Starfish-Derived Extracts Enhance Mitophagy and Suppress Senescence-Associated Markers in Human Dermal Fibroblasts
Abstract
1. Introduction
2. Results
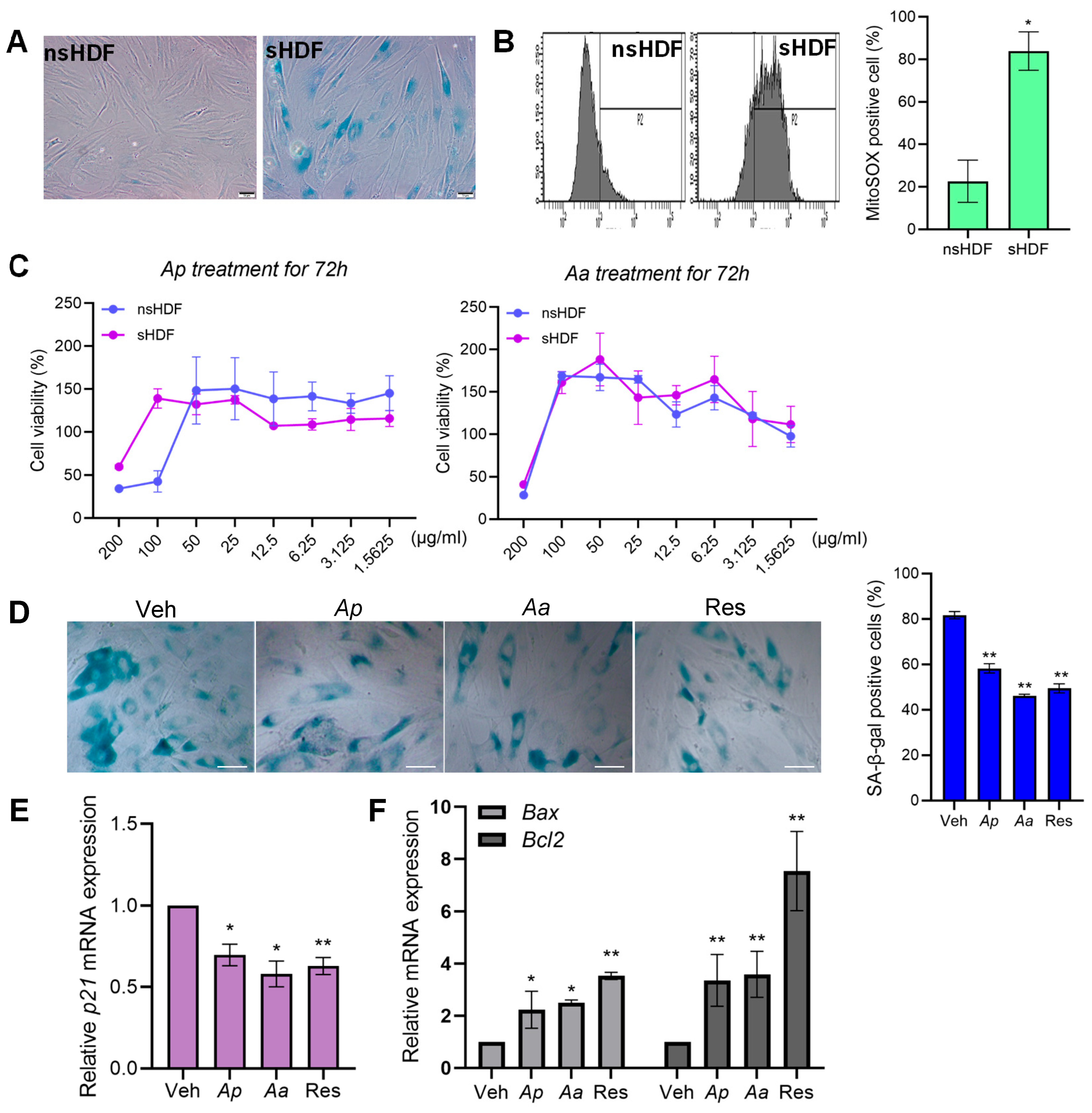
2.1. Asterias pectinifera (Ap) and Asterias amurensis (Aa) Treatment Reduce Senescence in Senescent Human Dermal Fibroblasts
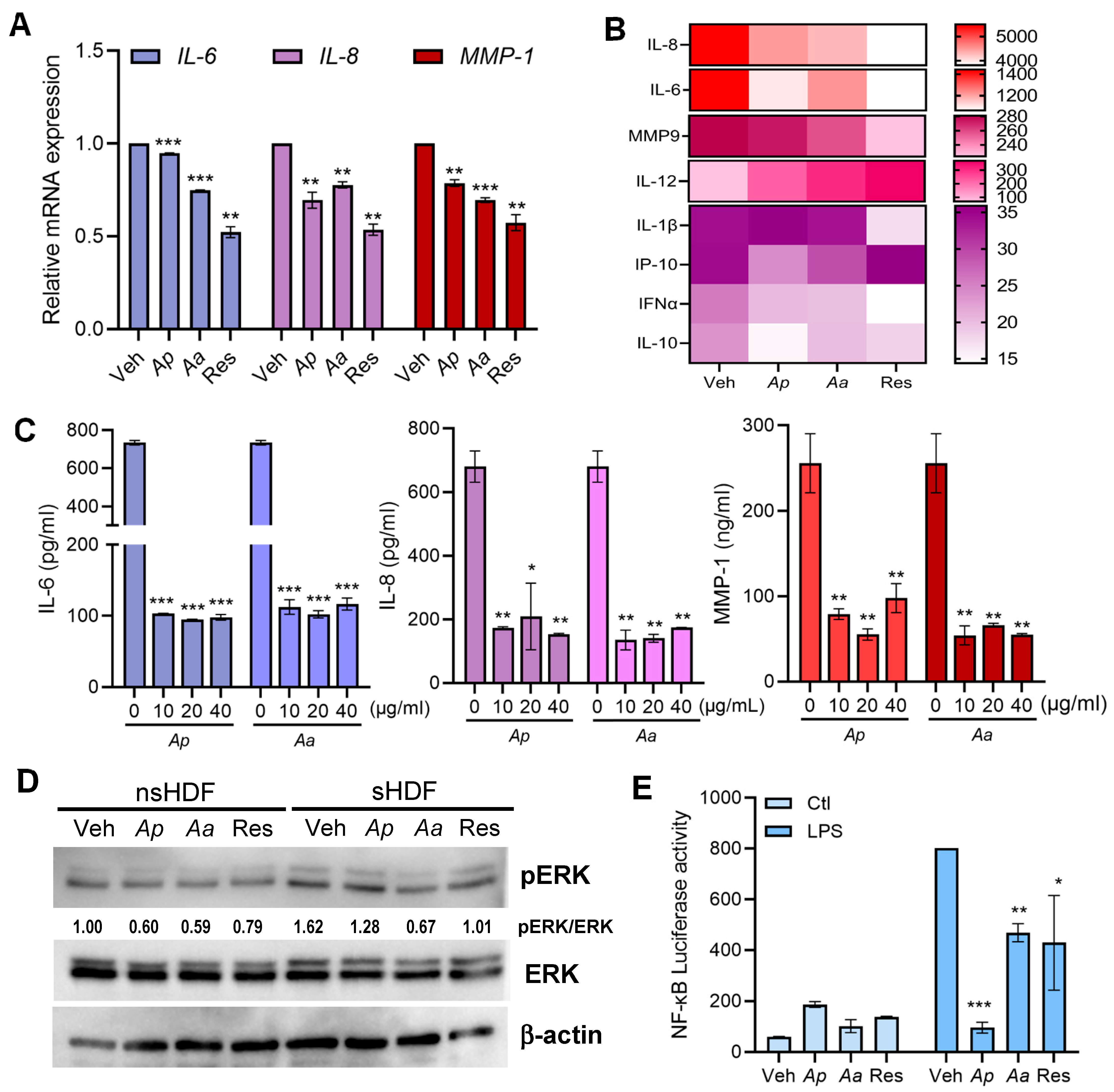
2.2. Anti-Inflammatory Effects of Ap or Aa-Derived Extracts in Human Dermal Fibroblasts
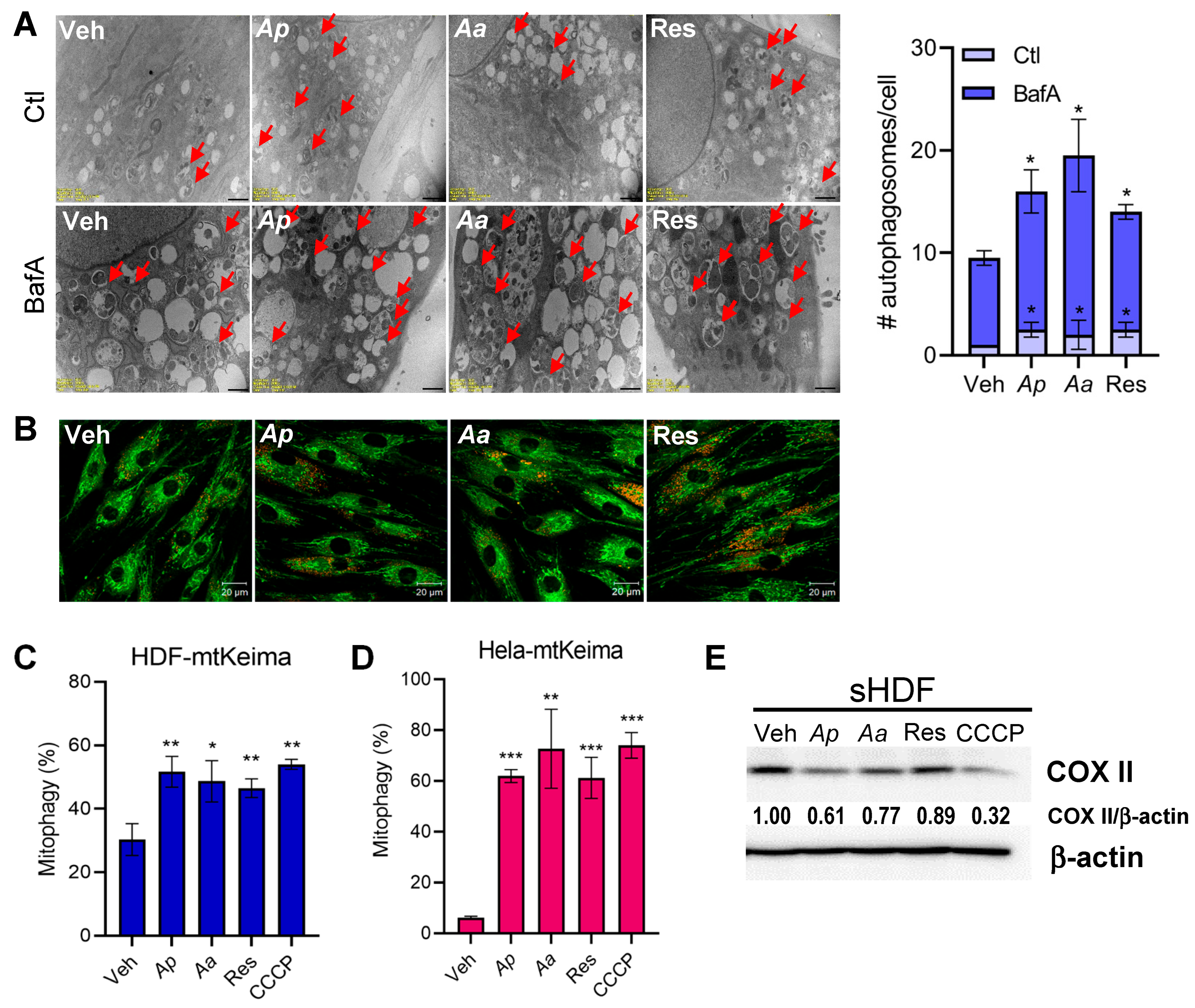
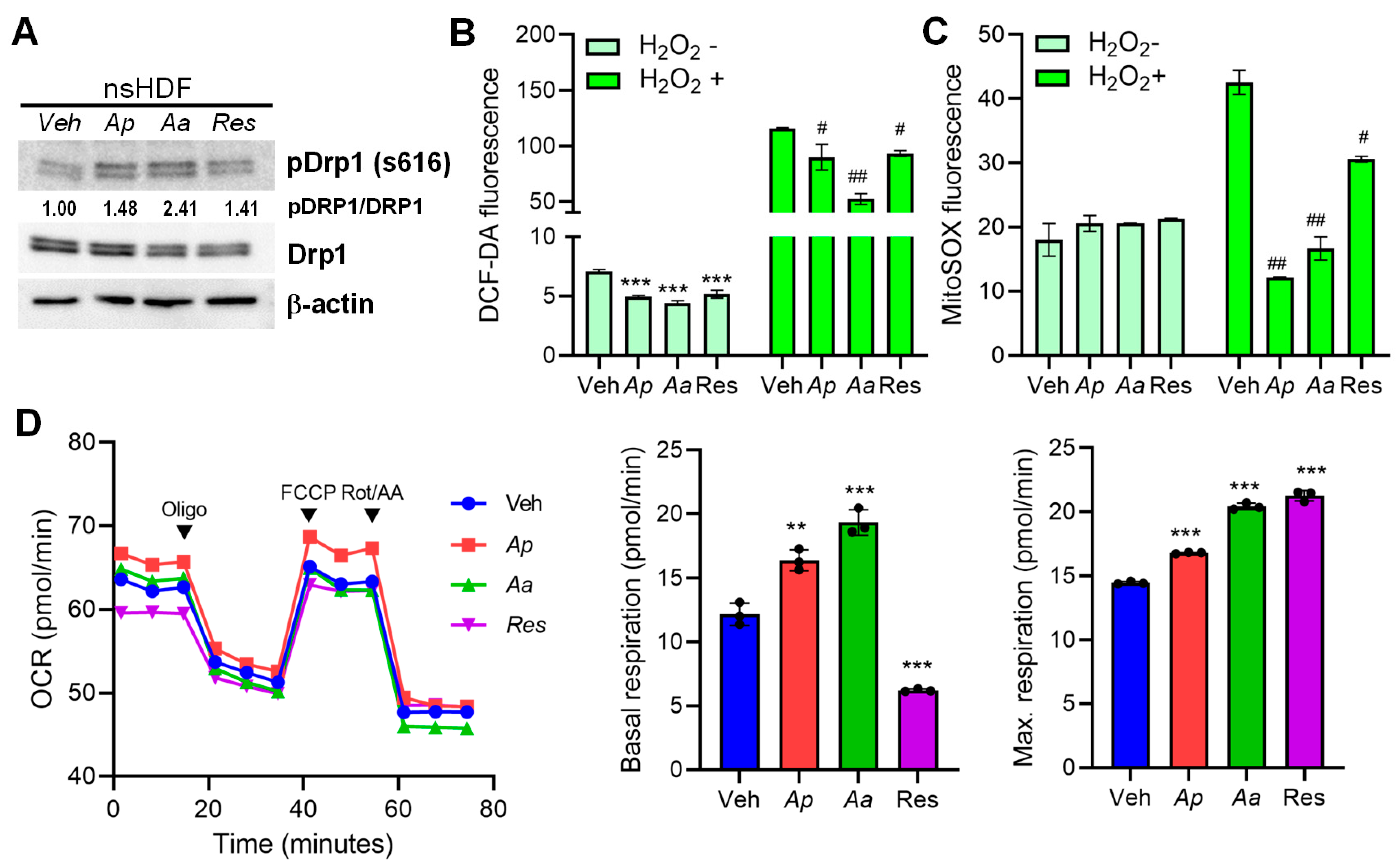
2.3. Starfish-Derived Extracts Promote Mitophagy in HDFs
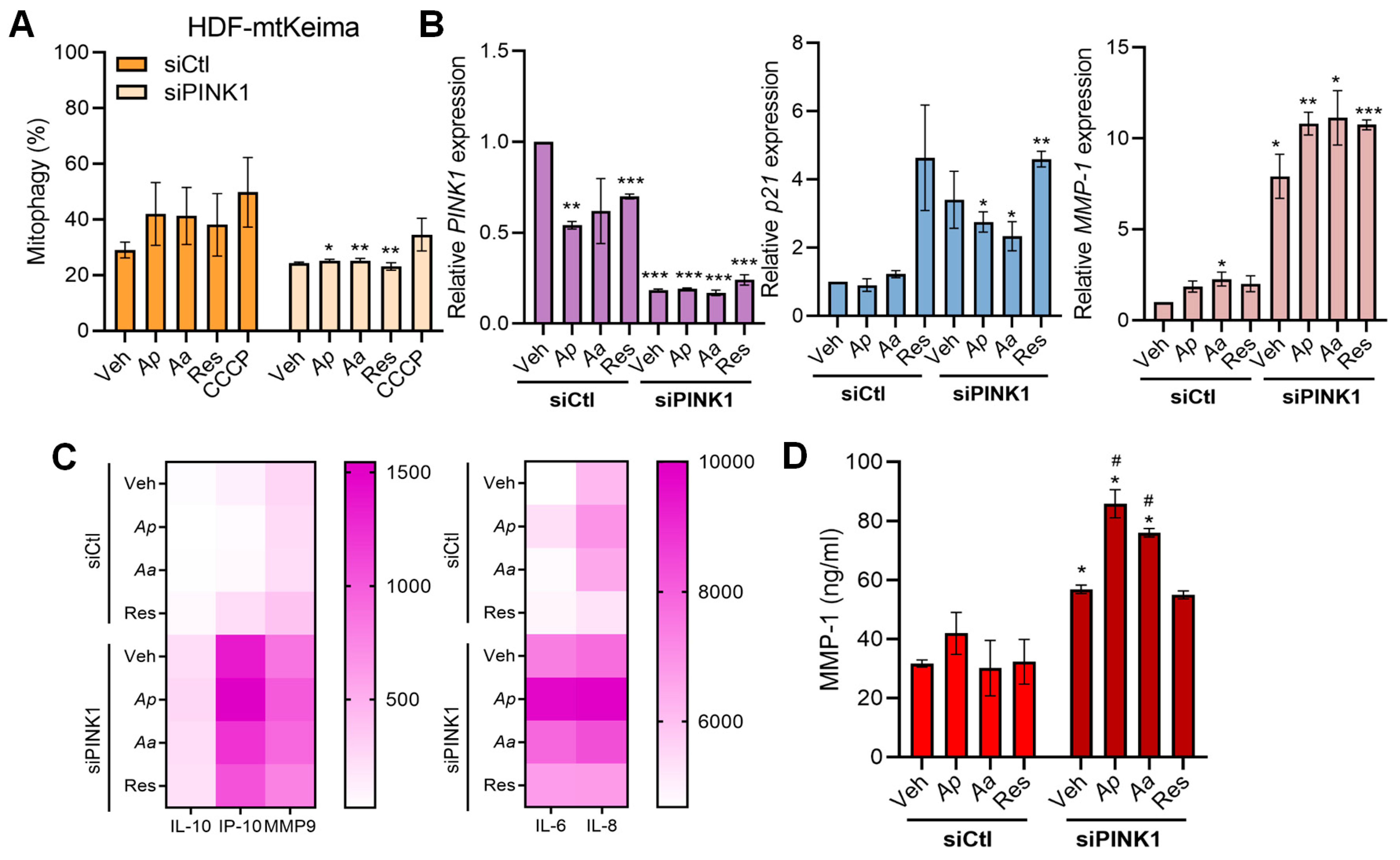
2.4. Starfish-Derived Extracts Improve Mitochondrial Function in Human Dermal Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Preparation of Ap or Aa-Derived Extracts
4.2. Replicative Senescence Cell Model
4.3. Cell Viability Assay
4.4. Quantification of Mitophagy Activity Using mtKeima
4.5. ROS Measurement Assay
4.6. NF-κB Luciferase Reporter Assay
4.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8. Measurement of Cytokines and Chemokine Secretion
4.9. siRNA Transfection
4.10. Immunoblot Analysis
4.11. Confocal Microscopy
4.12. Transmission Electron Microscopy (TEM)
4.13. Mitochondrial Respiration Analysis
