Integrated Regulation of Immunity and Nutritional Symbiosis in Deep-Sea Mussels
Abstract
1. Introduction
2. Innate Immune Defense in Bathymodiolus Mussels
2.1. Gill Defense
2.2. Hemocyte Defense
2.3. Compartmentalization of Immunity and Symbiosis
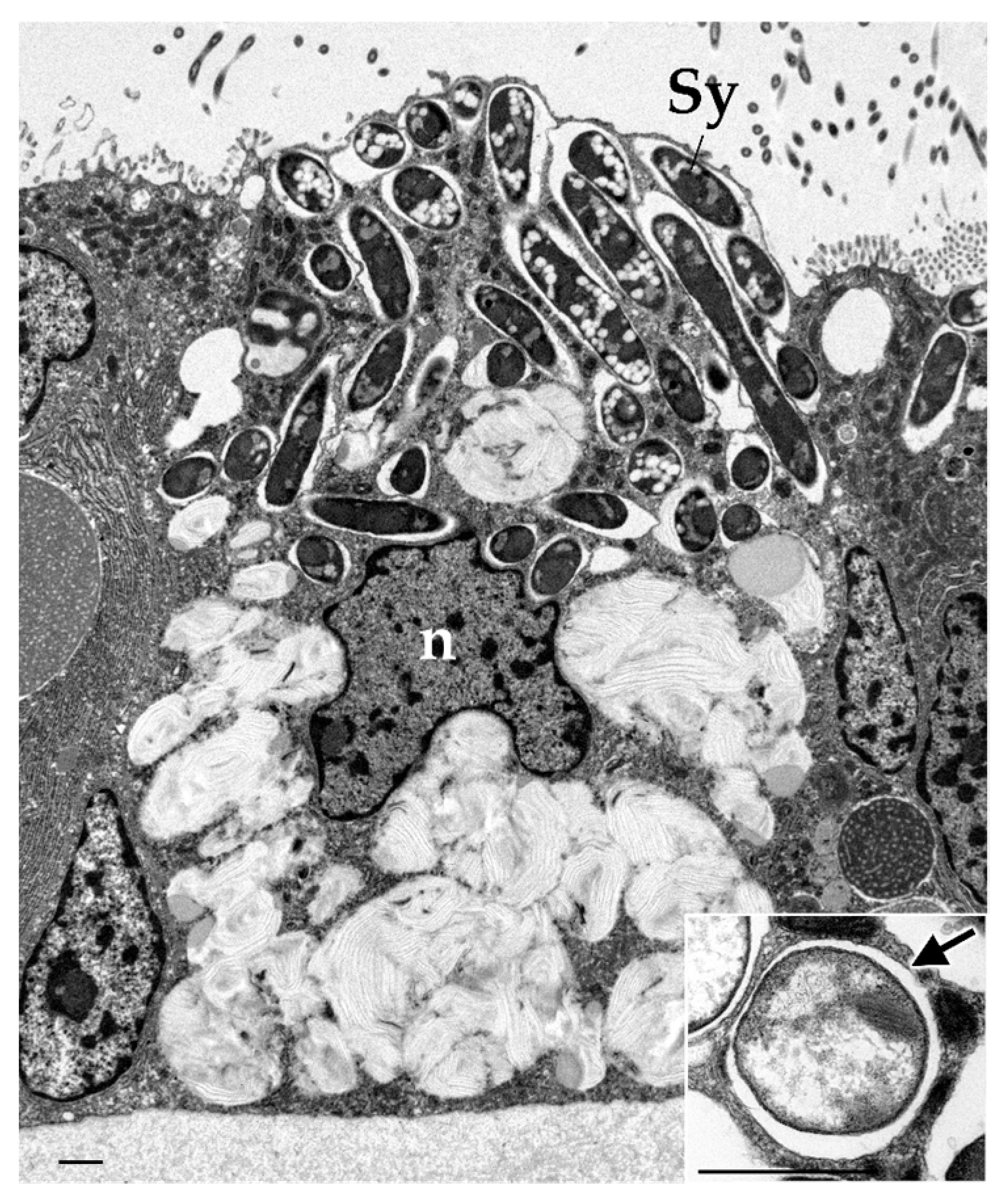
3. Gill Epithelial Cells as a Mediator of Symbiosis
3.1. Horizontal Acquisition and Developmental Distribution
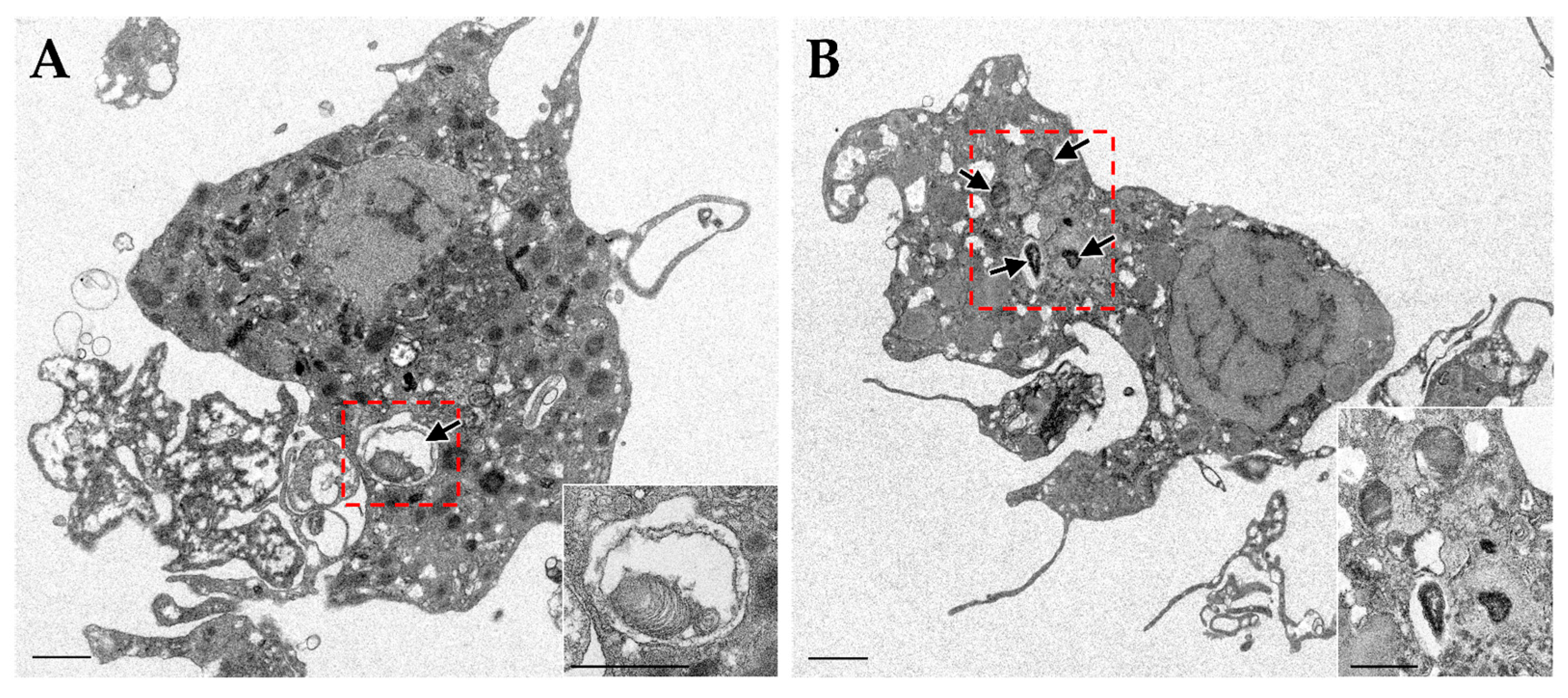
3.2. Disappearance and Reacquisition of Symbionts
3.3. Selective Specificity of Symbionts
4. Intracellular Nutritional Symbiotic Mechanism Through Phagocytosis
4.1. Phagocytosis for Symbiont Acquisition
4.2. Phagosome-Derived Symbiosome
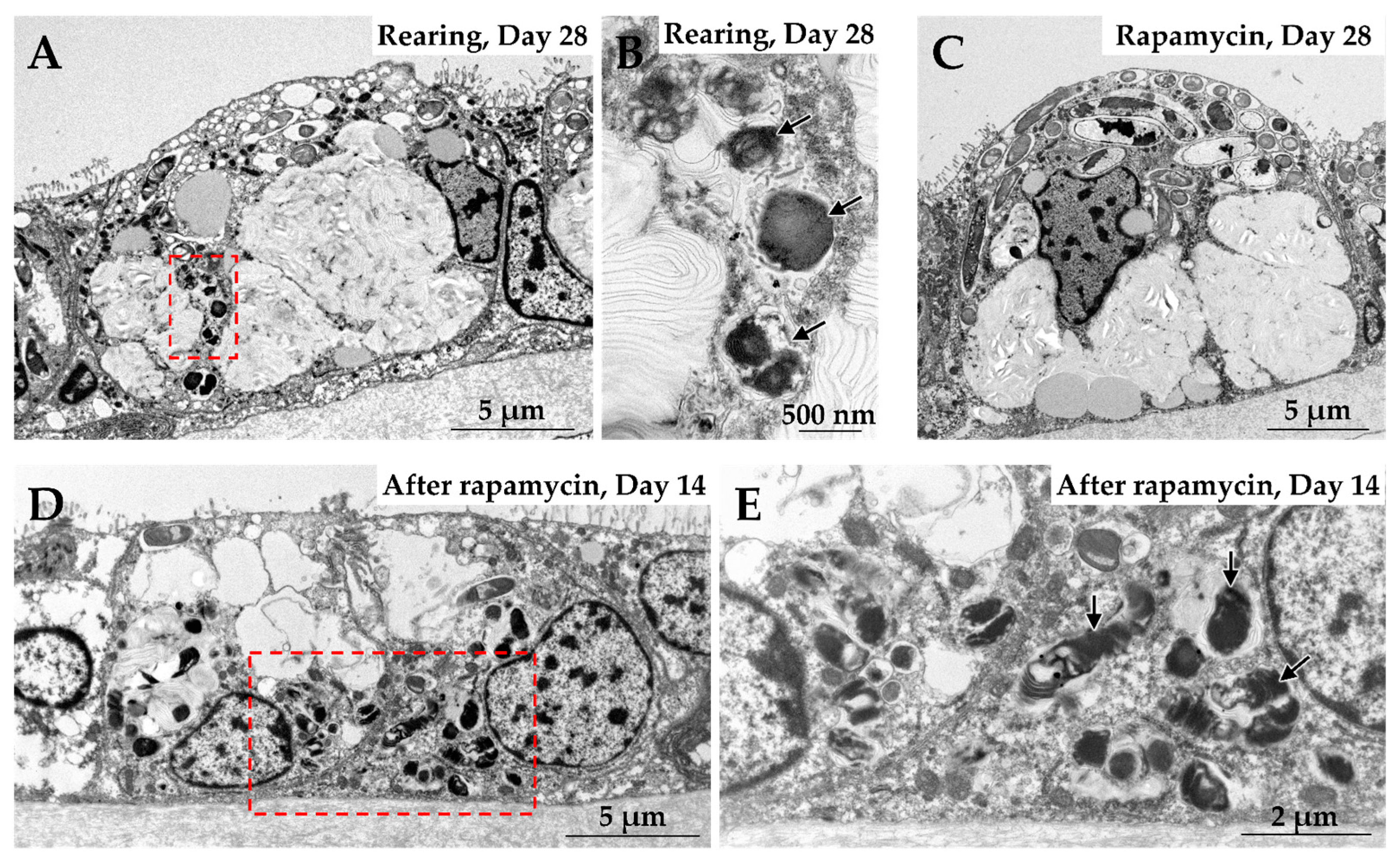
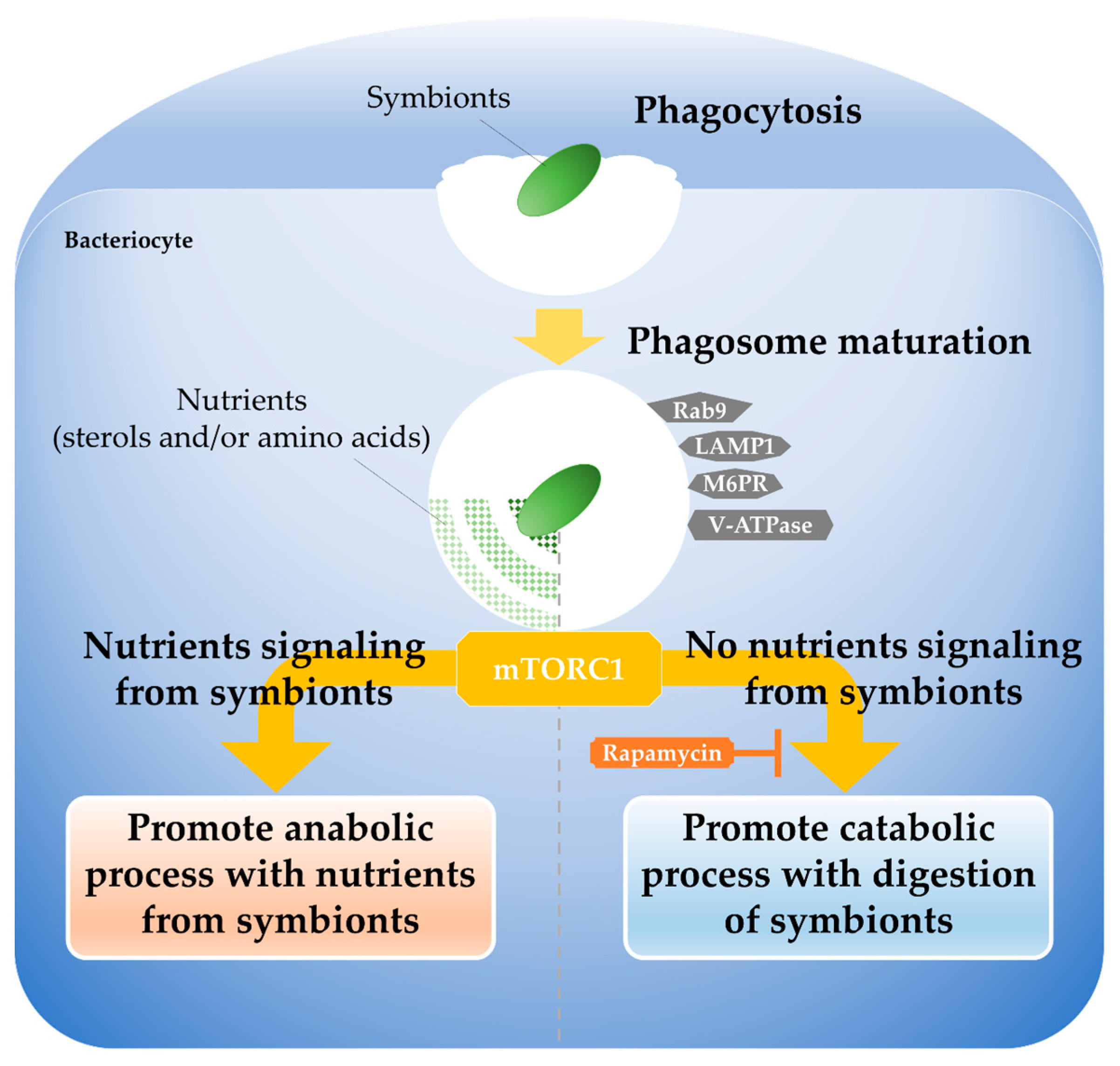
4.3. Integrated Regulation Between Phagocytosis and Nutrient Signaling by mTORC1
5. Discussion and Future Perspectives
- Deciphering the molecular basis of the immune–symbiosis interface: To understand how symbiotic bacteria are distinguished from foreign bacteria that should be eliminated, it is essential to identify key signaling molecules involved in the nutritional signaling pathways that control the transition from bacterial elimination to symbiont maintenance, and to pinpoint their intracellular localization.
- Elucidating bidirectional metabolic signals between symbionts and mTORC1: To gain a deeper understanding of the nutritional control network in symbiosis, it is necessary to clarify how symbiont-derived metabolites (e.g., amino acids, lipids, and sterols) affect host mTORC1 activity and downstream phagosome digestion processes, using methods such as metabolic inhibition experiments.
- Advancing symbiosis research from a comparative immunology and evolutionary perspective: Functional comparisons of symbiosis-related immune and metabolic regulatory factors across various host–symbiont systems will provide insights into the evolutionary conservation and plasticity of these networks. Furthermore, evolutionary tracing of the acquisition and modification processes of symbiosis-related factors is extremely important for understanding the universality and diversity of symbiosis.
- Exploring the minimal molecular basis for symbiont acquisition in non-symbiotic close relatives: Exploring symbiosis-related genes in non-symbiotic close relatives will be useful for identifying the minimal genetic components required for the establishment of symbiosis. This knowledge may potentially lead to the reconstruction of artificial symbiotic systems and their applications in various fields of biology.
