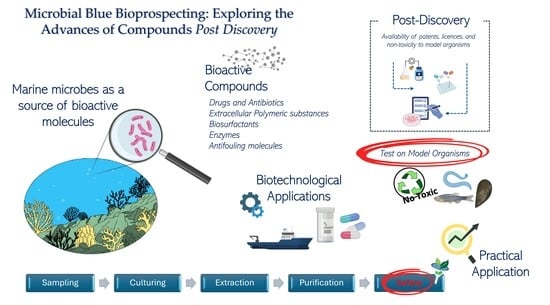
Microbial Blue Bioprospecting: Exploring the Advances of Compounds Post-Discovery
Abstract
1. Introduction
2. Materials and Methods
3. Marine Microbes as a Source of Bioactive Molecules
3.1. Drugs and Antibiotics
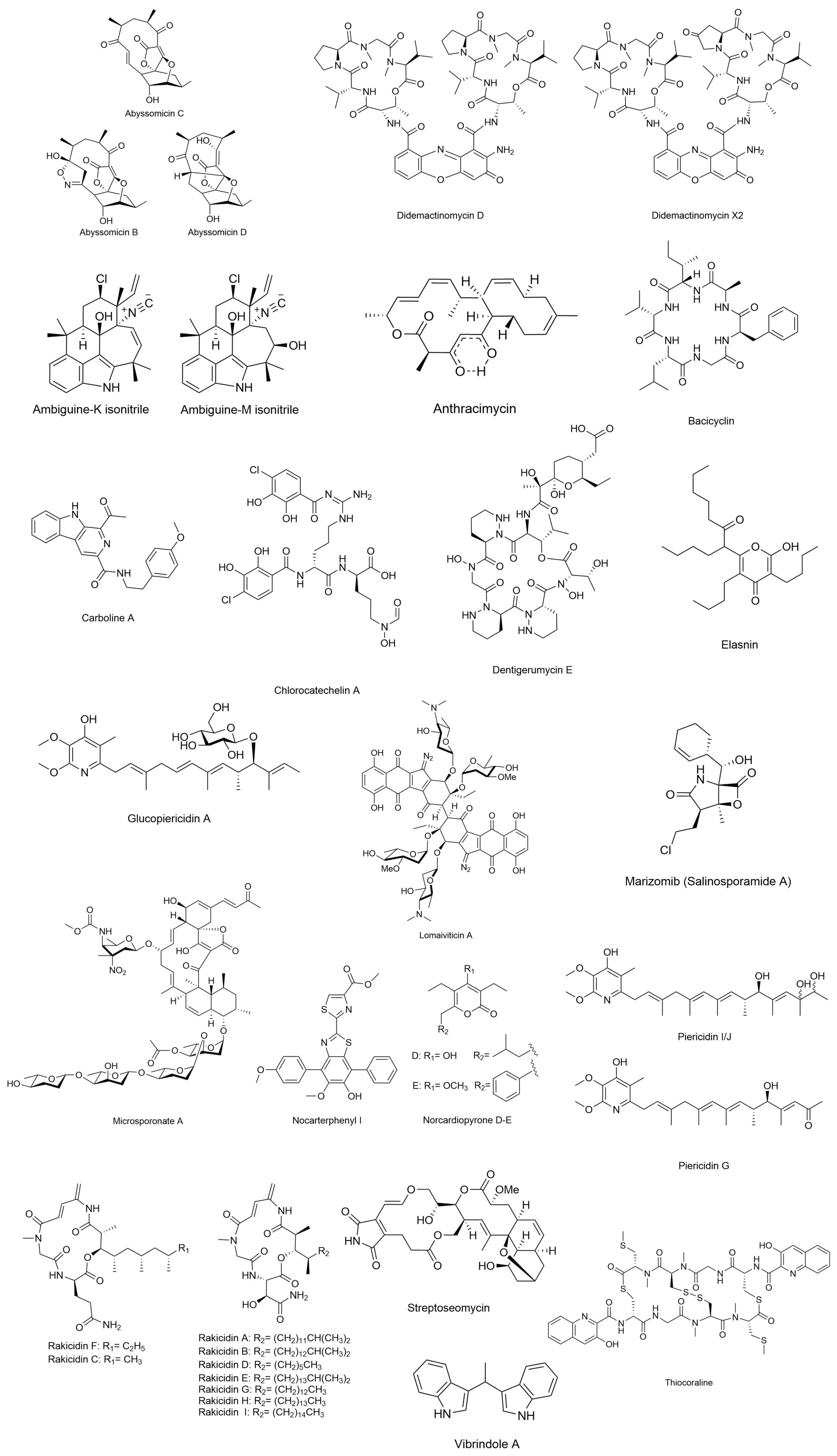
3.1.1. Abyssomicins
3.1.2. Carboline A
3.1.3. Didemactinomycin D
3.1.4. Glucopiericidin A
3.1.5. Lomaiviticin A
3.1.6. Marizomib (Salinosporamide A)
3.1.7. Piericidin
3.1.8. Polypeptide (PBN3)
3.1.9. Rakicidin
3.1.10. Thiocoraline
| Compound | Origin | Biological Activity | Type and Chemical Structure | Patent n° | References |
|---|---|---|---|---|---|
| Anthracimycin | Streptomyces sp. | Inhibitory effect against some pathogenic bacteria such as Bacillus anthracis, whose spores cause anthrax. | Macrolide polyketide; C25H32O4 | N/A | [86] |
| Nocarterphenyl I/Nocardiopyrone D | Nocardiopsis sp. | Antibacterial activity against B. subtilis and E. coli | p-terphenyl and α-pyrone | N/A | [63] |
| Vibrindole A | Vibrio parahaemolyticus | Antimicrobial activity against S. aureus, S. albus and B. subtilis | N,N-Diphenyl-p-phenylenediamine C18H16N2 | N/A | [87] |
| Dentigerumycin E | Streptomyces sp. and Bacillus sp. | Antiproliferative and antimetastatic activities against human carcinoma | Cyclic depsipeptide; C40H67N9O | N/A | [44] |
| Bacicyclin | Bacillus sp. | Antibacterial activity against E. faecalis and S. aureus | Cyclic hexapeptide; C31H48N6O6 | N/A | [88] |
| Chlorocatechelin A | Streptomyces sp. | Inhibited the growth of a wide range of bacterial and fungal pathogens | Acylguanidine-type siderophore; C26H30Cl2N6O11 | N/A | [89] |
| Ambiguine-K and M isonitrile | Fischerella ambigua | Antibacterial activity against Mycobacterium tuberculosis | Indole alkaloid with isonitrile group; C26H29ClN2O and C2H3N | N/A | [15] |
| Streptoseomycin | Streptomyces seoulensis | Antibacterial activity against Helicobacter pylori, Lactobacillus acidophilus, Bifidobacterium bifidum, Eubacterium brachy, Propionibacterium acnes, Staphylococcus aureus, Micrococcus luteus and Bacillus subtilis | Aminoglycoside; C31H37NO11 | N/A | [90] |
| Microsporonates A | Micromonospora harpali | Antibacterial activity against Gram-positive bacteria | Complex polycyclic macrolides | N/A | [90] |
| Patent or Clinical Trials Involved Compounds | |||||
| Abyssomicin | Verrucosispora strain AB 18-032 | Antibiotic, Antiretroviral | Polyketide | CN110092758A | [56] |
| Carboline A | Marine bacteria Actinoalloteichus cyanogriseus ZZ1866 | Anticancer | Alkaloid | CN111747955 | [60] |
| Didemactinomycin D | Streptomyces costaricanus SCSIO ZS0073 | Antibacterial activities against Staphylococcus aureus | Peptide lactone antibiotic | CN110669103 | [91] |
| Glucopiericidin A | Streptomyces sp. HBERC-58855 | Renal cancer | α-pyridone | CN109384823 | [63] |
| Lomaiviticin A | Salinispora pacifica DPJ-0019 | Induces DNA double-strand breaks | Dimeric diazofluorenes | JP2705789B2 | [65] |
| Marizomib (Salinosporamide A) | Salinispora tropica | Breast cancer, multiple myeloma | γ-lactam-β-lactone bicycle core C15H20ClNO4 | Clinical Trial stage (Phase III) | [36,72] |
| Piericidin | Streptomyces sp. HBERC-58855 | Renal carcinoma | α-pyridone | CN109384710A | [64] |
| Polypeptide PBN3 | Marine bacteria Brevibacillus sp. N189 | Lung cancer, Liver cancer, Pancreatic cancer, Breast cancer or Cervical cancer | Polypeptide | CN113150071 | [74] |
| Rakicidin I | Micromonospora sp. FIM 02-523 | Colon, Pancreatic cancer | Cyclic lipopeptide | CN108329280 | [79] |
| Rakicidin H | Micromonospora sp. FIM 02-523 | Colon, Pancreatic cancer | Cyclic lipopeptide | CN108586380 | [80] |
| Rakicidin B1-2 Depsipeptide | Marine bacteria sp. FIMR160609 | Colon, Pancreatic cancer | Cyclic lipopeptide | CN110698537 | [81] |
| Thiocoraline | Micromonospora marina | Colon cancer, Medullary thyroid carcinoma | Depsipeptide | Preclinical pharmacodynamic evaluation by PharmaMar | [82] |
3.2. Extracellular Polymeric Substances
3.2.1. EPS HE800 (Hyalurift®)
3.2.2. EPS HYD657 (Abyssine®)
3.2.3. EPS WO2015117985A1
3.2.4. EPS AU2016330332B2
3.2.5. EPS ES2585398B1
3.2.6. EPS US10993434B2
3.3. Biosurfactants
3.4. Enzymes
3.4.1. NucB
3.4.2. FlAly
3.5. Anti-Biofilm and Anti-Fouling Compounds
3.5.1. Elasnin
3.5.2. Tambjamines
4. Application of Marine Bacterial Bioactive Compounds
4.1. Pharmacological Treatments
4.2. Industrial Applications
4.3. Production of Cosmetic Products
4.4. Bioremediation
4.5. Anti-Fouling Strategies
5. Biodiscovery Pipeline: The Path for Concrete Application
5.1. Methods
5.2. Approval
6. Effects of Marine Bacterial Bioactive Compounds on Model Organisms
6.1. Mytilus galloprovincialis
6.2. Ciona Intestinalis
6.3. Danio rerio
| Bioactive Compounds | Effects | Type and Chemical Structure | References |
|---|---|---|---|
| Phloroglucinols | Contributes to the biological control of plant disease | Phenolic benzenetriol; C6H3(OH)3 | [191] |
| EPS produced by Bifidobacterium | Regulator of interlukin-10 secretion, plays a vital role during inflammation | Protein–lipid complex | [192] |
| Sebastenoic acid | Acts as an antimicrobial agent against various pathogenic bacteria | Medium-chain fatty acid; C10H18O4 | [192] |
| Extracellular materials produced by Pseudoalteromonas | Inhibited the settlement and metamorphosis of the ubiquitous fouling invertebrate larvae, Balanus amphitrite and Hydroides elegans | glycolipids/protein–polysaccharide complexes | [193] |
| 256-tribromo-1-methylgramine | Potent inhibitor against larval settlement of the barnacle Amphi-balanus amphitrite | Halogenated indole alkaloid; C12H13Br3N2 | [193] |
| Lichenicidin | Enterococcus faecalis, Salmonella typhimurium, Escherichia coli, Cronobacter sakazakii | Consists of two peptides: Lchα (approximately C130H191N35O39S5) and Lchβ (roughly C110H158N26O30S4) | [194] |
| Pentabromopseudilin | Deadly to small eukaryotes, such as protozoa and invertebrates | Highly brominated bromophenol-bromopyrrole hybrid; C10H4Br5NO | [128] |
| Soforolipid | Possess curative actions against the algae Phaeocystis globosa and Aeromicrobium tamlense | Glycolipid biosurfactant; C34H58O15 | [166] |
| Dimethylsulfoniopropionate | Aids in structuring the coral-associated bacterial community and enhancing the tolerance of corals to ROS and thermal stress | Organosulfur zwitterionic compound; C5H10O2S | [195] |
| Thallusin | Induces normal germination and morphogenesis of green macroalgae. | Sesquiterpenoid morphogen; C25H31NO7 | [196] |
7. New Approaches for Marine Biodiscovery
