Structural Characterization of Pinnatoxin Isomers
Abstract
1. Introduction
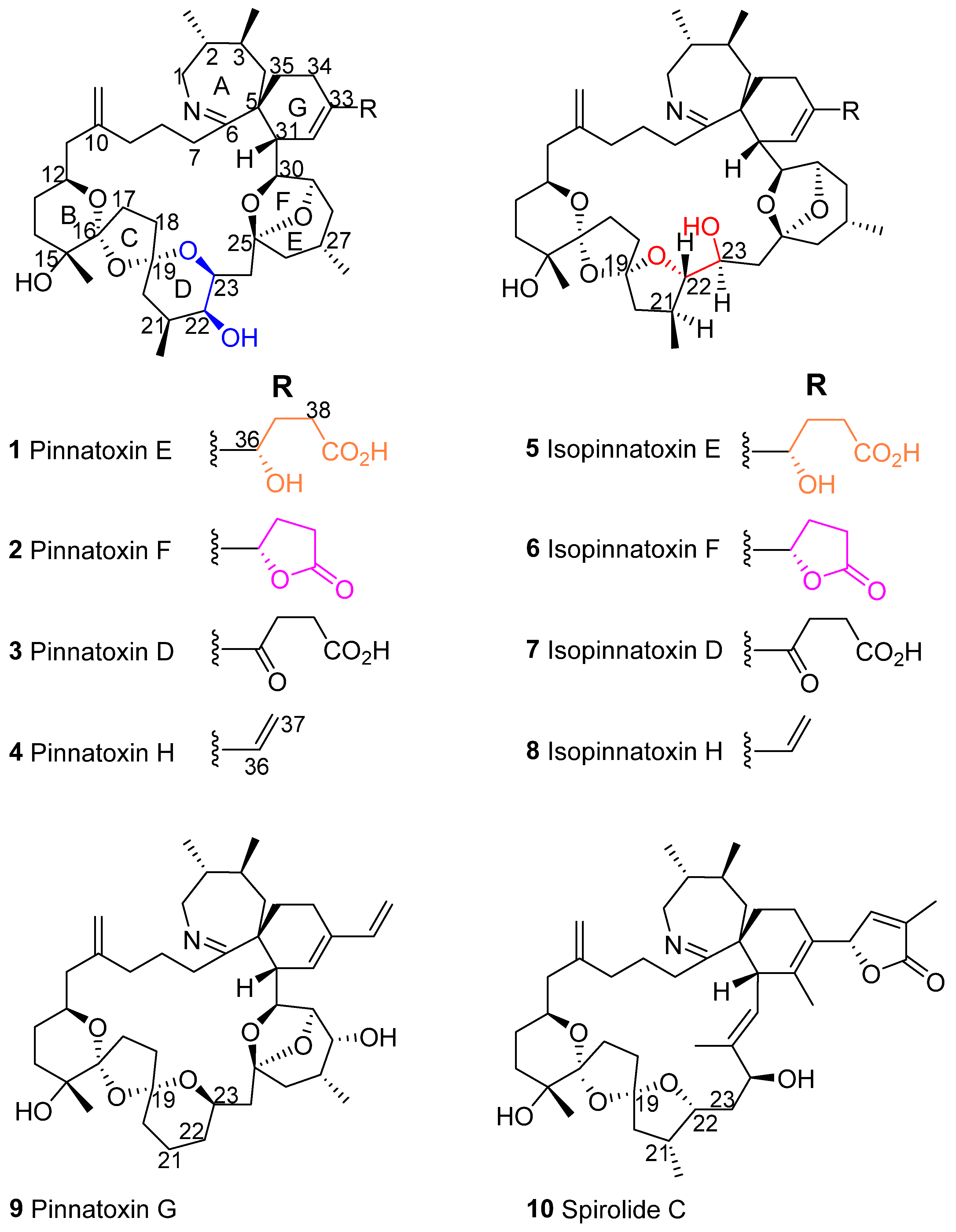
2. Results
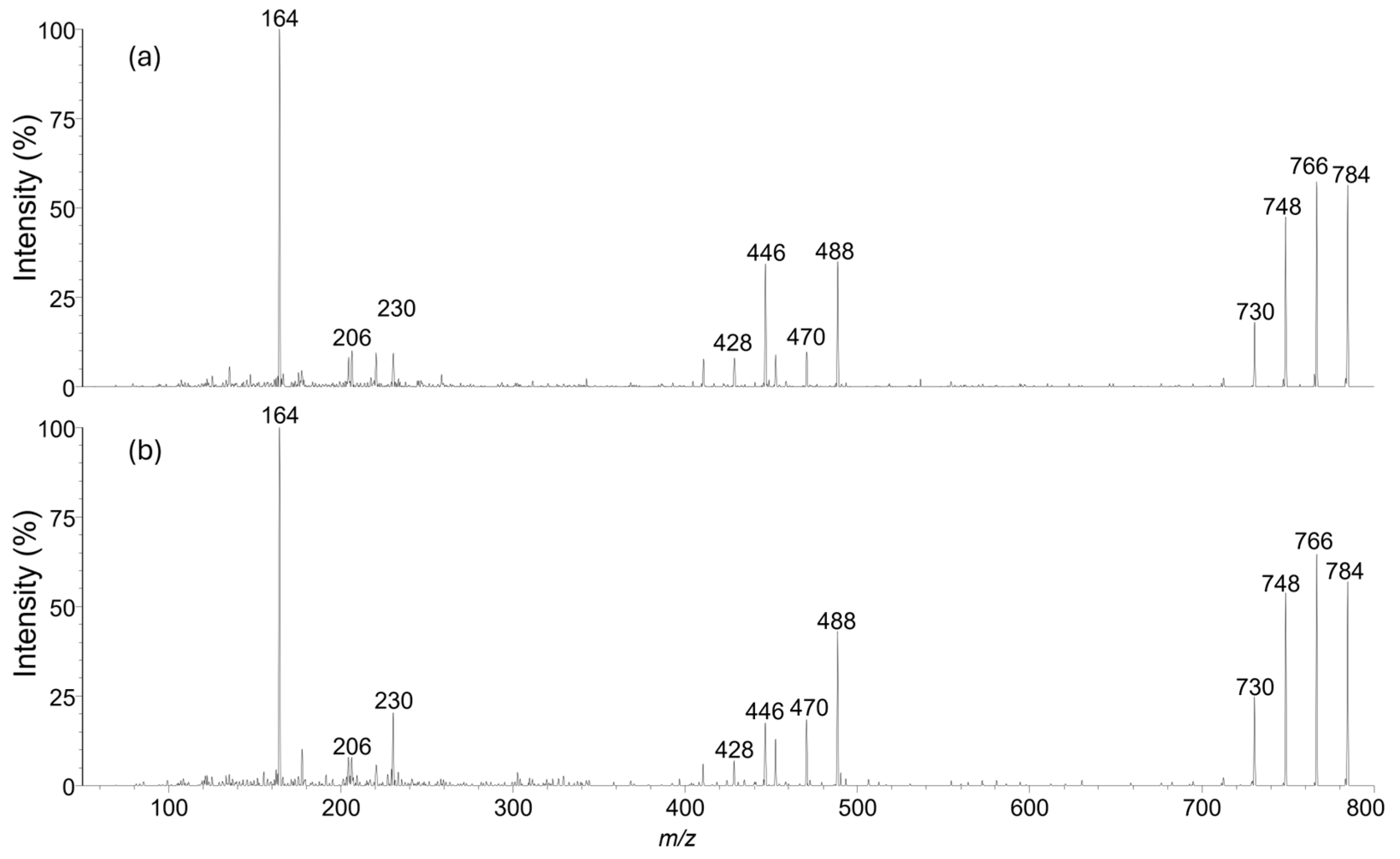
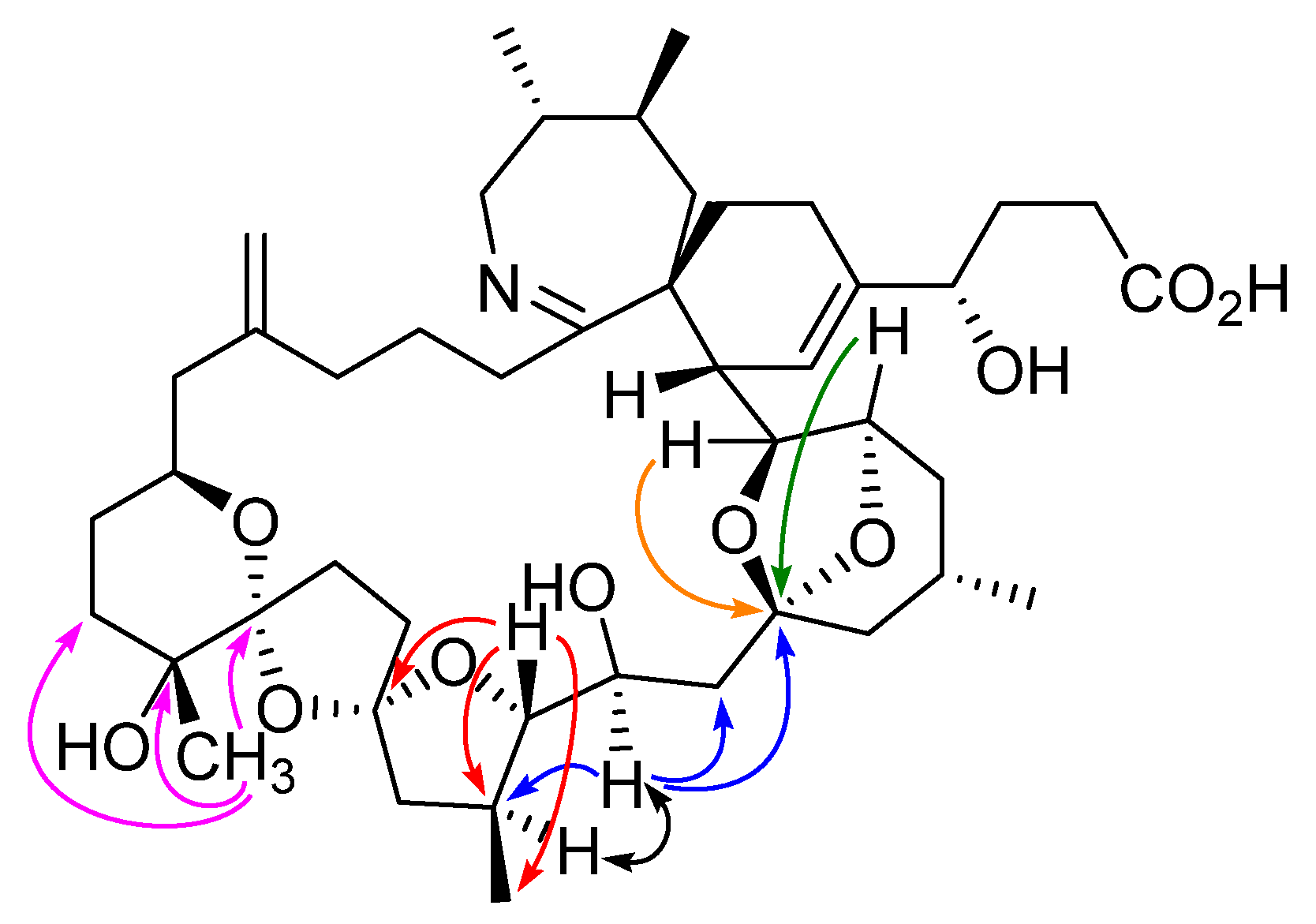
2.1. Structural Charaterization of Isopinnatoxin E
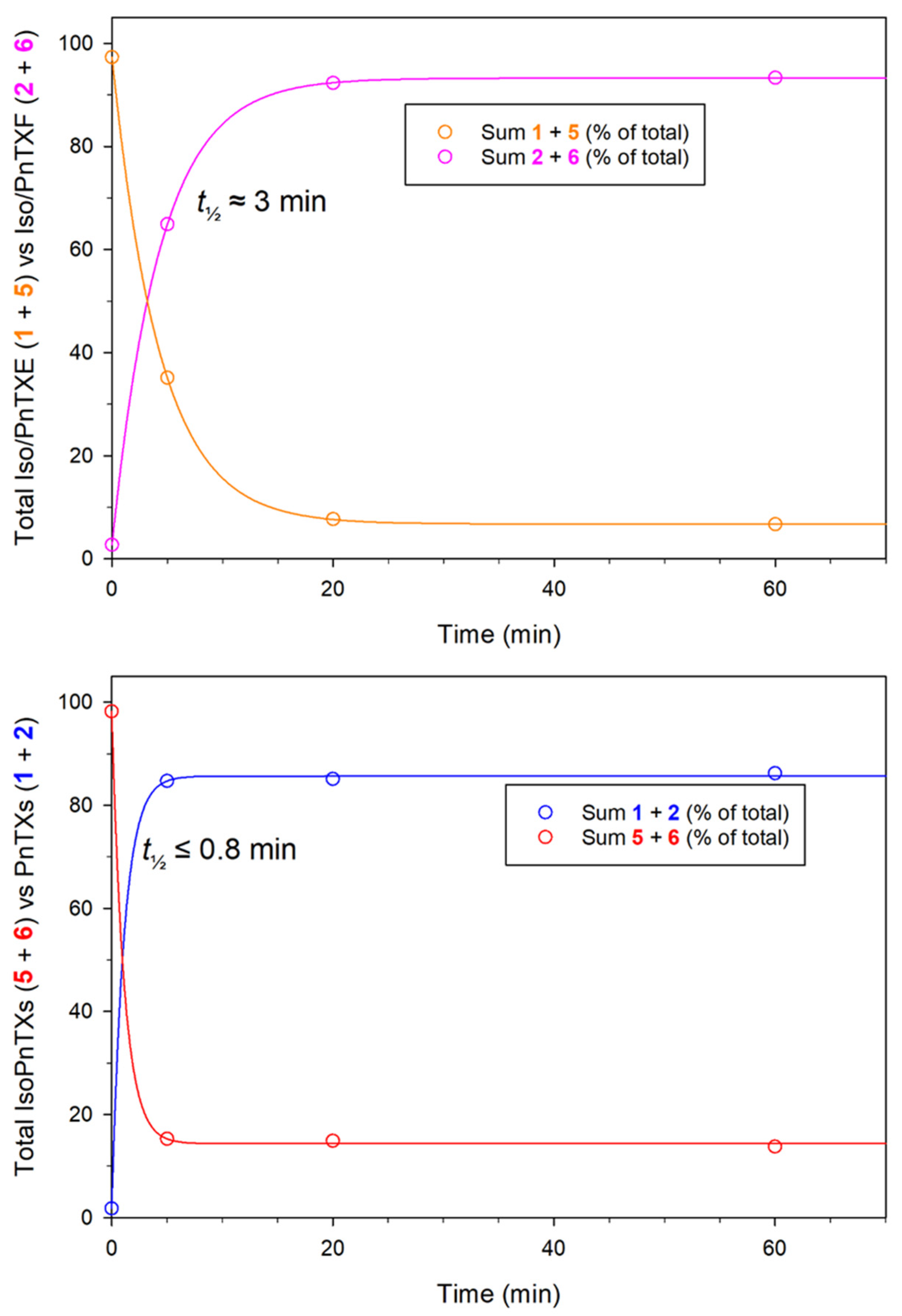
2.2. Isomerization
2.3. Toxicity of Isopinnatoxin E
3. Discussion
4. Materials and Methods
4.1. Purification of Isopinnatoxin E
4.2. LC–MS Analysis
4.3. Pinnatoxin Isomerization
4.4. NMR Spectroscopy
4.5. Toxicity Determination for Isopinnatoxin E
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uemura, D.; Chou, T.; Haino, T.; Nagatsu, A.; Fukuzawa, S.; Zheng, S.Z.; Chen, H.S. Pinnatoxin A: A toxic amphoteric macrocycle from the Okinawan bivalve Pinna muricata. J. Am. Chem. Soc. 1995, 117, 1155–1156. [Google Scholar] [CrossRef]
- Selwood, A.I.; Miles, C.O.; Wilkins, A.L.; Van Ginkel, R.; Munday, R.; Rise, F.; McNabb, P.S. Isolation, structural determination, and acute toxicity of novel pinnatoxins E, F and G. J. Agric. Food Chem. 2010, 58, 6532–6542. [Google Scholar] [CrossRef] [PubMed]
- Takada, N.; Umemura, N.; Suenaga, K.; Chou, T.; Nagatsu, A.; Haino, T.; Yamada, K.; Uemura, D. Pinnatoxins B and C, the most toxic components in the pinnatoxin series from the Okinawan bivalve Pinna muricata. Tetrahedron Lett. 2001, 42, 3491–3494. [Google Scholar] [CrossRef]
