Fingerprint Analysis and Comparison of Activity Differences of Crude Venom from Five Species of Vermivorous Cone Snail in the South China Sea
Abstract
1. Introduction
2. Results
2.1. Protein Components in the Crude Venoms
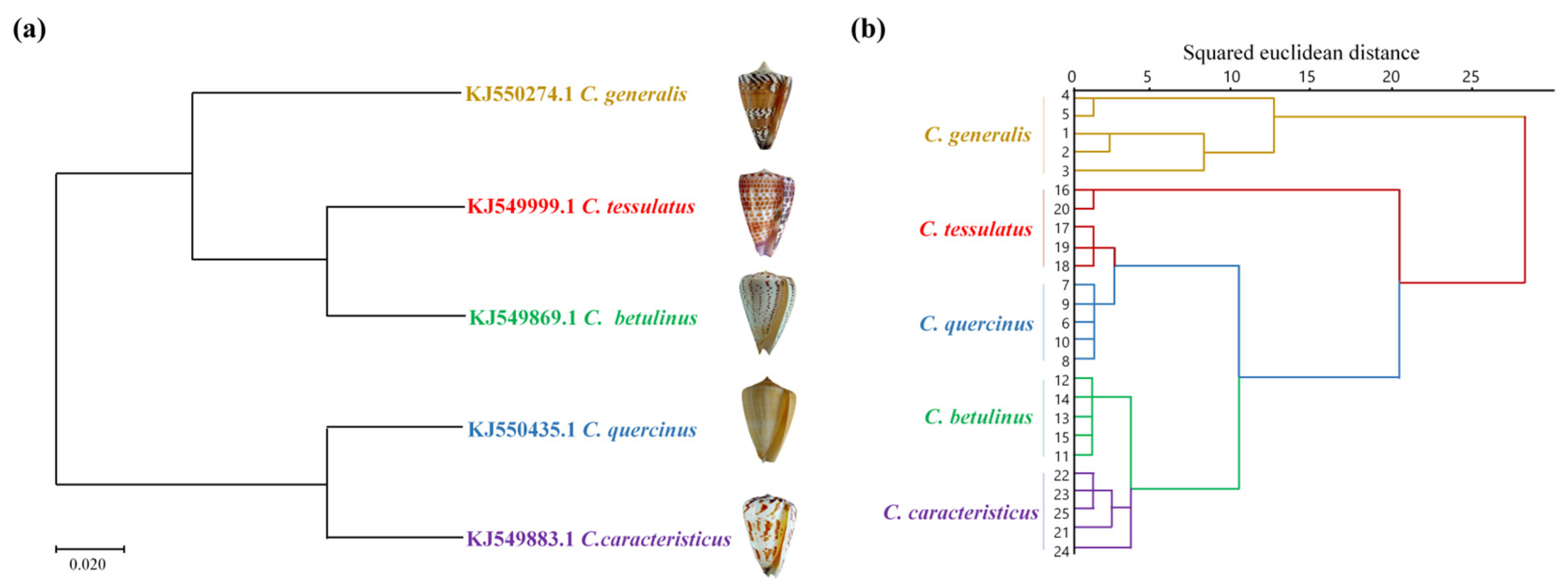
2.2. Similarity Evaluation and Phylogenetic Tree Analysis
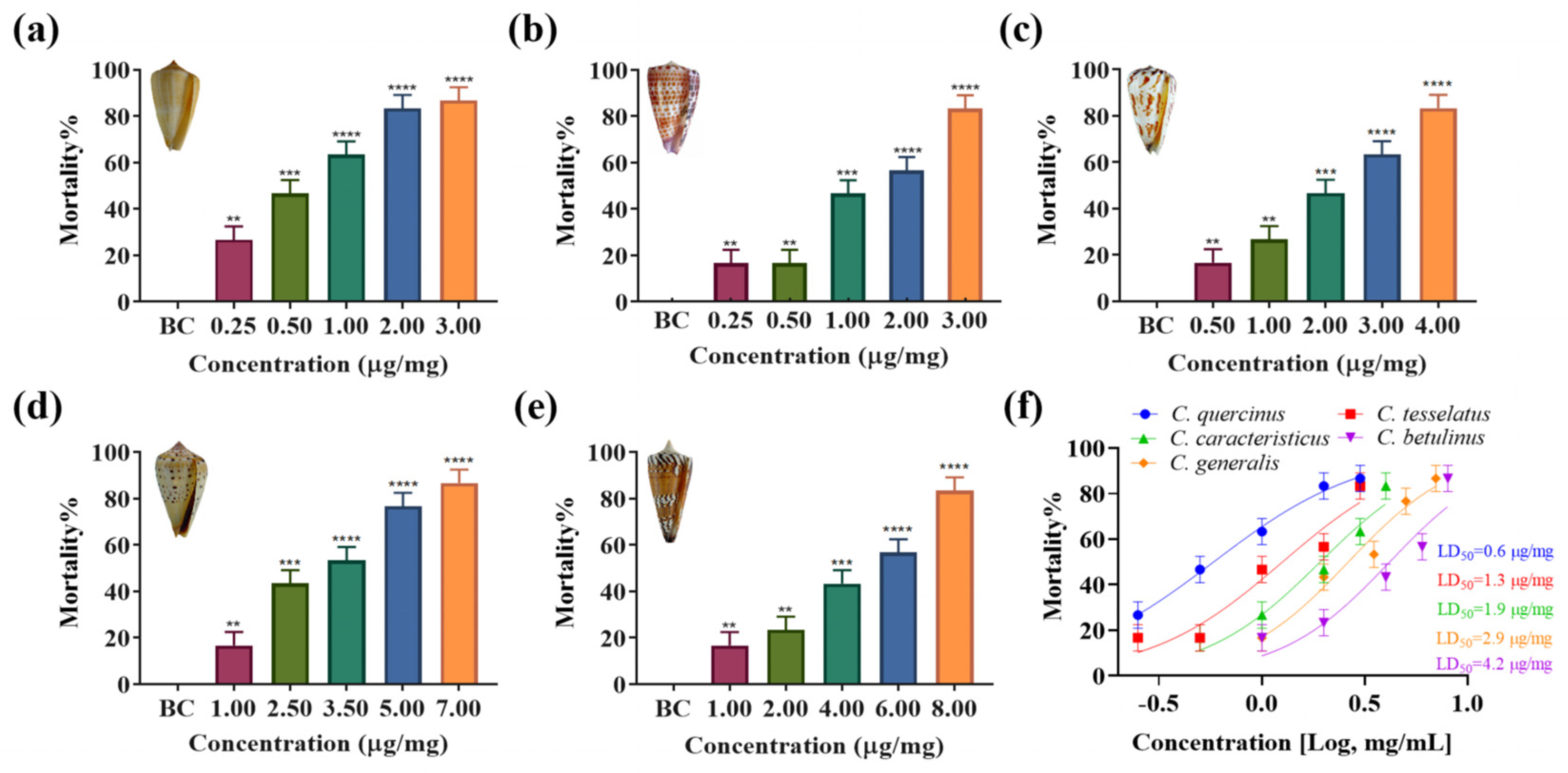
2.3. Insect Toxicity
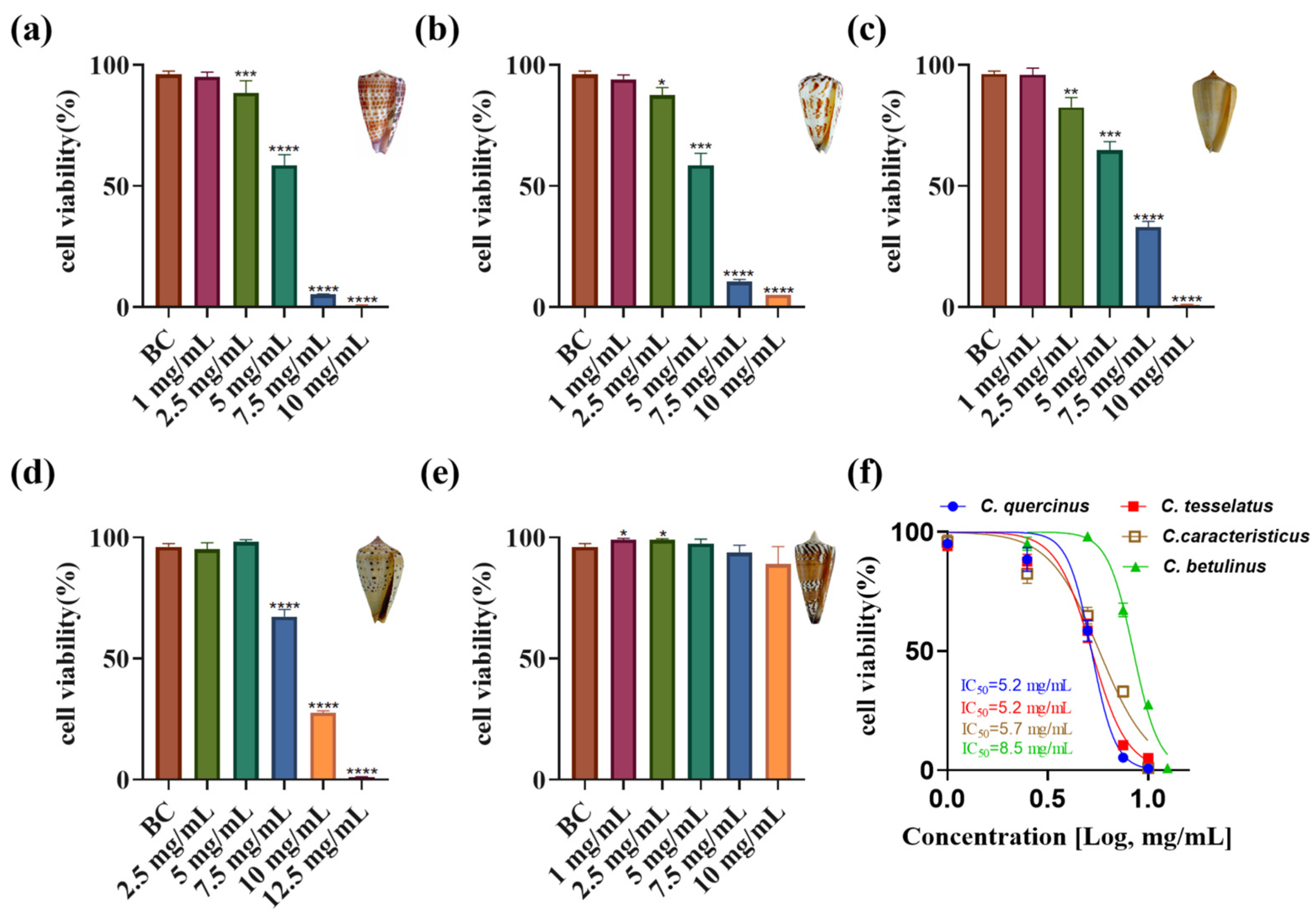
2.4. Cytotoxic Activity
2.5. Toxicity Assessment on Adult Zebrafish
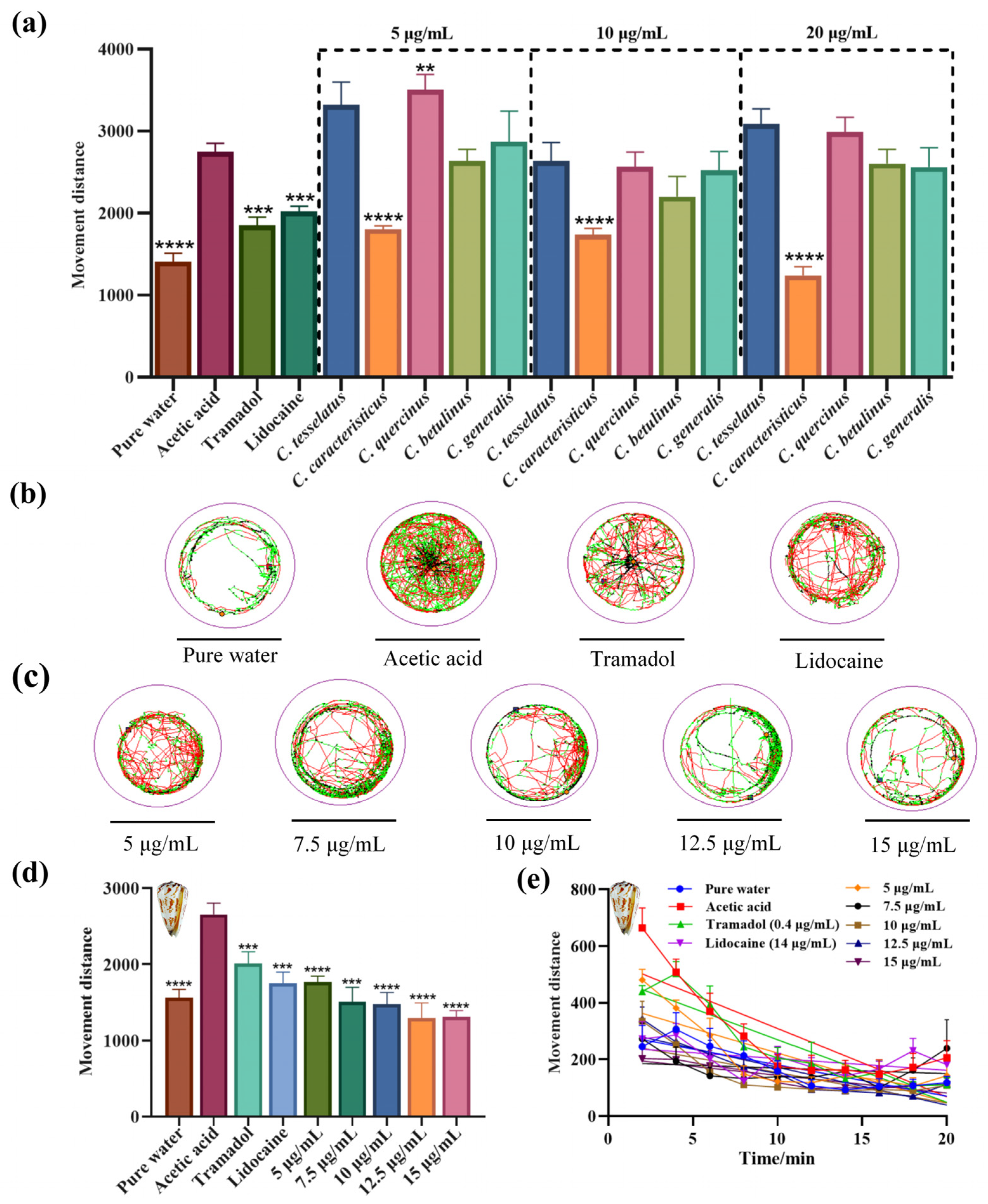
2.6. Assessment of Nociceptive-like Swimming Behavior in Larval Zebrafish
3. Discussion
4. Materials and Methods
4.1. Venom Extraction
4.2. Bicinchoninic Acid (BCA) Protein Assay
4.3. SDS-PAGE
4.4. Chromatographic Conditions
4.5. Establishment of Fingerprint and Similarity Analysis
4.6. Phylogenetic Tree Analysis
4.7. Insect Toxicity Test
4.8. Cytotoxicity Assays
4.9. Adult Zebrafish Toxicity Assay
4.10. Locomotor Behavior of Zebrafish Larval
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutthavilli, P.R.; Bharne, A.M.; Marimuthu, K.; Thiruchitrambalam, G. Unveiling the enigmatic cone snails along the coastal environments of the South Andaman Islands: Diversity, distribution and their habitat preference. Front. Mar. Sci. 2024, 11, 1477472. [Google Scholar] [CrossRef]
- Kumar, P.S.; Kumar, D.S.; Umamaheswari, S. A perspective on toxicology of Conus venom peptides. Asian Pac. J. Trop. Med. 2015, 8, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Laxmilatha, P.; Ameri, S.; Labeeb, K.A.; Ranjith, L.; Kathirvelpandian, A. A new species of cone snail, Conus laccadivensis sp. nov.: (Gastropoda: Conidae) from the Islands of Lakshadweep Archipelago. Reg. Stud. Mar. Sci. 2021, 44, 101783. [Google Scholar] [CrossRef]
- Fiorotti, H.B.; Figueiredo, S.G.; Campos, F.V.; Pimenta, D.C. Cone snail species off the Brazilian coast and their venoms: A review and update. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20220052. [Google Scholar] [CrossRef]
