1. Introduction
Global competition over genetic resources has sharpened as countries operationalize access-and-benefit sharing (ABS) frameworks under the Convention on Biological Diversity [
1]. The Nagoya Protocol, which entered into force on 12 October 2014, affirms national sovereignty over genetic resources and mandates fair, equitable benefit-sharing from their utilization, reshaping how bioresources are accessed and valorized [
2]. Ongoing negotiations around digital sequence information (DSI) further extend the ABS conversation from physical samples to digitized genetic data, underscoring both the economic value of biodiversity-derived knowledge and the urgency of locally anchored innovation pipelines [
3]. Together, these developments have intensified attention to high-seas marine resources, especially deep-sea resources.
The scope of the opportunity has been underscored by ocean metrics show that approximately 70% of Earth’s surface is ocean, and roughly 90% of that volume lies deeper than 200 m, conditions that define the deep-sea [
4]. Within this context, deep-sea ecosystems represent a compelling frontier for drug discovery and functional materials. The selection pressures of high hydrostatic pressure, low temperature, and oligotrophy foster unique microbial metabolisms that yield structurally distinct molecules. Translational precedents are already emerging in various studies. For example, the HE800 exopolysaccharide (EPS) from the hydrothermal-vent bacterium
Vibrio diabolicus has been assembled into glycosaminoglycan-mimetic biomaterials and shown efficacy in bone and skin regeneration, illustrating how extremophile-derived polymers can be engineered for clinical ends [
5,
6,
7]. Genomic analyses have begun to resolve the biosynthetic loci underpinning these architectures, offering roadmaps for scalable production.
Anti-inflammatory activities have likewise been documented across deep-sea–linked matrices and taxa using macrophage models relevant to innate immunity. An EPS (B3-15) suppressed inducible nitric oxide synthase (iNOS) expression, nitric oxide (NO) release, and pro-inflammatory cytokines in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells without cytotoxicity [
8]. Refined deep-sea water-based culture medium reduced iNOS/cyclooxygenase-2 (COX-2) expression and prostaglandin output in the same model via mitogen-activated protein kinase (MAPK)/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) modulation [
9]. Complementing these matrix-level effects, secondary metabolites from deep-sea–derived fungi have repeatedly inhibited LPS-induced NO production in RAW 264.7 macrophages, expanding the small-molecule chemical space for marine anti-inflammatories [
10]. Despite these promising findings, the chemical space of therapeutic small molecules from deep-sea bacterial extracts remains largely underexplored, particularly those derived from high-seas benthic bacteria that thrive in oligotrophic, high-pressure environments. This represents a critical opportunity for discovering structurally distinct bioactive compounds.
Building on these advances, we investigated an ethyl acetate (EtOAc) extract from
Galbibacter orientalis strain ROD011, isolated from continental-shelf sediments off 16.096898° S, 151.884181° W in the Pacific Ocean.
Galbibacter spp. (family
Flavobacteriaceae) was originally erected from marine sediments and now includes species recovered from deep-sea environments; recent taxonomic work also transferred
Joostella marina to
G. orientalis (nom. nov.), refining the genus and highlighting its marine provenance [
11,
12]. The sediment-associated lifestyles of these lineages suggest access to niche carbon sources and stressors that can drive uncommon metabolic chemistries [
13].
In this study, the EtOAc extract of
G. orientalis strain ROD011 (GOEE) was investigated for its protective role under LPS-triggered macrophage activation by remodeling metabolic circuits linked to inflammatory signaling. To test this, the phenotypic readouts [NO and reactive oxygen species [
14]] in RAW 264.7 macrophages were profiled. Subsequently, we coupled these measurements with metabolite-set enrichment and metabolite–pathway network mapping to delineate mechanism-level effects.
In designing this work, we deliberately focused on the extract-level investigation to establish reproducible anti-inflammatory activity while capturing a global metabolic signature that could guide subsequent compound-level studies. Rather than isolating single molecules at this stage, we aimed to define GOEE’s integrated pharmacological profile across cellular and organismal systems. This approach allows us to identify tractable biochemical nodes that can later be explored through bioassay-guided fractionation and structural characterization, which are currently underway in our laboratory.
By situating a deep-sea microorganism within the evolving ABS/DSI landscape, our study aims to (i) address the scarcity of structurally diverse small-molecule anti-inflammatory leads from high-seas bacteria and ref. [
15] provide a model for equitable, locally grounded valorization of national marine genetic resources.
3. Discussion
With the Nagoya Protocol foregrounding lawful access and fair benefit sharing for genetic resources under national control, the need to secure biological resources from areas beyond national jurisdiction has become more pressing [
1]. The high seas are still sparsely sampled, yet they harbor organisms adapted to extreme physicochemical niches that can provide unique enzymes, small molecules, and biomaterials of scientific and industrial value. Establishing compliant pipelines for sampling, archiving, and characterization, together with clear provenance records and open metadata for associated sequence information, will expand the global knowledge base while supporting equitable benefit sharing [
16]. Such stewardship ensures that discoveries from international waters translate into public value for research, conservation, and bioindustry.
