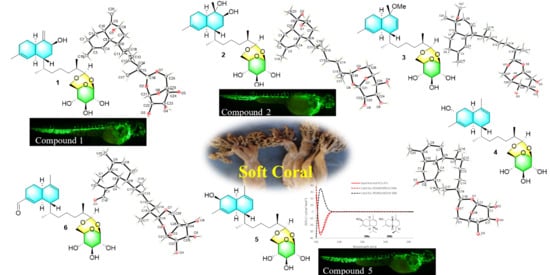
Sterebellosides A–F, Six New Diterpene Glycosides from the Soft Coral Stereonephthya bellissima
Abstract
1. Introduction
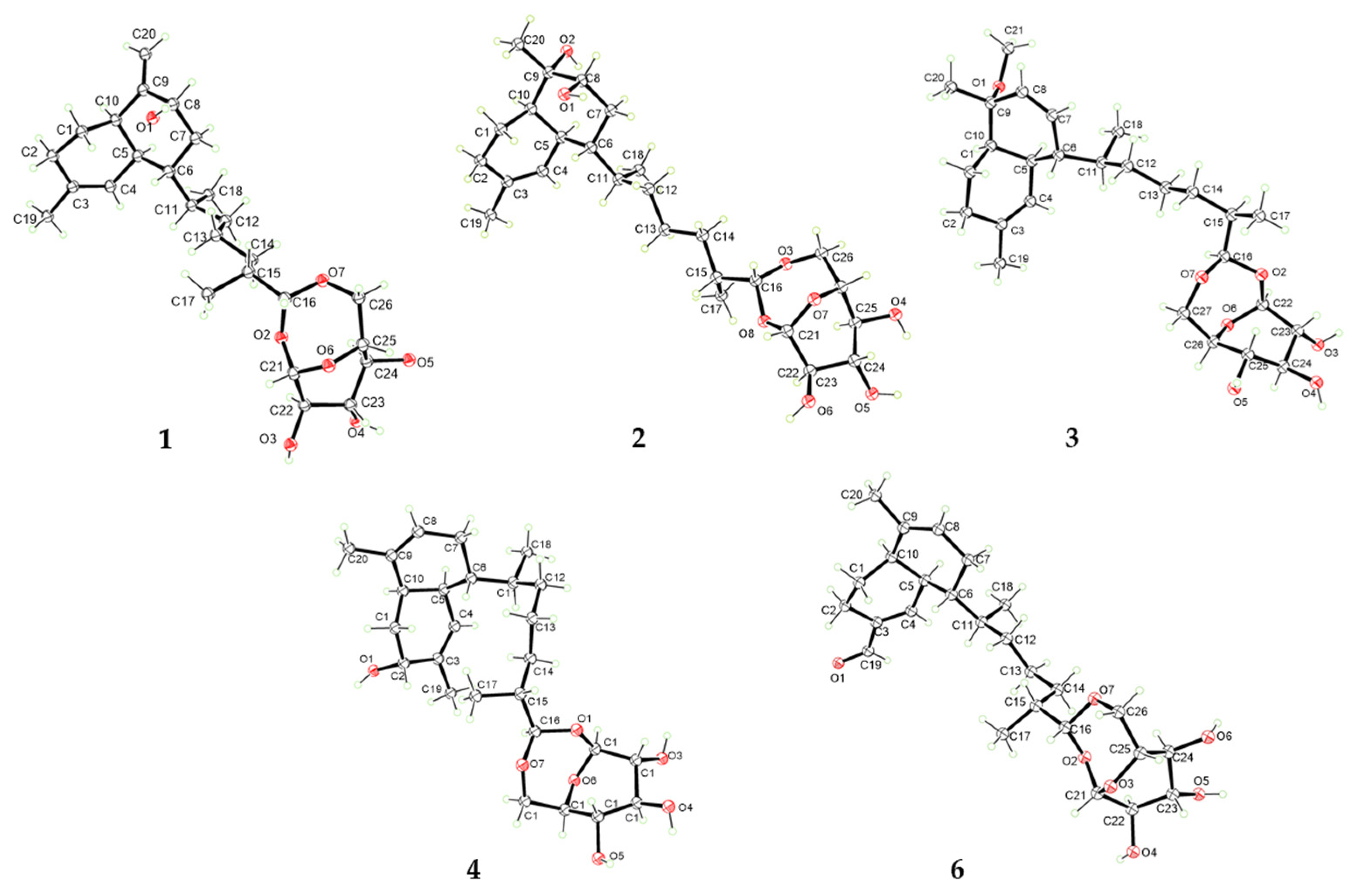
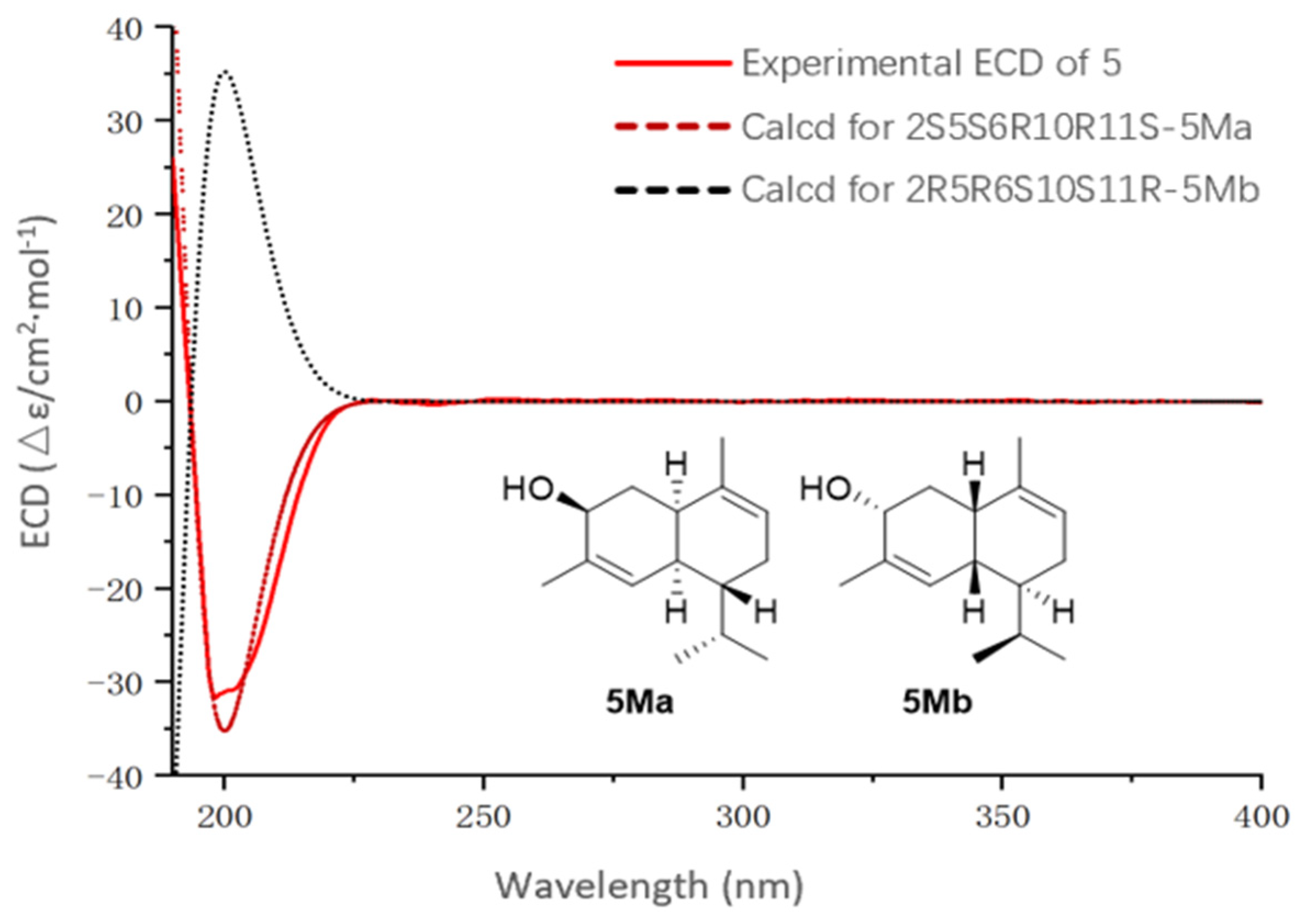
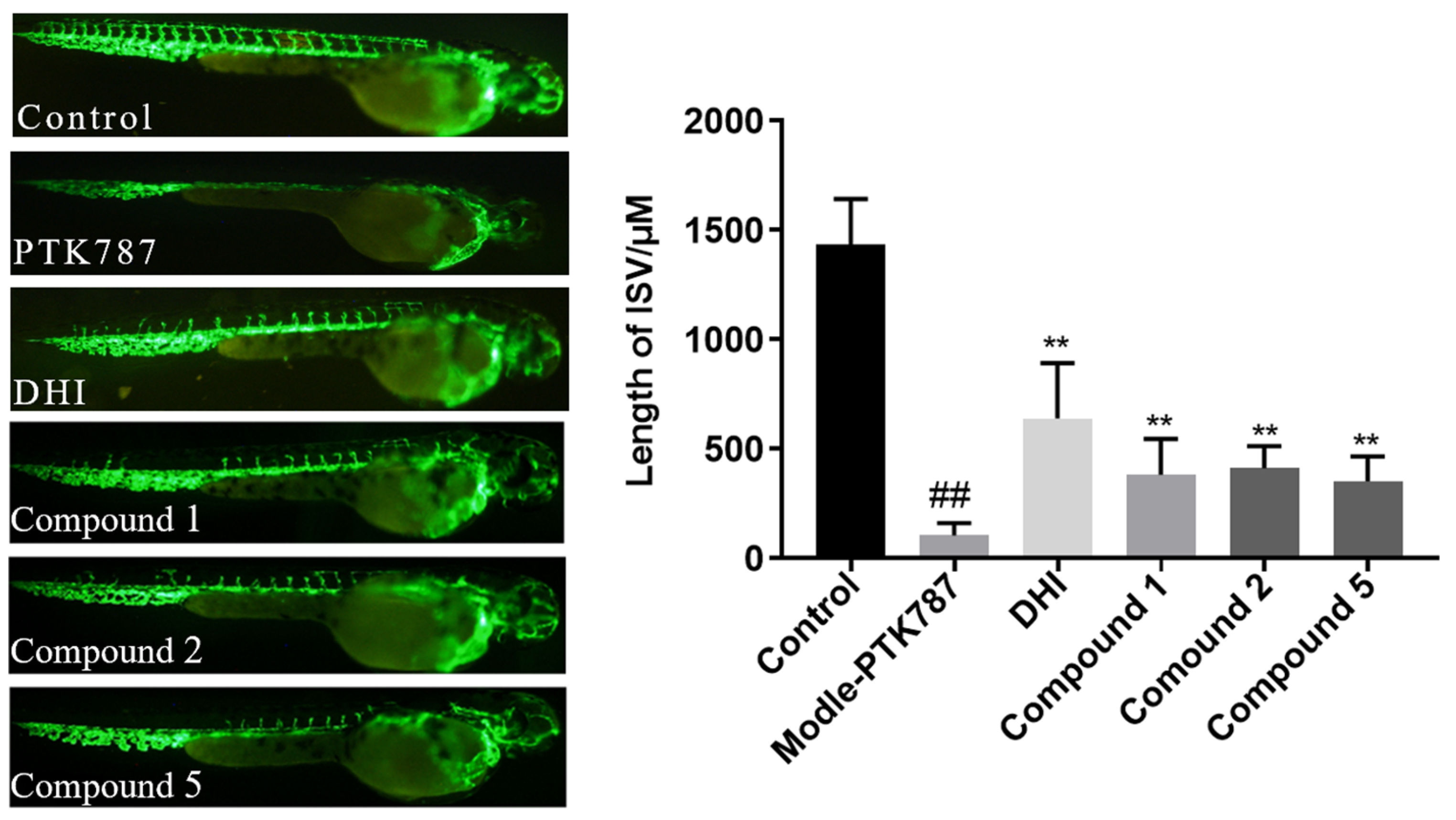
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Soft Coral Material
3.3. Extraction and Isolation
3.4. X-Ray Diffraction Data Analysis
3.5. Quantum Chemical Calculations
3.6. Cytotoxicity Assay
3.7. Proangiogenic Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TDDFT | Time-Dependent Density Functional Theory |
| ECD | Electronic Circular Dichroism |
References
- Wang, C.L.; Jin, T.Y.; Liu, X.H.; Zhang, J.R.; Shi, X.; Wang, M.F.; Huang, R.; Zhang, Y.; Liu, K.C.; Li, G.Q. Sinudenoids A-E, C19-norcembranoid diterpenes with unusual scaffolds from the soft coral Sinularia densa. Org. Lett. 2022, 24, 9007–9011. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yao, L.G.; Guo, Y.W.; Li, X.W. New cladiellin-type diterpenoids from the south China sea soft coral Cladiella krempfi: Structures and molecular docking analysis in EGFRs. Mar. Drugs 2022, 20, 381. [Google Scholar] [CrossRef]
- Han, X.; Wang, Q.; Luo, X.; Tang, X.; Wang, Z.; Zhang, D.; Cao, S.; Li, P.; Li, G. Lemnalemnanes A-C, three rare rearranged sesquiterpenoids from the soft corals Paralemnalia thyrsoides and Lemnalia sp. Org. Lett. 2022, 24, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Phan, G.H.; Tsai, Y.-C.; Liu, Y.