Improvement of Catalytic Activity and Thermostability of Alginate Lyase VxAly7B-CM via Rational Computational Design Strategies
Abstract
1. Introduction
2. Results
2.1. Rational Design of Mutants
2.2. Biochemical Characterization of VxAly7B-CM and Its Mutants
2.3. Optimal Temperature and Thermal Stability of VxAly7B-CM and the Mutant E188N/S204G
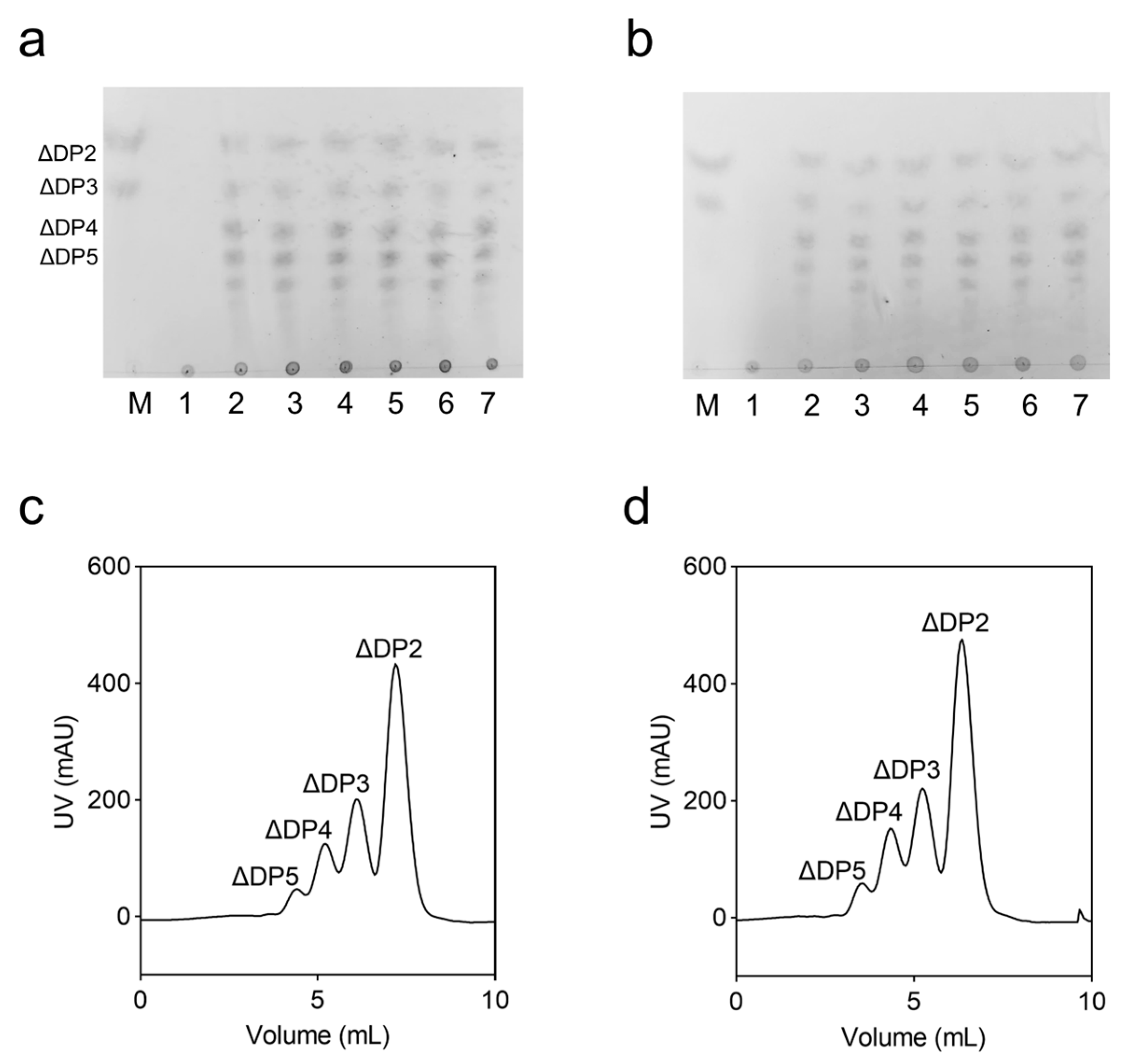
2.4. Analysis of Degradation Products
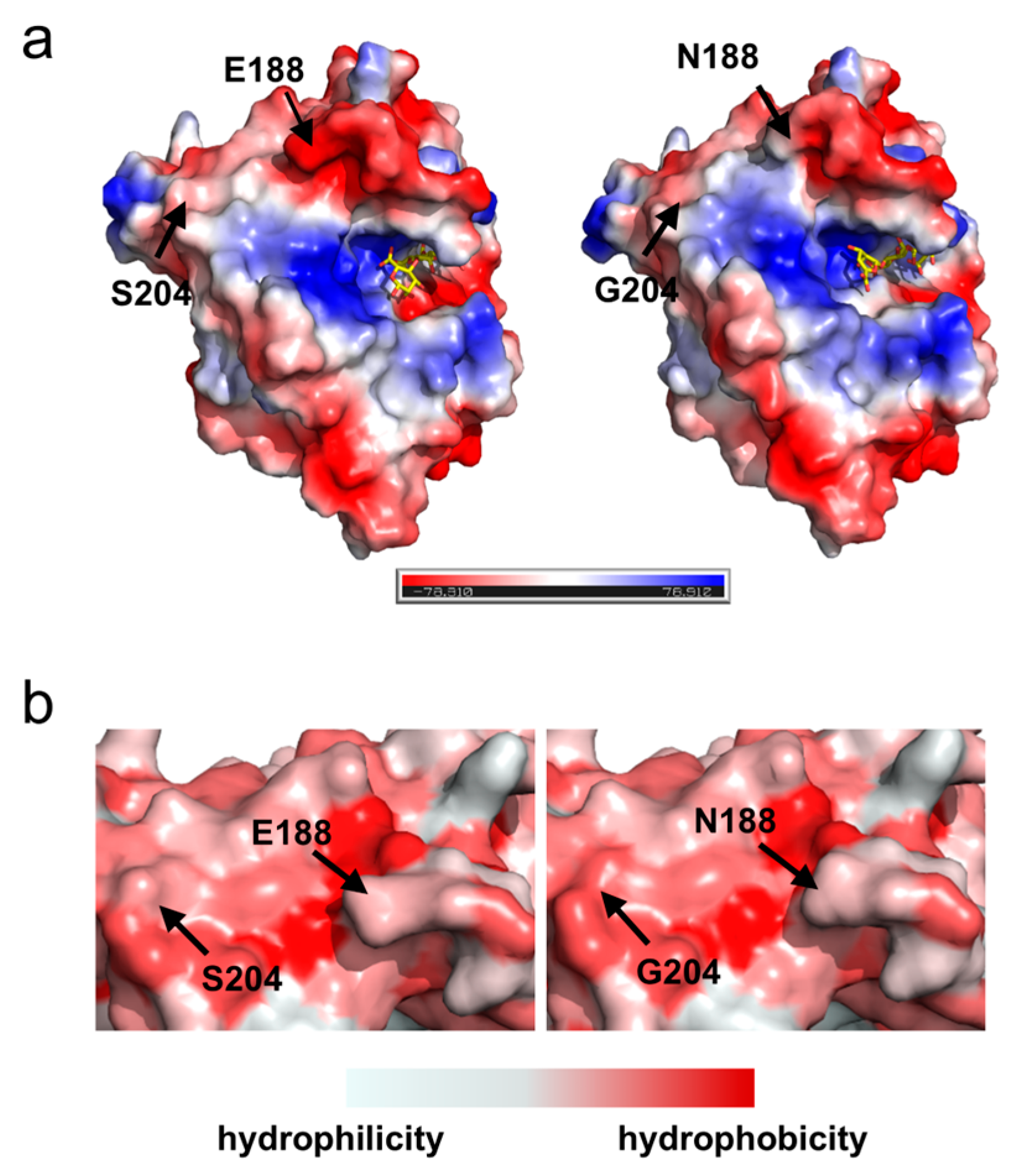
2.5. Surface Charge and Hydrophobic Distribution of Proteins
2.6. Analysis of Protein Intramolecular Interaction
2.7. MD Simulations Analysis
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Chemicals
4.2. Mutation Site Selection and Construction
4.2.1. Rational Design of Candidate Mutants
4.2.2. Construction of Mutants by Site-Directed Mutagenesis
4.2.3. Expression and Purification of VxAly7B-CM and Its Mutants
4.3. Biochemical Characterization of VxAly7B-CM and Its Mutants
4.3.1. Enzyme Activity Assay
4.3.2. Optimal Temperature and Thermal Stability Assay
4.4. Analysis of Degradation Product
4.5. Structural Analysis and Molecular Dynamics Simulations
4.5.1. Protein Structure Visualization and Analysis
4.5.2. Molecular Docking
4.5.3. Molecular Dynamics Simulations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOS | Alginate oligosaccharides |
| Tm | Melting temperature |
| DP | Degree of polymerization |
| UAOS | Unsaturated alginate oligosaccharides |
| DEH | 4-deoxy-L-erythro-5- hexoseulose uronate |
| ED | Entner–Doudoroff |
| PL | Polysaccharide lyase families |
| MD | Molecular dynamics |
| RMSD | Root-mean-square deviations |
| RMSF | Root-mean-square fluctuations |
| SASA | Solvent-accessible surface area |
| IPTG | Isopropyl β-D-1-thiogalactopyranoside |
| TLC | Thin-layer chromatography |
| FPLC | Fast protein liquid chromatography |
| ESI-MS | Electrospray ionization mass spectrometry |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
References
- Pawar, S.N.; Edgar, K.J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, C.; Yuan, J.; Qin, S. Alginate Oligosaccharide Improves Lipid Metabolism and Inflammation by Modulating Gut Microbiota in High-Fat Diet Fed Mice. Appl. Microbiol. Biotechnol. 2020, 104, 3541–3554. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, M.F.; Powell, L.C.; Jack, A.A.; Powell, K.; Beck, K.; Florance, H.; Forton, J.; Rye, P.D.; Dessen, A.; Hill, K.E.; et al. A Low-Molecular-Weight Alginate Oligosaccharide Disrupts Pseudomonal Microcolony Formation and Enhances Antibiotic Effectiveness. Antimicrob. Agents Chemother. 2017, 61, e00762-17. [Google Scholar] [CrossRef]