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, W.F.; Garg, S.; Zimorski, V. Endosymbiotic theories for eukaryote origin. Philos. Trans. R. Soc. B 2015, 370, 20140330. [Google Scholar] [CrossRef]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef]
- Dale, C.; Moran, N.A. Molecular interactions between bacterial symbionts and their hosts. Cell 2006, 126, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. How multi-partner endosymbioses function. Nat. Rev. Microbiol. 2016, 14, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.M.; Henry, L.M.; Cornwallis, C.K.; Kiers, E.T.; West, S.A. The evolution of host-symbiont dependence. Nat. Commun. 2017, 8, 15973. [Google Scholar] [CrossRef]
- Dubilier, N.; Bergin, C.; Lott, C. Symbiotic diversity in marine animals: The art of harnessing chemosynthesis. Nat. Rev. Microbiol. 2008, 6, 725–740. [Google Scholar] [CrossRef]
- Gerardo, N.M.; Hoang, K.L.; Stoy, K.S. Evolution of animal immunity in the light of beneficial symbioses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 1808. [Google Scholar] [CrossRef]
- Perreau, J.; Moran, N.A. Genetic innovations in animal-microbe symbioses. Nat. Rev. Genet. 2022, 1, 23–39. [Google Scholar] [CrossRef]
- O’Brien, P.A.; Webster, N.A.; Miller, D.J.; Bourne, D.G. Host-Microbe Coevolution: Applying Evidence from Model Systems to Complex Marine Invertebrate Holobionts. mBio 2019, 10, e02241-18. [Google Scholar] [CrossRef] [PubMed]
- Sievert, S.M.; Vetriani, C. Chemoautotrophy at Deep-Sea Vents: Past, Present, and Future. Oceanography 2012, 25, 218–233. [Google Scholar] [CrossRef]
- Van Dover, C.L. The Ecology of Deep-Sea Hydrothermal Vents, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2000. [Google Scholar]
- DeChaine, E.G.; Cavanaugh, C.M. Symbioses of methanotrophs and deep-sea mussels (Mytilidae: Bathymodiolinae). In Molecular Basis of Symbiosis; Springer: Berlin/Heidelberg, Germany, 2005; pp. 227–249. [Google Scholar]
- Duperron, S.; Fiala-Médioni, A.; Dubilier, N. The diversity of deep-sea chemosynthetic symbioses: Examples from marine invertebrates. In The Vent and Seep Biota: Aspects from Microbes to Ecosystems; Springer: Dordrecht, The Netherlands, 2010; pp. 137–167. [Google Scholar]
- Ponnudurai, R.; Kleiner, M.; Sayavedra, L.; Petersen, J.M.; Moche, M.; Otto, A.; Becher, D.; Takeuchi, T.; Satoh, N.; Dubilier, N.; et al. Metabolic and physiological interdependencies in the Bathymodiolus azoricus symbiosis. ISME J. 2017, 11, 463–477. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Xu, T.; Zhang, Y.; Mu, H.; Zhang, Y.; Lan, Y.; Fields, C.J.; Hui, J.H.L.; Zhang, W.; et al. Adaptation to deep-sea chemosynthetic environments as revealed by mussel genomes. Nat. Ecol. Evol. 2017, 1, 121. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Wang, M.; Li, C.; Sun, X.; Wang, X.; Sun, Y.; Sun, S. Insights into deep-sea adaptations and host-symbiont interactions: A comparative transcriptome study on Bathymodiolus mussels and their coastal relatives. Mol. Ecol. 2017, 26, 5133–5148. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.; Hallam, S.J.; O’Mullan, G.D.; Pan, I.L.; Buck, K.R.; Vrijenhoek, R.C. Environmental acquisition of thiotrophic endosymbionts by deep-sea mussels of the genus Bathymodiolus. Appl. Environ. Microbiol. 2003, 69, 6785–6792. [Google Scholar] [CrossRef] [PubMed]
- Bright, M.; Bulgheresi, S. A complex journey: Transmission of microbial symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230. [Google Scholar] [CrossRef]
- Wentrup, C.; Wendeberg, A.; Schimak, M.; Borowski, C.; Dubilier, N. Forever competent: Deep-sea bivalves are colonized by their chemosynthetic symbionts throughout their lifetime. Environ. Microbiol. 2014, 16, 3699–3713. [Google Scholar] [CrossRef]
- Picazo, D.R.; Dagan, T.; Ansorge, R.; Petersen, J.M.; Dubilier, N.; Kupczok, A. Horizontally transmitted symbiont populations in deep-sea mussels are genetically isolated. ISME J. 2019, 13, 2954–2968. [Google Scholar] [CrossRef]
- Breusing, C.; Genetti, M.; Russell, S.L.; Corbett-Detig, R.B.; Beinart, R.A. Horizontal transmission enables flexible associations with locally adapted symbiont strains in deep-sea hydrothermal vent symbioses. Proc. Natl. Acad. Sci. USA 2022, 119, e2115608119. [Google Scholar] [CrossRef]
- Tame, A.; Maruyama, T.; Yoshida, T. Phagocytosis of exogenous bacteria by gill epithelial cells in the deep-sea symbiotic mussel Bathymodiolus japonicus. R. Soc. Open Sci. 2022, 9, 211384. [Google Scholar] [CrossRef]
- Tame, A.; Maruyama, T.; Ikuta, T.; Chikaraishi, Y.; Ogawa, N.; Tsuchiya, M.; Takishita, K.; Tsuda, M.; Hirai, M.; Takaki, Y.; et al. mTORC1 regulates phagosome digestion of symbiotic bacteria for intracellular nutritional symbiosis in a deep-sea mussel. Sci. Adv. 2023, 9, adg8364. [Google Scholar] [CrossRef]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef]
- Levin, R.; Grinstein, S.; Canton, J. The life cycle of phagosomes: Formation, maturation, and resolution. Immunol. Rev. 2016, 273, 156–179. [Google Scholar] [CrossRef]
- Davy, S.K.; Allemand, D.; Weis, V.M. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. Rev. 2012, 76, 229–261. [Google Scholar] [CrossRef]