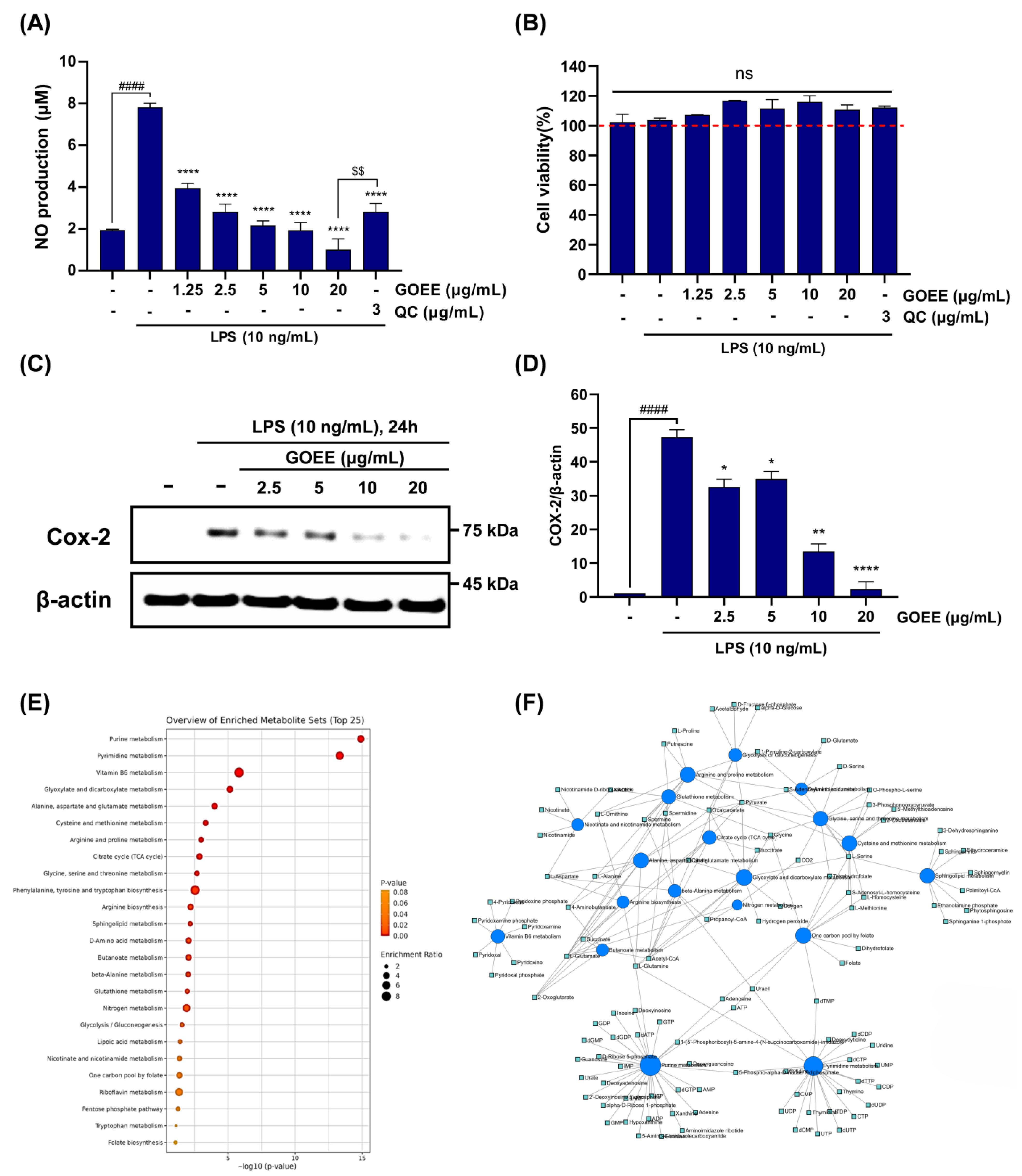
Against this backdrop, a marine biological resource from the high seas was secured using a remotely operated vehicle, and its bioactivity was elucidated through pro-inflammatory response assays in this study. In LPS-stimulated RAW 264.7 cells, NO output fell by 72–87% across 5–20 µg/mL, while MTT viability stayed at 111–116% of the LPS group, showing that activity was achieved without cytotoxicity (
Figure 3A,B). COX-2 protein, the enzyme that drives the NO/PGE
2 axis in the immune system, was also lowered in a dose-dependent way and was almost absent at 20 µg/mL (
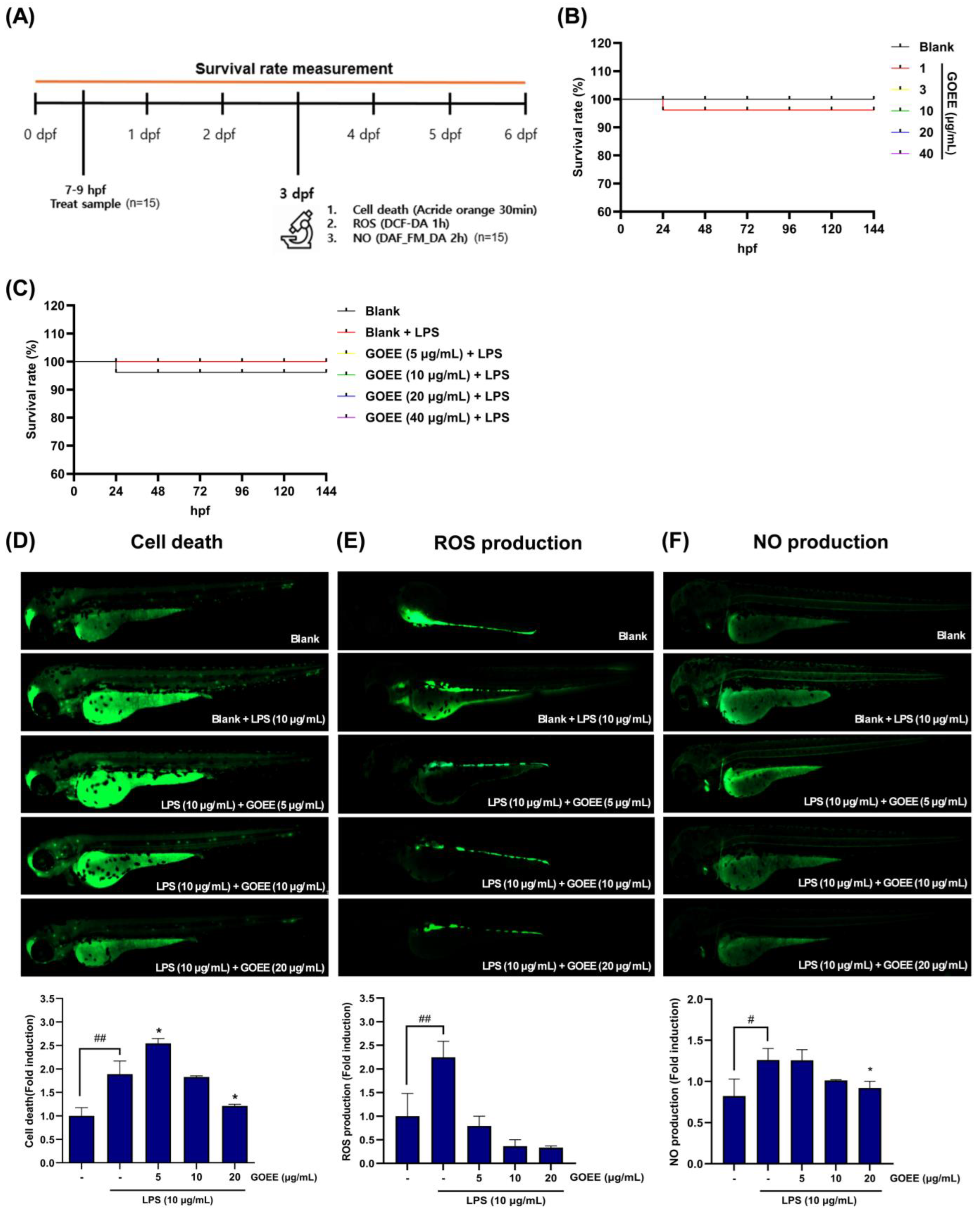
Figure 3C,D). In zebrafish, survival was maintained up to 40 µg/mL, and LPS-induced signals of cell death, ROS generation, and NO production were reduced by GOEE exposure by 65–85% at 10–20 µg/mL (
Figure 4A–F).