- Neves, J.L.B.; Imperial, J.S.; Morgenstern, D.; Ueberheide, B.; Gajewiak, J.; Antunes, A.; Robinson, S.D.; Espino, S.; Watkins, M.; Vasconcelos, V.; et al. Characterization of the First Conotoxin from Conus ateralbus, a Vermivorous Cone Snail from the Cabo Verde Archipelago. Mar. Drugs 2019, 17, 432. [Google Scholar] [CrossRef]
- Jin, A.-H.; Dutertre, S.; Dutt, M.; Lavergne, V.; Jones, A.; Lewis, R.J.; Alewood, P.F. Transcriptomic-Proteomic Correlation in the Predation-Evoked Venom of the Cone Snail, Conus imperialis. Mar. Drugs 2019, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Peng, C.; Lin, B.; Chen, Q.; Zhang, J.; Shi, Q. Screening and Validation of Highly-Efficient Insecticidal Conotoxins from a Transcriptome-Based Dataset of Chinese Tubular Cone Snail. Toxins 2017, 9, 214. [Google Scholar] [CrossRef]
- Lin, C.; Qin, H.; Liao, Y.; Chen, J.; Gao, B. Chemical Synthesis and Insecticidal Activity Research Based on α-Conotoxins. Molecules 2024, 29, 2846. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Lin, C.; Gao, B. Synthesis and insecticidal activity of cysteine-free conopeptides from Conus betulinus. Toxicon 2023, 233, 107253. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Fu, J.; Gao, B.; Tang, T. The complete mitochondrial DNA genome of a cone snail, Conus betulinus (Neogastropoda: Conidae), from the South China sea. Mitochondrial DNA Part B 2021, 6, 1696–1698. [Google Scholar] [CrossRef]
- Peng, C.; Yao, G.; Gao, B.-M.; Fan, C.-X.; Bian, C.; Wang, J.; Cao, Y.; Wen, B.; Zhu, Y.; Ruan, Z.; et al. High-throughput identification of novel conotoxins from the Chinese tubular cone snail (Conus betulinus) by multi-transcriptome sequencing. GigaScience 2016, 5, 17. [Google Scholar] [CrossRef]
- Espiritu, M.J.J.; Taylor, J.K.K.; Sugai, C.K.K.; Thapa, P.; Loening, N.M.M.; Gusman, E.; Baoanan, Z.G.G.; Baumann, M.H.H.; Bingham, J.-P. Characterization of the Native Disulfide Isomers of the Novel χ-Conotoxin PnID: Implications for Further Increasing Conotoxin Diversity. Mar. Drugs 2023, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Grandal, M.; Hoggard, M.; Neely, B.; Davis, W.C.; Mari, F. Proteogenomic Assessment of Intraspecific Venom Variability: Molecular Adaptations in the Venom Arsenal of Conus purpurascens. Mol. Cell. Proteom. 