Anti-Neuroinflammatory Eremophilane Sesquiterpenoids from Marine-Derived Fungus Phoma sp. DXH009
Abstract
1. Introduction
2. Results and Discussion
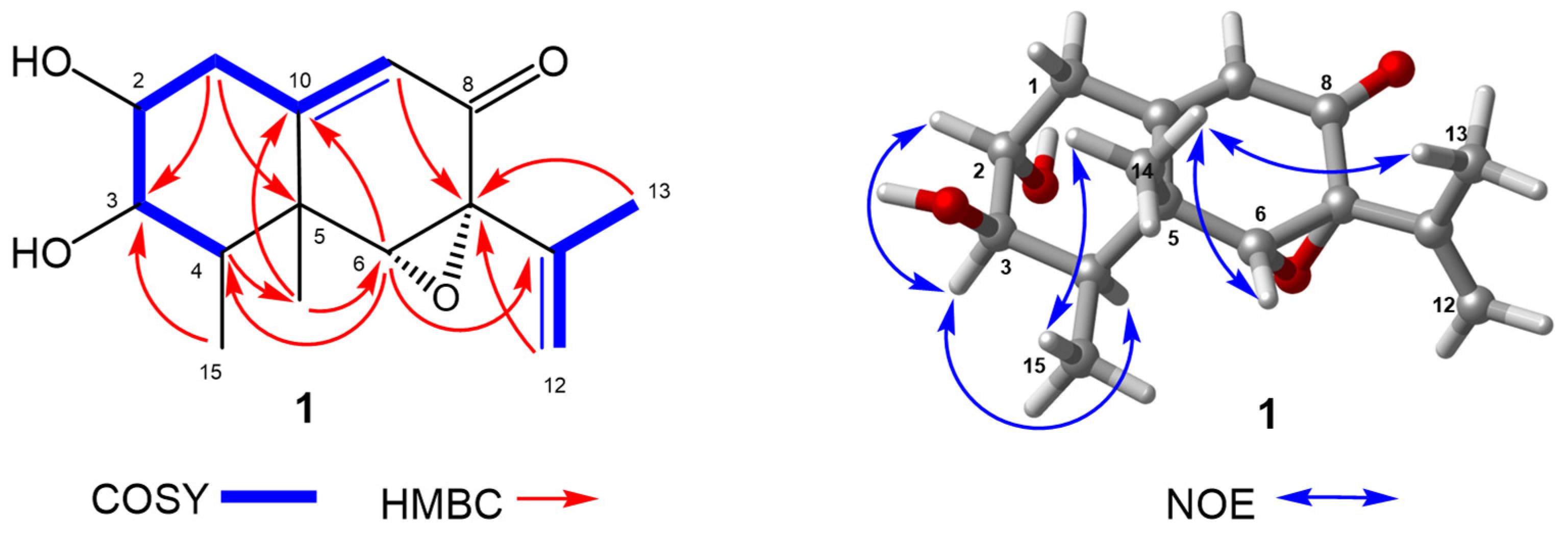
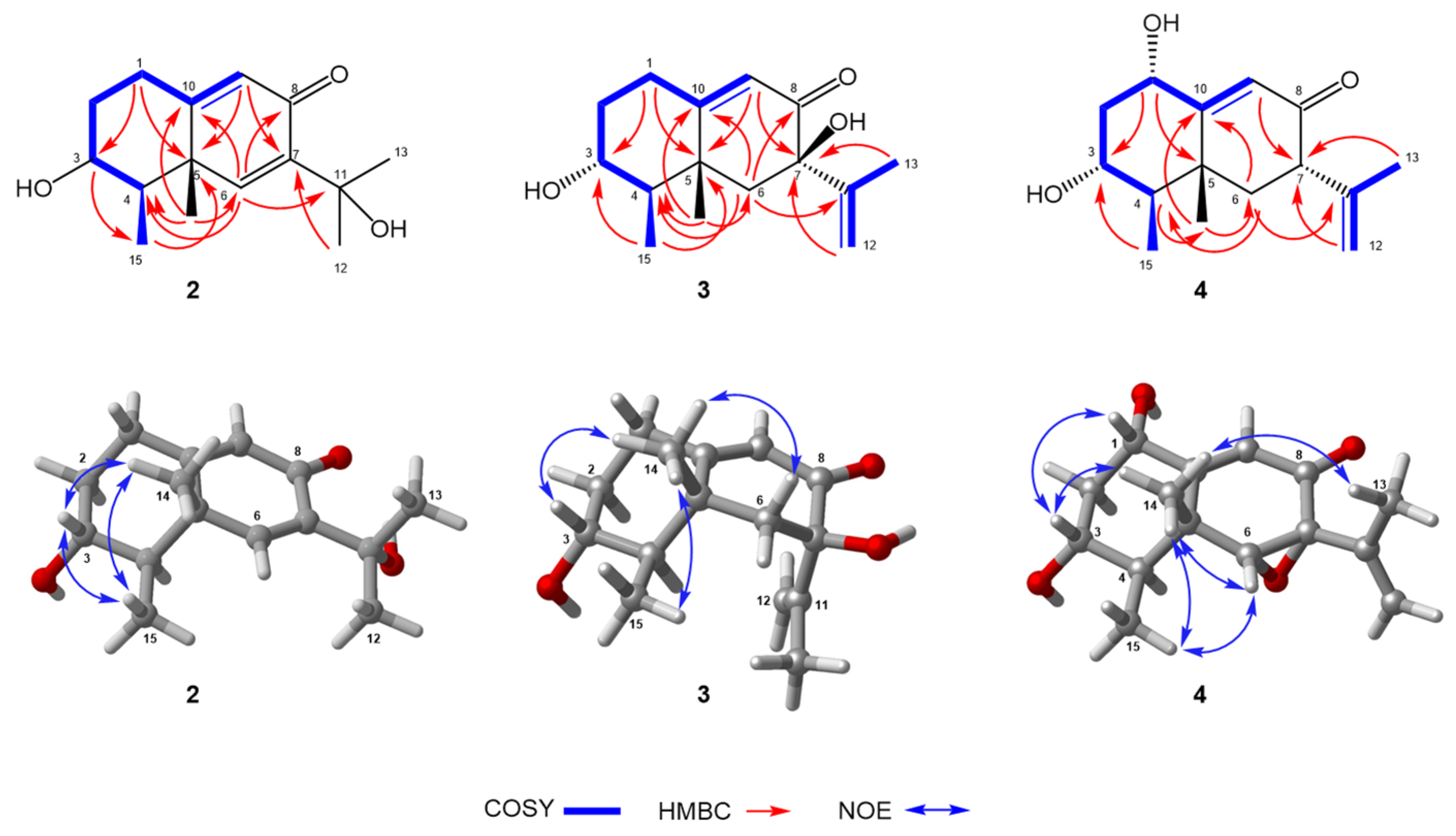
2.1. Structure Elucidation
2.2. Biological Activity
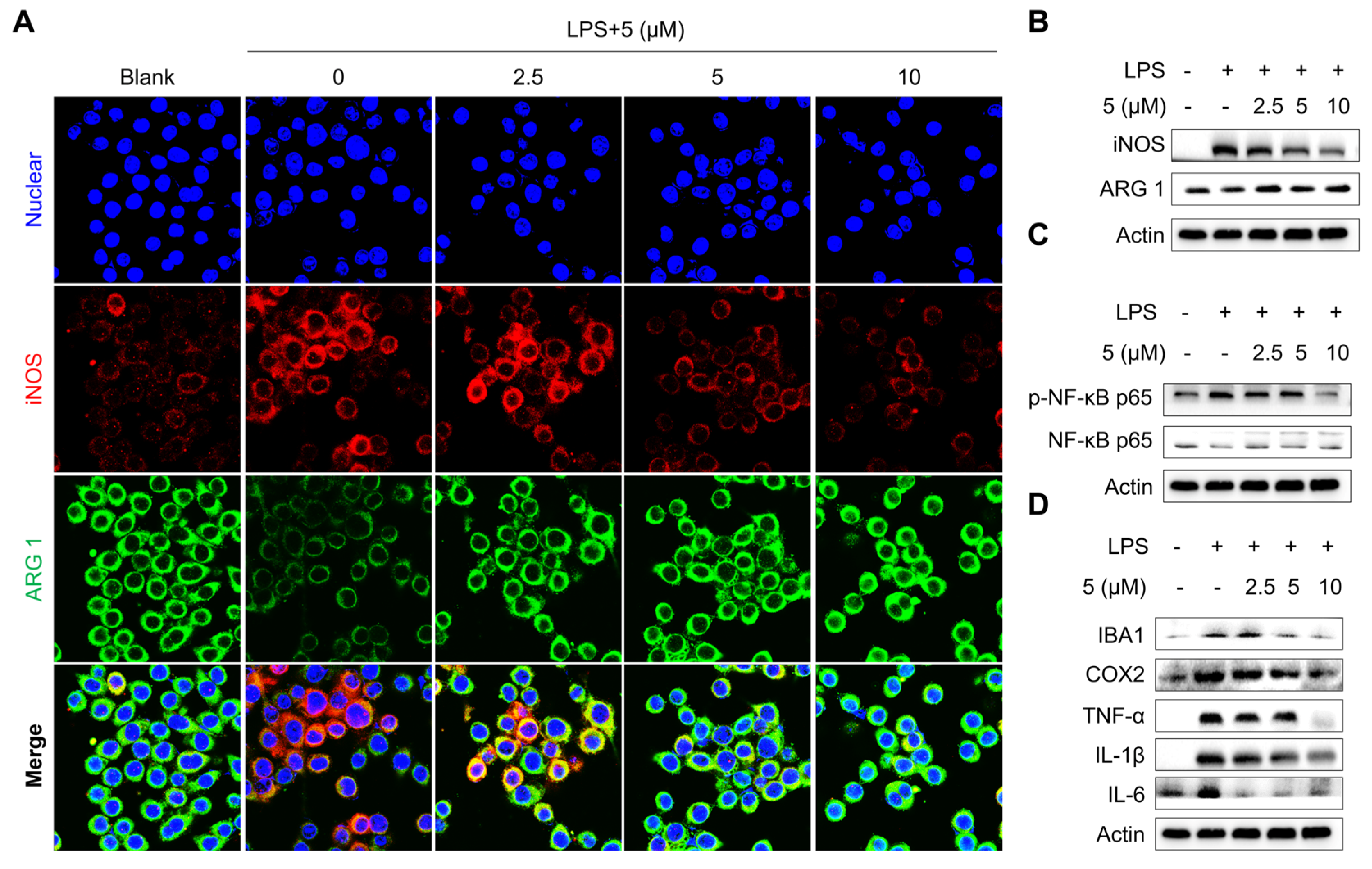
2.2.1. Evaluation of the Anti-Inflammatory Effects of Compounds in LPS-Treated Microglia Cells
2.2.2. Compound 5 Inhibits Microphage Depolarization Alongside NF-κB Signaling Suppression
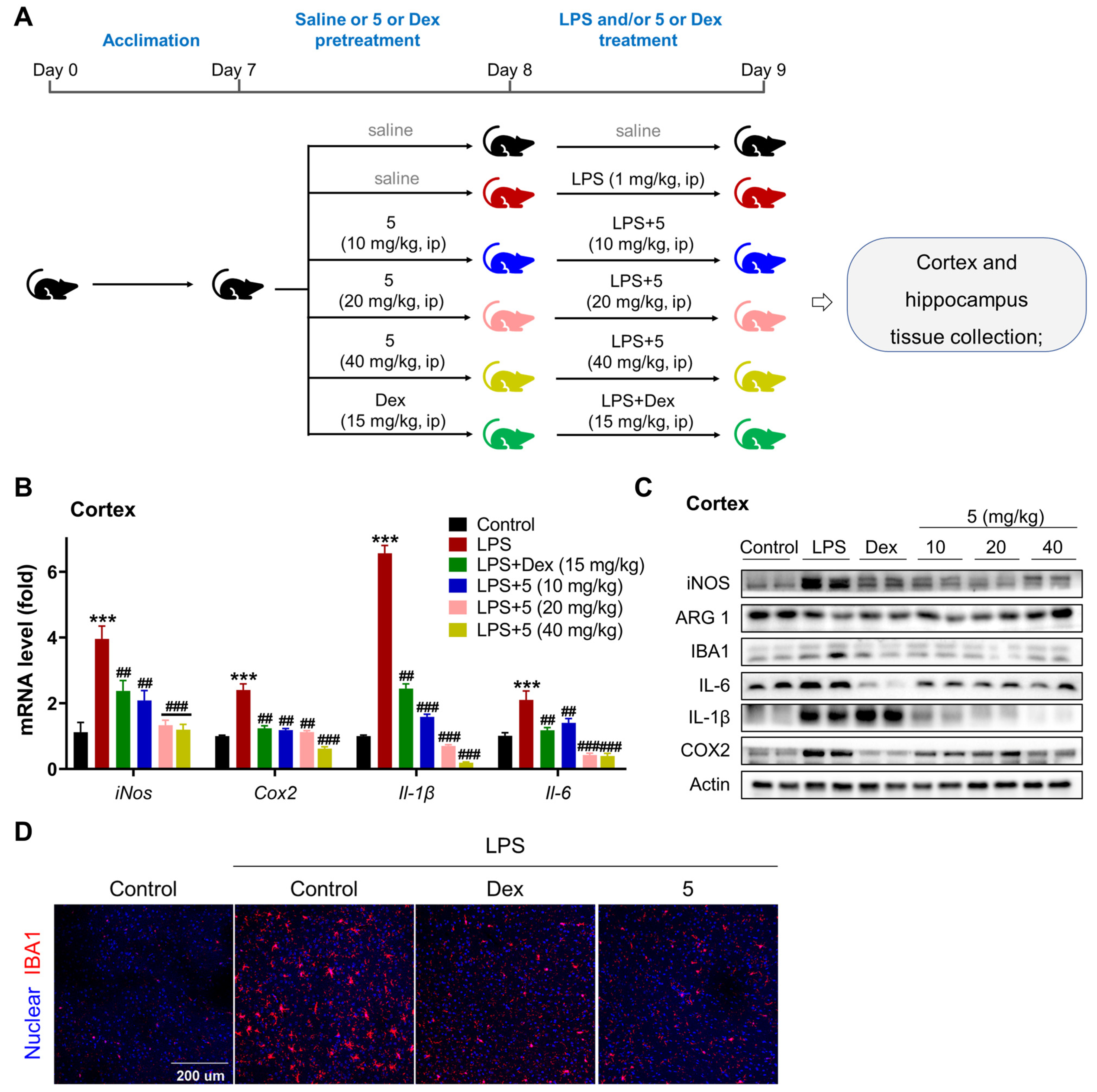
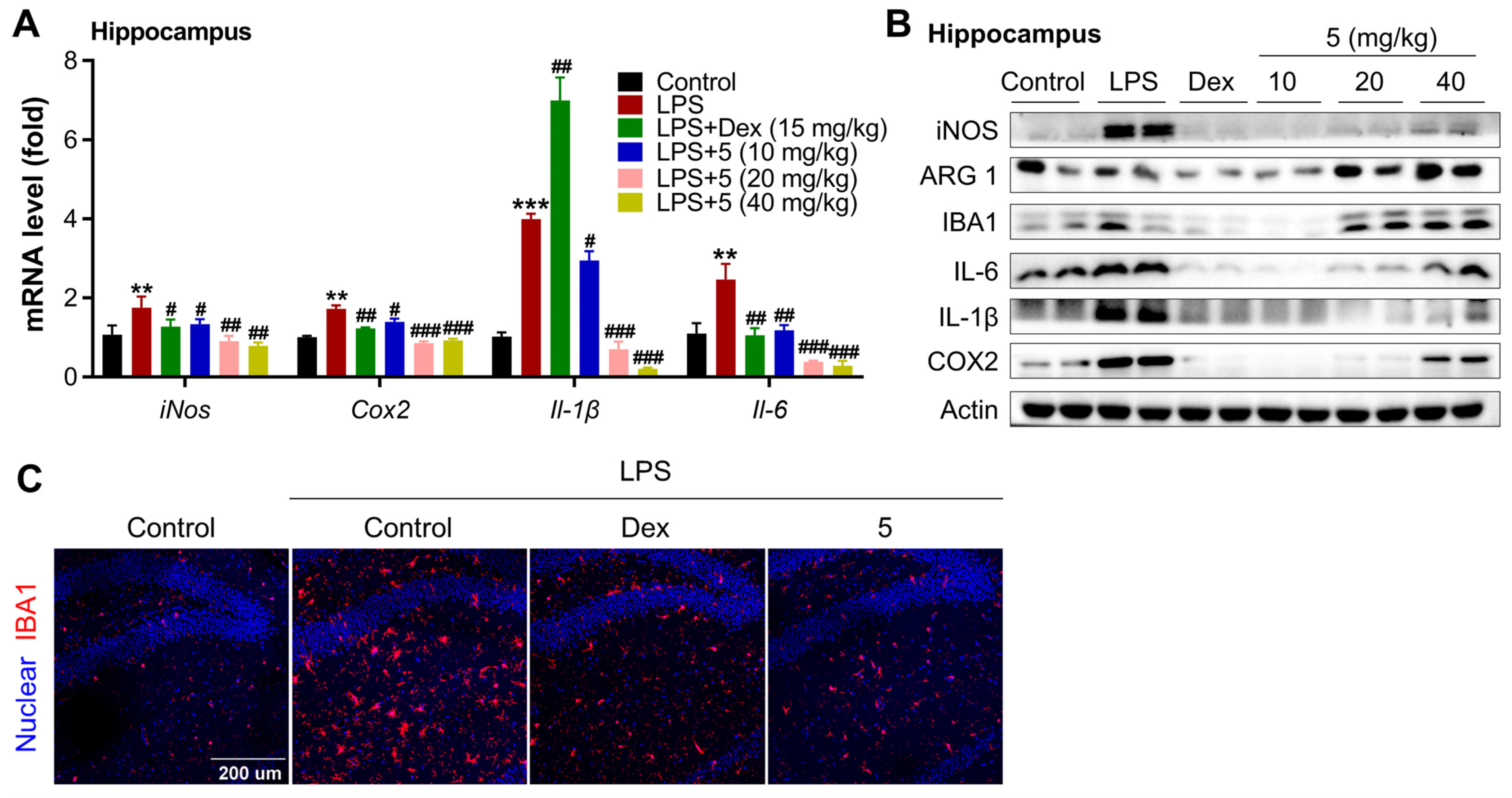
2.2.3. Compound 5 Attenuates Neuroinflammation in the Cortex and Hippocampus of LPS-Treated Mice
2.3. Structure–Activity Relationship (SAR) Analysis of Compounds 1–9
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.3.1. Phomenone C (1)
3.3.2. Phomenone D (2)
3.3.3. Hydroxypetasol (3)
3.3.4. Graphilane (4)
3.3.5. Dihydrosporogen AO-1 (5)
3.3.6. Sporogen AO-1 (6)
3.3.7. Phomenone (7)
3.3.8. Petasol (8)
3.3.9. 6-Dehydropetasol (9)
3.4. Quantum Chemical Calculation
3.5. Cell Culture
3.6. LPS Induction and Cellular NO Detection
3.7. Cell Viability Assay
3.8. Western Blot Assay
3.9. Immunofluorescence
3.10. LPS-Induced Neuroinflammation Models
3.11. Perfusion and Immunochemistry
3.12. mRNA Level of Inflammation Factor Detection with qPCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.Q.; Zhou, J.W. Neuroinflammation in the central nervous system: Symphony of glial cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Kölliker-Frers, R.; Udovin, L.; Otero-Losada, M.; Kobiec, T.; Herrera, M.I.; Palacios, J.; Razzitte, G.; Capani, F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. Mediat. Inflamm. 2021, 2021, 9999146. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 206. [Google Scholar] [CrossRef]