To account for these convergent outcomes, metabolomics was used to explain how these effects could arise at the systems level. Out of 58 KEGG pathways tested, 18 were significant, with leading signals including purine and pyraimidine metabolism, vitamin B6 metabolism, and the one-carbon pool by folate, plus coordinated shifts in amino-acid/TCA, glutathione and glyoxylate/dicarboxylate, and sphingolipid metabolism (
Figure 3E,F). These metabolisms are tightly linked to the role of GOEE, which acts through a coordinated reshaping of nucleotide supply, cofactor availability, redox handling, and membrane-linked signaling [
17,
18,
19,
20,
21]. In this framework, the fall in COX-2 protein expression and NO production was interpreted not as an isolated effect but as the visible consequence of a system whose metabolic levers had been shifted toward a less inflammatory state (
Figure 3).
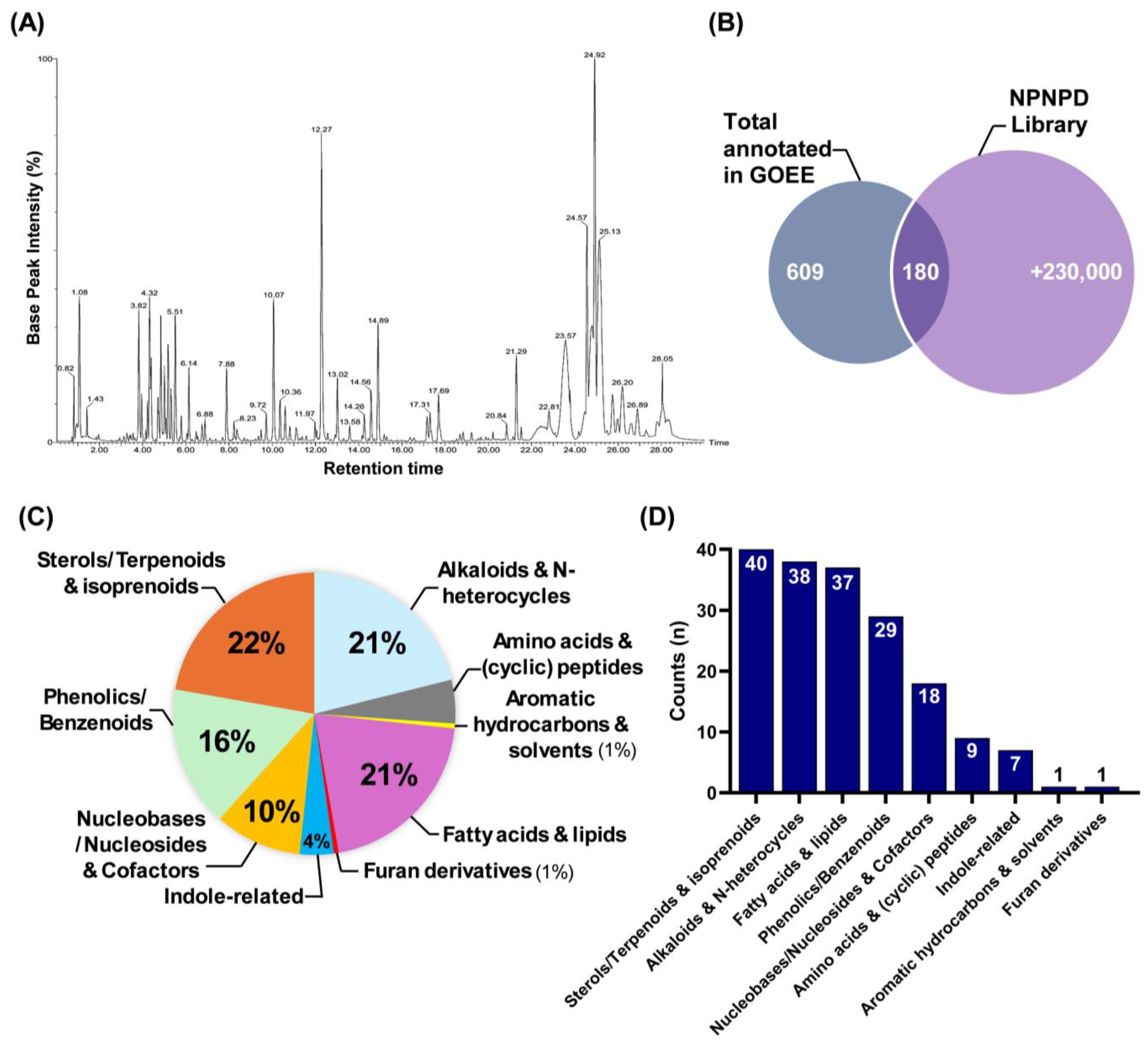
Compound classification analysis further clarifies how these pathway-level changes can be explained by the chemical diversity of GOEE (
Figure 5). The gradient-based polar/non-polar mobile phase shift revealed the presence of a wide range of primary metabolites with varying polarity in GOEE (
Figure 5A). In detail, sterols and terpenoids, alkaloids, fatty acids and lipids, phenolics, and indole derivatives together accounted for the majority of annotated compounds, whereas nucleosides, amino acid derivatives, and other minor classes contributed additional molecular diversity (
Figure 5C,D). The relatively high abundance of sterols, lipids, and phenolics also reflects the selective properties of EtOAc as an extraction solvent, which preferentially enriches mid- to low-polarity metabolites (
Figure 2A and
Figure 5C) [
22]. Such diversity is consistent with the multi-target phenotype observed in macrophages and zebrafish (
Figure 3,
Figure 4 and
Figure 5).