2021, 20, 100100. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zhangsun, D.; Luo, S. Diversity of Conopeptides and Their Precursor Genes of Conus Litteratus. Mar. Drugs 2020, 18, 464. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Karim, S.; Kamal, M.A.; Wilson, C.M.; Mirza, Z. Conotoxins: Structure, Therapeutic Potential and Pharmacological Applications. Curr. Pharm. Des. 2016, 22, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Ratibou, Z.; Inguimbert, N.; Dutertre, S. Predatory and Defensive Strategies in Cone Snails. Toxins 2024, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Espiritu, M.J.; Cabalteja, C.C.; Bingham, J.-P. Conotoxins and their regulatory considerations. Regul. Toxicol. Pharmacol. 2014, 70, 197–202. [Google Scholar] [CrossRef]
- Giribaldi, J.; Haufe, Y.; Evans, E.R.J.; Wilson, D.T.; Daly, N.L.; Enjalbal, C.; Nicke, A.; Dutertre, S. Synthesis, Structural and Pharmacological Characterizations of CIC, a Novel α-Conotoxin with an Extended N-Terminal Tail. Mar. Drugs 2021, 19, 141. [Google Scholar] [CrossRef]
- Margiotta, F.; Micheli, L.; Ciampi, C.; Ghelardini, C.; McIntosh, J.M.; Di Cesare Mannelli, L. Conus regius-Derived Conotoxins: Novel Therapeutic Opportunities from a Marine Organism. Mar. Drugs 2022, 20, 773. [Google Scholar] [CrossRef]
- Green, B.R.; Bulaj, G.; Norton, R.S. Structure and function of μ-conotoxins, peptide-based sodium channel blockers with analgesic activity. Future Med. Chem. 2014, 6, 1677–1698. [Google Scholar] [CrossRef]
- Jimenez, E.C. Pharmacological Classes of Conus Peptides Targeted to Calcium, Sodium, and Potassium Channels. Protein Pept. Lett. 2023, 30, 913–929. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.L.; Tran, H.N.T.; Deuis, J.R.; Lewis, R.J.; Vetter, I.; Schroeder, C.I. Discovery, Pharmacological Characterisation and NMR Structure of the Novel μ-Conotoxin SxIIIC, a Potent and Irreversible NaV Channel Inhibitor. Biomedicines 2020, 8, 391. [Google Scholar] [CrossRef]