- Chou, T.; Kamo, O.; Uemura, D. Relative stereochemistry of pinnatoxin A, a potent shellfish poison from Pinna muricata. Tetrahedron Lett. 1996, 37, 4023–4026. [Google Scholar] [CrossRef]
- Chou, T.; Haino, T.; Kuramoto, M.; Uemura, D. Isolation and structure of pinnatoxin D, a new shellfish poison from the Okinawan bilvalve Pinna muricata. Tetrahedron Lett. 1996, 37, 4027–4030. [Google Scholar] [CrossRef]
- Zeng, N.; Gu, H.; Smith, K.F.; Rhodes, L.; Selwood, A.I.; Yang, W. The first report of Vulcanodinium rugosum (Dinophyceae) from the South China Sea with a focus on the life cycle. N. Z. J. Mar. Freshw. Res. 2012, 46, 511–521. [Google Scholar] [CrossRef]
- Smith, K.F.; Rhodes, L.L.; Suda, S.; Selwood, A.I. A dinoflagellate producer of pinnatoxin G, isolated from sub-tropical Japanese waters. Harmful Algae 2011, 10, 702–705. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; Molenaar, S.; Munday, R.; Wilkinson, C.; Hallegraeff, G. Production of pinnatoxins E, F and G by scrippsielloid dinoflagellates isolated from Franklin Harbour, South Australia. N. Z. J. Mar. Freshw. Res. 2011, 45, 703–709. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; van Ginkel, R.; Holland, P.; Munday, R. Production of pinnatoxins by a peridinoid dinoflagellate isolated from Northland, New Zealand. Harmful Algae 2010, 9, 384–389. [Google Scholar] [CrossRef]
