Four New Pairs of MetO-Containing Diketopiperazine Enantiomers: Isolation, Synthesis and Potential Anti-Parkinson’s Disease Activity
Abstract
1. Introduction
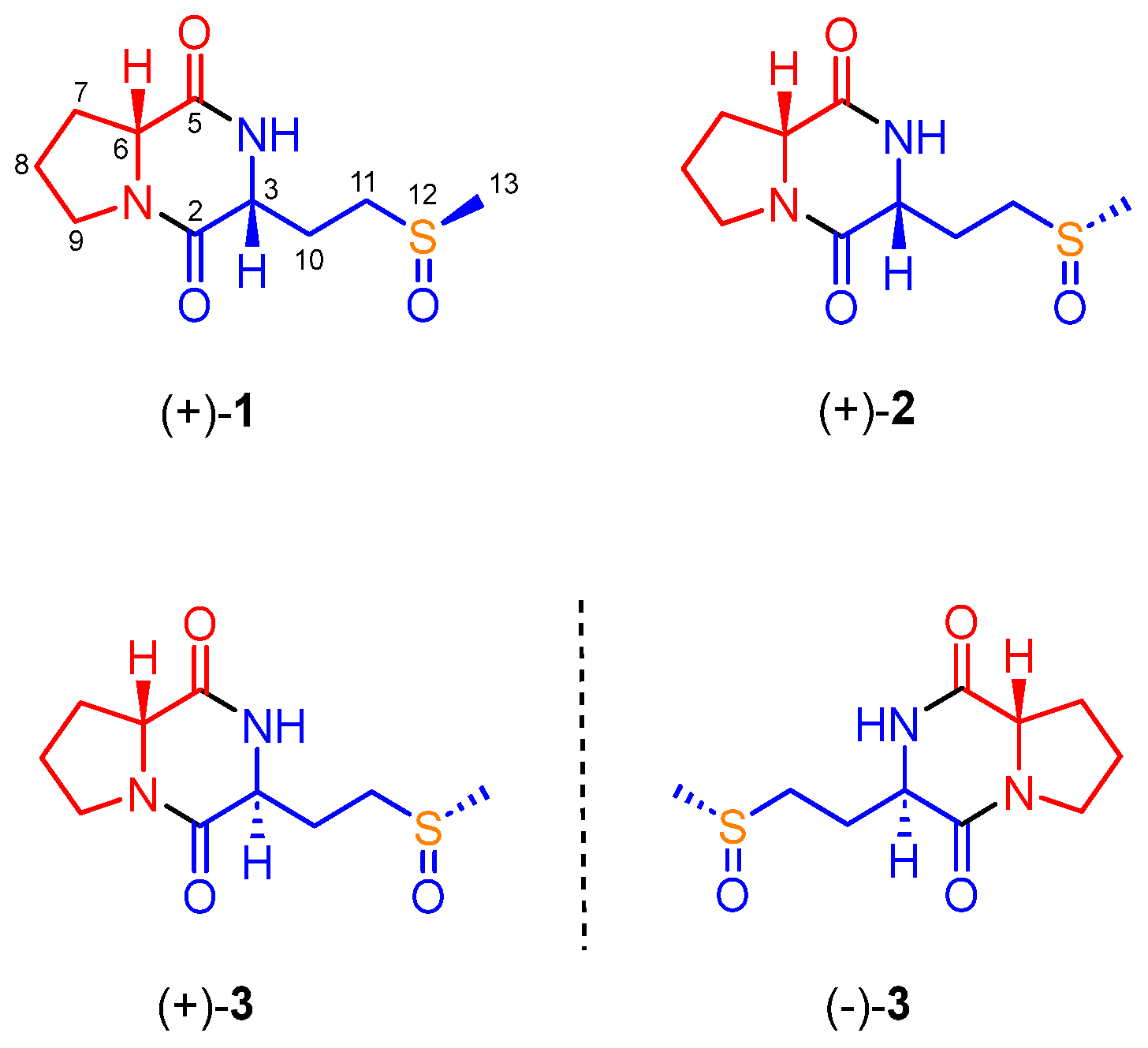
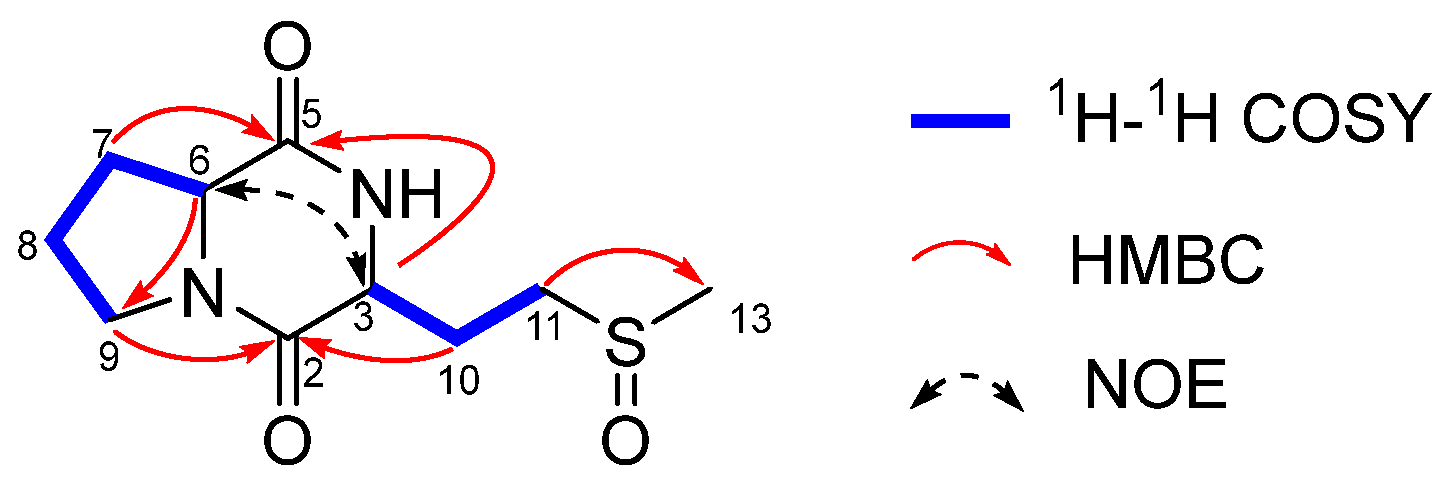
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
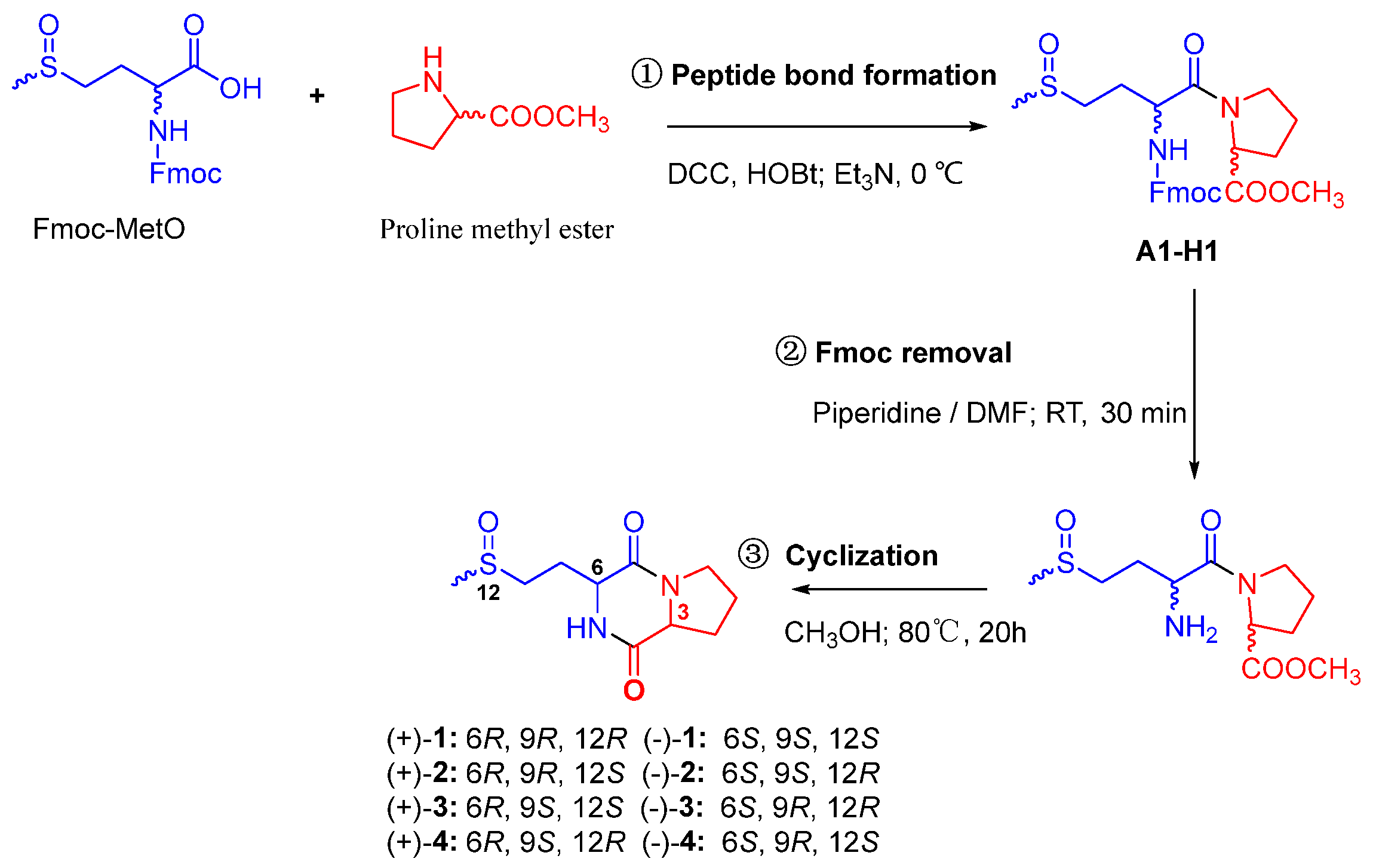
3.4. Total Synthesis of 1–4
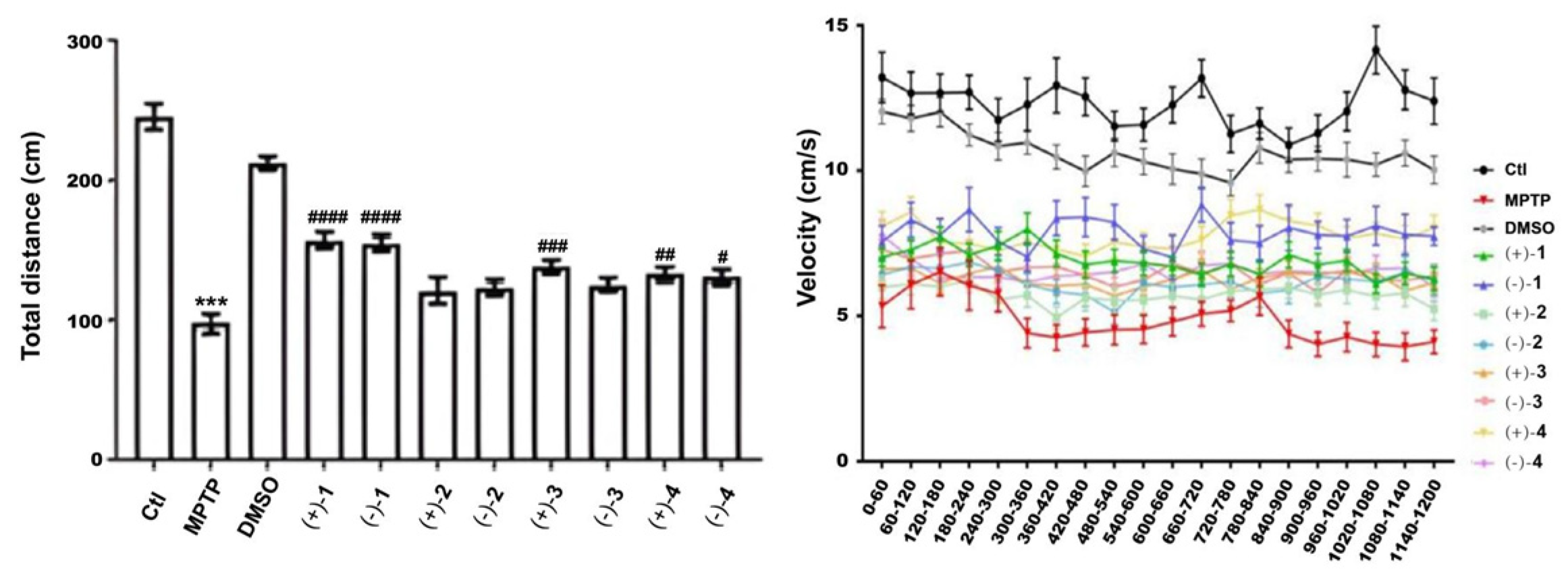
3.5. Anti-Parkinson’s Disease Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, N.Z.; Saidhareddy, P.; Jiang, X.F. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 2020, 37, 246–275. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636–1640. [Google Scholar] [CrossRef]
- Mau, C.M.; Nakao, Y.; Yoshida, W.Y.; Scheuer, P.J.; Kelly-Borges, M. Waiakeamide, a cyclic hexapeptide from the sponge Ircinia dendroides1. J. Org. Chem. 1996, 61, 6302–6304. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.M.; Zhang, S.S.; Liu, L.Y.; Sun, F.; Jiao, W.H.; Wang, S.P.; Lin, H.W. Axinellasins A-D, immunosuppressive cycloheptapeptide diastereomers, discovered via a precursor ion scanning-supercritical fluid chromatography strategy from the marine sponge Axinella species. Org. Lett. 2022, 24, 934–938. [Google Scholar] [CrossRef]
