Diterpenoids of Marine Organisms: Isolation, Structures, and Bioactivities
Abstract
1. Introduction
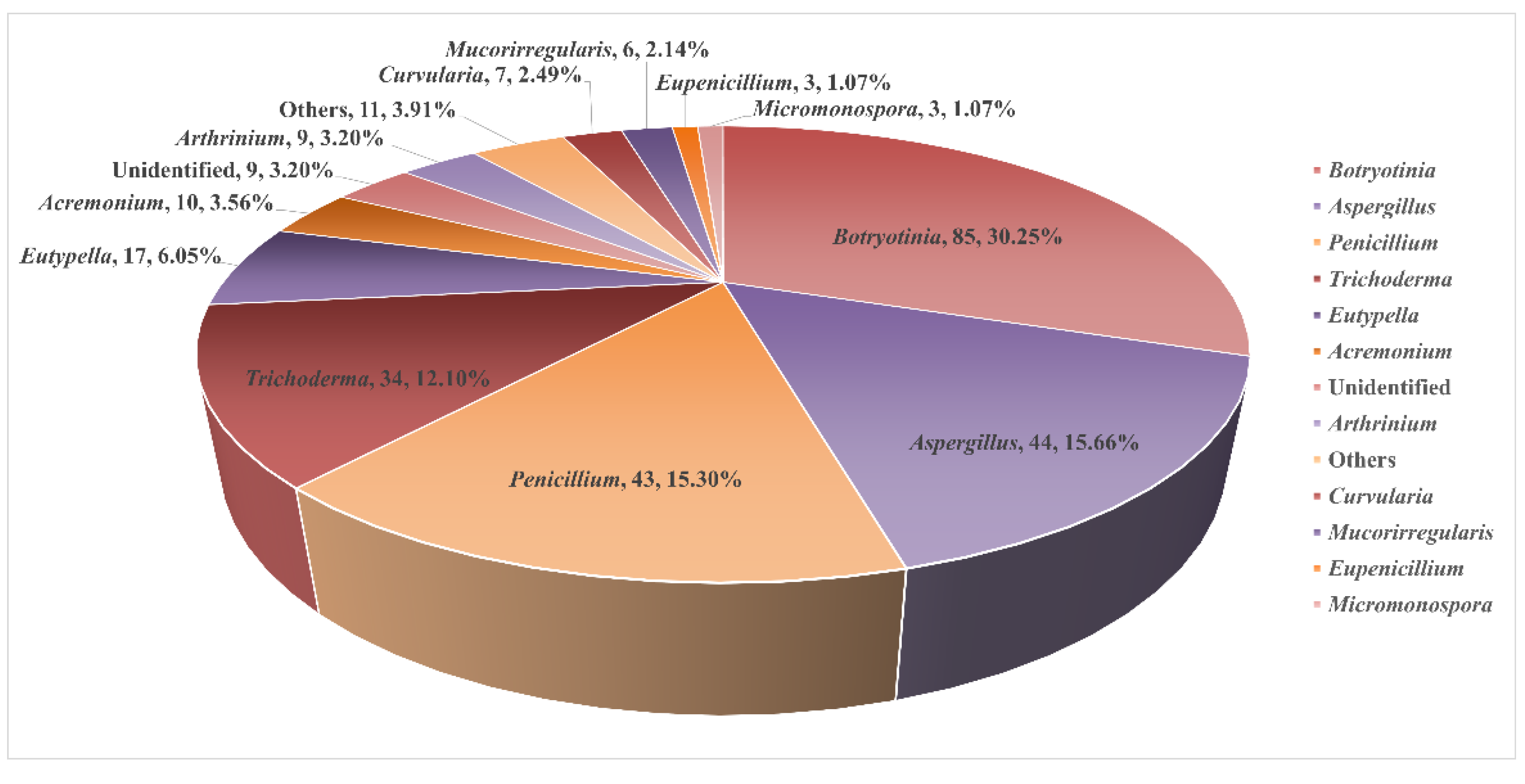
2. Diterpenoids from Marine Fungi
2.1. Sediment-Sourced Fungi
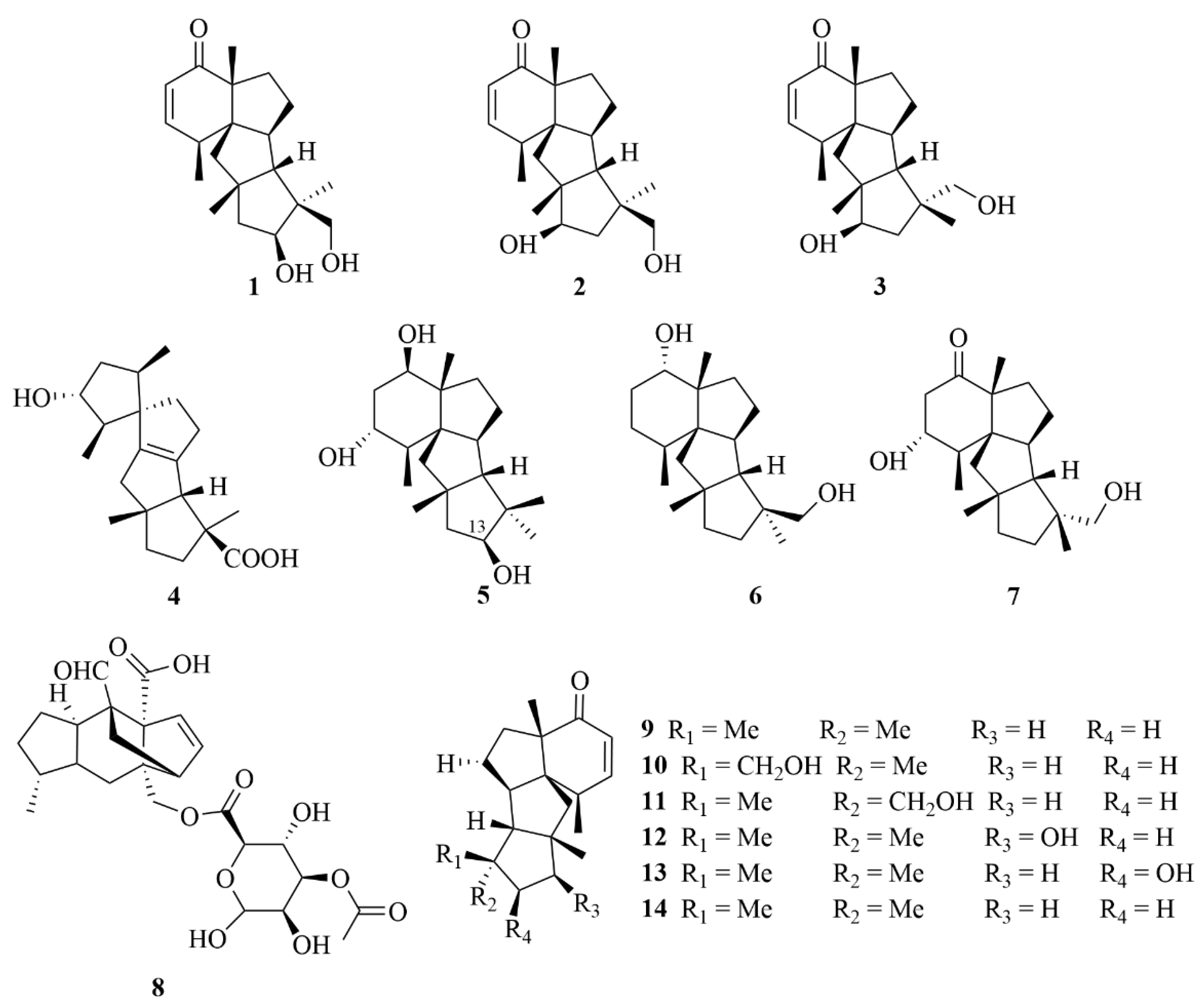
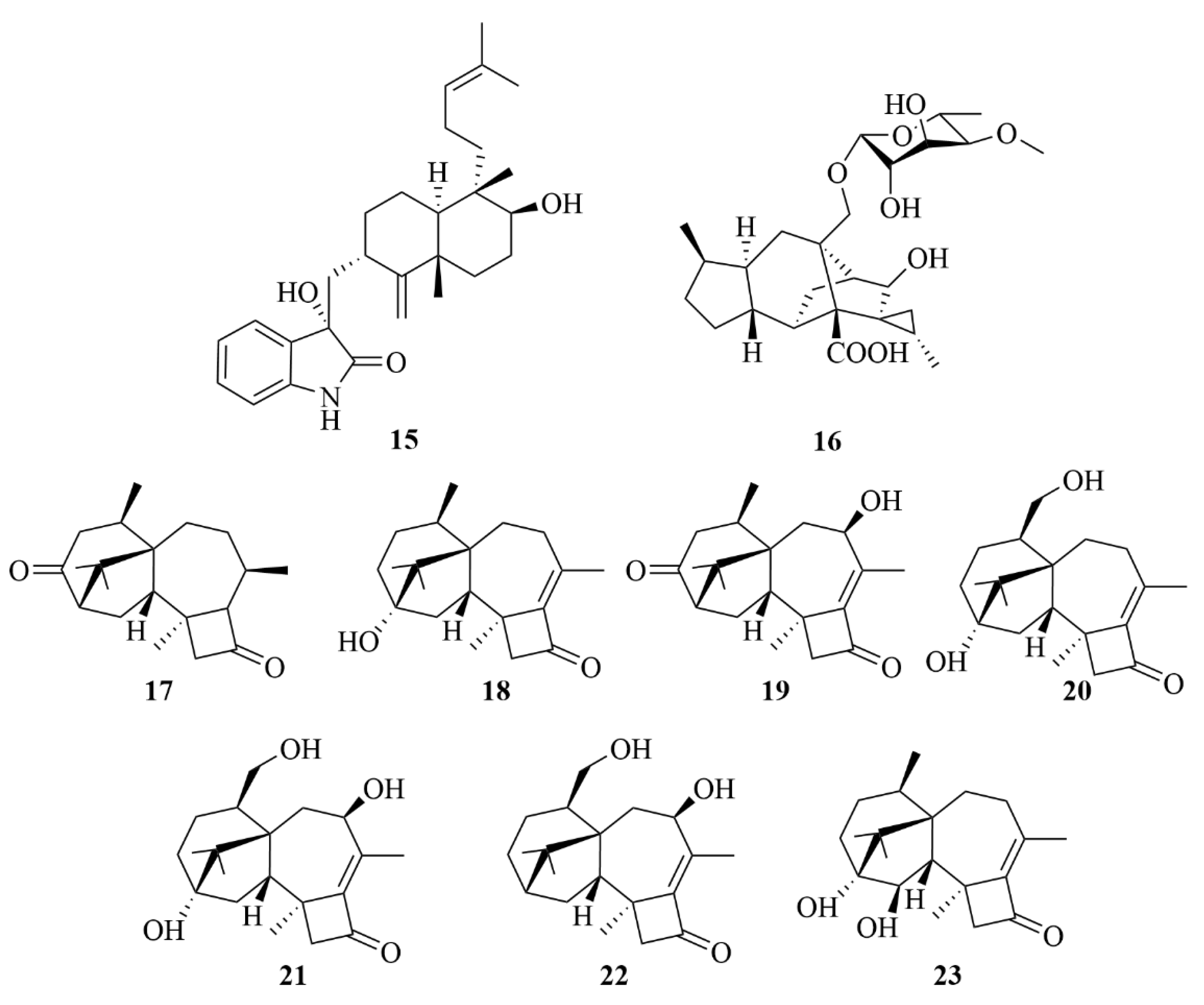
2.1.1. Penicillium sp.
2.1.2. Trichoderma sp.
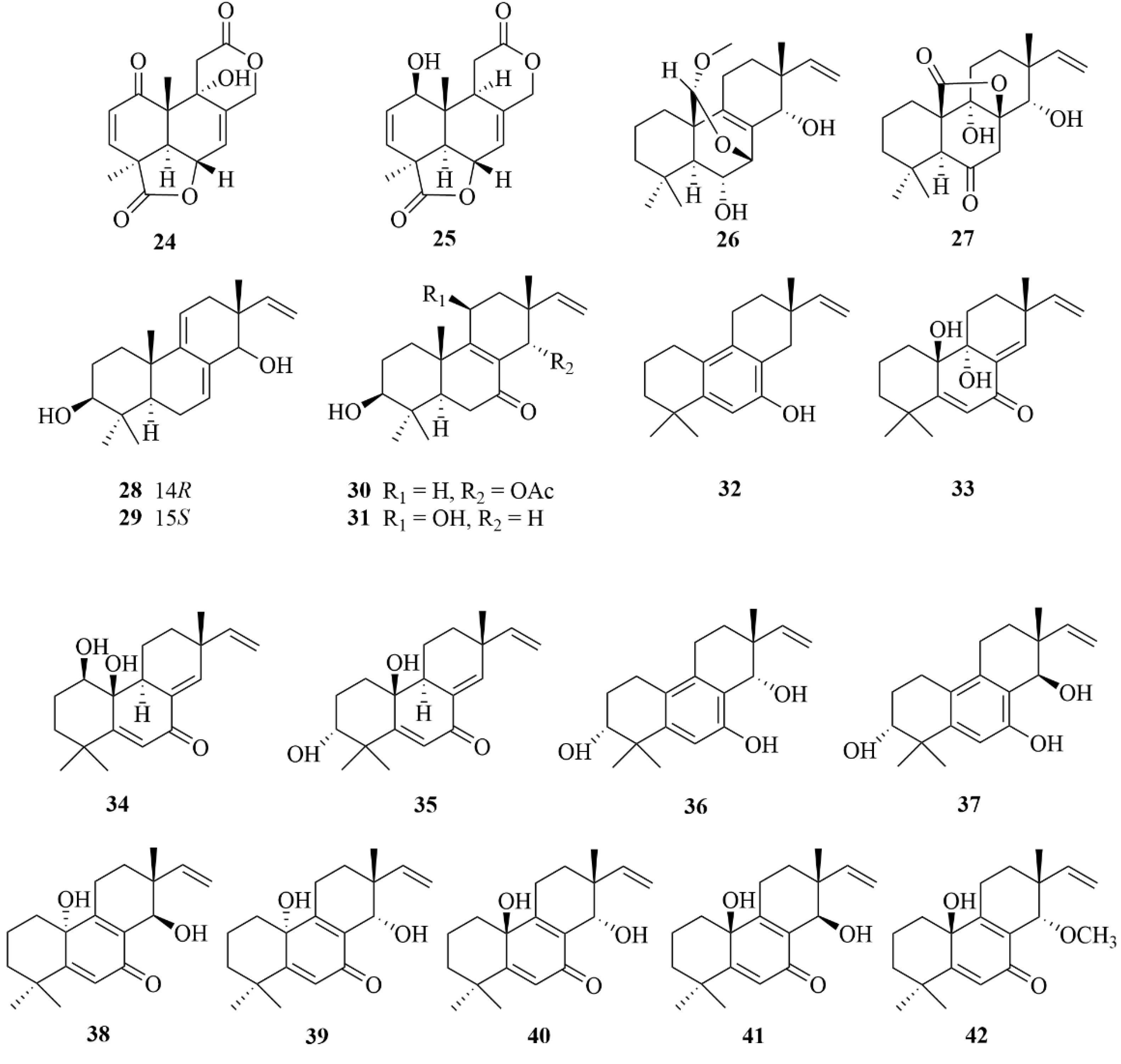
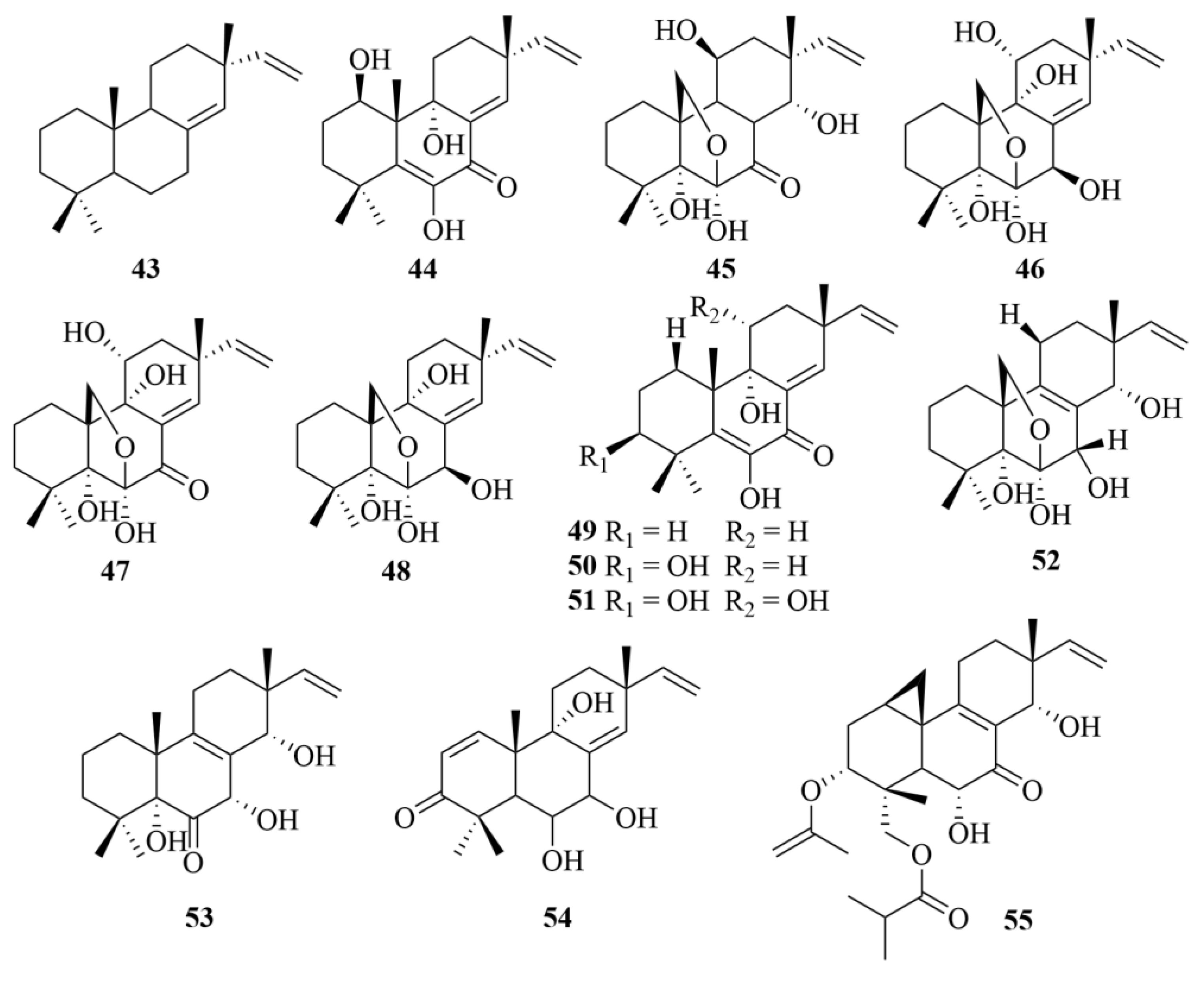
2.1.3. Aspergillus sp.
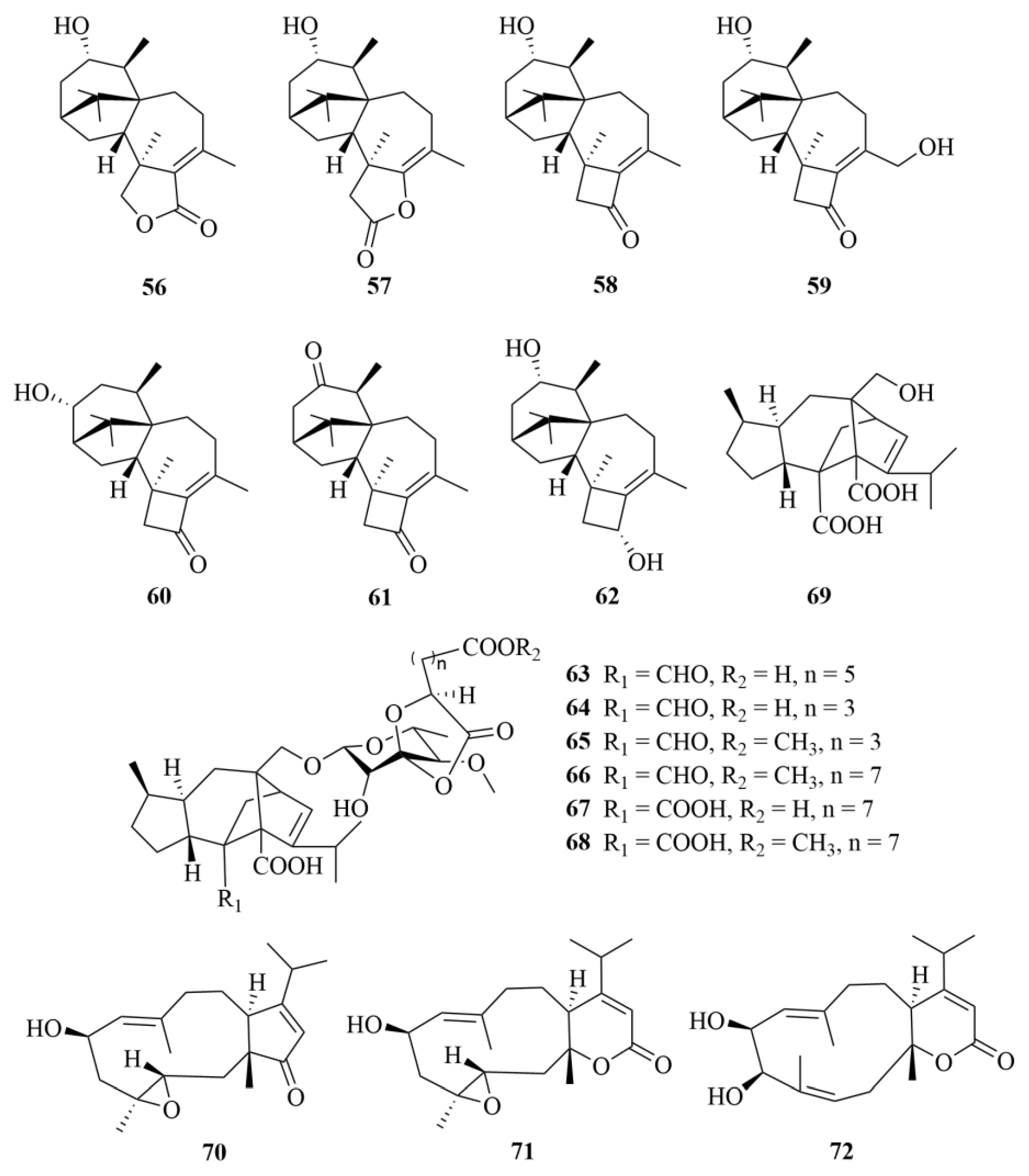
2.1.4. Eutypella sp.
2.2. Marine Invertebrates-Sourced Fungi
2.2.1. Coral-Sourced Fungi
2.2.2. Sponge-Sourced Fungi
2.3. Marine Algae-Derived Fungi
2.3.1. Trichoderma sp.
2.3.2. Aspergillus sp.
2.3.3. Penicillium sp.
2.3.4. Unidentified Fungi
2.4. Mangrove-Derived Fungi
2.5. Miscellaneous
2.5.1. Penicillium sp.
2.5.2. Botryotinia sp.
2.5.3. Micromonospora sp.
2.5.4. Acremonium sp.
2.5.5. Aspergillus sp.
2.5.6. Epicoccum sp.
2.5.7. Talaromyces sp.
2.5.8. Libertella sp.
2.5.9. Eutypella sp.
3. Diterpenoids from Marine Invertebrates
3.1. Sponge
3.2. Coral
3.2.1. Sarcophyton sp.
3.2.2. Nephthea sp.
3.2.3. Sinularia sp.
3.2.4. Lobophytum sp.
3.2.5. Junceella sp.
3.2.6. Briareum sp.
3.2.7. Anthelia sp.
3.2.8. Cespitularia sp.
3.2.9. Klyxum sp.
3.2.10. Vietnamese Soft Corals
3.3. Sea Hare
4. Diterpenoids from Marine Plants
4.1. Algae
4.2. Mangrove
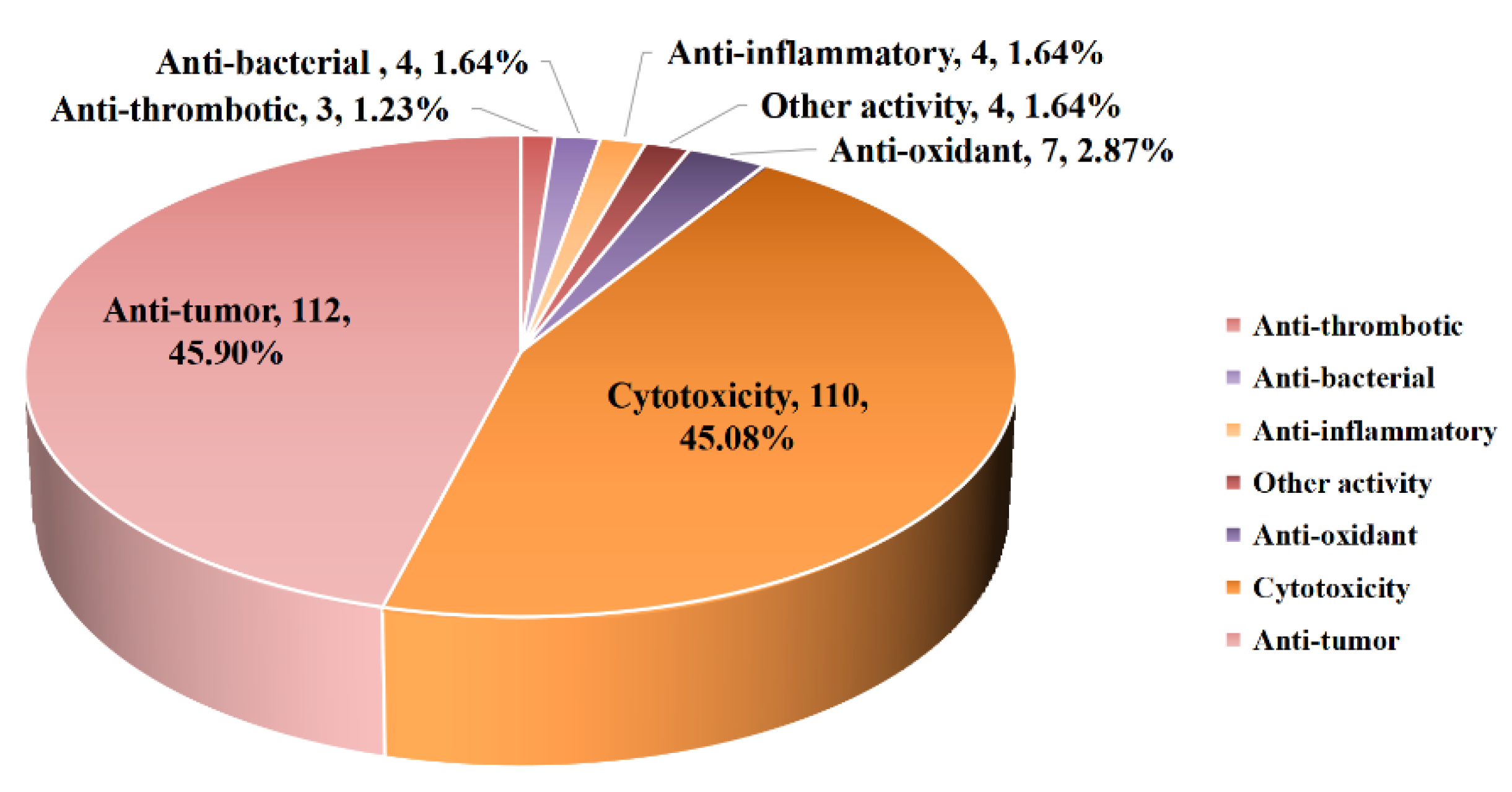
5. Bioactivities of Diterpenoids from Marine-Derived Fungi
| Source | NO. | Compound | Producing Organism | Extract/Fraction | Activity | References |
|---|---|---|---|---|---|---|
| Sediment | 1 | Harzianol J | Trichoderma sp. SCSIOW21 | BuOH extract | An anti-inflammatory effect with 81.8% and NO inhibition at 100 µM | [21] |
| Sediment | 2 | Harzianol A | Trichoderma sp. SCSIOW21 | BuOH extract | An anti-inflammatory effect with 46.8% and NO inhibition at 100 µM | [21] |
| Sediment | 7 | Harzianol O | Trichoderma sp. SCSIOW21 | BuOH extract | An anti-inflammatory effect with 50.5% and NO inhibition at 100 µM | [21] |
| Sediment | 8 | 13β-hydroxy conidiogenone C | Penicillium sp. TJ403-2 | EtOAc extract | A significant anti-inflammatory activity against LPS-induced NO production in RAW 264.7 cells, with an IC50 value of 2.19 µM | [14] |
| Sediment | 11 | Spirograterpene A | Penicillium granulatum MCCC 3A00475 | EtOAc extract | Anti-allergic effects on immunoglobulin E (IgE)-mediated rat mast RBL-2H3 cells with the inhibition rate of 18% at 20 µg/mL | [15] |
| Sediment | 12 | Conidiogenol C | Penicillium sp. YPGA11 | EtOAc extract | Weak inhibitory effects with inhibition rates below 36% at an initial concentration of 50 µM against five esophageal HTCLs (EC109, KYSE70, EC9706, KYSE30, and KYSE450) | [16] |
| Sediment | 13 | Conidiogenol D | Penicillium sp. YPGA11 | EtOAc extract | Weak inhibitory effects against five esophageal HTCLs (EC109, KYSE70, EC9706, KYSE30, and KYSE450) with an IC50 value ranging from 36.80 to 54.7 µM | [16] |
| Sediment | 14 | Conidiogenone L | Penicillium sp. YPGA11 | EtOAc extract | Weak inhibitory effects with inhibition rates below 36% at an initial concentration of 50 µM against five esophageal HTCLs (EC109, KYSE70, EC9706, KYSE30, and KYSE450) | [16] |
| Sediment | 15 | Xylarinonericin E | Penicillium sp. H1 | EtOAc extract | A moderate antifungal activity against Fusarium oxysporum f. sp. cubense, with an MIC value of 32.0 µM | [17] |
| Sediment | 16 | Conidiogenone B | Penicillium sp. F23-2 | EtOAc extract | Weak cytotoxicities against the A-549 cell line and HL-60 cell line with IC50 values of 40.3 and 28.2 µM, respectively | [19] |
| Sediment | 17 | Conidiogenone C | Penicillium sp. F23-2 | EtOAc extract | Exceptional potency against the HL-60 and BEL-7402 cell lines, with IC50 values of 0.038 and 0.97 µM | [19] |
| Sediment | 18 | Conidiogenone D | Penicillium sp. F23-2 | EtOAc extract | Cytotoxicities against the A-549, HL-60, BEL-7402, and MOLT-4 cell lines with IC50 values of 9.3, 5.3, 11.7, and 21.1 µM, respectively | [19] |
| Sediment | 19 | Conidiogenone E | Penicillium sp. F23-2 | EtOAc extract | Significant cytotoxicities against the A-549 cell line and HL-60 cell line with IC50 values of 15.1 and 8.5 µM, respectively | [19] |
| Sediment | 20 | Conidiogenone F | Penicillium sp. F23-2 | EtOAc extract | Cytotoxicities against the A-549, HL-60, BEL-7402, and MOLT-4 cell lines with IC50 values of 42.2, 17.8, 17.1, and 25.8 µM, respectively | [19] |
| Sediment | 21 | Conidiogenone G | Penicillium sp. F23-2 | EtOAc extract | Cytotoxicities against the A-549, HL-60, BEL-7402, and MOLT-4 cell lines with IC50 values of 8.3, 1.1, 43.8, and 4.7 µM, respectively | [19] |
| Sediment | 22 | Penicindopene A | Penicillium sp. YPCMAC1 | EtOAc extract | Moderate cytotoxicities against the A-549 and HeLa cell lines with IC50 values of 15.2 and 20.5 µM, respectively | [19] |
| Sediment | 23 | Trichosordarin A | Trichoderma harzianum R5 | CH2Cl2 and MeOH (1:1, v/v) extract | Toxicity towards the marine zooplankton A. salina with an LC50 value of 233 µM; weak inhibitory activities against two marine phytoplankton species (Amphidinium carterae and Phaeocysti globosa), with inhibition rates at 100 µg/mL of 20.6% and 8.1%, respectively | [20] |
| Sediment | 24 | Asperolide D | Aspergillus wentii SD-310 | EtOAc extract | Moderate inhibitory activities towards the aquatic pathogens Edwardsiella tarda and the plant bacteria Fusarium graminearum with MIC values of 16 and 2 µg/mL, respectively; inhibitory activities against aquatic bacteria Edwardsiella tarda, Micrococcus luteus, Pseudomonas aeruginosa, Vibrio harveyi, and V. parahemolyticus, with the same MIC value of 4.0 µg/mL | [22] |
| Sediment | 25 | Asperolide E | Aspergillus wentii SD-310 | EtOAc extract | Cytotoxicities against HeLa, MCF-7, and NCI-H446 cell lines, with IC50 values of 10.0, 11.0, and 16.0 µM, respectively, and moderate activity against Edwardsiella tarda, with an MIC value of 16 µg/mL | [22] |
| Sediment | 26 | Wentinoid A | Aspergillus wentii SD-310 | EtOAc extract | Inhibitory activities against aquatic bacteria Edwardsiella tarda, Micrococcus luteus, Pseudomonas aeruginosa, Vibrio harveyi, and V. parahemolyticus, with the same MIC value of 4.0 µg/mL; selective inhibition against four plant pathogenic fungi (Phytophthora parasitica, Fusarium oxysporum f. sp. lycopersici, Fusarium graminearum, and Botryosphaeria dothidea) | [23] |
| Sediment | 27 | Wentinoid B | Aspergillus wentii SD-310 | EtOAc extract | Inhibitory activities against aquatic bacteria Edwardsiella tarda, Micrococcus luteus, Pseudomonas aeruginosa, Vibrio harveyi, and V. parahemolyticus, with the same MIC value of 4.0 µg/mL | [23] |
| Sediment | 28 | Wentinoid C | Aspergillus wentii SD-310 | EtOAc extract | Inhibitory activities against aquatic bacteria Edwardsiella tarda, Micrococcus luteus, Pseudomonas aeruginosa, Vibrio harveyi, and V. parahemolyticus, with the same MIC value of 4.0 µg/mL; notable inhibitory activities towards the plant bacteria Fusarium graminearum with MIC values of 4.0 µg/mL | [23] |
| Sediment | 33 | Aspewentin D | Aspergillus wentii SD-310 | EtOAc extract | Significant inhibition against aquatic pathogens (M. luteus, E. tarda, V. harveyi, P. aeruginosa, and V. parahemolyticus), each with MIC values of 4.0 µg/mL, compared with the positive control chloramphenicol, with MIC values of 8.0 µg/mL; potent activity against plant pathogenic fungi F. graminearum with MIC values of 2.0 µg/mL. | [24] |
| Sediment | 35 | Aspewentin F | Aspergillus wentii SD-310 | EtOAc extract | Great inhibition against aquatic pathogens (M. luteus, E. tarda, V. harveyi, P. aeruginosa, and V. parahemolyticus), each with MIC values of 4.0 µg/mL, compared with the positive control chloramphenicol, with the MIC values of 8.0 µg/mL | [24] |
| Sediment | 36 | Aspewentin G | Aspergillus wentii SD-310 | EtOAc extract | Significant inhibition against aquatic pathogens (M. luteus, E. tarda, V. harveyi, P. aeruginosa, and V. parahemolyticus), each with MIC values of 4.0 µg/mL, compared with the positive control chloramphenicol, with the MIC values of 4.0 µg/mL | [24] |
| Sediment | 37 | Aspewentin H | Aspergillus wentii SD-310 | EtOAc extract | Significant inhibition against aquatic pathogens (M. luteus, E. tarda, V. harveyi, P. aeruginosa, and V. parahemolyticus), each with MIC values of 4.0 µg/mL, compared with the positive control chloramphenicol, with the MIC values of 4.0 µg/mL | [24] |
| Sediment | 38 | Aspewentin I | Aspergillus wentii SD-310 | EtOAc extract | Notable inhibitory activities against three marine bacteria (E. tarda, V. harveyi, and V. parahaemolyticus), with an MIC value of 8.0 µg/mL; inhibitory activities toward zoonotic pathogens between human and aquatic animals, such as Escherichia coli, Edwardsiella tarda, Vibrio harveyi, and V. parahaemolyticus; great inhibition against aquatic pathogens (M. luteus, E. tarda, V. harveyi, P. aeruginosa, and V. parahemolyticus), each with MIC values of 4.0 µg/mL, compared with the positive control chloramphenicol, with the MIC values of 1.0 µg/mL | [25] |
| Sediment | 39 | Aspewentin J | Aspergillus wentii SD-310 | EtOAc extract | Notable inhibitory activities against three marine bacteria (E. tarda, V. harveyi, and V. parahaemolyticus), with an MIC value of 8.0 µg/mL; inhibitory activities toward zoonotic pathogens between human and aquatic animals, such as Escherichia coli, Edwardsiella tarda, Vibrio harveyi, and V. parahaemolyticus; potent inhibition against aquatic pathogens (M. luteus, E. tarda, V. harveyi, P. aeruginosa, and V. parahemolyticus) | [25] |
| Sediment | 40 | Aspewentin K | Aspergillus wentii SD-310 | EtOAc extract | Activity against pathogenic bacteria | [25] |
| Sediment | 41 | Aspewentin L | Aspergillus wentii SD-310 | EtOAc extract | Activity against pathogenic bacteria | [25] |
| Sediment | 42 | Aspewentin M | Aspergillus wentii SD-310 | EtOAc extract | Activity against F. graminearum with an MIC value of 4.0 µg/mL | [25] |
| Sediment | 44 | Libertellenone A | Eutypella scoparia | EtOAc extract | Selective cytotoxic activities against SF-268, MCF-7, and NCI-H460 (IC50 = 20.5, 12.0, and 40.2 µM) | [26] |
| Sediment | 47 | Diaporthein B | Eutypella scoparia | EtOAc extract | Significant cytotoxicity against SF-268, MCF-7, and NCI-H460 (IC50 = 9.2, 4.4, and 9.9 µM) | [26] |
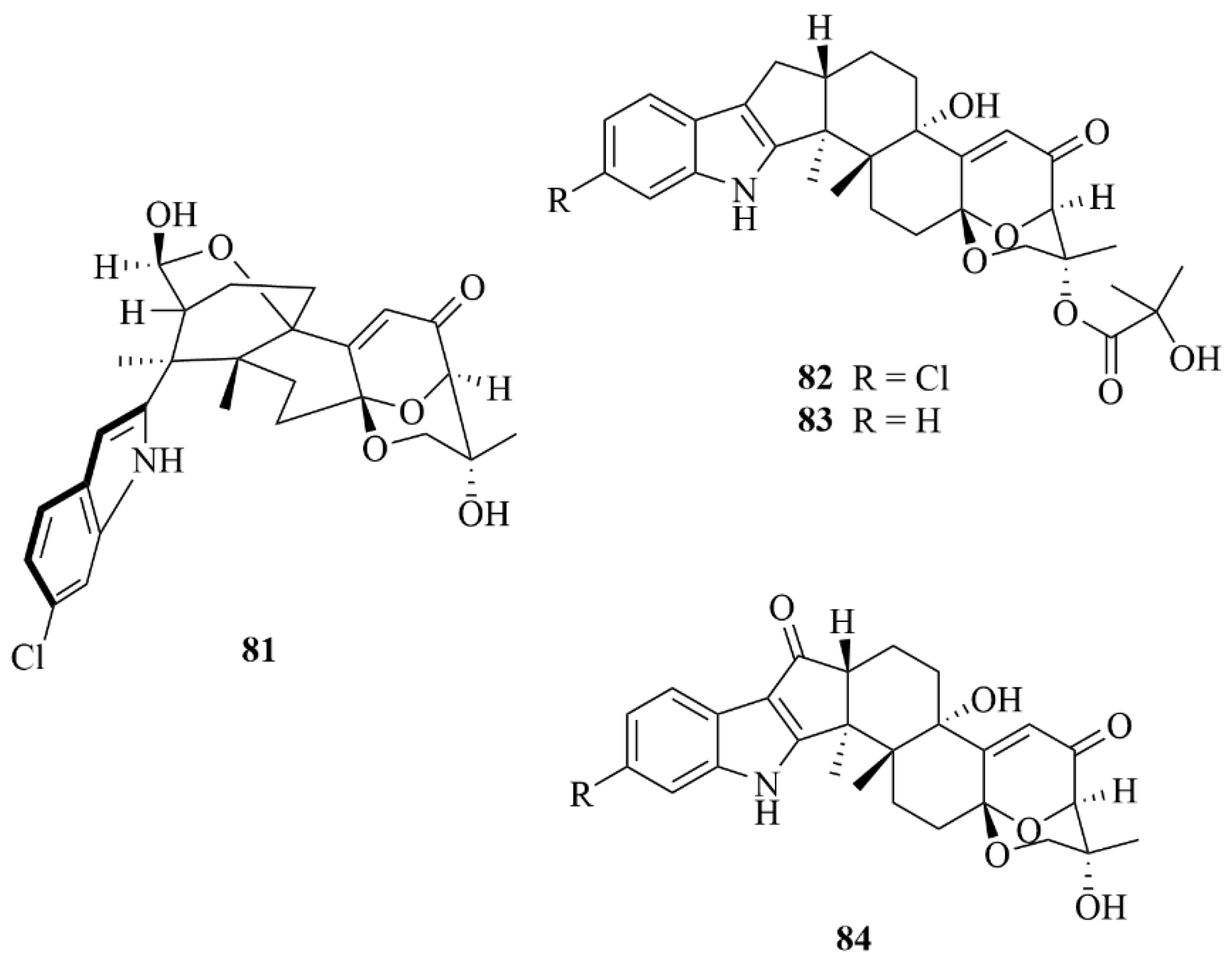