- Fiala-Médioni, A.; McKiness, Z.; Dando, P.; Boulegue, J.; Mariotti, A.; Alayse-Danet, A.; Robinson, J.; Cavanaugh, C. Ultrastructural, biochemical, and immunological characterization of two populations of the mytilid mussel Bathymodiolus azoricus from the Mid-Atlantic Ridge: Evidence for a dual symbiosis. Mar. Biol. 2002, 141, 1035–1043. [Google Scholar] [CrossRef]
- Duperron, S.; Bergin, C.; Zielinski, F.; Blazejak, A.; Pernthaler, A.; McKiness, Z.P.; DeChaine, E.; Cavanaugh, C.M.; Dubilier, N. A dual symbiosis shared by two mussel species, Bathymodiolus azoricus and Bathymodiolus puteoserpentis (Bivalvia: Mytilidae), from hydrothermal vents along the northern Mid-Atlantic Ridge. Environ. Microbiol. 2006, 8, 1441–1447. [Google Scholar] [CrossRef]
- Raulfs, E.; Macko, S.; Van Dover, C. Tissue and symbiont condition of mussels (Bathymodiolus thermophilus) exposed to varying levels of hydrothermal activity. J. Mar. Biol. Assoc. U. K. 1999, 84, 229–234. [Google Scholar] [CrossRef]
- Kádár, E.; Bettencourt, R.; Costa, V.; Santos, R.S.; Lobo-Da-Cunha, A.; Dando, P. Experimentally induced endosymbiont loss and re-acquirement in the hydrothermal vent bivalve Bathymodiolus azoricus. J. Exp. Mar. Biol. Ecol. 2005, 318, 99–110. [Google Scholar] [CrossRef]
- Allam, B.; Raftos, D. Immune responses to infectious diseases in bivalves. J. Invertebr. Pathol. 2015, 131, 121–136. [Google Scholar] [CrossRef]
- Balbi, T.; Auguste, M.; Ciacci, C.; Canesi, L. Immunological Responses of Marine Bivalves to Contaminant Exposure: Contribution of the -Omics Approach. Front. Immunol. 2021, 12, 618726. [Google Scholar] [CrossRef]
- Bettencourt, R.; Roch, P.; Stefanni, S.; Rosa, D.; Colaço, A.; Santos, R.S. Deep sea immunity: Unveiling immune constituents from the hydrothermal vent mussel Bathymodiolus azoricus. Mar. Environ. Res. 2007, 64, 108–127. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, R.; Dando, P.; Collins, P.; Costa, V.; Allam, B.; Serrão Santos, R. Innate immunity in the deep sea hydrothermal vent mussel Bathymodiolus azoricus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 152, 278–289. [Google Scholar] [CrossRef]
- Bettencourt, R.; Pinheiro, M.; Egas, C.; Gomes, P.; Afonso, M.; Shank, T.; Santos, R.S. High-throughput sequencing and analysis of the gill tissue transcriptome from the deep-sea hydrothermal vent mussel Bathymodiolus azoricus. BMC Genom. 2010, 11, 559. [Google Scholar] [CrossRef]
- Wong, Y.H.; Sun, J.; He, L.S.; Chen, L.G.; Qiu, J.W.; Qian, P.Y. High-throughput transcriptome sequencing of the cold seep mussel Bathymodiolus platifrons. Sci. Rep. 2015, 5, 16597. [Google Scholar] [CrossRef]