- Kwon, O.S.; Kim, C.K.; Byun, W.S.; Oh, J.; Lee, Y.J.; Lee, H.S.; Sim, C.J.; Oh, D.C.; Lee, S.K.; Oh, K.B.; et al. Cyclopeptides from the sponge Stylissa flabelliformis. J. Nat. Prod. 2018, 81, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Gustafson, K.R.; Boswell, J.L.; Boyd, M.R. Haligramides A and B, two new cytotoxic hexapeptides from the marine sponge Haliclona nigra. J. Nat. Prod. 2000, 63, 956–959. [Google Scholar] [CrossRef]
- Kobayashi, J.; Nakamura, T.; Tsuda, M. Hymenamide F, new cyclic heptapeptide from marine sponge Hymeniacidon sp. Tetrahedron 1996, 52, 6355–6360. [Google Scholar] [CrossRef]
- Pettit, G.R.; Tan, R. Isolation and structure of phakellistatin 14 from the western pacific marine sponge Phakellia sp. J. Nat. Prod. 2005, 68, 60–63. [Google Scholar] [CrossRef]
- Nakao, Y.; Kuo, J.; Yoshida, W.Y. More kapakahines from the marine sponge Cribrochalina olemda. Org. Lett. 2003, 5, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Matsunaga, S.; Yano, G.; Fusetani, N. Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J. Am. Chem. 2005, 127, 110–118. [Google Scholar] [CrossRef]
- Kalíková, K.; Šlechtová, T.; Vozka, J. Supercritical fluid chromatography as a tool for enantioselective separation; a review. Anal. Chim. Acta 2014, 821, 1–33. [Google Scholar] [CrossRef]