| Sediment | 48 | 11-deoxydiaporthein A | Eutypella scoparia | EtOAc extract | Moderate cytotoxicity against the MCF-7 cell line with IC50 = 38.8 µM | [26] |
| Sediment | 49 | Scopararane C | Eutypella scoparia | EtOAc extract | Moderate cytotoxicity against the MCF-7 cell line with IC50 = 16.4 µM | [26] |
| Sediment | 50 | Scopararane D | Eutypella scoparia FS26 | EtOAc extract | Cytotoxic activity towards the MCF-7 cell line with an IC50 value of 25.6 µM; moderate cytotoxic activities against SF-268 and NCI-H460 cell lines with IC50 values of 43.5 µM and 46.1 µM. | [27] |
| Sediment | 51 | Scopararane E | Eutypella scoparia FS26 | EtOAc extract | Cytotoxic activity towards the MCF-7 cell line with IC50 values of 74.1 µM | [27] |
| Sediment | 53 | Scopararane G | Eutypella scoparia FS26 | EtOAc extract | Cytotoxic activities towards the MCF-7 cell line with IC50 values of 85.5 µM | [27] |
| Sediment | 55 | Scopararane I | Eutypella sp. FS46 | EtOAc extract | Moderate inhibitory activity against NCI-H460 and SF268 cell lines with IC50 values of 13.59 and 25.31 µg/mL | [28] |
| Coral | 56 | Harzianelactone A | Trichoderma harzianum XS20090075 | EtOAc extract | Notable activities against seedling growth of amaranth and lettuce | [29] |
| Coral | 57 | Harzianelactone B | Trichoderma harzianum XS20090075 | EtOAc extract | Notable activities against seedling growth of amaranth and lettuce | [29] |
| Coral | 58 | Harzianone A | Trichoderma harzianum XS20090075 | EtOAc and CH2Cl2-MeOH (v/v, 1:1) extract | Notable activities against seedling growth of amaranth and lettuce | [29] |
| Coral | 59 | Harzianone B | Trichoderma harzianum XS20090075 | EtOAc and CH2Cl2-MeOH (v/v, 1:1) extract | Notable activities against seedling growth of amaranth and lettuce | [29] |
| Coral | 60 | Harzianone C | Trichoderma harzianum XS20090075 | EtOAc and CH2Cl2-MeOH (v/v, 1:1) extract | Notable activities against seedling growth of amaranth and lettuce | [29] |
| Coral | 61 | Harzianone D | Trichoderma harzianum XS20090075 | EtOAc and CH2Cl2-MeOH (v/v, 1:1) extract | Notable activities against seedling growth of amaranth and lettuce | [29] |
| Coral | 62 | Harziane | Trichoderma harzianum XS20090075 | EtOAc and CH2Cl2-MeOH (v/v, 1:1) extract | Notable activities against seedling growth of amaranth and lettuce | [29] |
| Coral | 63 | Moriniafungusn B | Curvularia hawaiiensis TA2615 | EtOAc extract | Diverse antifungal activity | [30] |
| Coral | 64 | Moriniafungusn C | Curvularia hawaiiensis TA2615 | EtOAc extract | Diverse antifungal activity | [30] |
| Coral | 65 | Moriniafungusn D | Curvularia hawaiiensis TA2615 | EtOAc extract | Diverse antifungal activity | [30] |
| Coral | 66 | Moriniafungusn E | Curvularia hawaiiensis TA2615 | EtOAc extract | Potent antifungal activity against Candida albicans ATCC10231 with an MIC value of 2.9 µM | [30] |
| Coral | 67 | Moriniafungusn F | Curvularia hawaiiensis TA2615 | EtOAc extract | Diverse antifungal activity | [30] |
| Coral | 68 | Moriniafungusn G | Curvularia hawaiiensis TA2615 | EtOAc extract | Diverse antifungal activity | [30] |
| Coral | 69 | Sordaricin B | Curvularia hawaiiensis TA2615 | EtOAc extract | Diverse antifungal activity | [30] |
| Coral | 71 | Stachatranone B | Stachybotrys chartarum TJ403-SS6 | EtOAc extract | An inhibitory effect against Acinetobacter baumannii (MIC = 16 µg/mL) and an inhibitory effect against Enterococcus faecalis (MIC = 32 µg/mL) | [31] |
| Sponge | 73 | Trichodermanin C | Trichoderma harzianum OUPS-111D-4 | EtOAc extract | Potent activities towards three cancer cell lines, P388, HL-60, and L1210, with IC50 values ranging from 6.8 to 7.9 µM | [32,33] |
| Sponge | 75 | Trichodermanin E | Trichoderma harzianum OUPS-111D-4 | EtOAc extract | Moderate activities towards three cancer cell lines, P388, HL-60, and L1210 | [32,33] |
| Sponge | 76 | Trichodermanin F | Trichoderma harzianum OUPS-111D-4 | EtOAc extract | Moderate activities towards three cancer cell lines, P388, HL-60, and L1210 | [32,33] |
| Sponge | 80 | Compound JBIR-65 | Actinomadura sp. | EtOAc extract | An ability to protect neuronal hybridoma N18-RE-105 cells from L-glutamate toxicity, with an EC50 value of 31 µM | [35] |
| Sponge | 83 | Ascandinine C | Aspergillus candidus HDN15-152 | EtOAc extract | Anti-influenza virus A (H1N1) activity with an IC50 value of 26 µM, with ribavirin served as the positive control (IC50 = 31 µM) | [36] |
| Sponge | 84 | Ascandinines D | Aspergillus candidus HDN15-152 | EtOAc extract | Strong cytotoxic activity against HL-60 cells with an IC50 value of 7.8 µM | [36] |
| Sponge | 85 | Myrocin A | Arthrinium sp. | Methanolic extract | Vascular endothelial growth factor A (VEGF-A)-dependent endothelial cell sprouting (IC50 = 3.7 µM); notable antiproliferative activities against L5178Y (mouse lymphoma) tumor cell line (IC50 = 2.05 µM); no inhibitory activity for the protein kinase and weak activities against K-562, A2780 (human ovarian cancer line), and A2780CisR (cisplatin-resistant human ovarian cancer cells) with IC50 values of 50.3, 41.3, and 66.0 µM, with cisplatin used as the positive control (IC50 = 7.80, 0.80, and 8.40 µM). | [37] |
| Sponge | 88 | Arthritis D | Arthrinium sp. | Methanolic extract | Vascular endothelial growth factor A (VEGF-A)-dependent endothelial cell sprouting (IC50 = 2.6 µM); notable antiproliferative activities against L5178Y (mouse lymphoma) tumor cell line (IC50 = 2.74 µM) | [37] |
| Sponge | 89 | Myrocin D | Arthrinium sp. | Methanolic extract | No inhibitory activity for the protein kinase and weak activities against K-562, A2780 (human ovarian cancer line), and A2780CisR (cisplatin-resistant human ovarian cancer cells) with IC50 values of 42.0, 28.2, and 154.7 µM, respectively, with cisplatin used as the positive control (IC50 = 7.80, 0.80, and 8.40 µM). | [37] |
| Algae | 95 | Trichocitrin | Trichoderma citrinoviride cf-27 | CH2Cl2 and MeOH (1:1, v/v) extract | An 8.0 mm inhibition zone against Escherichia coli at 20 µg/disk | [43,44] |
| Algae | 96 | Citrinovirin | Dictyopteris prolifera | EtOAc extract | Inhibitory activity towards S. aureus (MIC = 12.4 µg/mL); toxicity against the marine zooplankton Artemia salina (LC50 = 65.6 µg/mL); a 14.1–37.2% inhibition of three marine phytoplankton species (C. marina, H. akashiwo, and P. donghaiense) at 100 µg/mL. | [43,44] |
| Algae | 97 | (+)-wickerol A | Trichoderma asperellum d1-34 | EtOAc extract | Inhibitory activity against E. coli and S. aureus, with the same inhibitory diameters of 8.0 mm at 30 µg/disc; lethal activity against A. salina with an LC50 value of 12.0 µg/mL | [45] |
| Algae | 98 | 3R-hydroxy-9R,10R-dihydroharzianone | Trichoderma harzianum X-5 | CH2Cl2 and MeOH (1:1, v/v) extract | Inhibitory activity against Chattonella marina with an IC50 value of 7.0 µg/mL | [46] |
| Algae | 99 | 11Rmethoxy-5,9,13-proharzitrien-3-ol | Trichoderma harzianum X-5 | EtOAc extract | Notable inhibitory effect on the growth of all four kinds of phytoplankton, with IC50 values of 1.2, 1.3, 3.2, and 4.3 µg/mL, respectively, with K2Cr2O7 as a positive control (IC50 = 0.46, 0.98, 0.89, and 1.9 µM) | [46] |
| Algae | 103 | Deoxytrichoderma-erin | Trichoderma longibrachiatum A-WH-20-2 | EtOAc extract | Strong inhibition offour marine phytoplankton strains (C. marina, H. akashiwo, K. veneficum, and P. donghaiense) with IC50 values ranging from 0.53 to 2.7 µg/mL; toxicity against the marine zooplankton A. salina with a LC50 value of 19 µg/mL | [48] |
| Algae | 104 | 3S-hydroxyharzianone | Trichoderma asperellum A-YMD-9-2 | CH2Cl2 and MeOH (1:1, v/v) extract | Significant inhibition of four marine phytoplankton strains (C. marina, H. akashiwo, K. veneficum, and P. donghaiense) with IC50 values ranging from 3.1 to 7.7 µg/mL; weak inhibition against five marine-derived pathogenic bacteria (four different strains of Vibrio and a P. citrea), at 40 µg/disc | [49] |
| Algae | 105 | Harzianone | Trichoderma longibrachiatum | / | 82.6% of lethality in brine shrimp (Artemia salina L.) larvae at 100 µg/mL and exhibition of antibacterial activity against Escherichia coli and Staphylococcus aureus at 30 µg/disk, with inhibitory diameters of 8.3 and 7.0 mm, respectively | [50] |
| Algae | 113 | 19-hydroxypenitrem A | Aspergillus nidulans EN-330 | Acetone extract | Antibacterial activity against pathogens Edwardsiella tarda, Vibrio anguillarum, Escherichia coli, and Staphylococcus aureus, with MIC values of 16, 32, 16, and 16 µg/mL, respectively | [55] |
| Algae | 115 | Compound 115 | Aspergillus wentii na-3 | CHCl3 and MeOH (1:1, v/v) extract | Activities against two marine phytoplankton species (Chattonella marina and Heterosigma akashiwo) with LC50 values of 0.81 and 2.88 µM | [56] |
| Algae | 116 | Compound 116 | Aspergillus wentii na-3 | CHCl3 and MeOH (1:1, v/v) extract | Inhibitory activities against the marine zooplankton Artemia salina with an LC50 of 6.36 µM | [56] |
| Mangrove | 129 | (9R, 10R)-dihydro-harzianone | Trichoderma sp. Xy24 | / | Selective cytotoxicities toward the HeLa and MCF-7 cell lines with IC50 values of 30.1 and 30.7 µM | [59] |
| Mangrove | 130 | Harzianelactone | Trichoderma sp. Xy24 | / | Inactive cytotoxicities to the HeLa and MCF-7 cell lines with IC50 values of 10 mM | [59] |
| Mangrove | 132 | Anthcolorin H | Aspergillus versicolor | EtOAc extract | Weak activity against HeLa cells, with an IC50 value of 43.7 µM | [60] |
| Mangrove | 133 | Penicilindole A | Eupenicillium sp. HJ002 | EtOAc extract | Potent activities against human A-549 and HepG2 cell lines (IC50 = 5.5, 1.5 µM), with adriamycin used as the positive control (IC50 = 0.002, 0.1 µM), and 36.8 and 76.9 µM, respectively, for 5-fluoracil | [62] |
| Mangrove | 136 | Compound 141 | Penicillium camemberti OUCMDZ-1492 | EtOAc extract | Weak activities against the H1N1 virus, with IC50 values of 28.3 µM | [61] |
| Mangrove | 137 | Compound 142 | Penicillium camemberti OUCMDZ-1492 | EtOAc extract | Weak activities against the H1N1 virus, with IC50 values of 38.9 µM | [61] |
| Mangrove | 138 | Compound 143 | Penicillium camemberti OUCMDZ-1492 | EtOAc extract | Weak activities against the H1N1 virus, with IC50 values of 32.2 µM | [61] |
| Mangrove | 140 | Compound 145 | Penicillium camemberti OUCMDZ-1492 | EtOAc extract | Weak activities against the H1N1 virus, with IC50 values of 73.3 µM | [61] |
