Dental Resin Composites Modified with Chitosan: A Systematic Review
Abstract
1. Introduction
2. Results
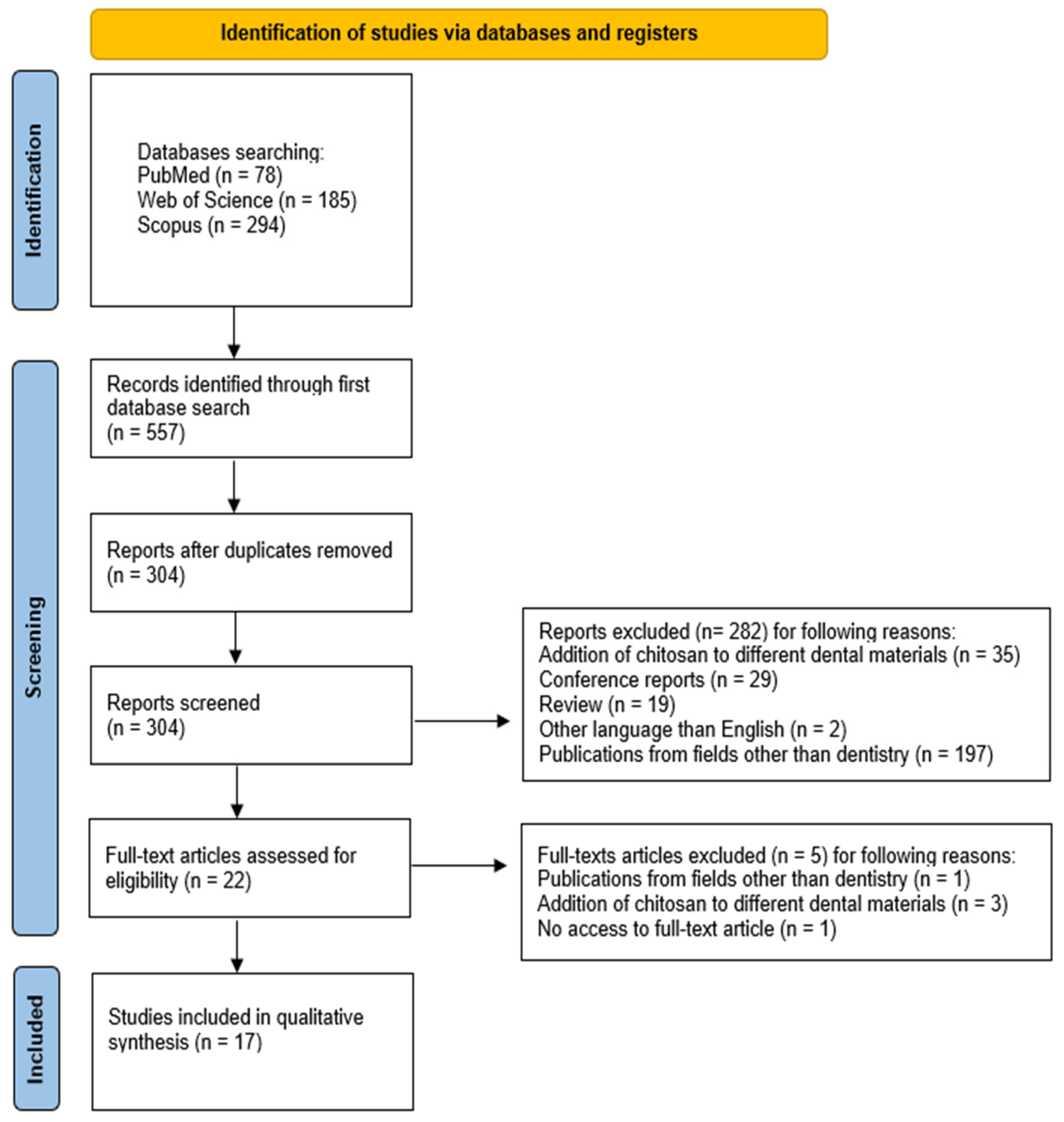
2.1. Study Selection
2.2. General Characteristics of the Included Studies
2.2.1. Antibacterial Activity
2.2.2. Shear Bond Strength
2.2.3. Hardness
2.2.4. Strength and Toughness Properties
2.2.5. Additional Material Properties
2.3. Main Study Outcomes
- Antimicrobial activity of chitosan incorporated in dental resins against S. mutans
- Addition of chitosan to dental resins either does not affect or lowers the shear bond strength
- The hardness of the material either does not change or lowers with the addition of chitosan
- The flexural modulus of chitosan-containing dental resins tends to follow a trend observed for flexural strength
2.4. Quality Assessment
3. Discussion
4. Materials and Methods
4.1. Focused Question
4.2. Protocol
4.3. Eligibility Criteria
- Addition of chitosan to the composite material;
- Research in the field of dentistry;
- In vitro studies;
- Studies in English;
- Full-text articles.
- Use of chitosan other than as an additive to the restorative material;
- Conference reports;
- Research in fields other than dentistry;
- Non-English papers
- Review articles;
- No full-text accessible;
- Duplicated publications.