- Martins, E.; Figueras, A.; Novoa, B.; Santos, R.S.; Moreira, R.; Bettencourt, R. Comparative study of immune responses in the deep-sea hydrothermal vent mussel Bathymodiolus azoricus and the shallow-water mussel Mytilus galloprovincialis challenged with Vibrio bacteria. Fish Shellfish Immunol. 2014, 40, 485–499. [Google Scholar] [CrossRef]
- Hine, R.M. The inter-relationships of bivalve haemocytes. Fish Shellfish Immunol. 1999, 1, 367–385. [Google Scholar] [CrossRef]
- Ballina, N.R.; Maresca, F.; Cao, A.; Villalba, A. Bivalve Haemocyte Subpopulations: A Review. Front. Immunol. 2022, 13, 826255. [Google Scholar] [CrossRef] [PubMed]
- Tame, A.; Yoshida, T.; Ohishi, K.; Maruyama, T. Phagocytic activities of hemocytes from the deep-sea symbiotic mussels Bathymodiolus japonicus, B. platifrons, and B. septemdierum. Fish Shellfish Immunol. 2015, 44, 146–156. [Google Scholar] [CrossRef]
- Bettencourt, R.; Dando, P.; Rosa, D.; Riou, V.; Calaco, A.; Sarrazin, J.; Sarradin, P.; Santos, R.S. Changes of gill and hemocyte-related bio-indicators during long term maintenance of the vent mussel Bathymodiolus azoricus held in aquaria at atmospheric pressure. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 150, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tame, A. Phagocytosis in the Deep-Sea Mussel, Bathymodiolus japonicus. Ph.D. Thesis, Kitasato University, Kanagawa, Japan, 2018. [Google Scholar]
- Nyholm, S.V.; Stewart, J.J.; Ruby, E.G.; McFall-Ngai, M.J. Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ. Microbiol. 2009, 11, 483–493. [Google Scholar] [CrossRef]
- McFall-Ngai, M.J.; Nyholm, S.V.; Castillo, M.G. The role of the immune system in the initiation and persistence of the Euprymna scolopes–Vibrio fischeri symbiosis. Semin. Immunol. 2010, 22, 48–53. [Google Scholar] [CrossRef]
- Rader, B.; McAnulty, S.j.; Nyholm, S.V. Persistent symbiont colonization leads to a maturation of hemocyte response in the Euprymna scolopes/Vibrio fischeri symbiosis. Microbiologyopen 2019, 8, e858. [Google Scholar] [CrossRef]
- Wentrup, C.; Wendeberg, A.; Huang, J.Y.; Borowski, C.; Dubilier, N. Shift from widespread symbiont infection of host tissues to specific colonization of gills in juvenile deep-sea mussels. ISME J. 2013, 7, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Franke, M.; Geier, B.; Hammel, J.U.; Dubilier, N.; Leisch, N. Coming together—Symbiont acquisition and early development in deep-sea bathymodioline mussels. Proc. R. Soc. B 2021, 288, 20211044. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, P.; Lu, J.; Wu, N.; Lin, G.; Zhang, X.; Cao, H.; Geng, W.; Zhai, B.; Xu, C.; et al. Gene expression profiles provide insights into the survival strategies in deep-sea mussel (Bathymodiolus platifrons) of different developmental stages. BMC Genom. 2022, 23, 311. [Google Scholar] [CrossRef]
