Improving the Value Utilization of Tuna Peptide Powder for the Cosmetics Field Through Ozone Oxidation
Abstract
1. Introduction
2. Results
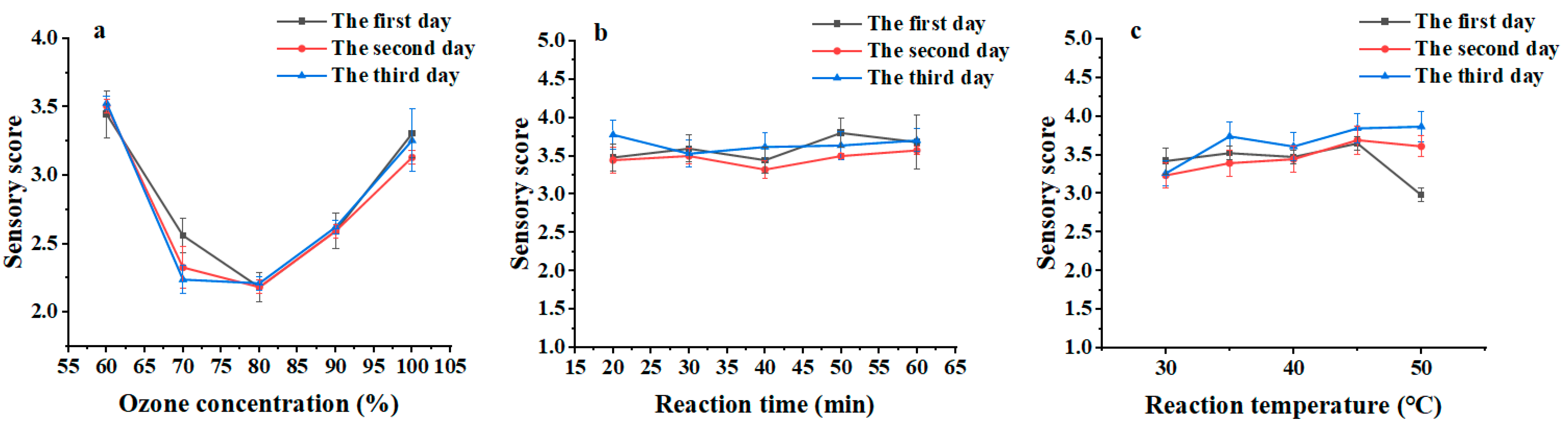
2.1. The Optimum Conditions for the Dry Ozonation
2.2. The Optimum Conditions for the Wet Ozonation
2.3. Low Loss of Total Nitrogen and Amino Acid Nitrogen
2.4. High Stability at Low and High Temperatures
2.5. Reduction in Fishy Odor
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
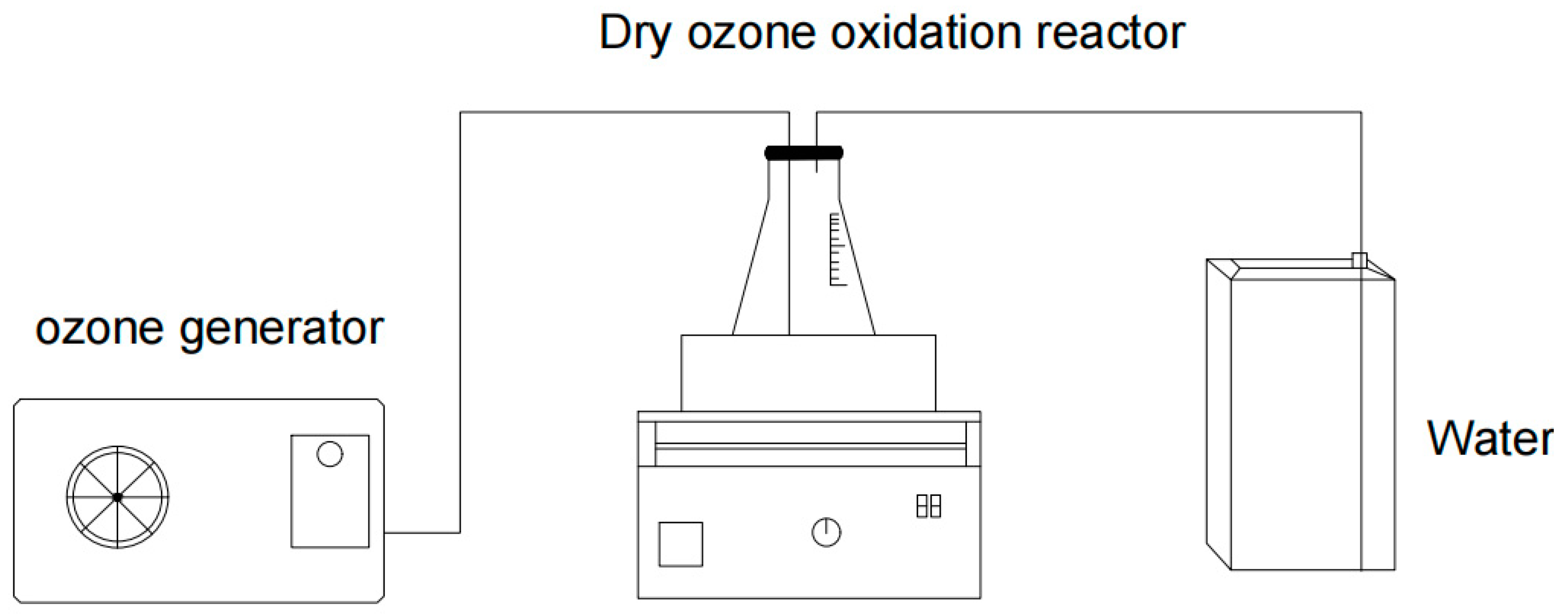
4.2. Dry Ozonation of Tuna Peptides
4.3. Wet Ozonation of Tuna Peptides
4.4. Sensory Evaluation
4.5. Low- and High-Temperature Stability Tests
4.6. Test Conditions for GC-MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, D.S. The Synergistic Effects of Fish Collagen Peptide, Bonito Elastin Peptide, Fish Maw Powder and Rose Water on The Improvement of Skin Conditions: A Mechanistic Study. Am. J. Biomed. Sci. Res. 2022, 17, 67–71. [Google Scholar] [CrossRef]
- Silva, T.; Moreira-Silva, J.; Marques, A.; Domingues, A.; Bayon, Y.; Reis, R. Marine Origin Collagens and Its Potential Applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Lim, Y.S.; Kim, J.-E.; Kang, H.Y.; Lee, C.; Rajbongshi, L.; Hwang, S.Y.; Oh, S.-O.; Kim, B.S.; Lee, D.; et al. A Marine Collagen-Based 3D Scaffold for In Vitro Modeling of Human Prostate Cancer Niche and Anti-Cancer Therapeutic Discovery. Mar. Drugs 2024, 22, 295. [Google Scholar] [CrossRef]
- Maqbool, A.; Strandvik, B.; Stallings, V.A. The skinny on tuna fat: Health implications. Public Health Nutr. 2013, 14, 2049–2054. [Google Scholar] [CrossRef]
- Amnuaikit, T.; Shankar, R.; Benjakul, S. Hydrolyzed fish collagen serum from by-product of food industry: Cosmetic product formulation and facial skin evaluation. Sustainability 2022, 14, 16553. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Liu, J.H.; Ji, J.J.; Wang, B.; Fang, Y.Z.; Ding, Y.T. Decolorization technology of enzymatic hydrolysate of tuna and its effect on odor substances and metal elements. J. Nucl. Agric. Sci. 2015, 29, 743–750. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, K.; Zhang, J.M.; Hu, L.; Huang, Y.; Fan, W.J. Optimization of Deodorization Technology for Cod by Using Response Surface Methodology. Food Res. Dev. 2019, 40, 178–184. [Google Scholar] [CrossRef]
- Bao, J.L.; Fang, X.B.; Chen, X.E.; Liu, S.T.; Zhang, F.; Shen, J.Y. Optimization of deodorization process and analysis of fishy aftertaste substances in Pangasius bocourti fillets. Sci. Technol. Food Ind. 2022, 43, 70–76. [Google Scholar] [CrossRef]