- Al Muftah, A.; Selwood, A.I.; Foss, A.J.; Al-Jabri, H.M.S.J.; Potts, M.; Yilmaz, M. Algal toxins and producers in the marine waters of Qatar, Arabian Gulf. Toxicon 2016, 122, 54–66. [Google Scholar] [CrossRef]
- Garrett, M.J.; Puchulutegui, C.; Selwood, A.I.; Wolny, J.L. Identification of the harmful dinoflagellate Vulcanodinium rugosum recovered from a ballast tank of a globally traveled ship in Port Tampa Bay, Florida, USA. Harmful Algae 2014, 39, 202–209. [Google Scholar] [CrossRef]
- Hess, P.; Abadie, E.; Hervé, F.; Berteaux, T.; Séchet, V.; Aráoz, R.; Molgó, J.; Zakarian, A.; Sibat, M.; Rundberget, T.; et al. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus galloprovincialis) and clams (Venerupis decussata) from Ingril, a French Mediterranean lagoon. Toxicon 2013, 75, 16–26. [Google Scholar] [CrossRef] [PubMed]
- McCarron, P.; Rourke, W.A.; Hardstaff, W.; Pooley, B.; Quilliam, M.A. Identification of pinnatoxins and discovery of their fatty acid ester metabolites in mussels (Mytilus edulis) from Eastern Canada. J. Agric. Food Chem. 2012, 60, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Munday, R.; Selwood, A.I.; Rhodes, L. Acute toxicity of pinnatoxins E, F and G to mice. Toxicon 2012, 60, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Chapela, M.J.; Atanassova, M.; Vieites, J.M.; Cabado, A.G. Cyclic imines: Chemistry and mechanism of action: A review. Chem. Res. Toxicol. 