Several of these annotated metabolites map directly onto the metabolic routes highlighted by enrichment analysis (
Table S2). 4-methylumbelliferone and herniarin, coumarins present in GOEE, are known to suppress NF-κB and JNK-dependent inflammatory signaling, aligning with the reduced COX-2 and iNOS expression observed in our metabolomic network (
Figure 3) [
23,
24]. Fatty acids such as γ-linolenic acid and α-linolenic acid inhibit NF-κB activation and downregulate iNOS and COX-2, consistent with the observed suppression of nitric oxide metabolism [
15,
25]. Pachymic acid, a triterpenoid identified in GOEE, reduces NO, PGE
2, and cytokine production by targeting NF-κB, in line with the downshift in pro-inflammatory amino acid and TCA-linked fluxes [
26]. Stigmasterol, a sterol found in the extract, modulates NF-κB/NLRP3 signaling and corresponds with the remodeling of sphingolipid metabolism [
27]. δ-Tocotrienol, a vitamin E isoform also annotated in GOEE, inhibits NF-κB and explains part of the antioxidant and redox-buffering reinforcement seen in glutathione-related pathways [
28]. Finally, nucleosides such as adenosine, detected in GOEE, are established activators of A2A/A2B receptors that suppress pro-inflammatory cytokines and shift macrophage polarization toward M2, consistent with the nucleotide economy and one-carbon pool reprogramming revealed by metabolomics [
29].
Together, these compound-level examples provide direct evidence that the chemical constituents of GOEE map coherently onto the pathway-level shifts detected by untargeted metabolomics. Rather than presenting two separate layers of data, the metabolite annotations and metabolic networks converge to show that GOEE acts through multiple, coordinated biochemical routes that collectively redirect macrophage physiology toward a less inflammatory state.
Within this system view, nucleotide economy was read as a primary brake. Enrichment of purine and pyrimidine pathways implied that the demand–supply balance for nucleotides was reset, a change that would be expected to constrain transcriptional throughput and the synthesis of enzymes central to inflammatory signaling [
19]. The large downward step in COX-2/β-actin ratios, together with the drop in cellular NO production rate, was therefore interpreted as a proximate manifestation of throttled nucleotide flow (
Figure 3C,D). A cofactor axis was read in parallel. The prominence of vitamin B6 and the folate one-carbon pool, together with the central placement of PLP and folate/dihydrofolate in the network (
Figure 3E,F), suggests that transamination capacity and methyl-group transfer were stabilized, supporting redox control and gene-regulatory balance under inflammatory pressure [
30]. In the same vein, co-enrichment across arginine and proline, alanine/aspartate and glutamate, and glycine/serine and threonine, with links into the TCA cycle, was read as a rerouting of carbon-nitrogen flux away from iNOS-dependent NO production and toward anaplerotic fates, consistent with the stepwise decline in NO production in both cells and embryos (
Figure 3A,C and
Figure 4F) [
14,
31].
Redox buffering was interpreted as a second stabilizing pillar. Signals in glutathione metabolism and glyoxylate/dicarboxylate pathways, together with the bridging of hydrogen peroxide nodes in the network map, indicated that oxidative stress handling had been reinforced; the 65–85% decreases in zebrafish ROS at 10–20 µg/mL of GOEE treatment were therefore read as the organismal imprint of a cell-intrinsic antioxidant program rather than as collateral toxicity (
Figure 3E and
Figure 4E). Finally, sphingolipid clustering around sphinganine, phytosphingosine, and dihydroceramide was interpreted as a membrane-embedded control point over stress and apoptotic signaling, and the concurrent fall in acridine orange-labeled cell-death fluorescence at effective doses was taken as supportive of that membrane-signal restraint (
Figure 3F and
Figure 4D). Finally, the provenance of
G. orientalis strain ROD011 from national deep-sea waters provides a tractable pathway for compliant development under ABS/DSI, aligning scientific opportunity with equitable resource governance.
Deep-sea microorganisms live under pressure, cold, and low nutrients, and their chemistry is often unlike that of land organisms [
32]. In this study, such chemistry translated into a coordinated anti-inflammatory effect: a key enzyme (COX-2) was lowered, NO and ROS generations were reduced, and multiple metabolic routes, such as nucleotide, one-carbon/vitamin B6, glutathione, and sphingolipids, were shifted toward a less inflammatory state (
Figure 3). Because these signals moved together while cell viability and embryo survival were maintained, deep-sea microbes can be viewed as practical sources of functional materials that act through several controllable pathways rather than a single target.
Several limits should be recognized. This study used a crude EtOAc extract, which selectively enriches mid- to low-polarity metabolites; highly polar constituents are likely underrepresented. Compound annotation relied on library matching and MS/MS inference, so many identifications remain putative and may include isomeric ambiguity; moreover, only a subset of the 609 detected features matched reference entries, leaving unknowns that could contribute to activity. The metabolomics links are associative and have not been proven causal. The biological scope was limited to one macrophage cell line and an embryonic zebrafish model, which, critically, lacked an established anti-inflammatory positive control in the in vivo setting, without disease-relevant mammalian validation. A clear path forward is apparent. Bioassay-guided fractionation should isolate the actives while the COX-2/NO readouts and the pathway signature are tracked. Future work should include the targeted tests of the highlighted nodes (nucleotide supply, one-carbon/PLP, glutathione/redox, and sphingolipids) to convert associations into mechanisms. Thereafter, extension to disease-relevant mammalian models will then allow efficacy and safety to be judged in settings where macrophage-driven inflammation is central.