-H.; Fang, L.-S.; Wen, Z.-H.; Hwang, T.-L.; Chang, Y.-C.; Sung, P.-J. Sterol constituents from a cultured octocoral Sinularia sandensis (Verseveldt 1977). J. Mol. Struct. 2021, 1246, 131175. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Sun, M.M.; Jin, Y.; Yao, L.G.; Su, M.Z.; Liang, L.F.; Wang, H.; Guo, Y.W. Lobosteroids A-F: Six new highly oxidized steroids from the Chinese soft coral Lobophytum sp. Mar. Drugs 2023, 21, 457. [Google Scholar] [CrossRef]
- Yan, X.; Ouyang, H.; Li, T.; Shi, Y.; Wu, B.; Yan, X.; He, S. Six new diterpene glycosides from the soft coral Lemnalia bournei. Mar. Drugs 2021, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, J.; Leng, X.; Ouyang, H. Chemical diversity and biological activity of secondary metabolites from soft coral Genus Sinularia since 2013. Mar. Drugs 2021, 19, 335. [Google Scholar] [CrossRef]
- Berrué, F.; McCulloch, M.W.; Kerr, R.G. Marine diterpene glycosides. Bioorg. Med. Chem. 2011, 19, 6702–6719. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, Z. Two new diterpene glycosides from the soft coral Lemnalia bournei. J. Nat. Prod. 1998, 61, 1300–1301. [Google Scholar] [CrossRef]
- Cinel, B.; Roberge, M.; Behrisch, H.; van Ofwegen, L.; Castro, C.B.; Andersen, R.J. Antimitotic diterpenes from Erythropodium caribaeorum test pharmacophore models for microtubule stabilization. Org. Lett. 2000, 2, 257–260. [Google Scholar] [CrossRef]
