Native Collagen and Total Lipid Extract Obtained from Caranx hyppos By-Products: Characterization for Potential Use in the Biomedical and Nutraceutical Fields
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of Caranx hippos By-Products
2.2. Collagen Extraction Yield
2.3. Chemical Characterization of Collagen
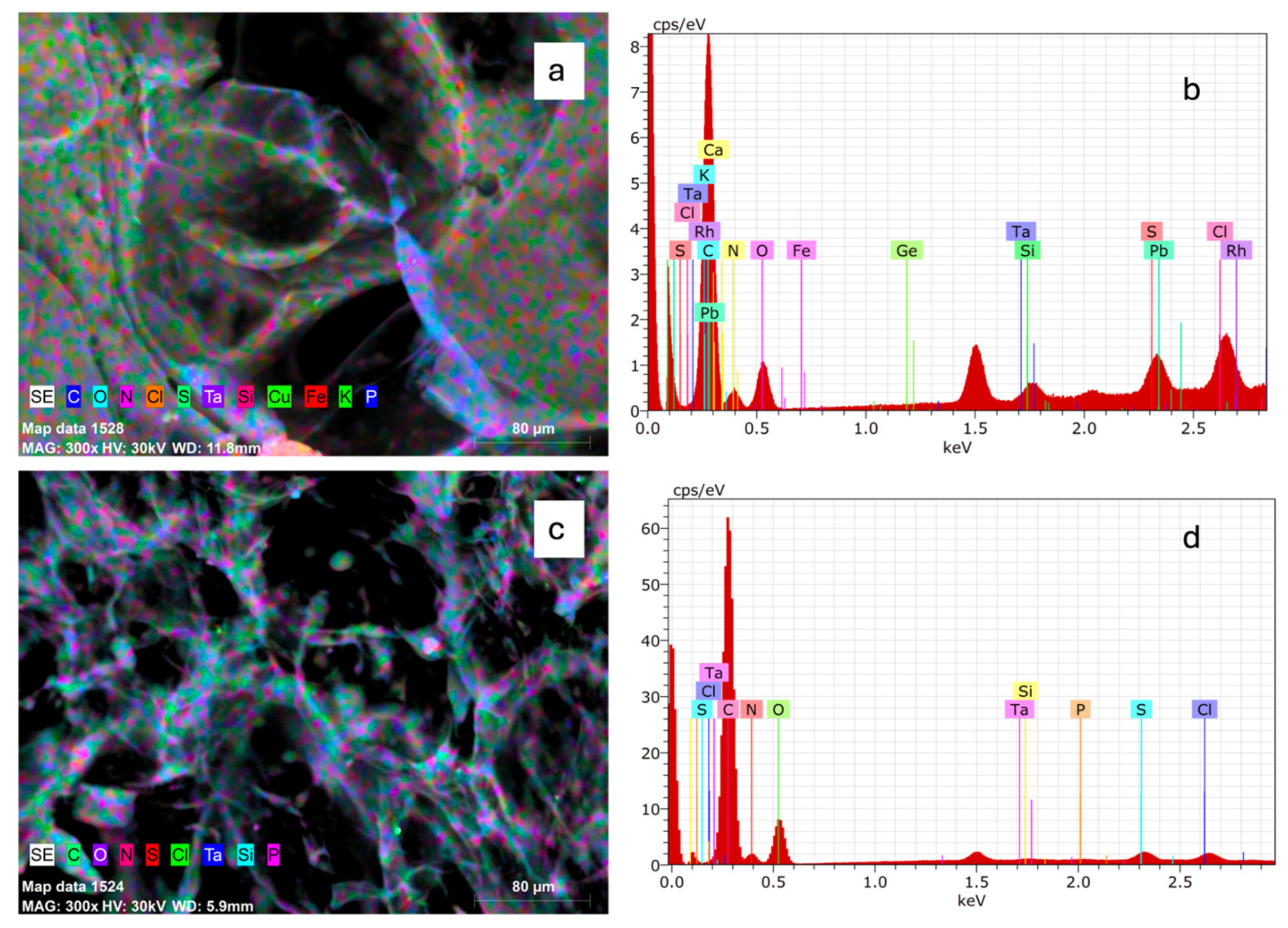
2.4. Element Composition Analysis of Collagen
2.5. Physicochemical Characterization of Collagen
2.5.1. Color of Collagen
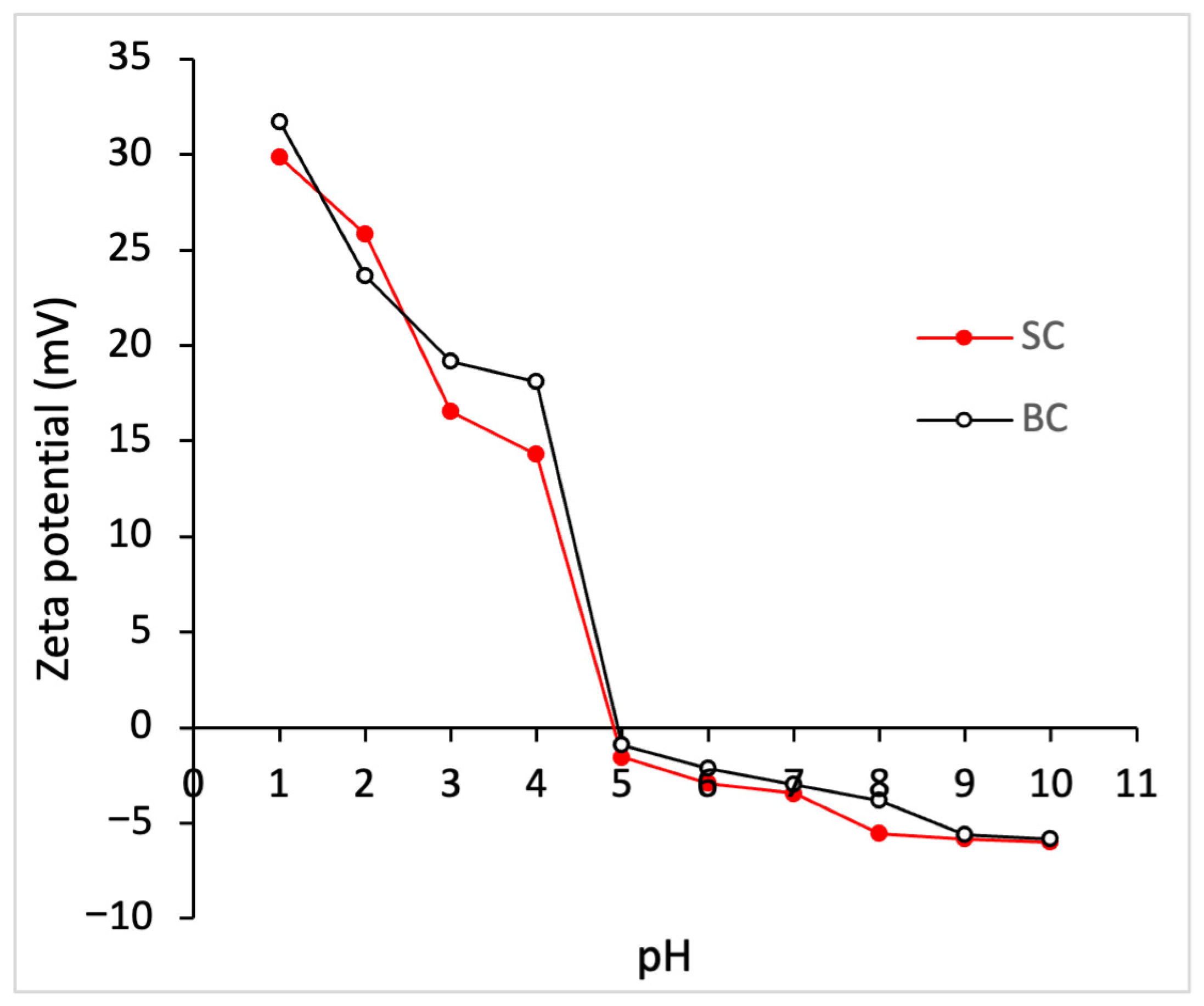
2.5.2. Zeta Potential (ζ) of Collagen
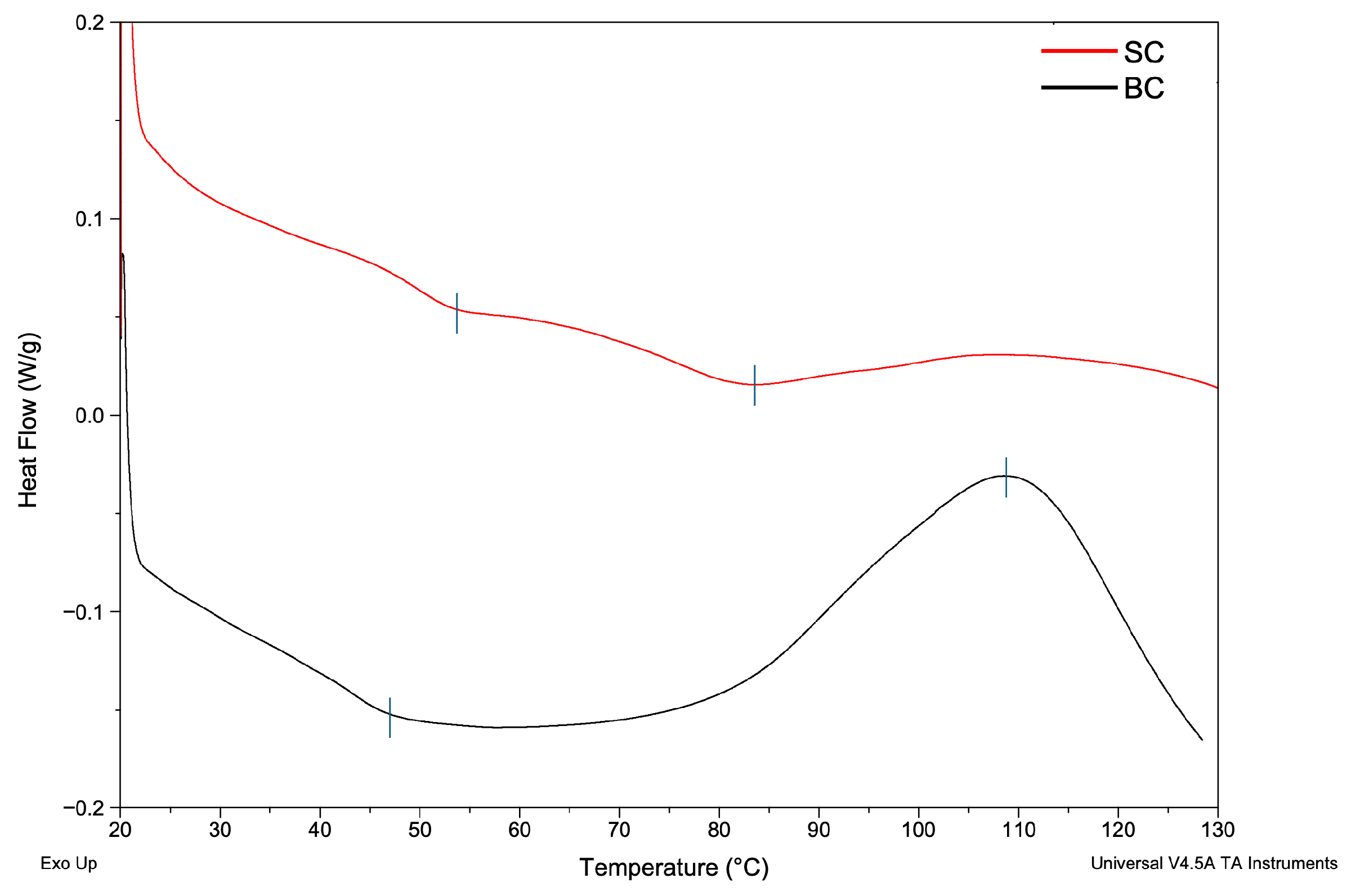
2.5.3. Differential Scanning Calorimetry (DSC) of Collagen
2.6. Structural Characterization of Collagen
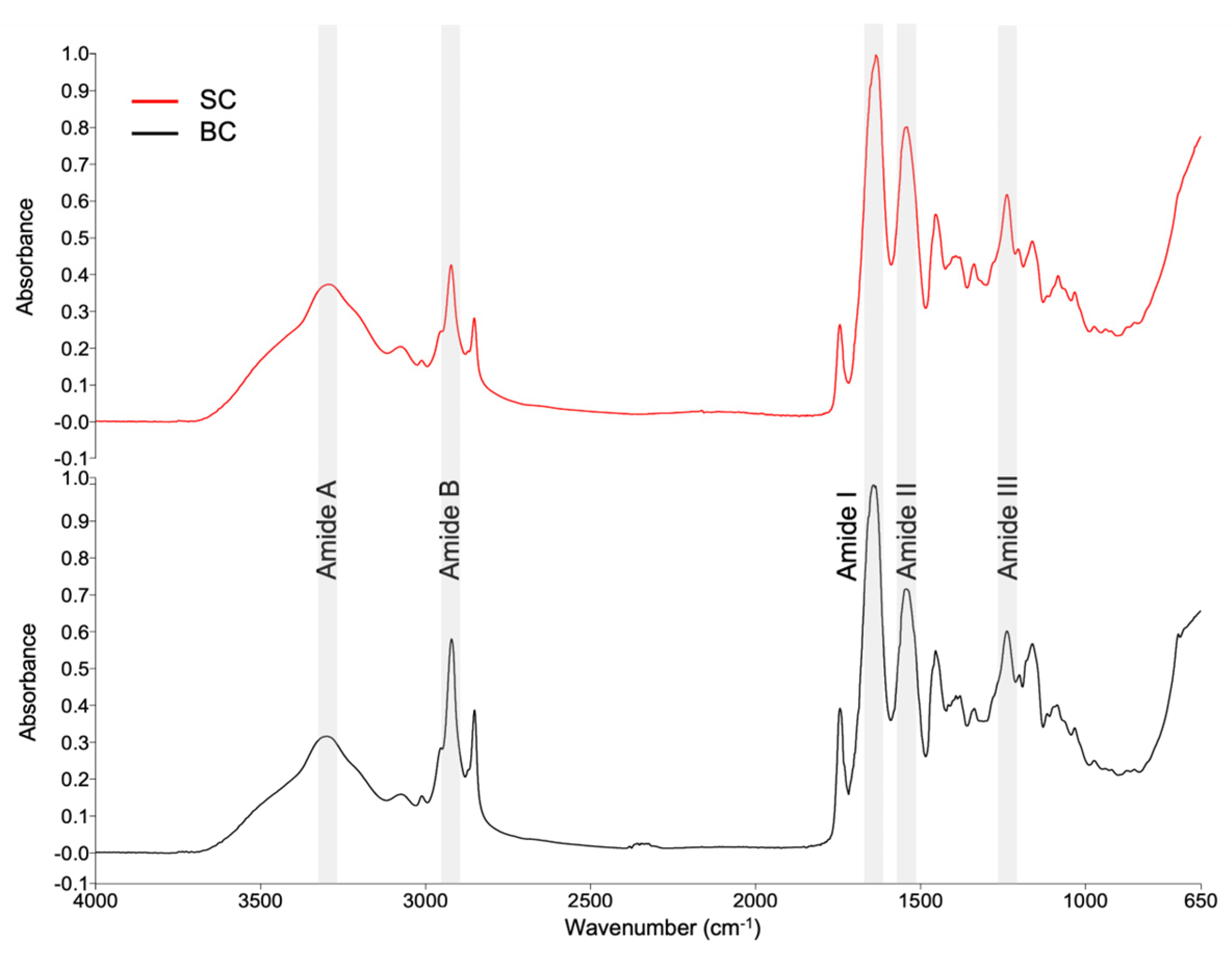