- Bi, D.; Lai, Q.; Cai, N.; Li, T.; Zhang, Y.; Han, Q.; Peng, Y.; Xu, H.; Lu, J.; Bao, W.; et al. Elucidation of the Molecular-Mechanisms and In Vivo Evaluation of the Anti-Inflammatory Effect of Alginate-Derived Seleno-Polymannuronate. J. Agric. Food Chem. 2018, 66, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-J.; Xu, F.-Q.; Li, Y.-H.; Li, J.; Liu, X.; Wang, X.-F.; Hu, L.-G.; An, Y. Alginate Oligosaccharide Alleviates Myocardial Reperfusion Injury by Inhibiting Nitrative and Oxidative Stress and Endoplasmic Reticulum Stress-Mediated Apoptosis. Drug Des. Devel Ther. 2017, 11, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium Oligomannate Therapeutically Remodels Gut Microbiota and Suppresses Gut Bacterial Amino Acids-Shaped Neuroinflammation to Inhibit Alzheimer’s Disease Progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Wang, Y.; Han, F.; Hu, B.; Li, J.; Yu, W. In Vivo Prebiotic Properties of Alginate Oligosaccharides Prepared through Enzymatic Hydrolysis of Alginate. Nutr. Res. 2006, 26, 597–603. [Google Scholar] [CrossRef]
- Garron, M.-L.; Cygler, M. Structural and Mechanistic Classification of Uronic Acid-Containing Polysaccharide Lyases. Glycobiology 2010, 20, 1547–1573. [Google Scholar] [CrossRef]
- Shen, Z.; Yin, H.; Sun, L.; Chen, L.; Li, J.; Zhang, X.; Zeng, M.; Jiang, X.; Yu, J. Influence of Consumption of Unsaturated Alginate Oligosaccharides on the Gut Microbiota and the Intestinal Mucosal Immunity Homeostasis in Immunocompromised Mice. Carbohydr. Polym. Technol. Appl. 2024, 8, 100604. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, M.; Ji, X.; Fan, L.; Zhao, L. Alginate Oligosaccharides Improve High-Fat Induced Hepatic Steatosis via PGC-1α-Mediated Lipophagy and Fatty Acid β-Oxidation Pathway. J. Funct. Foods 2023, 110, 105825. [Google Scholar] [CrossRef]
- Dong, S.; Wei, T.-D.; Chen, X.-L.; Li, C.-Y.; Wang, P.; Xie, B.-B.; Qin, Q.-L.; Zhang, X.-Y.; Pang, X.-H.; Zhou, B.-C.; et al. Molecular Insight into the Role of the N-Terminal Extension in the Maturation, Substrate Recognition, and Catalysis of a Bacterial Alginate Lyase from Polysaccharide Lyase Family 18*. J. Biol. Chem. 2014, 289, 29558–29569. [Google Scholar] [CrossRef] [PubMed]
