Sea Cucumber (Isostichopus badionotus): Bioactivity and Wound Healing Capacity In Vitro of Small Peptide Isolates from Digests of Whole-Body Wall or Purified Collagen
Abstract
1. Introduction
2. Results
2.1. Composition of I. badionotus
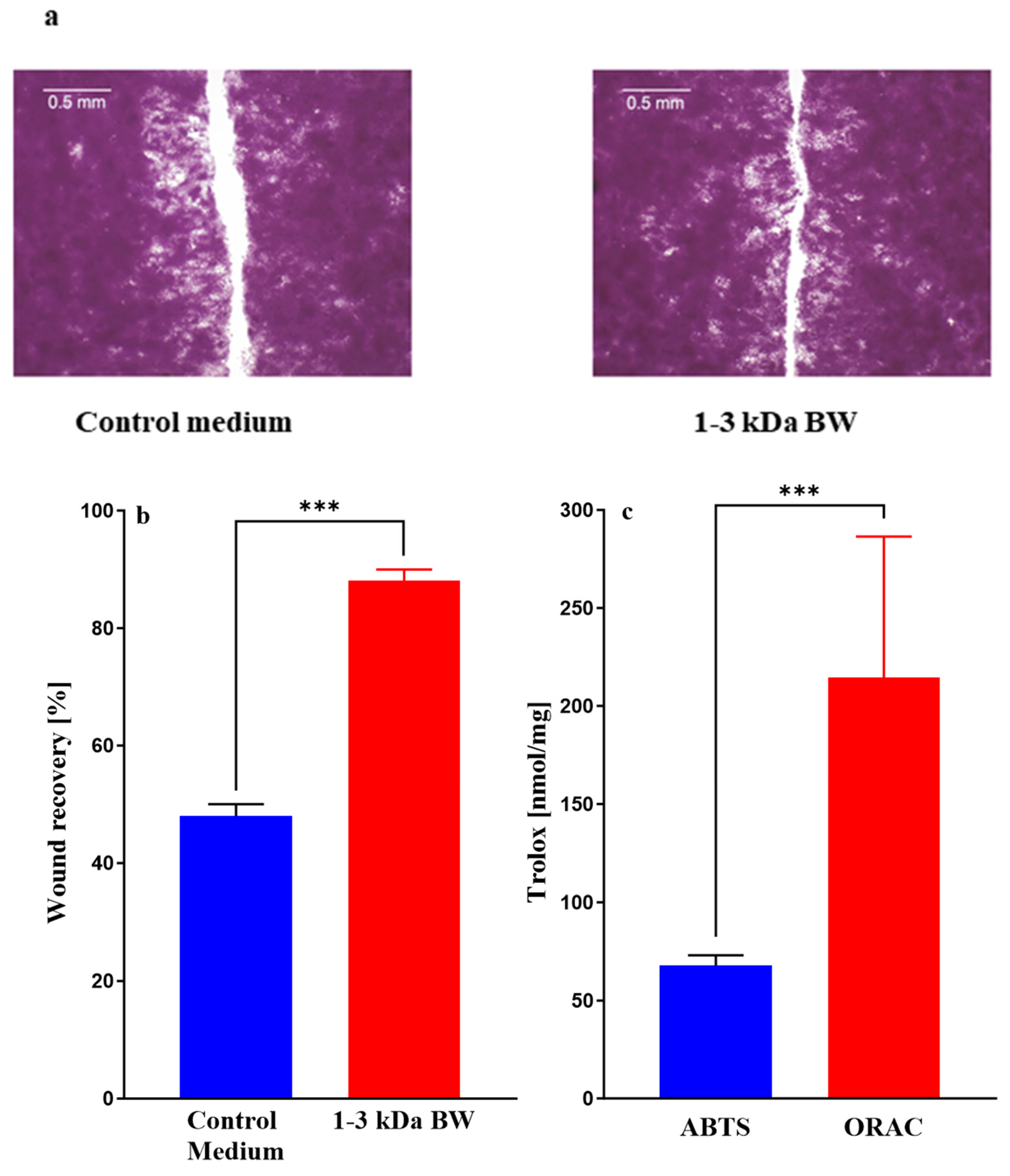
2.2. Initial Screening of Body Wall Fractions for Wound Healing Activity
2.3. Collagen Isolation and Characterization
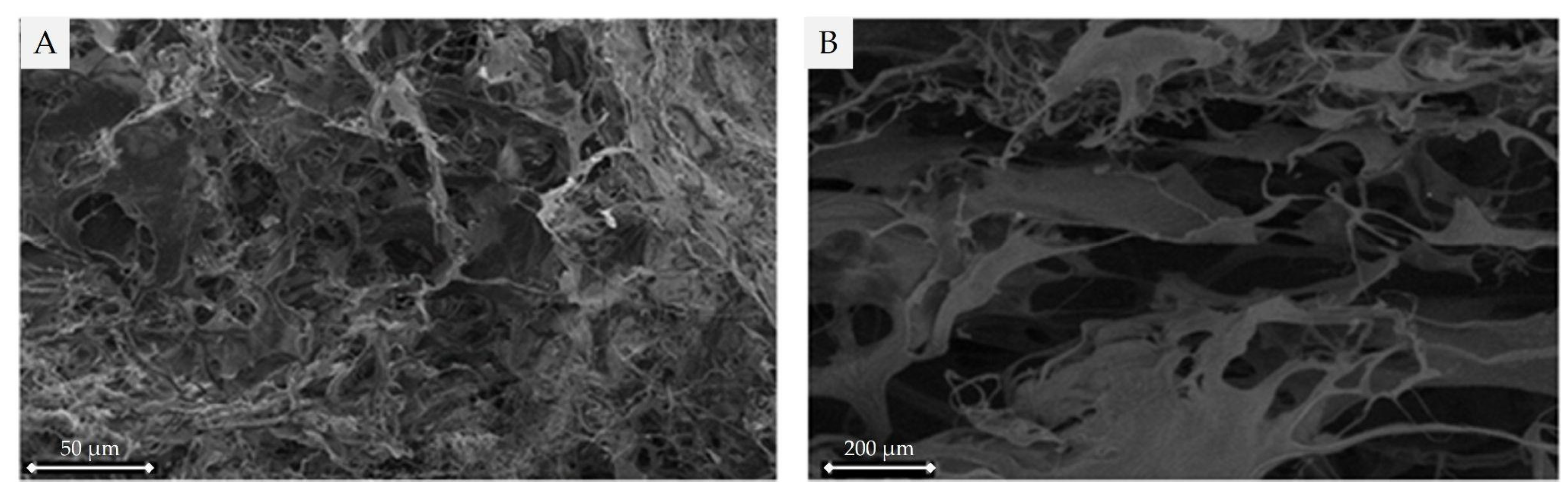
2.3.1. Scanning Electron Microscope
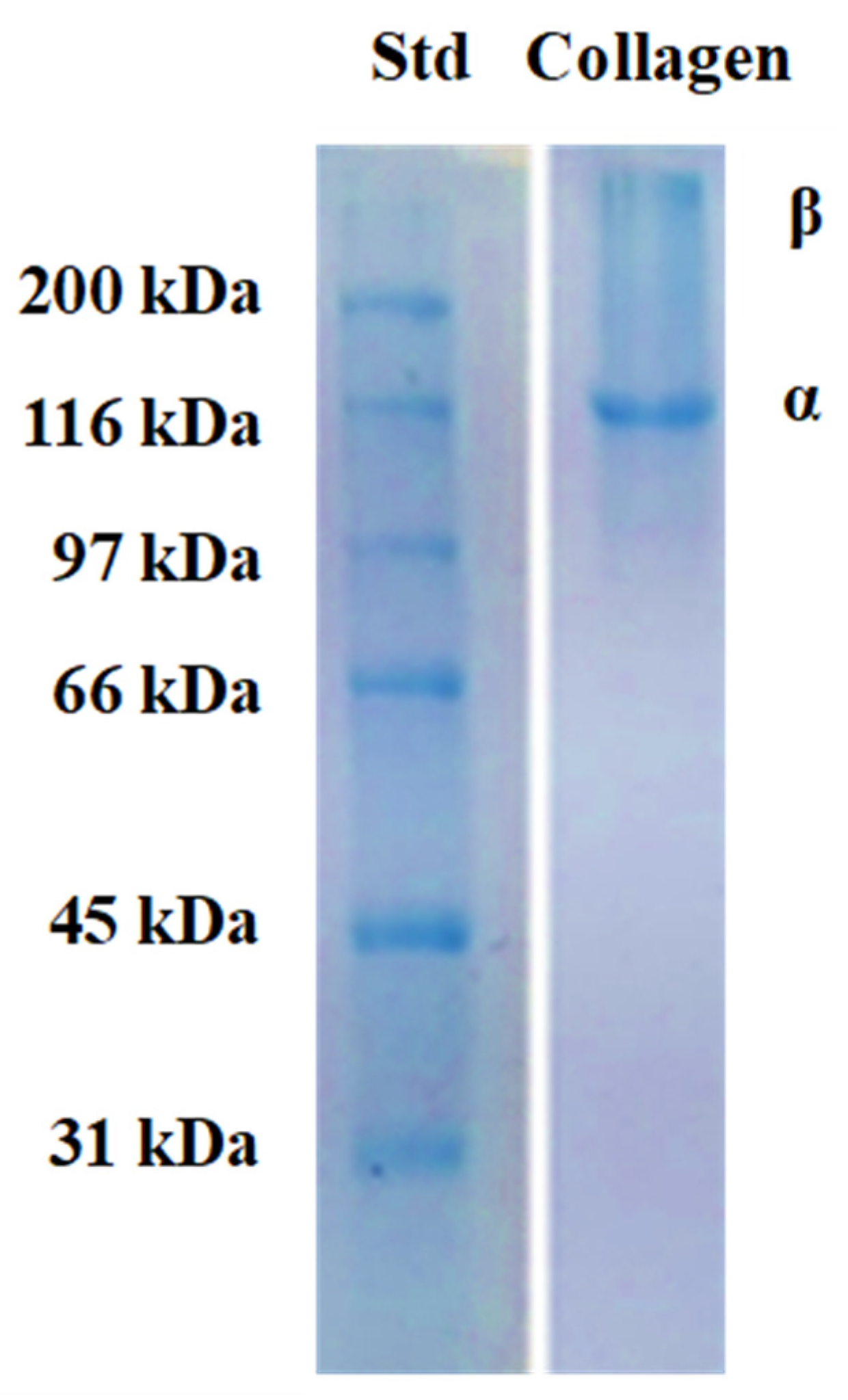
2.3.2. SDS-PAGE
2.3.3. Amino Acid Composition
2.3.4. UV–Visible Spectra and Fourier Transform Infrared Spectroscopy
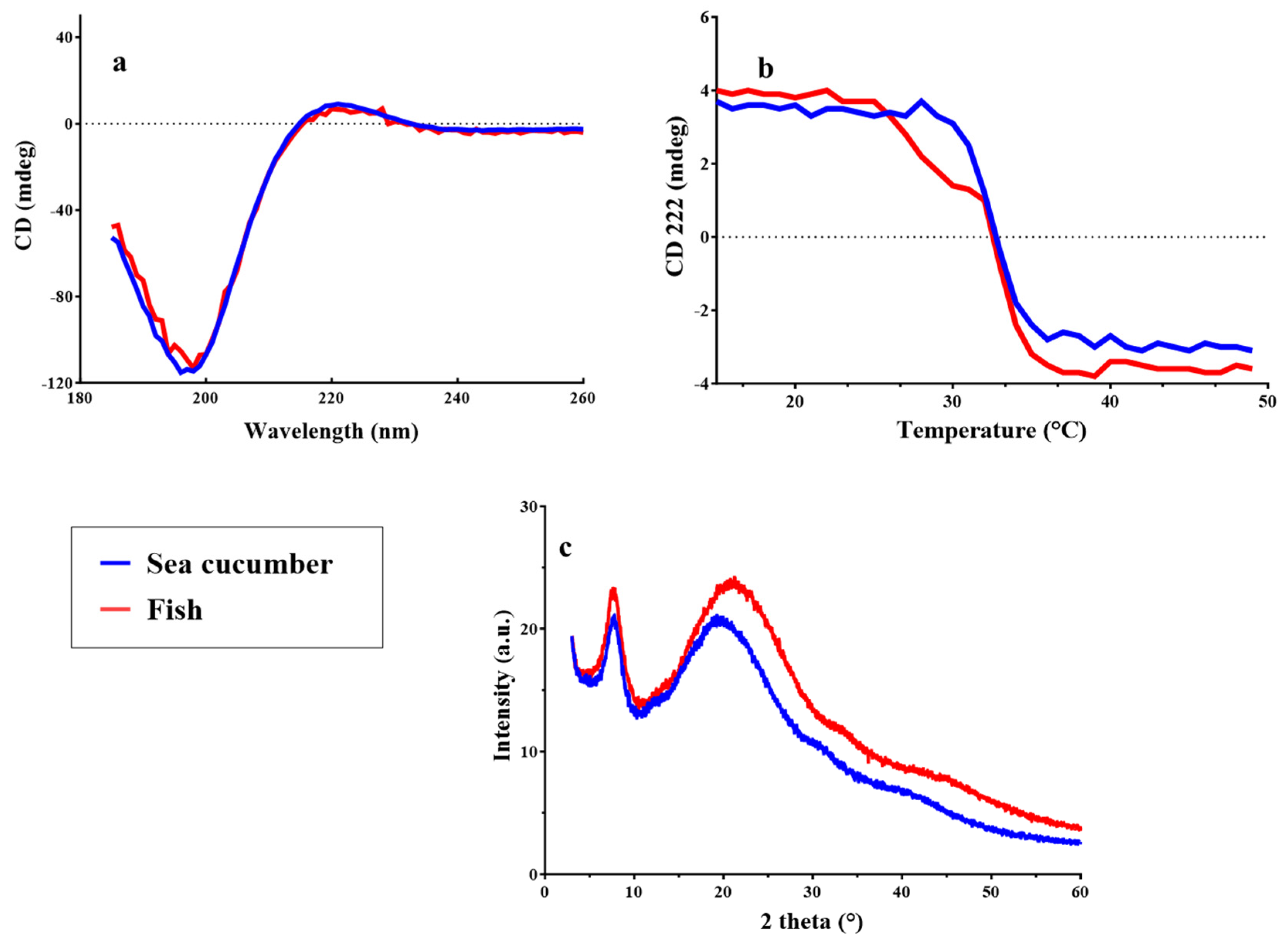
2.3.5. Circular Dichroism and X-Ray Diffraction
2.3.6. Collagen Hydrolysis and Ultrafiltration
2.3.7. Wound-Healing Assay
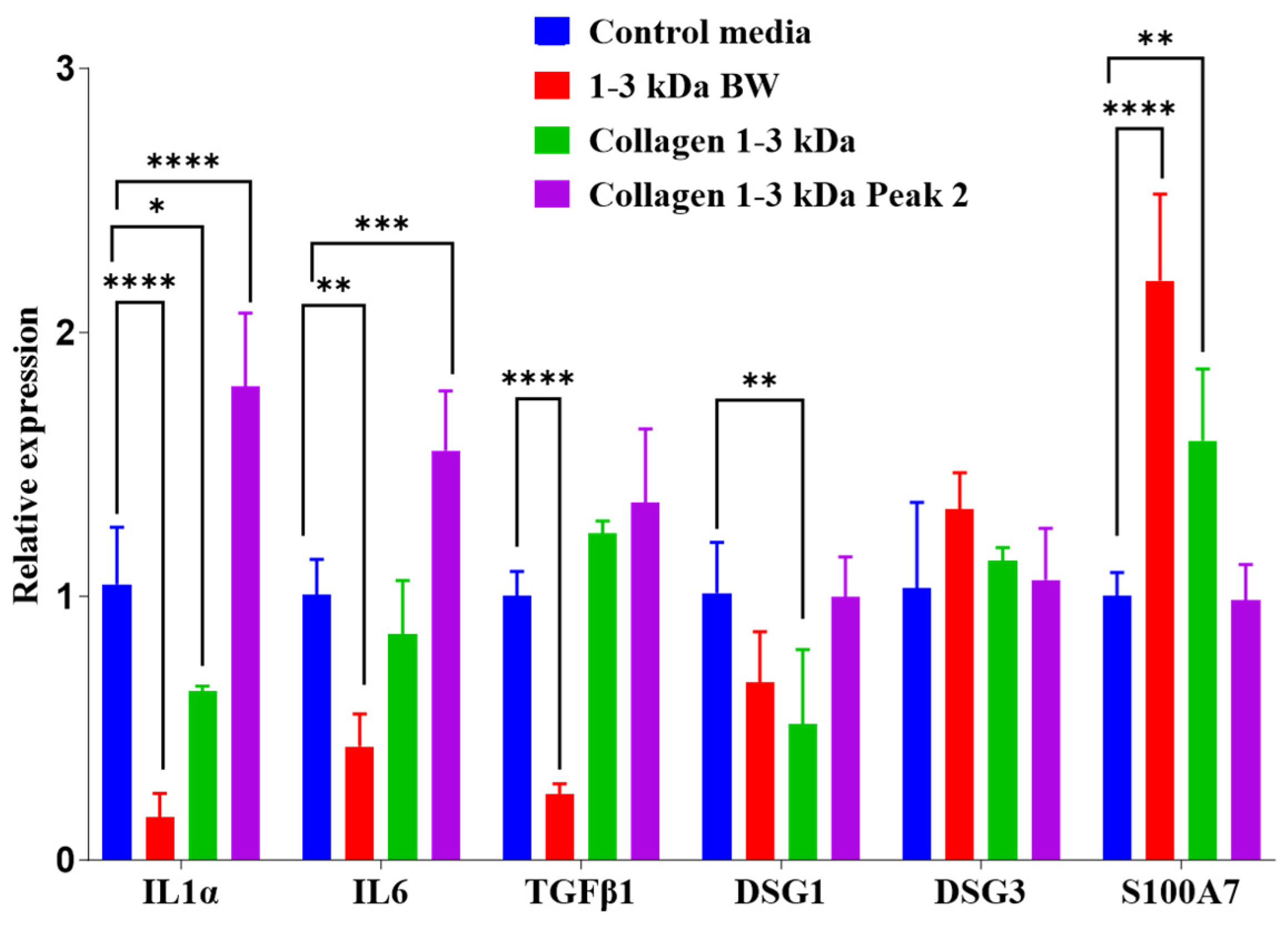
2.3.8. Gene Expression Analysis
2.3.9. Antioxidant Activities of Collagen Fractions
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Collection and Processing of Sea Cucumber
4.3. Proximate Composition
4.4. Skin-Free Body Wall Digest
4.5. Pepsin-Soluble Collagen Extraction
4.6. Collagen Characterization
4.6.1. Electrophoresis
4.6.2. Amino Acid Analysis
4.6.3. X-Ray Diffraction (XRD) and Circular Dichroism (CD)
4.6.4. UV–Vis and Fourier Transform Infrared (FTIR) Spectra
4.7. Collagen Hydrolysate Preparation
4.7.1. Ultrafiltration
4.7.2. Flash Chromatography of Collagen 1–3 kDa
4.7.3. Antioxidant Activity
ABTS+ Scavenging Activity
Oxygen Radical Absorbance Capacity (ORAC)
Further Analyses
4.8. Wound Healing In Vitro
4.8.1. Scratch Wound Healing Assay
4.8.2. Gene Expression
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPH | 2,2-Azobis (2-amidinopropane) dihydrochloride |
| ABTS | 2,2-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid] |
| ACE | Angiotensin I-converting enzyme |
| Β-actin | Beta actin |
| BW | Body wall |
| CD | Circular dichroism |
| CFU | Colony-forming units |
| CINVESTAV | Centro de Investigación y de Estudios Avanzados |
| CONAPESCA | Comisión Nacional de Pesca y Acuacultura |
| DGS1 | Desmoglein-1 |
| DGS3 | Desmoglein-3 |
| DH | Degree of hydrolysis |
| DPP4 | Dipeptidyl Peptidase-4 |
| EDTA | Ethylenediamine-tetra-acetic |
| EGF | Epidermal growth factor |
| FBS | Fetal bovine serum |
| FL | fluorescein (3,6-dihydroxyspiro [isobenzofuran-1[3H],9[9H]-xanthen]-3-one) |
| FTIR | Fourier transform infrared |
| HaCat | keratinocyte |
| IC50 | Half-maximal inhibitory concentration |
| IL1α | Interleukin 1α |
| IL6 | Interleukin 6 |
| KGF | Keratinocyte growth factor |
| MTT | Thiazolyl blue tetrazolium bromide |
| NAC | n-Acetyl-L-cysteine |
| ORAC | Oxygen radical absorbance capacity |
| PBS | Phosphate-buffered saline |
| PITC | Phenyl isothiocyanate |
| qPCR | Quantitative polychrome chain reaction |
| RP-UHPLC | Ultra high-pressure liquid chromatography |
| S100A7 | S100 calcium-binding protein A7 or psoriasin |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SEM | Scanning electron microscope |
| TE | Trolox equivalents |
| TGFα | Transforming growth factor alpha |
| TGFβ1 | Transforming growth factor beta one |
| XRD | X-ray diffraction |
References
- Mercier, A.; Gebruk, A.; Kremenetskaia, A.; Hamel, J.-F. An Overview of Taxonomic and Morphological Diversity in Sea Cucumbers (Holothuroidea: Echinodermata). In The World of Sea Cucumbers; Mercier, A., Hamel, J.-F., Suhrbier, A., Pearce, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–15. [Google Scholar]
- Mercier, A.; Purcell, S.W.; Montgomery, E.M.; Kinch, J.; Byrne, M.; Hamel, J.-F. Revered and Reviled: The Plight of the Vanishing Sea Cucumbers. Ann. Rev. Mar. Sci. 2025, 17, 115–142. [Google Scholar] [CrossRef]
