The Effect of Lipid Extract of Nannochloropsis oceanica Marine Microalgae on Glutathione and Thioredoxin-Dependent Antioxidant Systems in UVB-Irradiated Keratinocytes
Abstract
1. Introduction
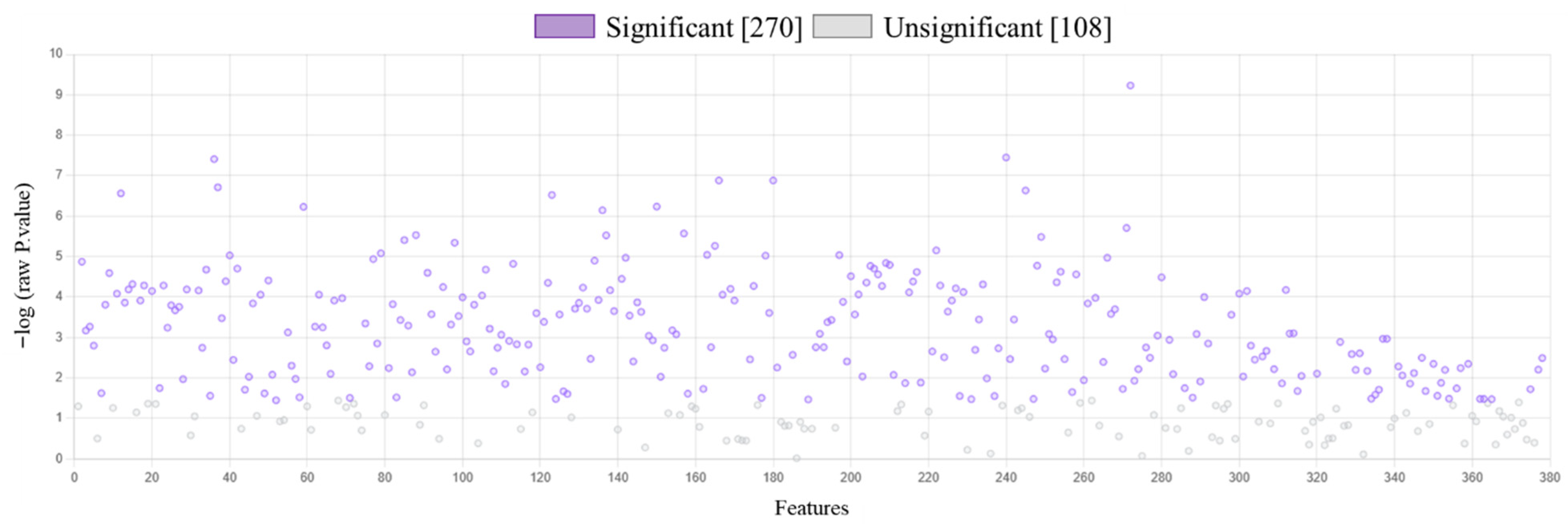
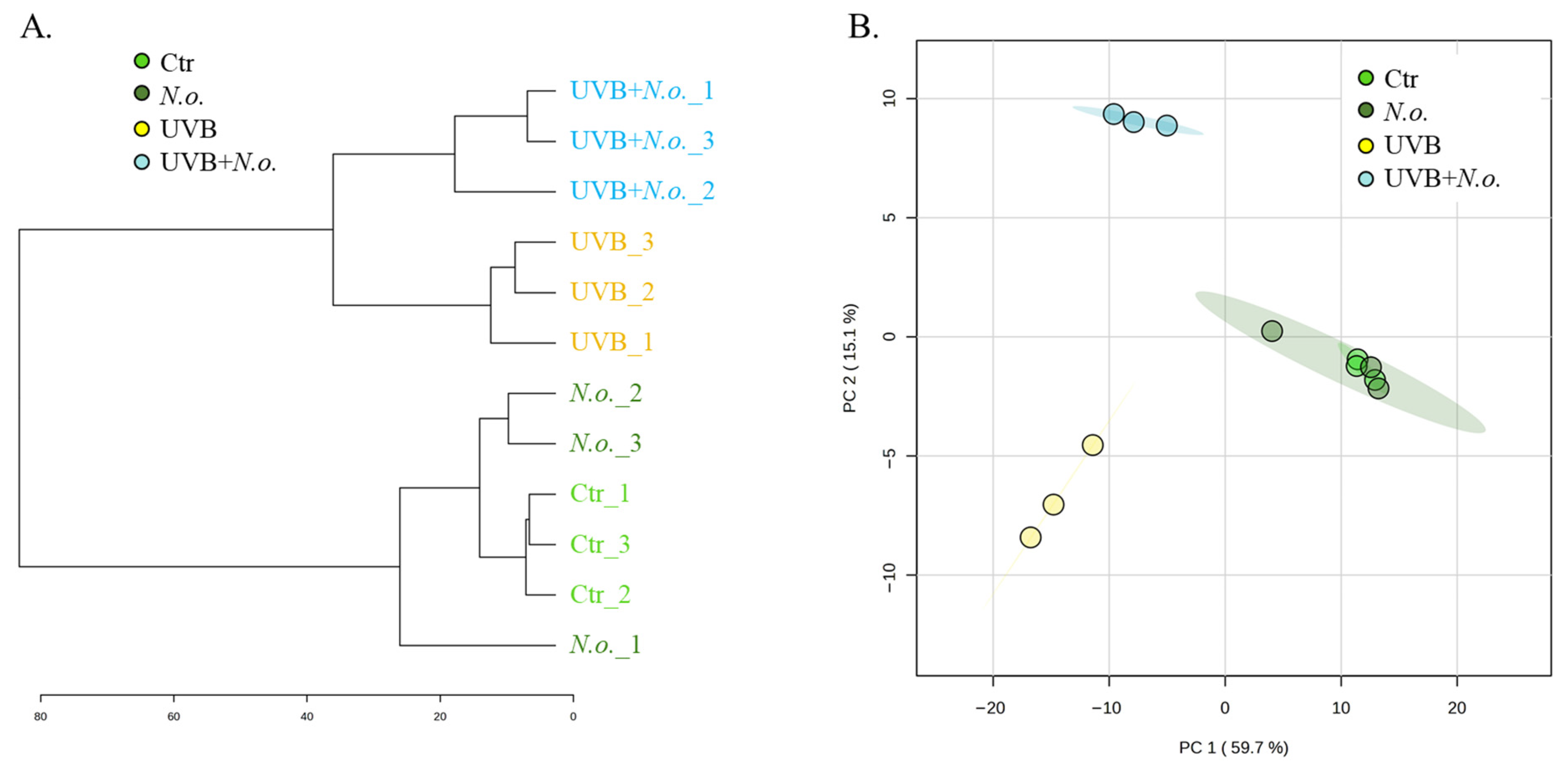
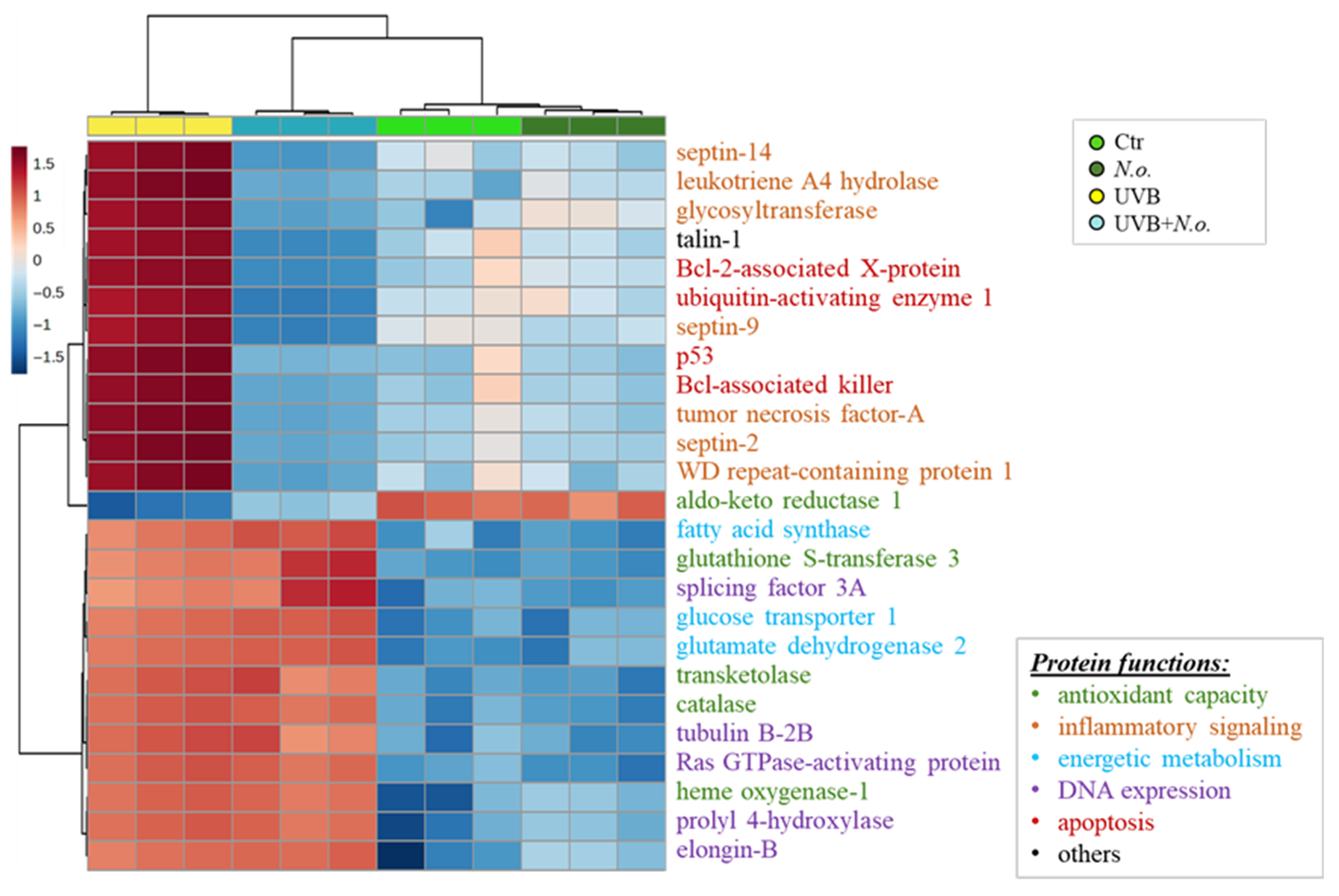
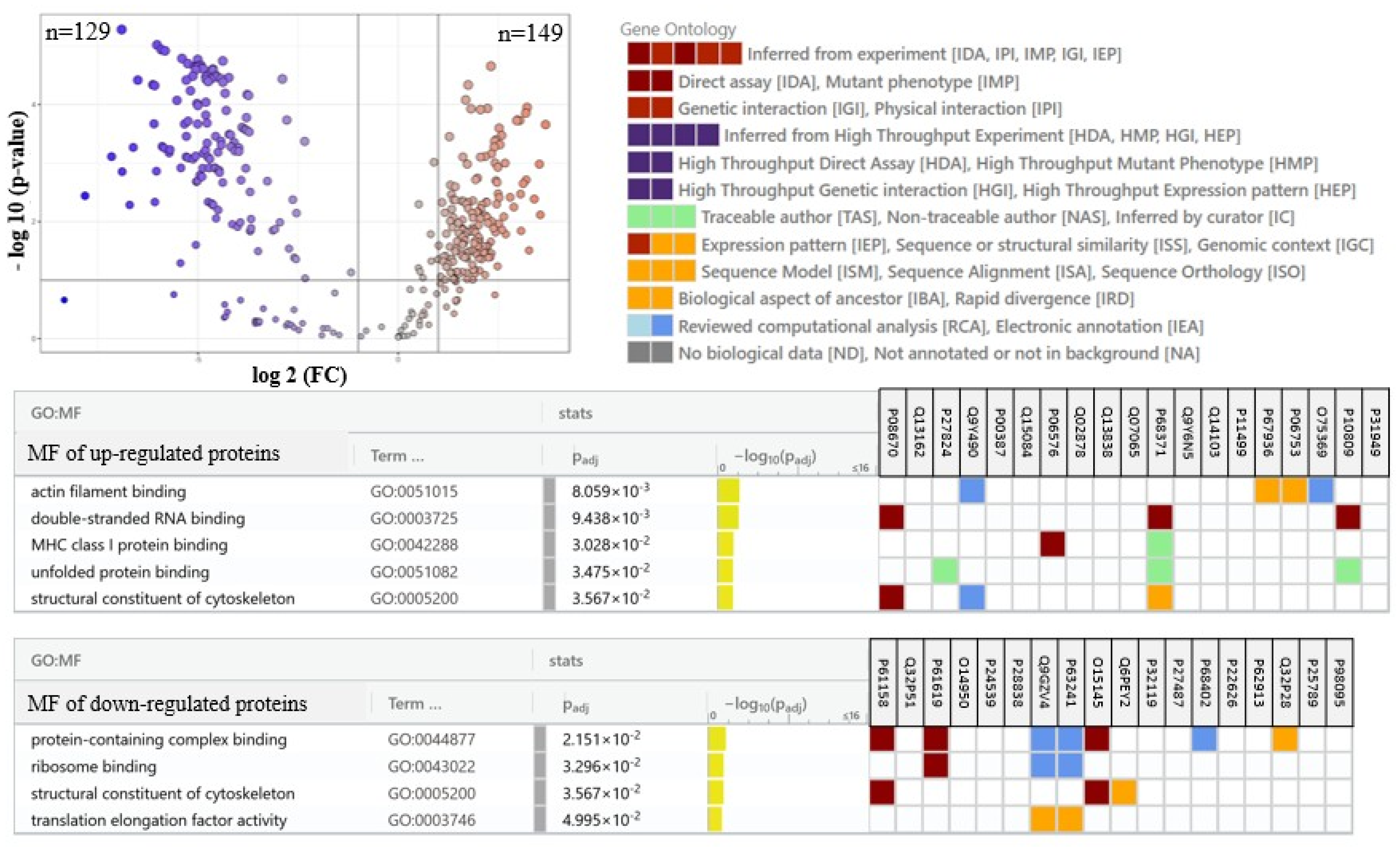
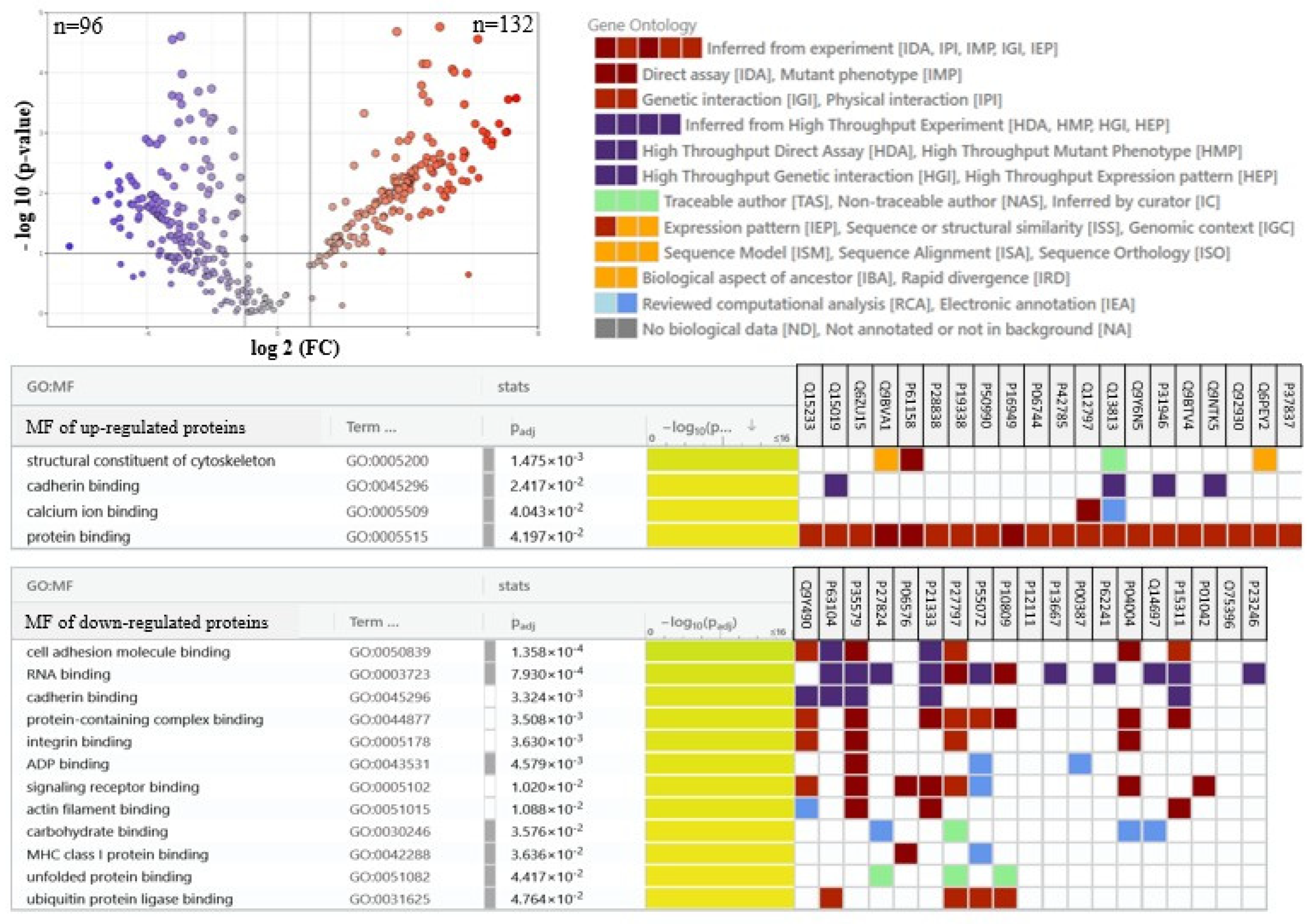
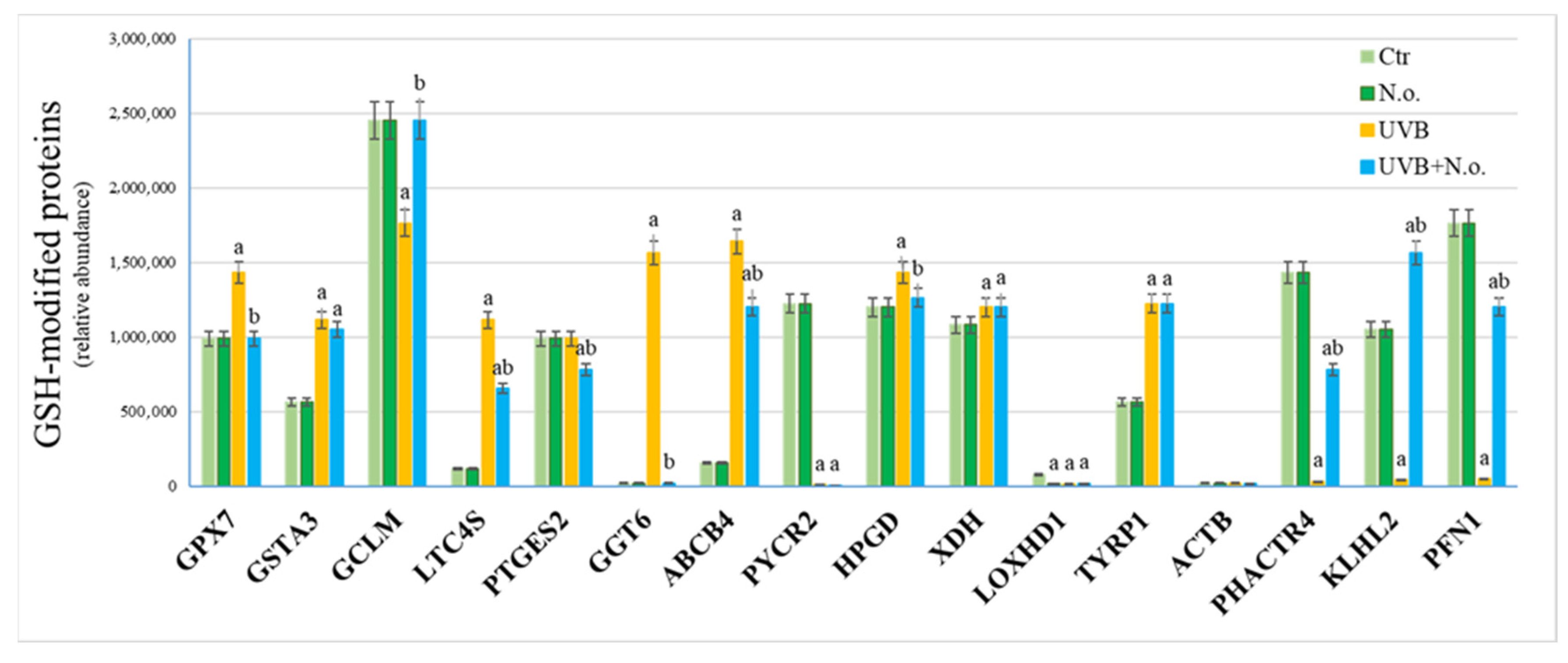
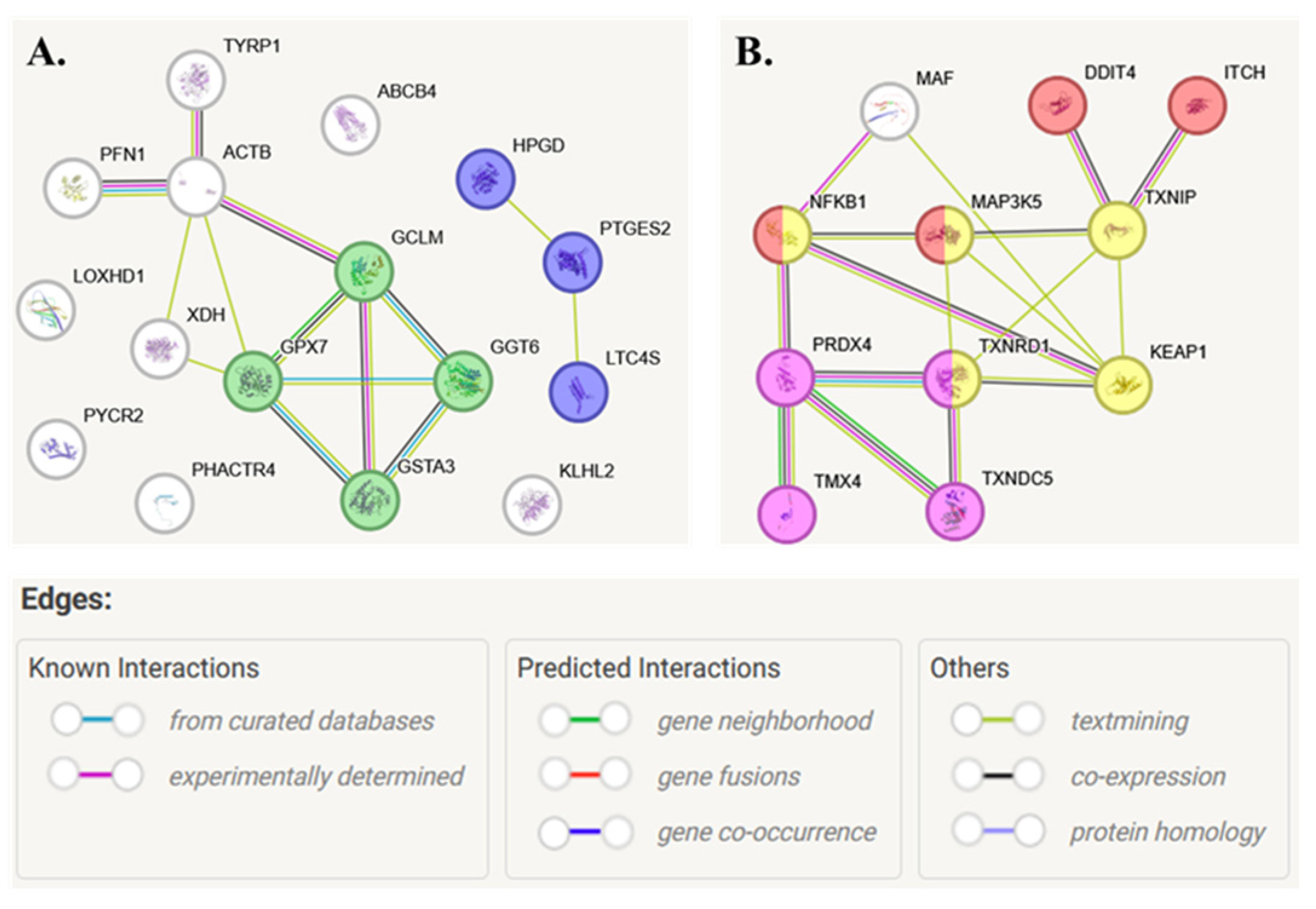
2. Results
3. Discussion
4. Materials and Methods
4.1. Microalgae Lipid Extracts
4.2. Cell Culture and Treatment
4.3. Isolation of GSH and Trx-Protein Adducts
4.4. In-Solution Protein Digestion and Peptide Analysis
4.5. Protein Identification and Label-Free Quantification
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Gu, X.; Yang, D.; Xu, S.; Wang, S.; Chen, X.; Wang, Z. Bioactive Substances and Potentiality of Marine Microalgae. Food Sci. Nutr. 2021, 9, 5279–5292. [Google Scholar] [CrossRef]
- Katiyar, R.; Arora, A. Health Promoting Functional Lipids from Microalgae Pool: A Review. Algal Res. 2020, 46, 101800. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for Oil: Strain Selection, Induction of Lipid Synthesis and Outdoor Mass Cultivation in a Low-Cost Photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Choo, W.-T.; Teoh, M.-L.; Phang, S.-M.; Convey, P.; Yap, W.-H.; Goh, B.-H.; Beardall, J. Microalgae as Potential Anti-Inflammatory Natural Product Against Human Inflammatory Skin Diseases. Front. Pharmacol. 2020, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Conde, T.A.; Zabetakis, I.; Tsoupras, A.; Medina, I.; Costa, M.; Silva, J.; Neves, B.; Domingues, P.; Domingues, M.R. Microalgal Lipid Extracts Have Potential to Modulate the Inflammatory Response: A Critical Review. Int. J. Mol. Sci. 2021, 22, 9825. [Google Scholar] [CrossRef]