- Takase, R.; Ochiai, A.; Mikami, B.; Hashimoto, W.; Murata, K. Molecular Identification of Unsaturated Uronate Reductase Prerequisite for Alginate Metabolism in Sphingomonas Sp. A1. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2010, 1804, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Preston, L.A.; Schiller, N.L. Alginate Lyase: Review of Major Sources and Enzyme Characteristics, Structure-Function Analysis, Biological Roles, and Applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, H.; Wang, X.; Dai, Q.; Liu, X.; Ni, D.; Zhu, Y.; Xu, W.; Mu, W. Rational Design for Thermostability Improvement of a Novel PL-31 Family Alginate Lyase from Paenibacillus sp. YN15. Int. J. Biol. Macromol. 2023, 253, 126919. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Guo, Z.; Tang, T.; Zhu, B. Alginate Degrading Enzymes: An Updated Comprehensive Review of the Structure, Catalytic Mechanism, Modification Method and Applications of Alginate Lyases. Crit. Rev. Biotechnol. 2021, 41, 953–968. [Google Scholar] [CrossRef]
- Gao, S.-K.; Yin, R.; Wang, X.-C.; Jiang, H.-N.; Liu, X.-X.; Lv, W.; Ma, Y.; Zhou, Y.-X. Structure Characteristics, Biochemical Properties, and Pharmaceutical Applications of Alginate Lyases. Mar. Drugs 2021, 19, 628. [Google Scholar] [CrossRef]
- Dobruchowska, J.M.; Bjornsdottir, B.; Fridjonsson, O.H.; Altenbuchner, J.; Watzlawick, H.; Gerwig, G.J.; Dijkhuizen, L.; Kamerling, J.P.; Hreggvidsson, G.O. Enzymatic Depolymerization of Alginate by Two Novel Thermostable Alginate Lyases from Rhodothermus Marinus. Front. Plant Sci. 2022, 13, 981602. [Google Scholar] [CrossRef]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and Utilization of Thermophiles and Thermostable Enzymes in Biorefining. Microb. Cell Fact. 2007, 6, 9. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Pan, L.; Yang, L.; Li, H.; Ji, F.; Zhang, Y.; Tang, H.; Yang, D. Improving the Thermostability of Alginate Lyase FlAlyA with High Expression by Computer-Aided Rational Design for Industrial Preparation of Alginate Oligosaccharides. Front. Bioeng. Biotechnol. 2022, 10, 1011273. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yang, M.; Liu, N.; Wang, S.; Sun, Y.; Sun, G.; Mou, H.; Zhou, D. Computer-Aided Rational Design Strategy to Improve the Thermal Stability of Alginate Lyase AlyMc. J. Agric. Food Chem. 2024, 72, 3055–3065. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Zhang, Y.; Chen, L. High-Level Expression of a Thermally Stable Alginate Lyase Using Pichia Pastoris, Characterization and Application in Producing Brown Alginate Oligosaccharide. Mar. Drugs 2018, 16, 158. [Google Scholar] [CrossRef]
