Novel Discorhabdin Derivatives from Antarctic Sponges of the Genus Latrunculia: Expanding the Chemical Diversity of Polar Marine Natural Products
Abstract
1. Introduction
2. Results and Discussion
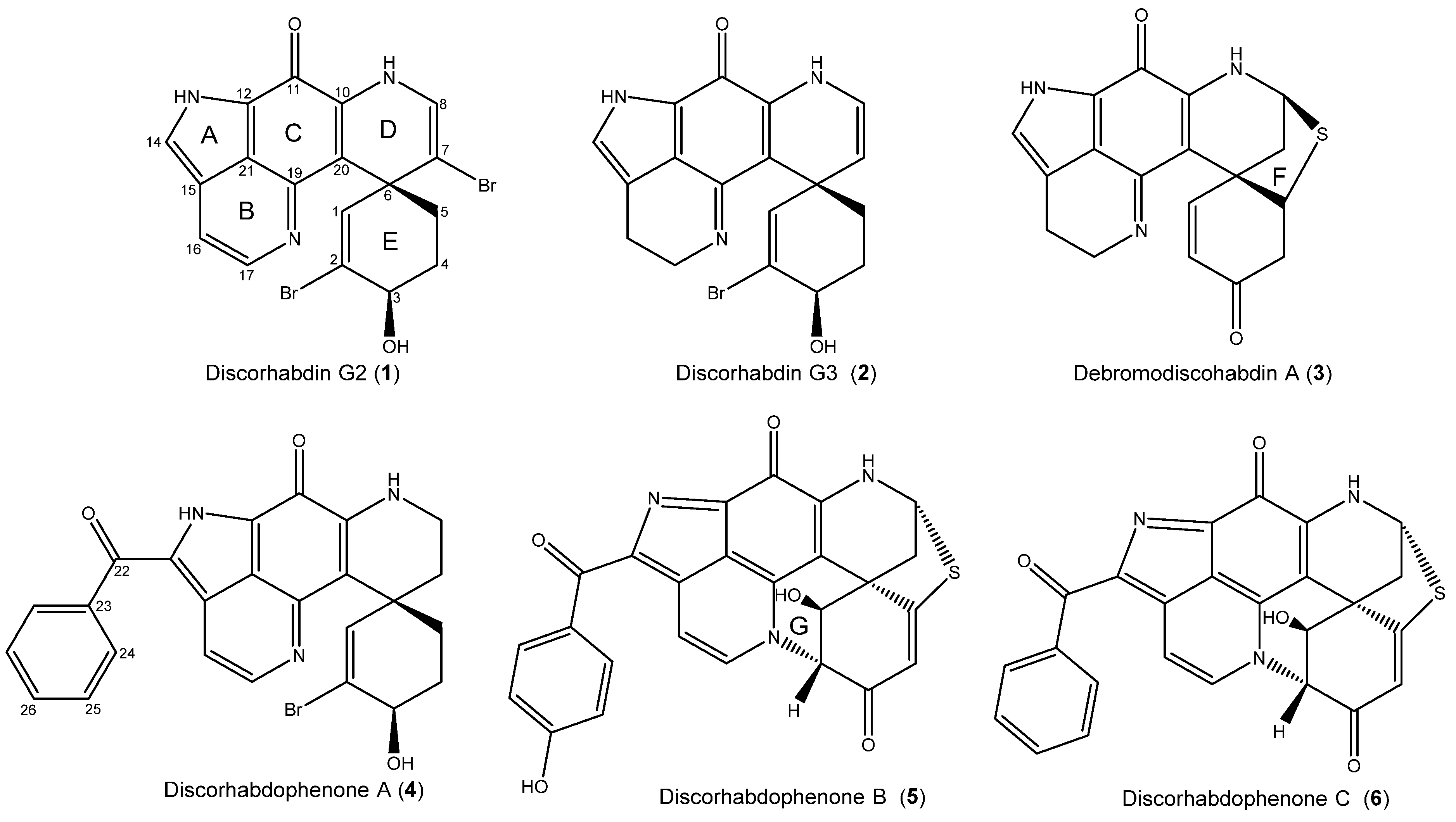