8. Challenges and Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmadniaye Motlagh, H.; Horie, Y.; Rashid, H.; Banaee, M.; Multisanti, C.R.; Faggio, C. Unveiling the effects of fennel (Foeniculum vulgare) seed essential oil as a diet supplement on the biochemical parameters and reproductive function in female common carps (Cyprinus carpio). Water 2023, 15, 2978. [Google Scholar] [CrossRef]
- Impellitteri, F.; Multisanti, C.R.; Riolo, K.; Zicarelli, G.; Porretti, M.; Cafeo, G.; Faggio, C. Bergamot (Citrus bergamia): A potential new nutraceutical against cellular and physiological alterations induced by emerging contaminants in sentinel organisms. Antioxidants 2025, 14, 539. [Google Scholar] [CrossRef] [PubMed]
- Jindal, R.; Sharma, R.; Kaur, P.; Kaur, S.; Multisanti, C.R.; Faggio, C. Mitigation of haemato-genotoxic and stress response effects in Cyprinus carpio via silymarin dietary supplementation following deltamethrin exposure. Heliyon 2024, 10, 7. [Google Scholar] [CrossRef]
- Rashidian, G.; Boldaji, J.T.; Rainis, S.; Prokić, M.D.; Faggio, C. Oregano (Origanum vulgare) extract enhances zebrafish (Danio rerio) growth performance, serum and mucus innate immune responses and resistance against Aeromonas hydrophila challenge. Animals 2021, 11, 299. [Google Scholar] [CrossRef]
- Multisanti, C.R.; Riolo, K.; Impellitteri, F.; Zicarelli, G.; Vazzana, I.; Cafeo, G.; Giannetto, A. Bergamot (Citrus bergamia) as a potential anti-stress agent: Counteracting cellular and physiological changes by sodium lauryl sulphate in Mytilus galloprovincialis. Environ. Pollut. 2025, 371, 125939. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Role of Bacillus licheniformis VS16-derived biosurfactant in mediating immune responses in Carp Rohu and its application to the food industry. Front. Microbiol. 2017, 8, 514. [Google Scholar] [CrossRef]
- Pastaki, N.J.; Abdollahpour, H.; Karimzadeh, M.; Zamani, H.; Multisanti, C.R.; Faggio, C. Physiological and immunological impact of methanolic lavender extract on female goldfish (Carassius auratus). Aquac. Rep. 2023, 33, 101841. [Google Scholar] [CrossRef]
- Van Doan, H.; Prakash, P.; Hoseinifar, S.H.; Ringø, E.; El-Haroun, E.; Faggio, C.; Dawood, M.A. Marine-derived products as functional feed additives in aquaculture: A review. Aquac. Rep. 2023, 31, 101679. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Piccione, G.; Multisanti, C.R.; Faggio, C. Synergistic interaction of nanoparticles and probiotic delivery: A review. J. Fish. Dis. 2024, 47, e13916. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Zhang, J.; Bian, Z.; Zhou, H.; Zhang, Z.; Lin, Z.; Xu, H. Bioactive natural compounds against human coronaviruses: A review and perspective. Acta Pharm. Sin. B 2020, 10, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef]
- Gill, B.S.; Qiu, F. Technologies for extraction and production of bioactive compounds. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–36. [Google Scholar]
- Rizzo, C.; Lo Giudice, A. Marine Invertebrates: Underexplored Sources of Bacteria Producing Biologically Active Molecules. Diversity 2018, 10, 52. [Google Scholar] [CrossRef]
- Andryukov, B.; Mikhailov, V.; Besednova, N. The biotechnological potential of secondary metabolites from marine bacteria. J. Mar. Sci. Eng. 2019, 7, 176. [Google Scholar] [CrossRef]
- Srinivasan, R.; Kannappan, A.; Gandhi, A.D.; Kannan, R.R. Marine microbes as a valuable source for bioactive compounds. Front. Microbiol. 2021, 12, 748621. [Google Scholar]
- Venter, J.C. Environmental genome shotgun sequencing of the Sargasso Sea. Science 2003, 304, 66–74. [Google Scholar] [CrossRef]
- Newman, D.J.; Hill, T. New drugs from marine microbes: The tide is turning. J. Ind. Microbiol. Biotechnol. 2006, 33, 539–544. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal mushrooms: Bioactive compounds, use, and clinical trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L.; Zhang, L. Natural products and drug discovery. In Natural Products: Drug Discovery and Therapeutic Medicine; Humana Press: Totowa, NJ, USA, 2005; pp. 3–29. [Google Scholar]
- Haefner, B. Drugs from the deep: Marine natural products as drug candidates. Drug Discov. Today 2003, 8, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Ghilan, A.K.M.; Esmail, G.A.; Arasu, M.V.; Duraipandiyan, V.; Ponmurugan, K. Bioactivity assessment of the Saudi Arabian Marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum beta-lactamase clinical bacterial pathogens. J. Infect. Public Health 2019, 12, 549–556. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Zaporozhets, T.S.; Besednova, N.N. Promising strategies for finding new means of fighting with infectious diseases. Antibiot. Khimioter. 2018, 63, 44–55. [Google Scholar]
- Böhringer, N.; Fisch, K.M.; Schillo, D.; Bara, R.; Hertzer, C.; Grein, F.; Schäberle, T.F. Antimicrobial potential of bacteria associated with marine sea slugs from North Sulawesi, Indonesia. Front. Microbiol. 2017, 8, 1092. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.G.; Briglia, M.; Zammuto, V.; Morganti, D.; Faggio, C.; Impellitteri, F.; Graziano, A.C.E. Innovation in osteogenesis activation: Role of marine-derived materials in bone regeneration. Curr. Issues Mol. Biol. 2025, 47, 175. [Google Scholar] [CrossRef]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef]
- Glaser, K.B.; Mayer, A. A renaissance in marine pharmacology: From preclinical curiosity to clinical reality. Biochem. Pharmacol. 2009, 78, 440–448. [Google Scholar] [CrossRef]
- Rizzo, C.; Poli, A.; Di Donato, P.; Nicolaus, B.; Lo Giudice, A. Biosurfactant-producing bacteria from extreme marine environments: The case of Idiomarina spp. Mar. Drugs 2022, 20, 684. [Google Scholar]
- Lo Giudice, A.; Gugliandolo, C. A special issue on microorganisms from extreme environments in Memory of Luigi Michaud (1974–2014). Diversity 2019, 12, 2. [Google Scholar] [CrossRef]
- Giordano, D. Bioactive molecules from extreme environments. Mar. Drugs 2020, 18, 640. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Kannappan, A.; Shi, C.; Lin, X. Marine bacterial secondary metabolites: A treasure house for structurally unique and effective antimicrobial compounds. Mar. Drugs 2021, 19, 530. [Google Scholar] [CrossRef] [PubMed]
- Raghukumar, S.; Raghukumar, S. Extreme marine environments. In Fungi in Coastal and Oceanic Marine Ecosystems: Marine Fungi; Springer: Cham, Switzerland, 2017; pp. 219–263. [Google Scholar]