- Tastan, B.; Heneka, M.T. The impact of neuroinflammation on neuronal integrity. Immunol. Rev. 2024, 327, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Lee, J.S. Mechanisms and Emerging Regulators of Neuroinflammation: Exploring New Therapeutic Strategies for Neurological Disorders. Curr. Issues Mol. Biol. 2025, 47, 8. [Google Scholar] [CrossRef] [PubMed]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Dong, Y.; Ma, J.; Pan, R.; Liao, Y.; Kong, X.; Li, X.; Li, S.; Chen, P.; Wang, L.; et al. Microglial Calhm2 regulates neuroinflammation and contributes to Alzheimer’s disease pathology. Sci. Adv. 2021, 7, eabe3600. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, S.K.L.; De Vries, N.M.; Helmich, R.C.; Verbeek, M.M.; Schwarzschild, M.A.; Bloem, B.R. Inhibition of neuroinflammation may mediate the disease-modifying effects of exercise: Implications for Parkinson’s disease. J. Park. Dis. 2022, 12, 1419–1422. [Google Scholar] [CrossRef]
- Henderson, M.X.; Trojanowski, J.Q.; Lee, V.M. α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci. Lett. 2019, 709, 134316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dhapola, R.; Sharma, P.; Nagar, P.; Medhi, B.; HariKrishnaReddy, D. The impact of cytokines in neuroinflammation-mediated stroke. Cytokine Growth Factor Rev. 2024, 78, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Robertson, A.A.B.; Chae, J.J.; Higgins, S.C.; Muñoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A Small-Molecule Inhibitor of the NLRP3 Inflammasome for the Treatment of Inflammatory Diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Azzini, E.; Peña-Corona, S.I.; Hernández-Parra, H.; Chandran, D.; Saleena, L.A.K.; Sawikr, Y.; Peluso, I.; Dhumal, S.; Kumar, M.; Leyva-Gómez, G.; et al. Neuroprotective and anti-inflammatory effects of curcumin in Alzheimer’s disease: Targeting neuroinflammation strategies. Phytother. Res. 2024, 38, 3169–3189. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Azargoonjahromi, A.; Abutalebian, F. Unraveling the therapeutic efficacy of resveratrol in Alzheimer’s disease: An umbrella review of systematic evidence. Nutr. Metab. 2024, 21, 15. [Google Scholar] [CrossRef]
- Lin, A.; Wu, G.; Gu, Q.; Zhu, T.; Li, D. New eremophilane-type sesquiterpenes from an Antarctic deepsea derived fungus, Penicillium sp. PR19 N-1. Arch. Pharm. Res. 2014, 37, 839–844. [Google Scholar] [CrossRef]
- Isaka, M.; Chinthanom, P.; Boonruangprapa, T.; Rungjindamai, N.; Pinruan, U. Eremophilane-type sesquiterpenes from the fungus Xylaria sp. BCC 21097. J. Nat. Prod. 2010, 73, 683–687. [Google Scholar] [CrossRef]
- Moreau, S.; Moulé, Y.; Bousquet, J.F. Relationship between chemical structure and biological properties of some fungal metabolites of the eremophilane type. Ann. Nutr. Aliment. 1977, 31, 881–884. [Google Scholar] [PubMed]