- Zeng, S.D.; Wu, J.Z.; Ou, S.Y.; Jin, J. Analysis of Volatile Components in Tilapia (Oreochromus niloticus) Enzymatic Hydrolysates. Food Sci. 2010, 31, 342–346. [Google Scholar] [CrossRef]
- Wu, S.F.; Hou, Y.M. Study on soft canned flavoured eel. Food Sci. Technol. 1998, 29–31. [Google Scholar] [CrossRef]
- Fu, X.J.; Xu, S.Y.; Kim, J. Removing the Fishy Flavor of Silver Carp Protein by Yeast Fermentation and the Mechanism. J. Food Sci. Biotechnol. 2009, 28, 57–62. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.Y. Technology of Enzyme Solution to Silver Carp Protein and Debittering and Deodorization Effects of Active Dry Yeast. Food Ind. 2012, 33, 45–47. [Google Scholar]
- Hanada, K.; Okuda, D.; Ogi, R.; Kojima, S.; Tsuruoka, R.; Shiota, G. Ozonized glycerin (OG)-based cosmetic products lighten age spots on human facial skin. J. Cosmet. Dermatol. 2022, 21, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Josephson, D.B.; Lindsay, R.C.; Stuiber, D.A. Variations in the occurrences of enzymically derived volatile aroma compounds in salt- and freshwater fish. J. Agric. Food Chem. 1984, 32, 1344–1347. [Google Scholar] [CrossRef]
- Karaca, H.; Velioglu, Y.S. Effects of Ozone Treatments on Microbial Quality and Some Chemical Properties of Lettuce, Spinach, and Parsley. Postharvest Biol. Technol. 2014, 88, 46–53. [Google Scholar] [CrossRef]
- Hassanin, S.O.; Hegab, A.M.M.; Mekky, R.H.; Said, M.A.; Khalil, M.G.; Hamza, A.A.; Amin, A. Combining In Vitro, In Vivo, and Network Pharmacology Assays to Identify Targets and Molecular Mechanisms of Spirulina-Derived Biomolecules against Breast Cancer. Mar. Drugs 2024, 22, 328. [Google Scholar] [CrossRef]
- Roh, S.S.; Choi, I.; Kim, H.-M.; Lee, M.S.; Jin, M.-H.; Kim, B.H.; Hwang, S.-J.; Kim, M.H. Clinical efficacy of herbal extract cream on the skin hydration, elasticity, thickness, and dermis density for aged skin: A randomized controlled double-blind study. J. Cosmet. Dermatol. 2019, 18, 1389–1394. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, H.; Wu, S.; Mao, C.; Dai, Y.; Yan, H. Marine Bioactive Peptides: Anti-Photoaging Mechanisms and Potential Skin Protective Effects. Curr. Issues Mol. Biol. 2024, 46, 990–1009. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, S.; Chen, J.; Wang, M.; Lin, Z.; Liu, Y. Synthesis and Characterization of a Novel Chitosan-Based Nanoparticle–Hydrogel Composite System Promising for Skin Wound Drug Delivery. Mar. Drugs 2024, 22, 428. [Google Scholar] [CrossRef]