- Pangestuti, R.; Arifin, Z. Medicinal and Health Benefit Effects of Functional Sea Cucumbers. J. Tradit. Complement. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef]
- Okada, A.; Udagawa, S.; Kohtsuka, H.; Hayashi, Y.; Miura, T. Gene-Expression Patterns during Regeneration of the Multi-Organ Complex after Evisceration in the Sea Cucumber Eupentacta quinquesemita. Front. Mar. Sci. 2024, 11, 1346172. [Google Scholar] [CrossRef]
- Liu, R.; Ren, X.; Wang, J.; Chen, T.; Sun, X.; Lin, T.; Huang, J.; Guo, Z.; Luo, L.; Ren, C.; et al. Transcriptomic Analysis Reveals the Early Body Wall Regeneration Mechanism of the Sea Cucumber Holothuria leucospilota after Artificially Induced Transverse Fission. BMC Genom. 2023, 24, 766. [Google Scholar] [CrossRef]
- Maskur, M.; Sayuti, M.; Widyasari, F.; Haryo Bimo Setiarto, R. Bioactive Compound and Functional Properties of Sea Cucumbers as Nutraceutical Products. Rev. Agric. Sci. 2024, 12, 45–64. [Google Scholar] [CrossRef]
- Shou, Y.; Feng, C.; Lu, Q.; Mao, X.; Huang, H.; Su, Z.; Guo, H.; Huang, Z. Research Progress on the Chemical Components and Biological Activities of Sea Cucumber Polypeptides. Front. Pharmacol. 2023, 14, 1290175. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Ahmed, F.; Zhang, M.; Sperou, N.; Franco, C.M.M.; Feng, Q.; Zhang, W. In Vivo and Clinical Studies of Sea Cucumber-Derived Bioactives for Human Health and Nutrition from 2012–2021. Front. Mar. Sci. 2022, 9, 917857. [Google Scholar] [CrossRef]
- Das, A.; Hossain, A.; Dave, D. The Effect of Pre-Treatment and the Drying Method on the Nutritional and Bioactive Composition of Sea Cucumbers—A Review. Appl. Sci. 2024, 14, 6475. [Google Scholar] [CrossRef]
- Sales, S.; Lourenço, H.M.; Bandarra, N.M.; Afonso, C.; Matos, J.; Botelho, M.J.; Pessoa, M.F.; Félix, P.M.; Veronez, A.; Cardoso, C. How Biological Activity in Sea Cucumbers Changes as a Function of Species and Tissue. Foods 2023, 13, 35. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, L.; Xia, X.; Hu, W.; Zhou, P. Effect of Geographic Variation on the Proteome of Sea Cucumber (Stichopus japonicus). Food Res. Int. 2020, 136, 109498. [Google Scholar] [CrossRef] [PubMed]
- Nuringtyas, T.R.; Hidayati, L.; Rohmah, Z.; Paramita, D.K.; Suparmin, A.; Prinanda, H.H.; Febryzalita, Q.N.; Utami, S.L.; Zulfa, L.F.; Ardiansyah, B.K.; et al. Bioactive Peptides from Sea Cucumbers and Sea Urchins: Therapeutic Roles and Mechanistic Insights. Trends Sci. 2025, 22, 9513. [Google Scholar] [CrossRef]
- Popov, A.; Kozlovskaya, E.; Rutckova, T.; Styshova, O.; Makhankov, V.; Vakhrushev, A.; Hushpulian, D.; Gazaryan, I.; Son, O.; Tekutyeva, L. Matrikines of Sea Cucumbers: Structure, Biological Activity and Mechanisms of Action. Int. J. Mol. Sci. 2024, 25, 12068. [Google Scholar] [CrossRef]
- Olivera-Castillo, L.; Grant, G.; Kantún-Moreno, N.; Barrera-Pérez, H.A.; Montero, J.; Olvera-Novoa, M.A.; Carrillo-Cocom, L.M.; Acevedo, J.J.; Puerto-Castillo, C.; May Solís, V.; et al. A Glycosaminoglycan-Rich Fraction from Sea Cucumber Isostichopus badionotus Has Potent Anti-Inflammatory Properties In Vitro and In Vivo. Nutrients 2020, 12, 1698. [Google Scholar] [CrossRef]
- Olivera-Castillo, L.; Grant, G.; Kantún-Moreno, N.; Acevedo-Fernández, J.J.; Puc-Sosa, M.; Montero, J.; Olvera-Novoa, M.A.; Negrete-León, E.; Santa-Olalla, J.; Ceballos-Zapata, J.; et al. Sea Cucumber (Isostichopus badionotus) Body-Wall Preparations Exert Anti-Inflammatory Activity In Vivo. PharmaNutrition 2018, 6, 74–80. [Google Scholar] [CrossRef]
- Becerra, J.; Rodriguez, M.; Leal, D.; Noris-Suarez, K.; Gonzalez, G. Chitosan-Collagen-Hydroxyapatite Membranes for Tissue Engineering. J. Mater. Sci. Mater. Med. 2022, 33, 18. [Google Scholar] [CrossRef]