4. Materials and Methods
4.1. Chemicals and Reagents
RAW 264.7 macrophages were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Culture media and supplements—Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin were sourced from Welgene Inc. (Daegu, Republic of Korea). Reagents used for viability and fluorescence assays, including 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), 2′,7′-dichlorofluorescein diacetate (DCF-DA), 4-amino-5-methylamino-2′,7′-dichlorofluorescein diacetate (DAF-FM DA), acridine orange, and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Unless otherwise specified, additional cell-culture plastics and consumables were obtained from SPL Life Science Inc. (Pocheon, Republic of Korea).
4.2. Microbial Strain Isolation
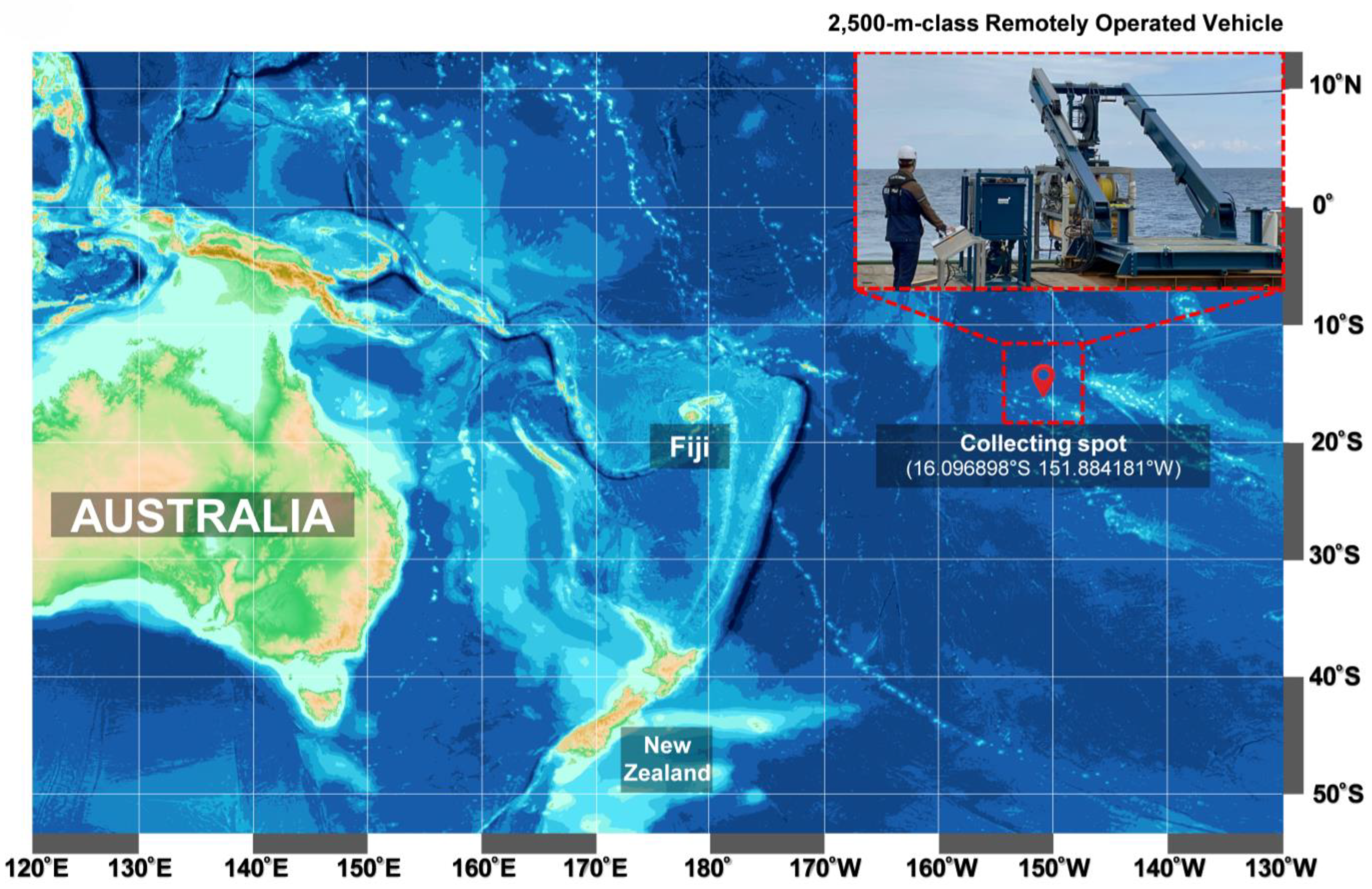
Strain ROD011 was isolated from a sediment sample collected in May 2021 from the Western Pacific Ocean high seas (16.096898° S 151.884181° W) at a depth of 1480 m (
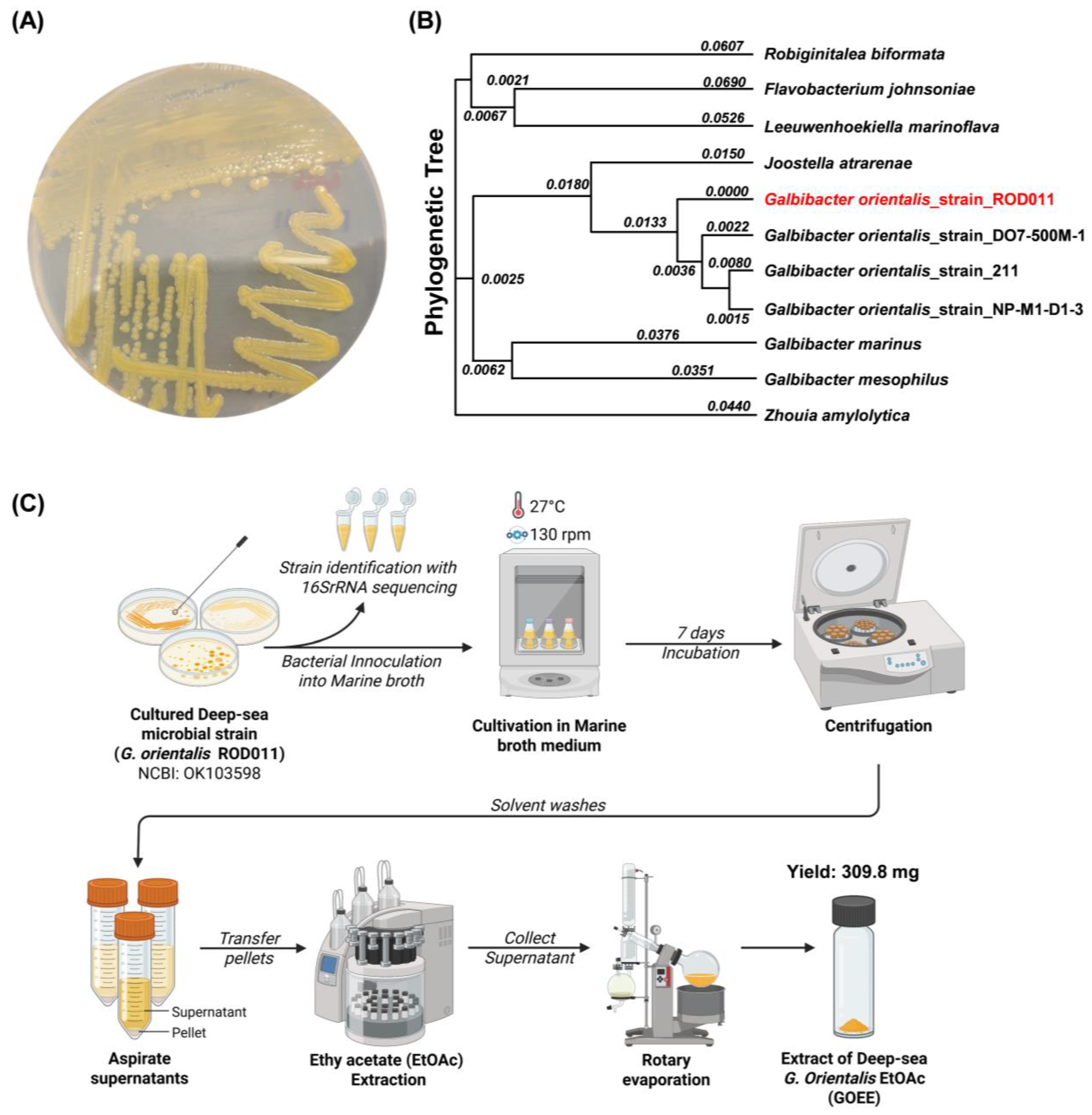
Figure 1). An underwater robot, a 2500 m class Remotely Operated Vehicle (URI-L, Redone Technologies Co., Ltd., Jangseong-gun, Republic of Korea), was used for sampling. The sediment samples were dried in air for 24 h on a clean bench and subjected to heat shock at 55 °C for 5 min to inhibit the growth of non-spore-forming bacteria. The sediment sample was suspended in sterile seawater, and a 100 μL aliquot of the sample was plated onto marine agar 2216 (Sigma-Aldrich Inc.). The plates were incubated at 27 °C for 1–3 months, allowing morphologically distinct colonies to develop. A single yellow-pigmented colony was selected, designated as strain ROD011, and purified by repeated subculturing on Marine Agar 2216 (
Figure 2A). The strain is routinely maintained on Marine Agar 2216 (Difco™, BD Diagnostics Inc., Sparks, MD, USA) and stored at −70 °C in Marine Broth 2216 (Difco™, BD Diagnostics Inc.) supplemented with 20% (
v/
v) glycerol for long-term preservation. The nearly complete 16S rRNA gene sequence of strain ROD011 was amplified using primers 27f and 1492r and deposited in GenBank under accession number OK103598. Sequence comparison revealed 100% identity with the type strain