- Robertson, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. The Anti-Inflammatory Effect of Algae-Derived Lipid Extracts on Lipopolysaccharide (LPS)-Stimulated Human THP-1 Macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Cunha, P.; Guerra, I.; Correia, N.; Silva, J.T.; Pereira, H.; et al. Effects of Outdoor and Indoor Cultivation on the Polar Lipid Composition and Antioxidant Activity of Nannochloropsis oceanica and Nannochloropsis limnetica: A Lipidomics Perspective. Algal Res. 2022, 64, 102718. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gęgotek, A.; Domingues, P.; Domingues, M.R.; Skrzydlewska, E. Comparison of Microalgae Nannochloropsis oceanica and Chlorococcum amblystomatis Lipid Extracts Effects on UVA-Induced Changes in Human Skin Fibroblasts Proteome. Mar. Drugs 2024, 22, 509. [Google Scholar] [CrossRef]
- Stasiewicz, A.; Conde, T.; Gęgotek, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Prevention of UVB Induced Metabolic Changes in Epidermal Cells by Lipid Extract from Microalgae Nannochloropsis oceanica. Int. J. Mol. Sci. 2023, 24, 11302. [Google Scholar] [CrossRef] [PubMed]
- Wroński, A.; Gęgotek, A.; Conde, T.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Nannochloropsis oceanica Lipid Extract Moderates UVB-Irradiated Psoriatic Keratinocytes: Impact on Protein Expression and Protein Adducts. Antioxidants 2024, 13, 1236. [Google Scholar] [CrossRef]