- Ebel, R. Terpenes from marine-derived fungi. Mar. Drugs 2010, 8, 2340–2368. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Fortkamp, D.; Abraham, W.R. Eremophilane-type sesquiterpenes from fungi and their medicinal potential. Biol. Chem. 2017, 399, 13–28. [Google Scholar] [CrossRef]
- Hou, C.; Kulka, M.; Zhang, J.; Li, Y.; Guo, F. Occurrence and biological activities of eremophilane-type sesquiterpenes. Mini Rev. Med. Chem. 2014, 14, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Li, Y.Y.; Huang, Y.J.; Su, W.J.; Shen, Y.M. Three new sesquiterpenoids from Xylaria sp. NCY2. Helv. Chim. Acta 2008, 91, 46–52. [Google Scholar] [CrossRef]
- Del Valle, P.; Figueroa, M.; Mata, R. Phytotoxic eremophilane sesquiterpenes from the coprophilous fungus Penicillium sp. G1-a14. J. Nat. Prod. 2015, 78, 339–342. [Google Scholar] [CrossRef]
- Zhai, B.; Li, H.; Hu, Y.; Wu, D.; Li, J.; Zhang, X.; Gao, Q.; Xie, C.; Yang, C. Anti-inflammatory sesquiterpenoids from Ligularia fischeri Turcz. Fitoterapia 2024, 177, 106088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.H.; Shen, T.; Yang, X.; Zhao, H.; Li, X.; Xie, W.D. Sesquiterpenoids from Farfugium japonicum and their inhibitory activity on NO production in RAW264.7 cells. Arch. Pharmacal Res. 2012, 35, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Arciniegas, A.; Pérez-Castorena, A.L.; Reyes, S.; Contreras, J.L.; De Vivar, A.R. New oplopane and eremophilane derivatives from Robinsonecio gerberifolius. J. Nat. Prod. 2003, 66, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, D.; Fan, A.; Huang, J.; Lin, W. Eremophilane-Type Sesquiterpenes from a Marine-Derived Fungus Penicillium Copticola with Antitumor and Neuroprotective Activities. Mar. Drugs 2022, 20, 712. [Google Scholar] [CrossRef]