- Quaranta, A.; Zöhrer, B.; Revol, C.J.; Benkestock, K.; Balas, L.; Oger, C.; Keyes, G.S.; Wheelock, Å.M.; Durand, T.; Galano, J.M.; et al. Development of a chiral supercritical fluid chromatography-tandem mass spectrometry and reversed-phase liquid chromatography-tandem mass spectrometry platform for the quantitative Metabolic Profiling of Octadecanoid Oxylipins. Anal. Chem. 2022, 94, 14618–14626. [Google Scholar] [CrossRef] [PubMed]
- He, P.X.; Zhang, Y.; Zhou, Y.; Li, G.H.; Zhang, J.W.; Feng, X.S. Supercritical fluid chromatography-a technical overview and its applications in medicinal plant analysis: An update covering 2012-2018. Analyst 2019, 144, 5324–5352. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.H.; Li, J.; Zhang, M.M.; Cui, J.; Gui, Y.H.; Zhang, Y.; Li, J.Y.; Liu, K.C.; Lin, H.W. Frondoplysins A and B, unprecedented terpene-alkaloid bioconjugates from Dysidea frondosa. Org. Lett. 2019, 21, 6190–6193. [Google Scholar] [CrossRef]
- Jiao, W.H.; Cheng, B.H.; Shi, G.H.; Chen, G.D.; Gu, B.B.; Zhou, Y.J.; Hong, L.L.; Yang, F.; Liu, Z.Q.; Qiu, S.Q.; et al. Dysivillosins A-D, unusual anti-allergic meroterpenoids from the marine sponge Dysidea villosa. Sci. Rep. 2017, 7, 8947. [Google Scholar] [CrossRef]
- Kim, C.K.; Woo, J.K.; Kim, S.H.; Cho, E.; Lee, Y.J.; Lee, H.S.; Sim, C.J.; Oh, D.C.; Oh, K.B.; Shin, J. Meroterpenoids from a tropical Dysidea sp. Sponge. J. Nat. Prod. 2015, 78, 2814–2821. [Google Scholar] [CrossRef]