- World Health Organization. Monitoring and Evaluation of the Global Action Plan on Antimicrobial Resistance: Framework and Recommended Indicators; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global contributors to antibiotic resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef]
- Wibowo, J.T.; Bayu, A.; Aryati, W.D.; Fernandes, C.; Yanuar, A.; Kijjoa, A.; Putra, M.Y. Secondary metabolites from marine-derived bacteria with antibiotic and antibiofilm activities against drug-resistant pathogens. Mar. Drugs 2023, 21, 50. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef] [PubMed]
- Stonik, V.A.; Makarieva, T.N.; Shubina, L.K. Antibiotics from marine bacteria. Biochemics 2020, 85, 1362–1373. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Zorzet, A. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- ul Hassan, S.S.; Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Tasneem, U. Emerging biopharmaceuticals from marine actinobacteria. Environ. Toxicol. Pharmacol. 2017, 49, 34–47. [Google Scholar] [CrossRef]
- Jang, K.H.; Nam, S.J.; Locke, J.B.; Kauffman, M.C.A.; Beatty, M.D.S.; Paul, M.L.A.; Fenical, W. Anthracimycin, a potent anthrax antibiotic from a marine-derived actinomycete. Angew. Chem. 2013, 52, 1000–1002. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, K.B.; Wang, W.; Bi, S.F.; Mei, Y.N.; Deng, X.Z. Discovery, biosynthesis, and heterologous production of streptoseomycin, an anti-microaerophilic bacteria macrodilactone. Org. Lett. 2018, 20, 2967–2971. [Google Scholar] [CrossRef]
- Karuppiah, V.; Sun, W.; Li, Z. Natural products of actinobacteria derived from marine organisms. Stud. Nat. Prod. Chem. 2016, 48, 417–446. [Google Scholar]
- Swain, S.S.; Paidesetty, S.K.; Padhy, R.N.; Singh, P.K. Computational approach for locating effective cyanobacterial compounds against Mycobacterium tuberculosis. Indian. J. Pharmac Edu Res. 2017, 51, 1–10. [Google Scholar] [CrossRef]
- Shin, D.; Byun, W.S.; Moon, K.; Kwon, Y.; Bae, M.; Um, S.; Lee, S.K.; Oh, D.C. Coculture of Marine Streptomyces sp. with Bacillus sp. produces a new piperazic acid-bearing cyclic peptide. Front. Chem. 2018, 6, 498. [Google Scholar] [CrossRef]
- Moncheva, P.; Tishkov, S.; Dimitrova, N.; Chipeva, V.; Antonova-Nikolova, S.; Bogatzevska, N. Characteristics of soil actinomycetes from Antarctica. J. Cult. Collect. 2002, 3, 3–14. [Google Scholar]
- O’Brien, A.; Sharp, R.; Russell, N.J.; Roller, S. Antarctic bacteria inhibit growth of food-borne microorganisms at low temperatures. FEMS Microbiol. Ecol. 2004, 48, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Nedialkova, D.; Naidenova, M. Screening the antimicrobial activity of Actinomycetes strains isolated from Antarctica. J. Cult. Collect. 2004, 4, 29–35. [Google Scholar]
- Biondi, N.; Tredici, M.R.; Taton, A.; Wilmotte, A.; Hodgson, D.; Losi, D.; Marinelli, F. Cyanobacteria from benthic mats of Antarctic lakes as a source of new bioactivities. J. Appl. Microbiol. 2008, 105, 105–115. [Google Scholar] [CrossRef]
- Lo Giudice, A.L.; Bruni, V.; Michaud, L. Characterization of Antarctic psychrotrophic bacteria with antibacterial activities against terrestrial microorganisms. J. Basic Microbiol. 2007, 47, 496–505. [Google Scholar] [CrossRef]
- Shekh, R.M.; Singh, P.; Singh, S.M.; Roy, U. Antifungal activity of Arctic and Antarctic bacteria isolates. Polar Biol. 2010, 34, 139–143. [Google Scholar] [CrossRef]
- Dong, N.; Di, Z.; Yu, Y.; Yuan, M.; Zhang, X.; Li, H. Extracellular enzyme activity and antimicrobial activity of culturable bacteria isolated from soil of Grove Mountains, East Antarctica. Acta Microbiol. Sin. 2013, 53, 1295–1306. [Google Scholar]
- Encheva, M.; Zaharieva, N.; Kenarova, A.; Chipev, N.; Chipeva, V.; Hristova, P.; Ivanova, I.; Moncheva, P. Abundance and activity of soil actinomycetes from Livingston Island, Antarctica. Bulg. J. Agric. Sci. 2013, 19, 68–71. [Google Scholar]
- Papaleo, M.C.; Fondi, M.; Maida, I.; Perrin, E.; Giudice, A.L.; Michaud, L.; Mangano, S.; Bartolucci, G.; Romoli, R.; Fani, R. Sponge-associated microbial Antarctic communities exhibiting antimicrobial activity against Burkholderia cepacia complex bacteria. Biotechnol. Adv. 2012, 30, 272–293. [Google Scholar] [CrossRef]
- Mohan, S.; Ajay Krishna, M.S.; Chandramouli, M.; Keri, R.S.; Patil, S.A.; Ningaiah, S.; Somappa, S.B. Antibacterial natural products from microbial and fungal sources: A decade of advances. Mol. Divers. 2023, 27, 517–541. [Google Scholar] [CrossRef]
- Banerjee, A.; Mohammed Breig, S.J.; Gómez, A.; Sánchez-Arévalo, I.; González-Faune, P.; Sarkar, S.; Cabrera-Barjas, G. Optimization and characterization of a novel exopolysaccharide from Bacillus haynesii CamB6 for food applications. Biomolecules 2022, 12, 834. [Google Scholar] [CrossRef]
- Bister, B.; Bischoff, D.; Ströbele, M.; Riedlinger, J.; Reicke, A.; Wolter, F. Abyssomicin C-A polycyclic antibiotic from a marine Verrucosispora strain as an inhibitor of the p-aminobenzoic acid/tetrahydrofolate biosynthesis pathway. Angew. Chem. Int. Ed. 2004, 43, 2574–2576. [Google Scholar] [CrossRef]
- Keller, S.; Nicholson, G.; Drahl, C.; Sorensen, E.; Fiedler, H.-P.; Süssmuth, R.D. Abyssomicins G and H and atrop-abyssomicin C from the marine Verrucosispora strain AB-18-032. J. Antibiot. 2007, 60, 391–394. [Google Scholar] [CrossRef]
- Wang, Q.; Song, F.; Xiao, X.; Huang, P.; Li, L.; Monte, A. Abyssomicins from the South China sea deep-sea sediment Verrucosispora sp.: Natural thioether michael addition adducts as antitubercular prodrugs. Angew. Chem. Int. Ed. 2013, 52, 1231–1234. [Google Scholar] [CrossRef]
- León, B.; Navarro, G.; Dickey, B.J.; Stepan, G.; Tsai, A.; Jones, G.S. Abyssomicin 2 reactivates latent HIV-1 by a PKC-and HDAC-independent mechanism. Org. Lett. 2015, 17, 262–265. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, J.; Qin, Q.; An, F.; Wang, S.; Li, L.; Lin, H. Marinacarboline glucuronide, a new member of β-carboline alkaloids from sponge-derived actinomycete Actinoalloteichus cyanogriseus LHW52806. J. Antibiot. 2022, 75, 523–525. [Google Scholar] [CrossRef]
- Waksman, S.A.; Woodruff, H.B. Bacteriostatic and Bactericidal Substances Produced by a Soil Actinomyces. Soc. Exp. Biol. Med. 1940, 45, 609–614. [Google Scholar] [CrossRef]
- Xianqin, S.; Jiang, X.; Sun, J.; Zhang, C.; Zhang, Y.; Lu, L.; Ju, J. Study on the secondary metabolites of antibacterial activity of the actinomycete Stremptmeces costaricanus SCSIOZS0073. Nat. Prod. Res. Dev. 2017, 29, 410–414. [Google Scholar]
- Zhou, L.; Chang, Y.; Yang, S. Antibacterial p-terphenyl and α-pyrone derivates isolated from the marine-derived actinomycete Nocardiopsis sp. HDN154086. J. Antibiot. 2024, 77, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tang, L.; Fang, W.; Lee, K.; Liu, Y.; Luo, X. Two Piericidin Glucosides and Application Thereof to Anti-Kidney Cancer Medicines. CN109384823, 26 February 2019. [Google Scholar]
