A New Algal Friendly Extract from Euglena cantabrica with Potential Applications in Biomedical Field
Abstract
1. Introduction
2. Results and Discussion
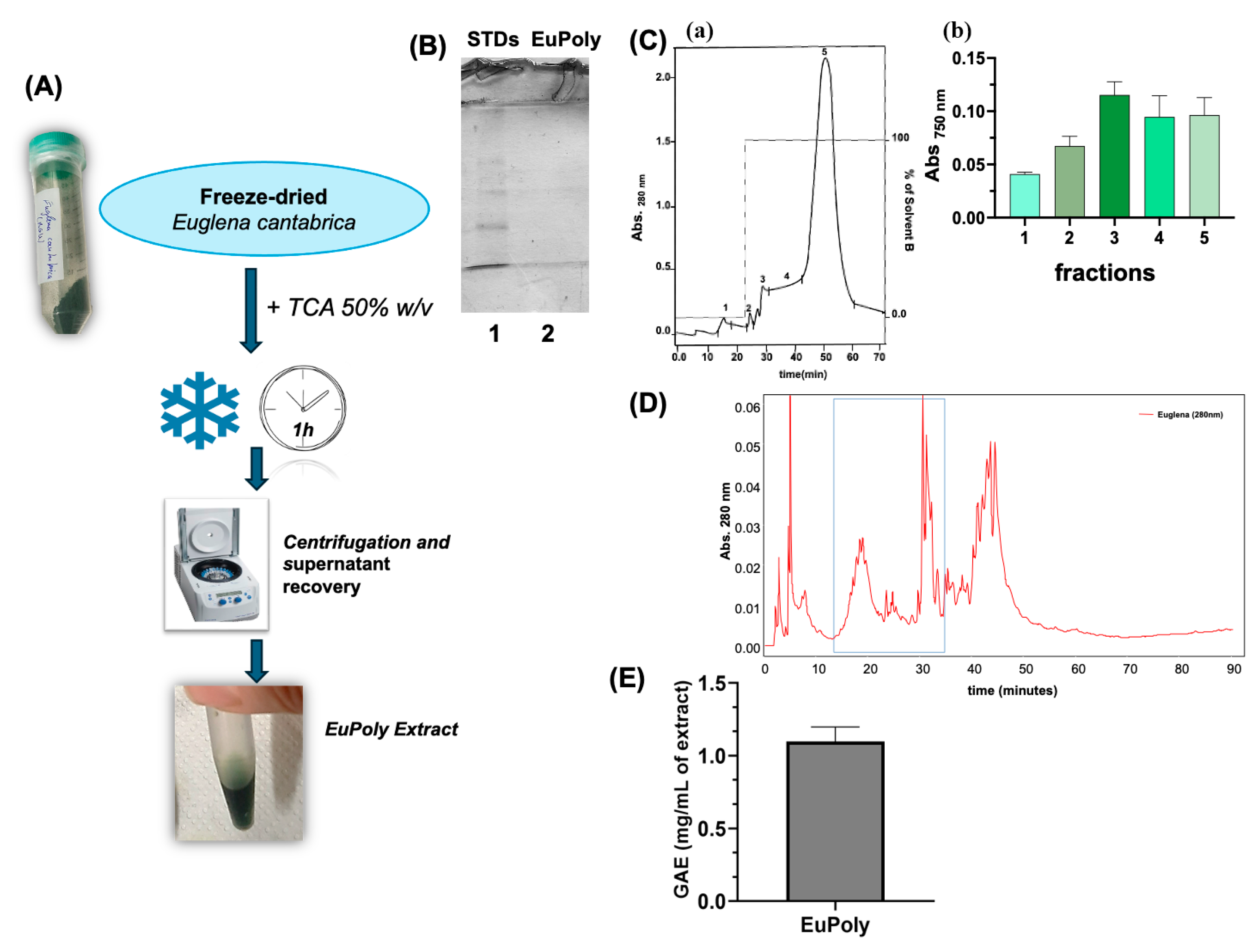
2.1. Production and Characterization of the E. cantabrica Extract
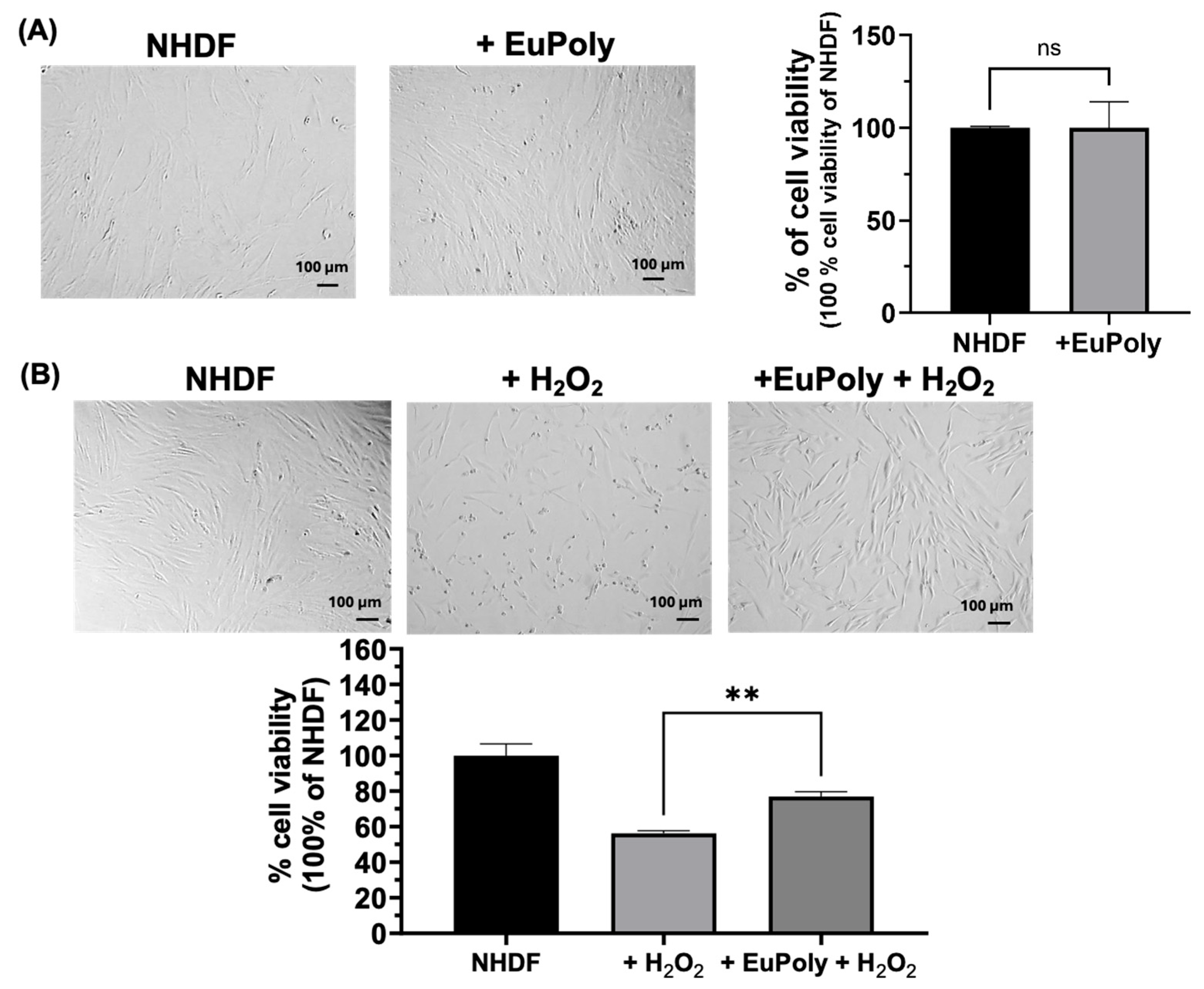
2.2. Effects of EuPoly on Human Dermal Fibroblasts (NHDFs)
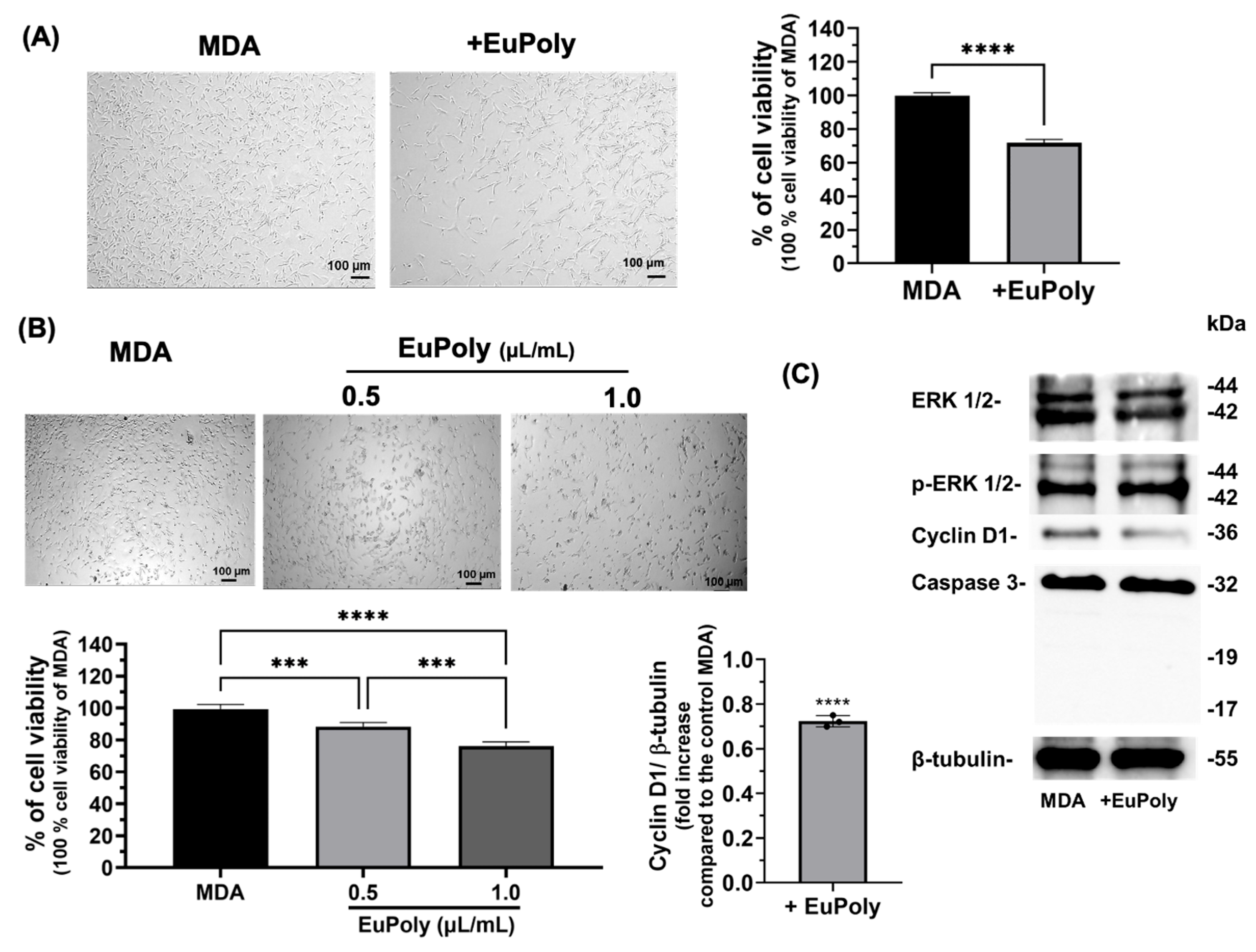
2.3. Effects of EuPoly on MDA-MB-231 Breast Cancer Cells
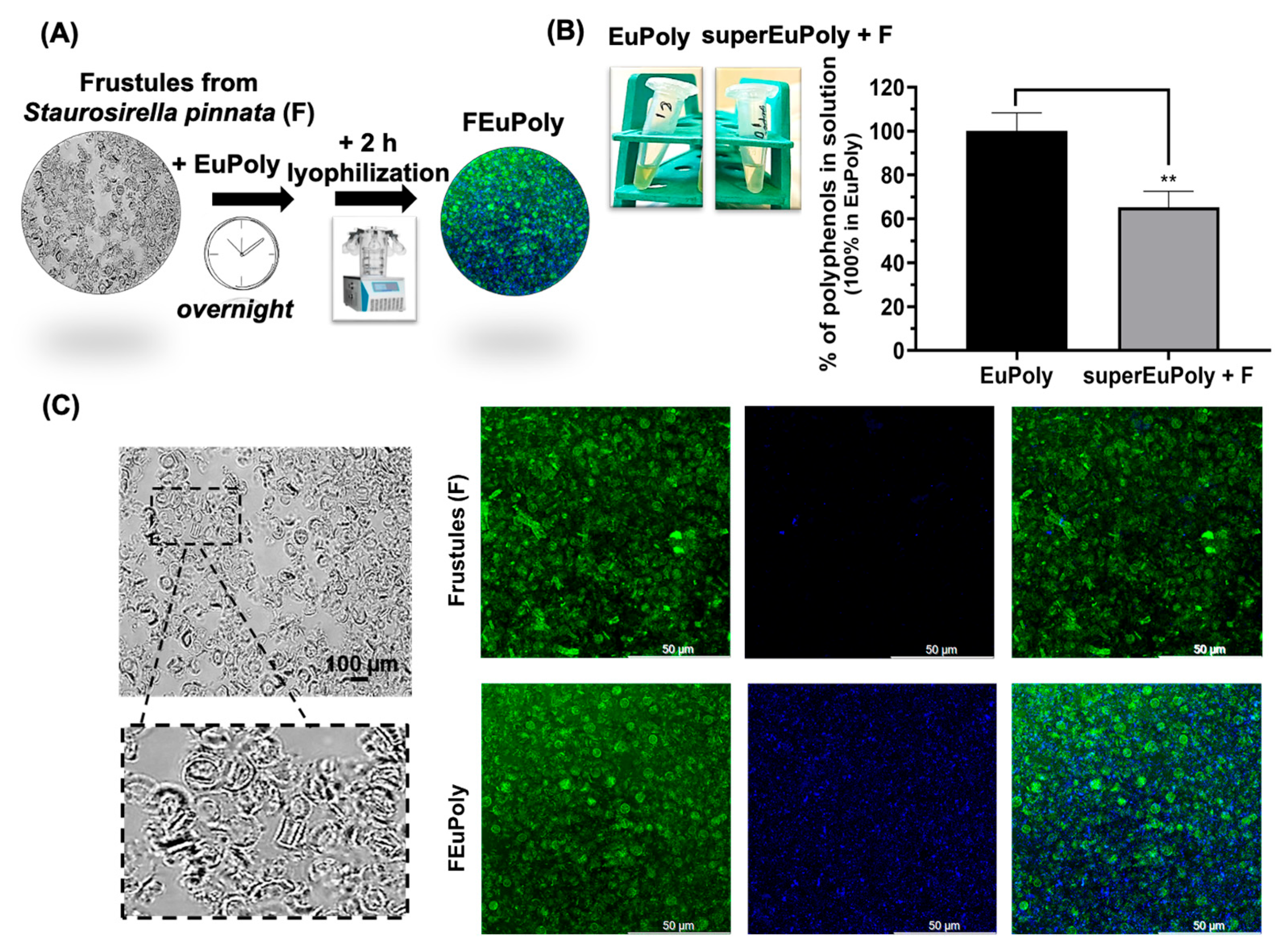
2.4. Diatoms Frustule Functionalization with EuPoly
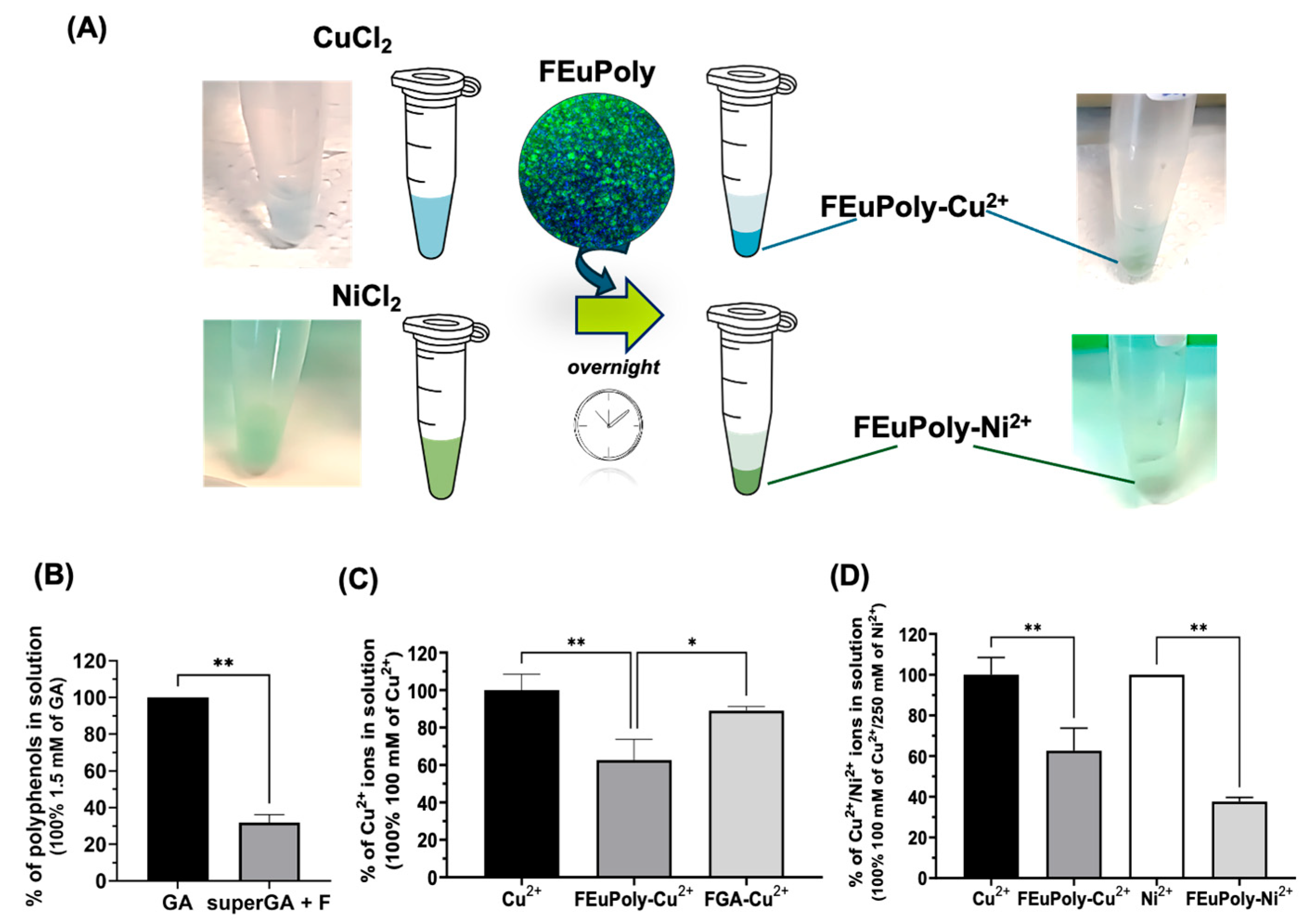
2.5. Metal Ion Trapping by FEuPoly
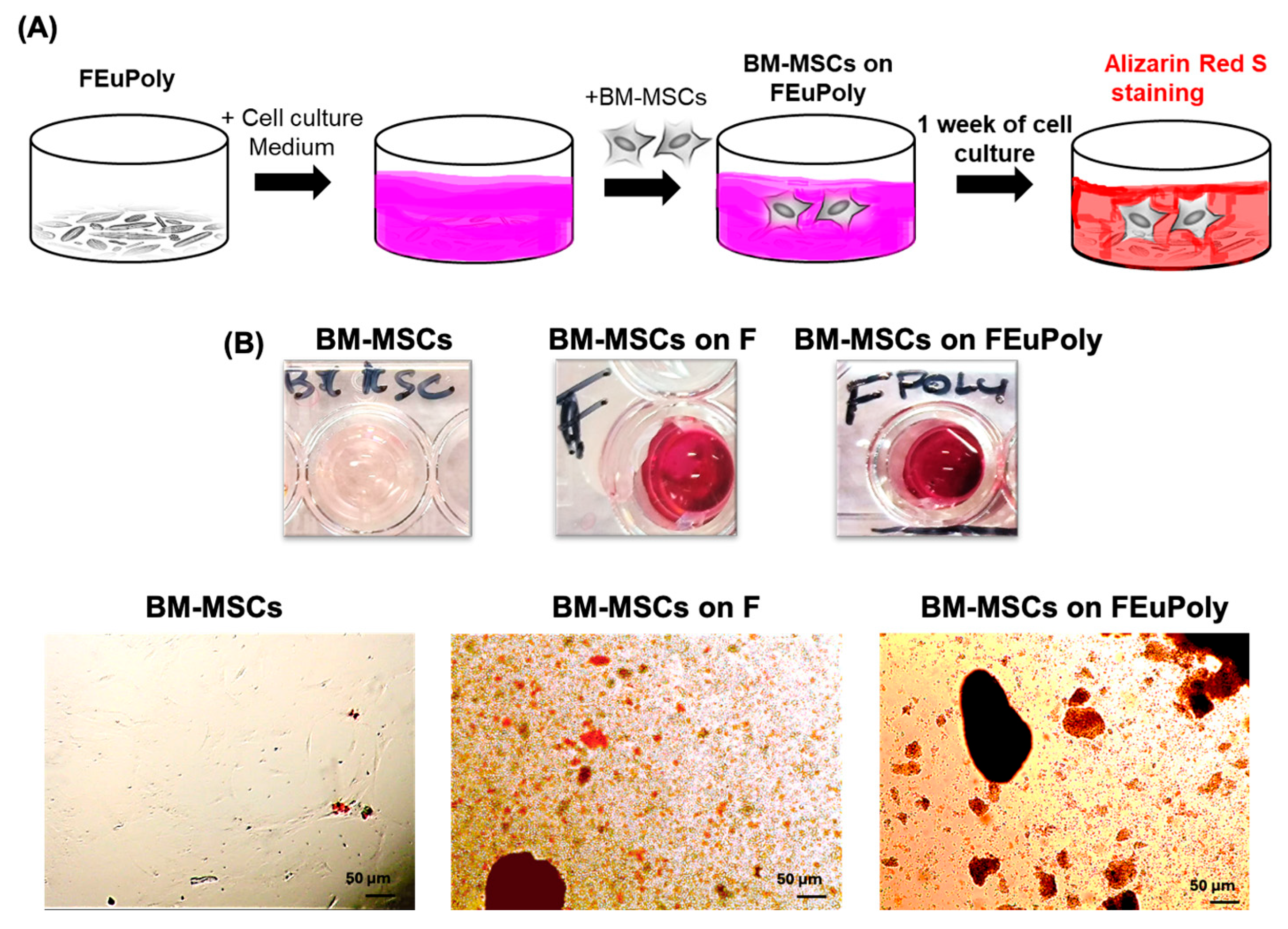
2.6. Evaluation of Stem Cell Scaffolding Properties of FEuPoly
3. Materials and Methods
3.1. Preparation of Dried Algal Biomass
3.2. Production of the E. cantabrica Extract (EuPoly) and Its Characterization by SPE, RP-HPLC and SDS-PAGE Analyses
3.3. Quantitative UV Spectrophotometric Analysis for Polyphenolic Content Determination
3.4. Cell Cultures and WST-1 Cell Viability Assay
3.5. Western Blotting Analysis of Protein Expression
3.6. Frustule Preparation, EuPoly Functionalization (FEuPoly), and Metal Ion Binding
3.7. Confocal Microscopy Analysis of FEuPoly
3.8. FEuPoly Validation for BM-MSC Growth and Alizarin Red S Staining
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline Phosphatase |
| BCA | Bicinchoninic acid |
| BM-MSCs | Bone marrow-mesenchymal stem cells |
| BMP | Bone morphogenetic protein |
| Cbfa1 | Core Binding Factor 1 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| ERK | Extracellular Signal-Regulated Kinase |
| EU | European Union |
| EuPoly | Euglena cantabrica extract |
| F | Frustules |
| FBS | Fetal Bovine Serum |
| FEuPoly | Frustules functionalized with Euglena cantabrica extract |
| GA | Gallic acid |
| GAE | Gallic acid equivalents |
| GelMA | Gelatin methacryloyl |
| HO-1 | Heme Oxygenase-1 |
| MAPKs | Mitogen-activated protein kinases |
| MSCs | Mesenchymal stem cells |
| NHDFs | Normal human dermal fibroblasts |
| NQO1 | NAD(P)H:quinone oxidoreductase 1 |
| Nrf2/ARE | Nuclear factor erythroid 2-related factor 2/ Antioxidant Response Element |
| Osx | Osterix |
| PAAM | Polyacrylamide |
| PBS | Phosphate Buffered Saline |
| PEG | Poly(ethylene glycol) |
| PFA | Paraformaldehyde |
| PI3K/Akt | Phosphatidylinositol 3-kinase/protein kinase B |
| PVA | Polyvinyl alcohol |