- Teng, S.; Lee, E.; Wang, P.; Shin, D.; Kim, H. Three-layered Membranes of Collagen/Hydroxyapatite and Chitosan for Guided Bone Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87B, 132–138. [Google Scholar] [CrossRef]
- Wiegand, C.; Dirksen, A.; Tittelbach, J. Treatment with a Red-laser-based Wound Therapy Device Exerts Positive Effects in Models of Delayed Keratinocyte and Fibroblast Wound Healing. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12926. [Google Scholar] [CrossRef] [PubMed]
- Kotian, S.R.; Bhat, K.M.R.; Padma, D.; Pai, K.S.R. Influence of Traditional Medicines on the Activity of Keratinocytes in Wound Healing: An in-Vitro Study. Anat. Cell Biol. 2019, 52, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sun, N.; Lu, Z.; Zheng, J.; Zhang, S.; Lin, S. The Potential Mechanisms of Skin Wound Healing Mediated by Tetrapeptides from Sea Cucumber. Food Biosci. 2023, 53, 102742. [Google Scholar] [CrossRef]
- Park, S.-Y.; Lim, H.K.; Lee, S.; Hwang, H.C.; Cho, S.K.; Cho, M. Pepsin-Solubilised Collagen (PSC) from Red Sea Cucumber (Stichopus japonicus) Regulates Cell Cycle and the Fibronectin Synthesis in HaCaT Cell Migration. Food Chem. 2012, 132, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-H.; Lu, W.-C.; Chan, Y.-J.; Ko, W.-C.; Jung, C.-C.; Le Huynh, D.T.; Ji, Y.-X. Extraction and Characterization of Collagen from Sea Cucumber (Holothuria cinerascens) and Its Potential Application in Moisturizing Cosmetics. Aquaculture 2020, 515, 734590. [Google Scholar] [CrossRef]
- Sánchez-Solís, M.J.; Gullian-Klanian, M.; Toledo-López, V.; Lora-Vilchis, M.C. Proximate composition and fatty acid profile of the sea cucumber Isostichopus badionotus and Holothuria floridana. Food Sci. Technol. Res. 2021, 27, 319–327. [Google Scholar] [CrossRef]
- Acosta, E.J.; Rodríguez-Forero, A.; Werding, B.; Kunzmann, A. Ecological and Reproductive Characteristics of Holothuroids Isostichopus badionotus and Isostichopus sp. in Colombia. PLoS ONE 2021, 16, e0247158. [Google Scholar] [CrossRef]
- Farooq, S.; Ahmad, M.I.; Zheng, S.; Ali, U.; Li, Y.; Shixiu, C.; Zhang, H. A Review on Marine Collagen: Sources, Extraction Methods, Colloids Properties, and Food Applications. Collagen Leather 2024, 6, 11. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Gkalogianni, E.Z.; Apostologamvrou, C.; Voulgaris, K.; Varkoulis, A.; Vafidis, D. Proximate Compositions and Fatty Acid Profiles of Raw and Processed Holothuria polii and Holothuria tubulosa from the Aegean Sea. Sustainability 2024, 16, 6048. [Google Scholar] [CrossRef]
- Talab, A.S.; Ghannam, H.E.; Hussein, A.M.S.; Zhang, T.-T.; Xue, C.; Wang, Y.; Abdelnaby, T. Proximate Composition and Quality Properties of Some Egyptian Sea Cucumber Species. Egypt. J. Aquat. Biol. Fish. 2024, 28, 379–396. [Google Scholar] [CrossRef]
- Wen, Y.; Dong, X.; Zamora, L.N.; Jeffs, A.G.; Quek, S.Y. Physicochemical Properties, Functionalities, and Antioxidant Activity of Protein Extracts from New Zealand Wild Sea Cucumbers (Australostichopus mollis). Foods 2024, 13, 2735. [Google Scholar] [CrossRef]
- Muhsin, M.F.; Fujaya, Y.; Hidayani, A.A.; Fazhan, H.; Wan Mahari, W.A.; Lam, S.S.; Shu-Chien, A.C.; Wang, Y.; Afiqah-Aleng, N.; Rukminasari, N.; et al. Bridging the Gap between Sustainability and Profitability: Unveiling the Untapped Potential of Sea Cucumber Viscera. PeerJ 2023, 11, e16252. [Google Scholar] [CrossRef]
- Pamungkas, S.Y.; Haryono, F.E.D. Bioprospecting of Sea Cucumber (Holothuria sp.) as Industries and Functional Foods for Human Health. Int. J. Sci. Res. Arch. 2023, 10, 669–690. [Google Scholar] [CrossRef]
- Purcell, S.W.; Lovatelli, A.; González-Wangüemert, M.; Solís-Marín, F.A.; Samyn, Y.; Conand, C. Commercially Important Sea Cucumbers of the World; FAO: Rome, Italy, 2023; No. 6, Revision 1; ISBN 978-92-5-137793-2. [Google Scholar]