- Janso, J.E.; Haltli, B.A.; Eustáquio, A.S.; Kulowski, K.; Waldman, A.J.; Zha, L.; Nakamura, H.; Bernan, V.S.; He, H.; Carter, G.T.; et al. Discovery of the lomaiviticin biosynthetic gene cluster in Salinispora pacifica. Tetrahedron 2014, 70, 4156–4164. [Google Scholar] [CrossRef]
- Kersten, R.D.; Lane, A.L.; Nett, M.; Richter, T.K.S.; Duggan, B.M.; Dorrestein, P.C.; Moore, B.S. Bioactivity-guided genome mining reveals the lomaiviticin biosynthetic gene cluster in Salinispora tropica. ChemBioChem 2013, 14, 955–962. [Google Scholar] [CrossRef]
- He, H.; Ding, W.D.; Bernan, V.S.; Richardson, A.D.; Ireland, C.M.; Greenstein, M.; Ellestad, G.A.; Carter, G.T. Lomaiviticins A and B, potent antitumor antibiotics from Micromonospora lomaivitiensis. J. Am. Chem. Soc. 2001, 123, 5362–5363. [Google Scholar] [CrossRef]
- Xue, M.; Herzon, S.B. Mechanism of nucleophilic activation of (-)-lomaiviticin A. J. Am. Chem. Soc. 2016, 138, 15559–15562. [Google Scholar] [CrossRef] [PubMed]
- Potts, B.C.; Albitar, M.X.; Anderson, K.C.; Baritaki, S.; Berkers, C.; Bonavida, B.; Chandra, J.; Chauhan, D.; Cusack, J.C., Jr.; Fenical, W.; et al. Marizomib, a proteasome inhibitor for all seasons: Preclinical profile and a framework for clinical trials. Curr. Cancer Drug Targets 2011, 11, 254–284. [Google Scholar] [CrossRef]
- Piskorz, W.M.; Kretowski, R.; Cechowska-Pasko, M. Marizomib (Salinosporamide A) Promotes Apoptosis in A375 and G361 Melanoma Cancer Cells. Mar. Drugs 2024, 22, 315. [Google Scholar] [CrossRef]
- Richardson, P.G.; Todd, M.; Zimmerman, C.C.; Hofmeister, M.; Talpaz, A.A.; Chanan-Khan, J.L.; Kaufman, J.P.; Laubach, D.; Chauhan, A.J.; Jakubowiak, S.; et al. Phase 1 study of marizomib in relapsed or relapsed and refractory multiple myeloma: NPI-0052-101 Part 1. Blood 2016, 127, 2693–2700. [Google Scholar] [CrossRef]
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem. Int. Ed. 2003, 42, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tang, L.; Fang, W.; Lee, K.; Liu, Y. Two Piericidin Glucosides and Application Thereof to Anti-Kidney Cancer Medicines. CN109384710A, 26 February 2019. [Google Scholar]
- Zheng, L.; Xu, Y.; Lin, X.; Ning, R.N.; Zhen, W.; Zhang, T. Marine Brevibacillus Brevis Anti-Tumor Active Polypeptide and Medicine and Application Thereof. CN113150071, 23 July 2021. [Google Scholar]
- McBrien, K.D.; Berry, R.L.; Lowe, S.E.; Neddermann, K.M.; Bursuker, I.; Huang, S.; Klohr, S.E.; Leet, J.E. Rakicidins, new cytotoxic lipopeptides from Micromonospora sp. fermentation, isolation and characterization. J. Antibiot. 1995, 48, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Shimasaki, R.; Miyanaga, S.; Oku, N.; Onaka, H.; Sakurai, H.; Saiki, I.; Kitani, S.; Nihira, T.; Wimonsiravude, W.; et al. Rakicidin D, an inhibitor of tumor cell invasion from marine-derived Streptomyces sp. J. Antibiot. 2010, 63, 563–565. [Google Scholar] [CrossRef]
- Oku, N.; Matoba, S.; Yamazaki, Y.M.; Shimasaki, R.; Miyanaga, S.; Igarashi, Y. Complete stereochemistry and preliminary structure-activity relationship of rakicidin A, a hypoxia-selective cytotoxin from Micromonospora sp. J. Nat. Prod. 2014, 77, 2561–2565, Erratum in: J. Nat. Prod. 2015, 78, 969. [Google Scholar] [CrossRef] [PubMed]
- Kitani, S.; Ueguchi, T.; Igarashi, Y.; Leetanasaksakul, K.; Thamchaipenet, A.; Nihira, T. Rakicidin F, a new antibacterial cyclic depsipeptide from a marine sponge-derived Streptomyces sp. J. Antibiot. 2018, 71, 139–141. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, W.; Jiang, H.; Kim, C.; Fung, R.L.; Hong, J.; Gang, L. Natural Rakicidins Compound Rakicidin I and Extraction Method Thereof. CN108329280, 31 August 2021. [Google Scholar]
- Chen, L.; Zhao, W.; Jiang, H.; Kim, C.; Fung, R.L.; Hong, J.; Gang, L. Natural Rakicidins Compound Namely Rakicidin H and Extraction Method Thereof. CN108586380, 31 August 2021. [Google Scholar]
- Chen, L.; Lin, F.; Jiang, H.; Zhao, V.; Kim, C.; Honglei, J.; Gang, L. Natural Rakicidins Compound Rakicidin B1-2 and Fermentation and Extraction Method Thereof. CN110698537, 31 August 2021. [Google Scholar]
- Romero, F.; Espliego, F.; Pérez Baz, J.; García de Quesada, T.; Grávalos, D.; De La Calle, F. Thiocoraline, a new depsipeptide with antitumor activity produced by a marine Micromonospora I. Taxonomy, fermentation, isolation, and biological activities. J. Antibiot. 1997, 50, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Wyche, T.P.; Hou, Y.; Braun, D.; Cohen, H.C.; Xiong, M.P.; Bugni, T.S. First natural analogs of the cytotoxic thiodepsipeptide thiocoraline A from a marine Verrucosispora sp. J. Org. Chem. 2011, 76, 6542–6547. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; AlNadhari, S.; Al-Homaidan, A.A. Marine microorganisms as an untapped source of bioactive compounds. Saudi J. Biol. Sci. 2021, 28, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.; Malkinson, J.P.; Paumier, D.; Searcey, M. Bisintercalator natural products with potential therapeutic applications: Isolation, structure determination, synthetic and biological studies. Nat. Prod. Rep. 2007, 24, 109–126. [Google Scholar] [CrossRef]
- Harunari, E.; Komaki, H.; Igarashi, Y. Biosynthetic origin of anthracimycin: A tricyclic macrolide from Streptomyces sp. J. Antibiot. 2016, 69, 403–405. [Google Scholar] [CrossRef]
- Corral, P.; Amoozegar, M.A.; Ventosa, A. Halophiles and Their Biomolecules: Recent Advances and Future Applications in Biomedicine. Mar. Drugs 2019, 18, 33. [Google Scholar] [CrossRef]
- Santos, J.D.; Vitorino, I.; Reyes, F.; Vicente, F.; Lage, O.M. From ocean to medicine: Pharmaceutical applications of metabolites from marine bacteria. Antibiotics 2020, 9, 455. [Google Scholar] [CrossRef]
- Barzkar, N.; Sukhikh, S.; Babich, O. Study of marine microorganism metabolites: New resources for bioactive natural products. Front. Microbiol. 2024, 14, 1285902. [Google Scholar] [CrossRef]
- Jianhua, J.; Liu, M.; Sun, Z.; Li, Q.; Ma, J. Didemethylated Actinomycin Derivative and Application Thereof in Preparation of Drugs for Resisting Drug-Resistant Bacteria Infection. CN110669103, 10 January 2020. [Google Scholar]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Finore, I.; Nicolaus, B.; Di Marco, G.; Michaud, L.; Giudice, A.L. Production and Biotechnological Potential of Extracellular Polymeric Substances from Sponge-Associated Antarctic Bacteria. Appl. Environ. Microbiol. 2017, 84, e01624–e01700. [Google Scholar] [CrossRef]