- Li-Bin, L.; Xiao, J.; Zhang, Q.; Han, R.; Xu, B.; Yang, S.X.; Han, W.B.; Tang, J.J.; Gao, J.M. Eremophilane Sesquiterpenoids with Antibacterial and Anti-inflammatory Activities from the Endophytic Fungus Septoria rudbeckiae. J. Agric. Food Chem. 2021, 69, 11878–11889. [Google Scholar] [CrossRef] [PubMed]
- Coxon, B. Developments in the Karplus equation as they relate to the NMR coupling constants of carbohydrates. Adv. Carbohydr. Chem. Biochem. 2009, 62, 17–82. [Google Scholar] [PubMed]
- Sugama, K.; Hayashi, K.; Nakagawa, T.; Mitsuhashi, H.; Yoshida, N. Sesquiterpenoids from petasites fragrans. Phytochemistry 1983, 22, 1619–1622. [Google Scholar] [CrossRef]
- Naya, K.; Takagi, I. The structure of petasitin, a new sesquiterpene from maxim. Tetrahedron Lett. 1968, 9, 629–632. [Google Scholar] [CrossRef]
- Vo, V.G.; Le, H.D.; Tran, T.N.; Nguyen, N.H.; Vo, T.P.G.; Sichaem, J.; Nguyen, V.K.; Duong, T.H. A new eremophilane-sesquiterpene from the cultured lichen mycobiont of Graphis sp. Nat. Prod. Res. 2022, 36, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Le, D.H.; Takenaka, Y.; Hamada, N.; Tanahashi, T. Eremophilane-type sesquiterpenes from cultured lichen mycobionts of Sarcographa tricosa. Phytochemistry 2013, 91, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Wada, K.; Marumo, S.; Hattori, H. Structure of sporogen-ao 1, a sporogenic substance of Aspergillus oryzae. Tetrahedron Lett. 1984, 25, 5907–5910. [Google Scholar] [CrossRef]
- Riche, C.; Pascard-Billy, C. The crystal and molecular structure of phomenone. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 1476–1477. [Google Scholar] [CrossRef]