- Biernacki, M.; Conde, T.; Stasiewicz, A.; Surażyński, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Restorative Effect of Microalgae Nannochloropsis oceanica Lipid Extract on Phospholipid Metabolism in Keratinocytes Exposed to UVB Radiation. Int. J. Mol. Sci. 2023, 24, 14323. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Biernacki, M.; Ambrożewicz, E.; Surażyński, A.; Wroński, A.; Skrzydlewska, E. The Cross-Talk between Electrophiles, Antioxidant Defence and the Endocannabinoid System in Fibroblasts and Keratinocytes after UVA and UVB Irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef]
- Mhamdi-Ghodbani, M.; Starzonek, C.; Degenhardt, S.; Bender, M.; Said, M.; Greinert, R.; Volkmer, B. UVB Damage Response of Dermal Stem Cells as Melanocyte Precursors Compared to Keratinocytes, Melanocytes, and Fibroblasts from Human Foreskin. J. Photochem. Photobiol. B Biol. 2021, 220, 112216. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Blarzino, C.; Foppoli, C.; Cini, C.; Giorgi, A.; Grillo, C.; De Marco, F.; Butterfield, D.A.; Schininà, M.E.; et al. Effects of UVB-Induced Oxidative Stress on Protein Expression and Specific Protein Oxidation in Normal Human Epithelial Keratinocytes: A Proteomic Approach. Proteome Sci. 2010, 8, 13. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Narzt, M.-S. The Impact of Recent Advances in Lipidomics and Redox Lipidomics on Dermatological Research. Free Radic. Biol. Med. 2019, 144, 256–265. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of Microalgal Compounds in Trending Natural Cosmetics: A Review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Ikeda, I.K.; Sydney, E.B.; Sydney, A.C.N. Potential Application of Spirulinain Dermatology. J. Cosmet. Dermatol. 2022, 21, 4205–4214. [Google Scholar] [CrossRef]