4.4. Information Sources, Search Strategy, and Study Selection
4.5. Data Collection Process and Data Items
4.6. Risk of Bias and Quality Assessment
4.7. Quality Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Peutzfeldt, A. Resin composites in dentistry: The monomer systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48 Pt A, 27280–27294. [Google Scholar] [CrossRef]
- Demarco, F.F.; Collares, K.; Coelho-de-Souza, F.H.; Correa, M.B.; Cenci, M.S.; Moraes, R.R.; Opdam, N.J. Anterior composite restorations: A systematic review on long-term survival and reasons for failure. Dent. Mater. 2015, 31, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Alomran, W.K.; Nizami, M.Z.I.; Xu, H.H.K.; Sun, J. Evolution of Dental Resin Adhesives—A Comprehensive Review. J. Funct. Biomater. 2025, 16, 104. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Resin composite restorative materials. Aust. Dent. J. 2011, 56 (Suppl. 1), 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.B.; Wu, D.; Holmes, B.N. An application of nanotechnology in advanced dental materials. J. Am. Dent. Assoc. 2003, 134, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A review of dental composites: Challenges, chemistry aspects, filler influences, and future insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Lehmann, A.; Nijakowski, K.; Jankowski, J.; Donnermeyer, D.; Ramos, J.C.; Drobac, M.; Martins, J.F.B.; Hatipoğlu, Ö.; Omarova, B.; Javed, M.Q.; et al. Clinical Difficulties Related to Direct Composite Restorations: A Multinational Survey. Int. Dent. J. 2025, 75, 797–806. [Google Scholar] [CrossRef]
- Ravandi, R.; Heris, S.Z.; Hemmati, S.; Aghazadeh, M.; Davaran, S.; Abdyazdani, N. Effects of chitosan and TiO2 nanoparticles on the antibacterial property and ability to self-healing of cracks and retrieve mechanical characteristics of dental composites. Heliyon 2024, 10, e27734. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Reche, A.; Agrawal, S.; Paul, P. Applications of Chitosan Nanoparticles in Dentistry: A Review. Cureus 2023, 15, e49934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Hui, D.; Du, C.; Sun, H.; Peng, W.; Pu, X.; Li, Z.; Sun, J.; Zhou, C. Preparation and application of chitosan biomaterials in dentistry. Int. J. Biol. Macromol. 2021, 167, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Pina, C.; Tavaria, F.K.; Pintado, M.M. Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens. Anaerobe 2012, 18, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Fayyad, A.A. What is the effectiveness of chitosan in promoting the healing of tooth extraction sockets? A systematic review. J. Oral Med. Oral Surg. 2024, 30, 25. [Google Scholar] [CrossRef]
- Surija, I.; Gunawan, H.A.; Amir, L.R. Effect of chitosan on the enamel demineralization process in vitro: An enamel solubility test. J. Phys. Conf. Ser. 2018, 1073, 052005. [Google Scholar] [CrossRef]
- Yamakami, S.A.; Faraoni, J.J.; Lia, N.S.N.D.; Regula, F.B.; Ohyama, H.; Palma-Dibb, R.G. Effect of an experimental chitosan/casein gel on demineralized enamel under a cariogenic challenge. Dent. Med. Probl. 2022, 59, 531–538. [Google Scholar] [CrossRef]
- Ali, S.; Sangi, L.; Kumar, N.; Kumar, B.; Khurshid, Z.; Zafar, M.S. Evaluating antibacterial and surface mechanical properties of chitosan modified dental resin composites. Technol. Health Care 2019, 28, 165–173. [Google Scholar] [CrossRef]
- Fadhila, D.N.; Ridwan, A.Z.; Amir, N.A.; Abdillah, A.; Kartini N, R.; Hasanuddin, H.; Djamaluddin, N. Mechanical properties and formulation of hydrophilic fiber and shrimp shell combination as a novel eco-friendly dental restoration material. Heliyon 2024, 10, e34180. [Google Scholar] [CrossRef]
- Krishna, N.; Lolayekar, N. Application of Chitosan Biomaterials in Dentistry—A Narrative Review. J. Health Allied Sci. NU 2023, 14, 152–156. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional fillers for dental resin composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Randolph, L.D.; Palin, W.M.; Leloup, G.; Leprince, J.G. Filler characteristics of modern dental resin composites and their influence on physico-mechanical properties. Dent. Mater. 2016, 32, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Habib, E.; Wang, R.; Wang, Y.; Zhu, M.; Zhu, X.X. Inorganic Fillers for Dental Resin Composites: Present and Future. ACS Biomater. Sci. Eng. 2016, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lee, Y.K.; Oguri, M.; Powers, J.M. Properties of a dental resin composite with a spherical inorganic filler. Oper. Dent. 2006, 31, 734–740. [Google Scholar] [CrossRef]
- Masouras, K.; Silikas, N.; Watts, D.C. Correlation of filler content and elastic properties of resin-composites. Dent. Mater. 2008, 24, 932–939. [Google Scholar] [CrossRef]
- Beyth, N.; Farah, S.; Domb, A.J.; Weiss, E.I. Antibacterial dental resin composites. React. Funct. Polym. 2014, 75, 81–88. [Google Scholar] [CrossRef]
- Chatzistavrou, X.; Fenno, J.C.; Faulk, D.; Badylak, S.; Kasuga, T.; Boccaccini, A.R.; Papagerakis, P. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta Biomater. 2014, 10, 3723–3732. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wu, R.; Fan, Y.; Liao, S.; Wang, Y.; Wen, Z.T.; Xu, X. Antibacterial dental composites with chlorhexidine and mesoporous silica. J. Dent. Res. 2014, 93, 1283–1289. [Google Scholar] [CrossRef]
- Aydin Sevinç, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94B, 22–31. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, B.; Chen, R.; Huang, Z.; Zhu, C.; Zhang, X. Effect of silver-supported materials on the mechanical and antibacterial properties of reinforced acrylic resin composites. Mater. Des. 2015, 65, 1245–1252. [Google Scholar] [CrossRef]
- Braga, R.R. Calcium phosphates as ion-releasing fillers in restorative resin-based materials. Dent. Mater. 2019, 5, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liang, Z.; Li, J.; Bai, Y.; He, J.; Lin, Z. Size Dependence of Particulate Calcium Phosphate Fillers in Dental Resin Composites. ACS Omega 2021, 6, 35057–35066. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, T.; Satheeshkumar, S.; Naveen, J. Glass fiber-reinforced polymer composites—A review. J. Reinf. Plast. Compos. 2014, 33, 1258–1275. [Google Scholar] [CrossRef]