- Luo, X.; Li, P.; Wang, K.; de Voogd, N.J.; Tang, X.; Li, G. Cytotoxic sesquiterpenoid quinones from South China Sea sponge Dysidea sp. Nat. Prod. Res. 2019, 35, 2866–2871. [Google Scholar] [CrossRef]
- Lai, W.; Qin, S.Y.; Zou, G.; Liao, X.J.; Chen, G.D.; Zhang, H.; Zhao, B.X.; Xu, S.H. Sinulaspirolactam A, a novel aza-spirocyclic valerenane sesquiterpenoid from soft coral Sinularia sp. J. Asian Nat. Prod. Res. 2019, 21, 494–501. [Google Scholar] [CrossRef]
- Yang, X.Q.; Yang, Y.B.; Zhou, H.; He, G.W.; Zhao, L.X.; Xu, L.H.; Ding, Z.T. New megastigmane glycoside and alkaloids from Streptomyces sp. YIM 63342. Nat. Prod. Res. 2013, 27, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Gamini, S.J.; Maureen, P.T.; Alan, C.L.; Julia, E.G.; Bill, J.B. Metabolites from an antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996, 59, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Scapinello, L.; Vavassori, F.; Ieronimo, G.; Ameta, K.L.; Cravotto, G.; Simonetti, M.; Tollari, S.; Palmisano, G.; Nicholas, K.; Penoni, A. Further developments on the regioselective synthesis of 3-aroylindole derivatives from C-nitrosoaromatics and alkynones: A novel synthetic approach to pravadoline, JWH-073, indothiazinone analogues and related compounds. Int. J. Org. Chem. 2022, 12, 127–142. [Google Scholar] [CrossRef]