2.6.1. Fourier Transform Infrared (FTIR) Spectra of Collagen
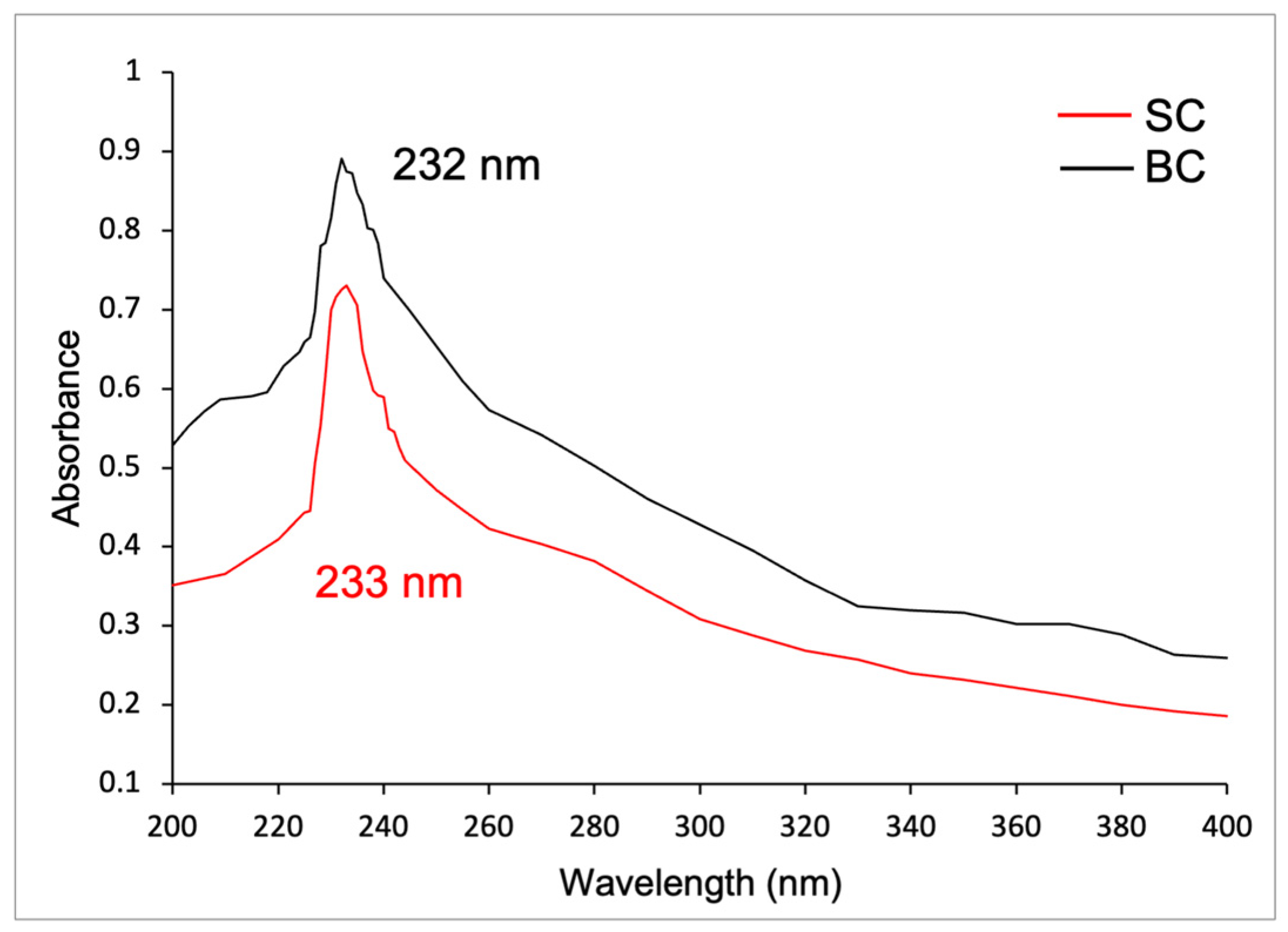
2.6.2. UV Spectra of Collagen
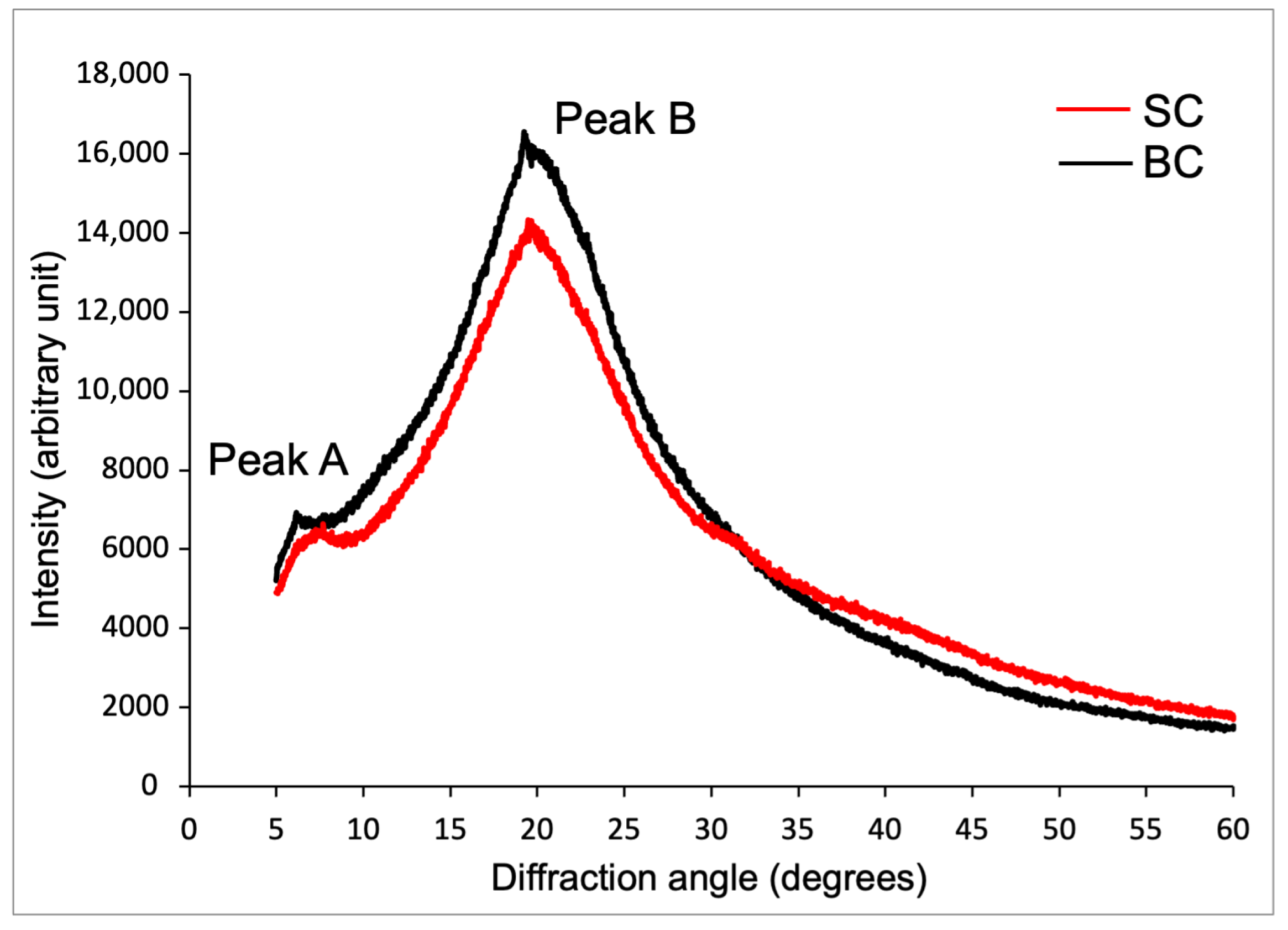
2.6.3. X-Ray Diffraction (XRD) Analysis of Collagen
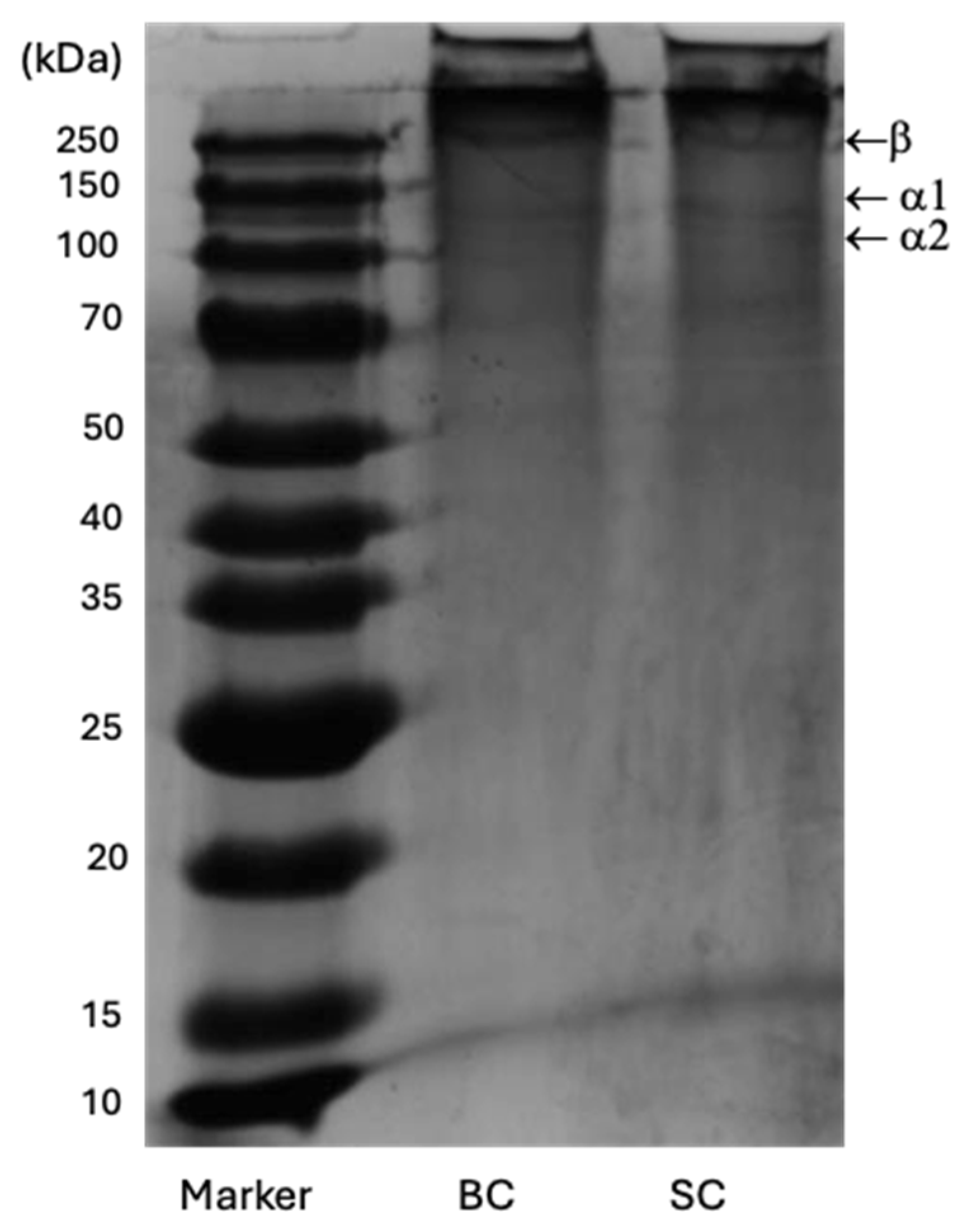
2.6.4. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) of Collagen
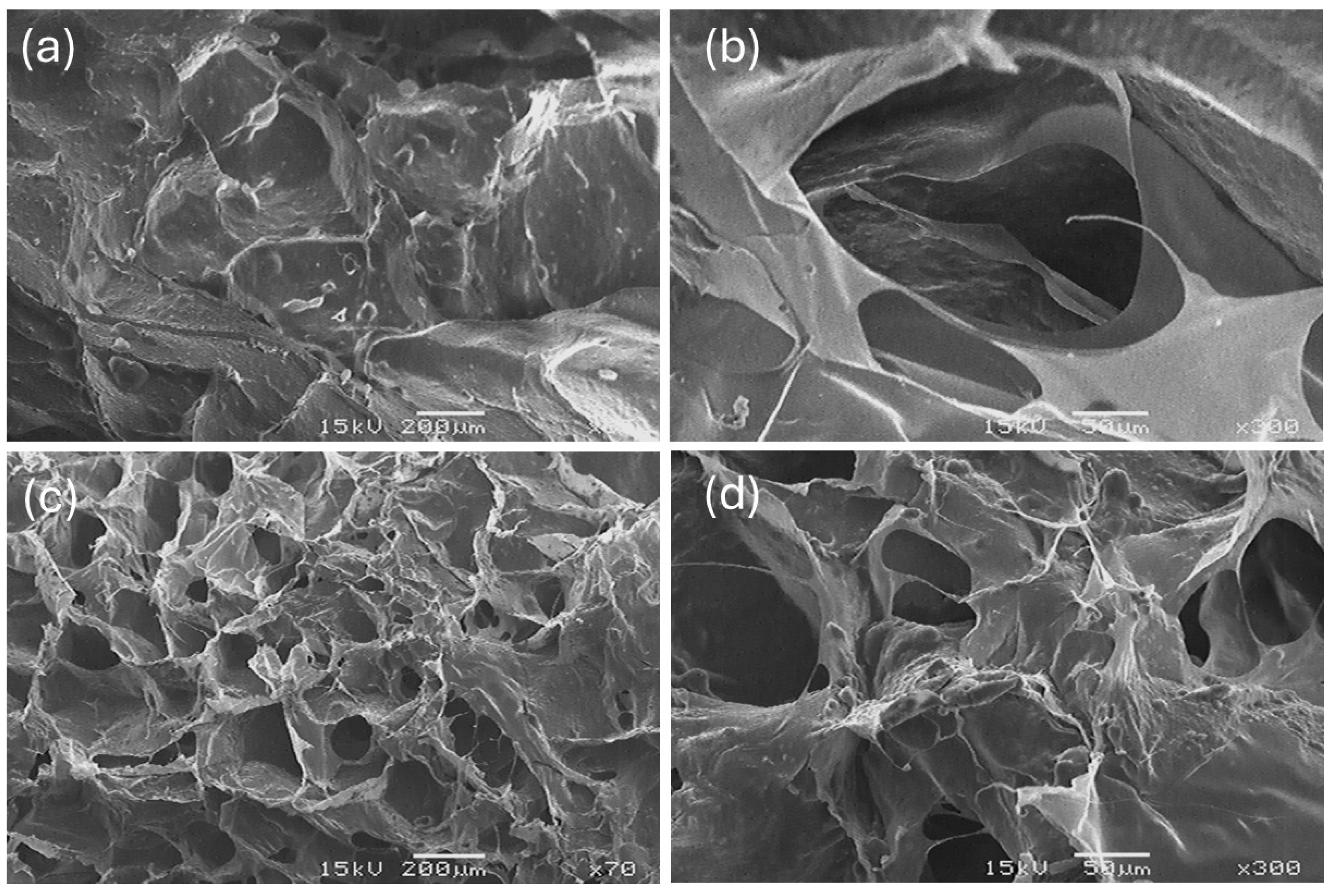
2.7. Environmental Scanning Electron Microscopy (ESEM) of Collagen
2.8. Total Lipid Extraction Yield
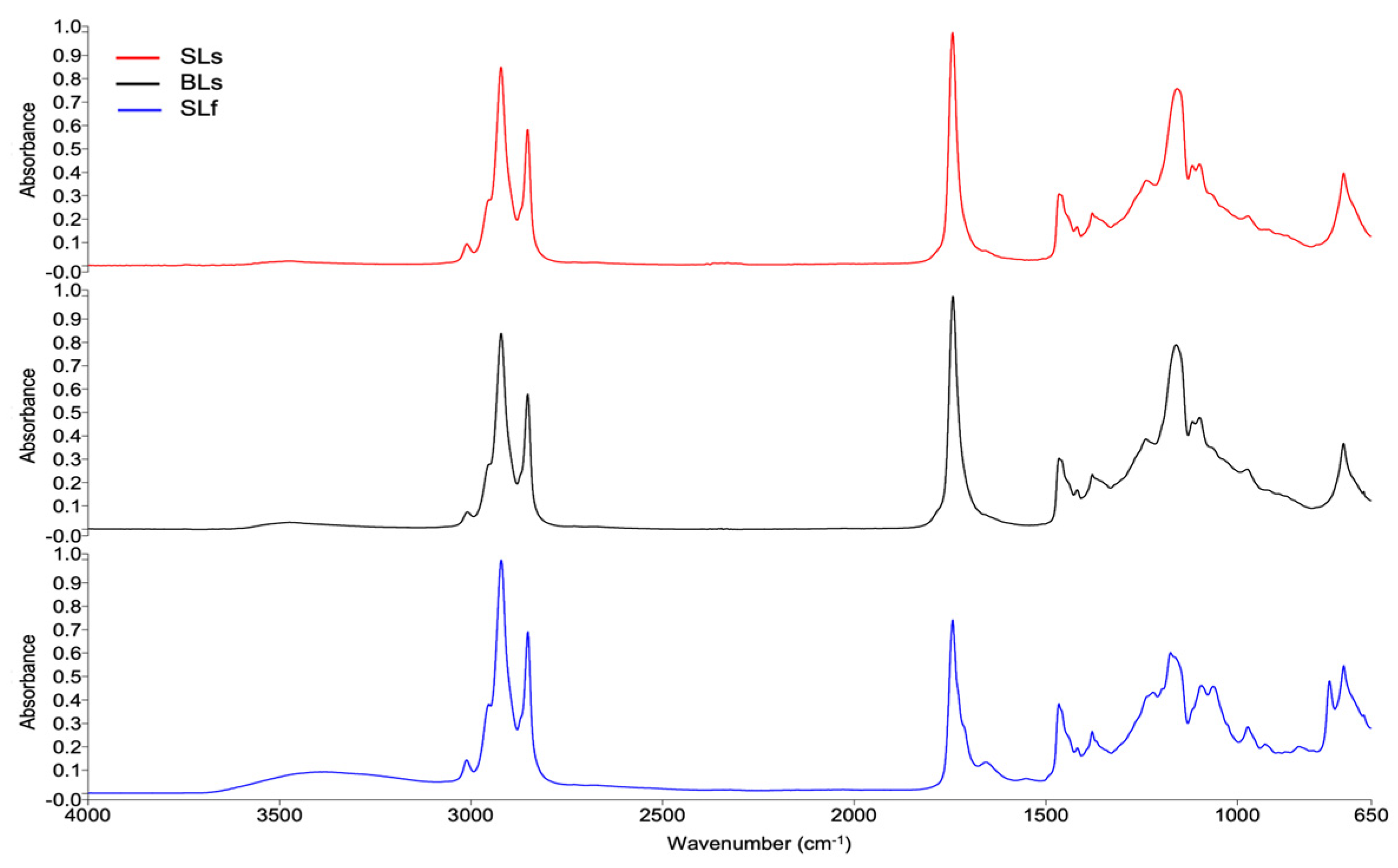
2.9. Fourier Transform Infrared (FTIR) Spectra of Total Lipid Extracts
2.10. Total Lipid Fatty Acids
2.11. Antioxidant Capacity of Total Lipid Extract
3. Discussion
3.1. Chemical Composition of Caranx hippos By-Products