2011, 24, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.C.; Harwood, D.T.; Boundy, M.J.; Selwood, A.I. A Review of cyclic imines in shellfish: Worldwide occurrence, toxicity and assessment of the risk to consumers. Mar. Drugs 2024, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Arnich, N.; Abadie, E.; Delcourt, N.; Fessard, V.; Fremy, J.M.; Hort, V.; Lagrange, E.; Maignien, T.; Molgó, J.; Peyrat, M.B.; et al. Health risk assessment related to pinnatoxins in French shellfish. Toxicon 2020, 180, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.; Baker, C.; Higgins, C.; Higman, W.; Swan, S.; Veszelovszki, A.; Turner, A.D. Potential threats posed by new or emerging marine biotoxins in UK waters and examination of detection methodologies used for their control: Cyclic imines. Mar. Drugs 2015, 13, 7087–7112. [Google Scholar] [CrossRef]
- Hu, T.; Burton, I.W.; Cembella, A.D.; Curtis, J.M.; Quilliam, M.A.; Walter, J.A.; Wright, J.L.C. Characterization of spirolides A, C, and 13-desmethyl C, new marine toxins isolated from toxic plankton and contaminated shellfish. J. Nat. Prod. 2001, 64, 308–312. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure; OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2022. [Google Scholar] [CrossRef]
