Metabolomic Insights into the Phytochemical Profiles and Seasonal Shifts of Fucus serratus and F. vesiculosus Harvested in Danish Coastal Waters (Aarhus Bay)—An Untargeted High-Resolution Mass-Spectrometry Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Environmental Conditions
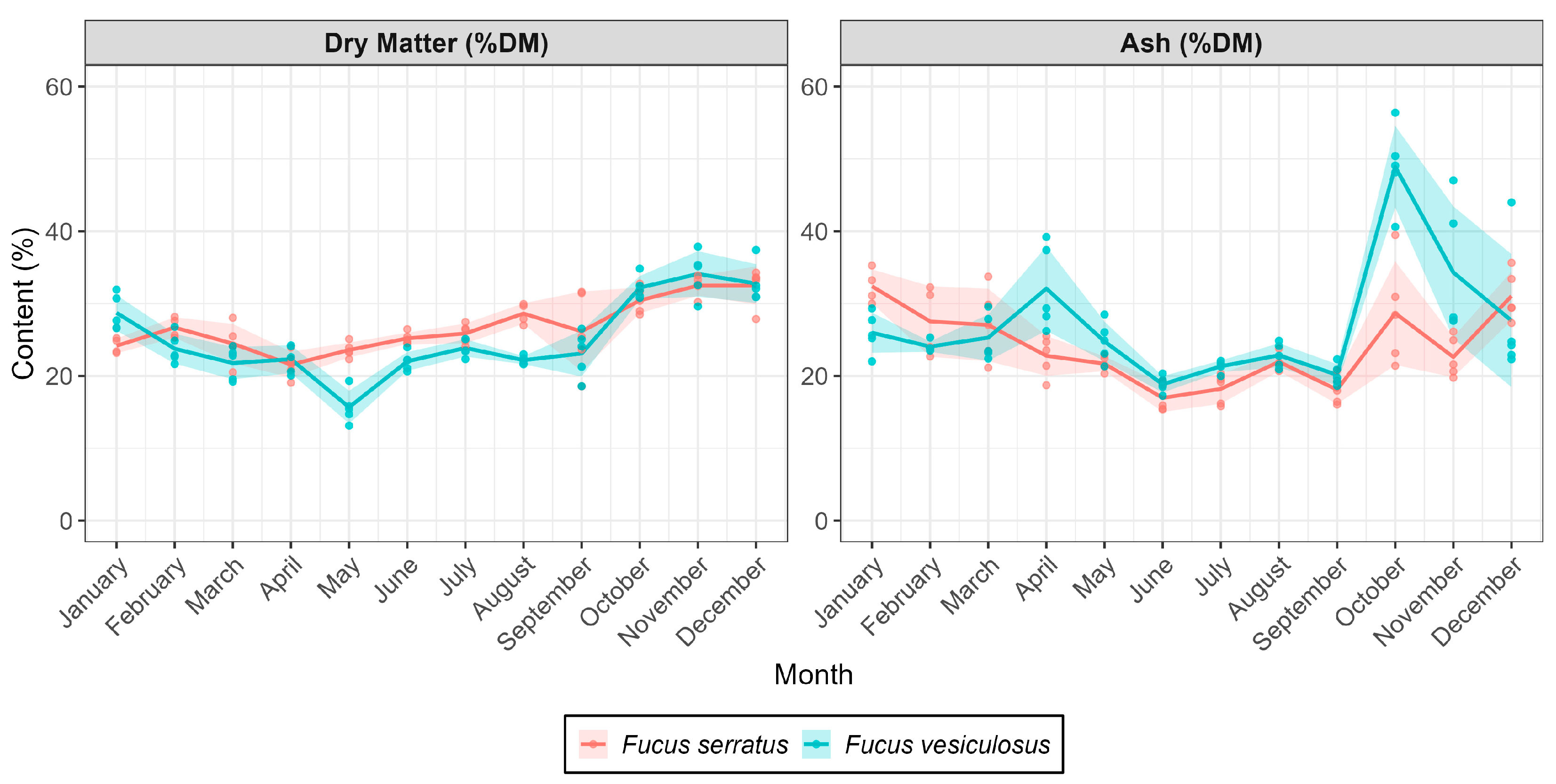
2.2. Proximate Composition and Polyphenol Content in Seaweeds
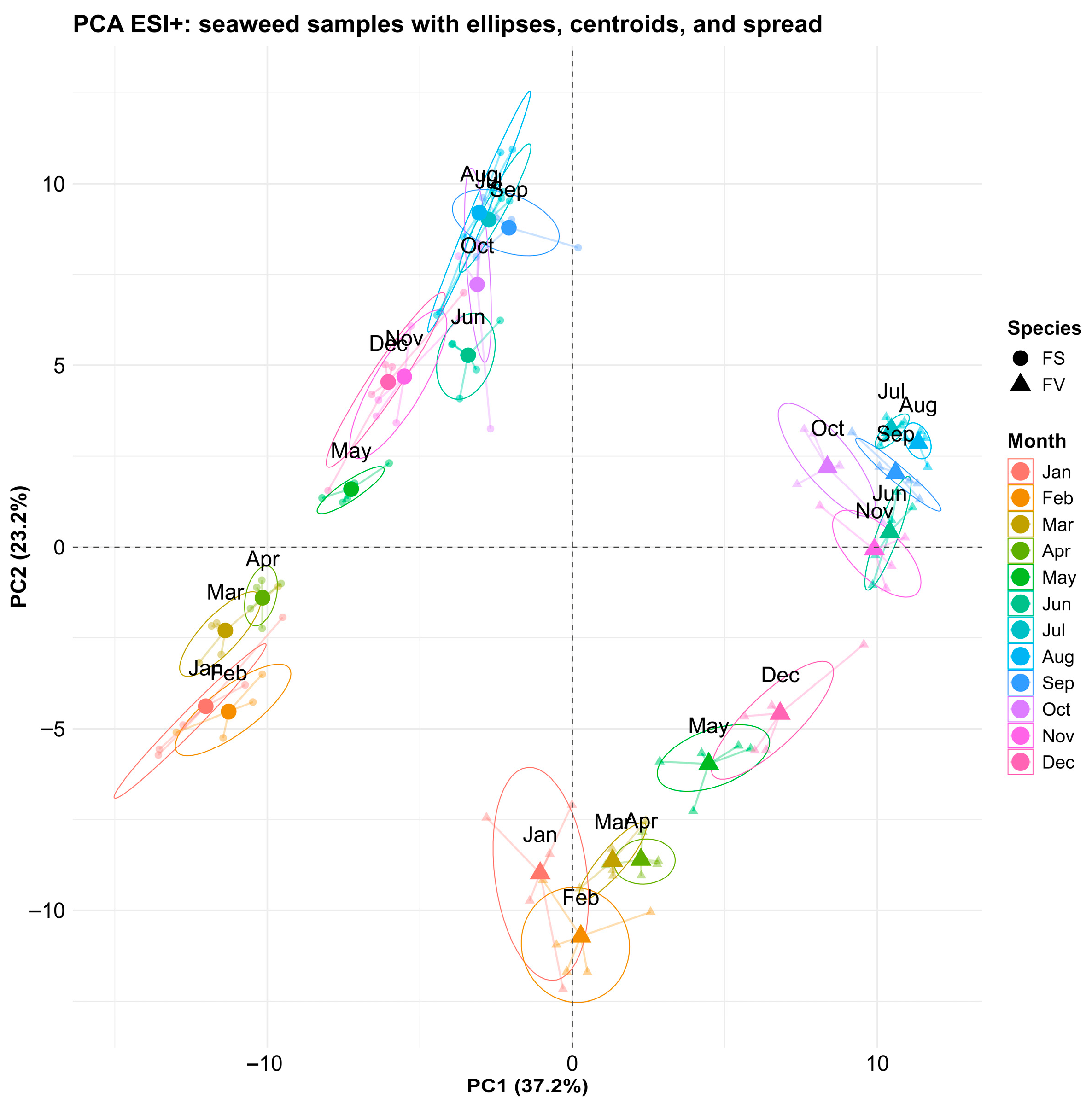
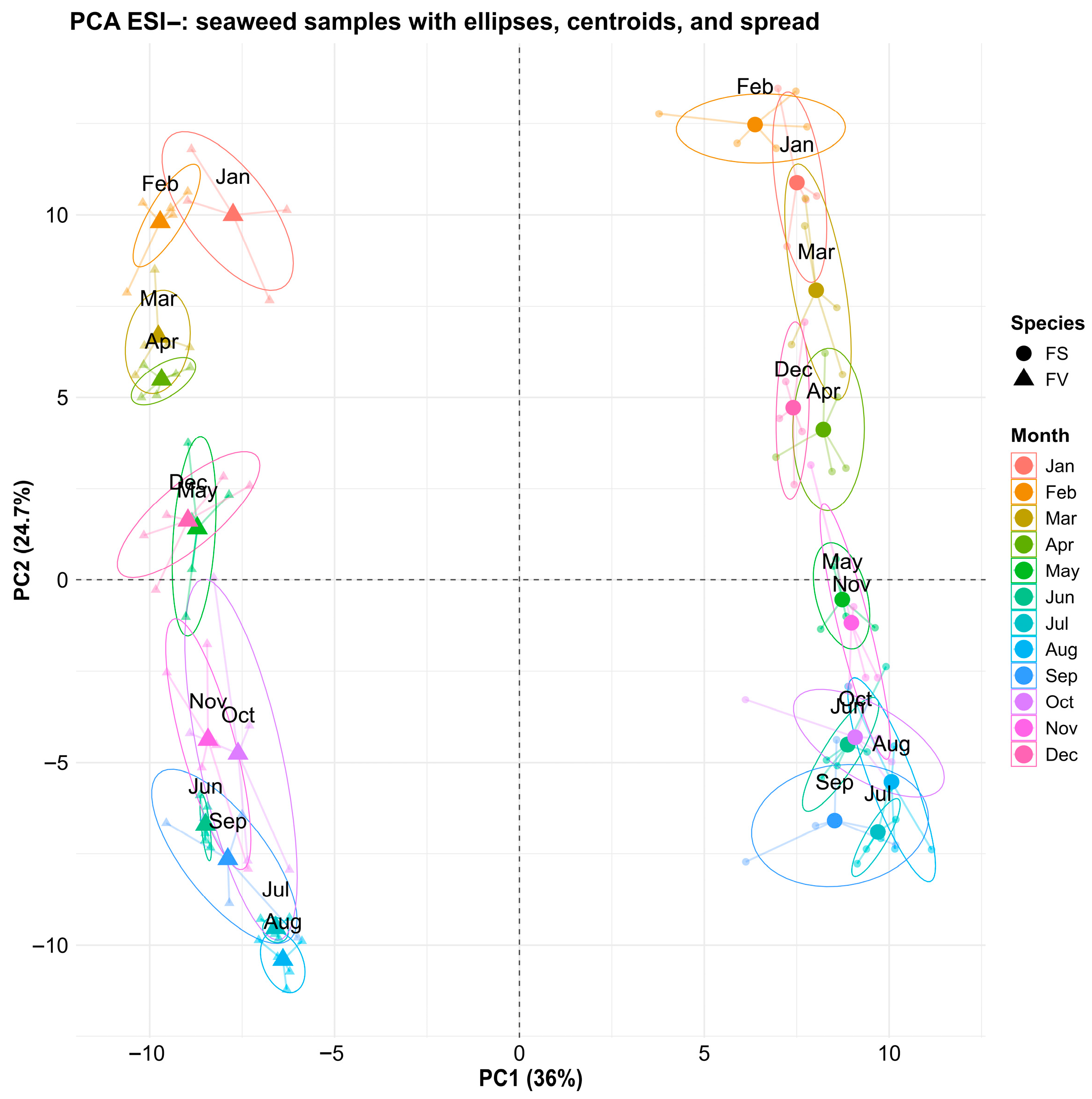
2.3. Untargeted Metabolomics Profiling—Principal Component Analysis Overview
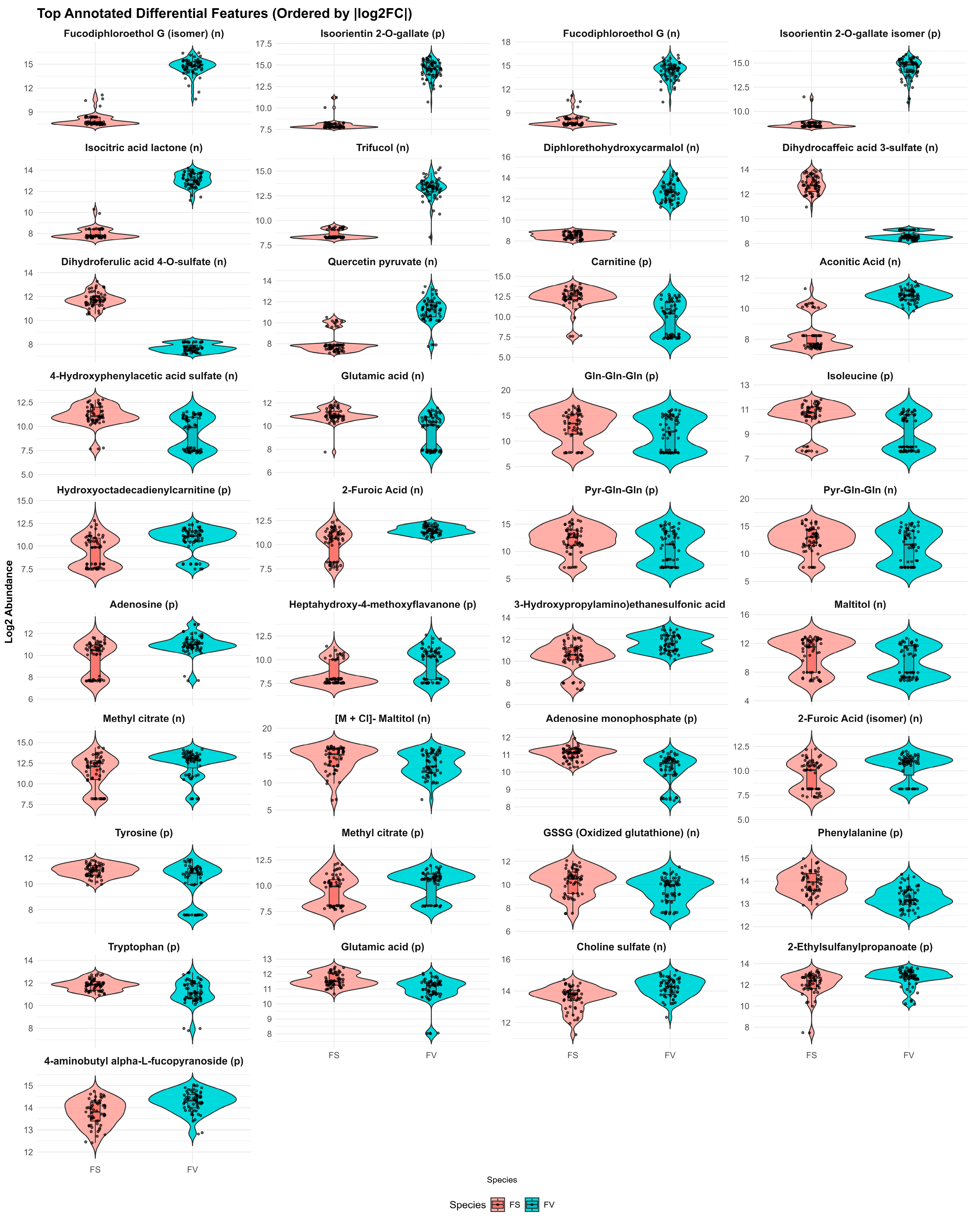
2.4. Species-Specific Metabolomic Differences
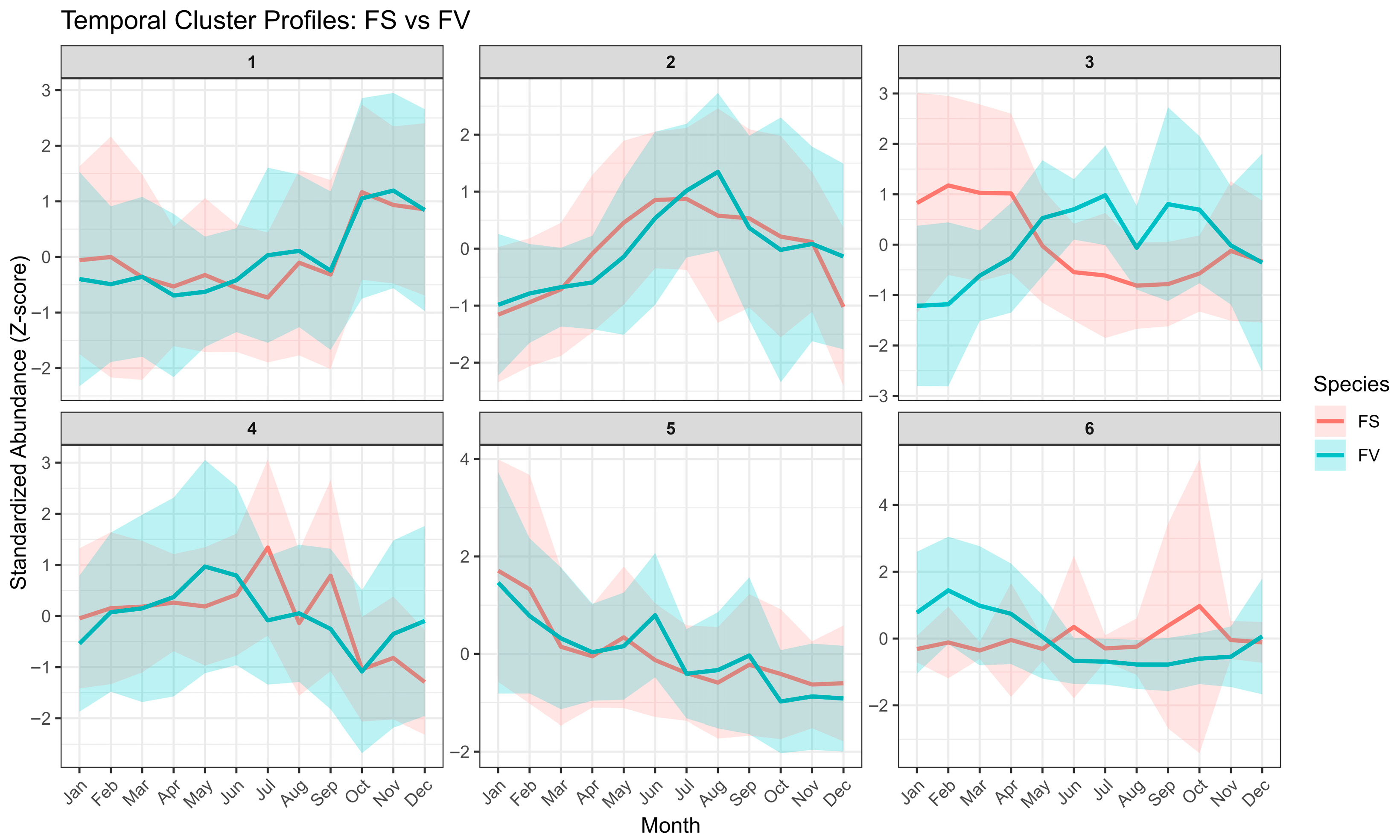
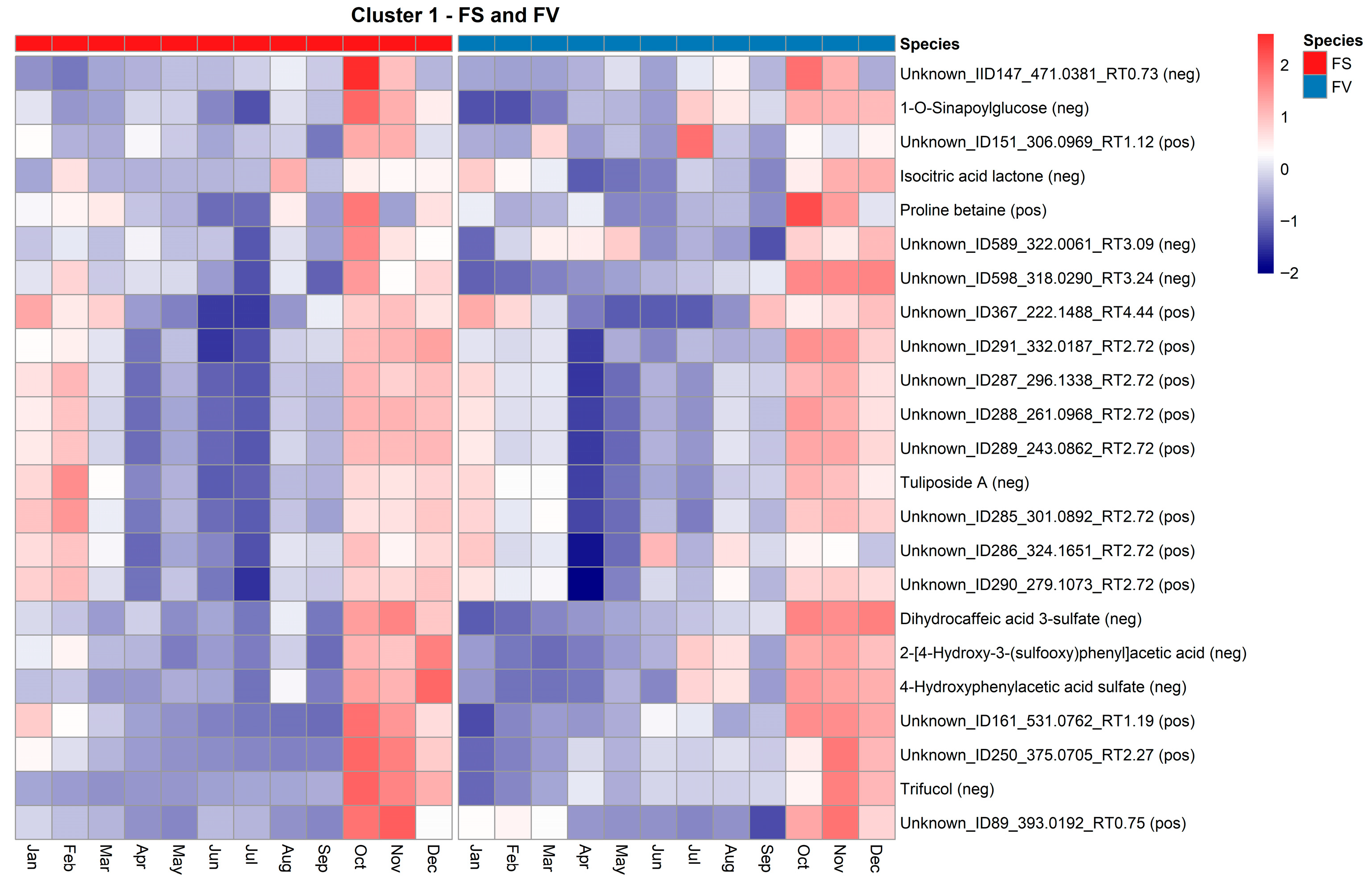
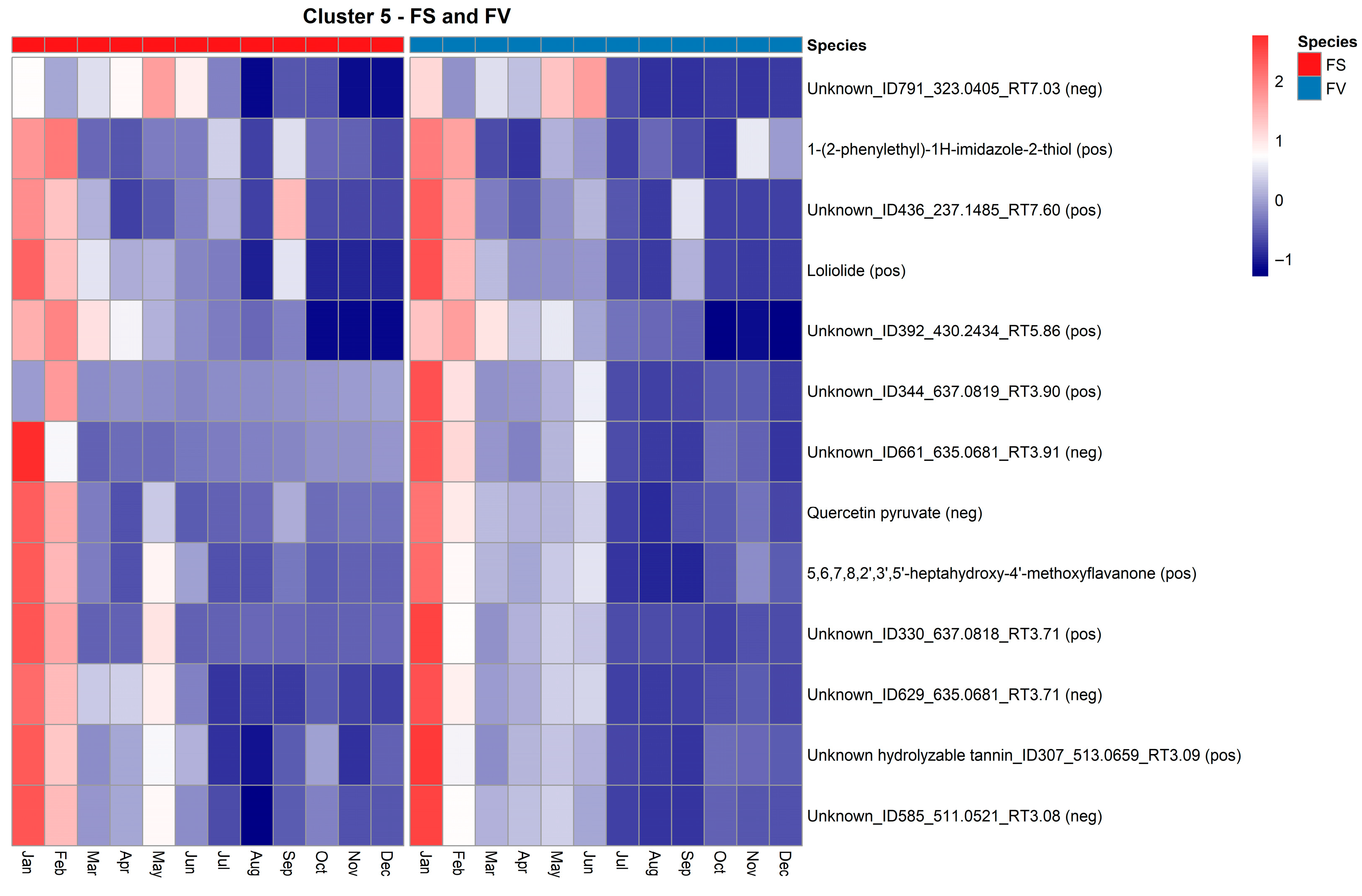
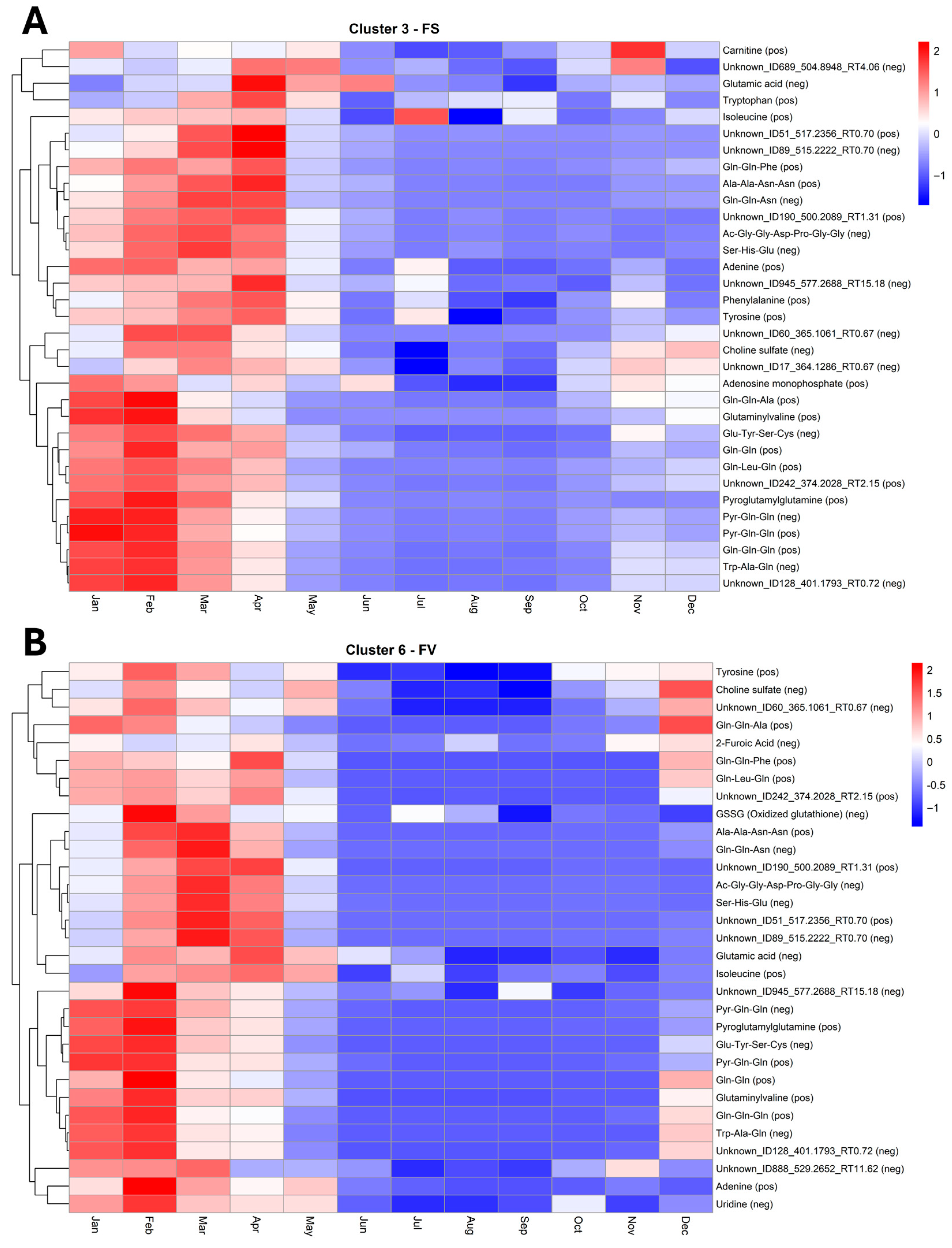
2.5. Temporal Variation of the Fucus Metabolic Profiles
3. Materials and Methods
3.1. Materials
3.2. Seaweed Collection
3.3. Environmental Parameters and Chemical Analysis of the Seawater Nutrient Concentrations
3.4. Analysis of Seaweed Material
3.5. Preprocessing of LC-QTOF-MS Data
3.6. Multivariate Data Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsson, J.; Engkvist, R.; Persson, L.E. Long-term decline and recent recovery of populations along the rocky shores of southeast Sweden, Baltic Sea. Aquat. Ecol. 2004, 38, 587–598. [Google Scholar] [CrossRef]
- Hatchett, W.J.; Coyer, J.A.; Sjotun, K.; Jueterbock, A.; Hoarau, G. A review of reproduction in the seaweed genus (Ochrophyta, Fucales): Background for renewed consideration as a model organism. Front. Mar. Sci. 2022, 9, 1051838. [Google Scholar] [CrossRef]