- Li, J.-W.; Dong, S.; Song, J.; Li, C.-B.; Chen, X.-L.; Xie, B.-B.; Zhang, Y.-Z. Purification and Characterization of a Bifunctional Alginate Lyase from Pseudoalteromonas sp. SM0524. Mar. Drugs 2011, 9, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Chang, Y.; Shen, J. Cloning, Expression and Characterization of an Endo-Acting Bifunctional Alginate Lyase of Marine Bacterium Wenyingzhuangia fucanilytica. Protein Expr. Purif. 2019, 154, 44–51. [Google Scholar] [CrossRef]
- Tang, L.; Guo, E.; Zhang, L.; Wang, Y.; Gao, S.; Bao, M.; Han, F.; Yu, W. The Function of CBM32 in Alginate Lyase VxAly7B on the Activity on Both Soluble Sodium Alginate and Alginate Gel. Front. Microbiol. 2022, 12, 798819. [Google Scholar] [CrossRef]
- Hopf, T.A.; Green, A.G.; Schubert, B.; Mersmann, S.; Schärfe, C.P.I.; Ingraham, J.B.; Toth-Petroczy, A.; Brock, K.; Riesselman, A.J.; Palmedo, P.; et al. The EVcouplings Python Framework for Coevolutionary Sequence Analysis. Bioinformatics 2019, 35, 1582–1584. [Google Scholar] [CrossRef]
- Tavakoli, Y.; Esmaeili, A.; Saber, H. Increasing Thermal Stability and Catalytic Activity of Glutamate Decarboxylase in E. Coli: An in Silico Study. Comput. Biol. Chem. 2016, 64, 74–81. [Google Scholar] [CrossRef]
- Musil, M.; Jezik, A.; Horackova, J.; Borko, S.; Kabourek, P.; Damborsky, J.; Bednar, D. FireProt 2.0: Web-Based Platform for the Fully Automated Design of Thermostable Proteins. Brief. Bioinform. 2023, 25, bbad425. [Google Scholar] [CrossRef]
- Vanella, R.; Küng, C.; Schoepfer, A.A.; Doffini, V.; Ren, J.; Nash, M.A. Understanding Activity-Stability Tradeoffs in Biocatalysts by Enzyme Proximity Sequencing. Nat. Commun. 2024, 15, 1807. [Google Scholar] [CrossRef]
- Zheng, C.; Ji, Z.; Mathews, I.I.; Boxer, S.G. Enhanced Active-Site Electric Field Accelerates Enzyme Catalysis. Nat. Chem. 2023, 15, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Gribenko, A.V.; Makhatadze, G.I. Role of the Charge–Charge Interactions in Defining Stability and Halophilicity of the CspB Proteins. J. Mol. Biol. 2007, 366, 842–856. [Google Scholar] [CrossRef]
- Gribenko, A.V.; Patel, M.M.; Liu, J.; McCallum, S.A.; Wang, C.; Makhatadze, G.I. Rational Stabilization of Enzymes by Computational Redesign of Surface Charge–Charge Interactions. Proc. Natl. Acad. Sci. USA 2009, 106, 2601–2606. [Google Scholar] [CrossRef] [PubMed]
- Tanford, C. The Hydrophobic Effect and the Organization of Living Matter. Science 1978, 200, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Vol’kenshtein, M.V. Hydrophobic Interactions and Protein Structure. In Enzyme Physics; Springer: Boston, MA, USA, 1969; pp. 18–29. ISBN 978-1-4899-2836-8. [Google Scholar]