- McMahon, K.L.; Vetter, I.; Schroeder, C.I. Voltage-Gated Sodium Channel Inhibition by μ-Conotoxins. Toxins 2024, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Wang, N.; Mei, Z.; Zhangsun, D.; Craik, D.J.; McIntosh, J.M.; Zhu, X.; Luo, S.; Wahlen, G.E. Conotoxins Targeting Voltage-Gated Sodium Ion Channels. Pharmacol. Rev. 2024, 76, 828–845. [Google Scholar] [CrossRef]
- Wilson, D.T.; Bansal, P.S.; Carter, D.A.; Vetter, I.; Nicke, A.; Dutertre, S.; Daly, N.L. Characterisation of a Novel A-Superfamily Conotoxin. Biomedicines 2020, 8, 128. [Google Scholar] [CrossRef]
- Lin, J.; Chen, S.; Butt, U.D.; Yan, M.; Wu, B. A comprehensive review on ziconotide. Heliyon 2024, 10, e31105. [Google Scholar] [CrossRef]
- Lin, Z.; Torres, J.P.; Watkins, M.; Paguigan, N.; Niu, C.; Imperial, J.S.; Tun, J.; Safavi-Hemami, H.; Finol-Urdaneta, R.K.; Neves, J.L.B.; et al. Non-Peptidic Small Molecule Components from Cone Snail Venoms. Front. Pharmacol. 2021, 12, 655981. [Google Scholar] [CrossRef]
- Zaig, S.; Scarpellini, C.d.S.; Montandon, G. Respiratory depression and analgesia by opioid drugs in freely behaving larval zebrafish. eLife 2021, 10, e63407. [Google Scholar] [CrossRef]
- Giribaldi, J.; Wilson, D.; Nicke, A.; El Hamdaoui, Y.; Laconde, G.; Faucherre, A.; Maati, H.M.O.; Daly, N.L.; Enjalbal, C.; Dutertre, S. Synthesis, Structure and Biological Activity of CIA and CIB, Two α-Conotoxins from the Predation-Evoked Venom of Conus catus. Toxins 2018, 10, 222. [Google Scholar] [CrossRef]
- Jin, A.-H.; Vetter, I.; Himaya, S.W.A.; Alewood, P.F.; Lewis, R.J.; Dutertre, S. Transcriptome and proteome of Conus planorbis identify the nicotinic receptors as primary target for the defensive venom. Proteomics 2015, 15, 4030–4040. [Google Scholar] [CrossRef] [PubMed]
- Pushpabai, R.R.; Alphonse, C.R.W.; Mani, R.; Apte, D.A.; Franklin, J.B. Diversity of Conopeptides and Conoenzymes from the Venom Duct of the Marine Cone Snail Conus bayani as Determined from Transcriptomic and Proteomic Analyses. Mar. Drugs 2021, 19, 202. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.H.; Craik, D.J.; Kaas, Q.; Schroeder, C.I. Bioactive Compounds Isolated from Neglected Predatory Marine Gastropods. Mar. Drugs 2018, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Zhang, Y.; Guo, X.; Yan, Q.; Liu, S.; Ma, B.; Zhang, M.; Bao, J.; Luo, S.; Fu, Y. Anti-Ovarian Cancer Conotoxins Identified from Conus Venom. Molecules 2022, 27, 6609. [Google Scholar] [CrossRef]