| PVDF | Polyvinylidene difluoride |
| ROS | Reactive oxygen species |
| RunX2 | RUNT-related transcription factor 2 |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TCA | Trichloroacetic acid |
| TFA | Trifluoroacetic acid |
| TGF-β | Transforming growth factor-beta |
| Wnt | Wingless-related integration site |
References
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Matin, M.; Koszarska, M.; Atanasov, A.G.; Król-Szmajda, K.; Jóźwik, A.; Stelmasiak, A.; Hejna, M. Bioactive Potential of Algae and Algae-Derived Compounds: Focus on Anti-Inflammatory, Antimicrobial, and Antioxidant Effects. Molecules 2024, 29, 4695. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of High Added-Value Compounds—A Brief Review of Recent Work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Soratur, A.; Kumar, S.; Venmathi Maran, B.A. A Review of Marine Algae as a Sustainable Source of Antiviral and Anticancer Compounds. Macromol 2025, 5, 11. [Google Scholar] [CrossRef]
- Antonacci, A.; Scognamiglio, V. Biotechnological Advances in the Design of Algae-Based Biosensors. Trends Biotechnol. 2020, 38, 334–347. [Google Scholar] [CrossRef]
- Allouzi, M.M.A.; Allouzi, S.; Al-Salaheen, B.; Khoo, K.S.; Rajendran, S.; Sankaran, R.; Sy-Toan, N.; Show, P.L. Current Advances and Future Trend of Nanotechnology as Microalgae-Based Biosensor. Biochem. Eng. J. 2022, 187, 108653. [Google Scholar] [CrossRef]
- Ciugulea, I.; Triemer, R.E. A Color Atlas of Photosynthetic Euglenoids; Michigan State University Press: East Lansing, MI, USA, 2010; ISBN 978-0-87013-879-9. [Google Scholar]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic Profile and Antioxidant Activity of Crude Extracts from Microalgae and Cyanobacteria Strains. J. Food Qual. 2017, 2017, 2924508. [Google Scholar] [CrossRef]
- Kapoor, S.; Singh, M.; Srivastava, A.; Chavali, M.; Chandrasekhar, K.; Verma, P. Extraction and Characterization of Microalgae-derived Phenolics for Pharmaceutical Applications: A Systematic Review. J. Basic Microbiol. 2022, 62, 1044–1063. [Google Scholar] [CrossRef]
- Muñóz-Almagro, N.; Gilbert-López, B.; Carmen, P.-R.M.; García-Fernandez, Y.; Almeida, C.; Villamiel, M.; Mendiola, J.A.; Ibáñez, E. Exploring the Microalga Euglena Cantabrica by Pressurized Liquid Extraction to Obtain Bioactive Compounds. Mar. Drugs 2020, 18, 308. [Google Scholar] [CrossRef]