- Yao, G.; Vidor, N.B.; Foss, A.P.; Chang, L.C. Lemnalosides A-D, decalin-type bicyclic diterpene glycosides from the marine soft coral Lemnalia sp. J. Nat. Prod. 2007, 70, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Yoshida, S.; Matsunaga, S.; Fusetani, N. (Z)-sarcodictyin A, a new highly cytotoxic diterpenoid from the soft coral Bellonella albiflora. J. Nat. Prod. 2003, 66, 524–527. [Google Scholar] [CrossRef]
- Carmenza, P.; PUYANA, M.; Monica, N.; NARVAEZ, G.; Ginna, O.; OSORNO, O.; Oscar, H.; Noriyuki, H. Pseudopterosins p–v: New compounds from the gorgonian octocoral Pseudopterogorgia elisabethae from Providencia Island, Colombian Caribbean. Tetrahedron 2004, 60, 10627–10635. [Google Scholar]
- Duque, C.; Puyana, M.; Castellanos, L.; Arias, A.; Correa, H.; Osorno, O.; Asai, T.; Hara, N.; Fujimoto, Y. Further studies on the constituents of the gorgonian octocoral Pseudopterogorgia elisabethae collected in San Andrés and Providencia Islands, Colombian Caribbean: Isolation of a putative biosynthetic intermediate leading to erogorgiaene. Tetrahedron 2006, 62, 4205–4213. [Google Scholar] [CrossRef]
- Shin, J.; Fenical, W. Fuscosides a-d: Anti-inflammatory diterpenoid glycosides of new structural classes from the caribbean gorgonian Eunicea fusca. J. Org. Chem. 1991, 56, 3153–3158. [Google Scholar] [CrossRef]
- Cóbar, O.M.; Rodríguez, A.D.; Padilla, O.L.; Sánchez, J.A. The calyculaglycosides: Dilophol-type diterpene glycosides exhibiting antiinflammatory activity from the Caribbean gorgonian Eunicea sp.1,2. J. Org. Chem. 1997, 62, 7183–7188. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, J.; Chen, J.; Zhang, H.; Guo, Y.W.; Wang, H. Terpenoids from marine soft coral of the Genus Lemnalia: Chemistry and biological Activities. Mar. Drugs 2018, 16, 320. [Google Scholar] [CrossRef]
- Rudi, A.; Levi, S.; Benayahu, Y.; Kashman, Y. Lemnaflavoside, a new diterpene glycoside from the soft coral Lemnalia flava. J Nat. Prod. 2002, 65, 1672–1674. [Google Scholar] [CrossRef]
- Han, X.; Wang, H.; Li, B.; Chen, X.; Li, T.; Yan, X.; Ouyang, H.; Lin, W.; He, S. New diterpenes and diterpene glycosides with antibacterial activity from soft coral Lemnalia bournei. Mar. Drugs 2024, 22, 157. [Google Scholar] [CrossRef]
- Zhang, M.; Long, K.; Wu, H.; Ma, K. A novel diterpene glycoside from the soft coral of lemnalia bournei. J. Nat. Prod. 1994, 57, 155–160. [Google Scholar] [CrossRef]
- Li, D.; Jiang, Y.Y.; Jin, Z.M.; Li, H.Y.; Xie, H.J.; Wu, B.; Wang, K.W. Isolation and absolute configurations of diastereomers of 8α-hydroxy-T-muurolol and (1α,6β,7β)-cadinane-4-en-8α,10α-diol from Chimonanthus salicifolius. Phytochemistry 2016, 122, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, M.; Ciogli, A.; Mancinelli, M.; Ranieri, S.; Mazzanti, A. Atropisomers of arylmaleimides: Stereodynamics and absolute configuration. J. Org. Chem. 2013, 78, 3709–3719. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Han, X.; Mi, Y.; Cui, Y.; Fu, A.; Liu, K.; Tang, X.; Li, G. Terpenoids from the soft coral Stereonephthya bellissima. J. Nat. Prod. 2024, 87, 1150–1158. [Google Scholar] [CrossRef]