- Cao, W.-J.; Liu, R.; Zhao, W.-X.; Li, J.; Wang, Y.; Yuan, X.-J.; Wang, H.-L.; Zhang, Y.-Z.; Chen, X.-L.; Zhang, Y.-Q. Potential of Marine Bacterial Metalloprotease A69 in the Preparation of Peanut Peptides with Angiotensin-Converting Enzyme (ACE)-Inhibitory and Antioxidant Properties. Mar. Drugs 2024, 22, 305. [Google Scholar] [CrossRef] [PubMed]
- Morakul, B.; Teeranachaideekul, V.; Wongrakpanich, A.; Leanpolchareanchai, J. The evidence from in vitro primary fibroblasts and a randomized, double-blind, placebo-controlled clinical trial of tuna collagen peptides intake on skin health. J. Cosmet. Dermatol. 2024, 23, 4255–4267. [Google Scholar] [CrossRef] [PubMed]
- Jawhari, F.Z.; El Moussaoui, A.; Bourhia, M.; Imtara, H.; Mechchate, H.; Es-Safi, I.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Grafov, A.; et al. Anacyclus pyrethrum (L): Chemical Composition, Analgesic, Anti-Inflammatory, and Wound Healing Properties. Molecules 2020, 25, 5469. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.B.; Liu, L.; Kotiw, M.; Benkendorff, K. Review of anti-inflammatory, immune-modulatory and wound healing properties of molluscs. J. Ethnopharmacol. 2018, 210, 156–178. [Google Scholar] [CrossRef]
- Luo, J.; Frank, D.; Arcot, J. Creating Alternative Seafood Flavour from Non-Animal Ingredients: A Review of Key Flavour Molecules Relevant to Seafood. Food Chem. X 2024, 22, 101400. [Google Scholar] [CrossRef]
- Ilie, G.-I.; Milea, Ș.-A.; Râpeanu, G.; Cîrciumaru, A.; Stănciuc, N. Sustainable Design of Innovative Kiwi Byproducts-Based Ingredients Containing Probiotics. Foods 2022, 11, 2334. [Google Scholar] [CrossRef]
- Güneş, Y.; Atav, R.; Namırtı, O. Effectiveness of ozone in decolorization of reactive dye effluents depending on the dye chromophore. Text. Res. J. 2012, 82, 994–1000. [Google Scholar] [CrossRef]
- Chen, H.B.; Liang, P.; Wang, Q.; Chen, X.H. Optimized deodorization process of Lateolabrax japonicas meat by response surface methodology. J. Food Saf. Qual. 2022, 13, 4203–4211. [Google Scholar] [CrossRef]
- Sun, J.; Mao, X. An environmental friendly process for Antarctic krill (Euphausia superba) utilization using fermentation technology. J. Clean. Prod. 2016, 127, 618–623. [Google Scholar] [CrossRef]
- Khazandi, M.; Deo, P.; Ferro, S.; Venter, H.; Pi, H.; Crabb, S.; Amorico, T.; Ogunniyi, A.D.; Trott, D.J. Efficacy evaluation of a new water sanitizer for increasing the shelf life of Southern Australian King George Whiting and Tasmanian Atlantic Salmon fillets. Food Microbiol. 2017, 68, 51–60. [Google Scholar] [CrossRef]