- Bijelic-Donova, J.; Garoushi, S.; Lassila, L.V.J.; Keulemans, F.; Vallittu, P.K. Mechanical and structural characterization of discontinuous fiber-reinforced dental resin composite. J. Dent. 2016, 52, 70–78. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, F.; Xie, H.; Gu, N. Nanoparticle-reinforced resin-based dental composites. J. Dent. 2008, 36, 450–455. [Google Scholar] [CrossRef]
- Sprenger, S. Epoxy resin composites with surface-modified silicon dioxide nanoparticles: A review. J. Appl. Polym. Sci. 2013, 130, 1421–1428. [Google Scholar] [CrossRef]
- Nakanishi, L.; Kaizer, M.R.; Brandeburski, S.; Cava, S.S.; Della Bona, A.; Zhang, Y.; Moraes, R.R. Non-silicate nanoparticles for improved nanohybrid resin composites. Dent. Mater. 2020, 36, 1314–1321. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Khaksar, S.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Salahshour, P.; Sadeghi, F.; Rostamnia, S.; Vahdat, S.M. Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review. Biomolecules 2022, 12, 155. [Google Scholar] [CrossRef]
- Mohamed, A.; Nabih, S.; Wakwak, M. Effect of chitosan nanoparticles on microtensile bond strength of resin composite to dentin: An in vitro study. Braz. Dent. Sci. 2020, 23, 1–10. [Google Scholar] [CrossRef]
- Tanaka, C.B.; Lopes, D.P.; Kikuchi, L.N.T.; Moreira, M.S.; Catalani, L.H.; Braga, R.R.; Kruzic, J.J.; Gonçalves, F. Development of novel dental restorative composites with dibasic calcium phosphate loaded chitosan fillers. Dent. Mater. 2020, 36, 551–559. [Google Scholar] [CrossRef]
- Zhu, Y.; Marin, L.M.; Xiao, Y.; Gillies, E.R.; Siqueira, W.L. pH-Sensitive Chitosan Nanoparticles for Salivary Protein Delivery. Nanomaterials 2021, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, H.A.; Soliman, N.K.; Al-Saudi, K.W. Antibacterial and preventive effects of newly developed modified nano-chitosan/glass-ionomer restoration on simulated initial enamel caries lesions: An in vitro study. Dent. Med. Probl. 2024, 61, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Biju, D.; Arumugam, P.; Kannan, S.; Yadalam, P.K.; Ronsivalle, V.; Cicciù, M.; Minervini, G. Development, characterization, and biocompatibility and corrosion analyses of a silver-decorated graphene oxide and chitosan surface coating for titanium dental implants: A preliminary report. Dent. Med. Probl. 2024, 61, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Lin, C.P.; Wang, Y.L. Development of antibacterial composite resin containing chitosan/fluoride microparticles as pit and fissure sealant to prevent caries. J. Oral Microbiol. 2021, 14, 2008615. [Google Scholar] [CrossRef]
- Ali, S.; Sangi, L.; Kumar, N. Exploring antibacterial activity and hydrolytic stability of resin dental composite restorative materials containing chitosan. Technol. Health Care 2017, 25, 11–18. [Google Scholar] [CrossRef]
- Kim, J.S.; Shin, D.H. Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin. Restor. Dent. Endod. 2013, 38, 36–42. [Google Scholar] [CrossRef]
- Kikuchi, L.N.T.; Freitas, S.R.M.; Amorim, A.F.; Delechiave, G.; Catalani, L.H.; Braga, R.R.; Moreira, M.S.; Boaro, L.C.C.; Gonçalves, F. Effects of the crosslinking of chitosan/DCPA particles in the antimicrobial and mechanical properties of dental restorative composites. Dent. Mater. 2022, 38, 1482–1491. [Google Scholar] [CrossRef]
- Masoumi, M.; Valizadeh, S.; Carvalho, R.M.; Moghaddam, A.A.; Ghodsi, S. The effect of adding chitosan nanoparticles on different properties of the adhesive and high-filled composite resin. Int. J. Adhes. Adhes. 2024, 134, 103766. [Google Scholar] [CrossRef]
- Chander, N.G.; Venkatraman, J. Mechanical properties and surface roughness of chitosan reinforced heat polymerized denture base resin. J. Prosthodont. Res. 2022, 66, 101–108. [Google Scholar] [CrossRef]
- Harmaji, A.; Saputri, N.D.; Sunendar, B. Chitosan-Modified Alumina–Zirconia–Carbonate Apatite Nanoparticles-Filled Dental Restorative Composite Materials: Characterization and Mechanical Properties. Nanosyst. Nanomater. Nanotechnol. 2023, 21, 817–828. [Google Scholar] [CrossRef]
- Halkai, R.; Gopinagaruri, S.; Halkai, K.R. Evaluation of fracture resistance of maxillary premolars of different geometrical cavities restored with different composite resins incorporated with chitosan nanoparticles. J. Conserv. Dent. Endod. 2024, 27, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Pai, V.; Nadig, R.R. Evaluation of Immediate and Delayed Microleakage of Class V Cavities Restored with Chitosan-incorporated Composite Resins: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2021, 14, 621–627. [Google Scholar] [PubMed]
- Deb, A.; Pai, V.; Akhtar, A.; Nadig, R.R. Evaluation of Microleakage of Micro Hybrid Composite Resins versus Chitosan-Incorporated Composite Resins When Restored in Class V Cavities Using Total Etch and Self-Etch Adhesives: An In vitro Study. Contemp. Clin. Dent. 2021, 12, 346–351. [Google Scholar] [CrossRef]
- Stenhagen, I.S.R.; Rukke, H.V.; Dragland, I.S.; Kopperud, H.M. Effect of methacrylated chitosan incorporated in experimental composite and adhesive on mechanical properties and biofilm formation. Eur. J. Oral Sci. 2019, 127, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Li, D.; Zhou, Z.; Zhang, J.; Liu, D.; Zhao, W.; Zhao, C.; Liu, X. The incorporation of phosphorylated chitosan/amorphous calcium phosphate nanocomplex into an experimental composite resin. Dent. Mater. J. 2021, 40, 422–430. [Google Scholar] [CrossRef]
- Halkai, R.S.; Gopinagaruri, S.P.; Halkai, K.R.; Hussain, A.; Rangappa, J.; Reshma, S.F. Evaluation of push-out bond strength of different concentrations of chitosan nanoparticles incorporated composite resin and eighth-generation bonding agent for class II restoration: An in vitro study. J. Conserv. Dent. 2022, 25, 666–671. [Google Scholar] [CrossRef]