- Lopez-Millan, J.M.; Coca-Gamito, C. Intrathecal Ziconotide Infusion Therapy for Oncologic Refractory Neuropathic Pain: Case Report with High-Dose Intraventricular Ziconotide After 15 Years of Treatment. Neural Interface 2024, 27, 1251–1253. [Google Scholar] [CrossRef]
- Monroe, L.K.; Truong, D.P.; Miner, J.C.; Adikari, S.H.; Sasiene, Z.J.; Fenimore, P.W.; Alexandrov, B.; Williams, R.F.; Nguyen, H.B. Conotoxin Prediction: New Features to Increase Prediction Accuracy. Toxins 2023, 15, 641. [Google Scholar] [CrossRef] [PubMed]
- Sukul, V.V. Intrathecal Pain Therapy for the Management of Chronic Noncancer Pain. Neurosurg. Clin. 2019, 30, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, G.; Fassio, G.; Smriglio, C.; Mariottini, P.; Albano, P.G.; Modica, M.V.; Zuccon, D.; Puillandre, N.; Oliverio, M. The polymorphic top-shell puzzle: Iterative taxonomy of Calliostoma Swainson, 1840 (Gastropoda: Calliostomatidae), in the Mediterranean Sea. J. Molluscan Stud. 2024, 90, eyae026. [Google Scholar] [CrossRef]
- Lawler, A.J.; Duda, T.F., Jr. Molecular and morphometric data suggest the presence of a neglected species in the marine gastropod family Conidae. Mol. Phylogenetics Evol. 2017, 109, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Bouchet, P.; Duda, T.F., Jr.; Kauferstein, S.; Kohn, A.J.; Olivera, B.M.; Watkins, M.; Meyer, C. Molecular phylogeny and evolution of the cone snails (Gastropoda, Conoidea). Mol. Phylogen. Evol. 2014, 78, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Stoecklin, R.; Favreau, P.; Bianchi, E.; Perret, F.; Rivasseau, A.; Limpalaer, L.; Monnier, E.; Bouchet, P. When everything converges: Integrative taxonomy with shell, DNA and venomic data reveals Conus conco, a new species of cone snails (Gastropoda: Conoidea). Mol. Phylogenetics Evol. 2014, 80, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Phuong, M.A.; Mahardika, G.N. Targeted Sequencing of Venom Genes from Cone Snail Genomes Improves Understanding of Conotoxin Molecular Evolution. Mol. Biol. Evol. 2018, 35, 1210–1224. [Google Scholar] [CrossRef]