- Johnson, L.E.; Brawley, S.H.; Adey, W.H. Secondary spread of invasive species: Historic patterns and underlying mechanisms of the continuing invasion of the European rockweed Fucus serratus in eastern North America. Biol. Invasions 2011, 14, 79–97. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical Constituents and Biological Activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Hayes, M. In Vitro Protein Digestibility of Selected Seaweeds. Foods 2022, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, A.; Luck, T.; Nash, R.; Touzet, N. Biochemical profiling and antioxidant activity analysis of commercially relevant seaweeds from northwest Europe. J. Sci. Food Agric. 2024, 104, 6746–6755. [Google Scholar] [CrossRef] [PubMed]
- Madden, M.; Mitra, M.; Ruby, D.; Schwarz, J. Seasonality of Selected Nutritional Constituents of Edible Delmarva Seaweeds. J. Phycol. 2012, 48, 1289–1298. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef]
- Overland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef]
- Thorsteinsson, M.; Weisbjerg, M.R.; Lund, P.; Battelli, M.; Chassé, E.; Bruhn, A.; Nielsen, M.O. Effects of seasonal and interspecies differences in macroalgae procured from temperate seas on the Northern hemisphere on in vitro methane mitigating properties and rumen degradability. Algal Res. 2023, 73, 103139. [Google Scholar] [CrossRef]
- Catarino, M.D.; Pires, S.M.G.; Silva, S.; Costa, F.; Braga, S.S.; Pinto, D.; Silva, A.M.S.; Cardoso, S.M. Overview of Phlorotannins’ Constituents in Fucales. Mar. Drugs 2022, 20, 754. [Google Scholar] [CrossRef]
- Rickert, E.; Wahl, M.; Link, H.; Richter, H.; Pohnert, G. Seasonal Variations in Surface Metabolite Composition of Fucus vesiculosus and Fucus serratus from the Baltic Sea. PLoS ONE 2016, 11, e0168196. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef]