3.2. Yield and Chemical Characterization of Collagen
3.3. Physicochemical Characterization of Collagen
3.4. Structural Characterization of Collagen
3.5. Yield and Spectral Characterization of Total Lipid Extracts
3.6. Total Lipid Fatty Acids
3.7. Antioxidant Capacity of Total Lipid Extracts
4. Materials and Methods
4.1. Raw Material
4.2. Chemical Reagents
4.3. Chemical Composition
4.4. Collagen Extraction
4.5. Physicochemical Characterization of Collagen
4.5.1. Color Analysis
4.5.2. Zeta Potential
4.5.3. Differential Scanning Calorimetry (DSC)—Thermal Analysis
4.6. Structural Characterization of Collagen
4.6.1. FTIR Spectra Acquisition
4.6.2. UV Spectra Acquisition
4.6.3. X-Ray Diffraction (XRD)
4.6.4. Electron Microscopy-Energy-Dispersive X-Ray Spectroscopy (SEM-EDX)
4.6.5. Structural Characterization by Gel Electrophoresis (SDS-PAGE)
4.7. Morphological Analysis of Collagen
Environmental Scanning Electron Microscopy (ESEM)
4.8. Lipid Extraction
4.9. Fatty Acid Methyl Ester Determination of Total Lipid Extracts
4.10. Antioxidant Capacity Determination of Total Lipid Extract
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024. Blue Transformation in Action; Food and Agriculture Organization (FAO): Rome, Italy, 2024; p. 75. [Google Scholar]
- Bachis, E. By-Product; IFFO, The Marine Ingredients Organization: London, UK, April 2024; Available online: https://www.iffo.com/by-product (accessed on 13 September 2025).