- Urbano, V.R.; Maniero, M.G.; Pérez-Moya, M.; Guimarães, J.R. Influence of pH and ozone dose on sulfaquinoxaline ozonation. J. Environ. Manag. 2017, 195, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, A.; Stylianou, S.K.; Kaffes, P.; Zouboulis, A.I.; Voutsa, D. Effects of ozonation pretreatment on natural organic matter and wastewater derived organic matter-Possible implications on the formation of ozonation by-products. Chemosphere 2017, 170, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Travagli, V.; Iorio, E.L. The Biological and Molecular Action of Ozone and Its Derivatives: State-of-the-Art, Enhanced Scenarios, and Quality Insights. Int. J. Mol. Sci. 2023, 24, 8465. [Google Scholar] [CrossRef] [PubMed]
- Deleebeeck, L.; Gil, V.; Ippolito, D.; Campana, R.; Hansen, K.K.; Holtappels, P. Direct Coal Oxidation in Modified Solid Oxide Fuel Cells. J. Electrochem. Soc. 2017, 164, F333–F337. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Pishbin, E. Ozone-Based Advanced Oxidation Processes in Water Treatment: Recent Advances, Challenges, and Perspective. Environ. Sci. Pollut. Res. 2025, 32, 3531–3570. [Google Scholar] [CrossRef]
- Jiang, W.; He, Y.; Xiong, S.; Liu, Y.; Yin, T.; Hu, Y.; You, J. Effect of Mild Ozone Oxidation on Structural Changes of Silver Carp (Hypophthalmichthys molitrix) Myosin. Food Bioprocess Technol. 2017, 10, 370–378. [Google Scholar] [CrossRef]
- Sharma, V.K.; Graham, N.J.D. Oxidation of Amino Acids, Peptides and Proteins by Ozone: A Review. Ozone Sci. Eng. 2010, 32, 81–90. [Google Scholar] [CrossRef]
- Leon, B.R.; Romary, D.J.; Landsberger, S.A.; Bradner, K.N.; Ramirez, M.; Lubitz, R.M. Risks of Ozonated Oil and Ozonated Water on Human Skin: A Systematic Review. Int. Wound J. 2022, 19, 1901–1910. [Google Scholar] [CrossRef]
- Moradi, N.; Vazquez, C.L.; Hernandez, H.G.; Brdjanovic, D.; van Loosdrecht, M.C.; Rincón, F.R. Removal of contaminants of emerging concern from the supernatant of anaerobically digested sludge by O3 and O3/H2O2: Ozone requirements, effects of the matrix, and toxicity. Environ. Res. 2023, 235, 116597. [Google Scholar] [CrossRef]
- Wilson, K.L.; Birks, J.W. Mechanism and elimination of a water vapor interference in the measurement of ozone by UV absorbance. Environ. Sci. Technol. 2006, 40, 6361–6367. [Google Scholar] [CrossRef]
- Wang, F.; Yang, J.S.; Shang, Y.L.; Xia, S.Y. The Analysis of Nutrition Components in Yellow-Fin Tuna. Food Ind. 2013, 34, 187–189. [Google Scholar]