- Spiezia, M.C.; Chiarabelli, C.; Polticelli, F. Recombinant expression and insecticidal properties of a Conus ventricosus conotoxin-GST fusion protein. Toxicon 2012, 60, 744–751. [Google Scholar] [CrossRef]
- Nakmode, D.D.; Singh, B.; Abdella, S.; Song, Y.; Garg, S. Long-acting parenteral formulations of hydrophilic drugs, proteins, and peptide therapeutics: Mechanisms, challenges, and therapeutic benefits with a focus on technologies. Drug Deliv. Transl. Res. 2024, 1–25. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Abdel-Nabi, I.M.; El-Naggar, M.S.; Abbas, O.A.; Strong, P.N. Conus vexillum venom induces oxidative stress in Ehrlich’s ascites carcinoma cells: An insight into the mechanism of induction. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 10. [Google Scholar] [CrossRef]
- Afsharirad, T.; Tahmasvand, R.; Amini, M.; Daraei, B.; Salimi, M. Two novel anticancer compounds with minimum cardiotoxic property. BMC Pharmacol. Toxicol. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Magdy, N.A.; Nafie, M.S.; El-Naggar, M.S.; Abu El-Regal, M.A.; Azeiz, A.Z.A.; Abdel-Rahman, M.A.; El-Zawahry, M. Cytotoxicity and apoptosis induction of the marine Conus flavidus venom in HepG2 cancer cell line. J. Biomol. Struct. Dyn. 2023, 41, 7786–7793. [Google Scholar] [CrossRef]
- Alburae, N.A.; Mohammed, A.E. Antiproliferative effect of the Red Sea cone snail, Conus geographus. Trop. J. Pharm. Res. 2020, 19, 577–581. [Google Scholar] [CrossRef]
- Ganesan, D.S.; Mohamed, M.A.R.; Lhanzin, P.; Neelan, K.; Vadakkuvaselvi, L.; Subramani, S.; Chinnasamy, A. Antiproliferative Effect of Crude Venom from Conus virgo on Human Lung Cancer Cell Line and Toxicity Assessment on Adult Zebra Fish (Danio rerio). Indian J. Pharm. Educ. Res. 2020, 54, 85–94. [Google Scholar] [CrossRef]
- Qiang, Y.; Wu, Y.; Zhao, D.; Zhao, B.; Wang, F.; Ren, S.; Wen, Y.; Gu, J.; Zhang, L.; Liu, K.; et al. Discovery and characterization of the novel conotoxin Lv1d from Conus lividus that presents analgesic activity. Toxicon 2021, 194, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Tabaraki, N.; Shahbazzadeh, D.; Moradi, A.M.; Vosughi, G.; Mostafavi, P.G. Analgesic effect of Persian Gulf Conus textile venom. Iran. J. Basic Med. Sci. 2014, 17, 793–797. [Google Scholar] [CrossRef]