- Ibrahim, N.’I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef]
- Pérez-Vega, J.A.; Olivera-Castillo, L.; Gómez-Ruiz, J.Á.; Hernández-Ledesma, B. Release of Multifunctional Peptides by Gastrointestinal Digestion of Sea Cucumber (Isostichopus badionotus). J. Funct. Foods 2013, 5, 869–877. [Google Scholar] [CrossRef]
- Atanassova, M.R.; Mildenberger, J.; Hansen, M.D.; Tamm, T. Microstructure of Sea Cucumber Parastichopus tremulus Peptide Hydrogels and Bioactivity in Caco-2 Cell Culture Model. Gels 2025, 11, 280. [Google Scholar] [CrossRef]
- Man, J.; Abd El-Aty, A.M.; Wang, Z.; Tan, M. Recent Advances in Sea Cucumber Peptide: Production, Bioactive Properties, and Prospects. Food Front. 2023, 4, 131–163. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Antioxidant Potential and Physicochemical Properties of Protein Hydrolysates from Body Parts of North Atlantic Sea Cucumber (Cucumaria frondosa). Food Prod. Process. Nutr. 2021, 3, 3. [Google Scholar] [CrossRef]
- Darya, M.; Sajjadi, M.M.; Yousefzadi, M.; Sourinejad, I.; Zarei, M. Antifouling and Antibacterial Activities of Bioactive Extracts from Different Organs of the Sea Cucumber Holothuria leucospilota. Helgol. Mar. Res. 2020, 74, 4. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Adibpour, N.; Nasr, F.; Nematpour, F.; Shakouri, A.; Ameri, A. Antibacterial and Antifungal Activity of Holothuria leucospilota Isolated From Persian Gulf and Oman Sea. Jundishapur J. Microbiol. 2014, 7, e8708. [Google Scholar] [CrossRef]
- Ghanbari, R.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Actinopyga lecanora Hydrolysates as Natural Antibacterial Agents. Int. J. Mol. Sci. 2012, 13, 16796–16811. [Google Scholar] [CrossRef]
- Barzkar, N.; Attaran-Fariman, G.; Taheri, A.; Venmathi Maran, B.A. Extraction and Characterization of Collagen and Gelatin from Body Wall of Sea Cucumbers Stichopus horrens and Holothuria arenicola. PeerJ 2024, 12, e18149. [Google Scholar] [CrossRef] [PubMed]
- Nabilla, N.; Shofiyah, I.; Sugiharto; Alvitasari, D.; Sumarsih, S.; Khaleyla, F.; Wirawati, I.; Winarni, D. Organization, Density, and Content of Collagen in the Body Wall of Sea Cucumbers Acaudina rosettis and Phyllophorus sp. Aquac. Fish. 2024, 10, 816–824. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Sea Cucumber Derived Type I Collagen: A Comprehensive Review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Q.; Tan, M.; Chen, Z.; Zheng, H.; Gao, J.; Lin, H.; Zhu, G.; Cao, W. Characterization and Film-Forming Properties of Collagen from Three Species of Sea Cucumber from the South China Sea: Emphasizing the Effect of Transglutaminase. Int. J. Biol. Macromol. 2025, 294, 139321. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Li, H.; Che, H.; Xie, W.; Ju, W.; Qi, H.; Dong, X. Effect of Ca2+ on the Structure of Collagen Fibers in Sea Cucumber (Apostichopus japonicus) under Low-Temperature Tenderization Condition. Food Chem. X 2025, 27, 102450. [Google Scholar] [CrossRef]
- Cruz-López, H.; Rodríguez-Morales, S.; Enríquez-Paredes, L.M.; Villarreal-Gómez, L.J.; True, C.; Olivera-Castillo, L.; Fernández-Velasco, D.A.; López, L.M. Swim Bladder of Farmed Totoaba macdonaldi: A Source of Value-Added Collagen. Mar. Drugs 2023, 21, 173. [Google Scholar] [CrossRef]
- Cruz-López, H.; Rodríguez-Morales, S.; Enríquez-Paredes, L.M.; Villarreal-Gómez, L.J.; Olivera-Castillo, L.; Cortes-Santiago, Y.; López, L.M. Comparison of Collagen Characteristic from the Skin and Swim Bladder of Gulf Corvina (Cynoscion othonopterus). Tissue Cell 2021, 72, 101593. [Google Scholar] [CrossRef]
