatohepatitis (NASH), improving the antioxidant capacity of the liver. Western blot analysis showed an increase in AMPK phosphorylation expression and a reduction in the expression levels of SREBP-1c and its target genes, ACC and FAS. Additionally, there was an increase in the expression of the transcription factor PPAR-α, regulated by AMPK, and its target gene CPT1. Phycocyanin promoted AMPK phosphorylation in hepatocytes, while increasing the phosphorylation levels of ACC both in vivo and in vitro. Furthermore, it improved liver inflammatory infiltration by upregulating PPAR-γ and downregulating CD36, IL6, and TNF-α. These results suggest that phycocyanin can improve lipid accumulation and inflammation in mice with non-alcoholic fatty liver disease via the AMPK pathway in hepatocytes. | [138] |
| Thermosynechococcus elongatus | Cytotoxic activity against breast cancer (MCF7, IC50 of 158.9 μM), colorectal cancer (Caco-2, IC50 of 258.3 μM), and liver cancer (HepG2, IC50 of 277.5 μM). | [139] |
| A. platensis (biomass) | An IC50 >150 μg/mL was reported for cancer cell lines of glioblastoma (SF295), colorectal cancer (HCT116), prostate cancer (PC3), and an IC50 of 112.6 μg/mL for leukemia (HL60). | [96] |
| S. platensis MK343101 | Inhibitory activity in HeLa cells of 64.1% at 500 μg/mL. | [99] |
| S. platensis | The expression of IRS-1 significantly decreased after treatment with 4.8 μM phycocyanin in lung cancer cells A549, H1299, and LTEP-a2, confirming transcriptome results via Western Blot. These findings suggest that IRS-1 may play a role in the antineoplastic function of phycocyanin in NSCLC cells. | [140] |
| N/D | Antitumor activity (50 mg/kg) in an in vivo 4T1 breast cancer model in Balb/c mice. Tumor growth was inhibited 12 days post-treatment, and by day 21, the tumor volume was 2.73 times smaller than the untreated control. Histopathological analysis showed inhibition of metastatic cancer cells in the lungs and liver compared to the control. Additionally, there was an increase in the survival rate of mice over 22 days. | [141] |
| S. platensis | Cytotoxic activity with an IC50 of 1.56 mg/mL in the MCF-7 breast cancer cell line. Treatment with 1.56 mg/mL induces upregulation of Bax and Cas-3 genes, along with downregulation of Bcl-2 expression. | [142] |
| S. platensis | Anticancer activity with IC50 values of 58.9, 48.1, and 44.7 µg/mL for the MCF-7, HCT-116, and HepG2 cell lines, respectively. C-PC induces apoptosis in the MCF7 cell line, with an increase in Cas9 protein (8.97 pg/mL) and a reduction in BCL2 (2.16 pg/mL) compared to the untreated control (Cas9 2.16 pg/mL and BCL2 5.10 pg/mL). | [102] |
| A. platensis | Commercial C-PC at 10 mg/mL reduced cell viability to <20% in fibroblasts and keratinocytes. Isolated C-PC reduced cell viability by >20% at 0.16 mg/mL for fibroblasts and at 0.62 mg/mL for keratinocytes. Isolated C-PC exhibited a pro-oxidant effect in keratinocytes when combined with UVA radiation exposure, increasing ROS levels, compared to commercial phycocyanin, which showed a protective effect with low ROS levels. | [104] |
| S. platensis SAM2021 | It showed cytotoxic activity with an IC50 of 108 μg/mL against the colorectal cancer cell line HT-29. | [143] |
| C-Phycocyanin (C-PC), Allophycocyanin (APC) | ||
| S. platensis | C-PC and purified APC can reverse TGFβ-induced migration of endometrial cancer cells and reduce peritoneal dissemination in a nude mouse model by modulating the TGFβ/SMAD4 signaling pathway. This involves the reduction of transcription factors such as TGFβR1, Smad4, Snail, SLUG, TWIST1/2, and ZEB1, followed by an increase in the expression of E-cadherin, while decreasing the expression of N-cadherin, vimentin, α-SMA, fibronectin, and TMEFF2 protein. | [144] |
| Phycoerythrin (PE) | ||
| G. corticata | Cellular inhibition at 4.8 μg in SW-620 (42%) and HCT-116 (39%) cell lines. | [111] |
| Organism | Assay Results | Reference |
|---|---|---|
| Phycocyanin (PC) | ||