- Sousa, S.R.; McArthur, J.R.; Brust, A.; Bhola, R.F.; Rosengren, K.J.; Ragnarsson, L.; Dutertre, S.; Alewood, P.F.; Christie, M.J.; Adams, D.J.; et al. Novel analgesic ω-conotoxins from the vermivorous cone snail Conus moncuri provide new insights into the evolution of conopeptides. Sci. Rep. 2018, 8, 13397. [Google Scholar] [CrossRef]
- Gao, B.; Zhangsun, D.; Hu, Y.; Wu, Y.; Sheng, L.; Fang, L.; Wu, X.; Yu, J.; Luo, S. Expression and secretion of functional recombinant μO-conotoxin MrVIB-His-tag in Escherichia coli. Toxicon 2013, 72, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Jergova, S.; Perez, C.; Imperial, J.S.; Gajavelli, S.; Jain, A.; Abin, A.; Olivera, B.M.; Sagen, J. Cannabinoid receptor agonists from Conus venoms alleviate pain-related behavior in rats. Pharmacol. Biochem. Behav. 2021, 205, 173182. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Costante, R.; Novellino, E.; Stefanucci, A.; Pieretti, S.; Zador, F.; Samavati, R.; Borsodi, A.; Benyhe, S.; Vetter, I.; et al. Design, Synthesis and Biological Evaluation of Two Opioid Agonist and Cav2.2 Blocker Multitarget Ligands. Chem. Biol. Drug Des. 2015, 86, 156–162. [Google Scholar] [CrossRef]
- Anonymous. Abstracts for the 21st Annual Meeting of the American Society for Neural Therapy and Repair. Cell Transplant. 2014, 23, 761–790. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yang, B.; Yan, L.; Dai, Q. Sensitive Detection of α-Conotoxin GI in Human Plasma Using a Solid-Phase Extraction Column and LC-MS/MS. Toxins 2017, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Y.; Jiang, X. Quantitative analysis of protein in thermosensitive hydroxypropyl chitin for biomedical applications. Anal. Biochem. 2020, 599, 113745. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Kong, W.-J.; Zhang, S.-S.; Zhao, Y.-L.; Wu, M.-Q.; Chen, P.; Wu, X.-R.; Ma, X.-P.; Guo, W.-Y.; Yang, M.-H. Combination of chemical fingerprint and bioactivity evaluation to explore the antibacterial components of Salvia miltiorrhizae. Sci. Rep. 2017, 7, 8112. [Google Scholar] [CrossRef]