- Cervantes-Garcia, D.; Troncoso-Rojas, R.; Sanchez-Estrada, A.; González-Mendoza, D.; Grimaldo-Juarez, O. Production of Phenolics and Flavonoids Compounds in Euglena Gracilis under Copper Stress. J. Pure Appl. Microbiol. 2013, 7, 93–100. [Google Scholar]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as Anti-Inflammatory Agents: Implications in Cancer and Cardiovascular Disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; De La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’aRchivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and Human Health: A Prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef]
- Zhang, R.; Ren, Y.; Ren, T.; Yu, Y.; Li, B.; Zhou, X. Marine-Derived Antioxidants: A Comprehensive Review of Their Therapeutic Potential in Oxidative Stress-Associated Diseases. Mar. Drugs 2025, 23, 223. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Gómez-Mejía, E.; Morante-Zarcero, S.; Rosales-Conrado, N.; Sierra, I. Analytical Strategies for Green Extraction, Characterization, and Bioactive Evaluation of Polyphenols, Tocopherols, Carotenoids, and Fatty Acids in Agri-Food Bio-Residues. Molecules 2025, 30, 1326. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, G.; Terenzi, C.; Medri, F.; Andrisano, V.; Montanari, S. Extraction and Analytical Methods for the Characterization of Polyphenols in Marine Microalgae: A Review. Mar. Drugs 2024, 22, 538. [Google Scholar] [CrossRef]
- Brun, P.; Piovan, A.; Caniato, R.; Dalla Costa, V.; Pauletto, A.; Filippini, R. Anti-Inflammatory Activities of Euglena Gracilis Extracts. Microorganisms 2021, 9, 2058. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Horio, Y.; Suzuki, K.; Isegawa, Y. Antiviral Activity and Underlying Action Mechanism of Euglena Extract against Influenza Virus. Nutrients 2021, 13, 3911. [Google Scholar] [CrossRef]
- Isegawa, Y. Activation of Immune and Antiviral Effects by Euglena Extracts: A Review. Foods 2023, 12, 4438. [Google Scholar] [CrossRef]
- Bedard, S.; Roxborough, E.; O’Neill, E.; Mangal, V. The Biomolecules of Euglena Gracilis: Harnessing Biology for Natural Solutions to Future Problems. Protist 2024, 175, 126044. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Z.; Du, M.; Chen, J.; Zhu, H.; Hu, Z.; Zhu, Y.; Wang, J. Euglena Gracilis and Its Aqueous Extract Constructed With Chitosan-Hyaluronic Acid Hydrogel Facilitate Cutaneous Wound Healing in Mice Without Inducing Excessive Inflammatory Response. Front. Bioeng. Biotechnol. 2021, 9, 713840. [Google Scholar] [CrossRef]