| Arthrospira sp. | The gelatin-C-phycocyanin (C-PC) formulation exhibited antibacterial activity against Staphylococcus aureus, Micrococcus luteus, E. coli, and Pseudomonas sp., in a dose-dependent manner, with the best activity observed at 5 mg/mL C-PC, resulting in inhibition halos of 12.5, 18, 13, and 9 mm, respectively. | [92] |
| N/D | A MIC of 125 μg/mL was determined against Enterococcus faecalis. | [146] |
| Oscillatoria minima | Inhibition at 16 μg/mL against Pseudomonas fragi, Pseudomonas vulgaris, Bacillus subtilis, Klebsiella oxytoca, Streptococcus pyogenes, and against the algae Nostoc, Gleocapsia, and Spirulina. | [94] |
| S. platensis MK343101 | It exhibited antibacterial activity with inhibition zones of 20 ± 0.9, 11 ± 0.7, 11 ± 0.5, and 11 ± 0.4 mm against Shigella dysenteriae, Salmonella typhi, Pseudomonas aeruginosa, and Bacillus subtilis, respectively. In comparison, tetracycline (30 μg) showed inhibition zones of 18 ± 0.3, 11 ± 0.5, 04 ± 0.5, and 11 ± 0.5 mm, respectively. | [99] |
| A. platensis | Antimicrobial activity against Bacillus subtilis ATCC 6633 (24.4% inhibition, 17.2% control), Enterococcus faecalis ATCC 29,212 (20.6% inhibition, 20.5% control), and Streptococcus agalactiae ATCC 13,813 (31.1% inhibition, 15.5% control). | [6] |
| Allophycocyanin (APC) | ||
| C. officinalis | S. aureus ATCC 25,923 33.7% (33.6% control), B. subtilis ATCC 6633 32.6% (17.2% control), E. faecalis ATCC 29,212 31.5% (20.5% control), S. agalactiae ATCC 13,813 33.7% (15.5% control) | [6] |
| Phycoerythrin (PE) | ||
| Nostoc sp. FA1 | It showed activity against B. subtilis with an inhibition zone of 10.5 ± 0.28 mm and against Candida albicans with an inhibition zone of 10.98 ± 0.006 mm. | [147] |
| Nostoc sp. A5 | Inhibition at 710 μg/mL with inhibition zones of 10.9 ± 0.16, 12.16 ± 0.44, 10.3 ± 0.88, 12.33 ± 0.33, 9.83 ± 0.44, and 12.5 ± 0.76 mm against B. subtilis, Bacillus cereus, E. coli, S. aureus, Pseudomonas aeruginosa, and Salmonella typhimurium, respectively. Antifungal activity (719 μg/mL) with inhibition zones of 13.55 μg/mL and 12.33 μg/mL against Aspergillus niger and Candida albicans. | [109] |
| G. corticata | Antifungal inhibition at 0.15 mg/mL against C. albicans (43 mm) and antimicrobial inhibition against Clostridium perfringens (35 mm), S. aureus (30 mm), Shigella sonnei (30 mm), S. typhi (30 mm), P. aeruginosa (29 mm), E. coli (27 mm), and Bacillus cereus (21 mm). | [111] |
| Method | Principle | Advantages | Disadvantages | Estimated Yield/Notes | References |
|---|---|---|---|---|---|
| FTC | Disruption via ice crystal formation | Simple, no reagents required | Time-consuming, incomplete lysis | Moderate yield | [154,157] |
| Sonication | Ultrasound waves to disrupt cell membranes | Fast, efficient for small volumes | Heat generation, protein denaturation risk | High yield if controlled properly | [154,155] |
| Enzymatic digestion | Cell wall degradation using lysozyme or cellulase | Gentle, preserves bioactivity | Expensive, slow | Variable, strain-dependent | [156] |
| Osmotic shock | Cell bursting via hypotonic solutions | Mild, no chemicals | Inefficient for thick-walled cells | Low yield | [157] |
| UAE | Acoustic cavitation facilitates cell disruption | High efficiency, scalable, less solvent | Requires optimization, may generate heat | High yield (up to 80% PBPs recovery) | [155,158] |
| MAE | Rapid heating of intracellular water | Fast, low solvent usage | Risk of overheating or protein denaturation | High, but may affect purity | [158] |
| PEF | Electroporation of membranes | Low temperature, gentle on proteins | Requires specialized equipment | Moderate to high, depends on strain | [158,172] |
| Mechanical homogenization | High-shear mechanical disruption | Common in industry, scalable | Heat generation, high energy cost | Moderate to high | [154,172] |
| Chemical lysis (detergents, buffers) | Use of surfactants and buffers | Effective in lysing cells, customizable | May affect protein integrity | Variable, depends on composition | [156] |
| Product Name | Application | Country | Commercial Brand | Reference |