- United Nations (UN). 17 Goals to Transform Our World; United Nations (UN): New York, NY, USA, 2025; Available online: https://www.un.org/sustainabledevelopment/ (accessed on 1 September 2025).
- Rotter, A.; Barbier, M.; Bertoni, F.; Bones, A.M.; Cancela, M.L.; Carlsson, J.; Carvalho, M.F.; Cegłowska, M.; Chirivella-Martorell, J.; Conk-Dalay, M.; et al. The essentials of marine biotechnology. Front. Mar. Sci. 2021, 8, 158. [Google Scholar] [CrossRef]
- Asharaf, F.; Rajasree S.R., R.; Rajan, R. Bioconversion of Eel Skin Waste into Valuable Collagen: Isolation, Spectral Characterization, and Biocompatibility Assessment. Waste Biomass Valorization 2024, 15, 4773–4783. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical applications of collagen and collagen-based materials. Curr. Med. Chem. 2019, 26, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef]
- Lozano Teruel, J.A. Bioquímica y Biología Molecular Para Ciencias de la Salud, 3rd ed.; McGraw-Hill Interamericana: Madrid, Spain, 2005; 804p. [Google Scholar]
- Lu, W.C.; Chiu, C.S.; Chan, Y.J.; Mulio, A.T.; Li, P.H. Characterization and biological properties of marine by-product collagen through ultrasound-assisted extraction. Aquac. Rep. 2023, 29, 101514. [Google Scholar] [CrossRef]
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen derived from fish industry waste: Progresses and challenges. Polymers 2023, 15, 544. [Google Scholar] [CrossRef]
- Prajaputra, V.; Isnaini, N.; Maryam, S.; Ernawati, E.; Deliana, F.; Haridhi, H.A.; Fadli, N.; Karina, S.; Agustina, S.; Nurfadillah, N.; et al. Exploring marine collagen: Sustainable sourcing, extraction methods, and cosmetic applications. S. Afr. J. Chem. Eng. 2024, 47, 197–211. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Characterization of Acid- and Pepsin-Soluble Collagen Extracted from the Skin of Purple-Spotted Bigeye Snapper. Gels 2022, 8, 665. [Google Scholar] [CrossRef]
- MarkNtel Advisors. Global Marine Collagen Market Research Report: Forecast (2024–2030); MarkNtel Advisors: Noida, Uttar Pradesh, India, 2025; Available online: https://www.marknteladvisors.com/research-library/marine-collagen-market.html#:~:text=The%20Global%20Marine%20Collagen%20Market,as%20a%20rich%20protein%20source (accessed on 21 August 2025).
- Alfio, V.G.; Manzo, C.; Micillo, R. From fish waste to value: An overview of the sustainable recovery of omega-3 for food supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef]
- Mgbechidinma, C.L.; Zheng, G.; Baguya, E.B.; Zhou, H.; Okon, S.U.; Zhang, C. Fatty acid composition and nutritional analysis of waste crude fish oil obtained by optimized milder extraction methods. Environ. Eng. Res. 2023, 28, 220034. [Google Scholar] [CrossRef]
- Swetha, N.; Mathanghi, S.K. Towards sustainable omega-3 fatty acids production—A comprehensive review on extraction methods, oxidative stability and bio-availability enhancement. Food Chem. Adv. 2024, 4, 100603. [Google Scholar] [CrossRef]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as sources of omega-3 polyunsaturated fatty acids: Biotechnological aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Grand View Research. Omega 3 Supplements Market Size Report: Forecast (2025–2030); Grand View Research: San Francisco, CA, USA, 2025; Available online: https://www.grandviewresearch.com/industry-analysis/omega-3-supplement-market#:~:text=The%20global%20omega%203%20supplements,%2C%20and%20anti%2Dinflammatory%20properties (accessed on 21 August 2025).
- Smith-Vaniz, W.F.; Carpenter, K.E. Review of the crevalle jacks, Caranx hippos complex (Teleostei: Carangidae), with a description of a new species from West Africa. Fish. Bull. 2007, 105, 207–233. Available online: https://digitalcommons.odu.edu/biology_fac_pubs/68 (accessed on 17 October 2025).
- Pacheco, C.; Cusba, J.; Bustamante, C. Fishery biology of the Crevalle jack Caranx hippos (L) from the Colombian Caribbean. Fish. Res. 2025, 285, 107334. [Google Scholar] [CrossRef]
- PROFECO, Procuraduría Federal del Consumidor (2024, marzo). De Temporada. Pescados y Mariscos [Seasonal. Fish and seafood]. Revista del Consumidor. Available online: https://www.profeco.gob.mx/revista/RevistaDelConsumidor_565_MARZO_2024.pdf (accessed on 1 September 2025).
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Valorisation of fish by-products against waste management treatments—Comparison of environmental impacts. Waste Manag. 2015, 46, 103–112. [Google Scholar] [CrossRef]
- Islam, J.; Mis Solval, K.E. Recent Advancements in Marine Collagen: Exploring New Sources, Processing Approaches, and Nutritional Applications. Mar. Drugs 2025, 23, 190. [Google Scholar] [CrossRef] [PubMed]
- Alcolea Ersinger, V.F.; Lamas, D.; Massa, Á. A review of marine collagens: Approaches on extractions, applications, market, and future trends. Environ. Sci. Pollut. Res. 2025, 32, 16077–16097. [Google Scholar] [CrossRef]
- Mamat, M.N.I.B.; Abdul Rahman, H.; Mohd Razali, N.S.; Syed Hussain, S.S.; Kasim, K.F.; Sofian-Seng, N.S. A Review on Fish Oil Extraction from Fish by-Product as Sustainable Practices and Resource Utilization in the Fish Processing Industry. Sains Malays. 2025, 54, 165–174. [Google Scholar] [CrossRef]
- Kalkan, E.; Keskin Çavdar, H.; Maskan, M. Health impacts and innovative extraction methods of fish oil: A review. Eur. J. Lipid Sci. Technol. 2025, 127, e20240017. [Google Scholar] [CrossRef]
- Martins, E.; Fernandes, R.; Alves, A.L.; Sousa, R.O.; Reis, R.L.; Silva, T.H. Skin byproducts of Reinhardtius hippoglossoides (Greenland Halibut) as ecosustainable source of marine collagen. Appl. Sci. 2022, 12, 11282. [Google Scholar] [CrossRef]
- Seixas, M.J.; Martins, E.; Reis, R.L.; Silva, T.H. Extraction and characterization of collagen from elasmobranch byproducts for potential biomaterial use. Mar. Drugs 2020, 18, 617. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK, 2006; 347p. [Google Scholar]
- Johny, L.C.; Vijaykumar, M.; Kudre, T.G.; Surech, P.V. Malabar sole (Cynoglossus macrostomus) skin as promising source of type I acid and pepsin solubilized collagens with potential bioactivity. J. Food Sci. Technol. 2022, 59, 157–167. [Google Scholar] [CrossRef]
- Wei, P.; Zheng, H.; Shi, Z.; Li, D.; Xiang, Y. Isolation and characterization of acid-soluble collagen and pepsin-soluble collagen from the skin of hybrid sturgeon. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2019, 34, 950–959. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, J.; Zhang, Y.; Zhang, H.; Chang, S.K.; Hong, H.; Luo, Y.; Tan, Y. Silver carp swim bladder collagen derived from deep eutectic solvents: Enhanced solubility against pH and NaCl stresses. Int. J. Biol. Macromol. 2024, 281, 136315. [Google Scholar] [CrossRef]
- Matarsim, N.N.; Jaziri, A.A.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Type I collagen from the skin of Barracuda (Sphyraena sp.) prepared with different organic acids: Biochemical, microstructural and functional properties. J. Funct. Biomater. 2023, 14, 87. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Qiao, Y.; Tian, Y.; Liu, J.; Qin, S.; Wu, W. Extraction and characterization of type I collagen from skin of tilapia (Oreochromis niloticus) and its potential application in biomedical scaffold material for tissue engineering. Process Biochem. 2018, 74, 156–163. [Google Scholar] [CrossRef]
- León, G.C.D.; Reyes, Z.P.X. Estandarización de la Técnica Blanqueamiento del Betacaroteno Para la Evaluación de la Actividad Antioxidante de Extractos Lipofílicos: Plantas Medicinales, Frutos y Microalgas. Bachelor’s Thesis, Universidad de Cuenca, Cuenca, Ecuador, 2017. Available online: https://rest-dspace.ucuenca.edu.ec/server/api/core/bitstreams/f9995e00-cd9f-42e5-b824-a5e7845f2257/content (accessed on 7 September 2025).