| Mangrove | 142 | Rhizovarin A | Mucor irregularis QEN-189 | MeOH and EtOAc extract | Moderate activities towards the A-549 cancer cell line, with IC50 values of 11.5 µM; notable activities against the HL-60 cancer cell line with IC50 values of 9.6 µM | [63] |
| Mangrove | 143 | Rhizovarin B | Mucor irregularis QEN-189 | MeOH and EtOAc extract | Moderate activities towards the A-549 cancer cell line, with IC50 values of 6.3 µM; notable activities against the HL-60 cancer cell line with IC50 values of 5.0 µM | [63] |
| Mangrove | 147 | Rhizovarin F | Mucor irregularis QEN-189 | MeOH and EtOAc extract | Moderate activities towards the A-549 cancer cell line, with an IC50 value of 9.2 µM | [63] |
| Miscellaneous | 151 | Penitholabene | Penicillium thomii YPGA3 | EtOAc extract | An inhibitory effect against the α-glucosidasewith an IC50 value of 282 µM | [65] |
| Miscellaneous | 153 | 6-hydroxylaspalinine | Penicillium sp. AS-79 | EtOAc extract | Activity against the aquatic pathogen Vibrio parahaemolyticus with an MIC of 64.0 µg/mL | [66] |
| Miscellaneous | 155 | Compound 155 | Penicillium sp. KFD28 | EtOAc extract | Potent inhibitory activities against protein tyrosine phosphatase (PTP1B) with IC50 values of 1.7 µM | [67] |
| Miscellaneous | 156 | Compound 156 | Penicillium sp. KFD28 | EtOAc extract | Potent inhibitory activities againstprotein tyrosine phosphatase (PTP1B) with IC50 values of 2.4 µM | [67] |
| Miscellaneous | 159 | Compound 159 | Penicillium sp. KFD28 | EtOAc extract | Potent inhibitory activities against protein tyrosine phosphatase (PTP1B) with IC50 values of 14 µM | [67] |
| Miscellaneous | 160 | Compound 160 | Penicillium sp. KFD28 | EtOAc extract | Potent inhibitory activities against protein tyrosine phosphatase (PTP1B) with IC50 values of 27 µM | [67] |
| Miscellaneous | 162 | Compound 162 | Penicillium sp. KFD28 | EtOAc extract | Potent inhibitory activities againstprotein tyrosine phosphatase (PTP1B) with IC50 values of 23 µM | [67] |
| Miscellaneous | 163 | Compound 163 | Penicillium sp. KFD28 | EtOAc extract | Potent inhibitory activities against protein tyrosine phosphatase (PTP1B) with IC50 values of 31.5 µM | [67] |
| Miscellaneous | 167 | Compound 167 | Penicillium sp. KFD28 | EtOAc extract | Weak activity against HeLa cells with an IC50 value of 36.3 µM | [67] |
| Miscellaneous | 168 | Compound 168 | Penicillium sp. KFD28 | EtOAc extract | Potent inhibitory activities against protein tyrosine phosphatase (PTP1B) with IC50 values of 9.5 µM | [67] |
| Miscellaneous | 171 | Botryotins A | Botryotinia fuckeliana MCCC | CHCl3/MeOH (1:1) extract | Being inactive against six HTCLs (HL-60, BEL-7402, BIU-87, PANC-1, HeLa-S3, and ECA109), each with the IC50 less than 20 µM; moderate antiallergic activity in RBL-2H3 cells with an IC50 value of 0.2 mM | [68,69] |
| Miscellaneous | 172 | Botryotins B | Botryotinia fuckeliana MCCC | CHCl3/MeOH (1:1) extract | Being inactive against six HTCLs (HL-60, BEL-7402, BIU-87, PANC-1, HeLa-S3, and ECA109), each with IC50 values less than 20 µM | [68,69] |
| Miscellaneous | 173 | Botryotins C | Botryotinia fuckeliana MCCC | CHCl3/MeOH (1:1) extract | Being inactive against six HTCLs (HL-60, BEL-7402, BIU-87, PANC-1, HeLa-S3, and ECA109), each with IC50 values less than 20 µM | [68,69] |
| Miscellaneous | 174 | Botryotins D | Botryotinia fuckeliana MCCC | CHCl3/MeOH (1:1) extract | Being inactive against six HTCLs (HL-60, BEL-7402, BIU-87, PANC-1, HeLa-S3, and ECA109), each with IC50 values less than 20 µM | [68,69] |
| Miscellaneous | 175 | Botryotins E | Botryotinia fuckeliana MCCC | CHCl3/MeOH (1:1) extract | Being inactive against six HTCLs (HL-60, BEL-7402, BIU-87, PANC-1, HeLa-S3, and ECA109), each with IC50 less than 20 µM | [68,69] |
| Miscellaneous | 176 | Botryotins F | Botryotinia fuckeliana MCCC | CHCl3/MeOH (1:1) extract | Being inactive against six HTCLs (HL-60, BEL-7402, BIU-87, PANC-1, HeLa-S3, and ECA109), each with IC50 less than 20 µM | [68,69] |
| Miscellaneous | 177 | Botryotins G | Botryotinia fuckeliana MCCC | CHCl3/MeOH (1:1) extract | Being inactive against six HTCLs (HL-60, BEL-7402, BIU-87, PANC-1, HeLa-S3, and ECA109), each with IC50 less than 20 µM | [68,69] |
| Miscellaneous | 178 | Botryotins H | Botryotinia fuckeliana MCCC | CHCl3/MeOH (1:1) extract | Being inactive against six HTCLs (HL-60, BEL-7402, BIU-87, PANC-1, HeLa-S3, and ECA109), each with IC50 less than 20 µM | [68,69] |
| Miscellaneous | 179 | A1 | Botryotinia fuckeliana MCCC | EtOAc extract | Useful as a potent cytotoxic lead compound due to its notable activities against T24 and HL-60 cells (IC50 = 2.5, 6.1 µM) | [70] |
| Miscellaneous | 252 | Micromonohalimane B | Micromonospora sp. WMMC-218 | Acetone extract | Inhibition of the methicillin-resistant Staphylococcus aureus with an MIC value of 40 µg/mL | [72] |
| Miscellaneous | 253 | Virescenosides Z9 | Acremonium striatisporum KMM 4401 | CHCl3-EtOH (2:1, v/v, 2.5 L) extract | Observably decreased ROS production in macrophages under 10 µM LPS stimulation. | [73] |
| Miscellaneous | 254 | Virescenoside Z10 | Acremonium striatisporum KMM 4401 | CHCl3-EtOH (2:1, v/v, 2.5 L) extract | Observably decreased ROS production in macrophages under 10 µM LPS stimulation, inducing downregulation of ROS production by 45%, and decreased NO production in LPS-stimulated macrophages at a concentration of 1 µM | [73] |
| Miscellaneous | 256 | Virescenosides Z12 | Acremonium striatisporum KMM 4401 | CHCl3-EtOH (2:1, v/v, 2.5 L) extrac | Observably decreased ROS production in macrophages under 10µM LPS stimulation | [73] |
| Miscellaneous | 257 | Virescenoside Z13 | Acremonium striatisporum KMM 4401 | CHCl3-EtOH (2:1, v/v, 2.5 L) extract | Observably decreased ROS production in macrophages under 10µM LPS stimulation, and decreased the NO production in LPS-stimulated macrophages at a concentration of 1 µM | [73] |
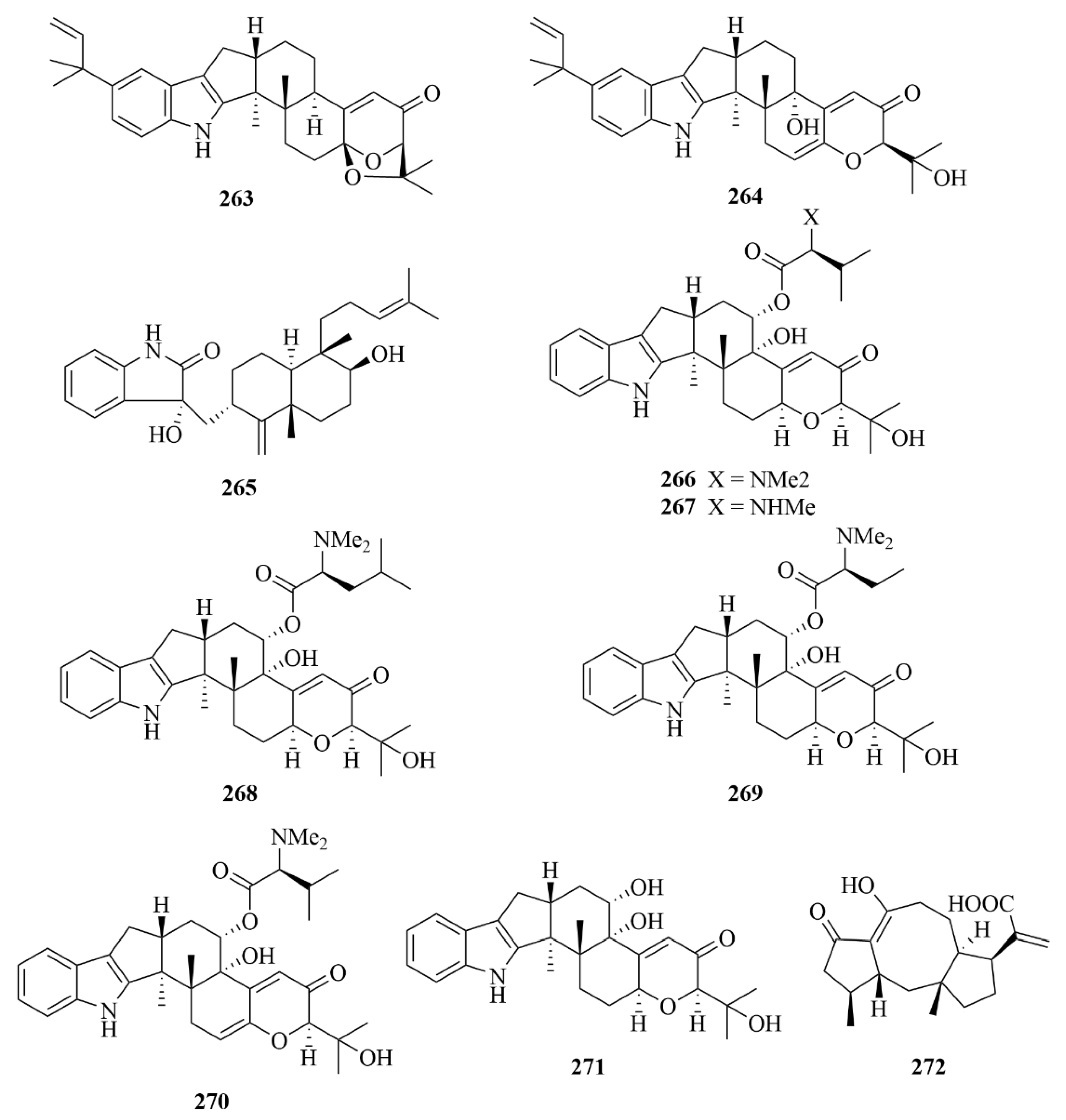
| Miscellaneous | 263 | (2R, 4bR, 6aS, 12bS, 12cS, 14aS)-4b-Deoxyβ-aflatrem | Aspergillus flavus OUCMDZ-2205 | EtOAc extract | Cytotoxicity against the A-549 cell cycle in the S phase with IC50 values of 10 µM; inhibition against the kinase PKC-β with an IC50 value of 15.6 µM | [74] |
| Miscellaneous | 264 | (2R, 4bS), 6aS, 12bS, 12cR)-9-Isopentenylpaxillin-e D | Aspergillus flavus OUCMDZ-2205i | EtOAc extract | Cytotoxicity against the A-549 cell cycle in the S phase with IC50 values of 10 µM | [74] |
| Miscellaneous | 265 | (3R, 9S, 12R, 13S, 17S, 18S)-2-carbonyl3hydroxylemeniveol | Aspergillus versicolor ZZ761 | / | Activity against Escherichia coli and Candida albicans with MIC values of 20.6 and 22.8 µM, respectively | [75] |
| Miscellaneous | 266 | Noonindole A | Aspergillus noonimiae CMB-M0339 | EtOAc extract | Moderate antifungal activity against the fungi Candida albicans | [76] |
| Miscellaneous | 273 | Compound 273 | Epicoccum sp. HS-1 | Ethyl acetate (1:1, v/v) extract | Inhibition of α-glucosidase with IC50 values of 4.6 µM, higher than the p.c. resveratrol, with IC50 = 31.2 µM | [78] |
| Miscellaneous | 274 | Roussoellol C | Talaromyces purpurogenus PP-414 | EtOAc extract | Cytotoxic activity against the MCF-7 cells with an IC50 of 6.5 µM | [79] |
| Miscellaneous | 275 | Libertellenone B | Libertella sp. | EtOAc extract | Weak activities against HCT-116 (human adenocarcinoma cell line) (IC50 = 15 µM) | [35] |
| Miscellaneous | 276 | Libertellenone C | Libertella sp. | EtOAc extract | Weak activities against HCT-116 (human adenocarcinoma cell line) (IC50 = 53 µM) | [35] |
| Miscellaneous | 277 | Libertellenone D | Libertella sp. | EtOAc extract | Significant cytotoxicity against HCT-116 (human adenocarcinoma cell line) (IC50 = 53 µM) | [35] |
| Miscellaneous | 279 | Eutypellenoid B | Eutypella sp. D-1 | CH2Cl2/CH3OH (1:1, v/v) extract | Antibacterial activities against Staphylococcus aureus and Escherichia coli with MIC values of 8 and 8 µg/mL; antifungal activities against Candida parapsilosis, Candida albicans, Candida glabrata, and Candida tropicalis with MIC values of 8, 8, 16, and 32 µg/mL, respectively; moderate cytotoxic activity against the HCT-116 cell line with IC50 value of 3.7 µM | [81] |
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| A2780CisR | Cisplatin-resistant human ovarian cancer cells |
| A-549 | Human non-small cell lung cancer cells |
| BEL-7402 | Human liver cancer cells |
| BuOH | Butanol |
| COX-2 | Cyclooxygenase-2 |
| ECD | Electronic circular dichroism |
| EtOAc | Ethyl acetate |
| GM-CSF | Granulocyte-macrophage colony stimulating factor |
| HepG2 | Human hepatocelular carcinomas |
| HL-60 | Human promyelocytic leukemia cells |
| HPLC | High-performance liquid chromatography |
| HR-ESI-MS | High-resolution electrospray ionization mass spectroscopy |
| HUVEC | Human umbilical vascular endothelial cell |
| IC50 | Half maximal inhibitory concentration |
| IgE | Immunoglobulin E |
| IL-13 | Interleukin-13 |
| IL-1β | Interleukin-1beta |
| iNOS | Inducible nitric oxide synthesis |
| LC-MS | Liquid chromatograph mass spectrometer |
| LPS | Lipopolysaccharide |
| MCF-7 | Human breast adenocarcinoma cell line |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MeOH | Methanol |
| MIC | Minimum inhibitory concentration |
| MIP-1β | Macrophage inflammatory protein |
| MOE | Murine oviductal epithelial |
| MOSE | Murine ovarian surface epithelial |
| MTT | Methyl thiazolyl tetrazolium |
| n-BuOH | N-Bromosuccinimide |
| NCI-H460 | Human non-small cell lung cancer cell line |
| NF-kB | Nuclear factor-kappa B |
| NHDF | Normal human dermal fibroblasts |
| NMR | Nuclear magnetic resonance spectroscopy |
| p.c. | Positive control |
| PE | Polyethylene |
| PTP1B | Protein tyrosine phosphatase |
| RBL-2H3 | Rat basophilic leukemia cells |
| ROS | Reactive oxygen species |
| SF-268 | Human glioma cell line |
| TB | Tuberculosis |
| TNF-α | Tumor necrosis factor-α |
| VEGF-A | Vascular endothelial growth factor A |
References