|---|---|---|---|---|
| Nutrex Hawaii Pure Hawaiian Spirulina Pacifica | Spirulina powder (Phycocyanin as main ingredient) | United States | Nutrex Hawaii Founded by Cyanotech | https://www.cyanotech.com/ |
| Spirulina Gold Plus | Spirulina Tablets | United States | Earthrise Nutritionals | https://www.earthrise.com/ |
| Organic Spirulina Powered Truth in Food | Phycocyanin Powder (as colorant or supplement) | Italy | Parry Nutraceuticals (sold by California Gold Nutrition®) | https://www.parrynutraceuticals.com/ |
| E3 AFA blue green algae | Blue-Green Algae Supplements | United States | E3Live | https://www.e3live.com/ |
| Klamin | Algae-based Food Supplements | Italy | NUTRIGEA Srl | https://nutrigea.com/ |
| Numerous brands and products | Phycocyanin Extracts (as an ingredient in high-performance supplements) | United States | Cyanotech | https://www.cyanotech.com/ |
| Spirulina algae powder | Phycobiliproteins naturally within the Spirulina algae. | United Kingdom | Organic Burst | https://www.organicburst.com/ |
| S. platensis | Sunfood | United States | Sunfood | https://www.sunfood.com/ |
| Spirulina maxima | Phycobiliproteins naturally within the Spirulina algae. | China | Hainan Super Biotech Co., Ltd. | https://www.super-biotech.com/ |
| Blue Spirulina E10, 18, and 25 | Spirulina extract (Phycocyanin as main ingredient) | China | Zhejiang Binmei Biotechnology Co., Ltd. | https://www.binmei-global.com/ |
| Product Name | Application | Country | Commercial Brand | Reference |
|---|---|---|---|---|
| Blue Spirulina Extract | Phycobiliproteins are extracted from Spirulina algae, providing a natural blue colorant. | United States | E3Live | https://www.e3live.com |
| Blue Majik Powder | Derived from Phycocyanin, a type of Phycobiliprotein found in Spirulina, providing a vibrant blue color. | United States | E3Live | https://www.e3live.com |
| Phycocyanin Extract | Extracted from Spirulina algae, Phycocyanin serves as a natural blue food colorant | China | Tianjin Norland Biotech Co., Ltd. | https://www.norlandbiotech.com |
| Blue Spirulina Extract Powder | Contains phycocyanin, a Phycobiliprotein sourced from Spirulina, providing a vivid blue hue to food products. | Taiwan | FEBICO | https://www.febico.com |
| Blue-Green Algae Powder | Phycobiliproteins extracted from Spirulina algae contribute to the blue-green color of this natural food coloring agent. | United States | Gourmet Imports | https://www.gourmetimports.com |
| Phycocyanin Blue Powder | Derived from phycocyanin, a Phycobiliprotein from Spirulina, this powder serves as a natural blue food colorant | China | Qingdao BNP BioScience Co., Ltd. | https://www.bnpharma.com |
| Blue Algae Powder | Phycobiliproteins from Spirulina algae contribute to the blue color of this natural food coloring product. | Australia | Southern Biological | https://www.southernbiological.com |
| Organic Blue Spirulina Powder | Contains phycocyanin, sourced from Spirulina, providing a natural blue hue to food and beverages | Mexico | NineLife | https://ninelife.mx |
| Blue Spirulina Blue Powder | Organic phycocyanin from Arthrospira platensisserve as a natural blue food colorant in this powder form. | China | Shaanxi honghao Bio-tech Co.,ltd, | https://www.herb-extract-supplier.com |
| Blue Phycocyanin Extract | Phycocyanin, derived from Spirulina, is used as a natural blue food colorant in various food applications. | Israel | Algatechnologies Ltd. | https://www.algatech.com |
| LinaBlue® | Pigment of phycocyanin derived from Spirulina, used as a natural food colorant as well as a functional food ingredient. | United States | Sun Chemical © | https://www.sunchemical.com/linablue/ |
| Organic Blue Spirulina | Spirulina powder extract (Phycocyanin as main ingredient) | China | Zhejiang Binmei Biotechnology Co., Ltd. | https://www.binmei-global.com/ |
| Product Name | Application | Country | Commercial Brand | Reference |
|---|---|---|---|---|