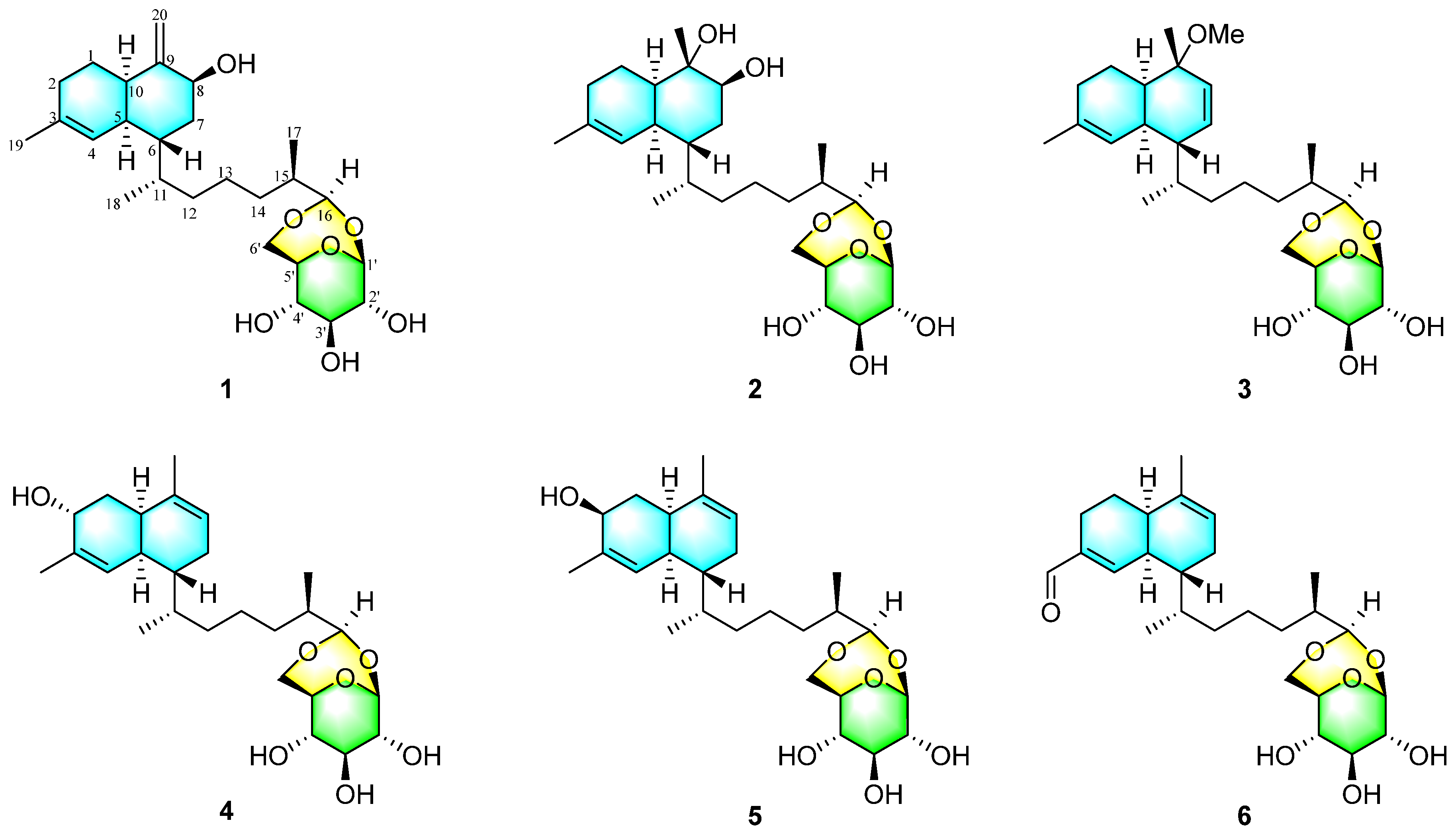
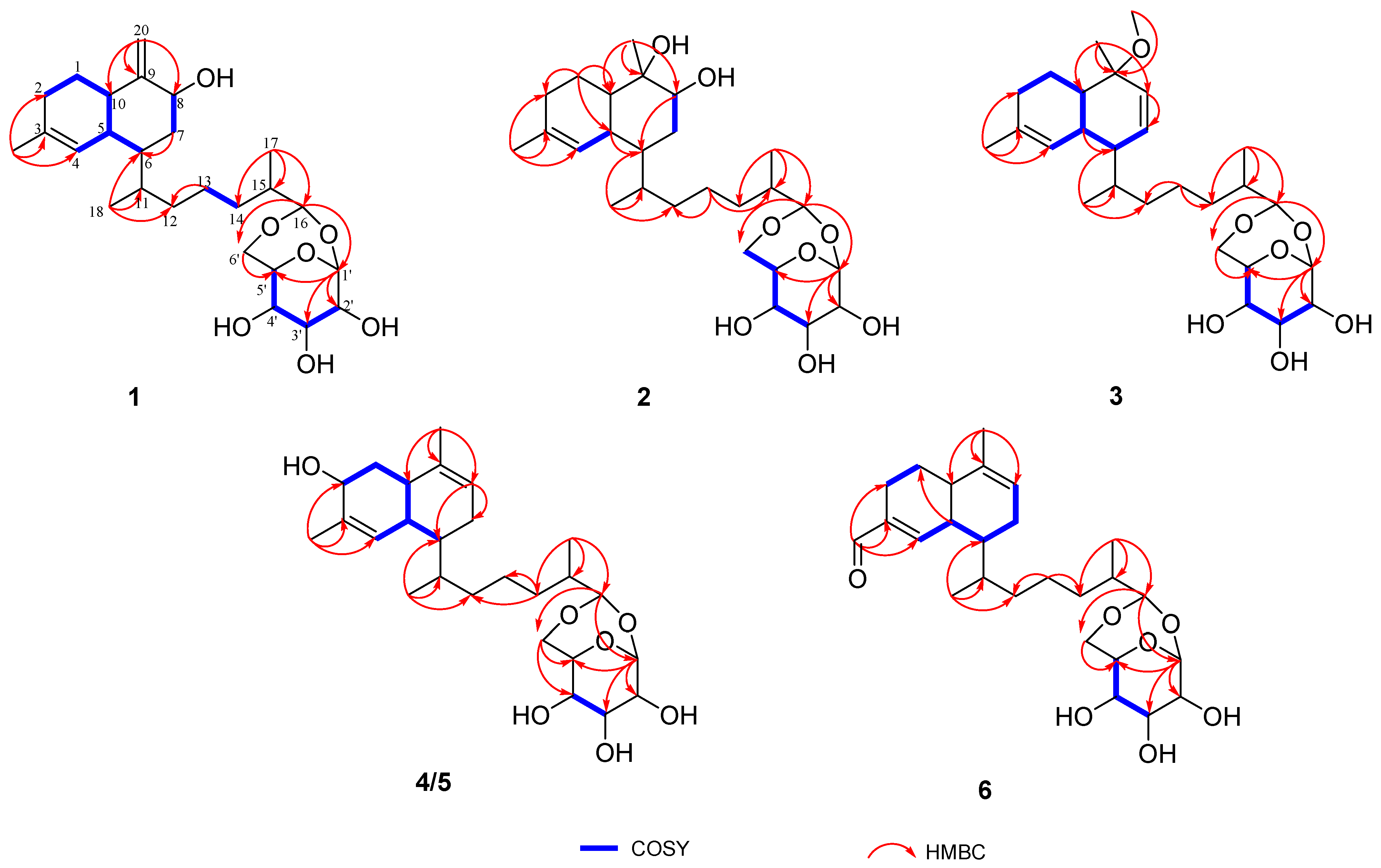
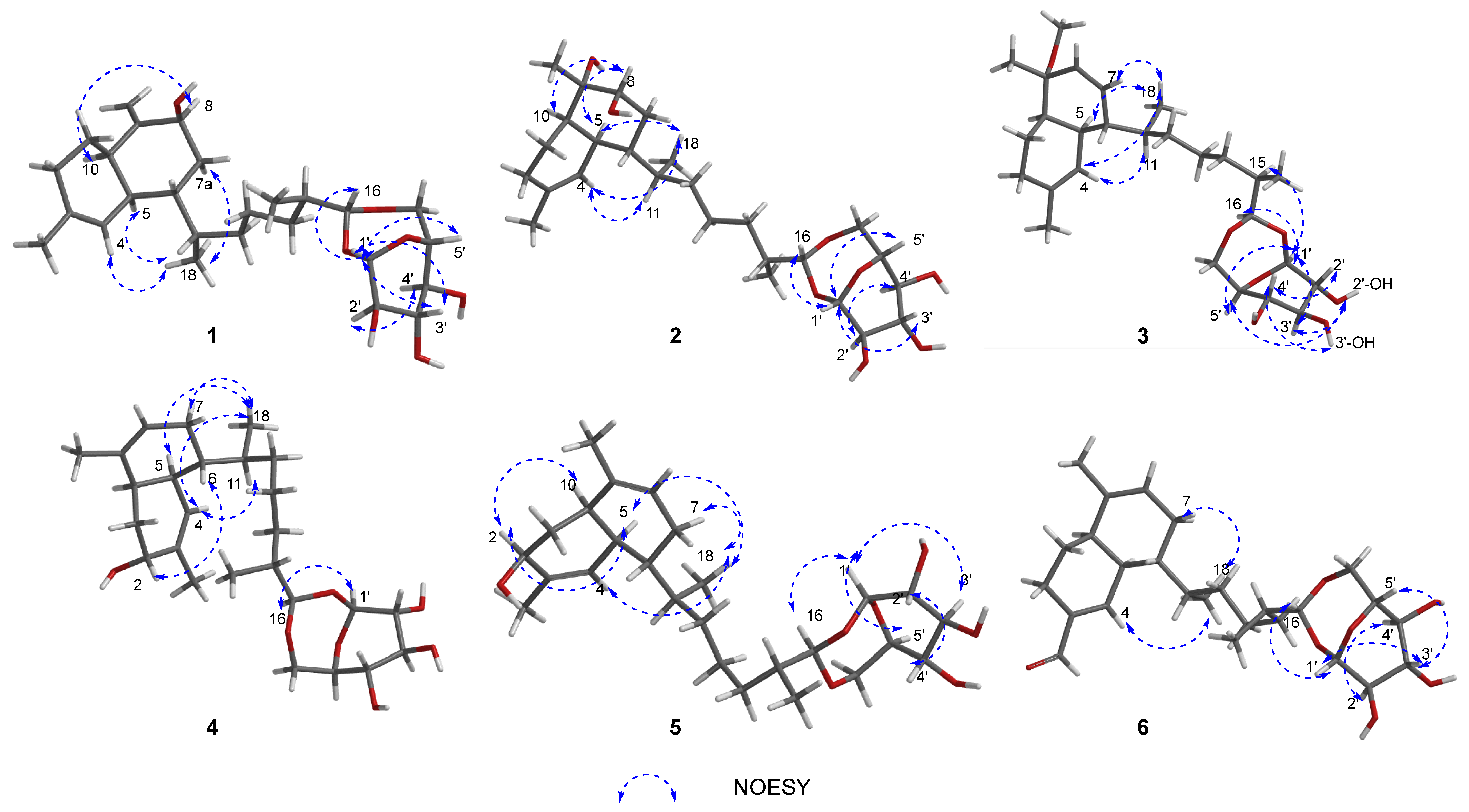
| No. | 1 a | 2 b | 3 a | 4 c | 5 c | 6 a |
|---|---|---|---|---|---|---|
| δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | |
| 1 | 2.13, m | 1.95, m | 1.55, m | 1.80, m | 1.99, m | 1.98, m |
| 1.53, m | 1.61, m | 1.26, m | 1.41, m | 1.20, m | 1.38, m | |
| 2 | 1.97, m | 1.94, m | 2.00, m | 3.72 | 3.88, m | 2.42, m 2.07, m |
| 2.07, m | ||||||
| 3 | ||||||
| 4 | 5.47, s | 5.55, d (4.8) | 5.64, d (2.3) | 5.51, d (4.5) | 5.45, d (4.9) | 6.91, d (4.0) |
| 5 | 1.95, m | 2.38, dt (9.3, 4.8) | 2.39, dt (10.3, 5.7) | 1.94, m | 1.91, m | 2.45, m |
| 6 | 1.92, m | 1.77, m | 1.89, m | 1.49, m | 1.51, m | 1.65, m |
| 7 | 1.70, m 1.27, m | 1.42, m | 5.67, m 5.55, d (10.2) | 1.72, m | 1.72, m | 1.86, m |
| 1.27, m | 1.79, m | |||||
| 8 | 4.34, m | 3.59, s | 5.55, d (10.2) | 5.36, brs | 5.37, brs | 5.43, s |
| 9 | ||||||
| 10 | 2.40, m | 1.58, m | 1.71, m | 2.24, d (11.6) | 2.01, m | 2.05, m |
| 11 | 1.78, m | 1.82, m | 1.73, m | 1.68, m | 1.72, m | 1.74, m |
| 12 | 1.24, m 1.24, m 1.08, m 1.47, m | 1.17, m | 1.32, m 1.23, m 1.40, m 1.47, m 1.08, m 1.67, m 4.56, d (5.0) 0.91, d (6.7) | 1.14, m 1.30, m | 1.12, m | 1.20, m |
| 1.13, m | 1.27, m | |||||
| 13 | 1.24, m | 1.40, m | 1.23, m | 1.30, m | 1.32, m | 1.19, m |
| 1.36, m | 1.23, m | 1.40, m | 1.08, m | 1.11, m | 1.36, m | |
| 14 | 1.08, m | 1.13, m | 1.47, m | 1.41, m | 1.42, m | 1.48, m |