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monmai, C.; Go, S.H.; Shin, I.S.; You, S.; Kim, D.O.; Kang, S.; Park, W.J. Anti-Inflammatory Effect of Asterias amurensis Fatty Acids through NF-kappaB and MAPK Pathways against LPS-Stimulated RAW264.7 Cells. J. Microbiol. Biotechnol. 2018, 28, 1635–1644. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsieh, H.J.; Hsieh, C.H.; Hwang, D.F. Antioxidative and anticancer activities of various ethanolic extract fractions from crown-of-thorns starfish (Acanthaster planci). Environ. Toxicol. Pharmacol. 2014, 38, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Jin, W.; Zhang, Q. The antioxidant activities and neuroprotective effect of polysaccharides from the starfish Asterias rollestoni. Carbohydr. Polym. 2013, 95, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.H.; Yang, K.M.; Kim, J.K.; Nam, B.H.; Kim, G.Y.; Lee, S.W.; Seo, S.Y.; Jo, W.S. Inhibitory effects of Asterina pectinifera extracts on melanin biosynthesis through tyrosinase activity. Int. J. Mol. Med. 2013, 31, 205–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thao, N.P.; Cuong, N.X.; Luyen, B.T.; Quang, T.H.; Hanh, T.T.; Kim, S.; Koh, Y.S.; Nam, N.H.; Van Kiem, P.; Van Minh, C.; et al. Anti-inflammatory components of the starfish Astropecten polyacanthus. Mar. Drugs 2013, 11, 2917–2926. [Google Scholar] [CrossRef]
- Oh, S.J.; Park, J.Y.; Won, B.; Oh, Y.T.; Yang, S.C.; Shin, O.S. Asterias pectinifera-Derived Collagen Peptides Mixed with Halocynthia roretzi Extracts Exhibit Anti-Photoaging Activities during Exposure to UV Irradiation, and Antibacterial Properties. J. Microbiol. Biotechnol. 2022, 32, 1382–1389. [Google Scholar] [CrossRef]