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Martins, E.; Diogo, G.S.; Pires, R.; Reis, R.L.; Silva, T.H. 3D biocomposites comprising marine collagen and silica-based materials inspired on the composition of marine sponge skeletons envisaging bone tissue regeneration. Mar. Drugs 2022, 20, 718. [Google Scholar] [CrossRef]
- Yu, D.; Chi, C.-F.; Wang, B.; Ding, G.-F.; Li, Z.-R. Characterization of acid-and pepsin-soluble collagens from spines and skulls of skipjack tuna (Katsuwonus pelamis). Chin. J. Nat. Med. 2014, 12, 712–720. [Google Scholar] [CrossRef]
- Cadar, E.; Pesterau, A.M.; Prasacu, I.; Ionescu, A.M.; Pascale, C.; Dragan, A.M.L.; Sirbu, R.; Tomescu, C.L. Marine Antioxidants from Marine Collagen and Collagen Peptides with Nutraceuticals Applications: A Review. Antioxidants 2024, 13, 919. [Google Scholar] [CrossRef]
- Abbas, A.A.; Shakir, K.A.; Walsh, M.K. Functional properties of collagen extracted from catfish (Silurus triostegus) waste. Foods 2022, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Emam, A.N. Collagen and collagen-derived materials: Synthesis, structure, classification, fundamental properties and biomedical applications. Discov Appl. Sci. 2025, 7, 1114. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the hierarchical organization of natural collagen: Toward the development of ideal scaffolding material for tissue regeneration. Front. Bioeng. Biotechnol. 2021, 9, 644595. [Google Scholar] [CrossRef]
- Jaziri, A.A.; Shapawi, R.; Mokhtar, R.A.M.; Noordin, W.N.M.; Huda, N. Physicochemical and microstructural analyses of pepsin-soluble collagens derived from lizardfish (Saurida tumbil Bloch, 1795) skin, bone and scales. Gels 2022, 8, 471. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Chinh, N.T.; Manh, V.Q.; Trung, V.Q.; Lam, T.D.; Huynh, M.D.; Tung, N.Q.; Trinh, N.D.; Hoang, T. Characterization of collagen derived from tropical freshwater carp fish scale wastes and its amino acid sequence. Nat. Prod. Commun. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Morales, S.M.; Chacón, A.; Mostue, M.; Prin, J. Análisis químico de colágeno en piel de cola de atún (Thunnus atlanticus) en medio ácido. [Chemical analysis of collagen in tuna tail skin (Thunnus atlanticus) in an acid medium]. Revista ESPAMCIENCIA 2023, 14, 47–55. [Google Scholar] [CrossRef]
- Mobarak, M.H.; Islam, M.A.; Hossain, N.; Al Mahmud, M.Z.; Rayhan, M.T.; Nishi, N.J.; Chowdhury, M.A. Recent advances of additive manufacturing in implant fabrication—A review. Appl. Surf. Sci. Adv. 2023, 18, 100462. [Google Scholar] [CrossRef]
- Yue, C.; Ding, C.; Xu, M.; Hu, M.; Zhang, R. Self-Assembly Behavior of Collagen and Its Composite Materials: Preparation, Characterizations, and Biomedical Engineering and Allied Applications. Gels 2024, 10, 642. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11507467/ (accessed on 17 October 2025). [CrossRef]
- Ahmad, M.; Benjakul, S.; Nalinanon, S. Compositional and physicochemical characteristics of acid solubilized collagen extracted from the skin of unicorn leatherjacket (Aluterus monoceros). Food Hydrocoll. 2010, 24, 588–594. [Google Scholar] [CrossRef]
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chem. 2011, 124, 97–105. [Google Scholar] [CrossRef]
- Matmaroh, K.; Benjakul, S.; Prodpran, T.; Encarnacion, A.B.; Kishimura, H. Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus). Food Chem. 2011, 129, 1179–1186. [Google Scholar] [CrossRef]
- Kozlowska, J.; Sionkowska, A.; Skopinska-Wisniewska, J.; Piechowicz, K. Northern pike (Esox lucius) collagen: Extraction, characterization and potential application. Int. J. Biol. Macromol. 2015, 81, 220–227. [Google Scholar] [CrossRef]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive assessment of Nile tilapia skin (Oreochromis niloticus) collagen hydrogels for wound dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef]
- Fernandes, R.M.T.; Couto Neto, R.G.; Paschoal, C.W.A.; Rohling, J.H.; Bezerra, C.W.B. Collagen films from swim bladders: Preparation method and properties. Colloids Surf. B 2008, 62, 17–21. [Google Scholar] [CrossRef]
- Dzorkpata, C. The Effects of NaCl on the Triple Helix Structure of Collagen and the Reinforcement of Tung Oil-Based Polymers with Collagen Peptides. Master’s Thesis, Georgia Southern University, Statesboro, GA, USA, 2021. Available online: https://digitalcommons.georgiasouthern.edu/etd/2261 (accessed on 10 September 2025).
- Vladu, A.F.; Albu Kaya, M.G.; Truşcă, R.D.; Motelica, L.; Surdu, V.A.; Oprea, O.C.; Constantinescu, R.R.; Cazan, B.; Ficai, D.; Andronescu, E.; et al. The Role of Crosslinking Agents in the Development of Collagen–Hydroxyapatite Composite Materials for Bone Tissue Engineering. Materials 2025, 18, 998. [Google Scholar] [CrossRef]
- Liao, W.; Guanghua, X.; Li, Y.; Shen, X.R.; Li, C. Comparison of characteristics and fibril-forming ability of skin collagen from barramundi (Lates calcarifer) and tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2018, 107, 549–559. [Google Scholar] [CrossRef]
- Uchida, D.T.; Volnistem, A.D.N.; Cook, M.T.; Bruschi, M.L. Effect of the Extraction Methods on the Physicochemical Characteristics of Collagen Derived from Tilapia (Oreochromis niloticus) Skin. ACS Omega 2025, 10, 35809–35826. [Google Scholar] [CrossRef]
- Reátegui-Pinedo, N.; Salirrosas, D.; Sánchez-Tuesta, L.; Quiñones, C.; Jáuregui-Rosas, S.R.; Barraza, G.; Cabrera, A.; Ayala-Jara, C.; Martínez, R.M.; Rolim-Baby, A.; et al. Characterization of collagen from three genetic lines (gray, red and F1) of Oreochromis niloticus (tilapia) skin in young and old adults. Molecules 2022, 27, 1123. [Google Scholar] [CrossRef]
- Xiao, L.; Lv, J.; Liang, Y.; Zhang, H.; Zheng, J.; Lin, F.; Wen, X. Structural, physicochemical properties and function of swim bladder collagen in promoting fibroblasts viability and collagen synthesis. LWT 2023, 173, 114294. [Google Scholar] [CrossRef]
- Tan, Y.; Chang, S.K. Isolation and characterization of collagen extracted from channel catfish (Ictalurus punctatus) skin. Food Chem. 2018, 242, 147–155. [Google Scholar] [CrossRef]
- Tapia-Vasquez, A.E.; Torres-Arreola, W.; Ezquerra-Brauer, J.M.; Márquez-Ríos, E.; Santacruz-Ortega, H.; Ramírez-Suárez, J.C.; García-Sánchez, G.; Suárez-Jiménez, G.M. Spectrometric determination of the collagen crosslinking degree through pyridinoline identification and evaluation of the viscosity properties of Octopus vulgaris and Dosidicus gigas arm muscles. Appl. Food Res. 2025, 5, 100832. [Google Scholar] [CrossRef]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ettiyappan, J.B.P. Extraction, optimization and characterization of collagen from sole fish skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- López, S.E. Andamios y Biomoléculas de Origen Marino Para Regeneración Tisular del Sistema Osteoarticular. Doctoral Thesis, Universidad de Vigo, Pontevedra, Spain, 2019. Available online: https://www.investigo.biblioteca.uvigo.es/xmlui/handle/11093/1416 (accessed on 18 October 2025).