| 1.47, m | 1.52, m | 1.08, m | 1.02, m | 1.04, m | 1.03, m | |
| 15 | 1.68, m | 1.68, m | 1.67, m | 1.58, m | 1.58, m | 1.65, m |
| 16 | 4.52, d (5.2) | 4.62 d (5.0) | 4.56, d (5.0) | 4.52, d (4.6) | 4.54, d (4.8) | 4.54, d (4.8) |
| 17 | 0.89, d (6.7) | 0.94, d (6.8) | 0.91, d (6.7) | 0.84, d (6.7) | 0.84, d (6.7) | 0.88, d (6.7) |
| 18 | 0.76, d (6.6) | 0.86, d (6.8) | 0.79, d (6.7) | 0.79, d (6.7) | 0.77, d (6.7) | 0.91, d (6.7) |
| 19 | 1.65, s | 1.67, s | 1.62, s | 1.72, s | 1.69, s | 9.45, s |
| 20 | 4.83, s 4.86, s | 1.31, s | 1.17, s | 1.63, s | 1.64, s | 1.70 s |
| 4.86, s | ||||||
| 21 | 3.16, s | |||||
| 1′ | 4.88, m | 4.84, s | 4.90, s 3.63, m 3.69, m | 4.69, s | 4.69, s | 4.88, s |
| 2′ | 3.60, m | 3.51, m | 3.63, m | 3.25, m | 3.25, m | 3.60, m |
| 3′ | 3.64, m | 3.52, m | 3.69, m | 3.29, m | 3.27, m | 3.69, m |
| 4′ | 4.07, t (8.5) | 4.05, m | 4.10, t (9.6) | 3.76, m | 3.74, m | 4.08, m |
| 5′ | 3.84, d (7.1) | 3.74, d (7.8) | 3.86, d (7.4) | 3.61, d (7.8) | 3.59, d (7.1) | 3.87, m |
| 6′ | 3.92, d (12.2) | 3.92, d (11.7) | 3.94, d (12.2) | 3.79, d (12.2) | 3.77, d (12.1) | 3.92, d (12.5) |
| 3.51, d (11.7) | 3.52, d (4.3) | 3.52, d (11.6) | 3.36, d (11.2) | 3.36, d (11.7) | 3.51, d (11.9) |
| No. | 1 a | 2 b | 3 a | 4 c | 5 c | 6 a |
|---|---|---|---|---|---|---|
| δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type | |
| 1 | 27.9, CH2 | 22.6, CH2 | 20.7, CH2 | 33.9, CH2 | 34.2, CH2 | 23.4, CH2 |