- Kalinovskaya, N.I.; Romanenko, L.A.; Kalinovsky, A.I.; Ermakova, S.P.; Dmitrenok, P.S.; Afiyatullov, S.S. The antitumor antibiotics complex of aureolic acids from the marine sediment-associated strain of Streptomyces sp. KMM 9048. Nat. Prod. Commun. 2017, 12, 1934578X1701200427. [Google Scholar] [CrossRef]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Nicolaus, B.; Lo Giudice, A. Isolation, characterization and optimization of EPSs produced by a cold-adapted Marinobacter isolate from Antarctic seawater. Antarct. Sci. 2019, 31, 69–79. [Google Scholar] [CrossRef]
- Gui, C.; Zhang, S.; Zhu, X.; Ding, W.; Huang, H.; Gu, Y.C.; Ju, J. Antimicrobial spirotetronate metabolites from marine-derived Micromonospora harpali SCSIO GJ089. J. Nat. Prod. 2017, 80, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Nicolaus, B.; Lo Giudice, A. Extracellular polymeric substances with metal adsorption capacity produced by Pseudoalteromonas sp. MER144 from Antarctic seawater. Environ. Sci. Pollut. Res. 2018, 25, 4667–4677. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Poli, A.; Finore, I.; Rizzo, C. Peculiarities of extracellular polymeric substances produced by Antarctic bacteria and their possible applications. Appl. Microbiol. Biotechnol. 2020, 104, 2923–2934. [Google Scholar] [CrossRef]
- Jeewon, R.; Aullybux, A.A.; Puchooa, D.; Nazurally, N.; Alrefaei, A.F.; Zhang, Y. Marine Microbial Polysaccharides: An Untapped Resource for Biotechnological Applications. Mar. Drugs 2023, 21, 420. [Google Scholar] [CrossRef] [PubMed]
- Lelchat, F.; Cozien, J.; Le Costaouec, T.; Brandilly, C.; Schmitt, S.; Baudoux, A.; Colliec-Jouault, S.; Boisset, C. Exopolysaccharide biosynthesis and biodegradation by a marine hydrothermal Alteromonas sp. Strain. Appl. Microb. Biotechnol. 2015, 99, 2637–2647. [Google Scholar] [CrossRef]
- Senni, K.; Gueniche, F.; Changotade, S.; Septier, D.; Sinquin, C.; Ratiskol, J.; Lutomski, D.; Godeau, G.; Guezennec, J.; Colliec-Jouault, S. Unusual glycosaminoglycans from a deep sea hydrothermal bacterium improve fibrillar collagen structuring and fibroblast activities in engineered connective tissues. Mar. Drugs. 2013, 11, 1351–1369. [Google Scholar] [CrossRef]
- Floris, R.; Rizzo, C.; Lo Giudice, A.L. Biosurfactants from marine microorganisms. In Metabolomics—New Insights into Biology and Medicine; InTech: London, UK, 2018; Volume 1, pp. 1–16. [Google Scholar]
- Franzetti, A.; Tamburini, E.; Banat, I.M. Applications of biological surface-active compounds in remediation technologies. Biosurfactants 2010, 1, 121–134. [Google Scholar]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef] [PubMed]
- Eras-Muñoz, E.; Farré, A.; Sánchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef] [PubMed]
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 11, 558–565. [Google Scholar] [CrossRef]
- Silva, N.M.P.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Screening of Pseudomonas species for biosurfactant production using low-cost substrates. Biocatal. Agric. Biotechnol. 2013, 3, 132–139. [Google Scholar] [CrossRef]
- Voulgaridou, G.P.; Mantso, T.; Anestopoulos, I.; Klavaris, A.; Katzastra, C.; Kiousi, D.E.; Pappa, A. Toxicity profiling of biosurfactants produced by novel marine bacterial strains. Int. J. Mol. Sci. 2021, 22, 2383. [Google Scholar] [CrossRef]
- Cheng, T.H.; Ismail, N.; Kamaruding, N.; Saidin, J.; Danish-Daniel, M.J.B.R. Industrial enzymes-producing marine bacteria from marine resources. Biotechnol. Rep. 2020, 27, e00482. [Google Scholar] [CrossRef]
- Dumorné, K.; Córdova, D.C.; Astorga-Eló, M.; Renganathan, P. Extremozymes: A Potential Source for Industrial Applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.; Alhuzani, M.R.; Kelany, M.S.; Hamed, M.M. Production and Partial Characterization of α-Amylase Enzyme from Marine Actinomycetes. Biomed. Res. Int. 2021, 2021, 5289848. [Google Scholar] [CrossRef]
- Raddadi, N.; Cherif, A.; Daffonchio, D.; Neifar, M.; Fava, F. Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl. Microbiol. Biotechnol. 2015, 99, 7907–7913. [Google Scholar] [CrossRef]
- Ghattavi, S.; Ahmad, H. Marine enzymes: Classification and application in various industries. Int. J. Biol. Macromol. 2023, 230, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Thebti, W.; Riahi, Y.; Gharsalli, R.; Belhadj, O. Screening and characterization of thermo-active enzymes of biotechnological interest produced by thermophilic Bacillus isolated from hot springs in Tunisia. Acta Biochim. Pol. 2016, 63, 581–587. [Google Scholar] [CrossRef]
- Choudhary, K.; Mankar, M.K.; Sahay, S. Extremophilic enzymes: Catalytic features and industrial applications. In Extremophilic Fungi: Ecology, Physiology and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 273–314. [Google Scholar]
- Mirete, S.; Morgante, V.; González-Pastor, J.E. Acidophiles: Diversity and mechanisms of adaptation to acidic environments. In Adaption of Microbial Life to Environmental Extremes: Novel Research Results and Application; Springer: Cham, Switzerland, 2017; pp. 227–251. [Google Scholar]
- de Lourdes Moreno, M.; Pérez, D.; García, M.T.; Mellado, E. Halophilic bacteria as a source of novel hydrolytic enzymes. Life 2013, 3, 38–51. [Google Scholar] [CrossRef]
- Daoud, L.; Ali, M.B. Halophilic microorganisms: Interesting group of extremophiles with important applications in biotechnology and environment. In Physiological and Biotechnological Aspects of Extremophiles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–64. [Google Scholar]
- Sharma, A.; Kawarabayasi, Y.; Satyanarayana, T. Acidophilic bacteria and archaea: Acid stable biocatalysts and their potential applications. Extremophiles 2012, 16, 1–19. [Google Scholar] [CrossRef]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef]
- Shields, R.C.; Mokhtar, N.; Ford, M.; Hall, M.J.; Burgess, J.G.; El Badawey, M.R.; Jakubovics, N.S. Efficacy of a marine bacterial nuclease against biofilm forming microorganisms isolated from chronic rhinosinusitis. PLoS ONE 2013, 8, e55339. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Breslawec, A.P.; Liang, T.; Deng, Z.; Kuperman, L.L.; Yu, Q. Strategy to combat biofilms: A focus on biofilm dispersal enzymes. npj Biofilms Microbiomes 2023, 9, 63. [Google Scholar] [CrossRef]
- Baslé, A.; Hewitt, L.; Koh, A.; Lamb, H.K.; Thompson, P.; Burgess, J.G.; Lewis, R.J. Crystal structure of NucB, a biofilm-degrading endonuclease. Nucleic Acids Res. 2018, 46, 473–484. [Google Scholar] [CrossRef]