- Sasaki, K.; Wright, J.L.; Yasumoto, T. Identification and characterization of pectenotoxin (PTX) 4 and PTX7 as spiroketal stereoisomers of two previously reported pectenotoxins. J. Org. Chem. 1998, 63, 2475–2480. [Google Scholar] [CrossRef] [PubMed]

| 5 | 1 a | 10 b | ||||
|---|---|---|---|---|---|---|
| No. | 13C | 1H | 13C | 1H | 13C | 1H |
| 1 | 53.7 | 3.49, 3.87 | 52.6 | 3.56, 4.10 | 53.3 | 3.44, 3.76 |
| 2 | 42.6 | 1.41 | 40.7 | 1.57 | 41.2 | 1.36 |
| 2-Me | 20.3 | 1.14 | 20.3 | 1.18 | 19.4 | 0.98 |
| 3 | 34.3 | 1.63 | 35.5 | 1.30 | 36.9 | 1.16 |
| 3-Me | 20.9 | 0.93 | 21.7 | 1.03 | 21.1 | 0.95 |
| 4 | 37.3 | 1.47, 1.74 | 36.5 | 1.66, 1.93 | 38.3 | 1.55, 1.73 |
| 5 | 51.1 | 51.9 | 50.8 | |||
| 6 | 179.9 | nd | 178.6 | |||
| 7 | 35.7 | 3.27 | 35.1 | 3.53 | 35.6 | 2.32, 2.43 |
| 8 | 23.1 | 1.43, 2.02 | 21.8 | 1.74, 2.15 | 23.4 | 1.40, 2.02 |
| 9 | 35.2 | 1.67, 2.35 | 33.8 | 1.82, 2.17 | 36.0 | 1.58, 2.10 |
| 10 | 148.3 | 146.5 | 147.8 | |||
| 10=CH2 | 110.6 | 4.74, 4.76 | 111.3 | 4.81, 4.86 | 111.3 | 4.75, 4.78 |
| 11 | 47.0 | 2.05, 2.40 | 46.9 | 2.15, 2.37 | 47.6 | 2.01, 2.37 |
| 12 | 69.8 | 4.25 | 69.5 | 4.06 | 69.3 | 3.97 |
| 13 | 29.8 | 1.23, 1.65 | 29.8 | 1.27, 1.64 | 30.2 | 1.24, 1.55 |