- Piquet, B.; Panse, S.L.; Lallier, L.H.; Duperron, S.; Andersen, A. “There and back again”—Ultrastructural changes in the gills of Bathymodiolus vent-mussels during symbiont loss: Back to a regular filter-feeding epidermis. Front. Mar. Sci. 2022, 9, 968331. [Google Scholar] [CrossRef]
- Détrée, C.; Haddad, I.; Demey-Thomas, E.; Vinh, J.; Lallier, F.H.; Tanguy, A.; Mary, J. Global host molecular perturbations upon in situ loss of bacterial endosymbionts in the deep-sea mussel Bathymodiolus azoricus assessed using proteomics and transcriptomics. BMC Genom. 2019, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Wang, M.; Chen, H.; Lian, C.; Li, C. Comparative transcriptomic analysis illuminates the host-symbiont interactions in the deep-sea mussel Bathymodiolus platifrons. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2019, 151, 103082. [Google Scholar] [CrossRef]
- Zhang, Y.; Nicholatos, J.; Dreier, J.R.; Ricoult, S.J.; Widenmaier, S.B.; Hotamisligil, G.S.; Kwiatkowski, D.J.; Manning, B.D. Coordinated regulation of protein synthesis and degradation by mTORC1. Nature 2014, 513, 440–443. [Google Scholar] [CrossRef]
- Petersen, J.M.; Wentrup, C.; Verna, C.; Knittel, K.; Dubilier, N. Origins and evolutionary flexibility of chemosynthetic symbionts from deep-sea animals. Biol. Bull. 2012, 223, 123–137. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Takai, K.; Uematsu, K.; Tsuchida, S.; Hunt, J.C.; Hashimoto, J. Phylogenetic characterization of endosymbionts in three hydrothermal vent mussels: Influence on host distributions. Mar. Ecol. Prog. Ser. 2000, 208, 147–155. [Google Scholar] [CrossRef]
- Petersen, J.M.; Dubilier, N. Methanotrophic symbioses in marine invertebrates. Environ. Microbiol. Rep. 2009, 1, 319–335. [Google Scholar] [CrossRef]
- Zielinski, F.U.; Pernthaler, A.; Duperron, S.; Raggi, L.; Giere, O.; Borowski, C.; Dubilier, N. Widespread occurrence of an intranuclear bacterial parasite in vent and seep bathymodiolin mussels. Environ. Microbiol. 2009, 11, 1150–1167. [Google Scholar] [CrossRef]
- Ikuta, T.; Takaki, Y.; Nagai, Y.; Shimamura, S.; Tsuda, M.; Kawagucci, S.; Aoki, Y.; Inoue, K.; Teruya, M.; Satou, K.; et al. Heterogeneous composition of key metabolic gene clusters in a vent mussel symbiont population. ISME J. 2016, 10, 990–1001. [Google Scholar] [CrossRef]
- Dale, C.; Plague, G.R.; Wang, B.; Ochman, H.; Moran, N.A. Type III secretion systems and the evolution of mutualistic endosymbiosis. Proc. Natl. Acad. Sci. USA 2002, 99, 12397–12402. [Google Scholar] [CrossRef]