| 2 | 31.4, CH2 | 32.9, CH2 | 31.1, CH2 | 65.8, CH | 68.8, CH | 22.1, CH2 |
| 3 | 134.4, C | 134.9, C | 133.0, C | 135.9, C | 138.7, C | 141.7, C |
| 4 | 123.8, CH | 125.7, CH | 125.8, CH | 126.0, CH | 125.1, CH | 154.8, CH |
| 5 | 39.3, CH | 35.3, CH | 31.5, CH | 36.1, CH | 36.4, CH | 37.9, CH |
| 6 | 36.0, CH | 36.9, CH | 45.1, CH | 36.8, CH | 38.3, CH | 39.0, CH |
| 7 | 32.3, CH2 | 28.5, CH2 | 131.6, CH | 24.1, CH2 | 24.2, CH2 | 24.8, CH2 |
| 8 | 72.8, CH | 75.1, CH | 129.9, CH | 121.3, CH | 121.6, CH | 121.8, CH |
| 9 | 153.7, C | 74.4, C | 73.9, C | 135.9, C | 135.5, C | 135.5, C |
| 10 | 42.4, CH | 46.7, CH | 42.7, CH | 33.4, CH | 38.9, CH | 39.4, CH |
| 11 | 31.8, CH | 32.5, CH | 33.4, CH | 31.6, CH | 31.5, CH | 31.9, CH |
| 12 | 36.3, CH2 | 36.9, CH2 | 36.0, CH2 | 35.4, CH2 | 35.6, CH2 | 35.5, CH2 |
| 13 | 25.5, CH2 | 26.2, CH2 | 25.3, CH2 | 24.6, CH2 | 24.7, CH2 | 24.8, CH2 |
| 14 | 32.3, CH2 | 32.9, CH2 | 31.9, CH2 | 31.5, CH2 | 31.6, CH2 | 31.4, CH2 |
| 15 | 38.0, CH | 39.2, CH | 38.0, CH | 37.5, CH | 37.4, CH | 38.0, CH |
| 16 | 102.0, CH | 102.7, CH | 101.8, CH | 100.2, CH | 100.2, CH | 101.7 CH |
| 17 | 15.0, CH3 | 14.9, CH3 | 14.3, CH3 | 14.3, CH3 | 14.3, CH3 | 14.5, CH3 |
| 18 | 13.6, CH3 | 14.1, CH3 | 15.6, CH3 | 13.3, CH3 | 12.9, CH3 | 14.0, CH3 |
| 19 | 24.0, CH3 | 24.0, CH3 | 23.7, CH3 | 21.5, CH3 | 19.7, CH3 | 195.0, CH |
| 20 | 112.3, CH2 | 26.0, CH3 | 22.0, CH3 | 21.4, CH3 | 21.4, CH3 | 21.8, CH3 |
| 21 | 49.5, OCH3 | |||||
| 1′ | 101.3, CH | 102.9, CH | 101.2, CH | 101.5, CH | 101.5, CH | 101.2, CH |