- Thorsteinsson, M.; Chasse, E.; Curtasu, M.V.; Battelli, M.; Bruhn, A.; Hellwing, A.L.F.; Weisbjerg, M.R.; Nielsen, M.O. Potential of 2 northern European brown seaweeds (Fucus serratus and Fucus vesiculosus) as enteric methane inhibitors in dairy cows. J. Dairy Sci. 2024, 107, 10628–10640. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Hansen, H.H.; Dhakal, R.; Aryal, N.; Rai, S.P.; Sapkota, R.; Nielsen, M.O.; Novoa-Garrido, M.; Khanal, P. Interspecies and seasonal variations in macroalgae from the Nordic region: Chemical composition and impacts on rumen fermentation and microbiome assembly. J. Clean. Prod. 2022, 363, 132456. [Google Scholar] [CrossRef]
- Maia, M.R.; Fonseca, A.J.; Oliveira, H.M.; Mendonca, C.; Cabrita, A.R. The Potential Role of Seaweeds in the Natural Manipulation of Rumen Fermentation and Methane Production. Sci. Rep. 2016, 6, 32321. [Google Scholar] [CrossRef] [PubMed]
- Künzel, S.; Yergaliyev, T.; Wild, K.J.; Philippi, H.; Petursdottir, A.H.; Gunnlaugsdottir, H.; Reynolds, C.K.; Humphries, D.J.; Camarinha-Silva, A.; Rodehutscord, M. Methane reduction potential of Brown seaweeds and their influence on nutrient degradation and microbiota composition in a rumen simulation technique. Front. Microbiol. 2022, 13, 889618. [Google Scholar] [CrossRef] [PubMed]
- Curtasu, M.V.; Battelli, M.; Chassé, É.; Bruhn, A.; Nielsen, M.O.; Nørskov, N.P. Seaweed extraction and fractionation as a strategy to identify bioactive compounds with potential methane inhibition in dairy cows. Algal Res. 2025, 92, 104379. [Google Scholar] [CrossRef]
- Carlson, L. Seasonal-Variation in Growth, Reproduction and Nitrogen-Content of Fucus Vesiculosus L in the Oresund, Southern Sweden. Bot. Mar. 1991, 34, 447–453. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2014, 27, 363–373. [Google Scholar] [CrossRef]
- Li, S.; Sun, Y.; Guo, T.; Liu, W.; Tong, X.; Zhang, Z.; Sun, J.; Yang, Y.; Yang, S.; Li, D.; et al. Sargassum mcclurei Mitigating Methane Emissions and Affecting Rumen Microbial Community in In Vitro Rumen Fermentation. Animals 2024, 14, 2057. [Google Scholar] [CrossRef]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef]