- Bunkers, G.J.; Kenfield, D.; Strobel, G.; Sugawara, F. Structure-activity relationships of the eremophilanes produced by Drechslera gigantea. Phytochemistry 1990, 29, 1471–1474. [Google Scholar] [CrossRef]
- Kono, Y.; Gardner, J.M.; Suzuki, Y.; Kondo, H.; Takeuchi, S. New minor components of host-selective ACTG-toxin and a novel sesquiterpene produced by a pathotype of Alternaria citri causing brown spot disease of mandarins. J. Pestic. Sci. 1989, 14, 223–228. [Google Scholar] [CrossRef][Green Version]
- Yurchenko, A.N.; Smetanina, O.F.; Kirichuk, N.N.; Yurchenko, E.A.; Afiyatullov, S.S. Biologically Active Metabolites of the Facultative Marine Fungus Aspergillus terreus. Chem. Nat. Compd. 2014, 49, 1123–1124. [Google Scholar] [CrossRef]
- Daengrot, C.; Rukachaisirikul, V.; Tansakul, C.; Thongpanchang, T.; Phongpaichit, S.; Bowornwiriyapan, K.; Sakayaroj, J. Eremophilane Sesquiterpenes and Diphenyl Thioethers from the Soil Fungus Penicillium copticola PSU-RSPG138. J. Nat. Prod. 2015, 78, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Vandermeersch, T.; Flynn, C.J.; Maguire, A.R.; Hutchison, G.R. Confab-Systematic generation of diverse low-energy conformers. J. Cheminform. 2011, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Bruhn, T.; Schaumloffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243. [Google Scholar] [CrossRef]