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in lipid extraction methods—A review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef]
- Hernández-Martínez, M.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Castañeda-Pérez, E.; Uribe-Hernández, K. Characterization of Mexican fishes according to fatty acid profile and fat nutritional indices. Int. J. Food Prop. 2016, 19, 1401–1412. [Google Scholar] [CrossRef]
- López-Puebla, S.; Arias-Santé, M.F.; Romero, J.; Costa de Camargo, A.; Rincón-Cervera, M.Á. Analysis of Fatty Acid Profile, α-Tocopherol, Squalene and Cholesterol Content in Edible Parts and By-Products of South Pacific Wild Fishes. Mar. Drugs 2025, 23, 104. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Villarreall-Rubio, M.B.; Valenzuela, R.; Valenzuela, A. Comparison of fatty acid profiles of dried and raw by-products from cultured and wild fishes. Eur. J. Lipid Technol. 2017, 119, 1600516. [Google Scholar] [CrossRef]
- Balikci, E. Influence of seasons and fish body parts on fatty acid profile and effect of seasons on proximate composition of Anatolian khramulya (Capoeta tinca) and Colchic khramulya (Capoeta sieboldii) captured from the Çekerek Dam in Yozgat, Turkey. J. Food Compos. Anal. 2024, 132, 106267. [Google Scholar] [CrossRef]
- Karabayır, E.S.; Öğütcü, M. Assessment and comparative analysis of the antioxidant capacity of some food waste for fish oils. Grasas Aceites 2024, 75, 2021. [Google Scholar] [CrossRef]
- Molla, M.T.H.; Hasan, S.; Al Bashera, M.; Kabir, B. Fatty Acid Composition, Antioxidant Activity and Thrombolytic Activity Analysis of Extracted Lipid from Colisa fasciatus. J. Sci. Eng. Pap. 2024, 1, 90–95. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Hernández-Martínez, M.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Almaraz-Abarca, N.; Ponce-Mendoza, A.; Vásquez-Murrieta, M.S. Prediction of total fat, fatty acid composition and nutritional parameters in fish fillets using MID-FTIR spectroscopy and chemometrics. LWT 2013, 52, 12–20. [Google Scholar] [CrossRef]
- Folch, J.; Less, M.; Sloane, G. A simple method for the isolation and purification of total lipides from animal tissues. JCB 1956, 226, 497–509. [Google Scholar] [CrossRef]
- Kabouche, A.; Kabouche, Z.; Öztürk, M.; Kolak, U.; Topçu, G. Antioxidant abietane diterpenoids from Salvia barrelieri. Food Chem. 2007, 102, 1281–1287. [Google Scholar] [CrossRef]
- Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
| SC | BC | |
|---|---|---|
| Moisture (g/100 g) | 1.30 ± 0.51 a | 0.91 ± 0.42 a |
| Ash (g/100 g) | 0.03 ± 0.01 b | 0.21 ± 0.37 a |
| Hydroxyproline (mg Hyp/g collagen) | 19.02 ± 1.31 a | 15.47 ± 1.65 b |
| L* | 62.66 ± 0.01 b | 86.48 ± 0.01 a |
| a* | 1.7 ± 0.01 a | −3.26 ± 0.03 b |
| b* | 15.16 ± 0.01 b | 20.95 ± 0.06 a |
| CI | 1.79 ± 0.01 a | −1.80 ± 0.02 b |
| W | 59.67 ± 0.02 b | 78.86 ± 0.05 a |
| C*ab | 15.25 ± 0.01 b | 21.20 ± 0.03 a |
| Element wt.% | SC | BC |
|---|---|---|
| Carbon (C) | 58.43 ± 5.31 | 64.42 ± 3.91 |
| Oxygen (O) | 22.99 ± 3.82 | 21.99 ± 3.26 |
| Nitrogen (N) | 15.0 ± 2.38 | 12.25 ± 0.76 |
| Tantalum (Ta) | 1.27 ± 1.18 | 0.43 ± 0.49 |
| Chlorine (Cl) | 0.60 ± 0.47 | 0.18 ± 0.14 |
| Iron (Fe) | 0.32 ± 0.00 | 0 |
| Copper (Cu) | 0.29 ± 0.30 | 0.058 ± 0.10 |
| Sulfur (S) | 0.25 ± 0.21 | 0.18 ± 0.15 |
| Fluorine (F) | 0.23 ± 0.15 | 0.29 ± 0.00 |
| Rhodium (Rh) | 0.22 ± 0.09 | 0.099 ± 0.17 |
| Lead (Pb) | 0.18 ± 0.16 | 0 |
| Silicon (Si) | 0.096 ± 0.19 | 0 |
| Potassium (K) | 0.059 ± 0.05 | 0.048 ± 0.08 |
| Sodium (Na) | 0.049 ± 0.03 | 0.039 ± 0.06 |
| Films | Thermal Transition 1 (Td), °C | ΔH Enthalpy of Td, J/g | Thermal Transition 2 (T2), °C | ΔH Enthalpy of T2, J/g |
|---|---|---|---|---|
| SC | ↓ 53.40 ± 1.28 a | 1.005 ± 0.153 | ↓ 82.39± 0.65 b | 2.19 ± 0.28 |
| BC | ↓ 46.88 ± 0.98 b | 1.428 ± 0.367 | ↑ 108.48 ± 0.01 a | 33.17 ± 3.487 |
| Region | SC | BC | Assignments | References |
|---|---|---|---|---|
| Wavenumber (cm−1) | ||||
| Amide A | 3299 ± 4.31 a | 3307 ± 0.35 a | N-H asymmetric stretching | [29] |
| - | 3077 ± 1.24 a | 3077 ± 0.70 a | N–H stretching overtone of amide II | [29] |
| - | 3013 ± 0.01 a | 3012 ± 0.02 a | C=CH stretching | [29] |
| - | 2954 ± 0.11 a | 2955 ± 0.20 a | -CH3 asymmetric stretching | [29] |
| Amide B | 2924 ± 5.72 a | 2922 ± 3.28 a | -CH2 asymmetric stretching | [12,27] |
| - | 2872 ± 0.03 a | 2872 ± 0.02 a | -CH3 symmetric stretching | [29] |
| - | 2856 ± 0.00 a | 2856 ± 0.00 a | -CH2 symmetric stretching | [29] |
| - | 1744 ± 0.15 a | 1743 ± 0.12 a | C=O stretching | [29] |
| Amide I | 1635 ± 0.60 a | 1636 ± 2.82 a | C=O stretching in peptide bond/Hydrogen bond coupled with COO- | [12,28,30] |
| Amide II | 1544 ± 0.23 a | 1543 ± 0.90 a | N-H bending/C-N stretching | [27,29] |
| - | 1454 ± 2.82 a | 1455 ± 0.21 a | N-H bending/-CH3 asymmetric bending | [29] |
| - | 1393 ± 2.05 a | 1392 ± 0.56 a | -CH3 symmetric bending/COO- symmetrical stretching | [29,31] |
| - | 1337 ± 0.46 a | 1336 ± 0.35 a | COO-symmetric stretching/-CH2 wagging | [12,30] |
| Amide III | 1238 ± 0.88 a | 1237 ± 0.64 a | C-N stretching/N-H in-plane bending/-CH2 wagging/CO stretching | [29,32] |
| - | 1203 ± 0.05 a | 1199 ± 0.03 a | - | - |
| - | 1160 ± 0.83 a | 1160 ± 0.40 a | C-N stretching | [29] |
| - | 1115 ± 0.39 a | 1114 ± 0.05 a | - | - |
| - | 1082 ± 0.15 a | 1085 ± 0.35 a | C-O stretching | [30] |
| - | 972 ± 0.23 a | 973 ± 0.42 a | C-C stretching | [29] |
| - | 922 ± 0.2 a | 921 ± 0.05 a | C-C stretching | [29] |
| - | 849 ± 0.04 a | 850 ± 0.01 a | C-N stretching | [29] |
| Freeze-Dried Samples | Band 1, kDa β dimer | Band 2, kDa Chain α1 | Band 3, kDa Chain α2 |
|---|---|---|---|
| SC | 260 | 141 | 106 |
| BC | 243 | 139 | 114 |
| Freeze-Dried Samples | Total Number of Pores (Per Micrograph) | Pore Diameter (µm) | Area Occupied by Pores (%) |
|---|---|---|---|
| SC | 1539 ± 329 a | 32.87 ± 12.13 a | 41.28 ± 23.43 a |
| BC | 1814 ± 696 a | 29.94 ± 10.66 a | 38.84 ± 15.23 a |
| SLf | SLs | BLs | Assignments | Reference |
|---|---|---|---|---|
| Wavenumber ( cm−1) | ||||
| 3385 ± 0.46 b | 3475 ± 0.89 a | 3475 ± 0.11 a | O-H stretching of water traces | [29] |
| 3013 ± 0.02 a | 3012 ± 0.02 a | 3011 ± 0.04 a | =C-H stretching | [29] |
| 2955 ± 0.51 a | 2954 ± 0.01 a | 2954 ± 0.03 a | -CH3 asymmetric stretching | [29] |
| 2922 ± 0.19 a | 2923 ± 0.01 a | 2922 ± 0.06 a | -CH2 asymmetric stretching | [29] |