- Ko, Y.; Baek, H.; Hwang, J.-H.; Kim, Y.; Lim, K.-M.; Kim, J.; Kim, J.W. Nonanimal Euglena Gracilis-Derived Extracellular Vesicles Enhance Skin-Regenerative Wound Healing. Adv. Mater. Interfaces 2023, 10, 2202255. [Google Scholar] [CrossRef]
- Kesherwani, R.; Kumar, R.; Minhas, U.; Rizvi, S.I. Euglena Tuba Extract Provides Protection against Lipopolysaccharide-Induced Inflammatory Response and Oxidative Stress in Mice. Biologia 2021, 76, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Attri, D.C.; Sati, P.; Dhyani, P.; Szopa, A.; Sharifi-Rad, J.; Hano, C.; Calina, D.; Cho, W.C. Recent Updates on Anticancer Mechanisms of Polyphenols. Front. Cell Dev. Biol. 2022, 10, 1005910. [Google Scholar] [CrossRef]
- Sharma, R.; Mondal, A.S.; Trivedi, N. Anticancer Potential of Algae-Derived Metabolites: Recent Updates and Breakthroughs. Future J. Pharm. Sci. 2023, 9, 44. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The Role of Polyphenols in Overcoming Cancer Drug Resistance: A Comprehensive Review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell. Longev. 2016, 2016, 6475624. [Google Scholar] [CrossRef]
- Panja, S.; Ghate, N.B.; Mandal, N. A Microalga, Euglena Tuba Induces Apoptosis and Suppresses Metastasis in Human Lung and Breast Carcinoma Cells through ROS-Mediated Regulation of MAPKs. Cancer Cell Int. 2016, 16, 51. [Google Scholar] [CrossRef]
- Upreti, D.; Ishiguro, S.; Phillips, M.; Nakashima, A.; Suzuki, K.; Comer, J.; Tamura, M. Euglena Gracilis Extract Protects From Tobacco Smoke Carcinogen-Induced Lung Cancer by Altering Gut Microbiota Metabolome. Integr. Cancer Ther. 2023, 22, 15347354231195323. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Volume 3: Environmental Toxicology; Luch, A., Ed.; Springer Basel: Basel, Switzerland, 2012; pp. 133–164. ISBN 978-3-7643-8340-4. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Jasso-Chávez, R.; Campos-García, M.L.; Vega-Segura, A.; Pichardo-Ramos, G.; Silva-Flores, M.; Santiago-Martínez, M.G.; Feregrino-Mondragón, R.D.; Sánchez-Thomas, R.; García-Contreras, R.; Torres-Márquez, M.E. Microaerophilia Enhances Heavy Metal Biosorption and Internal Binding by Polyphosphates in Photosynthetic Euglena Gracilis. Algal Res. 2021, 58, 102384. [Google Scholar] [CrossRef]