- Rao, Y.; Su, R.; Wu, C.; Chai, X.; Li, J.; Yang, G.; Wu, J.; Fu, T.; Jiang, Z.; Guo, Z.; et al. Identification of a natural PLA2 inhibitor from the marine fungus Aspergillus sp. c1 for MAFLD treatment that suppressed lipotoxicity by inhibiting the IRE-1α/XBP-1s axis and JNK signaling. Acta Pharm. Sin. B 2024, 14, 304–318. [Google Scholar] [CrossRef]
- Su, R.; Fu, H.L.; Zhang, Q.X.; Wu, C.Y.; Yang, G.Y.; Wu, J.J.; Cao, W.J.; Liu, J.; Jiang, Z.P.; Xu, C.J.; et al. Amplifying hepatic L-aspartate levels suppresses CCl4-induced liver fibrosis by reversing glucocorticoid receptor β-mediated mitochondrial malfunction. Pharmacol. Res. 2024, 206, 107294. [Google Scholar] [CrossRef] [PubMed]



| No. | 1 a | 2 b | 3 b | 4 a | ||||
|---|---|---|---|---|---|---|---|---|
| δC, Type | δH | δC, Type | δH | δC, Type | δH | δC, Type | δH | |
| 1 | 38.9, CH2 | 2.53, m 2.60, m | 31.4, CH2 | α 2.42, ddd (13.7, 4.6, 2.7) β 2.60, tdd (13.9, 5.0, 1.5) | 32.3, CH2 | α 2.36, ddd (14.0, 4.5, 2.7) β 2.61, tdd (14.4, 5.2, 1.9) | 74.0, CH | 4.48, t (2.8) |
| 2 | 74.1, CH | 3.48, m | 37.5, CH2 | 1.38, m 2.24, m | 37.0, CH2 | 1.39, dt (11.7, 4.0) 2.17, m | 41.7, CH2 | 1.65, m 2.32, m |
| 3 | 76.2, CH | 3.42, t (9.0) | 71.5, CH | 3.67, m | 71.8, CH | 3.58, td (10.9, 4.7) | 67.1, CH | 4.03, td (11.0, 4.0) |
| 4 | 40.5, CH | 1.87, br s | 48.6, CH | 1.32, m | 49.9, CH | 1.50, m | 44.3, CH | 1.80, m |
| 5 | 41.1, C | 44.5, C | 41.3, C | 40.9, C | ||||
| 6 | 68.0, CH | 3.32, s | 151.6, CH | 7.31, s | 45.9, CH2 | α 2.20, d (14.8) β 1.87, d (14.7) | 68.6, CH | 3.34, s |
| 7 | 63.6, C | 143.1, C | 78.2, C | 64.1, C | ||||
| 8 | 192.7, C | 188.2, C | 200.6, C | 194.2, C | ||||
| 9 | 123.0, CH | 5.81, d (1.6) | 125.6, CH | 6.04, d (1.5) | 123.1, CH | 5.86, d (1.7) | 123.8, CH | 5.86, s |