- Qiu, A.Y.; Song, J.; Tang, J. Determination of volatile constituents of fatty acid esters in fish oil. J. Wuxi Univ. Light Ind. 1997, 16, 20–25. [Google Scholar]
- Zhang, Z. Study on Preparation of Flavoring Base Materials for Aquatic Products from Tilapia Processing by-Products. Master’s Thesis, Ocean University of China, Qingdao, China, 2012. [Google Scholar]
- GB 5009.5-2016; National Food Safety Standard—Determination of Protein in Foods. National Health Commission of the People’s Republic of China & State Administration for Market Regulation: Beijing, China, 2016.
- GB 5009.235-2016; National Food Safety Standard—Determination of Amino Acid Nitrogen in Foods. National Health Commission of the People’s Republic of China & State Administration for Market Regulation: Beijing, China, 2016.
- Chen, D.K.; Chen, H.; Pan, J.Y.; Chen, X.G.; Liu, S.Y.; Sun, H.L. Stability of paphia undulate small-molecule peptides in the aqueous cosmetics. Hubei Agric. Sci. 2015, 54, 3208–3212. [Google Scholar] [CrossRef]
- Arya, P. Phytochemical Analysis and Antimicrobial Evaluation of Essential Oils from Eucalyptus tereticornis Sm. Int. J. Res. Appl. Sci. Eng. Technol. 2024, 12, 1196–1201. [Google Scholar] [CrossRef]
- Furdak, P.; Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Extracts of Common Vegetables Inhibit the Growth of Ovary Cancer Cells. Foods 2022, 11, 2518. [Google Scholar] [CrossRef] [PubMed]
- Šinu, A.; Kuštere, A.; Šķesters, A.; Vinceviča-Gaile, Z. Evaluation of Headspace Solid-Phase Microextraction and Static Headspace Methods Coupled with Gas Chromatography–Mass Spectrometry for the Determination of Volatile Compounds in Kombucha Beverages. Beverages 2023, 9, 88. [Google Scholar] [CrossRef]
- Tang, N. Insights into Chemical Structure-Based Modeling for New Sweetener Discovery. Foods 2023, 12, 2563. [Google Scholar] [CrossRef]
- Alali, H.; Ai, Y.; Pan, Y.-L.; Videen, G.; Wang, C. A Collection of Molecular Fingerprints of Single Aerosol Particles in Air for Potential Identification and Detection Using Optical Trapping-Raman Spectroscopy. Molecules 2022, 27, 5966. [Google Scholar] [CrossRef]

| Experiment | Time (min) | Ozone Concentration (mg/L) | Sensory Score |
|---|---|---|---|
| Sensory scores for samples in single-factor dry ozonation experiments | |||
| 1 | 30 | 50.2 | 4.7 ± 0.2 a |
| 2 | 60 | 50.2 | 4.7 ± 0.2 a |
| 3 | 90 | 50.2 | 4.5 ± 0.1 ab |
| 4 | 120 | 50.2 | 4.1 ± 0.2 bc |
| 5 | 180 | 50.2 | 4.1 ± 0.1 bc |