| Allophycocyanin-Conjugated Antibodies | Provides a variety of fluorescently labeled antibodies and reagents, including those incorporating phycobiliproteins, for flow cytometry and other research applications. | United States | BD Biosciences | https://www.bdbiosciences.com |
| Phycoerythrin-Conjugated Antibodies | Antibodies and proteins for research use, conjugated to phycobiliproteins for detection purposes. Immunofluorescence assays | United States | Santa Cruz Biotechnology, Inc. | https://www.scbt.com |
| Phycoerythrin-Conjugated Antibodies | Antibodies and protein conjugates for various research applications, Immunofluorescence assays | United States | Rockland Immunochemicals Inc. | https://www.rockland-inc.com |
| Phycoerythrin-Conjugated Antibodies | Antibodies and proteins, some of which may be conjugated with phycobiliproteins for fluorescence-based assays, Immunofluorescence assays | United States | ProSci Inc. | https://www.prosci-inc.com |
| Phycoerythrin-Conjugated Antibodies | Research antibodies and reagents, which may include some labeled with phycobiliproteins for use in fluorescence-based experiments. | United States | Cell Signaling Technology, Inc. | https://www.cellsignal.com |
| Phycoerythrin-Conjugated Antibodies | Wide range of research reagents, including antibodies and proteins, conjugated with phycobiliproteins for fluorescent labeling. | United States | Sigma-Aldrich Corporation (Merck). | https://www.sigmaaldrich.com |
| Phycoerythrin-Conjugated Antibodies | Extensive catalog of antibodies and research tools, including some labeled with phycobiliproteins for various applications. | United Kingdom | Abcam plc. | https://www.abcam.com |
| Cy7-Phycoerythrin Conjugate R-phycoerythrin | Diagnostic application: Immunofluorescence assay | Germany | Chroma Gesellschaft Schmidt & Co. GmbH. | https://www.chroma.de |
| Phycoerythrin-Conjugated Antibodies | Flow cytometry and immunofluorescence assays | United States | BioLegend | https://www.biolegend.com |
| Phycoerythrin-Antibody Conjugates | Flow cytometry and immunofluorescence assays | United States | Thermo Fisher Scientific. | https://www.thermofisher.com |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gómez, C.; Aguirre-Cavazos, D.E.; Chávez-Montes, A.; Ballesteros-Torres, J.M.; Orozco-Flores, A.A.; Reyna-Martínez, R.; Torres-Hernández, Á.D.; González-Meza, G.M.; Castillo-Hernández, S.L.; Gloria-Garza, M.A.; et al. Phycobilins Versatile Pigments with Wide-Ranging Applications: Exploring Their Uses, Biological Activities, Extraction Methods and Future Perspectives. Mar. Drugs 2025, 23, 201. https://doi.org/10.3390/md23050201
García-Gómez C, Aguirre-Cavazos DE, Chávez-Montes A, Ballesteros-Torres JM, Orozco-Flores AA, Reyna-Martínez R, Torres-Hernández ÁD, González-Meza GM, Castillo-Hernández SL, Gloria-Garza MA, et al. Phycobilins Versatile Pigments with Wide-Ranging Applications: Exploring Their Uses, Biological Activities, Extraction Methods and Future Perspectives. Marine Drugs. 2025; 23(5):201. https://doi.org/10.3390/md23050201
Chicago/Turabian StyleGarcía-Gómez, Celestino, Diana E. Aguirre-Cavazos, Abelardo Chávez-Montes, Juan M. Ballesteros-Torres, Alonso A. Orozco-Flores, Raúl Reyna-Martínez, Ángel D. Torres-Hernández, Georgia M. González-Meza, Sandra L. Castillo-Hernández, Marcela A. Gloria-Garza, and et al. 2025. "Phycobilins Versatile Pigments with Wide-Ranging Applications: Exploring Their Uses, Biological Activities, Extraction Methods and Future Perspectives" Marine Drugs 23, no. 5: 201. https://doi.org/10.3390/md23050201
APA StyleGarcía-Gómez, C., Aguirre-Cavazos, D. E., Chávez-Montes, A., Ballesteros-Torres, J. M., Orozco-Flores, A. A., Reyna-Martínez, R., Torres-Hernández, Á. D., González-Meza, G. M., Castillo-Hernández, S. L., Gloria-Garza, M. A., Kačániová, M., Ireneusz-Kluz, M., & Elizondo-Luevano, J. H. (2025). Phycobilins Versatile Pigments with Wide-Ranging Applications: Exploring Their Uses, Biological Activities, Extraction Methods and Future Perspectives. Marine Drugs, 23(5), 201. https://doi.org/10.3390/md23050201