- Canellas, A.L.B.; Dias, G.R.; Lopes, I.R.; Freitas-Silva, J.; Dobson, A.D.; Laport, M.S.; de Oliveira, B.F.R. Marine microbial enzymes as potential antibiofilm agents: Expanding the arsenal of bioactive agents targeting biofilm-forming microorganisms. Crit. Rev. Microbiol. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Cabra, N.; Paetzold, B.; Ferrar, T.; Mazzolini, R.; Torrents, E.; Serrano, L.; LLuch-Senar, M. Characterization of different alginate lyases for dissolving Pseudomonas aeruginosa biofilms. Sci. Rep. 2020, 10, 9390. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Huang, J.; Zhang, M.; Wang, X.; Wang, X.; Zhou, Y. Preliminary identification and semi-quantitative characterization of a multi-faceted high-stability alginate lyase from marine microbe Seonamhaeicola algicola with anti-biofilm effect on Pseudomonas aeruginosa. Enzym. Microb. Technol. 2024, 175, 110408. [Google Scholar] [CrossRef] [PubMed]
- Anjung, M.U.K.; Nursyam, H.; Prihanto, A.A.; Chasanah, E. Application of marine bacterial alginate lyases in wastewater treatment, biofilm removal and green technology. Glob. J. Environ. Sci. Manag. 2025, 11, 1343–1360. [Google Scholar]
- Inoue, A.; Nishiyama, R.; Ojima, T. The alginate lyases FlAlyA, FlAlyB, FlAlyC, and FlAlex from Flavobacterium sp. UMI-01 have distinct roles in the complete degradation of alginate. Algal Res. 2016, 19, 355–362. [Google Scholar] [CrossRef]
- Bowman, J.P. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Mar. Drugs 2007, 5, 220–241. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. S Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Rico-Virgen, E.G.; Ortiz-Aguirre, I.; Hellio, C.; Rangel-Dávalos, C.; Escobedo-Fregoso, A.; López-Fuerte, F.O.; Aguila-Ramírez, R.N. Antifouling activity of marine bacterial extracts: A non-toxic alternative as biocide. Rev. Biol. Mar. Oceanogr. 2024, 59, 39–50. [Google Scholar] [CrossRef]
- Tunkal, R.I.; El-Sheekh, M.M.; Soliman, A.M.; Soliman, M.S.; Abu El-Kheir, W.A.; Abdel-Raouf, N. Antifouling activity of bacterial extracts associated with soft coral and macroalgae from the Red Sea. Oceanol. Hydrobiol. Stud. 2023, 51, 325–336. [Google Scholar] [CrossRef]
- Singh, R.; Dubey, A.K. Isolation and characterization of a new endophytic actinobacterium Streptomyces californicus strain ADR1 as a promising source of anti-bacterial, anti-biofilm and antioxidant metabolites. Microorganisms 2020, 8, 929. [Google Scholar] [CrossRef]
- Alemán-Vega, M.; Sánchez-Lozano, I.; Hernández-Guerrero, C.J.; Hellio, C.; Quintana, E.T. Exploring antifouling activity of biosurfactants producing marine bacteria isolated from Gulf of California. Int. J. Mol. Sci. 2020, 21, 6068. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Cahill, P.L.; Moodie, L.W.; Hertzer, C.; Pinori, E.; Pavia, H.; Hellio, C.; Svenson, J. Creating new antifoulants using the tools and tactics of medicinal chemistry. Acc. Chem. Res. 2024, 57, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Sulaiman, J.E.; Xiao, Y.; Cheng, A.; Wang, R.; Malit, J.J.; Qian, P.Y. Mode of action of elasnin as biofilm formation eradicator of methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2022, 13, 967845. [Google Scholar] [CrossRef]
- Long, L.; Wang, R.; Chiang, H.Y.; Ding, W.; Li, Y.-X.; Chen, F.; Qian, P.-Y. Discovery of Antibiofilm Activity of Elasnin against Marine Biofilms and Its Application in the Marine Antifouling Coatings. Mar. Drugs 2021, 19, 19. [Google Scholar] [CrossRef]
- Setiyono, E.; Adhiwibawa, M.A.S.; Prabowo, M.R.; Brotosudarmo, T.H. Characterization of Tambjamines Pigment from Marine Bacterium Pseudoalteromonas sp. PM2 Indigenous from Alor Island, Indonesia. Indones. J. Nat. Pigment. 2021, 3, 16–23. [Google Scholar] [CrossRef]
- Sakai-Kawada, F.E.; Ip, C.G.; Hagiwara, K.A.; Awaya, J.D. Biosynthesis and bioactivity of prodiginine analogs in marine bacteria, Pseudoalteromonas: A mini review. Front. Microbiol. 2019, 10, 1715. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Kim, M.; Lee, C.; Yang, I.; Nam, S.J. Bioactive natural products from the genus Salinospora: A review. Arch. Pharm. Res. 2020, 43, 1230–1258. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Y.; Jiang, D. β-Lactone derivatives and their anticancer activities: A short review. Curr. Top. Med. Chem. 2021, 21, 1645–1656. [Google Scholar] [CrossRef]
- Qamar, H.; Hussain, K.; Soni, A.; Khan, A.; Hussain, T.; Chénais, B. Cyanobacteria as natural therapeutics and pharmaceutical potential: Role in antitumor activity and as nanovectors. Molecules 2021, 26, 247. [Google Scholar] [CrossRef]
- Öner, Ö.; Ekiz, G.; Hameş, E.; Demir, V.; Gübe, Ö.; Özkaya, F.; Yokeş, M.; Uzel, A.; Bedir, E. Cultivable sponge-associated actinobacteria from coastal area of Eastern Mediterranean Sea. Adv. Microbiol. 2014, 4, 306–316. [Google Scholar] [CrossRef]
- Asolkar, R.N.; Schroeder, D.; Heckmann, R.; Lang, S.; Wagner-Doebler, I.; Laatsch, H. Helquinoline, a new tetrahydroquinoline antibiotic from Janibacter limosus Hel 1. J. Antibiot. 2004, 57, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Flores, M.; Forteza, I.; Henriksen, N.M.; Concepcion, G.P.; Rosenberg, G.; Schmidt, E.W. Totopotensamides, polyketide–cyclic peptide hybrids from a mollusk-associated bacterium Streptomyces sp. J. Nat. Prod. 2012, 75, 644–649. [Google Scholar] [CrossRef]
- Wu, W.; Hu, C.Q.; Tao, B.H. Studies on marine bacterial metabolites with antibacterial activity. J. Trop. Oceanogr. 2001, 20, 80–87. [Google Scholar]
- Meisel, J.D.; Panda, O.; Mahanti, P.; Schroeder, F.C.; Kim, D.H. Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell 2014, 159, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Ray, S.; Patel, S.K.S.; Singh, M.; Singh, G.P. The dawn of novel biotechnological applications of polyhydroxyalkanoates. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–11. [Google Scholar]
- Mostafa, Y.S.; Alrumman, S.A.; Otaif, K.A.; Alamri, S.A.; Mostafa, M.S.; Sahlabji, T. Production and characterization of bioplastic by polyhydroxybutyrate-accumulating Erythrobacter aquimaris isolated from mangrove rhizosphere. Molecules 2020, 25, 179. [Google Scholar] [CrossRef] [PubMed]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Garti, N.; Leser, M.E. Natural hydrocolloids as food emulsifiers. In Design and Selection of Performance Surfactants; CRC Press: Boca Raton, FL, USA, 1999; Volume 2, pp. 104–145. [Google Scholar]
- Johansson, I.; Kjellin, M. (Eds.) Surfactants from Renewable Resources; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Imhoff, J.F.; Labes, A.; Wiese, J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol. Adv. 2011, 29, 468–482. [Google Scholar] [CrossRef]