G. orientalis strain En5 (NR_044346).
4.3. Preparation of GOEE
GOEE was prepared according to the workflow (
Figure 2C).
G. orientalis Strain ROD011 was cultivated at 27 °C in two 2.5 L Ultra Yield Flasks, each containing 1 L of medium, shaking at 130 rpm for seven days. The medium was composed of 10 g/L soluble starch, 2 g/L yeast extract, and 4 g/L peptone, dissolved in distilled water containing 34.75 g artificial sea salt. After the seven-day incubation period, the culture broth (2 L) was harvested by centrifugation (4 °C, 10,000×
g for 15 min) to separate the microbial biomass. The resulting microbial cell pellet was then subjected to extraction by adding pure EtOAc (100%). The mixture was vigorously agitated by shaking at 130 rpm for 24 h to facilitate cell wall disruption and elution of intracellular compounds. This extraction process was repeated three times to maximize metabolite recovery. The EtOAc-added extracts were pooled, and residual cell debris was removed by a final centrifugation (4 °C, 10,000×
g for 15 min). EtOAc solvent was subsequently removed under reduced pressure using a rotary evaporator to yield 309.8 mg of the crude extract.
4.4. Phylogenetic Tree
The 16S rRNA gene sequence of
G. orientalis strain ROD011 (NCBI accession OK103598) and reference sequences from closely related taxa in the family
Flavobacteriaceae were retrieved from GenBank (full list in
Table S1). Entire sequences (1374 bp) were aligned with MUSCLE (default parameters) as implemented in MEGA X (v11). After alignment, positions containing gaps or ambiguous bases were treated by pairwise deletion. Genetic distances were computed using the Kimura 2-parameter (K2P) model, and a Neighbor-Joining tree was inferred in MEGA. Bootstrap support was assessed with 1000 pseudoreplicates. Branch lengths are reported as substitutions per site. For display, the tree was midpoint-rooted, and the tip corresponding to strain ROD011 was highlighted (
Figure 2B).