- Abdelmohsen, U.R.; Balasubramanian, S.; Oelschlaeger, T.A.; Grkovic, T.; Pham, N.B.; Quinn, R.J.; Hentschel, U. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect. Dis. 2017, 17, e30–e41. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Chen, X. Screening of Marine Bioactive Antimicrobial Compounds for Plant Pathogens. Mar. Drugs 2021, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Nweze, J.A.; Mbaoji, F.N.; Li, Y.M.; Yang, L.Y.; Huang, S.S.; Chigor, V.N.; Eze, E.A.; Pan, L.X.; Zhang, T.; Yang, D.F. Potentials of marine natural products against malaria, leishmaniasis, and trypanosomiasis parasites: A review of recent articles. Infect. Dis. Poverty 2021, 10, 9. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Varela Coelho, A.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramšak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, M.L.; Jiang, R.; Pang, T.; Zhang, C.J. The roles of fungus in CNS autoimmune and neurodegeneration disorders. Front. Immunol. 2022, 13, 1077335. [Google Scholar] [CrossRef]
- Yang, B.; Huang, J.; Zhou, X.; Lin, X.; Liu, J.; Liao, S.; Wang, J.; Liu, F.A.; Tao, H.; Liu, Y. The Fungal Metabolites with Potential Antiplasmodial Activity. Curr. Med. Chem. 2018, 25, 3796–3825. [Google Scholar] [CrossRef]
- Wiese, J.; Imhoff, J.F. Marine bacteria and fungi as promising source for new antibiotics. Drug Dev. Res. 2019, 80, 24–27. [Google Scholar] [CrossRef]
- Imhoff, J.F. Natural Products from Marine Fungi—Still an Underrepresented Resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Prakash, V.; Ranjan, N. Marine Fungi: A Source of Potential Anticancer Compounds. Front. Microbiol. 2017, 8, 2536. [Google Scholar] [CrossRef]
- Elissawy, A.M.; El-Shazly, M.; Ebada, S.S.; Singab, A.B.; Proksch, P. Bioactive terpenes from marine-derived fungi. Mar. Drugs 2015, 13, 1966–1992. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, D.; Chen, X.; Zhao, F. Marine-Derived Diterpenes from 2019 to 2024: Structures, Biological Activities, Synthesis and Potential Applications. Mar. Drugs 2025, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xu, W.; Yan, Y.; Chen, M.; Li, H.; Chen, L. Cembrane diterpenoids: Chemistry and pharmacological activities. Phytochemistry 2023, 212, 113703. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sun, W.; Zhang, S.; Gao, W.; Lin, S.; Yang, B.; Chai, C.; Li, H.; Wang, J.; Hu, Z.; et al. New cyclopiane diterpenes with anti-inflammatory activity from the sea sediment-derived fungus Penicillium sp. TJ403-2. Chin. Chem. Lett. 2020, 31, 197–201. [Google Scholar] [CrossRef]
- Niu, S.; Fan, Z.W.; Xie, C.L.; Liu, Q.; Luo, Z.H.; Liu, G.; Yang, X.W. Spirograterpene A, a Tetracyclic Spiro-Diterpene with a Fused 5/5/5/5 Ring System from the Deep-Sea-Derived Fungus Penicillium granulatum MCCC 3A00475. J. Nat. Prod. 2017, 80, 2174–2177. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y.; Xu, W.; Liu, W.; Liu, L.; Zhu, D.; Kang, Y.; Luo, Z.; Li, Q. Three new cyclopiane-type diterpenes from a deep-sea derived fungus Penicillium sp. YPGA11 and their effects against human esophageal carcinoma cells. Bioorg. Chem. 2019, 91, 103129. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Lei, F.; Tan, X.; Yi, X. A new glycosyl ester isolated from marine-derived Penicillium sp. Chin. Tradit. Herb. Drugs 2019, 50, 2518–2523. [Google Scholar]
- Du, L.; Li, D.; Zhu, T.; Cai, S.-X.; Wang, F.; Xiao, X.; Gu, Q. New alkaloids and diterpenes from a deep ocean sediment derived fungus Penicillium sp. Tetrahedron 2009, 65, 1033–1039. [Google Scholar] [CrossRef]
- Liu, L.; Xu, W.; Li, S.; Chen, M.; Cheng, Y.; Yuan, W.; Cheng, Z.; Li, Q. Penicindopene A, a new indole diterpene from the deep-sea fungus Penicillium sp. YPCMAC1. Nat. Prod. Res. 2019, 33, 2988–2994. [Google Scholar] [CrossRef]
- Liang, X.R.; Ma, X.Y.; Ji, N.Y. Trichosordarin A, a norditerpene glycoside from the marine-derived fungus Trichoderma harzianum R5. Nat. Prod. Res. 2020, 34, 2037–2042. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Li, X.; Hu, Z.; Wang, L. Novel Harziane Diterpenes from Deep-Sea Sediment Fungus Trichoderma sp. SCSIOW21 and Their Potential Anti-Inflammatory Effects. Mar. Drugs 2021, 19, 689. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Li, X.; Li, X.-M.; Xu, G.-M.; Zhang, P.; Meng, L.-H.; Wang, B.-G. Tetranorlabdane Diterpenoids from the Deep Sea Sediment-Derived Fungus Aspergillus wentii SD-310. Planta Med. 2016, 82, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.-D.; Li, X.-M.; Xu, G.-M.; Liu, Y.; Wang, B.-G. Wentinoids A–F, six new isopimarane diterpenoids from Aspergillus wentii SD-310, a deep-sea sediment derived fungus. RSC Adv. 2017, 7, 4387–4394. [Google Scholar] [CrossRef]
- Li, X.-D.; Li, X.-M.; Li, X.; Xu, G.-M.; Liu, Y.; Wang, B.-G. Aspewentins D–H, 20-Nor-isopimarane Derivatives from the Deep Sea Sediment-Derived Fungus Aspergillus wentii SD-310. J. Nat. Prod. 2016, 79, 1347–1353. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.; Li, X.M.; Xu, G.M.; Liu, Y.; Wang, B.G. 20-Nor-Isopimarane Epimers Produced by Aspergillus wentii SD-310, a Fungal Strain Obtained from Deep Sea Sediment. Mar. Drugs 2018, 16, 440. [Google Scholar] [CrossRef]
- Sun, L.; Li, D.L.; Chen, Y.C.; Tao, M.H.; Dan, F.J.; Zhang, W.M. Secondary metabolites of marine fungus Eutypella scoparia from the South China Sea and their antitumor activities. Chin. Tradit. Herb. Drugs 2011, 42, 432–436. [Google Scholar]
- Sun, L.; Li, D.; Tao, M.; Chen, Y.; Dan, F.; Zhang, W. Scopararanes C–G: New oxygenated pimarane diterpenes from the marine sediment-derived fungus Eutypella scoparia FS26. Mar. Drugs 2012, 10, 539–550. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Chen, Y.; Li, S.; Tan, G.; Sun, Z.; Pan, Q.; Ye, W.; Li, H.; Zhang, W. Cytotoxic pimarane-type diterpenes from the marine sediment-derived fungus Eutypella sp. FS46. Nat. Prod. Res. 2017, 31, 404–410. [Google Scholar] [CrossRef]
- Zhao, D.L.; Yang, L.J.; Shi, T.; Wang, C.Y.; Shao, C.L.; Wang, C.Y. Potent Phytotoxic Harziane Diterpenes from a Soft Coral-Derived Strain of the Fungus Trichoderma harzianum XS-20090075. Sci. Rep. 2019, 9, 13345. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Xu, K.X.; Xue, Y.; Cao, F.; Yang, L.J.; Hou, X.M.; Wang, C.Y.; Shao, C.L. Sordarin Diterpene Glycosides with an Unusual 1,3-Dioxolan-4-one Ring from the Zoanthid-Derived Fungus Curvularia hawaiiensis TA26-15. J. Nat. Prod. 2019, 82, 2477–2482. [Google Scholar] [CrossRef]
- Yang, B.; He, Y.; Lin, S.; Zhang, J.; Li, H.; Wang, J.; Hu, Z.; Zhang, Y. Antimicrobial Dolabellanes and Atranones from a Marine-Derived Strain of the Toxigenic Fungus Stachybotrys chartarum. J. Nat. Prod. 2019, 82, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Suzue, M.; Arai, T.; Kikuchi, T.; Tanaka, R. Trichodermanins C–E, New Diterpenes with a Fused 6-5-6-6 Ring System Produced by a Marine Sponge-Derived Fungus. Mar. Drugs 2017, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Fujii, A.; Kikuchi, T. New Diterpenes with a Fused 6-5-6-6 Ring System Isolated from the Marine Sponge-Derived Fungus Trichoderma harzianum. Mar. Drugs 2019, 17, 480. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Kito, K.; Ooi, T.; Kanoh, K.; Shizuri, Y.; Kusumi, T. Four Pimarane Diterpenes from Marine Fungus: Chloroform Incorporated in Crystal Lattice for Absolute Configuration Analysis by X-ray. Chem. Lett. 2007, 36, 1386–1387. [Google Scholar] [CrossRef]
- Takagi, M.; Motohashi, K.; Khan, S.T.; Hashimoto, J.; Shin-Ya, K. JBIR-65, a new diterpene, isolated from a sponge-derived Actinomadura sp. SpB081030SC-15. J. Antibiot. 2010, 63, 401–403. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, C.; Hou, X.; Che, Q.; Zhang, G.; Gu, Q.; Liu, C.; Zhu, T.; Li, D. Ascandinines A–D, Indole Diterpenoids, from the Sponge-Derived Fungus Aspergillus candidus HDN15-152. J. Org. Chem. 2021, 86, 2431–2436. [Google Scholar] [CrossRef]
- Ebada, S.S.; Schulz, B.; Wray, V.; Totzke, F.; Kubbutat, M.H.G.; Müller, W.E.G.; Hamacher, A.; Kassack, M.U.; Lin, W.; Proksch, P. Arthrinins A–D: Novel diterpenoids and further constituents from the sponge derived fungus Arthrinium sp. Bioorg. Med. Chem. 2011, 19, 4644–4651. [Google Scholar] [CrossRef]
- Tsukada, M.; Fukai, M.; Miki, K.; Shiraishi, T.; Suzuki, T.; Nishio, K.; Sugita, T.; Ishino, M.; Kinoshita, K.; Takahashi, K.; et al. Chemical Constituents of a Marine Fungus, Arthrinium sacchari. J. Nat. Prod. 2011, 74, 1645–1649. [Google Scholar] [CrossRef]
- Gomes, N.M.; Bessa, L.J.; Buttachon, S.; Costa, P.M.; Buaruang, J.; Dethoup, T.; Silva, A.M.; Kijjoa, A. Antibacterial and antibiofilm activities of tryptoquivalines and meroditerpenes isolated from the marine-derived fungi Neosartorya paulistensis, N. laciniosa, N. tsunodae, and the soil fungi N. fischeri and N. siamensis. Mar. Drugs 2014, 12, 822–839. [Google Scholar] [CrossRef]
- Liu, T.; Li, Q.; Xu, X.; Li, G.; Tian, C.; Zhang, T. Molecular mechanisms of anti-cancer bioactivities of seaweed polysaccharides. Chin. Herb. Med. 2022, 14, 528–534. [Google Scholar] [CrossRef]
- Behzadnia, A.; Moosavi-Nasab, M.; Oliyaei, N. Anti-biofilm activity of marine algae-derived bioactive compounds. Front. Microbiol. 2024, 15, 1270174. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, A.; Kesharwani, K.; Deka, R.; Muthukumar, S.; Khan, M.J.; Rai, A.; Vinayak, V.; Varjani, S.; Joshi, K.B.; Morjaria, S. Microalgal drugs: A promising therapeutic reserve for the future. J. Biotechnol. 2022, 349, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.R.; Miao, F.P.; Song, Y.P.; Guo, Z.Y.; Ji, N.Y. Trichocitrin, a new fusicoccane diterpene from the marine brown alga-endophytic fungus Trichoderma citrinoviride cf-27. Nat. Prod. Res. 2016, 30, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.R.; Miao, F.P.; Song, Y.P.; Liu, X.H.; Ji, N.Y. Citrinovirin with a new norditerpene skeleton from the marine algicolous fungus Trichoderma citrinoviride. Bioorg. Med. Chem. Lett. 2016, 26, 5029–5031. [Google Scholar] [CrossRef]
- Liang, X.R.; Miao, F.P.; Song, Y.P.; Guo, Z.Y.; Ji, N.Y. Diterpenes and steroids from marine alga-endophytic fungus Trichoderma asperellum dl-34. Chem. Bioeng. 2016, 33, 32–36. [Google Scholar]
- Song, Y.P.; Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. Diterpenes and Sesquiterpenes from the Marine Algicolous Fungus Trichoderma harzianum X-5. J. Nat. Prod. 2018, 81, 2553–2559. [Google Scholar] [CrossRef]
- Zou, J.X.; Song, Y.P.; Zeng, Z.Q.; Ji, N.Y. Proharziane and Harziane Derivatives from the Marine Algicolous Fungus Trichoderma asperelloides RR-dl-6-11. J. Nat. Prod. 2021, 84, 1414–1419. [Google Scholar] [CrossRef]