- RodrÍguez-Zavala, J.S.; GarcÍa-GarcÍa, J.D.; Ortiz-Cruz, M.A.; Moreno-Sánchez, R. Molecular Mechanisms of Resistance to Heavy Metals in the Protist Euglena Gracilis. J. Environ. Sci. Health Part A 2007, 42, 1365–1378. [Google Scholar] [CrossRef]
- Khatiwada, B.; Hasan, M.T.; Sun, A.; Kamath, K.S.; Mirzaei, M.; Sunna, A.; Nevalainen, H. Proteomic Response of Euglena Gracilis to Heavy Metal Exposure—Identification of Key Proteins Involved in Heavy Metal Tolerance and Accumulation. Algal Res. 2020, 45, 101764. [Google Scholar] [CrossRef]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel Allergy and Allergic Contact Dermatitis: A Clinical Review of Immunology, Epidemiology, Exposure, and Treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef]
- Sailer, J.; Nagel, J.; Akdogan, B.; Jauch, A.T.; Engler, J.; Knolle, P.A.; Zischka, H. Deadly Excess Copper. Redox Biol. 2024, 75, 103256. [Google Scholar] [CrossRef]
- Mizzi, L.; Chatzitzika, C.; Gatt, R.; Valdramidis, V. HPLC Analysis of Phenolic Compounds and Flavonoids with Overlapping Peaks. Food Technol. Biotechnol. 2020, 58, 12–19. [Google Scholar] [CrossRef]
- Buonvino, S.; Ciocci, M.; Nanni, F.; Cacciotti, I.; Melino, S. New Vegetable-Waste Biomaterials by Lupin albus L. as Cellular Scaffolds for Applications in Biomedicine and Food. Biomaterials 2023, 293, 121984. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Zenjari, L.; Hali, F.; Chiheb, S. Trichloroacetic Acid (50%) in the Treatment of Venous Leg Ulcers. JMV J. Médecine Vasc. 2021, 46, 139–143. [Google Scholar] [CrossRef]
- Sitohang, I.B.S.; Legiawati, L.; Suseno, L.S.; Safira, F.D. Trichloroacetic Acid Peeling for Treating Photoaging: A Systematic Review. Dermatol. Res. Pract. 2021, 2021, 3085670. [Google Scholar] [CrossRef]
- Yu, Z.; Hong, Y.; Xie, K.; Fan, Q. Research Progresses on the Physiological and Pharmacological Benefits of Microalgae-Derived Biomolecules. Foods 2022, 11, 2806. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Cotas, J.; Valado, A. Antioxidants from Microalgae and Their Potential Impact on Human Well-Being. Explor. Drug Sci. 2024, 2, 292–321. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. 1—Overview of Polyphenols and Their Properties. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 3–44. ISBN 978-0-12-813572-3. [Google Scholar]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Ambrosino, L.; Albini, A.; Noonan, D.M.; Sansone, C.; and Brunet, C. Insights into Phenolic Compounds from Microalgae: Structural Variety and Complex Beneficial Activities from Health to Nutraceutics. Crit. Rev. Biotechnol. 