- Farzanegan, F.; Shafaee, H.; Darroudi, M.; Rangrazi, A. Effect of the Incorporation of Chitosan and TiO2 Nanoparticles on the Shear Bond Strength of an Orthodontic Adhesive: An In Vitro Study. J. Adv. Oral Res. 2021, 12, 261–266. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan Biomaterials for Current and Potential Dental Applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef]
- Zalega, M.; Bociong, K. Antibacterial Agents Used in Modifications of Dental Resin Composites: A Systematic Review. Appl. Sci. 2024, 14, 3710. [Google Scholar] [CrossRef]
- Bazli, L.; Nargesi khoramabadi, H.; Modarresi Chahardehi, A.; Arsad, H.; Malekpouri, B.; Asgari Jazi, M.; Azizabadi, N. Factors influencing the failure of dental implants: A systematic review. J. Compos. Compd. 2020, 2, 18–25. [Google Scholar] [CrossRef]
- Dionysopoulos, D.; Gerasimidou, O.; Papadopoulos, C. Current modifications of dental adhesive systems for composite resin restorations: A review in literature. J. Adhes. Sci. Technol. 2021, 36, 453–468. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Pakseresht, A.H.; Shahedi Asl, M.; Esmaeilkhanian, A.; Nargesi khoramabadi, H.; Won Jang, H.; Shokouhimehr, M. Hydroxyapatite consolidated by zirconia: Applications for dental implant. J. Compos. Compd. 2020, 2, 26–34. [Google Scholar] [CrossRef]
- Tebidze, N.; Jincharadze, M.; Margvelasvili, V. Oral complications of palliative patients with advanced cancer. Transl. Clin. Med.-Georgian Med. J. 2017, 2, 20–23. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a Knowledge Representation for Clinical Questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Homa, K.; Zakrzewski, W.; Dobrzyński, W.; Piszko, P.J.; Piszko, A.; Matys, J.; Wiglusz, R.J.; Dobrzyński, M. Surface Functionalization of Titanium-Based Implants with a Nanohydroxyapatite Layer and Its Impact on Osteoblasts: A Systematic Review. J. Funct. Biomater. 2024, 15, 45. [Google Scholar] [CrossRef]
- Rygas, J.; Matys, J.; Wawrzyńska, M.; Szymonowicz, M.; Dobrzyński, M. The Use of Graphene Oxide in Orthodontics—A Systematic Review. J. Funct. Biomater. 2023, 14, 500. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, S.; Kiryk, J.; Horodniczy, T.; Struzik, N.; Wiśniewska, K.; Matys, J.; Dobrzyński, M. Bone Regeneration Capabilities of Scaffolds Containing Chitosan and Nanometric Hydroxyapatite—Systematic Review Based on In Vivo Examinations. Biomimetics 2024, 9, 503. [Google Scholar] [CrossRef]
- Kowalski, J.; Rygas, J.; Homa, K.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. Antibacterial Activity of Endodontic Gutta-Percha—A Systematic Review. Appl. Sci. 2024, 14, 388. [Google Scholar] [CrossRef]
- Kensy, J.; Dobrzyński, M.; Wiench, R.; Grzech-Leśniak, K.; Matys, J. Fibroblasts Adhesion to Laser-Modified Titanium Surfaces—A Systematic Review. Materials 2021, 14, 7305. [Google Scholar] [CrossRef] [PubMed]
- Kiryk, J.; Kiryk, S.; Kensy, J.; Świenc, W.; Palka, B.; Zimoląg-Dydak, M.; Dobrzyński, W.; Matys, J.; Dobrzyński, M. Effectiveness of Laser-Assisted Teeth Bleaching: A Systematic Review. Appl. Sci. 2024, 14, 9219. [Google Scholar] [CrossRef]
- Struzik, N.; Wiśniewska, K.; Piszko, P.J.; Piszko, A.; Kiryk, J.; Matys, J.; Dobrzyński, M. SEM Studies Assessing the Efficacy of Laser Treatment for Primary Teeth: A Systematic Review. Appl. Sci. 2024, 14, 1107. [Google Scholar] [CrossRef]
- Piszko, P.J.; Piszko, A.; Kiryk, J.; Lubojański, A.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. The Influence of Fluoride Gels on the Physicochemical Properties of Tooth Tissues and Dental Materials—A Systematic Review. Gels 2024, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Struzik, N.; Kensy, J.; Piszko, P.J.; Kiryk, J.; Wiśniewska, K.; Kiryk, S.; Korjat, Ł.; Horodniczy, T.; Sobierajska, P.; Matys, J.; et al. Contamination in Bone Substitute Materials: A Systematic Review. Appl. Sci. 2024, 14, 8266. [Google Scholar] [CrossRef]
- Woś, P.; Kiryk, S.; Dyl, T.; Kiryk, J.; Horodniczy, T.; Szablińska, M.; Dubowik, M.A.; Dobrzyński, W.; Mikulewicz, M.; Matys, J.; et al. Laser Applications in Metal Orthodontic Bracket Debonding: A Systematic Review. Appl. Sci. 2025, 15, 927. [Google Scholar] [CrossRef]
- Watson, P.F.; Petrie, A. Method Agreement Analysis: A Review of Correct Methodology. Theriogenology 2010, 73, 1167–1179. [Google Scholar] [CrossRef]

| Study | Aim of the study | Materials and Methods | Results | Conclusions |
|---|---|---|---|---|
| Lai et al. [44] | Assessment of the antibacterial properties, fluoride release, and recharge of fissure sealant containing C/F microparticles. | C/F microparticles were incorporated into the Bis-GMA matrix. The experimental group consisted of samples with the addition of 0%, 2%, and 4% C/F, with ClinproTM fissure sealant as a control group. Antibacterial activity was detected by Alamar Blue assay and colony-forming units (CFU). Biocompatibility was evaluated using WST-1 and LDH tests. ISO standards were applied to measure curing depth, flowability, tensile and flexural strength, while microhardness was determined by the Vickers test. Fluoride release and recharge were analyzed using ionic chromatography. | 2% and 4% C/F demonstrated antibacterial properties, reducing CFU ratios to 10% and 25%, respectively (p < 0.01). However, 4% C/F showed cytotoxicity (p < 0.001). 2% C/F outperformed ClinproTM in curing depth (p < 0.001), microhardness, and tensile strength (p < 0.01), with comparable fluoride release and recharge ability (p = 0.67). | Resin with the addition of 2% C/F was indicated as an antibacterial sealant with satisfactory mechanical strength, present fluoride release, and the ability to recharge. |