- Han, S.-B.; Won, B.; Yang, S.-C.; Kim, D.-H. Asterias pectinifera derived collagen peptide-encapsulating elastic nanoliposomes for the cosmetic application. J. Ind. Eng. Chem. 2021, 98, 289–297. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Man, M.Q.; Hu, L. Aging in the dermis: Fibroblast senescence and its significance. Aging Cell 2024, 23, e14054. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in oxidative stress, inflammation and aging: From mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Xu, T.; Gan, D.; Ma, Z.; Zhang, H.; Zhang, J.; Zeng, Q.; Xu, D. PINK1-mediated mitophagy attenuates pathological cardiac hypertrophy by suppressing the mtDNA release-activated cGAS-STING pathway. Cardiovasc. Res. 2025, 121, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Rao, Z.; Xu, J.; Sun, Y.; Hu, H.; Wang, P.; Xia, Y.; Pan, X.; Tang, W.; Chen, Z.; et al. Defective mitophagy in aged macrophages promotes mitochondrial DNA cytosolic leakage to activate STING signaling during liver sterile inflammation. Aging Cell 2022, 21, e13622. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Pei, Y.; Li, H.; Jiang, Y.; Jin, W.; Gao, Y.; Liu, W.; Guan, X.; Qiao, Y.; Gao, X.; et al. PINK1 Deficiency Facilitates Palmitic Acid-Induced Inflammation by Disrupting Mitochondrial Function to Activate mtDNA-cGAS-STING Signaling. Cell Biochem. Funct. 