- Rancès, E.; Voronin, D.; Tran-Van, V.; Mavingui, P. Genetic and functional characterization of the type IV secretion system in Wolbachia. J. Bacteriol. 2008, 190, 5020–5030. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Huang, H.M.; Yu, M.; Lai, E.M.; Chien, H.L.; Liu, C.T. Functional exploration of the bacterial type VI secretion system in mutualism: Azorhizobium caulinodans ORS571–Sesbania rostrata as a research model. Mol. Plant-Microbe Interact. 2018, 31, 856–867. [Google Scholar] [CrossRef]
- Hirayama, H.; Takaki, Y.; Abe, M.; Imachi, H.; Ikuta, T.; Miyazaki, J.; Tasumi, E.; Uematsu, K.; Tame, A.; Tsuda, M.; et al. Multispecies populations of methanotrophic Methyloprofundus and cultivation of a likely dominant species from the Iheya North deep-sea hydrothermal field. Appl. Environ. Microbiol. 2021, 88, e0075821. [Google Scholar] [CrossRef]
- Takishita, K.; Takaki, Y.; Chikaraishi, Y.; Ikuta, T.; Ozawa, G.; Yoshida, T.; Ohkouchi, N.; Fujikura, K. Genomic evidence that methanotrophic endosymbionts likely provide deep-sea Bathymodiolus mussels with a sterol intermediate in cholesterol biosynthesis. Genome Biol. Evol. 2017, 9, 1148–1160. [Google Scholar] [CrossRef]
- Ikuta, T.; Tame, A.; Takahashi, T.; Nomaki, H.; Nakajima, R. Microplastic particles are phagocytosed in gill cells of deep-sea and coastal mussels. Front. Mar. Sci. 2022, 9, 1034950. [Google Scholar] [CrossRef]
- Saco, A.; Ray-Campos, M.; Novoa, B.; Figueras, A. Transcriptomic response of mussel gills after a Vibrio splendidus infection demonstrates their role in the immune response. Front. Immunol. 2020, 11, 615580. [Google Scholar] [CrossRef] [PubMed]
- Musella, M.; Wathsala, R.; Tavella, T.; Rampelli, S.; Barone, M.; Palladino, G.; Biagi, E.; Brigidi, P.; Turroni, S.; Franzellitti, S.; et al. Tissue-scale microbiota of the Mediterranean mussel (Mytilus galloprovincialis) and its relationship with the environment. Sci. Total Environ. 2020, 717, 137209. [Google Scholar] [CrossRef]
- Voss, P.A.; Gornik, S.G.; Jacobovitz, M.R.; Rupp, S.; Dörr, M.; Maegele, I.; Guse, A. Host nutrient sensing is mediated by mTOR signaling in cnidarian-dinoflagellate symbiosis. Curr. Biol. 2023, 33, 3634–3647. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef] [PubMed]
- McGourty, K.; Thurston, T.L.; Matthews, S.A.; Pinaud, L.; Mota, L.J.; Holden, D.W. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science 2012, 338, 963–967. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Chua, C.E.; Tang, B.L. Role of Rab GTPases and their interacting proteins in mediating metabolic signalling and regulation. Cell. Mol. Life Sci. 2015, 72, 2289–2304. [Google Scholar] [CrossRef] [PubMed]