| 14 | 35.3 | 1.48, 1.93 | 35.5 | 1.50, 1.90 | 35.8 | 1.49, 1.81 |
| 15 | 71.5 | 71.1 | 71.1 | |||
| 15-Me | 22.8 | 1.22 | 22.9 | 1.22 | 22.5 | 1.19 |
| 16 | 112.4 | 113.7 | 112.5 | |||
| 17 | 32.5 | 1.81, 2.24 | 31.4 | 1.78, 2.20 | 31.5 | 1.74, 2.11 |
| 18 | 36.1 | 1.99, 2.17 | 38.7 | 1.85, 2.15 | 36.5 | 2.04, 2.22 |
| 19 | 116.8 | 110.1 | 117.4 | |||
| 20 | 46.9 | 1.71, 2.40 | 37.7 | 1.66, 1.66 | 45.8 | 2.14, 2.26 |
| 21 | 35.7 | 2.51 | 32.1 | 2.09 | 35.4 | 2.41 |
| 21-Me | 18.1 | 1.09 | 18.3 | 0.97 | 15.6 | 1.19 |
| 22 | 91.4 | 3.32 | 69.4 | 3.44 | 81.7 | 4.31 |
| 23 | 70.8 | 3.83 c | 73.1 | 4.09 | ||
| 24 | 44.0 | 1.82, 1.82 | 40.1 | 1.92, 2.21 | ||
| 25 | 110.1 | 109.6 | ||||
| 26 | 44.2 | 1.31, 2.21 | 45.4 | 1.41, 1.87 | ||
| 27 | 25.8 | 2.35 | 26.0 | 2.29 | ||
| 27-Me | 22.8 | 0.98 | 22.7 | 0.99 | ||
| 28 | 34.1 | 1.52, 2.01 | 33.8 | 1.55, 2.04 | ||
| 29 | 77.7 | 4.57 | 77.4 | 4.62 | ||
| 30 | 81.8 | 3.87 | 81.1 | 3.86 | ||
| 31 | 43.5 | 3.36 | 44.7 | 3.52 | ||
| 32 | 121.7 | 5.14 | 121 | 5.21 | ||
| 33 | 144.2 | 144.3 | ||||
| 34 | 21.4 | 2.16, 2.16 | 21.9 | 2.26, 2.26 | ||
| 35 | 33.7 | 1.68, 1.84 | 34.1 | 1.82, 1.92 | ||
| 36 | 76.6 | 3.98 | 75.7 | 4.02 | ||
| 37 | 32.8 | 1.82, 1.82 | 32.1 | 1.84, 1.84 | ||
| 38 | 35.6 | 2.21, 2.21 | 33.7 | 2.25, 2.25 | ||
| 39 | 182.3 | 180.3 | ||||