- Zou, J.X.; Song, Y.P.; Ji, N.Y. Deoxytrichodermaerin, a harziane lactone from the marine algicolous fungus Trichoderma longibrachiatum A-WH-20-2. Nat. Prod. Res. 2021, 35, 216–221. [Google Scholar] [CrossRef]
- Song, Y.-P.; Miao, F.-P.; Liang, X.-R.; Yin, X.-L.; Ji, N.-Y. Harziane and cadinane terpenoids from the alga-endophytic fungus Trichoderma asperellum A-YMD-9-2. Phytochem. Lett. 2019, 32, 38–41. [Google Scholar] [CrossRef]
- Miao, F.P.; Liang, X.R.; Yin, X.L.; Wang, G.; Ji, N.Y. Absolute configurations of unique harziane diterpenes from Trichoderma species. Org. Lett. 2012, 14, 3815–3817. [Google Scholar] [CrossRef]
- Song, Y.P.; Liu, X.H.; Shi, Z.Z.; Miao, F.P.; Fang, S.T.; Ji, N.Y. Bisabolane, cyclonerane, and harziane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Phytochemistry 2018, 152, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.F.; Li, X.M.; Meng, L.; Cui, C.M.; Gao, S.S.; Li, C.S.; Huang, C.G.; Wang, B.G. Asperolides A–C, tetranorlabdane diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. J. Nat. Prod. 2012, 75, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Ishino, M.; Kamauchi, H.; Takatori, K.; Kinoshita, K.; Sugita, T.; Koyama, K. Three novel phomactin-type diterpenes from a marine-derived fungus. Tetrahedron Lett. 2016, 57, 4341–4344. [Google Scholar] [CrossRef]
- Qiao, M.F.; Ji, N.Y.; Liu, X.H.; Li, K.; Zhu, Q.M.; Xue, Q.Z. Indoloditerpenes from an algicolous isolate of Aspergillus oryzae. Bioorg. Med. Chem. Lett. 2010, 20, 5677–5680. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.-M.; Li, X.; Wang, B.-G. New indole-diterpenoids from the algal-associated fungus Aspergillus nidulans. Phytochem. Lett. 2015, 12, 182–185. [Google Scholar] [CrossRef]
- Miao, F.P.; Liang, X.R.; Liu, X.H.; Ji, N.Y. Aspewentins A–C, norditerpenes from a cryptic pathway in an algicolous strain of Aspergillus wentii. J. Nat. Prod. 2014, 77, 429–432. [Google Scholar] [CrossRef]
- Gao, S.S.; Li, X.M.; Zhang, Y.; Li, C.S.; Wang, B.G. Conidiogenones H and I, two new diterpenes of Cyclopiane class from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S. Chem. Biodivers. 2011, 8, 1748–1753. [Google Scholar] [CrossRef]
- Ishino, M.; Kinoshita, K.; Takahashi, K.; Sugita, T.; Shiro, M.; Hasegawa, K.; Koyama, K. Phomactins K–M, three novel phomactin-type diterpenes from a marine-derived fungus. Tetrahedron 2012, 68, 8572–8576. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.-M.; Zhao, J.-L.; Li, N.; Chen, R.-D.; Xie, K.-B.; Zhang, W.-J.; Feng, K.-P.; Yan, Z.; Wang, N.; et al. Two new diterpenoids from the endophytic fungus Trichoderma sp. Xy24 isolated from mangrove plant Xylocarpus granatum. Chin. Chem. Lett. 2016, 27, 957–960. [Google Scholar] [CrossRef]
- Elsbaey, M.; Tanaka, C.; Miyamoto, T. New secondary metabolites from the mangrove endophytic fungus Aspergillus versicolor. Phytochem. Lett. 2019, 32, 70–76. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Y.; Liu, P.; Fu, P.; Zhu, T.; Wang, W.; Zhu, W. Indole-diterpenoids with anti-H1N1 activity from the aciduric fungus Penicillium camemberti OUCMDZ-1492. J. Nat. Prod. 2013, 76, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Bai, M.; Zhou, X.M.; Huang, G.L.; Shao, T.M.; Luo, Y.P.; Niu, Z.G.; Niu, Y.Y.; Chen, G.Y.; Han, C.R. Penicilindoles A-C, Cytotoxic Indole Diterpenes from the Mangrove-Derived Fungus EuPenicillium sp. HJ002. J. Nat. Prod. 2018, 81, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Li, X.M.; Williams, K.; Proksch, P.; Ji, N.Y.; Wang, B.G. Rhizovarins A–F, Indole-Diterpenes from the Mangrove-Derived Endophytic Fungus Mucor irregularis QEN-189. J. Nat. Prod. 2016, 79, 2066–2074. [Google Scholar] [CrossRef]
- Niu, S.; Fan, Z.; Tang, X.; Liu, Q.; Shao, Z.; Liu, G.; Yang, X.-W. Cyclopiane-type diterpenes from the deep-sea-derived fungus Penicillium commune MCCC 3A00940. Tetrahedron Lett. 2018, 59, 375–378. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Han, S.; Zhang, J.; Xu, W.; Li, Q.; Cheng, Z. Penitholabene, a rare 19-nor labdane-type diterpenoid from the deep-sea-derived fungus Penicillium thomii YPGA3. Fitoterapia 2020, 146, 104691. [Google Scholar] [CrossRef]
- Hu, X.Y.; Meng, L.H.; Li, X.; Yang, S.Q.; Li, X.M.; Wang, B.G. Three New Indole Diterpenoids from the Sea-Anemone-Derived Fungus Penicillium sp. AS-79. Mar. Drugs 2017, 15, 137. [Google Scholar] [CrossRef]
- Kong, F.D.; Fan, P.; Zhou, L.M.; Ma, Q.Y.; Xie, Q.Y.; Zheng, H.Z.; Zheng, Z.H.; Zhang, R.S.; Yuan, J.Z.; Dai, H.F.; et al. Four Indole Terpenoids with Potent Protein Tyrosine Phosphatase Inhibitory Activity from the Marine-Derived Fungus Penicillium sp. KFD28. Org. Lett. 2019, 21, 4864–4867. [Google Scholar] [CrossRef]
- Niu, S.; Peng, G.; Xia, J.M.; Xie, C.L.; Li, Z.; Yang, X.W. A New Pimarane Diterpenoid from the Botryotinia fuckeliana Fungus Isolated from Deep-Sea Water. Chem. Biodivers. 2019, 16, e1900519. [Google Scholar] [CrossRef]
- Niu, S.; Xie, C.L.; Xia, J.M.; Liu, Q.M.; Peng, G.; Liu, G.M.; Yang, X.W. Botryotins A–H, Tetracyclic Diterpenoids Representing Three Carbon Skeletons from a Deep-Sea-Derived Botryotinia fuckeliana. Org. Lett. 2020, 22, 580–583. [Google Scholar] [CrossRef]
- Niu, S.; Xia, J.M.; Li, Z.; Yang, L.H.; Yi, Z.W.; Xie, C.L.; Peng, G.; Luo, Z.H.; Shao, Z.; Yang, X.W. Aphidicolin Chemistry of the Deep-Sea-Derived Fungus Botryotinia fuckeliana MCCC 3A00494. J. Nat. Prod. 2019, 82, 2307–2331. [Google Scholar] [CrossRef]
- Mullowney, M.W.; Ó hAinmhire, E.; Tanouye, U.; Burdette, J.E.; Pham, V.C.; Murphy, B.T. A Pimarane Diterpene and Cytotoxic Angucyclines from a Marine-Derived Micromonospora sp. in Vietnam’s East Sea. Mar. Drugs 2015, 13, 5815–5827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Adnani, N.; Braun, D.R.; Ellis, G.A.; Barns, K.J.; Parker-Nance, S.; Guzei, I.A.; Bugni, T.S. Micromonohalimanes A and B: Antibacterial Halimane-Type Diterpenoids from a Marine Micromonospora Species. J. Nat. Prod. 2016, 79, 2968–2972. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Antonov, A.S.; Oleinikova, G.K.; Khudyakova, Y.V.; Popov, R.S.; Denisenko, V.A.; Pislyagin, E.A.; Chingizova, E.A.; Afiyatullov, S.S. Virescenosides From the Holothurian-Associated Fungus Acremonium striatisporum Kmm 4401. Mar. Drugs 2019, 17, 616. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, Y.; Guo, L.; Wang, Y.; Liu, P.; Zhu, W. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs 2014, 12, 3970–3981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yi, W.; Ge, H.; Zhang, Z.; Wu, B. A new antimicrobial indoloditerpene from a marine-sourced fungus aspergillus versicolor ZZ761. Nat. Prod. Res. 2021, 35, 3114–3119. [Google Scholar] [CrossRef]
- Kankanamge, S.; Khalil, Z.G.; Bernhardt, P.V.; Capon, R.J. Noonindoles A–F: Rare Indole Diterpene Amino Acid Conjugates from a Marine-Derived Fungus, Aspergillus noonimiae CMB-M0339. Mar. Drugs 2022, 20, 698. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; He, L.M.; Xue, Y.Q.; Jia, J.; Wang, S.B.; Zhu, K.K.; Hong, K.; Cai, Y.S. A New Fusicoccane-Type Norditerpene and a New Indone from the Marine-Derived Fungus Aspergillus aculeatinus WHUF0198. Chem. Biodivers. 2021, 18, e2100562. [Google Scholar] [CrossRef]
- Xia, X.; Qi, J.; Liu, Y.; Jia, A.; Zhang, Y.; Liu, C.; Gao, C.; She, Z. Bioactive isopimarane diterpenes from the fungus, Epicoccum sp. HS-1, associated with Apostichopus japonicus. Mar. Drugs 2015, 13, 1124–1132. [Google Scholar] [CrossRef]
- Wang, W.; Wan, X.; Liu, J.; Wang, J.; Zhu, H.; Chen, C.; Zhang, Y. Two New Terpenoids from Talaromyces purpurogenus. Mar. Drugs 2018, 16, 150. [Google Scholar] [CrossRef]
- Oh, D.-C.; Jensen, P.R.; Kauffman, C.A.; Fenical, W. Libertellenones A–D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorg. Med. Chem. 2005, 13, 5267–5273. [Google Scholar] [CrossRef]
- Yu, H.B.; Wang, X.L.; Xu, W.H.; Zhang, Y.X.; Qian, Y.S.; Zhang, J.P.; Lu, X.L.; Liu, X.Y. Eutypellenoids A–C, New Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1. Mar. Drugs 2018, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- González, Y.; Torres-Mendoza, D.; Jones, G.E.; Fernandez, P.L. Marine Diterpenoids as Potential Anti-Inflammatory Agents. Mediat. Inflamm. 2015, 2015, 263543. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.E.; Collins, J.E.; Roberts, B.; Roberts, J.C.; Winder, P.L.; Reed, J.K.; Diaz, M.C.; Pomponi, S.A.; Chakrabarti, D. Antiplasmodial Compounds from Deep-Water Marine Invertebrates. Mar. Drugs 2021, 19, 179. [Google Scholar] [CrossRef]
- Santacruz, L.; Thomas, O.P.; Duque, C.; Puyana, M.; Tello, E. Comparative Analyses of Metabolomic Fingerprints and Cytotoxic Activities of Soft Corals from the Colombian Caribbean. Mar. Drugs 2019, 17, 37. [Google Scholar] [CrossRef]
- Torres-García, I.; López-Martínez, J.L.; Muñoz-Dorado, M.; Rodríguez-García, I.; Álvarez-Corral, M. Marine Terpenic Endoperoxides. Mar. Drugs 2021, 19, 661. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Thawabteh, A.; Lelario, F.; Bufo, S.A.; Scrano, L. Classification, Toxicity and Bioactivity of Natural Diterpenoid Alkaloids. Molecules 2021, 26, 4103. [Google Scholar] [CrossRef]
- Tai, C.J.; Ahmed, A.F.; Chao, C.H.; Yen, C.H.; Hwang, T.L.; Chang, F.R.; Huang, Y.M.; Sheu, J.H. Spongenolactones A–C, Bioactive 5,5,6,6,5-Pentacyclic Spongian Diterpenes from the Red Sea Sponge Spongia sp. Mar. Drugs 2022, 20, 498. [Google Scholar] [CrossRef]
- Chen, Q.; Mao, Q.; Bao, M.; Mou, Y.; Fang, C.; Zhao, M.; Jiang, W.; Yu, X.; Wang, C.; Dai, L.; et al. Spongian Diterpenes Including One with a Rearranged Skeleton from the Marine Sponge Spongia officinalis. J. Nat. Prod. 2019, 82, 1714–1718. [Google Scholar] [CrossRef]
- El-Desoky, A.H.; Kato, H.; Angkouw, E.D.; Mangindaan, R.E.; de Voogd, N.J.; Tsukamoto, S. Ceylonamides A–F, Nitrogenous Spongian Diterpenes That Inhibit RANKL-Induced Osteoclastogenesis, from the Marine Sponge Spongia ceylonensis. J. Nat. Prod. 2016, 79, 1922–1928. [Google Scholar] [CrossRef]
- El-Desoky, A.H.; Kato, H.; Kagiyama, I.; Hitora, Y.; Losung, F.; Mangindaan, R.E.; de Voogd, N.J.; Tsukamoto, S. Ceylonins A–F, Spongian Diterpene Derivatives That Inhibit RANKL-Induced Formation of Multinuclear Osteoclasts, from the Marine Sponge Spongia ceylonensis. J. Nat. Prod. 2017, 80, 90–95. [Google Scholar] [CrossRef]