- Roskam, E.; Kirwan, S.F.; Kenny, D.A.; O’Donnell, C.; O’Flaherty, V.; Hayes, M.; Waters, S.M. Effect of brown and green seaweeds on diet digestibility, ruminal fermentation patterns and enteric methane emissions using the rumen simulation technique. Front. Anim. Sci. 2022, 3, 1021631. [Google Scholar] [CrossRef]
- Wanapat, M.; Prachumchai, R.; Dagaew, G.; Matra, M.; Phupaboon, S.; Sommai, S.; Suriyapha, C. Potential use of seaweed as a dietary supplement to mitigate enteric methane emission in ruminants. Sci. Total. Environ. 2024, 931, 173015. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Taubner, I.; Böhm, F.; Winde, V.; Böttcher, M.E. Effect of temperature rise and ocean acidification on growth of calcifying tubeworm shells (Spirorbis spirorbis): An in situ benthocosm approach. Biogeosciences 2018, 15, 1425–1445. [Google Scholar] [CrossRef]
- Saderne, V.; Wahl, M. Differential responses of calcifying and non-calcifying epibionts of a brown macroalga to present-day and future upwelling pCO2. PLoS ONE 2013, 8, e70455. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Coelho, M.; Machado, M.; Costa, E.; Gomes, A.M.; Pocas, F.; Sperotto, R.A.; Rosa-Martinez, E.; Vasconcelos, M.; Pintado, M.E.; et al. Integrated Valorization of Fucus spiralis Alga: Polysaccharides and Bioactives for Edible Films and Residues as Biostimulants. Foods 2024, 13, 2938. [Google Scholar] [CrossRef]
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Gall, E.A. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot. Mar. 2004, 47, 410–416. [Google Scholar] [CrossRef]
- Cruces, E.; Huovinen, P.; Gomez, I. Phlorotannin and antioxidant responses upon short-term exposure to UV radiation and elevated temperature in three south Pacific kelps. Photochem. Photobiol. 2012, 88, 58–66. [Google Scholar] [CrossRef]