- Rahban, M.; Zolghadri, S.; Salehi, N.; Ahmad, F.; Haertlé, T.; Rezaei-Ghaleh, N.; Sawyer, L.; Saboury, A.A. Thermal Stability Enhancement: Fundamental Concepts of Protein Engineering Strategies to Manipulate the Flexible Structure. Int. J. Biol. Macromol. 2022, 214, 642–654. [Google Scholar] [CrossRef]
- Wong, E.T.C.; Na, D.; Gsponer, J. On the Importance of Polar Interactions for Complexes Containing Intrinsically Disordered Proteins. PLoS Comput. Biol. 2013, 9, e1003192. [Google Scholar] [CrossRef]
- Guo, Q.; Dan, M.; Zheng, Y.; Shen, J.; Zhao, G.; Wang, D. Improving the Thermostability of a Novel PL-6 Family Alginate Lyase by Rational Design Engineering for Industrial Preparation of Alginate Oligosaccharides. Int. J. Biol. Macromol. 2023, 249, 125998. [Google Scholar] [CrossRef]
- Su, J.; Wu, H.; Yin, C.; Zhang, F.; Han, F.; Yu, W. The Hydrophobic Cluster on the Surface of Protein Is the Key Structural Basis for the SDS-Resistance of Chondroitinase VhChlABC. Mar. Life Sci. Technol. 2023, 6, 93–101. [Google Scholar] [CrossRef]
- Ali, S.A.; Hassan, M.I.; Islam, A.; Ahmad, F. A Review of Methods Available to Estimate Solvent-Accessible Surface Areas of Soluble Proteins in the Folded and Unfolded States. Curr. Protein Pept. Sci. 2014, 15, 456–476. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Tan, G.-Y.; Xin, Z.; Wang, W. In Vitro Allosteric Transcription Factor-Based Biosensing. Trends Biotechnol. 2023, 41, 1080–1095. [Google Scholar] [CrossRef]
- Zheng, N.; Cai, Y.; Zhang, Z.; Zhou, H.; Deng, Y.; Du, S.; Tu, M.; Fang, W.; Xia, X. Tailoring Industrial Enzymes for Thermostability and Activity Evolution by the Machine Learning-Based iCASE Strategy. Nat. Commun. 2025, 16, 604. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, S.-X.; Liu, Z.-M.; Li, N.-N.; Li, L.; Mou, H.-J. Rational Design of Alginate Lyase from Microbulbifer sp. Q7 to Improve Thermal Stability. Mar. Drugs 2019, 17, 378. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wu, D.; Xu, X.; Xu, L.; Wang, L.; Lin, J. Design of a PL18 Alginate Lyase with Flexible Loops and Broader Entrance to Enhance the Activity and Thermostability. Enzym. Microb. Technol. 2021, 151, 109916. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Panchenko, A.R. Structural and Functional Roles of Coevolved Sites in Proteins. PLoS ONE 2010, 5, e8591. [Google Scholar] [CrossRef]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting Functional Effect of Human Missense Mutations Using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 7, Unit7.20. [Google Scholar] [CrossRef]