- Yi, Y.; Lena, H. Optimized Fmoc-removal strategy to suppress the traceless and conventional diketopiperazine formation in solid-phase peptide synthesis. ACS Omega 2022, 7, 12015–12020. [Google Scholar]
- Yasuo, S.; Fumitaka, H. Method for Producing Cyclic Dipeptide. JP. Patent 5,456,876, 01 April 2014. [Google Scholar]
- Kamil, J.K.; Michał, Z.; Jakub, S.; Gniewomir, L.; Agnieszka, O.M.; Piotr, J.; Agata, D.P.; Andreas, B.; Christa, E.M. Novel, dual target-directed annelated xanthine derivatives acting on adenosine receptors and monoamine oxidase B. ChemMedChem 2020, 15, 772–786. [Google Scholar]
| No. | (+)-1 | (+)-2 | 3 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 2 | - | 167.2 | - | 167.2 | - | 167.3 |
| 3 | 4.36 t (4.9) | 55.2 | 4.39 t (5.3) | 55.3 | 3.96 m | 57.7 |
| 4 | - | - | - | - | - | - |
| 5 | - | 172.7 | - | 172.7 | - | 171.2 |
| 6 | 4.26 t (7.9) | 60.4 | 4.30 t (7.9) | 60.4 | 4.31 dd (9.9, 6.5) | 59.4 |
| 7 | a 2.31 m | 29.2 | a 2.35 m | 29.3 | a 2.35 m | 29.9 |
| b 2.03 m | b 2.08 m | b 2.04 m | ||||
| 8 | 2.01~1.98 m | 23.5 | 2.04~2.02 m | 23.5 | 1.94~1.92 m | 23.0 |
| 9 | 3.57~3.48 m | 46.4 | 3.61~3.53 m | 46.5 | a 3.63~3.58 m | 46.8 |
| b 3.55~3.48 m | ||||||
| 10 | 2.30~2.26 m | 23.9 | 2.34~2.29 m | 23.8 | 2.24~2.20 m | 28.0 |