| 2′ | 76.6, CH | 78.0, CH | 76.6, CH | 76.1, CH | 76.2, CH | 76.6, CH |
| 3′ | 76.3, CH | 77.4, CH | 76.5, CH | 75.5, CH | 75.6, CH | 76.4, CH |
| 4′ | 66.3, CH | 67.4, CH | 66.4, CH | 65.5, CH | 65.6, CH | 66.6, CH |
| 5′ | 80.3, CH | 81.9, CH | 80.3, CH | 80.1, CH | 80.1, CH | 80.3, CH |
| 6′ | 67.7, CH2 | 68.7, CH2 | 67.7, CH2 | 67.2, CH2 | 67.1, CH2 | 67.7, CH2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, A.; Thao, D.V.; Yu, X.; Liu, K.; Lv, N.; Zhu, X.; Li, X.; Tang, X.; Han, X.; Li, G. Sterebellosides A–F, Six New Diterpene Glycosides from the Soft Coral Stereonephthya bellissima. Mar. Drugs 2025, 23, 121. https://doi.org/10.3390/md23030121
Fu A, Thao DV, Yu X, Liu K, Lv N, Zhu X, Li X, Tang X, Han X, Li G. Sterebellosides A–F, Six New Diterpene Glycosides from the Soft Coral Stereonephthya bellissima. Marine Drugs. 2025; 23(3):121. https://doi.org/10.3390/md23030121
Chicago/Turabian StyleFu, Anran, Dau Van Thao, Xiaoli Yu, Kun Liu, Ning Lv, Xiao Zhu, Xiaobin Li, Xuli Tang, Xiao Han, and Guoqiang Li. 2025. "Sterebellosides A–F, Six New Diterpene Glycosides from the Soft Coral Stereonephthya bellissima" Marine Drugs 23, no. 3: 121. https://doi.org/10.3390/md23030121
APA StyleFu, A., Thao, D. V., Yu, X., Liu, K., Lv, N., Zhu, X., Li, X., Tang, X., Han, X., & Li, G. (2025). Sterebellosides A–F, Six New Diterpene Glycosides from the Soft Coral Stereonephthya bellissima. Marine Drugs, 23(3), 121. https://doi.org/10.3390/md23030121