| Ali et al. [45] | Exploration of antibacterial activity and hydrolytic stability of resin dental composite restorative materials (RDCRM) containing CS. | The antibacterial activity of microhybrid and flowable RDCRM with 0, 0.25, 0.5, and 1% wt/wt of CS against L. casei bacteria was evaluated via agar diffusion test and direct contact methods. Hydrolytic stability was assessed using gravimetric analysis. | The control and experimental flowable and microhybrid RDCRM showed no growth inhibition zone in the lawn growth of L. casei. The direct contact test revealed comparable CFU count of L. casei among the experimental and control RDCRM. Water sorption and solubility values for all RDCRM formulations remained within ISO standards, with no statistically significant differences between experimental groups. | The addition of one percent of CS in RDCRM had no antibacterial activity against L. casei. The hydrolytic stability of RDCRM containing CS was within the acceptable level. |
| Tanaka et al. [40] | Development of experimental composite resins with CS or CS loaded with DCPA possessing antimicrobial potential while preserving their mechanical properties and biocompatibility. | CS and CS/DCPA particles were synthesized using the electrospray method. The experimental biomaterials were formulated by adding particles (0, 0.5, or 1.0 wt%) into a resin matrix. Conversion degree and mechanical properties were assessed after 1 and 90 days of water aging post-photoactivation. Dental pulp fibroblasts in conditioned medium were used to evaluate cytotoxicity and genotoxicity. S. mutans antimicrobial activity was determined via crystal violet biofilm assay. | Experimental composites showed no cytotoxicity or genotoxicity. All chitosan-containing formulations reduced biofilm by 20% (p < 0.001) compared to the control group. With no detectable CS release, antimicrobial effects likely resulted from direct bacterial contact with exposed surface particles. The addition of the CS and CS/DCPA submicrometer particles to restorative materials maintained the degree of conversion, flexural strength, elastic modulus, and fracture toughness comparable to the control group after 90 days of aging in water. | The addition of CS or CS/DCPA particles provided antimicrobial properties to the composites without compromising mechanical properties or biocompatibility. |
| Ali et al. [18] | Evaluation of the antibacterial activity and the hardness of microhybrid and flowable resin-based composites (RBC) modified with CS. | RBC (flowable and microhybrid composites) were doped by CS in the form of flakes or powder. Number of 40 specimens were subjected to testing (20 flow, 20 microhybrid, both with 0, 0.25, 0.5, and 1.0% of CS addition) | No growth inhibition zones were observed for actinomyces, with comparable CFU counts between experimental and control groups. CS-modified composites demonstrated higher VH (Vickers Hardness), indicating increased surface resistance. | The addition of CS improved mechanical capabilities without improving antibacterial activity. |
| Kim and Shin [46] | Evaluation of antibacterial effect and mechanical properties of RBC incorporating CS. | S. mutans CS powder was inoculated in Brain Heart Infusion (BHI) solution, centrifuged (12 h), and absorbance measured for antibacterial assessment. Mechanical properties were evaluated via Vickers hardness and 3-point bending tests after 1 and 3 weeks of storage, respectively. | CS powder inhibited S. mutans growth, reducing CFU. Mechanical properties showed no significant differences, though one resin exhibited slightly decreased flexural strength and maximum load. | CS addition to restorative materials, provides antibacterial benefits without compromising mechanical properties. |
| Kikuchi et al. [47] | Evaluation of the influence of CS particles loaded with DCPA on the mechanical properties, degree of conversion, and mechanical properties of resin-based composites. | CS particles were added to experimental resins and separately characterized by Minimum inhibitory concentration (MIC) against S. mutans. | Composites demonstrated significantly higher biofilm inhibition. Particle-containing composites showed no significant decrease in flexural strength. | CS enhanced antibacterial activity without affecting mechanical properties compared to the control group |
| Fadhila et al. [19] | The impact of CS and CA on composite resin’s mechanical properties, toxicity, and antibacterial activity was investigated. | 9 resin samples with CA+ CS were tested for: dissolution/water absorption in saliva; microleakage via methylene blue penetration; compressive strength, SBS, and tensile strength; hardness via VH; thermal properties by oven incubation. Toxicity assessed by shrimp larvae viability; antibacterial activity against S. mutans. | CS+CA inhibits S. mutans growth and is non-toxic at low doses. CS+CA addition reduces SBS, hardness, and water absorption; increases solubility, compressive, and tensile strength. No significant difference in microleakage or shrinkage compared to the control. | CS and CA addition enhances antibacterial and mechanical properties without inducing toxicity in the filling material. |
| Masoumi et al. [48] | Assessment of the effect of adding CS to composite resin on material properties. | Composite resin without CS and with 1% CS in the form of discs was obtained. Antibacterial effect—samples were incubated in S. mutans suspension. Biofilm and growth inhibition zones were assessed; resin conversion was measured using a spectrophotometer. Water solubility and sorption assessment of sample mass before and after immersion in water; SBS assessment - resin cylinders were glued to maxillary molars, and the wire and loop method was used. | The addition of CS increased the inhibition of biofilm development on the resin surface and significantly increased the water sorption of the composite. No significant effects were observed on composite conversion degree or SBS. | Incorporating CS into composites confers antibacterial properties without affecting mechanical properties. |
| Chander and Venkatraman [49] | Investigation of the influence of CS addition on the mechanical properties of denture base resin. | Four composite material samples were prepared: without CS and three samples with CS (5, 10, 15%). They were tested for: flexural and fracture strength; impact strength; surface roughness. | After the addition of CS to the resin, flexural strength, fracture strength, and impact strength increased. When the resin reached 15% of CS, the surface roughness decreased. | The addition of CS to dental resin can improve the mechanical parameters and increase its smoothness. |