- Kasting, G.B.; Miller, M.A.; LaCount, T.D.; Jaworska, J. A Composite Model for the Transport of Hydrophilic and Lipophilic Compounds Across the Skin: Steady-State Behavior. J. Pharm. Sci. 2019, 108, 337–349. [Google Scholar] [CrossRef]
- Łuczaj, W.; Gęgotek, A.; Conde, T.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Lipidomic Assessment of the Impact of Nannochloropsis oceanica Microalga Lipid Extract on Human Skin Keratinocytes Exposed to Chronic UVB Radiation. Sci. Rep. 2023, 13, 22302. [Google Scholar] [CrossRef]
- Stasiewicz, A.; Conde, T.; Domingues, M.d.R.; Domingues, P.; Biernacki, M.; Skrzydlewska, E. Comparison of the Regenerative Metabolic Efficiency of Lipid Extracts from Microalgae Nannochloropsis oceanica and Chlorococcum amblystomatis on Fibroblasts. Antioxidants 2024, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Conde, T.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Impact of Nannochloropsis oceanica and Chlorococcum amblystomatis Extracts on UVA-Irradiated on 3D Cultured Melanoma Cells: A Proteomic Insight. Cells 2024, 13, 1934. [Google Scholar] [CrossRef]
- Zhao, H.; Ruan, H.; Li, H. Progress in the Research of GSH in Cells. Chin. Sci. Bull. 2011, 56, 3057. [Google Scholar] [CrossRef]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Niu, C.; Dong, M.; Niu, Y. Role of Glutathione in Parkinson’s Disease Pathophysiology and Therapeutic Potential of Polyphenols. Phytother. Res. 2024, 38, 5567–5582. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Goldstone, J.V.; Imhoff, B.R.; Stegeman, J.J.; Hahn, M.E.; Hansen, J.M. Glutathione Redox Dynamics and Expression of Glutathione-Related Genes in the Developing Embryo. Free Radic. Biol. Med. 2013, 65, 89–101. [Google Scholar] [CrossRef]
- Yao, S.; Xu, J.; Zhao, K.; Song, P.; Yan, Q.; Fan, W.; Li, W.; Lu, C. Down-Regulation of HPGD by miR-146b-3p Promotes Cervical Cancer Cell Proliferation, Migration and Anchorage-Independent Growth through Activation of STAT3 and AKT Pathways. Cell Death Dis. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Riazuddin, S.A.; Parker, D.S.; McGlumphy, E.J.; Oh, E.C.; Iliff, B.W.; Schmedt, T.; Jurkunas, U.; Schleif, R.; Katsanis, N.; Gottsch, J.D. Mutations in LOXHD1, a Recessive-Deafness Locus, Cause Dominant Late-Onset Fuchs Corneal Dystrophy. Am. J. Hum. Genet. 2012, 90, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xiao, L.; Yin, X.; Xiang, S.; Wang, C. Comprehensive Analysis Reveals an Arachidonic Acid Metabolism-Related Gene Signature in Patients with Pancreatic Ductal Adenocarcinoma. Biocell 2022, 46, 2241–2256. [Google Scholar] [CrossRef]
- Lalier, L.; Cartron, P.-F.; Olivier, C.; Logé, C.; Bougras, G.; Robert, J.-M.; Oliver, L.; Vallette, F.M. Prostaglandins Antagonistically Control Bax Activation during Apoptosis. Cell Death Differ. 2011, 18, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Perng, D.-W.; Wu, Y.-C.; Chang, K.-T.; Wu, M.-T.; Chiou, Y.-C.; Su, K.-C.; Perng, R.-P.; Lee, Y.-C. Leukotriene C4 Induces TGF-Β1 Production in Airway Epithelium via P38 Kinase Pathway. Am. J. Respir. Cell Mol. Biol. 2006, 34, 101–107. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione Metabolism in Cancer Progression and Treatment Resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and Ascorbic Acid Cooperation in Antioxidant and Antiapoptotic Effect on Human Skin Keratinocytes and Fibroblasts Exposed to UVA and UVB Radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef]
- Hsiao, P.-F.; Huang, Y.-T.; Lu, P.-H.; Chiu, L.-Y.; Weng, T.-H.; Hung, C.-F.; Wu, N.-L. Thioredoxin-Interacting Protein Regulates Keratinocyte Differentiation: Implication of Its Role in Psoriasis. FASEB J. 2022, 36, e22313. [Google Scholar] [CrossRef]
- Biswas, R.; Bagchi, A. NFkB Pathway and Inhibition: An Overview. Comput. Mol. Biol. 2016, 6, 1–20. [Google Scholar] [CrossRef]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of Ultraviolet B Exposure-Mediated Activation of NF-κB in Normal Human Keratinocytes by Resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef]
- Fuchs, J. Environmental Stressors in Health and Disease; CRC Press: Boca Raton, FL, USA, 2001; ISBN 978-0-203-90478-7. [Google Scholar]
- Moran, L.K.; Gutteridge, J.; Quinlan, G.J. Thiols in Cellular Redox Signalling and Control. Curr. Med. Chem. 2001, 8, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, A.; Srivenugopal, K.S. Degradation of NF-κB, P53 and Other Regulatory Redox-Sensitive Proteins by Thiol-Conjugating and -Nitrosylating Drugs in Human Tumor Cells. Carcinogenesis 2013, 34, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Spandau, D.F. UVB Activation of NF-κB in Normal Human Keratinocytes Occurs via a Unique Mechanism. Arch. Dermatol. Res. 2007, 299, 93–101. [Google Scholar] [CrossRef]