- Sim, N.-L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT Web Server: Predicting Effects of Amino Acid Substitutions on Proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef]
- Hopf, T.A.; Ingraham, J.B.; Poelwijk, F.J.; Schärfe, C.P.I.; Springer, M.; Sander, C.; Marks, D.S. Mutation Effects Predicted from Sequence Co-Variation. Nat. Biotechnol. 2017, 35, 128–135. [Google Scholar] [CrossRef]
- Facciotti, M.T.; Bertain, P.B.; Yuan, L. Improved Stearate Phenotype in Transgenic Canola Expressing a Modified Acyl-Acyl Carrier Protein Thioesterase. Nat. Biotechnol. 1999, 17, 593–597. [Google Scholar] [CrossRef]
- Lin, W.; Wang, Q.; Han, R.; Zhou, J.; Xu, G.; Ni, Y. Engineering of Methionine Adenosyltransferase Reveals Key Roles of Electrostatic Interactions in Enhanced Catalytic Activity. Appl. Biochem. Biotechnol. 2024, 196, 3246–3259. [Google Scholar] [CrossRef]
- Georgiev, D.D.; Glazebrook, J.F. Thermal Stability of Solitons in Protein α-Helices. Chaos Solitons Fractals 2022, 155, 111644. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, M.; Wang, Y.; Ma, R.; Guo, C.; Feng, L.; Wu, J.; Yao, H.; Lin, D. Structural Basis for the Complete Resistance of the Human Prion Protein Mutant G127V to Prion Disease. Sci. Rep. 2018, 8, 13211. [Google Scholar] [CrossRef] [PubMed]
- Lüthy, R.; Bowie, J.U.; Eisenberg, D. Assessment of Protein Models with Three-Dimensional Profiles. Nature 1992, 356, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Guerois, R.; Nielsen, J.E.; Serrano, L. Predicting Changes in the Stability of Proteins and Protein Complexes: A Study of More than 1000 Mutations. J. Mol. Biol. 2002, 320, 369–387. [Google Scholar] [CrossRef]
- Yao, Y.; Li, S.; Cao, J.; Liu, W.; Qi, F.; Xiang, W.; Yang, K.; Wang, W.; Zhang, L. A Novel Signal Transduction System for Development of Uric Acid Biosensors. Appl. Microbiol. Biotechnol. 2018, 102, 7489–7497. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Y.; Gao, S.; Wu, H.; Wang, D.; Yu, W.; Han, F. Biochemical Characteristics and Molecular Mechanism of an Exo-Type Alginate Lyase VxAly7D and Its Use for the Preparation of Unsaturated Monosaccharides. Biotechnol. Biofuels 2020, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Peytam, F.; Takalloobanafshi, G.; Saadattalab, T.; Norouzbahari, M.; Emamgholipour, Z.; Moghimi, S.; Firoozpour, L.; Bijanzadeh, H.R.; Faramarzi, M.A.; Mojtabavi, S.; et al. Design, Synthesis, Molecular Docking, and in Vitro α-Glucosidase Inhibitory Activities of Novel 3-Amino-2,4-Diarylbenzo[4,5]Imidazo[1,2-a]Pyrimidines against Yeast and Rat α-Glucosidase. Sci. Rep. 2021, 11, 11911. [Google Scholar] [CrossRef]