- Heavisides, E.; Rouger, C.; Reichel, A.F.; Ulrich, C.; Wenzel-Storjohann, A.; Sebens, S.; Tasdemir, D. Seasonal Variations in the Metabolome and Bioactivity Profile of Fucus vesiculosus Extracted by an Optimised, Pressurised Liquid Extraction Protocol. Mar. Drugs 2018, 16, 503. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Creis, E.; Delage, L.; Charton, S.; Goulitquer, S.; Leblanc, C.; Potin, P.; Ar Gall, E. Constitutive or Inducible Protective Mechanisms against UV-B Radiation in the Brown Alga Fucus vesiculosus? A Study of Gene Expression and Phlorotannin Content Responses. PLoS ONE 2015, 10, e0128003. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of phenolic acids. Phytochem. Rev. 2007, 7, 301–311. [Google Scholar] [CrossRef]
- Oppong-Danquah, E.; Blumel, M.; Tasdemir, D. Metabolomics and Microbiomics Insights into Differential Surface Fouling of Three Macroalgal Species of Fucus (Fucales, Phaeophyceae) That Co-Exist in the German Baltic Sea. Mar. Drugs 2023, 21, 595. [Google Scholar] [CrossRef]
- Birkemeyer, C.; Osmolovskaya, N.; Kuchaeva, L.; Tarakhovskaya, E. Distribution of natural ingredients suggests a complex network of metabolic transport between source and sink tissues in the brown alga Fucus vesiculosus. Planta 2019, 249, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, S.E. Developing Sustainable Strategies for Cultivation and Harvest of Fucus vesiculosus. Master’s Thesis, Aarhus University, Aarhus, Denmark, 2019; p. 96. [Google Scholar]
- Herrmann, K.M. The shikimate pathway as an entry to aromatic secondary metabolism. Plant Physiol. 1995, 107, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Rempt, M.; Gebser, B.; Grueneberg, J.; Pohnert, G.; Weinberger, F. Dimethylsulphopropionate (DMSP) and proline from the surface of the brown alga Fucus vesiculosus inhibit bacterial attachment. Biofouling 2012, 28, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Flavonoid sulphates: A new class of sulphur compounds in higher plants. Phytochemistry 1975, 14, 1147–1155. [Google Scholar] [CrossRef]