2025, 43, e70092. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Huang, W.; Xiong, L.; Ma, C.; Wang, Y.; Sun, P.; Shi, D.; Li, L.; Yan, H.; Wu, Y. FUNDC1-mediated mitophagy regulates photodamage independently of the PINK1/Parkin-dependent pathway. Free Radic. Biol. Med. 2024, 225, 630–640. [Google Scholar] [CrossRef]
- He, H.; Xiong, L.; Jian, L.; Li, L.; Wu, Y.; Qiao, S. Role of mitochondria on UV-induced skin damage and molecular mechanisms of active chemical compounds targeting mitochondria. J. Photochem. Photobiol. B 2022, 232, 112464. [Google Scholar] [CrossRef]
- Li, C.; Zhu, Y.; Liu, W.; Xiang, W.; He, S.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Impaired mitophagy causes mitochondrial DNA leakage and STING activation in ultraviolet B-irradiated human keratinocytes HaCaT. Arch. Biochem. Biophys. 2023, 737, 109553. [Google Scholar] [CrossRef]
- Yuan, X.; Li, H.; Lee, J.S.; Lee, D.H. Role of Mitochondrial Dysfunction in UV-Induced Photoaging and Skin Cancers. Exp. Dermatol. 2025, 34, e70114. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Zhang, S.; Wang, Y.; Pei, X.; Chen, Y.; Zhang, J.; Zhang, Y.; Du, Y.; Hao, S.; et al. Mitochondrial dysfunction in the regulation of aging and aging-related diseases. Cell Commun. Signal 2025, 23, 290. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiang, W.; He, S.; San, Z.; Liu, W.; Wu, J.