| Harmaji et al. [50] | Determination of how varying CS concentrations affect the mechanical properties of alumina–zirconia–carbonate apatite nanoparticle composites. | Alumina-zirconia synthesized by sol-gel (900°C calcination); carbonate apatite from calcium nitrate, ammonium hydrogen phosphate, sodium hydrogen carbonate (700 °C calcination); CS solutions (2–6% in acetic acid); dental composite: dimethacrylate matrix and fillers (alumina-zirconia/carbonate apatite 50:50) at 70:30 ratio; light-cured samples tested for hardness, morphology, and composition. | When CS concentration was higher, particle sizes increased. The addition of 2% CS provided the most uniform particle size distribution; higher concentrations caused aggregation. Hardness decreased with increasing CS addition. Only 2% chitosan met dental material hardness standards (30–90 VHN). | The incorporation of CS has been demonstrated to exert an influence on the mechanical properties of the composite. A lower concentration of CS (2%) was found to be optimal for composite manufacturing, producing smaller nanoparticle sizes, improved distribution of the particles, and higher hardness values. |
| Halkai et al. [51] | Evaluation of whether the addition of 0.2% CSN (chitosan nanoparticles) to either universal or bulk-fill composites would improve fracture resistance across various cavity geometries in maxillary premolars. | Number of 130 maxillary premolars were divided as follows: control (n = 10), class I (n = 40), class II MO (n = 40), class II MOD (n = 40). Each cavity group is subdivided: A: Neo spectra ST-Universal composite, B: Tetric N-Ceram Bulk-fill composite, C: NST+0.2% CSN, D: TNC+0.2% CSN. CSN prepared in distilled water/acetic acid. All cavities received G-Premio Bond adhesive. | MOD cavities exhibited the highest fracture resistance, followed by MO cavities, then class I. TNC bulk-fill with CSN consistently demonstrated the highest fracture resistance across all cavity designs. | The addition of CSN improved fracture resistance in all cavity designs. TNC bulk-fill with 0.25% CSN showed the best overall performance. |
| Deb et al. [52] | Microleakage evaluation of microhybrid composite and 0.2% CS-doped composite in Class V cavities. | 60 maxillary premolars with Class V cavities were divided into two groups (n = 30): microhybrid or CS-incorporated composite. Each group contained two subgroups (n = 15): immediate testing or testing after three months in artificial saliva. Microleakage was evaluated via dye extraction and spectrophotometry. | After immediate restoration, no significant microleakage difference existed between CS-modified and unmodified composites. Following 3 months in artificial saliva, microleakage significantly increased in unmodified composites while remaining stable in CS-modified composites. | CS-incorporated composite demonstrated superior stability, enhanced mechanical properties, and antibacterial advantages versus the unmodified microhybrid composite. |
| Deb et. Al. [53] | In vitro microleakage evaluation of 0.2% CS-enriched composite resin compared to microhybrid composite resin using a two-etching system. | Class V cavities in 60 human teeth were divided into 4 groups: two with the total-etch system and two with the self-etch system. Each group received either traditional or experimental material. Microleakage was measured using dye and spectrophotometry. | A significant difference favoring the CS-enriched composite was observed with the total-etch method. No significant difference was found between total-etch and self-etching systems. | The addition of CS did not interfere with the bonding of the composite resin to dentin. |
| Stenhagen et al. [54] | Synthesis of methacrylated chitosan (CH-MA) and its addition to dental composite. Assessment of mechanical properties and biofilm inhibition of the biomaterial. | The experimental composite was prepared by partial filler replacement with CH-MA. Resulting disks were used for S. mutans biofilm testing, Kaiser test analysis, and mechanical property evaluation. | CS was observed to have a significant impact on the reduction of S. mutans biofilm formation and composite hardness, as compared with the control group. | Incorporation of CH-MA into the experimental composite reduced S. mutans biofilm formation. An increased amount of CH-MA negatively affected the mechanical properties of the material. |
| Niu et al. [55] | Investigation of the mechanical and chemical properties of a bioactive composite resin that contains phosphorylated CS and amorphous calcium phosphate (Pchi/ACP). | Pchi was synthesized by the Nashi method. Composite was prepared by incorporating freeze-dried Pchi/ACP. The material was employed in the fabrication of disks for the purpose of conducting a series of experiments. The experiments conducted in this study encompassed the assessment of calcium ion release, hardness, wetting angle, modulus of elasticity, flexibility, and remineralization properties. Moreover, scanning electron microscopy (SEM) images were obtained to enable subsequent analysis. | Increasing Pchi/ACP content produced smoother surfaces, reduced wetting angles, decreased hardness and elasticity, while enhancing calcium ion release and dentin remineralization. | The mechanical and chemical properties of the composite deteriorate with the addition of Pchi/ACP. |
| Halkai et al. [56] | Bond strength evaluation of a composite containing different concentrations of chitosan nanoparticles (CSN). | Class II cavities were made in 70 molars and then filled with composite containing 0.25% and 2% CSN. Push-out bond strength was also tested. | The highest bond strength was observed when using the composite with 0.25% CSN content. However, it was not statistically different from the control group. | The investigation revealed that the incorporation of 0.25% CSN into a composite resin did not exert any significant influence on the push-out bond strength of class II restorations, as compared to the control material. |