2021, 41, 155–171. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Ghate, N.B.; Deb, S.; Panja, S.; Sarkar, R.; Rout, J.; Mandal, N. Assessment of the Phytochemical Constituents and Antioxidant Activity of a Bloom Forming Microalgae Euglena Tuba. Biol. Res. 2014, 47, 24. [Google Scholar] [CrossRef]
- Nisar, M.F.; Li, M.; Xu, J.; Wan, C. Anti-Diabetic Effects of Marine Natural Products through Redox Modulation via Nrf2/HO-1 Cytoprotective Pathways. Front. Mar. Sci. 2024, 11, 1438955. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Cao, X.; Chen, X.; Zou, T.; You, J. Plant-Derived Polyphenols as Nrf2 Activators to Counteract Oxidative Stress and Intestinal Toxicity Induced by Deoxynivalenol in Swine: An Emerging Research Direction. Antioxidants 2022, 11, 2379. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting Antioxidants for Cancer Therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Frazzini, S.; Rossi, L. Anticancer Properties of Macroalgae: A Comprehensive Review. Mar. Drugs 2025, 23, 70. [Google Scholar] [CrossRef]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef]
- Buonvino, S.; Arciero, I.; Martinelli, E.; Seliktar, D.; Melino, S. Modelling the Disease: H2S-Sensitivity and Drug-Resistance of Triple Negative Breast Cancer Cells Can Be Modulated by Embedding in Isotropic Micro-Environment. Mater. Today Bio 2023, 23, 100862. [Google Scholar] [CrossRef]
- Buonvino, S.; Di Giuseppe, D.; Filippi, J.; Martinelli, E.; Seliktar, D.; Melino, S. 3D Cell Migration Chip (3DCM-Chip): A New Tool toward the Modeling of 3D Cellular Complex Systems. Adv. Healthc. Mater. 2024, 13, 2400040. [Google Scholar] [CrossRef] [PubMed]
- Arciero, I.; Buonvino, S.; Palumbo, V.; Scimeca, M.; Melino, S. A 3D-Printable Cell Array for In Vitro Breast Cancer Modeling. Int. J. Mol. Sci. 2024, 25, 13068. [Google Scholar] [CrossRef]
- Gupta, S.P.; Siddiqi, N.J.; Khan, H.A.; Alrokayan, S.H.; Alhomida, A.S.; Singh, R.K.; Verma, P.K.; Kumar, S.; Acharya, A.; Sharma, B. Phytochemical Profiling of Microalgae Euglena Tuba and Its Anticancer Activity in Dalton’s Lymphoma Cells. FBL 2022, 27, 120. [Google Scholar] [CrossRef]
- Das, B.; Pradhan, J.; Mahammad, S.; Nayak, K.K. In Vitro Cytotoxic and Antibacterial Activity of Ethanolic Extract of Euglena Viridis. Int. J. Pharma. Bio Sci. 2012, 3, 321–331. [Google Scholar]