- Tai, C.J.; Huang, C.Y.; Ahmed, A.F.; Orfali, R.S.; Alarif, W.M.; Huang, Y.M.; Wang, Y.H.; Hwang, T.L.; Sheu, J.H. An Anti-Inflammatory 2,4-Cyclized-3,4-Secospongian Diterpenoid and Furanoterpene-Related Metabolites of a Marine Sponge Spongia sp. from the Red Sea. Mar. Drugs 2021, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Lhullier, C.; de Oliveira Tabalipa, E.; Nienkotter Sarda, F.; Sandjo, L.P.; Zanchett Schneider, N.F.; Carraro, J.L.; Oliveira Simoes, C.M.; Schenkel, E.P. Clerodane Diterpenes from the Marine Sponge Raspailia bouryesnaultae Collected in South Brazil. Mar. Drugs 2019, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Abdjul, D.B.; Yamazaki, H.; Kanno, S.; Takahashi, O.; Kirikoshi, R.; Ukai, K.; Namikoshi, M. Structures and Biological Evaluations of Agelasines Isolated from the Okinawan Marine Sponge Agelas nakamurai. J. Nat. Prod. 2015, 78, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Cho, Y.; Son, A.; Shin, S.W.; Lee, Y.J.; Park, H.C. Therapeutic Potential of (-)-Agelamide D, a Diterpene Alkaloid from the Marine Sponge Agelas sp. as a Natural Radiosensitizer in Hepatocellular Carcinoma Models. Mar. Drugs 2020, 18, 500. [Google Scholar] [CrossRef]
- Shingaki, M.; Wauke, T.; Ahmadi, P.; Tanaka, J. Four Cytotoxic Spongian Diterpenes from the Sponge Dysidea cf. arenaria. Chem. Pharm. Bull. 2016, 64, 272–275. [Google Scholar] [CrossRef]
- Lee, J.S.; Abdjul, D.B.; Yamazaki, H.; Takahashi, O.; Kirikoshi, R.; Ukai, K.; Namikoshi, M. Strongylophorines, new protein tyrosine phosphatase 1B inhibitors, from the marine sponge Strongylophora strongilata collected at Iriomote Island. Bioorg. Med. Chem. Lett. 2015, 25, 3900–3902. [Google Scholar] [CrossRef]
- Lee, H.Y.; Jang, E.J.; Bae, S.Y.; Jeon, J.E.; Park, H.J.; Shin, J.; Lee, S.K. Anti-Melanogenic Activity of Gagunin D, a Highly Oxygenated Diterpenoid from the Marine Sponge Phorbas sp. via Modulating Tyrosinase Expression and Degradation. Mar. Drugs 2016, 14, 212. [Google Scholar] [CrossRef]
- Hong, L.L.; Yu, H.B.; Wang, J.; Jiao, W.H.; Cheng, B.H.; Yang, F.; Zhou, Y.J.; Gu, B.B.; Song, S.J.; Lin, H.W. Unusual Anti-allergic Diterpenoids from the Marine Sponge Hippospongia lachne. Sci. Rep. 2017, 7, 43138. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A.; Perinu, C.; Cirino, G.; De Gruttola, L.; Roviezzo, F. Tedanol: A potent anti-inflammatory ent-pimarane diterpene from the Caribbean Sponge Tedania ignis. Bioorg. Med. Chem. 2009, 17, 7542–7547. [Google Scholar] [CrossRef]
- El-Desoky, A.H.; Kato, H.; Tsukamoto, S. Ceylonins G–I: Spongian diterpenes from the marine sponge Spongia ceylonensis. J. Nat. Med. 2017, 71, 765–769. [Google Scholar] [CrossRef]
- Pech-Puch, D.; Forero, A.M.; Fuentes-Monteverde, J.C.; Lasarte-Monterrubio, C.; Martinez-Guitian, M.; González-Salas, C.; Guillén-Hernández, S.; Villegas-Hernández, H.; Beceiro, A.; Griesinger, C.; et al. Antimicrobial Diterpene Alkaloids from an Agelas citrina Sponge Collected in the Yucatán Peninsula. Mar. Drugs 2022, 20, 298. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Yang, M.; Yan, X.Y.; Li, S.W.; Ge, Z.Y.; Zhang, L.; Yao, L.G.; Guo, Y.W.; Liang, L.F. Mililatensols A–C, New Records of Sarsolenane and Capnosane Diterpenes from Soft Coral Sarcophyton mililatensis. Mar. Drugs 2022, 20, 566. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Q.; Chen, J.; Wu, M.J.; Zhang, H.Y.; Liang, L.F.; Guo, Y.W. Uncommon Capnosane Diterpenes with Neuroprotective Potential from South China Sea Soft Coral Sarcophyton boettgeri. Mar. Drugs 2022, 20, 602. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Elshamy, A.I.; Abdel-Tawab, A.M.; AbdelMohsen, M.M.; Ohta, S.; Pare, P.W.; Hegazy, M.F. Oxygenated Cembrene Diterpenes from Sarcophyton convolutum: Cytotoxic Sarcoconvolutum A–E. Mar. Drugs 2021, 19, 519. [Google Scholar] [CrossRef]
- Shaaban, M.; Issa, M.Y.; Ghani, M.A.; Hamed, A.; Abdelwahab, A.B. New pyranosyl cembranoid diterpenes from Sarcophyton trocheliophorum. Nat. Prod. Res. 2019, 33, 24–33. [Google Scholar] [CrossRef]
- Sun, H.S.; Horgen, F.D.; Romo, D.; Hull, K.G.; Kiledal, S.A.; Fleig, A.; Feng, Z.P. Waixenicin A, a marine-derived TRPM7 inhibitor: A promising CNS drug lead. Acta Pharmacol. Sin. 2020, 41, 1519–1524. [Google Scholar] [CrossRef]
- Hsiao, T.H.; Cheng, C.H.; Wu, T.Y.; Lu, M.C.; Chen, W.F.; Wen, Z.H.; Dai, C.F.; Wu, Y.C.; Sung, P.J. New cembranoid diterpenes from the cultured octocoral Nephthea columnaris. Molecules 2015, 20, 13205–13215. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Yang, Y.C.; Wang, S.K.; Duh, C.Y. Numerosol A–D, new cembranoid diterpenes from the soft coral Sinularia numerosa. Mar. Drugs 2014, 12, 3371–3380. [Google Scholar] [CrossRef]
- Ye, F.; Zhu, Z.D.; Gu, Y.C.; Li, J.; Zhu, W.L.; Guo, Y.W. Further New Diterpenoids as PTP1B Inhibitors from the Xisha Soft Coral Sinularia polydactyla. Mar. Drugs 2018, 16, 103. [Google Scholar] [CrossRef]
- Wright, A.D.; Nielson, J.L.; Tapiolas, D.M.; Liptrot, C.H.; Motti, C.A. A great barrier reef Sinularia sp. yields two new cytotoxic diterpenes. Mar. Drugs 2012, 10, 1619–1630. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.; Ngan, N.T.; Song, S.B.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Kim, Y.H.; Minh, C.V. New anti-inflammatory cembranoid diterpenoids from the Vietnamese soft coral Lobophytum crassum. Bioorg. Med. Chem. Lett. 2014, 24, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cheng, S.; Yuan, W.; Xi, Y.; Li, X.; Dong, J.; Huang, K.; Gustafson, K.R.; Yan, P. Cembranoids from a Chinese Collection of the Soft Coral Lobophytum crassum. Mar. Drugs 2016, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Cuong, N.X.; Thao, N.P.; Luyen, B.T.; Ngan, N.T.; Thuy, D.T.; Song, S.B.; Nam, N.H.; Kiem, P.V.; Kim, Y.H.; Minh, C.V. Cembranoid diterpenes from the soft coral Lobophytum crassum and their anti-inflammatory activities. Chem. Pharm. Bull. 2014, 62, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, J.; E, H.C.; Liu, B.S.; Tang, H.; Gerwick, W.H.; Hua, H.M.; Zhang, W. Briarane diterpenes from the South China Sea gorgonian coral, Junceella gemmacea. Mar. Drugs 2014, 12, 589–600. [Google Scholar] [CrossRef]
- Gutierrez, M.; Santamaria, R.; Gomez-Reyes, J.F.; Guzman, H.M.; Avila-Roman, J.; Motilva, V.; Talero, E. New Eunicellin-Type Diterpenes from the Panamanian Octocoral Briareum Asbestinum. Mar. Drugs 2020, 18, 84. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Lin, S.C.; Feng, C.W.; Chen, P.C.; Su, Y.D.; Li, C.M.; Yang, S.N.; Jean, Y.H.; Sung, P.J.; Duh, C.Y.; et al. Anti-Inflammatory and Analgesic Effects of the Marine-Derived Compound Excavatolide B Isolated from the Culture-Type Formosan Gorgonian Briareum excavatum. Mar. Drugs 2015, 13, 2559–2579. [Google Scholar] [CrossRef]
- Hanif, N.; Murni, A.; Tanaka, J. Sangiangols A and B, Two New Dolabellanes from an Indonesian Marine Soft Coral, Anthelia sp. Molecules 2020, 25, 3803. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lin, C.C.; Chu, Y.C.; Fu, C.W.; Sheu, J.H. Bioactive Diterpenes, Norditerpenes, and Sesquiterpenes from a Formosan Soft Coral Cespitularia sp. Pharmaceuticals 2021, 14, 1252. [Google Scholar] [CrossRef]
- Wu, S.L.; Su, J.H.; Huang, C.Y.; Tai, C.J.; Sung, P.J.; Liaw, C.C.; Sheu, J.H. Simplexins P–S, eunicellin-based diterpenes from the soft coral Klyxum simplex. Mar. Drugs 2012, 10, 1203–1211. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.; Brun, R.; Kaiser, M.; Van Kiem, P.; Van Minh, C.; Schmidt, T.J.; Kang, J.S.; Kim, Y.H. Anti-Protozoal Activities of Cembrane-Type Diterpenes from Vietnamese Soft Corals. Molecules 2015, 20, 12459–12468. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Michaud, D.P.; Schmidt, P.G. Marine natural products: Parguerol, deoxyparguerol, and isoparguerol. New brominated diterpenes with modified pimarane skeletons from the sea hare Aplysia dactylomela. J. Am. Chem. Soc. 1982, 104, 6415–6423. [Google Scholar] [CrossRef]
- Bian, W.-T.; You, Z.-J.; Wang, C.-Y.; Shao, C.-L. Brominated Pimarane Diterpenoids from the sea Hare Aplysia pulmonica from the South China Sea. Chem. Nat. Compd. 2014, 50, 557–559. [Google Scholar] [CrossRef]
- Zerbe, P.; Bohlmann, J. Plant diterpene synthases: Exploring modularity and metabolic diversity for bioengineering. Trends Biotechnol. 2015, 33, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Bathe, U.; Tissier, A. Cytochrome P450 enzymes: A driving force of plant diterpene diversity. Phytochemistry 2019, 161, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, B.; Xing, J.; Li, C. Endophytes: The novel sources for plant terpenoid biosynthesis. Appl. Microbiol. Biotechnol. 2021, 105, 4501–4513. [Google Scholar] [CrossRef]
- Pelot, K.A.; Chen, R.; Hagelthorn, D.M.; Young, C.A.; Addison, J.B.; Muchlinski, A.; Tholl, D.; Zerbe, P. Functional Diversity of Diterpene Synthases in the Biofuel Crop Switchgrass. Plant Physiol. 2018, 178, 54–71. [Google Scholar] [CrossRef]
- Zidorn, C. Secondary metabolites of seagrasses (Alismatales and Potamogetonales; Alismatidae): Chemical diversity, bioactivity, and ecological function. Phytochemistry 2016, 124, 5–28. [Google Scholar] [CrossRef]
- Suzuki, T.; Takeda, S.; Hayama, N.; Tanaka, I.; Komiyama, K. The Structure of Brominated Diterpene from the Marine Red Alga Laurencia obtusa (Hudson) Lamouroux. Chem. Lett. 2006, 18, 969–970. [Google Scholar] [CrossRef]
- Pardo-Vargas, A.; de Barcelos Oliveira, I.; Stephens, P.R.; Cirne-Santos, C.C.; de Palmer Paixao, I.C.; Ramos, F.A.; Jimenez, C.; Rodriguez, J.; Resende, J.A.; Teixeira, V.L.; et al. Dolabelladienols A–C, new diterpenes isolated from Brazilian brown alga Dictyota pfaffii. Mar. Drugs 2014, 12, 4247–4259. [Google Scholar] [CrossRef]
- Campbell, S.; Murray, J.; Delgoda, R.; Gallimore, W. Two New Oxodolastane Diterpenes from the Jamaican Macroalga Canistrocarpus cervicornis. Mar. Drugs 2017, 15, 150. [Google Scholar] [CrossRef]