| Farzanegan et al. [57] | Assessment of how varying concentrations of CSN and TiO2 NPs influence the shear bond strength (SBS) of an orthodontic adhesive. | 72 extracted human premolars embedded in an acrylic resin and randomly allocated into four groups (n = 18). Gr. 1 (control): Brackets bonded with Transbond XT orthodontic adhesive Gr. 2: Bonding with Transbond XT containing 0.5% CSN and 0.5% TiO2 (anatase) NPs; Gr 3.: Bonding with Transbond XT containing 1% CSN and 1% TiO2 (anatase) NPs; Gr. 4: Bonding with Transbond XT containing 1.5% CSN and 1.5% TiO2 (anatase) NPs. SBS and ARI were evaluated using a universal testing machine. | No statistically significant differences in SBS were found among groups 1, 2, and 3, while group 4 showed significantly decreased SBS. Increasing concentration to 1% (both chitosan and TiO₂) did not significantly affect SBS; however, at 1.5% concentration, SBS was lower compared to other groups. No significant differences in ARI scores were observed between groups. | The orthodontic composite containing 1% CSN and 1% TiO2 NPs has adequate SBS for use in the clinical setting. |
| Authors | Chitosan Concentration | Tested Properties | Effect on Bacterial Biofilm | Influence on Mechanical Properties | Influence on Shear Bond Strength | Fluoride Release Capacity |
|---|---|---|---|---|---|---|
| Lai et al. [44] | 0, 2, 4% C/F microparticles addition into the resin | Particle size, antibacterial properties, cell survival, and mechanical properties | Bis-GMA reduces metabolic activity and biofilm biomass. | The incorporation of 2% of C/F particles has resulted in an increase in the curing depth, microhardness, and tensile strength of the material. There was a decrease in flowability, whilst flexural strength was observed to remain unchanged. Mechanical strength 73.18 MPa | No data | Comparable to sole sealant |
| Ali et al. [45] | RDCRM containing 0, 0.25, 0.5, and 1 wt% of chitosan | Antibacterial properties and water sorption | No antibacterial effect against L. casei bacteria. | No data | No data | No data |
| Tanaka et al. [40] | 0.5 and 1 wt% addition of chitosan and CS/DCPA particles | Cytotoxicity and genotoxicity, chitosan release, antimicrobial activity, mechanical properties, degree of conversion, elemental analysis | Both the CS and CS/DCPA containing composites demonstrated antimicrobial properties against S. mutans. | Following a 90-day hydration period, the mechanical properties of all composites were found to be equivalent, indicating that the presence of CS or CS/DCPA submicrometer fillers did not exert a detrimental influence on the composites’ mechanical properties. Flexural strength 85 MPa | No data | No data |
| Ali et al. [18] | 0, 0.25, 0.5, and 1% | Antibacterial properties and hardness | No antibacterial activity has been presented. The control and experimental groups were comparable. | VH was significantly higher in the group with flowable RBC modified with 1% chitosan. Group consisting of microhybrid RBC modified with 0, 5% CS demonstrated significantly higher VH. Vickers hardness: Control (MH0) = 49 VHN MH0.25 = 48 VHN MH0.5 = 50 VHN MH1.0 = 52 VHN Control (FA0) = 82 VHN FA0.25 = 77 VHN FA0.5 = 83 VHN FA1.0 = 75 VHN | No data | No data |
| Kim and Shin [46] | Three CS powders with different molecular weights: low, medium, and high (75–85% deacylated powder) | Antibacterial properties, inhibitory effect on S. mutans, bending test, hardness, flexural strength, flexural modulus | The effect of CS on S. mutans: absorbance was significantly higher. CFUs in chitosan-modified groups were significantly lower. | There was no significant difference in VH and flexural modulus. Maximum load and flexural strength were significantly lower in CS modified resins. Flexural strength: Control = 108.3 MPa L = 86.28 MPa M = 64.02 MPa H = 89.36 MPa Vickers hardness: Control = 32.63 VHN L = 32.04 VHN M = 29.63 VHN H = 29.72 VHN | No data | No data |
| Kikuchi et al. [47] | CS/DCPA was added in 0, 5% concentration to the composite resin. Crosslinking times were 0, 8, and 16 h. | Mechanical properties, degree of conversion, and antibacterial properties | Composite resins modified with CS/DCPA crosslinked for 16 h showed the highest percentage of dead bacteria. The amount of dead cells was higher in all modified resins in comparison with the control group. | Flexural strength of modified resins was lower for the group crosslinked for 8 h. The storage of the material in water has been shown to reduce the flexural strength of the material with non-crosslinked particles in comparison with storage in a dry state after 24 h and seven days. The storage in acid reduced the flexural strength in the material with particles crosslinked for 8 h after 7 days. Regarding elastic modulus, it was determined that neither the degree of crosslinking of the particles nor the storage medium had a significant impact on the flexural strength of the investigated materials. Flexural strength: Control = 81 MPa 0 h = 74 MPa 8 h = 83 MPa 16 h = 77 MPa Elastic modulus: Control = 3.2 GPa 0 h = 3.1 GPa 8 h = 2.8 GPa 16 h = 3.4 GPa | No data | No data |
| Fadhila et al. [19] | 3, 5, 7% of CS addition in the matrix containing CA | Solubility, water absorption, microleakage, compressive strength, shear bond strength, tensile strength, hardness, toxicity, antibacterial properties, thermal expansion/shrinkage | Mean zone of inhibition of S. mutans growth (mm): Cellulose (12,80); CS (8,70); Cellulose + CS (7,57); negative control (0,00); positive control (20,10) | Reduction in SBS, hardness, and water absorption; Increase in solubility, compressive strength, and tensile strength. No change: microleakage, shrinkage Vickers hardness: Control = 33.9 VHN Samples with chitosan: 0.4–1.17 VHN | No significant differences between the control and experimental groups were observed | No data |