- Uthappa, U.T.; Brahmkhatri, V.; Sriram, G.; Jung, H.-Y.; Yu, J.; Kurkuri, N.; Aminabhavi, T.M.; Altalhi, T.; Neelgund, G.M.; Kurkuri, M.D. Nature Engineered Diatom Biosilica as Drug Delivery Systems. J. Control. Release 2018, 281, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Khatami, M.; Jamalipour Soufi, G.; Fatahi, Y.; Iravani, S.; Varma, R.S. Diatoms with Invaluable Applications in Nanotechnology, Biotechnology, and Biomedicine: Recent Advances. ACS Biomater. Sci. Eng. 2021, 7, 3053–3068. [Google Scholar] [CrossRef]
- Bhat, K.; Ajees, M.A.; Kumar, P.; Vibha, B.V.G.; Nayak, R.; Mazumder, N. Diatoms: Harnessing Nature’s Microscopic Marvels for Biosensing and Multifaceted Applications. Biophys. Rev. 2025, 17, 103–125. [Google Scholar] [CrossRef]
- Fuhrmann, T.; Landwehr, S.; El Rharbi-Kucki, M.; Sumper, M. Diatoms as Living Photonic Crystals. Appl. Phys. B 2004, 78, 257–260. [Google Scholar] [CrossRef]
- De Tommasi, E.; Rea, I.; Mocella, V.; Moretti, L.; Stefano, M.D.; Rendina, I.; Stefano, L.D. Multi-Wavelength Study of Light Transmitted through a Single Marine Centric Diatom. Opt. Express 2010, 18, 12203–12212. [Google Scholar] [CrossRef]
- Anouar, E.H.; Gierschner, J.; Duroux, J.-L.; Trouillas, P. UV/Visible Spectra of Natural Polyphenols: A Time-Dependent Density Functional Theory Study. Food Chem. 2012, 131, 79–89. [Google Scholar] [CrossRef]
- Terracciano, M.; De Stefano, L.; Rea, I. Diatoms Green Nanotechnology for Biosilica-Based Drug Delivery Systems. Pharmaceutics 2018, 10, 242. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Loya, M.; Ghosh, S.; Atta, A.K. A Review on Dual Detection of Cu2+ and Ni2+ Ions by Using Single Fluorometric and Colorimetric Organic Molecular Probes. J. Mol. Struct. 2023, 1278, 134949. [Google Scholar] [CrossRef]
- Chen, Z.; Świsłocka, R.; Choińska, R.; Marszałek, K.; Dąbrowska, A.; Lewandowski, W.; Lewandowska, H. Exploring the Correlation Between the Molecular Structure and Biological Activities of Metal–Phenolic Compound Complexes: Research and Description of the Role of Metal Ions in Improving the Antioxidant Activities of Phenolic Compounds. Int. J. Mol. Sci. 2024, 25, 11775. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/water-safety-and-quality/drinking-water-quality-guidelines (accessed on 24 May 2025).