- Newman, L.; Duffus, A.L.J.; Lee, C. Using the Free Program MEGA to Build Phylogenetic Trees from Molecular Data. Am. Biol. Teach. 2016, 78, 608–612. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef] [PubMed]
- Horzmann, K.A.; Freeman, J.L. Making Waves: New Developments in Toxicology with the Zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef]
- Lantz-McPeak, S.; Guo, X.; Cuevas, E.; Dumas, M.; Newport, G.D.; Ali, S.F.; Paule, M.G.; Kanungo, J. Developmental toxicity assay using high content screening of zebrafish embryos. J. Appl. Toxicol. 2015, 35, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, P.J.; Bardine, N. Antinociceptive effects of buprenorphine in zebrafish larvae: An alternative for rodent models to study pain and nociception? Appl. Anim. Behav. Sci. 2014, 152, 92–99. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Liao, Y.; Fu, J.; Liang, Y.; Chen, Y.; Mao, K.; Gao, B. Fingerprint Analysis and Comparison of Activity Differences of Crude Venom from Five Species of Vermivorous Cone Snail in the South China Sea. Mar. Drugs 2025, 23, 102. https://doi.org/10.3390/md23030102
Sun S, Liao Y, Fu J, Liang Y, Chen Y, Mao K, Gao B. Fingerprint Analysis and Comparison of Activity Differences of Crude Venom from Five Species of Vermivorous Cone Snail in the South China Sea. Marine Drugs. 2025; 23(3):102. https://doi.org/10.3390/md23030102
Chicago/Turabian StyleSun, Shibo, Yanling Liao, Jinxing Fu, Yanxia Liang, Yurong Chen, Kailin Mao, and Bingmiao Gao. 2025. "Fingerprint Analysis and Comparison of Activity Differences of Crude Venom from Five Species of Vermivorous Cone Snail in the South China Sea" Marine Drugs 23, no. 3: 102. https://doi.org/10.3390/md23030102
APA StyleSun, S., Liao, Y., Fu, J., Liang, Y., Chen, Y., Mao, K., & Gao, B. (2025). Fingerprint Analysis and Comparison of Activity Differences of Crude Venom from Five Species of Vermivorous Cone Snail in the South China Sea. Marine Drugs, 23(3), 102. https://doi.org/10.3390/md23030102