| 11 | 3.00~2.26 m | 50.0 | a 3.09~3.02 m | 49.8 | a 2.99~2.93 m | 50.6 |
| b 2.91 m | b 2.87 m | |||||
| 13 | 2.66 s | 38.2 | 2.71 s | 37.9 | 2.67 s | 38.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Yang, Z.; Li, D.; Liao, X.; Hettiarachchi, C.; Zhao, B.; Xu, S. Four New Pairs of MetO-Containing Diketopiperazine Enantiomers: Isolation, Synthesis and Potential Anti-Parkinson’s Disease Activity. Mar. Drugs 2025, 23, 477. https://doi.org/10.3390/md23120477
Lei Y, Yang Z, Li D, Liao X, Hettiarachchi C, Zhao B, Xu S. Four New Pairs of MetO-Containing Diketopiperazine Enantiomers: Isolation, Synthesis and Potential Anti-Parkinson’s Disease Activity. Marine Drugs. 2025; 23(12):477. https://doi.org/10.3390/md23120477
Chicago/Turabian StyleLei, Yu, Zhenyu Yang, Daichun Li, Xiaojian Liao, Chamari Hettiarachchi, Bingxin Zhao, and Shihai Xu. 2025. "Four New Pairs of MetO-Containing Diketopiperazine Enantiomers: Isolation, Synthesis and Potential Anti-Parkinson’s Disease Activity" Marine Drugs 23, no. 12: 477. https://doi.org/10.3390/md23120477
APA StyleLei, Y., Yang, Z., Li, D., Liao, X., Hettiarachchi, C., Zhao, B., & Xu, S. (2025). Four New Pairs of MetO-Containing Diketopiperazine Enantiomers: Isolation, Synthesis and Potential Anti-Parkinson’s Disease Activity. Marine Drugs, 23(12), 477. https://doi.org/10.3390/md23120477