- Blunden, G.; Morse, P.F.; Mathe, I.; Hohmann, J.; Critchleye, A.T.; Morrell, S. Betaine yields from marine algal species utilized in the preparation of seaweed extracts used in agriculture. Nat. Prod. Commun. 2010, 5, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Blunden, G.; Smith, B.E.; Irons, M.W.; Yang, M.-H.; Roch, O.G.; Patel, A.V. Betaines and tertiary sulphonium compounds from 62 species of marine algae. Biochem. Syst. Ecol. 1992, 20, 373–388. [Google Scholar] [CrossRef]
- Wen, J.; Shi, J.; Meng, M.; Xu, K.; Xu, Y.; Ji, D.; Wang, W.; Xie, C. Metabolic Responses of Pyropia haitanensis to Dehydration-Rehydration Cycles Revealed by Metabolomics. Mar. Drugs 2025, 23, 203. [Google Scholar] [CrossRef]
- Graiff, A.; Bartsch, I.; Glaser, K.; Karsten, U. Seasonal Photophysiological Performance of Adult Western Baltic (Phaeophyceae) Under Ocean Warming and Acidification. Front. Mar. Sci. 2021, 8, 666493. [Google Scholar] [CrossRef]
- Pearson, G.; Kautsky, L.; Serrao, E. Recent evolution in Baltic: Reduced tolerance to emersion stresses compared to intertidal (North Sea) populations. Mar. Ecol. Prog. Ser. 2000, 202, 67–79. [Google Scholar] [CrossRef]
- Reed, R.H.; Davison, I.R.; Chudek, J.A.; Foster, R. The Osmotic Role of Mannitol in the Phaeophyta—An Appraisal. Phycologia 1985, 24, 35–47. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Seasonal Variability of the Biochemical Composition and Antioxidant Properties of Fucus spiralis at Two Azorean Islands. Mar. Drugs 2018, 16, 248. [Google Scholar] [CrossRef]