- Xu, L.Q.; Neoh, K.-G.; Kang, E.-T. Natural Polyphenols as Versatile Platforms for Material Engineering and Surface Functionalization. Prog. Polym. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Cao, H.; Yang, L.; Tian, R.; Wu, H.; Gu, Z.; Li, Y. Versatile Polyphenolic Platforms in Regulating Cell Biology. Chem. Soc. Rev. 2022, 51, 4175–4198. [Google Scholar] [CrossRef]
- Cheng, W.; Wen, J. Now and Future: Development and Perspectives of Using Polyphenol Nanomaterials in Environmental Pollution Control. Coord. Chem. Rev. 2022, 473, 214825. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, H.; Richardson, J.J.; Xu, W.; Chen, J.; Zhou, J.; Caruso, F. Metal–Phenolic Network Composites: From Fundamentals to Applications. Chem. Soc. Rev. 2024, 53, 10800–10826. [Google Scholar] [CrossRef]
- Gerosa, C.; Fanni, D.; Congiu, T.; Piras, M.; Cau, F.; Moi, M.; Faa, G. Liver Pathology in Wilson’s Disease: From Copper Overload to Cirrhosis. J. Inorg. Biochem. 2019, 193, 106–111. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy Metals: Toxicity and Human Health Effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Yang, P.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Polyphenol Scaffolds in Tissue Engineering. Mater. Horiz. 2021, 8, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Zulkefli, N.; Che Zahari, C.N.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhang, M.; Han, F.; Liao, W.; Duan, X. Natural Polyphenols for Drug Delivery and Tissue Engineering Construction: A Review. Eur. J. Med. Chem. 2024, 266, 116141. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-B.; Kim, J.-K.; Lee, G.; Kim, D.-H. Mechanical Properties of Materials for Stem Cell Differentiation. Adv. Biosyst. 2020, 4, 2000247. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ruan, M.; Bu, Q.; Zhao, C. Signaling Pathways Driving MSC Osteogenesis: Mechanisms, Regulation, and Translational Applications. Int. J. Mol. Sci. 2025, 26, 1311. [Google Scholar] [CrossRef]
- Han, Y.; Du, L.; Wu, J.; Zhang, H.; Yang, G.; Zheng, Y.; Wu, C. Diatomaceous Cross-Species Constructs for Tendon-to-Bone Regeneration. Mat. Today 2025, 83, 64–84. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural Products for Treatment of Osteoporosis: The Effects and Mechanisms on Promoting Osteoblast-Mediated Bone Formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, B.; Zheng, G.; Du, S.; Li, X. Quercetin Stimulates Osteogenic Differentiation of Bone Marrow Stromal Cells through miRNA-206/Connexin 43 Pathway. Am. J. Transl. Res. 2020, 12, 2062. [Google Scholar] [PubMed]
- Zhang, J.; Liu, Z.; Luo, Y.; Li, X.; Huang, G.; Chen, H.; Li, A.; Qin, S. The Role of Flavonoids in the Osteogenic Differentiation of Mesenchymal Stem Cells. Front. Pharmacol. 2022, 13, 2022. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, J.S.; Lee, M.S.; An, S.; Yang, K.; Lee, K.; Yang, H.S.; Lee, H.; Cho, S.-W. Plant Flavonoid-Mediated Multifunctional Surface Modification Chemistry: Catechin Coating for Enhanced Osteogenesis of Human Stem Cells. Chem. Mater. 2017, 29, 4375–4384. [Google Scholar] [CrossRef]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of Polyphenols Using Liquid Chromatography–Tandem Mass Spectrometry Technique (LC–MS/MS): A Review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Koyanagi, M.; Kawakabe, S.; Arimura, Y. A Comparative Study of Colorimetric Cell Proliferation Assays in Immune Cells. Cytotechnology 2016, 68, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, R.; Melino, S.; Prosposito, P.; Casalboni, M.; Lamastra, F.R.; Nanni, F.; Bruno, L.; Congestri, R. The Diatom Staurosirella Pinnata for Photoactive Material Production. PLoS ONE 2016, 11, e0165571. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonvino, S.; Trinca, C.; Leu, S.; Licoccia, S.; Melino, S. A New Algal Friendly Extract from Euglena cantabrica with Potential Applications in Biomedical Field. Mar. Drugs 2025, 23, 453. https://doi.org/10.3390/md23120453
Buonvino S, Trinca C, Leu S, Licoccia S, Melino S. A New Algal Friendly Extract from Euglena cantabrica with Potential Applications in Biomedical Field. Marine Drugs. 2025; 23(12):453. https://doi.org/10.3390/md23120453
Chicago/Turabian StyleBuonvino, Silvia, Carolina Trinca, Stefan Leu, Silvia Licoccia, and Sonia Melino. 2025. "A New Algal Friendly Extract from Euglena cantabrica with Potential Applications in Biomedical Field" Marine Drugs 23, no. 12: 453. https://doi.org/10.3390/md23120453
APA StyleBuonvino, S., Trinca, C., Leu, S., Licoccia, S., & Melino, S. (2025). A New Algal Friendly Extract from Euglena cantabrica with Potential Applications in Biomedical Field. Marine Drugs, 23(12), 453. https://doi.org/10.3390/md23120453