| 10 | 159.6, C | 169.9, C | 172.3, C | 160.6, C | ||||
| 11 | 139.0, C | 72.4, C | 148.8, C | 138.7, C | ||||
| 12 | 114.8, CH2 | 5.11, br s | 29.1, CH3 | 1.46, s | 113.5, CH2 | 5.00, d (1.9) 5.04, s | 115.0, CH2 | 5.12, m |
| 13 | 19.9, CH3 | 1.87, br s | 29.1, CH3 | 1.47, s | 19.4, CH3 | 1.74, s | 19.9, CH3 | 1.85, s |
| 14 | 19.0, CH3 | 1.24, s | 19.0, CH3 | 1.20, s | 22.3, CH3 | 1.27, s | 20.8, CH3 | 1.40, s |
| 15 | 11.2, CH3 | 1.27, d (6.8) | 12.2, CH3 | 1.28, d (2.3) | 11.7, CH3 | 1.10, d (6.7) | 11.3, CH3 | 1.27, d (6.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Qin, M.; Chen, M.; Shi, Y.; Liu, S.; Rao, Y.; Huang, L.; Fu, Y. Anti-Neuroinflammatory Eremophilane Sesquiterpenoids from Marine-Derived Fungus Phoma sp. DXH009. Mar. Drugs 2025, 23, 94. https://doi.org/10.3390/md23030094
Yang G, Qin M, Chen M, Shi Y, Liu S, Rao Y, Huang L, Fu Y. Anti-Neuroinflammatory Eremophilane Sesquiterpenoids from Marine-Derived Fungus Phoma sp. DXH009. Marine Drugs. 2025; 23(3):94. https://doi.org/10.3390/md23030094
Chicago/Turabian StyleYang, Guanyu, Mengwei Qin, Mingbin Chen, Yujia Shi, Siyi Liu, Yong Rao, Ling Huang, and Ying Fu. 2025. "Anti-Neuroinflammatory Eremophilane Sesquiterpenoids from Marine-Derived Fungus Phoma sp. DXH009" Marine Drugs 23, no. 3: 94. https://doi.org/10.3390/md23030094
APA StyleYang, G., Qin, M., Chen, M., Shi, Y., Liu, S., Rao, Y., Huang, L., & Fu, Y. (2025). Anti-Neuroinflammatory Eremophilane Sesquiterpenoids from Marine-Derived Fungus Phoma sp. DXH009. Marine Drugs, 23(3), 94. https://doi.org/10.3390/md23030094