- Indriani, S.; Benjakul, S.; Quan, T.H.; Sitanggang, A.B.; Chaijan, M.; Kaewthong, P.; Petcharat, T.; Karnjanapratum, S. Effect of Different Ultrasound-Assisted Process Modes on Extraction Yield and Molecular Characteristics of Pepsin-Soluble Collagen from Asian Bullfrog Skin. Food Bioprocess Technol. 2023, 16, 3019–3032. [Google Scholar] [CrossRef]
- Zhong, M.; Chen, T.; Hu, C.; Ren, C. Isolation and Characterization of Collagen from the Body Wall of Sea Cucumber Stichopus monotuberculatus. J. Food Sci. 2015, 80, C671–C679. [Google Scholar] [CrossRef]
- Liu, Z.; Su, Y.; Zeng, M. Amino Acid Composition and Functional Properties of Giant Red Sea Cucumber (Parastichopus californicus) Collagen Hydrolysates. J. Ocean Univ. China 2011, 10, 80–84. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, W.; Meng, Y.; Tian, Q.; Hao, L.; Hou, H. Thermal Stability of Sea Cucumber Collagen and Effects of Gallic Acid Crosslinking. Int. J. Food Sci. Technol. 2023, 58, 2280–2288. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of Bioactive Peptides from Sea Cucumber and Its Potential Health Benefits: A Comprehensive Review. J. Agric. Food Chem. 2022, 70, 7607–7625. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Bai, X.; Dai, X.; Li, Y. The Biological Processes During Wound Healing. Regen. Med. 2021, 16, 373–390. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. Methods Mol. Biol. 2019, 2109, 225–229. [Google Scholar] [CrossRef]
- Aw, Y.B.; Chen, S.; Yeo, A.; Dangerfield, J.A.; Mok, P. Development and Functional Testing of a Novel in Vitro Delayed Scratch Closure Assay. Histochem. Cell Biol. 2024, 162, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Hipler, U.-C.; Elsner, P.; Tittelbach, J. Keratinocyte and Fibroblast Wound Healing In Vitro Is Repressed by Non-Optimal Conditions but the Reparative Potential Can Be Improved by Water-Filtered Infrared A. Biomedicines 2021, 9, 1802. [Google Scholar] [CrossRef]
- Farhangniya, M.; Samadikuchaksaraei, A. A Review of Genes Involved in Wound Healing. Med. J. Islam. Repub. Iran 2023, 37, 1094–1100. [Google Scholar] [CrossRef]
- Morgner, B.; Husmark, J.; Arvidsson, A.; Wiegand, C. Effect of a DACC-Coated Dressing on Keratinocytes and Fibroblasts in Wound Healing Using an In Vitro Scratch Model. J. Mater. Sci. Mater. Med. 2022, 33, 22. [Google Scholar] [CrossRef]
- Ågren, M.S.; Litman, T.; Eriksen, J.O.; Schjerling, P.; Bzorek, M.; Gjerdrum, L.M.R. Gene Expression Linked to Reepithelialization of Human Skin Wounds. Int. J. Mol. Sci. 2022, 23, 15746. [Google Scholar] [CrossRef]
- Costantini, E.; Aielli, L.; Serra, F.; De Dominicis, L.; Falasca, K.; Di Giovanni, P.; Reale, M. Evaluation of Cell Migration and Cytokines Expression Changes under the Radiofrequency Electromagnetic Field on Wound Healing In Vitro Model. Int. J. Mol. Sci. 2022, 23, 2205. [Google Scholar] [CrossRef]
- Marinelli, L.; Cacciatore, I.; Costantini, E.; Dimmito, M.P.; Serra, F.; Di Stefano, A.; Reale, M. Wound-Healing Promotion and Anti-Inflammatory Properties of Carvacrol Prodrugs/Hyaluronic Acid Formulations. Pharmaceutics 2022, 14, 1468. [Google Scholar] [CrossRef]
- Kawano, Y.; Patrulea, V.; Sublet, E.; Borchard, G.; Iyoda, T.; Kageyama, R.; Morita, A.; Seino, S.; Yoshida, H.; Jordan, O.; et al. Wound Healing Promotion by Hyaluronic Acid: Effect of Molecular Weight on Gene Expression and In Vivo Wound Closure. Pharmaceuticals 2021, 14, 301. [Google Scholar] [CrossRef]
- Yuksel, S.N.; Dikmen, M.; Canturk, Z. Evaluation of Real Time Cell Proliferation, Anti-Inflammatory and Wound Healing Potential of Helenalin on HaCaT Keratinocytes Treated with Lipopolysaccharide Stimulated Monocytes. Indian J. Pharm. Sci. 2021, 83, 219–229. [Google Scholar] [CrossRef]