- Rodrigues, C.H.; Pires, D.E.; Ascher, D.B. DynaMut: Predicting the Impact of Mutations on Protein Conformation, Flexibility and Stability. Nucleic Acids Res. 2018, 46, W350–W355. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega. Curr. Protoc. Bioinform. 2014, 48, 3.13.1–3.13.16. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering Key Features in Protein Structures with the New ENDscript Server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Mikami, B.; Ban, M.; Suzuki, S.; Yoon, H.-J.; Miyake, O.; Yamasaki, M.; Ogura, K.; Maruyama, Y.; Hashimoto, W.; Murata, K. Induced-Fit Motion of a Lid Loop Involved in Catalysis in Alginate Lyase A1-III. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 1207–1216. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved Side-Chain Torsion Potentials for the Amber ff99SB Protein Force Field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]






| Name | Tm (°C) | Specific Activity (U/mg) |
|---|---|---|
| WT | 47.0 | 2935.76 ± 37.40 |
| E188D | 48.1 | 3096.17 ± 77.98 |
| E188N | 48.9 | 3701.02 ± 118.17 |
| S194M | 46.4 | 2799.74 ± 57.95 |
| S204G | 50.2 | 2812.01 ± 76.20 |
| Q214S | 46.6 | 1975.93 ± 55.40 |
| S296L | 48.5 | 2492.81 ± 141.51 |
| K368D | 47.8 | 1945.44 ± 32.79 |
| V370I | 48.4 | 2163.29 ± 96.81 |
| H375K | 47.2 | 2429.83 ± 80.60 |
| A384G | 43.7 | 2661.22 ± 90.39 |
| E188N/S204G | 52.0 | 3832.80 ± 17.84 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhu, K.; Wang, K.; Liao, W.; Yang, X.; Yu, W.; Wang, W.; Han, F. Improvement of Catalytic Activity and Thermostability of Alginate Lyase VxAly7B-CM via Rational Computational Design Strategies. Mar. Drugs 2025, 23, 198. https://doi.org/10.3390/md23050198
Ma X, Zhu K, Wang K, Liao W, Yang X, Yu W, Wang W, Han F. Improvement of Catalytic Activity and Thermostability of Alginate Lyase VxAly7B-CM via Rational Computational Design Strategies. Marine Drugs. 2025; 23(5):198. https://doi.org/10.3390/md23050198
Chicago/Turabian StyleMa, Xin, Ke Zhu, Kaiyang Wang, Wenhui Liao, Xiaohan Yang, Wengong Yu, Weishan Wang, and Feng Han. 2025. "Improvement of Catalytic Activity and Thermostability of Alginate Lyase VxAly7B-CM via Rational Computational Design Strategies" Marine Drugs 23, no. 5: 198. https://doi.org/10.3390/md23050198
APA StyleMa, X., Zhu, K., Wang, K., Liao, W., Yang, X., Yu, W., Wang, W., & Han, F. (2025). Improvement of Catalytic Activity and Thermostability of Alginate Lyase VxAly7B-CM via Rational Computational Design Strategies. Marine Drugs, 23(5), 198. https://doi.org/10.3390/md23050198