| Masoumi et al. [48] | 1% of CS addition | Antibacterial properties, water absorption, solubility, and SBS | CFU: 1. S. mutans—control: 13.800; composite with CS 5.971 2. S. sanguinis—control: 19.400; composite with CS 7.014 3. L. acidophilus—control: 16.885; composite with CS: 7.728 | No data | Micro SBS values ( MPa): 1. Control: 6.122 2. Pretreatment: 5.382 3. Adhesive + CS: 6.304 4. Composite + CS: 5.739 | No data |
| Chander and Venkatraman [49] | 5, 10, 15% | Flexural strength, fracture strength, impact strength, surface roughness | No data | Improvement of flexural strength, fracture strength, and impact strength Flexural strength 0% = 67.890 MPa 5% = 73.019 MPa 10% = 71.903 MPa 15% = 69.253 MPa | No data | No data |
| Harmaji et al. [50] | 2, 4, and 6% addition of CS | Hardness, particle size, morphology, and phase composition | No data | Lower chitosan concentration improves the mechanical properties of composites: 2%—51.3 VHN, 4%—28.24 VHN, 6%—25.48 VHN | No data | No data |
| Halkai et al. [51] | 0.2% addition of CSN | Fracture resistance | No data | No data | No data | No data |
| Deb et al. [52] | 0.2% addition of CS | Microleakage | No data | The composite demonstrated a statistically significant increase in microleakage, from 0.0105 nm to 0.0158 nm over a duration of three months. In contrast, CS + composite demonstrated a comparatively minor increase from 0.0096 nm to 0.0117 nm, exhibiting no statistically significant difference | No data | No data |
| Deb et al. [53] | 0.2% addition of CS | Microleakage | No data | No data | No data | No data |
| Stenhagen et al. [54] | 5, 10, 20% addition of CH-MA | Antibacterial effect, flexural strength, hardness, amount of free amino groups, SBS | The addition of 10–20% CH-MA to composites resulted in a reduction in S. mutans biofilm formation at pH 5.9 after 24 h and two weeks (in comparison with control groups), with no effect observed at pH 7. However, 20% CH-MA in adhesives led to a decrease in biofilm at pH 7 after 24 h. | Flexural strength Control = 131.0 MPa 5% = 87.0 MPa 10% = 72.0 MPa 20% = 65.8 MPa Hardness Control = 56.8 (3.0) HV 5% = 43.7 (1.2) HV 10% = 39.5 (1.2) HV 20% = 25.9 (1.1) HV | Control = 24.0 MPa 10% = 22.8 Mpa 20% = 22.4 MPa | No data |
| Niu et al. [55] | 15, 25, 35% addition of Pchi/ACP | Morphology, contact angle, flexural strength, elastic modulus, hardness, calcium ion release, remineralization | No data | Flexural strength, elastic modulus, and hardness decreased with the addition of CS No numeric data | No data | No data |
| Halkai et al. [56] | 0,25 or 2% | Push-out bond strength | No data | No data | Mean value Control = 568.8 (139.80)N 0,25% = 623.2 (60.52)N 2% = 475.4 (33.85)N | No data |
| Farzanegan et al. [57] | 0.5, 1.0, and 1.5% of CSN and TiO2 NPs | SBS, ARI | No data | ARI scores were not statistically significant between groups | The addition of 1.5% CSN and 1.5% TiO2 NPs resulted in a decrease in SBS compared to the other three groups | No data |
|
Authors/ Criteria | Randomization | Sample Size Calculation | Control Group | Detailed Description of the Percentage of Chitosan in the Material | Description of Specimen Manufacturing |
Application of Standardized Procedures (ISO) | Blinding | Number of Research Methods Applied (Microbiological/Mechanical/ Physicochemical): 1 method— 1 point 2 methods— 2 points 3 methods— 3 points | Total Points | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Lai et al. [44] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 3 | 7 | moderate |
| Ali et al. [45] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 2 | 6 | moderate |
| Tanaka et al. [40] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 3 | 7 | moderate |
| Ali et al. [18] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 2 | 6 | moderate |
| Kim and Shin [46] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 2 | 6 | moderate |
| Kukuchi et al. [47] | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 3 | 6 | moderate |
| Fadhila et al. [19] | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 3 | 7 | moderate |
| Masoumi et al. [48] | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 3 | 8 | low |
| Chander and Venkatraman [49] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 2 | 6 | moderate |
| Harmaji et al. [50] | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 4 | high |
| Halkai et al. [51] | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 4 | high |
| Deb et al. [52] | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 4 | high |
| Deb et al. [53] | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 5 | moderate |
| Stenhagen et al. [54] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 3 | 7 | moderate |
| Niu et al. [55] | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 5 | moderate |
| Halkai et al. [56] | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 4 | high |
| Farzanegan et al. [57] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 | moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzyński, W.; Piszko, P.J.; Kiryk, J.; Kiryk, S.; Michalak, M.; Kotela, A.; Kensy, J.; Świenc, W.; Grychowska, N.; Matys, J.; et al. Dental Resin Composites Modified with Chitosan: A Systematic Review. Mar. Drugs 2025, 23, 199. https://doi.org/10.3390/md23050199
Dobrzyński W, Piszko PJ, Kiryk J, Kiryk S, Michalak M, Kotela A, Kensy J, Świenc W, Grychowska N, Matys J, et al. Dental Resin Composites Modified with Chitosan: A Systematic Review. Marine Drugs. 2025; 23(5):199. https://doi.org/10.3390/md23050199
Chicago/Turabian StyleDobrzyński, Wojciech, Paweł J. Piszko, Jan Kiryk, Sylwia Kiryk, Mateusz Michalak, Agnieszka Kotela, Julia Kensy, Witold Świenc, Natalia Grychowska, Jacek Matys, and et al. 2025. "Dental Resin Composites Modified with Chitosan: A Systematic Review" Marine Drugs 23, no. 5: 199. https://doi.org/10.3390/md23050199
APA StyleDobrzyński, W., Piszko, P. J., Kiryk, J., Kiryk, S., Michalak, M., Kotela, A., Kensy, J., Świenc, W., Grychowska, N., Matys, J., & Dobrzyński, M. (2025). Dental Resin Composites Modified with Chitosan: A Systematic Review. Marine Drugs, 23(5), 199. https://doi.org/10.3390/md23050199