- Rodrigues, D.; Alves, C.; Horta, A.; Pinteus, S.; Silva, J.; Culioli, G.; Thomas, O.P.; Pedrosa, R. Antitumor and antimicrobial potential of bromoditerpenes isolated from the red alga, Sphaerococcus coronopifolius. Mar. Drugs 2015, 13, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; de Andrade Tomaz, A.C.; Vanderlei de Souza, M.F.; Leitao da Cunha, E.V.; Kiss, R.; Mathieu, V.; Ioannou, E.; Roussis, V. Halogenated Diterpenes with In Vitro Antitumor Activity from the Red Alga Sphaerococcus coronopifolius. Mar. Drugs 2019, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Smyrniotopoulos, V.; Firsova, D.; Fearnhead, H.; Grauso, L.; Mangoni, A.; Tasdemir, D. Density Functional Theory (DFT)-Aided Structure Elucidation of Linear Diterpenes from the Irish Brown Seaweed Bifurcaria bifurcata. Mar. Drugs 2021, 19, 42. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Merten, C.; Firsova, D.; Fearnhead, H.; Tasdemir, D. Oxygenated Acyclic Diterpenes with Anticancer Activity from the Irish Brown Seaweed Bifurcaria bifurcata. Mar. Drugs 2020, 18, 581. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and Neuroprotective Potential of the Brown Seaweed Bifurcaria bifurcata in an in vitro Parkinson’s Disease Model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Merten, C.; Kaiser, M.; Tasdemir, D. Bifurcatriol, a New Antiprotozoal Acyclic Diterpene from the Brown Alga Bifurcaria bifurcata. Mar. Drugs 2017, 15, 245. [Google Scholar] [CrossRef]
- Pereira, R.C.; Lourenco, A.L.; Terra, L.; Abreu, P.A.; Laneuville Teixeira, V.; Castro, H.C. Marine Diterpenes: Molecular Modeling of Thrombin Inhibitors with Potential Biotechnological Application as an Antithrombotic. Mar. Drugs 2017, 15, 79. [Google Scholar] [CrossRef]
- Abou-El-Wafa, G.S.; Shaaban, M.; Shaaban, K.A.; El-Naggar, M.E.; Maier, A.; Fiebig, H.H.; Laatsch, H. Pachydictyols B and C: New diterpenes from Dictyota dichotoma Hudson. Mar. Drugs 2013, 11, 3109–3123. [Google Scholar] [CrossRef]
- Wu, J.; Xi, Y.; Li, G.; Zheng, Y.; Wang, Z.; Wang, J.; Fang, C.; Sun, Z.; Hu, L.; Jiang, W.; et al. Hydroazulene Diterpenes from a Dictyota Brown Alga and Their Antioxidant and Neuroprotective Effects Against Cerebral Ischemia-Reperfusion Injury. J. Nat. Prod. 2021, 84, 1306–1315. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Vyrla, D.; Harizani, M.; Doxaki, C.; Eliopoulos, A.G.; Roussis, V.; Ioannou, E.; Tsatsanis, C.; Kampranis, S.C. Neorogioltriol and Related Diterpenes from the Red Alga Laurencia Inhibit Inflammatory Bowel Disease in Mice by Suppressing M1 and Promoting M2-Like Macrophage Responses. Mar. Drugs 2019, 17, 97. [Google Scholar] [CrossRef]
- Wang, X.B.; Sun, Z.H.; Fan, L.X.; Liu, Y.Y.; Feng, J.; Ma, G.X.; Chen, D.L. Two novel diterpenes from the stems and leaves of tropical seagrass Enhalus acoroides in the South China sea. Nat. Prod. Res. 2021, 35, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, W.; Shen, L.; Wu, J. Four new diterpenes from the mangrove Ceriops tagal and structure revision of four dolabranes with a 4,18-epoxy group. Fitoterapia 2018, 124, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chacha, M.; Mapitse, R.; Afolayan, A.J.; Majinda, R.R.T. Antibacterial Diterpenes from the Roots of Ceriops tagal. Nat. Prod. Commun. 2008, 3, 1934578X0800300104. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.; Sattler, I.; Dahse, H.M.; Fu, H.; Grabley, S.; Lin, W. Three new pimaren diterpenoids from marine mangrove plant, Bruguiera gymnorrhiza. Pharmazie 2005, 60, 705–707. [Google Scholar] [CrossRef]
- Wang, J.-D.; Guo, Y.-W. Agallochaols A and B, Two New Diterpenes from the Chinese Mangrove Excoecaria agallocha L. Helv. Chim. Acta 2004, 87, 2829–2833. [Google Scholar] [CrossRef]
- Li, X.; Lei, J.; Zheng, Y.-N.; Sattler, I.; Lin, W.-H. New ent-Isopimarane Diterpene from Mangrove Excoecaria agallocha L. Chem. Res. Chin. Univ. 2007, 23, 541–543. [Google Scholar] [CrossRef]
- Kang, J.; Chen, R.Y.; Yu, D.Q. A new isopimarane-type diterpene and a new natural atisane-type diterpene from Excoecaria agallocha. J. Asian Nat. Prod. Res. 2005, 7, 729–734. [Google Scholar] [CrossRef]
- Dai, L.P.; Li, X.F.; Feng, Q.M.; Zhang, L.X.; Liu, Q.Y.; Xu, E.P.; Wu, H.; Wang, Z.M. Isolation and identification of two pairs of cytotoxic diterpene tautomers and their tautomerization mechanisms. Sci. Rep. 2020, 10, 1442. [Google Scholar] [CrossRef]
- Mohammed, A.; Tajuddeen, N.; Ibrahim, M.A.; Isah, M.B.; Aliyu, A.B.; Islam, M.S. Potential of diterpenes as antidiabetic agents: Evidence from clinical and pre-clinical studies. Pharmacol. Res. 2022, 179, 106158. [Google Scholar] [CrossRef]
- Saha, P.; Rahman, F.I.; Hussain, F.; Rahman, S.M.A.; Rahman, M.M. Antimicrobial Diterpenes: Recent Development From Natural Sources. Front. Pharmacol. 2021, 12, 820312. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Xu, F.; Gao, X.; Li, J.; Xu, S.; Zhang, D.; Wu, X.; Xu, J.; Hua, H.; et al. Diterpenoid lead stevioside and its hydrolysis products steviol and isosteviol: Biological activity and structural modification. Eur. J. Med. Chem. 2018, 156, 885–906. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Takeda, K.; Haraguchi, M.; Abe, Y.; Kuwahara, N.; Suzuki, S.; Terui, A.; Masaka, T.; Munakata, N.; Uchida, M.; et al. Four new diterpenoid alkaloids from Aconitum japonicum subsp. subcuneatum. J. Nat. Med. 2018, 72, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Jin, P.; Huang, L.; Zhang, Q.; Meng, L.; Yao, G. Structurally diverse diterpenoids from Pieris japonica as potent analgesics. Bioorg. Chem. 2020, 99, 103794. [Google Scholar] [CrossRef]
- Prieto, J.M.; Silveira, D. Natural Cytotoxic Diterpenoids, a Potential Source of Drug Leads for Melanoma Therapy. Curr. Pharm. Des. 2018, 24, 4237–4250. [Google Scholar] [CrossRef]
- Ndjoubi, K.O.; Sharma, R.; Hussein, A.A. The Potential of Natural Diterpenes Against Tuberculosis: An Updated Review. Curr. Pharm. Des. 2020, 26, 2909–2932. [Google Scholar] [CrossRef]
- Sarkar, S.; Gopal, P.K.; Paul, S. Diterpenoids-potential chemopreventive and chemotherapeutic agents in leukemia. Curr. Pharm. Biotechnol. 2014, 15, 127–142. [Google Scholar] [CrossRef]
- Luan, S.; Gao, Y.; Liang, X.; Zhang, L.; Yin, L.; He, C.; Liu, S.; Yin, Z.; Yue, G.; Zou, Y.; et al. Synthesis and structure-activity relationship of lipo-diterpenoid alkaloids with potential target of topoisomerase IIα for breast cancer treatment. Bioorg. Chem. 2021, 109, 104699. [Google Scholar] [CrossRef]











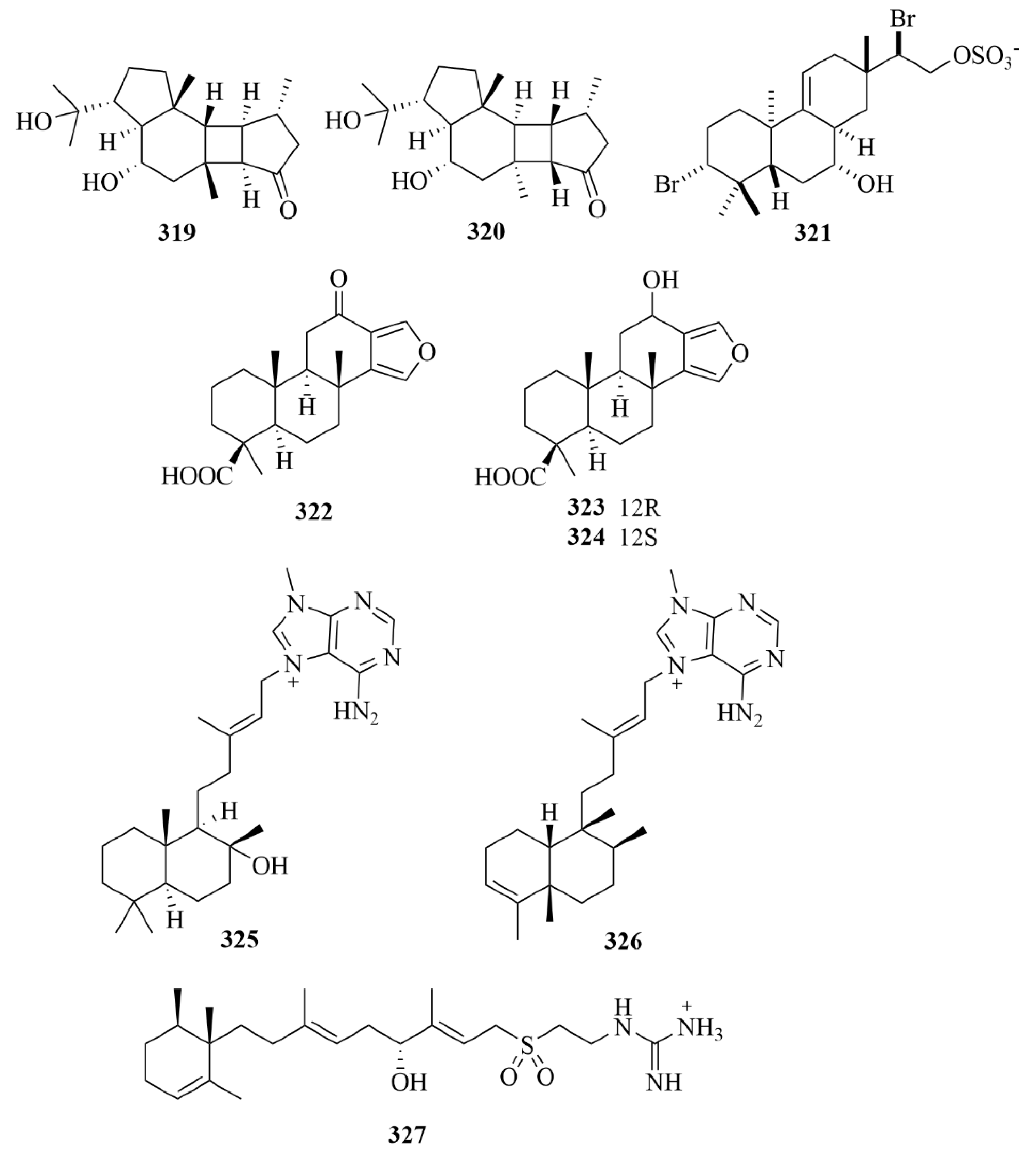

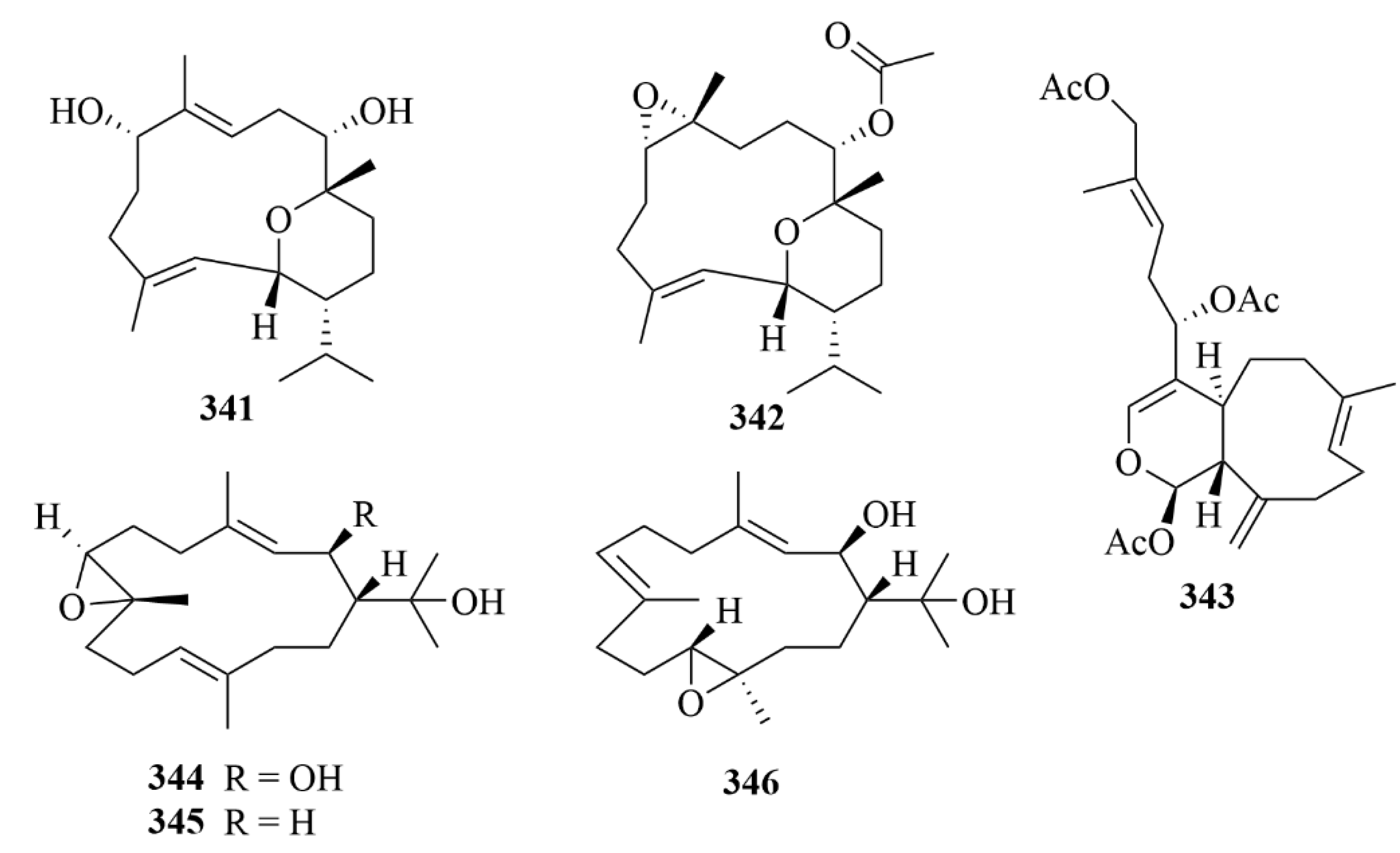
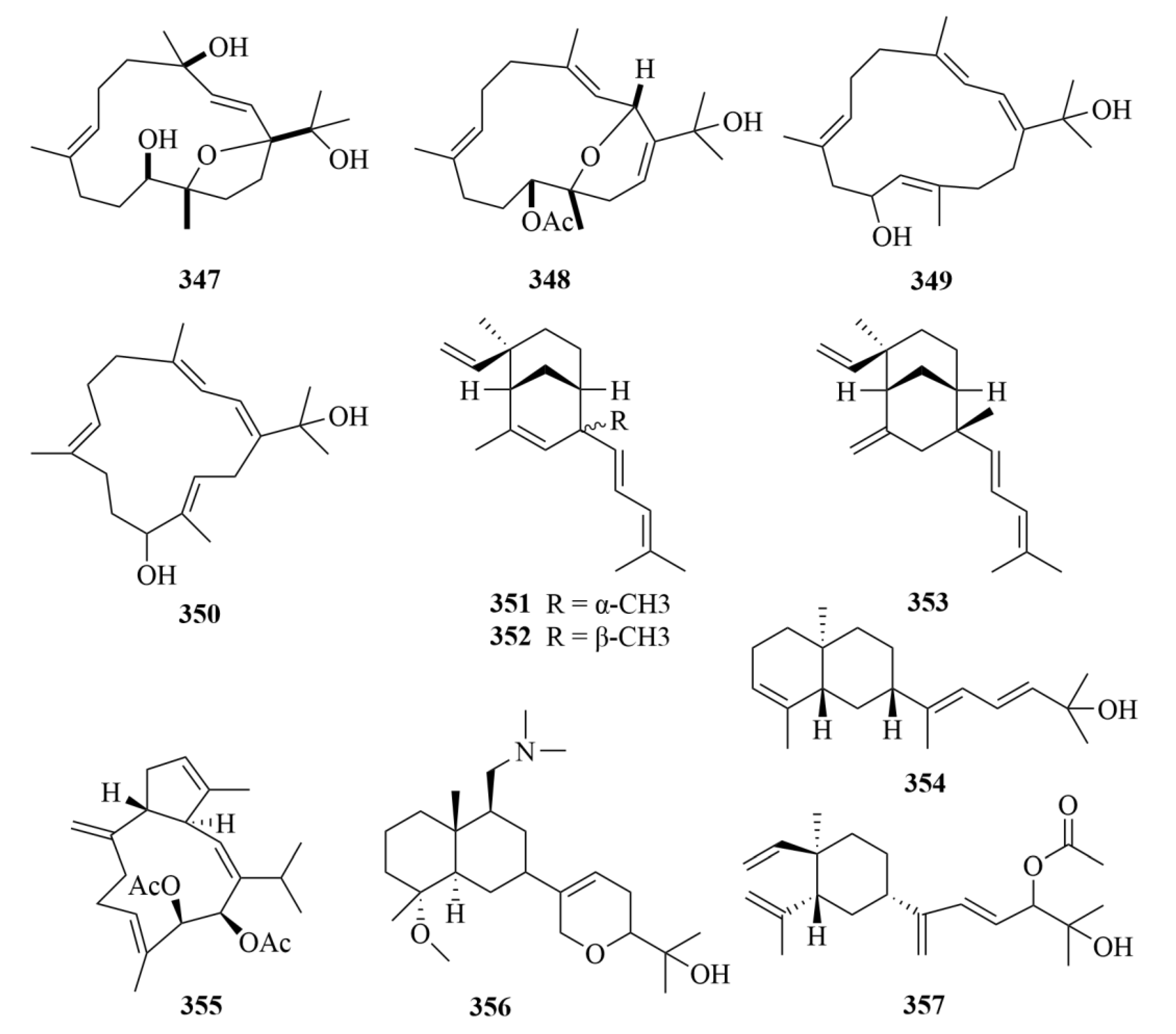
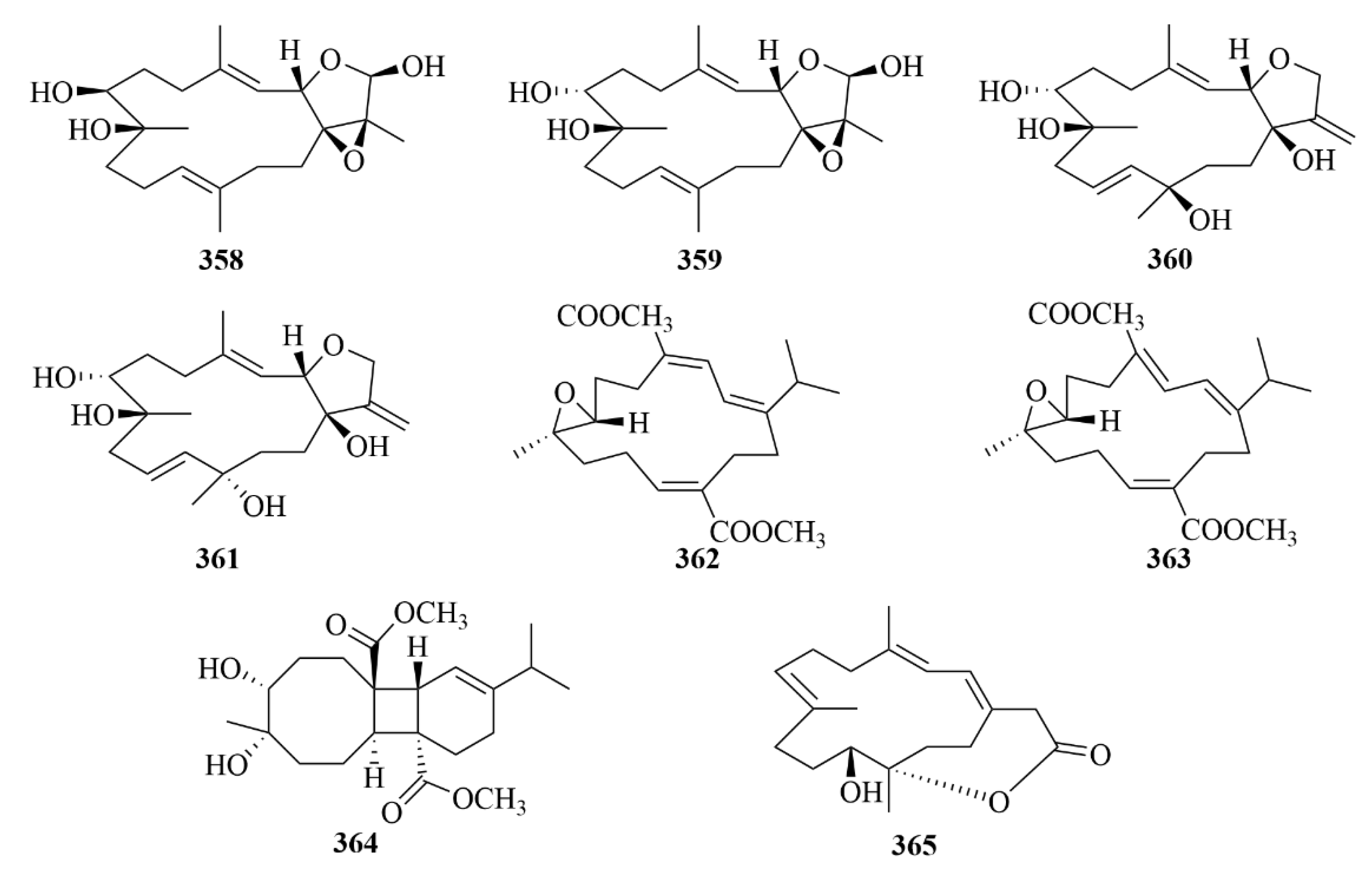


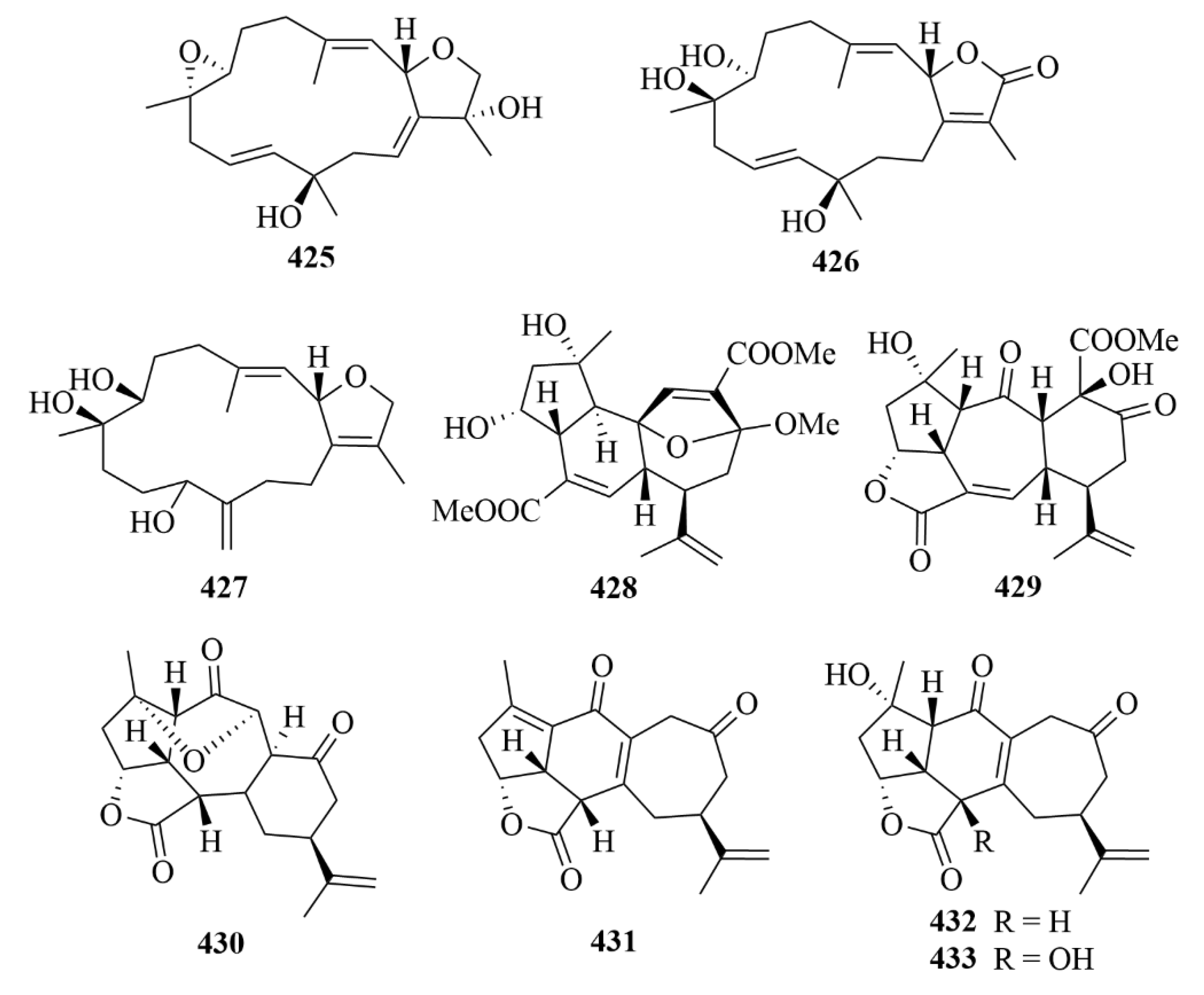
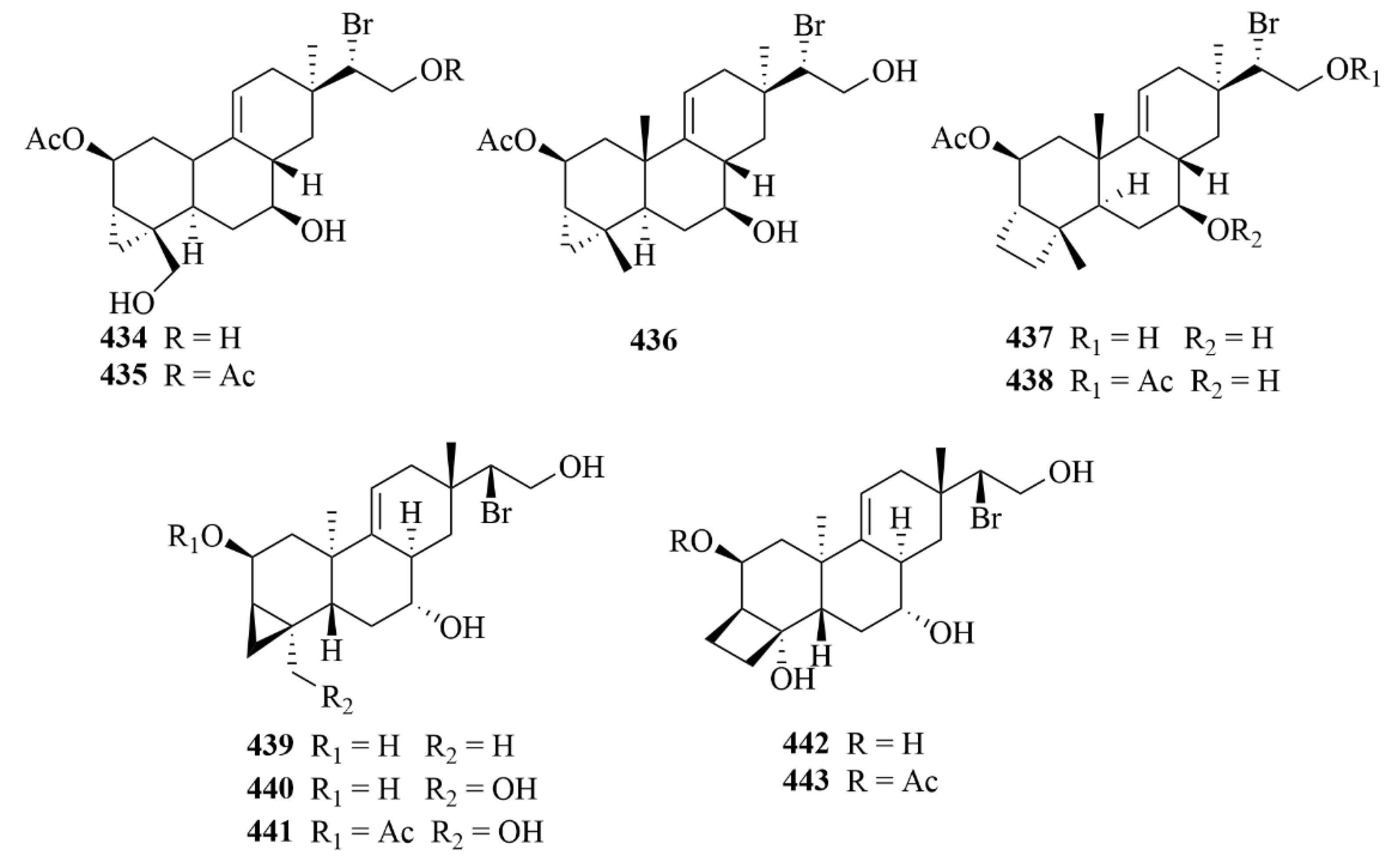
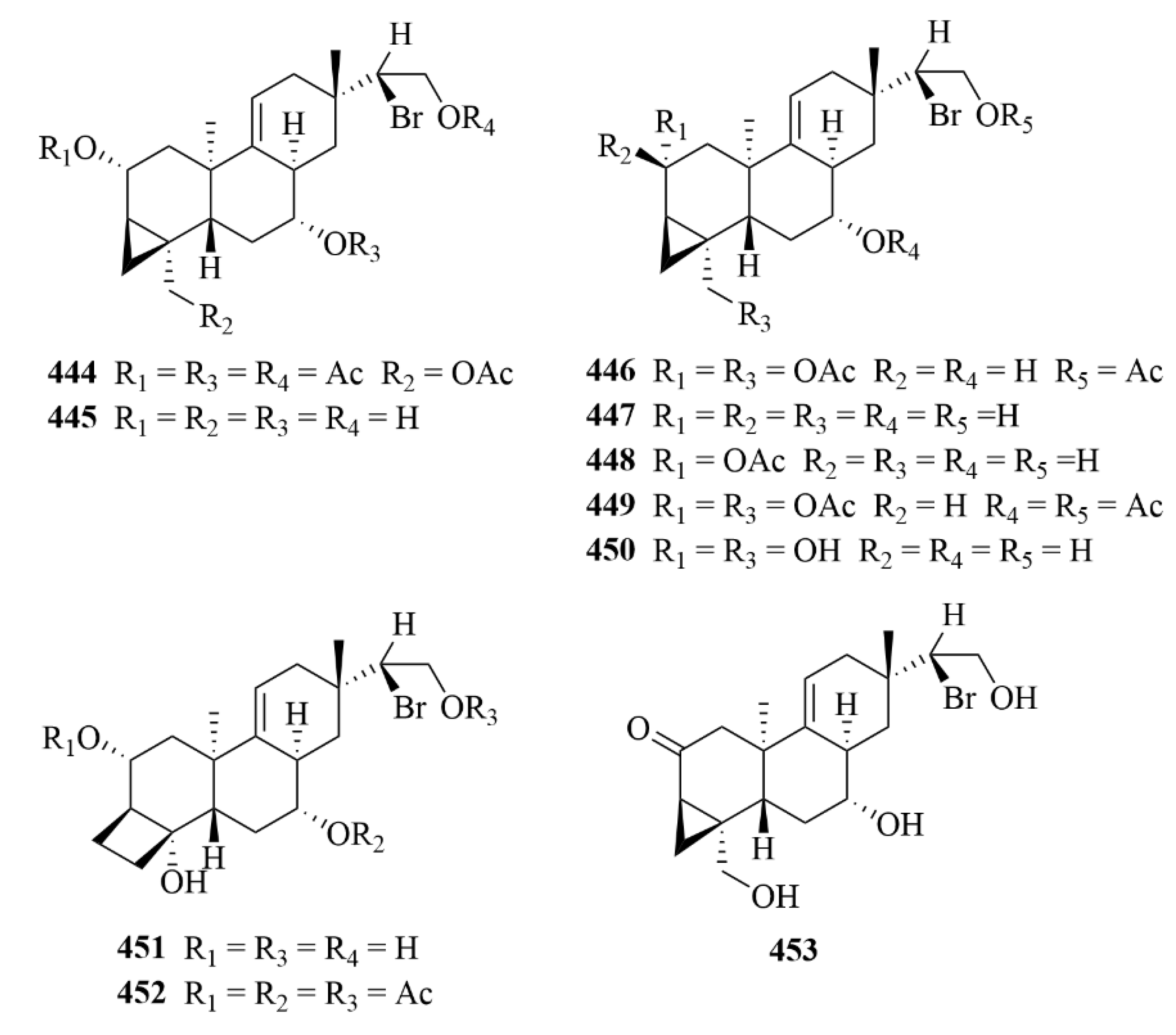

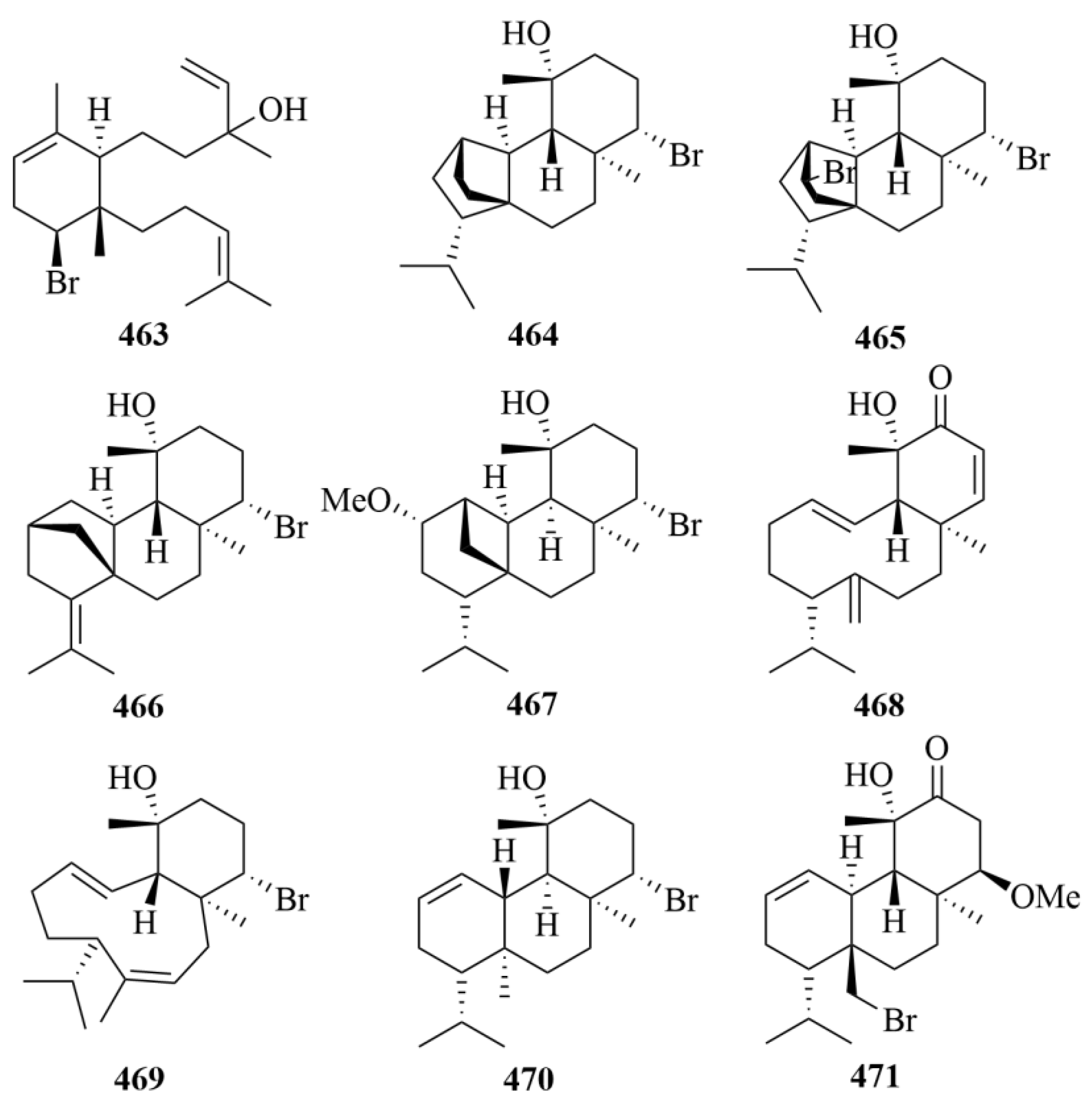


| Source | NO. | Compound | Producing Organism | Extract/Fraction | Activity | References |
|---|---|---|---|---|---|---|
| Sponge | 282–284 | Spongenolactones A-C | Red Sea sponge Spongia sp. | EtOAc/MeOH/CH2Cl2 (1:1:0.5) extract | An inhibitory effect against superoxide anion generation in fMLF/CB-stimulated human neutrophils; spongenolactone A (282) was more active against the growth of Staphylococcus aureus than spongenolactone B (283). | [87] |
| 285–289 | Sponalactone (285), 17-O-acetylepispongiatriol (286) and 17-O-acetylspongiatriol (287), together with two novel spongian diterpene artifacts, namely 15α,16α-dimethoxy-15,16-dihydroepispongiatriol (288) and 15α-ethoxyepispongiatriol-16(15H)-one (289) | The South China Sea sponge Spongia officinalis | 95% EtOH extract | Moderate inhibitory activities against LPS-induced NO production in RAW264.7 macrophages, with IC50 values of 12–32 µM. | [88] | |
| 290 and 291 | Ceylonamides A and B | the Indonesian marine sponge Spongia ceylonensis | EtOH extract | Significant inhibition of RANKL-induced osteoclastogenesis in RAW264 macrophages, with IC50 values of 13 µM and 18 µM, respectively. | [89,90] | |
| 297 | 17-dehydroxysponalactone | Red Sea sponge Spongia sp. | EtOAc/MeOH/CH2Cl2 (1:1:0.5) extract | No cytotoxicity but strong inhibitory activity against the superoxide anion generation and elastase release in the fMLF/CB-induced neutrophils. | [91] | |
| 298–302 | Raspadiene (298), kerlinic acid (299), kerlinic acid methyl ester (300), annonene (301), and 6-hydroxyannonene (302) | Marine sponge Raspailia bouryesnaultae | Ethanol extract | Moderate cytotoxic activity against the human cancer cell line A549, with IC50 values lower than 25 µM; Compound 286 exhibits inhibitory activities against HSV-1 (KOS and 29R strains) replication by 83% and 74%, respectively, which proved that it may be a promising compound against herpes simplex virus type 1 (HSV-1, KOS, and 29R strains). | [92] | |
| 303 and 304 | 2oxoagelasines A and F | marine sponge Agelas nakamurai Hoshino | EtOH extract | Inhibition against the growth of Mycobacterium smegmatis with inhibition zones of 10 mm at 20 µg/disc. | [93] | |
| 305 | 10-hydro-9-hydroxyagelasine F | marine sponge Agelas nakamurai Hoshino | EtOH extract | Significant activities against M. smegmatis. | [93] | |
| 306 | (-)-Agelamide D | marine sponge Agelas sp. | Methanol (1 L × 2) and dichloromethan-e (1 L × 1) extract | Activity toward tumor growth inhibition by radiation without systemic toxicities and enhanced radiation-induced ATF4 expression and apoptotic cell death. | [94] | |
| 307–310 | Compounds 307–310 | marine sponge Dysidea cf. arenaria | Acetone (1 L) extract | Cytotoxicity against NBT-T2 cells, with IC50 values of 3.1, 1.9, 8.4, and 3.1 µM, respectively. | [95] | |
| 311–317 | 26-O-ethylstrongylophorine-14 (311), 26-O-methylstrongylophorine-16 (312) and strongylophorines-2 (313), -3 (314), -8 (315), -15 (316), and -17 (317) | The Okinawan marine sponge Strongylophora strongilata | Ethanol extract | Inhibition against protein tyrosine phosphatase 1B (PTP1B) with IC50 values of 8.7, 8.5, >24.4, 9.0, 21.2, 11.9, and 14.8 lM, respectively. | [96] | |
| 318 | Gagunin D (GD) | marine sponge Phorbas sp. | / | Cytotoxicity against human leukemia cells, suppressing the expression of tyrosinase and increasing the degradation rate of tyrosinase, and inhibition activity against tyrosinase enzymatic. | [97] | |
| 319 and 320 | Hipposponlachnins A and B | marine sponge Hippospongia lachne | 95% EtOH extract | Inhibition activity against the release of biomarker β-hexosaminidase and the production of pro-inflammatory cytokine IL-4 and lipid mediator LTB4 in DNP-IgE stimulated RBL-2H3 cells. | [98] | |
| 321 | Tedanol | the Caribbean sponge Tedania ignis | MeOH and CHCl3 extract | Great anti-inflammatory activity at 1 mg/kg in the mouse model of inflammation in vivo, and potent reduction of the carrageenan-induced inflammation in acute (4 h) and subchronic (48 h) phases. | [99] | |