- Gao, Q.; Garcia-Pichel, F. Microbial ultraviolet sunscreens. Nat. Rev. Microbiol. 2011, 9, 791–802. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Suen, Y.L.; Tang, H.; Huang, J.; Chen, F. Enhanced production of fatty acids and astaxanthin in Aurantiochytrium sp. by the expression of Vitreoscilla hemoglobin. J. Agric. Food Chem. 2014, 62, 12392–12398. [Google Scholar] [CrossRef]
- Courtois, A.; Berthou, C.; Guézennec, J.; Boisset, C.; Bordron, A. Exopolysaccharides isolated from hydrothermal vent bacteria can modulate the complement system. PLoS ONE 2014, 9, e94965. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef]
- Aliko, V.; Multisanti, C.R.; Turani, B.; Faggio, C. Get rid of marine pollution: Bioremediation an innovative, attractive, and successful cleaning strategy. Sustainability 2022, 14, 11784. [Google Scholar] [CrossRef]
- Rizzo, C.; Michaud, L.; Graziano, M.; De Domenico, E.; Syldatk, C.; Hausmann, R.; Lo Giudice, A. Biosurfactant activity, heavy metal tolerance and characterization of Joostella strain A8 from the Mediterranean polychaete Megalomma claparedei (Gravier, 1906). Ecotoxicology 2015, 24, 1294–1304. [Google Scholar] [CrossRef]
- Rizzo, C.; Michaud, L.; Hörmann, B.; Gerçe, B.; Syldatk, C.; Hausmann, R.; Lo Giudice, A. Bacteria associated with sabellids (Polychaeta: Annelida) as a novel source of surface active compounds. Mar. Pollut. Bull. 2013, 70, 125–133. [Google Scholar] [CrossRef]
- Rizzo, C.; Papale, M.; Lo Giudice, A. Idiomarina sp. isolates from cold and temperate environments as biosurfactant producers. J. Mar. Sci. Eng. 2022, 10, 1135. [Google Scholar] [CrossRef]
- Zykwinska, A.; Marchand, L.; Bonnetot, S.; Sinquin, C.; Colliec-Jouault, S.; Delbarre-Ladrat, C. Deep-sea hydrothermal vent bacteria as a source of glycosaminoglycan-mimetic exopolysaccharides. Molecules 2019, 24, 1703. [Google Scholar] [CrossRef]
- Neu, A.K.; Månsson, M.; Gram, L.; Prol-García, M.J. Toxicity of bioactive and probiotic marine bacteria and their secondary metabolites in Artemia sp. and Caenorhabditis elegans as eukaryotic model organisms. Appl. Environ. Microbiol. 2014, 80, 146–153. [Google Scholar] [CrossRef]
- Ayuningrum, D.; Liu, Y.; Riyanti; Sibero, M.T.; Kristiana, R.; Asagabaldan, M.A.; Schaeberle, T.F. Tunicate-associated bacteria show a great potential for the discovery of antimicrobial compounds. PLoS ONE 2019, 14, e0213797. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Choi, J.K.; Kim, E.K. A preliminary study on the mechanism of harmful algal bloom mitigation by use of sophorolipid treatment. J. Exp. Mar. Biol. Ecol. 2004, 304, 35–49. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Chen, X. Screening of marine bioactive antimicrobial compounds for plant pathogens. Mar. Drugs 2021, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Valença, C.A.S.; Hollanda, L.M.; Jain, S.; Caramão, E.B. Chromatographic Profiles of Ethyl Acetate Extracts Produced by Bacillus sp. Collected from the Mangroves in the Brazilian Northeast. J. Braz. Chem. Soc. 2022, 33, 1375–1385. [Google Scholar] [CrossRef]
- Baümler, E.R.; Carrín, M.E.; Carelli, A.A. Extraction of sunflower oil using ethanol as solvent. J. Food Eng. 2016, 178, 190–197. [Google Scholar] [CrossRef]
- Raynie, D.E. Modern extraction techniques. Anal. Chem. 2006, 78, 3997–4004. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Herath, K.H.I.N.M.; Kim, Y.S.; Jeon, Y.J.; Kim, S.K. Enzyme-assisted extraction of bioactive compounds from seaweeds and microalgae. TrAC Trends Anal. Chem. 2023, 167, 117266. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of ultrasound-assisted extraction. In Water Extraction of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar]
- Luthria, D.; Vinjamoori, D.; Noel, K.; Ezzell, J. Accelerated solvent extraction. In Oil Extraction and Analysis; AOCS Publishing: Champaign, IL, USA, 2019; pp. 25–38. [Google Scholar]
- Kumari, V.C.; Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Kumar, N.; Sowmya, B.P.; Patrick-Iwuanyanwu, K.C. Chromatographic techniques: Types, principles, and applications. In Analytical Techniques in Biosciences; Academic Press: Cambridge, MA, USA, 2022; pp. 73–101. [Google Scholar]
- De Hoffmann, E. Mass spectrometry. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-Interscience: Hoboken, NJ, USA, 2000. [Google Scholar]
- Hore, P.J. Nuclear Magnetic Resonance; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Belma, P.; Dina, F.; Emina, A.; Nermina, Ž.; Fahir, B. Animal models in modern biomedical research. Eur. J. Pharm. Med. Res. 2019, 6, 35–38. [Google Scholar]
- Tagkalidou, N.; Multisanti, C.R.; Bleda, M.J.; Bedrossiantz, J.; Prats, E.; Faggio, C.; Raldúa, D. Analyzing the effects of age, time of day, and experiment on the basal locomotor activity and light-off visual motor response assays in zebrafish larvae. Toxics 2024, 12, 349. [Google Scholar] [CrossRef]
- Stevanović, M.; Tagkalidou, N.; Multisanti, C.R.; Pujol, S.; Aljabasini, O.; Prats, E.; Faggio, C.; Raldúa, D. Zebra_K, a kinematic analysis automated platform for assessing sensitivity, habituation and prepulse inhibition of the acoustic startle response in adult zebrafish. Sci. Total Environ. 2025, 958, 178028. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Impellitteri, F.; Riolo, K.; Multisanti, C.R.; Zicarelli, G.; Piccione, G.; Faggio, C.; Giannetto, A. Evaluating quaternium-15 effects on Mytilus galloprovincialis: New insights on physiological and cellular responses. Sci. Total Environ. 2024, 918, 170568. [Google Scholar] [CrossRef] [PubMed]
- Multisanti, C.R.; Riolo, K.; Impellitteri, F.; Chebbi, I.; Faggio, C.; Giannetto, A. Short-term in vitro exposure of Pinctada imbricata’s haemocytes to quaternium-15: Exploring physiological and cellular responses. Environ. Toxicol. Pharmacol. 2023, 101, 104198. [Google Scholar] [CrossRef] [PubMed]
- Multisanti, C.R.; Zicarelli, G.; Caferro, A.; Filice, M.; Faggio, C.; Vazzana, I.; Impellitteri, F. From personal care to coastal concerns: Investigating polyethylene glycol impact on mussel’s antioxidant, physiological, and cellular responses. Antioxidants 2024, 13, 734. [Google Scholar] [CrossRef]
- Multisanti, C.R.; Impellitteri, F.; Cannatà, G.; Cotugno, A.; Perugini, M.; Piccione, G.; Faggio, C.; Rizzo, M.G. Discovering the effects of octylisothiazolinone: Analysis of physiological changes in the Mediterranean mussel (Mytilus galloprovincialis). Ecotoxicol. Environ. Saf. 2025, 302, 118563. [Google Scholar] [CrossRef]