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; et al. Collagen I protects human keratinocytes HaCaT against UVB injury via restoring PINK1/parkin-mediated mitophagy. Arch. Biochem. Biophys. 2024, 753, 109905. [Google Scholar] [CrossRef]
- Zhou, D.D.; Luo, M.; Huang, S.Y.; Saimaiti, A.; Shang, A.; Gan, R.Y.; Li, H.B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Wu, X.; Xu, Y.; Tan, Q. Resveratrol activates autophagy and protects from UVA-induced photoaging in human skin fibroblasts and the skin of male mice by regulating the AMPK pathway. Biogerontology 2024, 25, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Li, R.; Gao, T. Role of Mitochondrial Dynamics in Skin Homeostasis: An Update. Int. J. Mol. Sci. 2025, 26, 1803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, X.; Li, M.; Yu, X.; Huang, F.; Wang, Y.; Yan, Y.; Zhang, H.; Shi, Y.; He, X. The role of mitochondrial quality surveillance in skin aging: Focus on mitochondrial dynamics, biogenesis and mitophagy. Ageing Res. Rev. 2023, 87, 101917. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Diniz, L.P.; Araujo, A.P.B.; Carvalho, C.F.; Matias, I.; de Sa Hayashide, L.; Marques, M.; Pessoa, B.; Andrade, C.B.V.; Vargas, G.; Queiroz, D.D.; et al. Accumulation of damaged mitochondria in aging astrocytes due to mitophagy dysfunction: Implications for susceptibility to mitochondrial stress. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167470. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef]