- Suzuki, T.; Takahashi, J.; Yamamoto, M. Molecular Basis of the KEAP1-NRF2 Signaling Pathway. Mol. Cells 2023, 46, 133–141. [Google Scholar] [CrossRef]
- Gao, S.; Guo, K.; Chen, Y.; Zhao, J.; Jing, R.; Wang, L.; Li, X.; Hu, Z.; Xu, N.; Li, X. Keratinocyte Growth Factor 2 Ameliorates UVB-Induced Skin Damage via Activating the AhR/Nrf2 Signaling Pathway. Front. Pharmacol. 2021, 12, 655281. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Z.; Wu, Z. Four-Octyl Itaconate Attenuates UVB-Induced Melanocytes and Keratinocytes Apoptosis by Nrf2 Activation-Dependent ROS Inhibition. Oxidative Med. Cell. Longev. 2022, 2022, 9897442. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.; Pereira, H.; Campos, J.; Marques, A.; Varela, J.; Silva, J. Heterotrophy as a Tool to Overcome the Long and Costly Autotrophic Scale-up Process for Large Scale Production of Microalgae. Sci. Rep. 2019, 9, 13935. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In Vitro Cytotoxicity Assays: Comparison of LDH, Neutral Red, MTT and Protein Assay in Hepatoma Cell Lines Following Exposure to Cadmium Chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Medzihradszky, K.F. In-Solution Digestion of Proteins for Mass Spectrometry. Methods Enzym. 2005, 405, 50–65. [Google Scholar] [CrossRef]
- Gęgotek, A.; Domingues, P.; Wroński, A.; Wójcik, P.; Skrzydlewska, E. Proteomic Plasma Profile of Psoriatic Patients. J. Pharm. Biomed. Anal. 2018, 155, 185–193. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant Computational Platform for Mass Spectrometry-Based Shotgun Proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS Spectra Processing, Multi-Omics Integration and Covariate Adjustment of Global Metabolomics Data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. G:Profiler—Interoperable Web Service for Functional Enrichment Analysis and Gene Identifier Mapping (2023 Update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gęgotek, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. The Effect of Lipid Extract of Nannochloropsis oceanica Marine Microalgae on Glutathione and Thioredoxin-Dependent Antioxidant Systems in UVB-Irradiated Keratinocytes. Mar. Drugs 2025, 23, 454. https://doi.org/10.3390/md23120454
Gęgotek A, Domingues MR, Domingues P, Skrzydlewska E. The Effect of Lipid Extract of Nannochloropsis oceanica Marine Microalgae on Glutathione and Thioredoxin-Dependent Antioxidant Systems in UVB-Irradiated Keratinocytes. Marine Drugs. 2025; 23(12):454. https://doi.org/10.3390/md23120454
Chicago/Turabian StyleGęgotek, Agnieszka, Maria Rosario Domingues, Pedro Domingues, and Elżbieta Skrzydlewska. 2025. "The Effect of Lipid Extract of Nannochloropsis oceanica Marine Microalgae on Glutathione and Thioredoxin-Dependent Antioxidant Systems in UVB-Irradiated Keratinocytes" Marine Drugs 23, no. 12: 454. https://doi.org/10.3390/md23120454
APA StyleGęgotek, A., Domingues, M. R., Domingues, P., & Skrzydlewska, E. (2025). The Effect of Lipid Extract of Nannochloropsis oceanica Marine Microalgae on Glutathione and Thioredoxin-Dependent Antioxidant Systems in UVB-Irradiated Keratinocytes. Marine Drugs, 23(12), 454. https://doi.org/10.3390/md23120454