| 6 | 240 | 50.2 | 4.0 ± 0.1 bcd |
| 7 | 300 | 50.2 | 4.0 ± 0.1 bcd |
| 8 | 60 | 0.1 | 5.0 ± 0.2 a |
| 9 | 60 | 4.2 | 4.8 ± 0.3 a |
| 10 | 60 | 50.2 | 4.5 ± 0.3 ab |
| 11 | 60 | 99.1 | 3.7 ± 0.3 cd |
| 12 | 60 | 104.6 | 3.5 ± 0.4 d |
| Sensory scores for samples in the orthogonal dry ozonation experiments | |||
| 1 | 60 | 60.8 | 4.1 ± 0.3 ab |
| 2 | 60 | 99.1 | 4.2 ± 0.4 a |
| 3 | 60 | 101.2 | 3.8 ± 0.4 ab |
| 4 | 120 | 60.8 | 3.6 ± 0.3 ab |
| 5 | 120 | 99.1 | 3.8 ± 0.4 ab |
| 6 | 120 | 101.2 | 3.7 ± 0.3 ab |
| 7 | 180 | 60.8 | 3.6 ± 0.4 ab |
| 8 | 180 | 99.1 | 3.4 ± 0.2 b |
| 9 | 180 | 101.2 | 3.5 ± 0.3 ab |
| Experiment | Time (min) | Temperature (°C) | Ozone Concentration (mg/L) | Sensory Score |
|---|---|---|---|---|
| Sensory scores for samples in the single-factor wet ozonation experiments | ||||
| 1 | 30 | 35 | 50.2 | 4.6 ± 0.1 ab |
| 2 | 60 | 35 | 50.2 | 4.6 ± 0.1 ab |
| 3 | 90 | 35 | 50.2 | 4.5 ± 0.1 bc |
| 4 | 120 | 35 | 50.2 | 4.3 ± 0.2 c |
| 5 | 180 | 35 | 50.2 | 4.3 ± 0.1 c |
| 6 | 240 | 35 | 50.2 | 4.4 ± 0.1 bc |
| 7 | 300 | 35 | 50.2 | 4.5 ± 0.1 bc |
| 8 | 60 | 35 | 0.1 | 5.0 ± 0.3 a |
| 9 | 60 | 35 | 4.2 | 4.8 ± 0.3 ab |
| 10 | 60 | 35 | 50.2 | 4.5 ± 0.3 bc |
| 11 | 60 | 35 | 99.1 | 2.2 ± 0.3 e |
| 12 | 60 | 35 | 104.6 | 2.8 ± 0.2 d |
| 13 | 60 | 30 | 50.2 | 4.2 ± 0.2 c |
| 14 | 60 | 40 | 50.2 | 4.2 ± 0.1 c |
| 15 | 60 | 50 | 50.2 | 4.0 ± 0.2 c |
| 16 | 60 | 60 | 50.2 | 4.2 ± 0.2 c |
| 17 | 60 | 70 | 50.2 | 4.4 ± 0.3 bc |
| Sensory scores for the samples in the orthogonal wet ozonation experiments | ||||
| 1 | 20 | 30 | 50.2 | 4.6 ± 0.3 a |
| 2 | 30 | 35 | 50.2 | 4.4 ± 0.3 ab |
| 3 | 40 | 40 | 50.2 | 4.7 ± 0.3 a |
| 4 | 50 | 45 | 50.2 | 4.3 ± 0.3 abc |
| 5 | 60 | 50 | 50.2 | 4.4 ± 0.3 ab |
| 6 | 20 | 35 | 60.8 | 3.7 ± 0.3 bcd |
| 7 | 30 | 40 | 60.8 | 3.6 ± 0.1 cde |
| 8 | 40 | 45 | 60.8 | 3.6 ± 0.1 cde |
| 9 | 50 | 50 | 60.8 | 3.5 ± 0.4 def |
| 10 | 60 | 30 | 60.8 | 3.6 ± 0.5 cde |
| 11 | 20 | 40 | 99.1 | 3.4 ± 0.2 defg |
| 12 | 30 | 45 | 99.1 | 2.9 ± 0.2 efghi |
| 13 | 40 | 50 | 99.1 | 2.3 ± 0.3 i |
| 14 | 50 | 30 | 99.1 | 2.6 ± 0.3 hi |
| 15 | 60 | 35 | 99.1 | 2.8 ± 0.3 fghi |
| 16 | 20 | 45 | 101.2 | 3.1 ± 0.3 defgh |
| 17 | 30 | 50 | 101.2 | 2.8 ± 0.3 fghi |
| 18 | 40 | 30 | 101.2 | 2.9 ± 0.3 efghi |
| 19 | 50 | 35 | 101.2 | 2.7 ± 0.3 ghi |
| 20 | 60 | 40 | 101.2 | 3.1 ± 0.3 defgh |
| 21 | 20 | 50 | 104.6 | 3.2 ± 0.2 defgh |
| 22 | 30 | 30 | 104.6 | 2.8 ± 0.4 fghi |
| 23 | 40 | 35 | 104.6 | 2.8 ± 0.5 fghi |
| 24 | 50 | 40 | 104.6 | 2.7 ± 0.4 ghi |