- Shamilov, R.; Ackley, T.W.; Aneskievich, B.J. Enhanced Wound Healing- and Inflammasome-Associated Gene Expression in TNFAIP3-Interacting Protein 1-(TNIP1-) Deficient HaCaT Keratinocytes Parallels Reduced Reepithelialization. Mediat. Inflamm. 2020, 2020, 5919150. [Google Scholar] [CrossRef]
- Patruno, A.; Ferrone, A.; Costantini, E.; Franceschelli, S.; Pesce, M.; Speranza, L.; Amerio, P.; D’Angelo, C.; Felaco, M.; Grilli, A.; et al. Extremely low-frequency electromagnetic fields accelerates wound healing modulating MMP-9 and inflammatory cytokines. Cell Prolif. 2018, 51, e12432. [Google Scholar] [CrossRef]
- Peplow, P.V.; Chatterjee, M.P. A Review of the Influence of Growth Factors and Cytokines in In Vitro Human Keratinocyte Migration. Cytokine 2013, 62, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, L.; Jiang, G.; Häkkinen, L.; Chan, B.; Larjava, H. HaCaT Keratinocyte Migration Is Dependent on Epidermal Growth Factor Receptor Signaling and Glycogen Synthase Kinase-3α. Exp. Cell Res. 2006, 312, 2791–2805. [Google Scholar] [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Ho, C.-T.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing Meanings in Food Science and Health Science. J. Agric. Food Chem. 2018, 66, 3063–3068. [Google Scholar] [CrossRef] [PubMed]
- Mfotie Njoya, E. Medicinal Plants, Antioxidant Potential, and Cancer. In Cancer; Preedy, V.R., Patel, V.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 349–357. [Google Scholar]
- Sakurai, S.; Kawakami, Y.; Kuroki, M.; Gotoh, H. Structure–Antioxidant Activity (Oxygen Radical Absorbance Capacity) Relationships of Phenolic Compounds. Struct. Chem. 2022, 33, 1055–1062. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Rowley, A.T.; Meli, V.S.; Wu-Woods, N.J.; Chen, E.Y.; Liu, W.F.; Wang, S.-W. Effects of Surface-Bound Collagen-Mimetic Peptides on Macrophage Uptake and Immunomodulation. Front. Bioeng. Biotechnol. 2020, 8, 747. [Google Scholar] [CrossRef] [PubMed]
- Szarka, E.; Neer, Z.; Balogh, P.; Ádori, M.; Angyal, A.; Prechl, J.; Kiss, A.; Kövesdi, D.; Sármay, G. Exacerbation of Collagen Induced Arthritis by Fcγ Receptor targeted Collagen Peptide due to Enhanced Inflammatory Chemokine and Cytokine Production. Biologics 2012, 6, 101–115. [Google Scholar] [CrossRef]
- Pilus, N.S.M.; Muhamad, A.; Shahidan, M.A.; Yusof, N.Y.M. Potential of Epidermal Growth Factor-like Peptide from the Sea Cucumber Stichopus horrens to Increase the Growth of Human Cells: In Silico Molecular Docking Approach. Mar. Drugs 2022, 20, 596. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-H.; Song, S.; Yang, J.-F. Oral Administration of Sea Cucumber (Stichopus japonicus) Protein Exerts Wound Healing Effects via the PI3K/AKT/MTOR Signaling Pathway. Food Funct. 2022, 13, 9796–9809. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Hagiwara, M. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blotting Analyses via Colored Stacking Gels. Anal. Biochem. 2022, 652, 114751. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Binlateh, T.; Hutamekalin, P.; Aluko, R.E.; Tepaamorndech, S.; Zhang, B.; Benjakul, S. Impact of Hydrolyzed Collagen from Defatted Sea Bass Skin on Proliferation and Differentiation of Preosteoblast MC3T3-E1 Cells. Foods 2021, 10, 1476. [Google Scholar] [CrossRef]
- Vieira, R.P.; Mourão, P.A. Occurrence of a Unique Fucose-Branched Chondroitin Sulfate in the Body Wall of a Sea Cucumber. J. Biol. Chem. 1988, 263, 18176–18183. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Spellman, D.; McEvoy, E.; O’Cuinn, G.; FitzGerald, R.J. Proteinase and Exopeptidase Hydrolysis of Whey Protein: Comparison of the TNBS, OPA and PH Stat Methods for Quantification of Degree of Hydrolysis. Int. Dairy J. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Miralles, B.; Amigo, L.; Ramos, M.; Recio, I. Identification of Antioxidant and ACE-inhibitory Peptides in Fermented Milk. J. Sci. Food Agric. 2005, 85, 1041–1048. [Google Scholar] [CrossRef]