- Cavinato, M.; Martic, I.; Wedel, S.; Pittl, A.; Koziel, R.; Weinmmullner, R.; Schosserer, M.; Jenewein, B.; Bobbili, M.R.; Arcalis, E.; et al. Elimination of damaged mitochondria during UVB-induced senescence is orchestrated by NIX-dependent mitophagy. Aging Cell 2024, 23, e14186. [Google Scholar] [CrossRef]
- Kelly, G.; Kataura, T.; Panek, J.; Ma, G.; Salmonowicz, H.; Davis, A.; Kendall, H.; Brookes, C.; Ayine-Tora, D.M.; Banks, P.; et al. Suppressed basal mitophagy drives cellular aging phenotypes that can be reversed by a p62-targeting small molecule. Dev. Cell 2024, 59, 1924–1939.e7. [Google Scholar] [CrossRef]
- Yang, D.; Ying, J.; Wang, X.; Zhao, T.; Yoon, S.; Fang, Y.; Zheng, Q.; Liu, X.; Yu, W.; Hua, F. Mitochondrial Dynamics: A Key Role in Neurodegeneration and a Potential Target for Neurodegenerative Disease. Front. Neurosci. 2021, 15, 654785. [Google Scholar] [CrossRef]
- Slim, C.; Zaouali, M.A.; Nassrallah, H.; Ammar, H.H.; Majdoub, H.; Bouraoui, A.; Abdennebi, H.B. Protective potential effects of fucoidan in hepatic cold ischemia-rerfusion injury in rats. Int. J. Biol. Macromol. 2020, 155, 498–507. [Google Scholar] [CrossRef]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Fucoidan Suppresses Mitochondrial Dysfunction and Cell Death against 1-Methyl-4-Phenylpyridinum-Induced Neuronal Cytotoxicity via Regulation of PGC-1alpha Expression. Mar. Drugs 2019, 17, 518. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Jeong, D.J.; Kim, E.; Choi, J.H.; Jang, H.J.; Kim, Y.Y.; Um, J.H.; Lee, J.; Lee, Y.J.; Lee, K.M.; et al. A novel marine-derived mitophagy inducer ameliorates mitochondrial dysfunction and thermal hypersensitivity in paclitaxel-induced peripheral neuropathy. Br. J. Pharmacol. 2024, 181, 4012–4027. [Google Scholar] [CrossRef] [PubMed]
- Riccio, G.; Martinez, K.A.; Martin, J.; Reyes, F.; D’Ambra, I.; Lauritano, C. Jellyfish as an Alternative Source of Bioactive Antiproliferative Compounds. Mar. Drugs 2022, 20, 350. [Google Scholar] [CrossRef] [PubMed]
- Merquiol, L.; Romano, G.; Ianora, A.; D’Ambra, I. Biotechnological Applications of Scyphomedusae. Mar. Drugs 2019, 17, 604. [Google Scholar] [CrossRef]
- Park, S.; Choi, S.G.; Yoo, S.M.; Son, J.H.; Jung, Y.K. Choline dehydrogenase interacts with SQSTM1/p62 to recruit LC3 and stimulate mitophagy. Autophagy 2014, 10, 1906–1920. [Google Scholar] [CrossRef]
- Kim, J.A.; Seong, R.K.; Shin, O.S. Enhanced Viral Replication by Cellular Replicative Senescence. Immune Netw. 2016, 16, 286–295. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, Y.Y.; Ma, R.; Choi, S.T.; Choi, S.M.; Cho, J.H.; Hur, J.Y.; Yoo, Y.; Han, K.; Park, H.; et al. Pharmacological targeting of mitophagy via ALT001 improves herpes simplex virus 1 (HSV1)-mediated microglial inflammation and promotes amyloid beta phagocytosis by restricting HSV1 infection. Theranostics 2025, 15, 4890–4908. [Google Scholar] [CrossRef]