| 25 | 60 | 45 | 104.6 | 2.7 ± 0.4 ghi |
| Original Sample | Dry Sample | Wet Sample | |
|---|---|---|---|
| Total nitrogen content (calculated as N) (g/100 g) | 15.4 ± 0.3 a | 14.3 ± 0.1 b | 15.0 ± 0.2 a |
| Amino acid nitrogen (g/100 g) | 2.5 ± 0.1 a | 2.2 ± 0.2 a | 2.3 ± 0.1 a |
| Sample | Sample Returned to Room Temperature Within | ||||
|---|---|---|---|---|---|
| 1 Day | 3 Days | 7 Days | 21 Days | 28 Days | |
| Cosmetic containing 1% untreated tuna peptide powder | Barely acceptable | Unacceptable | Unacceptable | Unacceptable | Unacceptable |
| Cosmetic containing 1% ozone-treated tuna peptide powder | Moderately acceptable | Moderately acceptable | Moderately acceptable | Moderately acceptable | Moderately acceptable |
| Volatile Substance | Original Concentration (µg/L) | Concentration of Treated Sample (µg/L) | OT (µg/L) | The OAV of Original Sample | The OAV of Treated Sample | Reduction Level (%) |
|---|---|---|---|---|---|---|
| n-Hexaldehyde | 5.9 ± 0.2 | 2.0 ± 0.1 | 0.02 | 295 | 100 | 66.5 ± 0.2 |
| Nonaldehyde | 0.8 ± 0.1 | 1.9 ± 0.2 | 1.2 | 0.7 | 1.6 | −59.3 ± 0.1 |
| Octanal | 0.3 ± 0.2 | 0.5 ± 0.1 | 5.0 | 0.06 | 0.1 | −33.3 ± 0.1 |
| Volatile Substance | Retention Time (min) | Qualitative Factor (m/z) | Categorical Factor (m/z) |
|---|---|---|---|
| n-Hexaldehyde | 19.797 | 57 | 71 |
| Nonaldehyde | 16.080 | 57 | 43 |
| Octanal | 12.238 | 57 | 83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Gu, S.; Huang, S.; Zhang, Y.; Xu, F.; Lv, D.; Yuan, W.; Zhu, K.; Chen, X. Improving the Value Utilization of Tuna Peptide Powder for the Cosmetics Field Through Ozone Oxidation. Mar. Drugs 2025, 23, 191. https://doi.org/10.3390/md23050191
Zheng H, Gu S, Huang S, Zhang Y, Xu F, Lv D, Yuan W, Zhu K, Chen X. Improving the Value Utilization of Tuna Peptide Powder for the Cosmetics Field Through Ozone Oxidation. Marine Drugs. 2025; 23(5):191. https://doi.org/10.3390/md23050191
Chicago/Turabian StyleZheng, Haolong, Shiyang Gu, Shiqi Huang, Yan Zhang, Feng Xu, Daofei Lv, Wenbing Yuan, Kongyu Zhu, and Xin Chen. 2025. "Improving the Value Utilization of Tuna Peptide Powder for the Cosmetics Field Through Ozone Oxidation" Marine Drugs 23, no. 5: 191. https://doi.org/10.3390/md23050191
APA StyleZheng, H., Gu, S., Huang, S., Zhang, Y., Xu, F., Lv, D., Yuan, W., Zhu, K., & Chen, X. (2025). Improving the Value Utilization of Tuna Peptide Powder for the Cosmetics Field Through Ozone Oxidation. Marine Drugs, 23(5), 191. https://doi.org/10.3390/md23050191