- Garrett, A.R.; Weagel, E.G.; Martinez, A.D.; Heaton, M.; Robison, R.A.; O’Neill, K.L. A Novel Method for Predicting Antioxidant Activity Based on Amino Acid Structure. Food Chem. 2014, 158, 490–496. [Google Scholar] [CrossRef]
- Mani, S.; Swargiary, G. In Vitro Cytotoxicity Analysis: MTT/XTT, Trypan Blue Exclusion. In Animal Cell Culture: Principles and Practice; Springer International Publishing: Cham, Switzerland, 2023; pp. 267–284. [Google Scholar]
- Kadeřábková, N.; Mahmood, A.J.S.; Mavridou, D.A.I. Antibiotic Susceptibility Testing Using Minimum Inhibitory Concentration (MIC) Assays. npj Antimicrob. Resist. 2024, 2, 37. [Google Scholar] [CrossRef]
- Nagy, G.; Kiraly, G.; Banfalvi, G. Optimization of Cell Cycle Measurement by Time-Lapse Microscopy. In Methods in Cell Biology; Conn, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 112, pp. 143–161. [Google Scholar]
- Wallace, S.E.; Wilcox, W.R. Camurati-Engelmann Disease; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; GeneReviews® [Internet]: Seattle, WA, USA, 2023. [Google Scholar]
- Rebrikov, D.V.; Trofimov, D.Y. Real-Time PCR: A Review of Approaches to Data Analysis. Appl. Biochem. Microbiol. 2006, 42, 455–463. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Artika, I.M.; Dewi, Y.P.; Nainggolan, I.M.; Siregar, J.E.; Antonjaya, U. Real-Time Polymerase Chain Reaction: Current Techniques, Applications, and Role in COVID-19 Diagnosis. Genes 2022, 13, 2387. [Google Scholar] [CrossRef] [PubMed]
- Abedin, M.Z.; Karim, A.A.; Latiff, A.A.; Gan, C.-Y.; Ghazali, F.C.; Barzideh, Z.; Ferdosh, S.; Akanda, M.J.H.; Zzaman, W.; Karim, M.R.; et al. Biochemical and Radical-Scavenging Properties of Sea Cucumber (Stichopus vastus) Collagen Hydrolysates. Nat. Prod. Res. 2014, 28, 1302–1305. [Google Scholar] [CrossRef]
- Abedin, M.Z.; Karim, A.A.; Ahmed, F.; Latiff, A.A.; Gan, C.; Che Ghazali, F.; Islam Sarker, M.Z. Isolation and Characterization of Pepsin-solubilized Collagen from the Integument of Sea Cucumber (Stichopus vastus). J. Sci. Food Agric. 2013, 93, 1083–1088. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, Y.; Yang, Z.; Jin, H. Physicochemical Properties of Collagen from Acaudina molpadioides and Its Protective Effects Against H2O2-Induced Injury in RAW264.7 Cells. Mar. Drugs 2020, 18, 370. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Oliveira, A.C.M.; Su, Y.-C. Purification and Characterization of Pepsin-Solubilized Collagen from Skin and Connective Tissue of Giant Red Sea Cucumber (Parastichopus californicus). J. Agric. Food Chem. 2010, 58, 1270–1274. [Google Scholar] [CrossRef]
- Adibzadeh, N.; Aminzadeh, S.; Jamili, S.; Karkhane, A.A.; Farrokhi, N. Purification and Characterization of Pepsin-Solubilized Collagen from Skin of Sea Cucumber Holothuria parva. Appl. Biochem. Biotechnol. 2014, 173, 143–154. [Google Scholar] [CrossRef]
- Saallah, S.; Roslan, J.; Julius, F.S.; Saallah, S.; Mohamad Razali, U.H.; Pindi, W.; Sulaiman, M.R.; Pa’ee, K.F.; Mustapa Kamal, S.M. Comparative Study of The Yield and Physicochemical Properties of Collagen from Sea Cucumber (Holothuria scabra), Obtained through Dialysis and the Ultrafiltration Membrane. Molecules 2021, 26, 2564. [Google Scholar] [CrossRef]
- Syahputra, G.; Hariyatun, H.; Firdaus, M.; Santoso, P. Extraction and Characterization of Collagen from Sand Sea Cucumber (Holothuria scabra). J. Ilmu Pertan. Indones. 2021, 26, 319–327. [Google Scholar] [CrossRef]