- Multisanti, C.R.; Impellitteri, F.; Zicarelli, G.; Perugini, M.; Iovine, M.A.; Filice, M.C.; Faggio, C. Toxicological assessment of 2-methylisothiazol-3 (2H)-one on physiological and antioxidant parameters in Mytilus galloprovincialis. Environ. Pollut. 2025, 384, 126982. [Google Scholar] [CrossRef] [PubMed]
- Avelar-Freitas, B.A.; Almeida, V.G.; Pinto, M.C.X.; Mourão, F.A.G.; Massensini, A.R.; Martins-Filho, O.A.; Brito-Melo, G.E.A. Trypan blue exclusion assay by flow cytometry. Braz. J. Med. Biol. Res. 2014, 47, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Murgarella, M.; Puiu, D.; Novoa, B.; Figueras, A.; Posada, D.; Canchaya, C. A first insight into the genome of the filter-feeder mussel Mytilus galloprovincialis. PLoS ONE 2016, 11, e0151561. [Google Scholar]
- Liu, L.; Xiang, J.; Dong, B.; Natarajan, P.; Yu, K.; Cai, N. Ciona intestinalis as an emerging model organism: Its regeneration under controlled conditions and methodology for egg dechorionation. J. Zhejiang Univ. Sci. B 2006, 7, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Mizotani, Y.; Itoh, S.; Hotta, K.; Tashiro, E.; Oka, K.; Imoto, M. Evaluation of drug toxicity profiles based on the phenotypes of ascidian Ciona intestinalis. Biochem. Biophys. Res. Commun. 2015, 463, 656–660. [Google Scholar] [CrossRef]
- Khan, F.R.; Alhewairini, S. Zebrafish (Danio rerio) as a model organism. Curr. Trends Cancer Manag. 2018, 27, 3–18. [Google Scholar]
- Keswani, C.; Singh, H.B.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Sansinenea, E. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Appl. Microbiol. Biotechnol. 2020, 104, 1013–1034. [Google Scholar] [CrossRef]
- Bhattarai, H.D.; Ganti, V.S.; Paudel, B.; Lee, Y.K.; Lee, H.K.; Hong, Y.K.; Shin, H.W. Isolation of antifouling compounds from the marine bacterium, Shewanella oneidensis SCH0402. World J. Microbiol. Biotechnol. 2007, 23, 243–249. [Google Scholar] [CrossRef]
- Xin, X.; Huang, G.; Zhou, X.; Sun, W.; Jin, C.; Jiang, W.; Zhao, S. Potential antifouling compounds with antidiatom adhesion activities from the sponge-associated bacteria, Bacillus pumilus. J. Adhes. Sci. Technol. 2017, 31, 1028–1043. [Google Scholar] [CrossRef]
- Rather, I.A.; Galope, R.; Bajpai, V.K.; Lim, J.; Paek, W.K.; Park, Y.H. Diversity of marine bacteria and their bacteriocins: Applications in aquaculture. Rev. Fish. Sci. Aquacult. 2017, 25, 257–269. [Google Scholar] [CrossRef]
- Wang, J.; Curson, A.R.; Zhou, S.; Carrión, O.; Liu, J.; Vieira, A.R.; Todd, J.D. Alternative dimethylsulfoniopropionate biosynthesis enzymes in diverse and abundant microorganisms. Nat. Microbiol. 2024, 9, 1979–1992. [Google Scholar] [CrossRef]
- Matsuo, Y.; Imagawa, H.; Nishizawa, M.; Shizuri, Y. Isolation of an algal morphogenesis inducer from a marine bacterium. Science 2005, 307, 1598. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.K.; Haygood, M.G. Identification of sibling species of the bryozoan Bugula neritina that produce different anticancer bryostatins and harbor distinct strains of the bacterial symbiont “Candidatus Endobugula sertula”. Biol. Bull. 1999, 196, 273–280. [Google Scholar] [CrossRef]
- Reen, F.J.; Gutiérrez-Barranquero, J.A.; Dobson, A.D.; Adams, C.; O'Gara, F. Emerging concepts promising new horizons for marine biodiscovery and synthetic biology. Mar. Drugs. 2015, 13, 2924–2954. [Google Scholar] [CrossRef]
- Rocha-Martin, J.; Harrington, C.; Dobson, A.D.; O’Gara, F. Emerging strategies and integrated systems microbiology technologies for biodiscovery of marine bioactive compounds. Mar. Drugs. 2014, 12, 3516–3559. [Google Scholar] [CrossRef]
- Paddon, C.J.; Keasling, J.D. Semi-synthetic artemisinin: A model for the use of synthetic biology in pharmaceutical development. Nat. Rev. Microbiol. 2014, 12, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.A.; Feng, Z.; Brady, S.F. Biocatalysts and small molecule products from metagenomic studies. Curr. Opin. Chem. Biol. 2012, 16, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kumar, D. AI-Driven Bioprospecting of Microbial Resources. In Genomic Intelligence; CRC Press: Boca Raton, FL, USA, 2024; pp. 257–275. [Google Scholar]
- Blin, K.; Shaw, S.; Kautsar, S.A.; Medema, M.H.; Weber, T. The antiSMASH database version 3: Increased taxonomic coverage and new query features for modular enzymes. Nucleic Acids Res. 2020, 49, D639–D643. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Prihoda, D.; Palicka, A.; Soukup, J.; Klempir, O.; Rampula, L.; Bitton, D.A. A deep learning genome-mining strategy for biosynthetic gene cluster prediction. Nucleic Acids Res. 2021, 47, e110. [Google Scholar] [CrossRef]
- Basnet, B.B.; Zhou, Z.Y.; Wei, B.; Wang, H. Advances in AI-based strategies and tools to facilitate natural product and drug development. Crit. Rev. Biotechnol. 2025, 45, 1527–1558. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.; Liu, G.; Catacutan, D.B.; Arnold, A.; Zou, J.; Stokes, J.M. Generative AI for designing and validating easily synthesizable and structurally novel antibiotics. Nat. Mach. Intell. 2024, 6, 338–353. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, S.; Sun, H.; Li, Y.; Yao, Z. Exploration of Novel Antimicrobial Agents against Foodborne Pathogens via a Deep Learning Approach. J. Agric. Food Chem. 2025, 73, 7456–7469. [Google Scholar] [CrossRef] [PubMed]
- Olayo-Alarcon, R.; Amstalden, M.K.; Zannoni, A.; Bajramovic, M.; Sharma, C.M.; Brochado, A.R.; Müller, C.L. Pre-trained molecular representations enable antimicrobial discovery. Nat. Commun. 2025, 16, 3420. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Multisanti, C.R.; Celi, V.; Dibra, A.; Pintus, A.; Calogero, R.; Rizzo, C.; Faggio, C. Microbial Blue Bioprospecting: Exploring the Advances of Compounds Post-Discovery. Mar. Drugs 2025, 23, 406. https://doi.org/10.3390/md23100406
Multisanti CR, Celi V, Dibra A, Pintus A, Calogero R, Rizzo C, Faggio C. Microbial Blue Bioprospecting: Exploring the Advances of Compounds Post-Discovery. Marine Drugs. 2025; 23(10):406. https://doi.org/10.3390/md23100406
Chicago/Turabian StyleMultisanti, Cristiana Roberta, Valeria Celi, Aurora Dibra, Angela Pintus, Rosario Calogero, Carmen Rizzo, and Caterina Faggio. 2025. "Microbial Blue Bioprospecting: Exploring the Advances of Compounds Post-Discovery" Marine Drugs 23, no. 10: 406. https://doi.org/10.3390/md23100406
APA StyleMultisanti, C. R., Celi, V., Dibra, A., Pintus, A., Calogero, R., Rizzo, C., & Faggio, C. (2025). Microbial Blue Bioprospecting: Exploring the Advances of Compounds Post-Discovery. Marine Drugs, 23(10), 406. https://doi.org/10.3390/md23100406