4.5. Conditions of UPLC-QTOF-MS/MS and Identification of Metabolites Profile
The UPLC–QTOF–MS/MS system comprised a SYNAPT XS mass spectrometer (Waters Inc., Milford, MA, USA) coupled to a Waters ACQUITY UPLC system equipped with a ZSpray™ electrospray ionization source and LockSpray™ interface. Prior to LC–MS/MS analysis, the dried GOEE was re-dissolved in 100% methanol at 1 mg/mL, vortex-mixed, sonicated for 5 min, and filtered through a 0.22 µm PTFE membrane. Chromatographic separation was performed on a Waters BEH C18 column (2.1 × 100 mm, 1.7 µm; Waters Inc.) at a flow rate of 0.3 mL/min. The injection volume was 1 µL. Solvent A consisted of water with 0.1% formic acid, and solvent B consisted of acetonitrile with 0.1% formic acid. The gradient program was as follows: 0–1 min, 95% A; 1–25 min, linear gradient to 100% B; 25–27 min, 100% B; 27.01–30 min, re-equilibration at 95% A. The mass spectrometer was operated in positive-ion mode over a scan range of 100–1200 m/z. The capillary voltage was set to 3.0 kV, the sampling cone at 40 V, the source temperature at 120 °C, and the desolvation temperature at 350 °C. The cone gas flow was maintained at 50 L/h, and the desolvation gas flow at 600 L/h. Data were acquired in MSe continuum mode with two functions: low-energy trap collision energy (6 V) and high-energy ramp trap collision energy (20–45 V).
Accurate precursor and fragment spectra were processed using MassLynx software v4.2 (Waters Inc.). Profiles were preprocessed by subtracting solvent blanks and aligning retention times to a pooled QC run. Peak detection used an absolute intensity threshold of 2000 counts, a retention time window of 0–30 min, and an adduct filter including [M+H]+. A combined feature matrix was assembled to ensure peak correspondence across samples, with absent features recorded as zero (no imputation applied).
To minimize interference from medium-derived compounds, LC–MS/MS data were pre-filtered using a custom reference library constructed from the known composition of Marine Broth 2216 (Difco™, BD Diagnostics Inc.). The library contained 28 representative amino acids, peptides, organic acids, carbohydrates, vitamins, and minor lipids typically detected in uninoculated media. Features matching library entries (
m/
z ± 5 ppm, RT ± 0.05 min, MS/MS similarity ≥ 0.8) were automatically flagged and excluded through the UNIFI™ software v1.9 SR4 (Waters Inc.) library-filtering module, following the background-subtraction precedent described by the previous literature [
33]. This procedure enabled retention of microbially derived features while computationally removing medium-borne background peaks.
Metabolic constituents of GOEE were tentatively annotated using UNIFI™ software (Waters Inc.) by matching exact
m/
z values, retention times, and fragmentation patterns to curated reference files for compounds reported from the extract (
Table S2). The filtered feature list was then processed in UNIFI™ (Waters Inc.) for tentative metabolite identification using a tiered-matching workflow combining exact mass (±5 ppm), retention-time tolerance (±0.05 min), and fragment pattern similarity (cosine ≥ 0.8) criteria. Each feature was searched against an in-house library of known natural products and subsequently cross-referenced to the NCI Program for Natural Products Discovery (NPNPD) prefractionated library [
34]. The NPNPD dataset was accessed through the U.S. National Cancer Institute Developmental Therapeutics Program (DTP) collaborative repository (
https://dctd.cancer.gov/programs/dtp/organization/npb/npnpd) (accessed on 1 September 2025) via a formal data-sharing request (dtpmail:
dtpinfo@nih.gov). Metadata, including fraction identifiers, molecular formulas, and LC–MS/MS reference spectra, were used for spectral cross-validation following the hierarchical matching protocol [
34]. Features with no corresponding matches in either the medium-compound or NPNPD libraries were considered putatively novel metabolites derived from
G. orientalis strain ROD011. All raw and processed LC–MS/MS data, including the Marine Broth 2216 compound library, are archived in the public repository cited in the Data Availability Statement to enable independent verification and re-analysis.
4.6. Metabolite-Based Pathway Enrichment and Interaction Network Analysis
A list of confidently identified metabolites from the untargeted LC-MS experiment, including normalized relative intensities and group labels, was uploaded to MetaboAnalyst (version 6.0;
https://www.metaboanalyst.ca/MetaboAnalyst/) (accessed on 16 August 2025) [
35]. Compound identifiers were mapped using the built-in Compound ID conversion tool, prioritizing KEGG Compound IDs; unmatched synonyms were manually resolved, and duplicates were collapsed. The organism was set to
Mus musculus. Pathway over-representation was assessed against KEGG pathways using the hypergeometric test, and pathway topology was evaluated by degree centrality to estimate node impact. Multiple testing was controlled by the Benjamini–Hochberg procedure, and statistical significance was defined as FDR < 0.05. Bubble plots were generated within MetaboAnalyst, with pathway impact on the
x-axis, −log10(FDR) on the
y-axis, bubble size proportional to impact, and color indicating significance.
The same metabolite list was analyzed using the Network Explorer module in MetaboAnalyst v6.0 to construct a Metabolite–Metabolite Interaction Network based on the integrated HMDB/STITCH knowledge base. After node mapping, edges were filtered using a confidence cutoff of ≥0.5. The network was visualized with the iGraph force-directed layout. Node centralities (degree and betweenness) were computed to highlight hubs, and communities were inspected to annotate functionally coherent modules. Final network graphics were exported directly from MetaboAnalyst at publication resolution.
4.7. In Vitro Evaluation of the Anti-Inflammatory Potential of GOEE Using LPS-Induced RAW 264.7
4.7.1. Cell Culture and Evaluation of Cytotoxicity of GOEE
The murine macrophage—RAW 264.7 cells were cultured in DMEM containing 10% FBS and 1% P/S at 37 °C in a 5% CO
2 humidified incubator (Sanyo Electric Co., Ltd., Tokyo, Japan). The cytotoxicity of GOEE was assessed by the MTT assay [
36]. RAW 264.7 cells were seeded into a 96-well plate at a density of 1 × 10
5 cells per well and incubated at 37 °C with 5% CO
2 for 24 h. Subsequently, the cells were exposed to various concentrations of GOEE (1.25, 2.5, 5, 10, and 20 μg/mL) or QC as a positive control (3 μg/mL), and the cells were stimulated with LPS (10 ng/mL) and incubated for another 24 h. For statistical robustness, all cytotoxicity experiments were performed in triplicate biological replicates. After the 24 h exposure to the samples, 20 μL of 5 mg/mL MTT reagent dissolved in PBS was added to each well. The plate was then incubated at 37 °C for 1 h at 5% CO
2. Following incubation, the solution was carefully aspirated from all the wells, and 100 μL of DMSO was added to dissolve the formazan crystals that had formed. The absorbance of each well was subsequently measured at 540 nm using a Synergy™ HTX microplate reader (Agilent Technologies Inc., Santa Clara, CA, USA).