- Lin, S.; Xue, Y.-P.; San, E.; Keong, T.C.; Chen, L.; Zheng, Y.-G. Extraction and Characterization of Pepsin Soluble Collagen from the Body Wall of Sea Cucumber Acaudina leucoprocta. J. Aquat. Food Prod. Technol. 2017, 26, 502–515. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and Hydroxyproline Metabolism: Implications for Animal and Human Nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef]
- Ratoe Oedjoe, M.D. Composition of Nutritional Content of Sea Cucumbers (Holothuroidea) in Mania Waters, Sabu Raijua Regency, East Nusa Tenggara. J. Aquac. Res. Dev. 2017, 8, 1–3. [Google Scholar] [CrossRef]
- Chen, J.; Yao, M.; Yi, Y. Studies on the Amino Acid Composition and Antioxidant Activities of Collagen Polypeptides from Holothuria nobilis Selenka. Sch. Acad. J. Pharm. (SAJP) 2016, 5, 421–424. [Google Scholar]




| Amino Acid | Residues * | Amino Acid | Residues * |
|---|---|---|---|
| Aspartic acid | 93 | Tyrosine | 6 |
| Glutamine | 157 | Valine | 20 |
| Hydroxyproline | 97 | Methionine | 5 |
| Serine | 21 | Cysteine | 1 |
| Glycine | 289 | Isoleucine | 10 |
| Histidine | 3 | Leucine | 15 |
| Arginine | 54 | Hydroxylysine | 5 |
| Threonine | 23 | Phenylalanine | 9 |
| Alanine | 110 | Lysine | 5 |
| Proline | 75 | Imino acids ** | 172 |
| Target Gene | Forward 5′—3′ Reverse 3′—5′ | Reference |
|---|---|---|
| ILlα | F: CGCCAATGACTCAGAGGAAGA R: AGGGCGTCATTCAGGATGAA | Wiegand et al., 2021 [56] |
| IL6 | F: AGACAGCCACTCACCTCTTCAG R: TTCTGCCAGTGCCTCTTTGCTG | NM_000600.5 |
| TGFβ1 | F: GAGCCCTGGATACCAACTATT R: AGGACCTTGCTGTACTGTGTG | Wallace et al., 2023 [90] |
| DDSG1 | F: TCCCCACATTTCGGCACTAC R: GCCCAGAGGATCGAGAATAGG | Wiegand et al., 2021 [56] |
| DSG3 | F: GTCAGAACAATCGGTGTGAGATG R: TGCGGCCTGCCATACCT | Wiegand et al., 2021 [56] |
| SI00A7 | F: GTCCAAACACACACATCTCACT R: TCATCATCGTCAGCAGGCTT | Wiegand et al., 2021 [56] |
| β-actin | F: GATCATTGCTCCTCCTGAGC R: GTCATAGTCCGCCTAGAAGCAT | NM_001101.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivera-Castillo, L.; Grant, G.; Medina-Contreras, O.; Cruz-López, H.; Carrillo-Cocom, L.; Cruz-Córdova, A.; Segura-Cadiz, F.; Fernández-Velasco, D.A.; Rodríguez-Morales, S.; Cauich-Rodríguez, J.V.; et al. Sea Cucumber (Isostichopus badionotus): Bioactivity and Wound Healing Capacity In Vitro of Small Peptide Isolates from Digests of Whole-Body Wall or Purified Collagen. Mar. Drugs 2025, 23, 411. https://doi.org/10.3390/md23110411
Olivera-Castillo L, Grant G, Medina-Contreras O, Cruz-López H, Carrillo-Cocom L, Cruz-Córdova A, Segura-Cadiz F, Fernández-Velasco DA, Rodríguez-Morales S, Cauich-Rodríguez JV, et al. Sea Cucumber (Isostichopus badionotus): Bioactivity and Wound Healing Capacity In Vitro of Small Peptide Isolates from Digests of Whole-Body Wall or Purified Collagen. Marine Drugs. 2025; 23(11):411. https://doi.org/10.3390/md23110411
Chicago/Turabian StyleOlivera-Castillo, Leticia, George Grant, Oscar Medina-Contreras, Honorio Cruz-López, Leydi Carrillo-Cocom, Ariadnna Cruz-Córdova, Frank Segura-Cadiz, Daniel Alejandro Fernández-Velasco, Sergio Rodríguez-Morales, Juan Valerio Cauich-Rodríguez, and et al. 2025. "Sea Cucumber (Isostichopus badionotus): Bioactivity and Wound Healing Capacity In Vitro of Small Peptide Isolates from Digests of Whole-Body Wall or Purified Collagen" Marine Drugs 23, no. 11: 411. https://doi.org/10.3390/md23110411
APA StyleOlivera-Castillo, L., Grant, G., Medina-Contreras, O., Cruz-López, H., Carrillo-Cocom, L., Cruz-Córdova, A., Segura-Cadiz, F., Fernández-Velasco, D. A., Rodríguez-Morales, S., Cauich-Rodríguez, J. V., Moo-Puc, R. E., Puerto-Castillo, C., Moo-Pech, G. d. J., Uuh-Narvaez, J. J., Olvera-Novoa, M. A., & Rodriguez-Canul, R. (2025). Sea Cucumber (Isostichopus badionotus): Bioactivity and Wound Healing Capacity In Vitro of Small Peptide Isolates from Digests of Whole-Body Wall or Purified Collagen. Marine Drugs, 23(11), 411. https://doi.org/10.3390/md23110411