- Lee, J.K.; Oh, S.J.; Gim, J.A.; Shin, O.S. miR-10a, miR-30c, and miR-451a Encapsulated in Small Extracellular Vesicles Are Prosenescence Factors in Human Dermal Fibroblasts. J. Investig. Dermatol. 2022, 142, 2570–2579.e6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Kim, J.; Won, B.; Lee, D.H.; Shin, O.S. Starfish-Derived Extracts Enhance Mitophagy and Suppress Senescence-Associated Markers in Human Dermal Fibroblasts. Mar. Drugs 2025, 23, 418. https://doi.org/10.3390/md23110418
Lee HJ, Kim J, Won B, Lee DH, Shin OS. Starfish-Derived Extracts Enhance Mitophagy and Suppress Senescence-Associated Markers in Human Dermal Fibroblasts. Marine Drugs. 2025; 23(11):418. https://doi.org/10.3390/md23110418
Chicago/Turabian StyleLee, Hyun Jung, Junhee Kim, Bada Won, Dong Hun Lee, and Ok Sarah Shin. 2025. "Starfish-Derived Extracts Enhance Mitophagy and Suppress Senescence-Associated Markers in Human Dermal Fibroblasts" Marine Drugs 23, no. 11: 418. https://doi.org/10.3390/md23110418
APA StyleLee, H. J., Kim, J., Won, B., Lee, D. H., & Shin, O. S. (2025). Starfish-Derived Extracts Enhance Mitophagy and Suppress Senescence-Associated Markers in Human Dermal Fibroblasts. Marine Drugs, 23(11), 418. https://doi.org/10.3390/md23110418