4.7.2. Evaluation of LPS-Induced NO Production
The inhibitory effect of GOEE on NO production was measured using the Griess assay [
37]. RAW 264.7 cells were seeded into a 96-well plate at a density of 1 × 10
5 cells/mL and incubated at 37 °C with 5% CO
2 for 24 h. Subsequently, the cells were divided into the following experimental groups: (1) Control (Vehicle), (2) LPS alone (10 ng/mL), (3) GOEE + LPS, and (4) Positive control (3 μg/mL) + LPS. Cells were exposed to various concentrations of GOEE (1.25−20 μg/mL), and the cells were stimulated with LPS (10 ng/mL) and incubated for another 24 h. After the 24 h exposure to samples, equal volumes of the cell culture medium and Griess reagent were combined in a 96-well plate and incubated for 10 min. Then, the absorbance was measured at 540 nm using a Synergy™ HTX microplate reader (Agilent Technologies Inc.).
4.7.3. Western Blotting
Western blotting was performed to quantify key inflammatory mediators. RAW 264.7 cells were seeded in 6-well plates (1 × 105 cells/well) and cultured for 24 h, pretreated with GOEE (2.5, 5, 10, or 20 µg/mL) for 1 h, then stimulated with LPS (10 ng/mL) and incubated for an additional 24 h. Whole-cell lysates were prepared in RIPA buffer (89901, Thermo Fisher Scientific Inc., Waltham, MA, USA) and protein concentrations determined using the Pierce BCA assay. Equal protein amounts were mixed with sample buffer, heated at 100 °C for 5 min, resolved by 12% SDS-PAGE, and transferred to 0.45 µm nitrocellulose membranes. Membranes were blocked with 5% skim milk in TBST (room temperature, 2 h), probed with primary antibodies to the indicated targets, followed by HRP-conjugated secondary antibodies, and developed using an enhanced chemiluminescent substrate (RPN2105). Fluorescent signals were captured on a FUSION SOLO imaging system Vilber Lourment system (Vilber Lourmat Deutschland GmbH, Wielandstrasse 2, Eberhardzell, Germany) and band intensities quantified in ImageJ v1.54p (NIH, Bethesda, MD, USA) after normalization to β-actin.
4.8. In Vivo Evaluation of the Anti-Inflammatory Potential of GOEE Using Zebrafish
4.8.1. Maintenance of Zebrafish
Adult zebrafish (
Danio rerio) were obtained from a licensed supplier (Jeju Aquarium Inc., Jeju, Republic of Korea) and maintained as described previously [
38]. Fish were housed in 3 L acrylic tanks at 28.5 °C on a 14:10 h light–dark cycle with conditioned, aerated water, and fed TetraMin
® flake and live brine shrimp three times daily, six days a week. Spawning was triggered by lights-on in the morning; fertilized embryos were collected within 30 min, staged, and allocated to experiments.
4.8.2. Survival Rate Analysis in Zebrafish
Zebrafish embryos at 7–9 h post-fertilization were arrayed in 12-well plates (15 per well) and pre-exposed to GOEE (5, 10, or 20 μg/mL) for 1 h; LPS (10 μg/mL) was then added and cultures maintained to 24 hpf, after which survival was recorded daily from 1 to 5 day post-fertilization (dpf). The survival rate analysis was performed using independent biological replicates (n = 3).
4.8.3. Evaluation of LPS-Induced Cell Death, NO Generation, and ROS Generation in Zebrafish
Fluorescence images analysis for cell death, nitric oxide (NO), and reactive oxygen species using LPS-evoked zebrafish embryos was adapted from those described by a previous study [
39]. At 3 dpf, morphologically normal larvae were transferred into embryo medium in 12- or 24-well plates at five larvae per well and allowed to equilibrate for 20 min at 28 °C. To test protection against inflammation, larvae were pre-exposed to GOEE at 5, 10, or 20 µg/mL for 1 h, after which LPS (10 µg/mL) was added to the same wells. The experiment included the following four core groups for comparison: (1) Vehicle, (2) LPS alone, (3) GOEE + LPS, and (4) Positive control (3 μg/mL) + LPS. Vehicle (0.01% DMSO contained DMEM) and LPS-only controls were included on each plate. All fluorescence image analyses were conducted with independent biological replicates (n = 3). Unless otherwise specified, all incubations were performed in the dark at 28 °C, larvae were rinsed three times in fresh embryo medium between steps, and anesthesia with 0.016% (
v/
v) tricaine preceded imaging. For cell-death measurements, larvae exposed to LPS for 24 h were stained with acridine orange at 7 µg/mL for 30 min. For NO measurements, larvae were incubated with DAF-FM DA at 5 µM for 1 h, allowing de-esterification during the final rinse to minimize background. For ROS measurements, larvae were first challenged with LPS for 24 h and then stained with DCF-DA at 20 µM for 1 h. Fluorescence images were acquired on a Lionheart FX microscope controlled by Gen5 v3.03 (Agilent Technologies Inc.) using appropriate filter sets and identical exposure, gain, and binning across groups to avoid saturation. And the mean fluorescence intensity was quantified in ImageJ (NIH).
4.9. Statistical Analysis
For antioxidant and anti-inflammatory activity, dates are presented as the mean ± standard error of the mean, and the means of each treatment were compared using one-way ANOVA from GraphPad PRISM Version 10 (GraphPad Software Inc., San Diego, CA, USA). Significance levels were denoted as # p < 0.05, ## p < 0.01, and ### p < 0.001 compared to the control, and those compared with the LPS group are denoted as * p < 0.05, ** p < 0.01, and *** p < 0.001.