- Yang, X.; Kang, M.-C.; Lee, K.-W.; Kang, S.-M.; Lee, W.-W.; Jeon, Y.-J. Antioxidant activity and cell protective effect of loliolide isolated from Sargassum ringgoldianum subsp. coreanum. Algae 2011, 26, 201–208. [Google Scholar] [CrossRef]
- Kimura, J.; Maki, N. New loliolide derivatives from the brown alga Undaria pinnatifida. J. Nat. Prod. 2002, 65, 57–58. [Google Scholar] [CrossRef]
- Felline, S.; Del Coco, L.; Kaleb, S.; Guarnieri, G.; Fraschetti, S.; Terlizzi, A.; Fanizzi, F.P.; Falace, A. The response of the algae Fucus virsoides (Fucales, Ochrophyta) to Roundup(R) solution exposure: A metabolomics approach. Environ. Pollut. 2019, 254, 112977. [Google Scholar] [CrossRef]
- Rugiu, L.; Panova, M.; Pereyra, R.T.; Jormalainen, V. Gene regulatory response to hyposalinity in the brown seaweed Fucus vesiculosus. BMC Genom. 2020, 21, 42. [Google Scholar] [CrossRef]
- Sjøtun, K. Seaweeds of Denmark. Phycologia 2023, 62, 394–395. [Google Scholar] [CrossRef]
- Hansen, H.P.; Koroleff, F. Determination of nutrients. In Methods of Seawater Analysis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1999; pp. 159–228. [Google Scholar]
- Curtasu, M.V.; Norskov, N.P. Quantitative distribution of flavan-3-ols, procyanidins, flavonols, flavanone and salicylic acid in five varieties of organic winter dormant Salix spp. by LC-MS/MS. Heliyon 2024, 10, e25129. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative analysis of multimodal mass spectrometry data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef]
- Heuckeroth, S.; Damiani, T.; Smirnov, A.; Mokshyna, O.; Brungs, C.; Korf, A.; Smith, J.D.; Stincone, P.; Dreolin, N.; Nothias, L.F.; et al. Reproducible mass spectrometry data processing and compound annotation in MZmine 3. Nat. Protoc. 2024, 19, 2597–2641. [Google Scholar] [CrossRef]
- Duhrkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Bocker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, D.I. QC:MXP Repeat Injection Based Quality Control, Batch Correction, Exploration & Data Cleaning (Version 2); Zendono: Geneva, Switzerland, 2025. [Google Scholar] [CrossRef]
- Kumar, L.; Futschik, M.E. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5–7. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curtasu, M.V.; Levinsen, J.U.G.; Bruhn, A.; Nielsen, M.O.; Nørskov, N.P. Metabolomic Insights into the Phytochemical Profiles and Seasonal Shifts of Fucus serratus and F. vesiculosus Harvested in Danish Coastal Waters (Aarhus Bay)—An Untargeted High-Resolution Mass-Spectrometry Approach. Mar. Drugs 2025, 23, 417. https://doi.org/10.3390/md23110417
Curtasu MV, Levinsen JUG, Bruhn A, Nielsen MO, Nørskov NP. Metabolomic Insights into the Phytochemical Profiles and Seasonal Shifts of Fucus serratus and F. vesiculosus Harvested in Danish Coastal Waters (Aarhus Bay)—An Untargeted High-Resolution Mass-Spectrometry Approach. Marine Drugs. 2025; 23(11):417. https://doi.org/10.3390/md23110417
Chicago/Turabian StyleCurtasu, Mihai Victor, Jørgen Ulrik Graudal Levinsen, Annette Bruhn, Mette Olaf Nielsen, and Natalja P. Nørskov. 2025. "Metabolomic Insights into the Phytochemical Profiles and Seasonal Shifts of Fucus serratus and F. vesiculosus Harvested in Danish Coastal Waters (Aarhus Bay)—An Untargeted High-Resolution Mass-Spectrometry Approach" Marine Drugs 23, no. 11: 417. https://doi.org/10.3390/md23110417
APA StyleCurtasu, M. V., Levinsen, J. U. G., Bruhn, A., Nielsen, M. O., & Nørskov, N. P. (2025). Metabolomic Insights into the Phytochemical Profiles and Seasonal Shifts of Fucus serratus and F. vesiculosus Harvested in Danish Coastal Waters (Aarhus Bay)—An Untargeted High-Resolution Mass-Spectrometry Approach. Marine Drugs, 23(11), 417. https://doi.org/10.3390/md23110417