| Pinnatoxin Used | Pinnatoxin | Isopinnatoxin | Carboxylic Acid | Lactone |
|---|---|---|---|---|
| Pinnatoxin E (1) | 84.4 | 15.6 | 5.4 | 94.6 |
| Pinnatoxin F (2) | 84.8 | 15.2 | 5.4 | 94.6 |
| Pinnatoxin D (3) | 85.5 | 14.5 | N/A | N/A |
| Pinnatoxin H (4) | 85.9 | 14.1 | N/A | N/A |
| Isopinnatoxin E (5) | 85.7 | 14.3 | 6.8 | 93.2 |
| Average for 1–5 | 85.3 | 14.7 | 5.9 | 94.1 |
| Pinnatoxin G (9) | 100.0 | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selwood, A.I.; Miles, C.O.; Wilkins, A.L.; Rise, F.; Finch, S.C.; van Ginkel, R. Structural Characterization of Pinnatoxin Isomers. Mar. Drugs 2025, 23, 103. https://doi.org/10.3390/md23030103
Selwood AI, Miles CO, Wilkins AL, Rise F, Finch SC, van Ginkel R. Structural Characterization of Pinnatoxin Isomers. Marine Drugs. 2025; 23(3):103. https://doi.org/10.3390/md23030103
Chicago/Turabian StyleSelwood, Andrew I., Christopher O. Miles, Alistair L. Wilkins, Frode Rise, Sarah C. Finch, and Roel van Ginkel. 2025. "Structural Characterization of Pinnatoxin Isomers" Marine Drugs 23, no. 3: 103. https://doi.org/10.3390/md23030103
APA StyleSelwood, A. I., Miles, C. O., Wilkins, A. L., Rise, F., Finch, S. C., & van Ginkel, R. (2025). Structural Characterization of Pinnatoxin Isomers. Marine Drugs, 23(3), 103. https://doi.org/10.3390/md23030103