| 2852 ± 0.15 a | 2853 ± 0.01 a | 2853 ± 0.04 a | -CH2 symmetric stretching | [29] |
| 1742 ± 0.11 a | 1743 ± 0.02 a | 1742 ± 0.01 a | C=O stretching in triglycerides, phospholipids | [29] |
| 1657 ± 0.54 a | 1659 ± 0.39 a | 1659 ± 0.59 a | C=C stretching in cis fatty acids | [29] |
| 1553 ± 0.67 | - | - | - | - |
| 1465 ± 0.03 a | 1464 ± 0.06 a | 1464 ± 0.01 a | -CH2 and -CH3 bending | [15,29] |
| 1416 ± 0.50 a | 1417 ± 0.04 a | 1417 ± 0.11 a | COO- symmetric stretching in carboxylate group/-CH2 bending | [29] |
| 1378 ± 0.02 a | 1377 ± 0.01 a | 1377 ± 0.01 a | -CH3 symmetric bending | [29] |
| 1219 ± 0.54 c | 1235 ± 0.36 b | 1237 ± 0.06 a | C-O stretching in trigliceridos/-CH2 bending | [15] |
| 1173 ± 0.38 | - | - | C-O stretching | [15] |
| - | 1156 ± 0.03 a | 1159 ± 0.13 a | C-O stretching | [29] |
| - | 1115 ± 0.68 a | 1116 ± 0.00 a | C-O stretching | [29] |
| 1093 ± 0.54 a | 1097 ± 0.00 a | 1097 ± 0.00 a | C-O stretching | [15] |
| 1061 ± 0.23 | - | - | - | - |
| 971 ± 0.04 a | 971 ± 0.02 a | 972 ± 0.02 a | C-N asymmetric stretching | [29] |
| 926 ± 0.14 | - | - | =CH2 bending out of plane | [15] |
| 890 ± 0.16 | - | - | -CH2 rocking | [29] |
| 837 ± 0.06 | - | - | =CH2 wagging | [15] |
| 758 ± 0.43 | - | - | - | - |
| 721 ± 0.29 a | 721 ± 0.02 a | 721 ± 0.01 a | CH2 rocking | [15,29] |
| Fatty Acid (% of Total Fatty Acids) | SLs | BLs | SLf | CFO |
|---|---|---|---|---|
| C6:0 | n.d. | n.d. | n.d. | 0.68 ± 0.01 |
| C8:0 | n.d. | n.d. | n.d. | 0.10 ± 0.02 |
| C12:0 | n.d. | n.d. | n.d. | 0.08 ± 0.03 |
| C14:0 | 5.52 ± 0.39 a | 5.60 ± 0.49 a | 3.04 ± 0.02 b | 6.42 ± 0.96 |
| C16:0 | 28.64 ± 0.60 a | 28.74 ± 0.79 a | 26.78 ±1.24 a | 15.31 ± 2.04 |
| C17:0 | 1.84 ± 0.005 ab | 1.93 ± 0.18 a | 1.66 ± 0.03 b | 0.52 ± 0.04 |
| C18:0 | 11.81 ± 0.28 a | 11.42 ± 0.17 a | 11.63 ± 0.09 a | 3.17 ± 0.05 |
| C20:0 | 0.99 ± 0.04 a | 0.91 ± 0.03 a | 0.75 ± 0.01 b | 1.3 ± 0.16 |
| C21:0 | 0.26 ± 0.004 b | 0.25 ± 0.02 b | 0.38 ± 0.02 a | 0.08 ± 0.02 |
| C22:0 | 0.59 ± 0.03 a | 0.50 ± 0.11 ab | 0.41 ± 0.01 b | 2.81 ± 0.11 |
| C23:0 | 0.15 ± 0.03 a | 0.15 ± 0.03 a | 0.17 ± 0.02 a | 0.23 ± 0.15 |
| C24:0 | 0.73 ± 0.02 a | 0.67 ± 0.08 a | 0.51 ± 0.02 b | 3.74 ± 0.85 |
| Σ SFA | 50.53 ± 0.60 a | 50.19 ± 0.71 a | 45.33 ± 1.04 b | 34.44 ± 2.64 |
| C14:1 (n-5) | 1.44 ± 0.05 a | 1.38 ± 0.18 a | 0.75 ± 0.01 b | 0.45 ± 0.04 |
| C16:1 (n-7) | 4.91 ± 0.12 ab | 5.21 ± 0.13 a | 4.55 ± 0.19 b | 8.56 ± 0.76 |
| C17:1 (n-7) | 0.68 ± 0.01 a | 0.56 ± 0.18 a | 0.62 ± 0.01 a | 1.13 ± 0.01 |
| C18:1t (n-9) | 0.37 ± 0.01 a | 0.23 ± 0.22 a | 0.38 ± 0.005 a | 1.20 ± 0.02 |
| C18:1c9 (n-9) | 18.12 ± 0.34 a | 19.11 ± 1.62 a | 17.61 ± 0.03 a | 10.81 ± 1.46 |
| C18:1c14 (n-7) | 2.96 ± 0.05 a | 2.14 ± 1.30 a | 2.18 ± 0.10 a | 1.52 ± 0.08 |
| C20:1c | 0.64 ± 0.02 a | 0.50 ± 0.13 a | 0.23 ± 0.01 a | 0.22 ± 0.05 |
| C22:1n (n-9) | 0.15 ± 0.01 a | 0.16 ± 0.005 a | 0.13 ± 0.01 a | 0.28 ± 0.01 |
| C24:1 (n-9) | 0.59 ± 0.05 a | 0.60 ± 0.06 a | 0.98 ± 0.03 a | 0.25 ± 0.01 |
| Σ MUFA | 31.24 ± 0.28 a | 31.24 ± 0.28 a | 27.43 ± 0.08 b | 24.42 ± 0.78 |
| C18:2t (n-6) | 0.56 ± 0.02 a | 0.56 ± 0.10 a | 0.38 ± 0.04 a | 0.26 ± 0.06 |
| C18:2c (n-6) | 1.44 ± 0.01 a | 1.53 ± 0.02 a | 0.83 ± 0.06 b | 2.09 ± 0.51 |
| C18:3c 6,9 (n-6) | 0.11 ± 0.02 b | 0.17 ± 0.004 a | 0.11 ± 0.005 b | 0.11 ± 0.01 |
| C18:3c9,12 (n-3) | 1.77 ± 0.07 a | 1.87 ± 0.28 a | 0.88 ± 0.01 b | 3.10 ± 0.27 |
| C20:2 (n-6) | 0.61 ± 0.03 a | 0.63 ± 0.12 a | 0.27 ± 0.005 b | 0.01 ± 0.01 |
| C20:3c8 (n-6) | 0.17 ± 0.01 a | 0.09 ± 0.02 c | 0.13 ± 0.00 b | 0.13 ± 0.01 |
| C20:3c11(n-9) | 0.20 ± 0.02 a | 0.19 ± 0.07 a | 0.13 ± 0.01 a | 0.09 ± 0.01 |
| C20:4 (n-6) | 1.50 ± 0.05 b | 1.57 ± 0.01 b | 2.80 ± 0.13 a | 3.74 ± 0.85 |
| C20:5 EPA (n-3) | 1.26 ± 0.05 b | 1.62 ± 0.35 b | 3.16 ± 0.04 a | 17.59 ± 0.28 |
| C22:2 (n-6) | 0.35 ± 0.01 a | 0.37 ± 0.05 a | 0.36 ± 0.01 a | 0.79 ± 0.07 |
| C22:6 DHA (n-3) | 3.94 ± 0.20 b | 4.57 ± 0.58 b | 9.78 ± 0.03 a | 11.23 ± 0.76 |
| Σ PUFA | 11.93 ± 0.42 b | 13.15 ± 1.50 b | 18.70 ± 0.36 a | 39.14 ± 1.12 |
| n-3/n-6 ratio | 1.47:1 | 1.64:1 | 2.85:1 | 4.54:1 |
| SFA:MUFA:PUFA | 1:0.62:0.24 | 1:0.62:0.26 | 1:0.6:0.4 | 1:0.71:1.14 |
| Assay | Sample | ||
|---|---|---|---|
| SLf | CFO | ||
| β-carotene bleaching | % inhibition 1 | 70.83 ± 0.18 b | 80.71 ± 0.64 a |
| DR | 0.0161 ± 0.0001 a | 0.0106 ± 0.0003 b | |
| ABTS | % inhibition | 32.70 ± 3.61 a | 32.90 ± 1.14 a |
| Concentration 2 | 0.039 ± 0.001 a | 0.039 ± 0.005 a | |
| DPPH | % inhibition | 19.60 ± 0.69 b | 19.98 ± 0.79 a |
| Concentration 2 | 0.0061 ± 0.001 b | 0.0087 ± 0.001 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menéndez-Tasé, S.; Gaeta-Leal, E.; Téllez-Medina, D.I.; Tapia-Maruri, D.; López-Villegas, E.O.; Calderón-Domínguez, G.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Hernández-Botello, M.T.; Hernández-Martínez, D.M. Native Collagen and Total Lipid Extract Obtained from Caranx hyppos By-Products: Characterization for Potential Use in the Biomedical and Nutraceutical Fields. Mar. Drugs 2025, 23, 432. https://doi.org/10.3390/md23110432
Menéndez-Tasé S, Gaeta-Leal E, Téllez-Medina DI, Tapia-Maruri D, López-Villegas EO, Calderón-Domínguez G, Gallardo-Velázquez T, Osorio-Revilla G, Hernández-Botello MT, Hernández-Martínez DM. Native Collagen and Total Lipid Extract Obtained from Caranx hyppos By-Products: Characterization for Potential Use in the Biomedical and Nutraceutical Fields. Marine Drugs. 2025; 23(11):432. https://doi.org/10.3390/md23110432
Chicago/Turabian StyleMenéndez-Tasé, Sheyza, Evelin Gaeta-Leal, Darío Iker Téllez-Medina, Daniel Tapia-Maruri, Edgar Oliver López-Villegas, Georgina Calderón-Domínguez, Tzayhri Gallardo-Velázquez, Guillermo Osorio-Revilla, Mayuric Teresa Hernández-Botello, and Diana Maylet Hernández-Martínez. 2025. "Native Collagen and Total Lipid Extract Obtained from Caranx hyppos By-Products: Characterization for Potential Use in the Biomedical and Nutraceutical Fields" Marine Drugs 23, no. 11: 432. https://doi.org/10.3390/md23110432
APA StyleMenéndez-Tasé, S., Gaeta-Leal, E., Téllez-Medina, D. I., Tapia-Maruri, D., López-Villegas, E. O., Calderón-Domínguez, G., Gallardo-Velázquez, T., Osorio-Revilla, G., Hernández-Botello, M. T., & Hernández-Martínez, D. M. (2025). Native Collagen and Total Lipid Extract Obtained from Caranx hyppos By-Products: Characterization for Potential Use in the Biomedical and Nutraceutical Fields. Marine Drugs, 23(11), 432. https://doi.org/10.3390/md23110432