- Rabanal-Ruiz, Y.; Korolchuk, V.I. mTORC1 and nutrient homeostasis: The central role of the lysosome. Int. J. Mol. Sci. 2018, 19, 818. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Lund-Ricard, Y.; Cormier, P.; Morales, J.; Boutet, A. mTOR Signaling at the Crossroad between Metazoan Regeneration and Human Diseases. Int. J. Mol. Sci. 2020, 21, 2718. [Google Scholar] [CrossRef]
- Valvezan, A.J.; Manning, B.D. Molecular logic of mTORC1 signalling as a metabolic rheostat. Nat. Metab. 2019, 3, 321–333. [Google Scholar] [CrossRef]
- Nyholm, S.V.; McFall-Ngai, M.J. A lasting symbiosis: How the Hawaiian bobtail squid finds and keeps its bioluminescent bacterial partner. Nat. Rev. Microbiol. 2021, 10, 666–679. [Google Scholar] [CrossRef]
- Sachdvena, P.; Sundaramurthy, V. The interplay of host lysosomes and intracellular pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 595502. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Powell, J.D. More TOR: The expanding role of mTOR in regulating immune responses. Immunity 2025, 58, 1629–1645. [Google Scholar] [CrossRef] [PubMed]
- Mannur, G.; Taepakdee, A.; Ho, J.P.; Xiang, T. Leveraging Functional Genomics and Engineering Approaches to Uncover the Molecular Mechanisms of Cnidarian–Dinoflagellate Symbiosis and Broaden Biotechnological Applications. Phycology 2025, 5, 14. [Google Scholar] [CrossRef]
- Smith, C.A.; Ashby, B. Tolerance-conferring defensive symbionts and the evolution of parasite virulence. Evol. Lett. 2023, 7, 262–272. [Google Scholar] [CrossRef]
- Wang, H.; He, K.; Zhang, H.; Zhang, Q.; Cao, L.; Li, J.; Zhong, Z.; Chen, H.; Zhou, L.; Lian, C.; et al. Deciphering deep-sea chemosynthetic symbiosis by single-nucleus RNA-sequencing. eLife 2024, 12, RP88294. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tame, A. Integrated Regulation of Immunity and Nutritional Symbiosis in Deep-Sea Mussels. Mar. Drugs 2025, 23, 425. https://doi.org/10.3390/md23110425
Tame A. Integrated Regulation of Immunity and Nutritional Symbiosis in Deep-Sea Mussels. Marine Drugs. 2025; 23(11):425. https://doi.org/10.3390/md23110425
Chicago/Turabian StyleTame, Akihiro. 2025. "Integrated Regulation of Immunity and Nutritional Symbiosis in Deep-Sea Mussels" Marine Drugs 23, no. 11: 425. https://doi.org/10.3390/md23110425
APA StyleTame, A. (2025). Integrated Regulation of Immunity and Nutritional Symbiosis in Deep-Sea Mussels. Marine Drugs, 23(11), 425. https://doi.org/10.3390/md23110425