| 325–327 | (+)-8-epiagelasine T (325), (+)-10-epiagelasine B (326), and (+)-12-hydroxyagelasidine C (327) | sponge Agelas citrina | CH3OH-CH2Cl2 (1:1, 3 × 1.5 L) extract | Compound 326 exhibited the most activity against the Gram-positive pathogens (Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus faecalis) with an MIC in the range of 1–8 µg/mL, while other compounds showed lower activities. | [101] | |
| Coral | 328–330 | Mililatensols A–C | soft coral Sarcophyton mililatensis | Acetone extract | Great activities in the preliminary virtual screening of inhibitory potential against SARS-CoV-2. | [102] |
| 335 | Sarboettgerin E | the South China Sea soft coral Sarcophyton boettgeri | Acetone extract | Significant anti-neuroinflammatory activity against LPS-induced NO release in BV-2 microglial cells. | [103] | |
| 339 | Sarcoconvolutum D | the red sea soft coral Sarcophyton convolutum | Ethyl acetate extract | Cytotoxic activity against cell lines A549 and HSC-2 with IC50 values of 49.70 and 53.17 µM, respectively. | [104] | |
| 343 | Waixenicin A | soft coral Sarcothelia edmondsoni | / | Reduces hypoxic-ischemic brain injury and preserves long-term behavioral outcomes in mouse neonates. | [106] | |
| 354 and 355 | Sinupol (354) and sinulacetate (355) | Xisha soft coral Sinularia polydactyla | Acetone extract | Notable activity against protein tyrosine phosphatase 1B (PTP1B). | [109] | |
| 356 | Compound 356 | soft coral Sinularia sp. | MeOH (3 × 400 mL) and a butanol:CH2Cl2: H2O (150:50:100 mL) extract | Inhibition of the growth of three human tumor cell lines (SF-268, MCF-7, and H460) with a GI50 value of 70–175 µM | [110] | |
| 358–361 | Lobocrasols A–D | soft coral Lobophytum crassum | MeOH extract | Compounds 358 and 359 showed potent inhibition against TNFa-induced NF-jB transcriptional activity in HepG2 cells in a dose-dependent manner (IC50 = 6.30 ± 0.42, 6.63 ± 0.11 lM); decreased the gene expression levels in HepG2 cells in cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) to inhibit transcription. | [111] | |
| 362 and 368 | Locrassumins A (362) and G (368) | soft coral Lobophytum crassum | 95% EtOH extract | Moderate inhibition against lipopolysaccharide (LPS)-induced nitric oxide (NO) production with IC50 values of 8–24 µM. | [112] | |
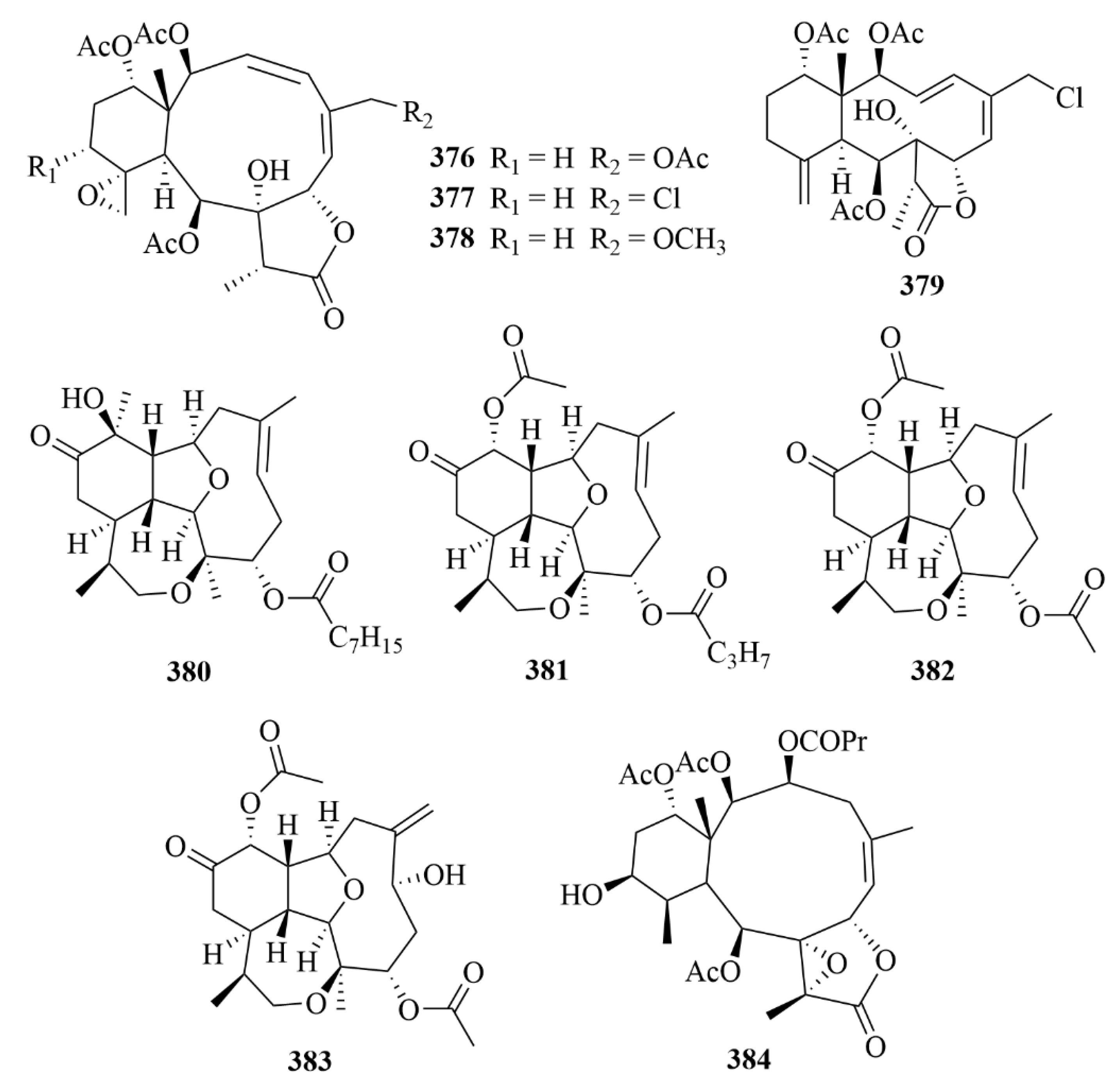
| 380–383 | Briarellin T (380), asbestinin 27 (381) and asbestinin 28 (382), asbestinin 17 (383) | octocoral Briareum asbestinum | N-hexane, ethyl acetate, and methanol extract | Well-proven anti-inflammatory activity through downregulation of the pro-inflammatory cytokines TNF-α, IL-6, IL-1β, and IL-8 as well as reduction of COX-2 expression in LPS-induced THP-1 macrophages. | [115] | |
| 384 | Excavatolide B | Formosan gorgonian Briareum excavatum | Methanol and dichloromethan-e (1:1) extract | Significant inhibition against the mRNA expression of the proinflammatory mediators, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), in lipopolysaccharide (LPS)-challenged murine macrophages (RAW 264.7); deaden carrageenan-induced nociceptive behaviors, mechanical allodynia, thermal hyperalgesia, weight-bearing deficits, and paw edema; inhibitory activity against iNOS and the infiltration of immune cells in carrageenan-induced inflammatory paw tissue. | [116] | |
| 385 and 386 | Sangiangol A and B | soft coral Anthelia sp. | EtOH extract | Moderate cytotoxicity against an NBT-T2 cell line (0.5–10 µg/mL). | [117] | |
| 387–389 | cespitulins H-J | soft coral Cespitularia sp. | EtOAc extract | Great anti-inflammatory activities; inhibition of the production of TNF-α and NO; suppression of the expression of iNOS and COX-2 gene. | [118] | |
| 399, 401–403 | Simplexins P (399) and R and S (401 and 402), simplexin A (403) | soft coral Klyxum simplex | EtOAc extract | Cytotoxicity against a limited panel of cancer cell lines. | [119] | |
| 409 and 411 | lobocrasol A and C | Vietnamese soft corals | / | Activities against bloodstream forms of T. brucei; elective activity against L. donovani. | [120] | |
| 404, 405, 409, 411, 417, and 420 | Compounds 404, 405, and lobocrasol A (409), lobocrasol C (411), sinumaximol C (417), and 13-Epi-scabrolide C (420) | Vietnamese soft corals | / | Activities against bloodstream forms of T. brucei. | [120] | |
| Sea hare | 434–438 | parguerol (434), parguerol 16-acetate (435), deoxyparguerol (436), isoparguerol (437), and isoparguerol 16-acetate (438) | sea hare Aplysia. Dactylomela | Chloroform-methanol (2:1) extract | Inhibition against P388 murine leukemia cells (IC50 = 8.3, 8.6, 0.86, 10.1, 1.0 µM). | [121] |
| 442 and 443 | Compounds 442 and 443 | sea hare Aplysia pulmonica | 95% EtOH extract | Toxicity against Artemia salina at a concentration of 0.5 µM. | [122] |
| Source | NO. | Compound | Producing Organism | Extract/Fraction | Activity | References |
|---|---|---|---|---|---|---|
| Red algae | 444 and 445 | 15-bromo-2,7,16,19-tetraacetoxy-9(11)-parguerene (444) and 15-bromo-2,7,16-tetraacetoxy-9(11)-parguerene (445) | the marine red algae Laurencia obtusa (Hudson) Lamouroux | / | Cytotoxicity. | [128] |
| 446, 450, 451, 453 | Compounds 446, 450, 451, 453 | the marine red algae Laurencia obtusa (Hudson) Lamouroux | / | Cytotoxic activity against HeLa with IC50 values of 5.7, 0.68, 10.8, and 11.6 µM, respectively, and against P388 cell lines with IC50 values of 6.5, 2.5, 14.6, and 18.3 µM, respectively. | [128] | |
| 463 | Sphaerodactylomelol | the red algae Sphaerococcus coronopifolius | MeOH and CH2Cl2 extract | Antimicrobial activity against S. aureus with IC50 value of 96.3 µM; showed cytotoxicity to HepG-2 cells with IC50 value of 720 µM; induced inhibition of cell proliferation with IC50 value of 280 µM. | [131] | |
| 468 and 471 | Compounds 468 and 471 | the red algae Sphaerococcus coronopifolius | CH2Cl2/MeOH (3/1) extract | Antitumor activity on one murine cancer cell line (one murine cancer cell line, B16F10, and five human cancer cell lines, A549, Hs683, MCF7, U373) with IC50 values 15 and 16 µM, respectively, and doxorubicin used as a positive control. | [132] | |
| 495–497 | Neorogioltriol (495), neorogioldiol (496), and O11,15-cyclo-14-bromo-14,15-dihydrorogiol-3,11-diol (497) | the red algae Laurencia | CH2Cl2/MeOH extract | Suppressed macrophage activation and promoted an M2-like anti-inflammatory phenotype. | [140] | |
| Brown algae | 454 and 455 | Compounds 454 and 455 | the marine brown algae Dictyota pfaffii | CH2Cl2 extract | Greater anti-HIV-1 activities than compound 457, with IC50 values of 2.9 and 4.1 µM, while its cytotoxic activity against MT-2 lymphocyte tumor cells was lower. | [129] |
| 473 | Compound 473 | the brown seaweed Bifurcaria bifurcate | CH2Cl2/MeOH extract | Inhibition against the growth of cancer cells (78.8%) at 100 µg/mL test concentration. | [133] | |
| 476–481 | Compounds 476–481 | the brown seaweed Bifurcaria bifurcate | CH2Cl2/MeOH extract | Inhibiton against the growth of the MDA-MB-231 cell line with IC50 values ranging from 11.6 to 32.0 µg/mL. | [134] | |
| 482 and 483 | Eleganolone and eleganonal | the brown seaweed Bifurcaria bifurcate | CH2Cl2/MeOH extract | Antioxidant potential by FRAP and ORAC assays. | [135] | |
| 484 | Bifurcatriol | the brown seaweed Bifurcaria bifurcate | CH2Cl2/MeOH extract | High activity against the malaria parasite P. falciparum (IC50 = 0.65 µg/mL) with low cytotoxicity (IC50 = 56.6 µg/mL). | [136] | |
| 485a | Pachydictyol B | the brown algae Dictyota dichotoma | Dichloromethan-e extract | Weak antimicrobial properties. | [138] | |
| 487–489 | Pachydictyol A (487), isopachydictyol A (488), and dichotomanol (489) | the brown algae Dictyota dichotoma | Dichloromethan-e extract | Useful to the studies of more active antithrombotic prototypes. | [137] | |
| 490–494 | Compounds 490–494 | the Dictyota brown algae | 95% EtOH extract | Potent antioxidant activities against H2O2-induced oxidative damage in neuron-like PC12 cells at a low concentration of 2 µM. | [139] | |
| Another algae | 498 and 499 | Enhoidin A and B | Tropical seagrass Enhalus acoroides | 95% ethyl alcohol extract | Moderate cytotoxic activities against four human cancer cell lines (MCF-7, HCT-116, HepG-2, and HeLa). | [141] |
| 468–470 | Compounds 468–470 | Jamaican macroalga Canistrocarpus cervicornis | Hexane, methylene chloride, ethyl acetate, and methanol extract | Moderate and concentration-dependent cytotoxic activity against human tumor cell lines PC3 and HT29. | [130] | |
| Mangrove | 502 and 503 | Tagalons C and D | the Chinese mangrove Ceriops tagal | 95% EtOH extract | Selective cytotoxicities with IC50 values of 3.75 and 8.07 µM against the human breast cancer cell line MT-1. | [142] |
| 504 | Isopimar-8(14)-en-16-hydroxy- 15-one | Maruhubi mangrove Ceriops tagal | Chloroform extract | Antibacterial activity against Bacillus cereus, Staphylococcus aureus, and Micrococcus kristinae (each with MIC values of 100 µg/mL); activity towards Streptococcus pyrogens and Salmonella pooni (MIC = 500, 250 µg/mL), with chloramphenicol serving as the positive control (each with MIC values of 1.0 µg/mL). | [143] | |
| 506 | Compound 506 | marine mangrove Bruguiera gymnorrhiza | / | Moderate cytotoxicity against K562 chronic myeloid leukemia cells with an IC50 value of 22.9 µM. | [144] | |
| 510 | Compound 510 | the stems of marine mangrove Bruguiera gymnorrhiza from Xiamen China | / | Weak cytotoxicity on L-929 (IC50 = 30.6 µM) | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Yu, S.; Zhou, M.; Wang, P.; Li, W.; Jin, X.; Pan, Y.; Sheng, Y.; Li, H.; Qin, L.; et al. Diterpenoids of Marine Organisms: Isolation, Structures, and Bioactivities. Mar. Drugs 2025, 23, 131. https://doi.org/10.3390/md23030131
Shi Q, Yu S, Zhou M, Wang P, Li W, Jin X, Pan Y, Sheng Y, Li H, Qin L, et al. Diterpenoids of Marine Organisms: Isolation, Structures, and Bioactivities. Marine Drugs. 2025; 23(3):131. https://doi.org/10.3390/md23030131
Chicago/Turabian StyleShi, Qi, Shujie Yu, Manjia Zhou, Peilu Wang, Wenlong Li, Xin Jin, Yiting Pan, Yunjie Sheng, Huaqiang Li, Luping Qin, and et al. 2025. "Diterpenoids of Marine Organisms: Isolation, Structures, and Bioactivities" Marine Drugs 23, no. 3: 131. https://doi.org/10.3390/md23030131
APA StyleShi, Q., Yu, S., Zhou, M., Wang, P., Li, W., Jin, X., Pan, Y., Sheng, Y., Li, H., Qin, L., & Meng, X. (2025). Diterpenoids of Marine Organisms: Isolation, Structures, and Bioactivities. Marine Drugs, 23(3), 131. https://doi.org/10.3390/md23030131