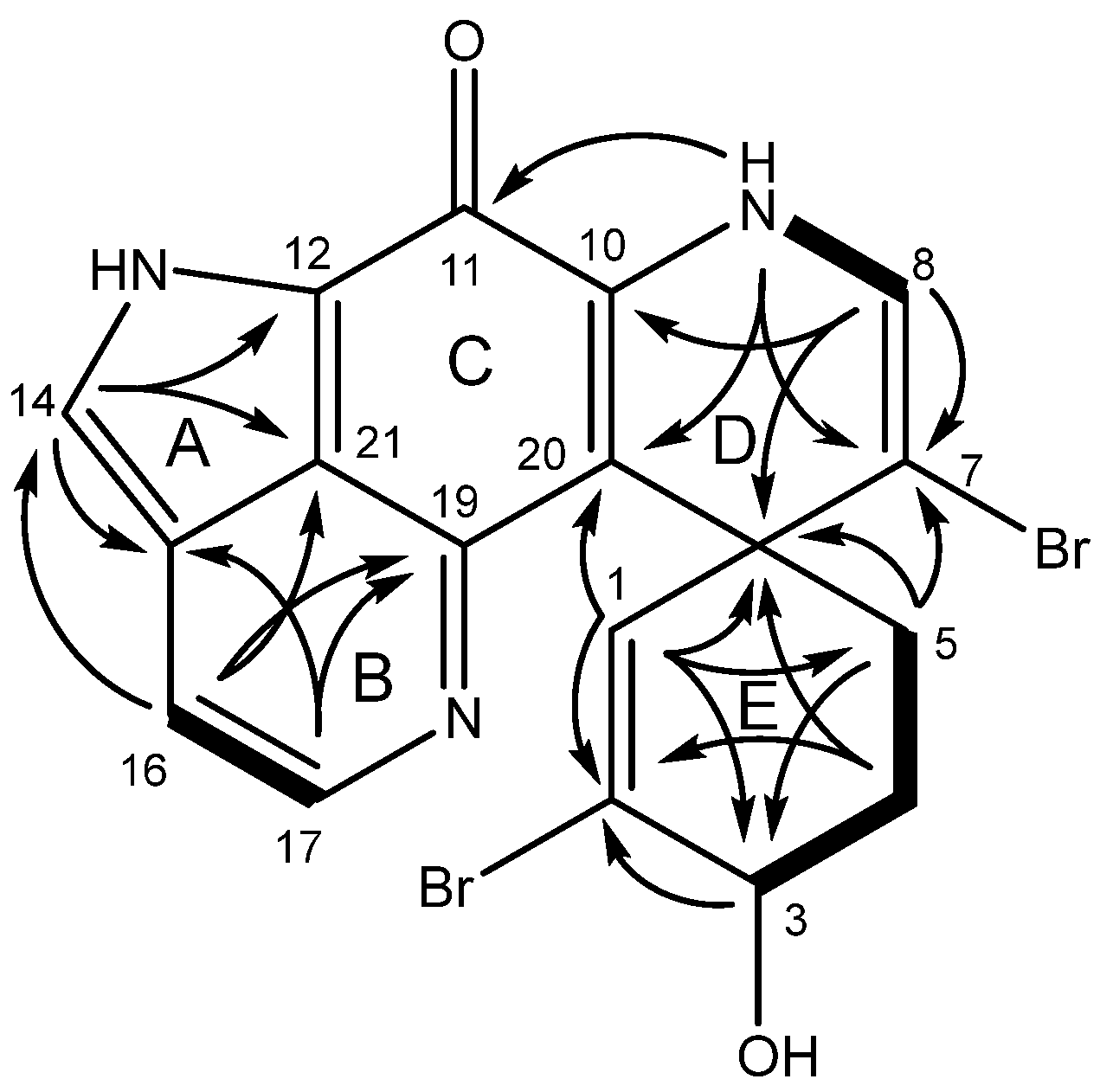
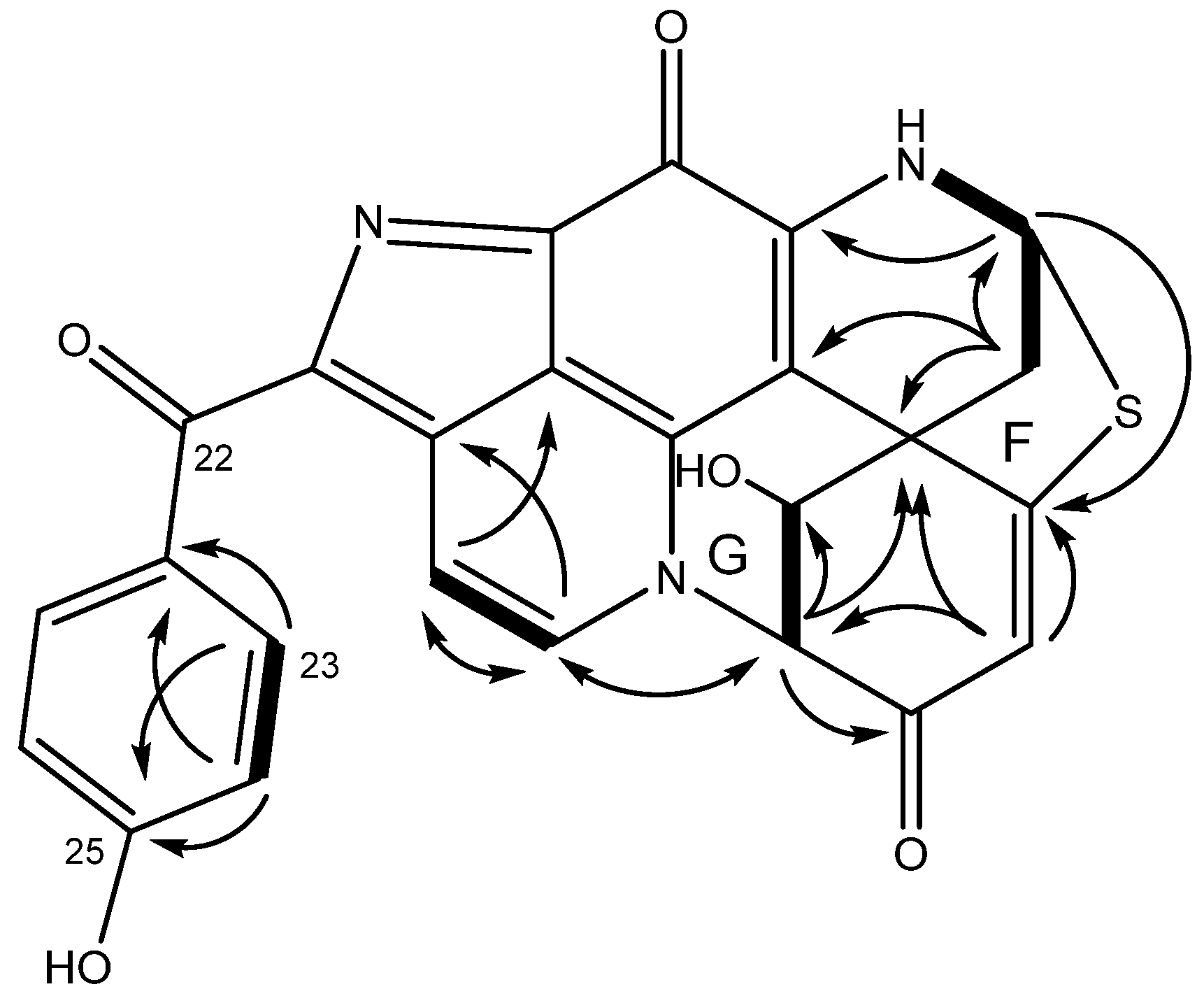
2.1. Structure Elucidation
2.2. Stereochemical Analysis
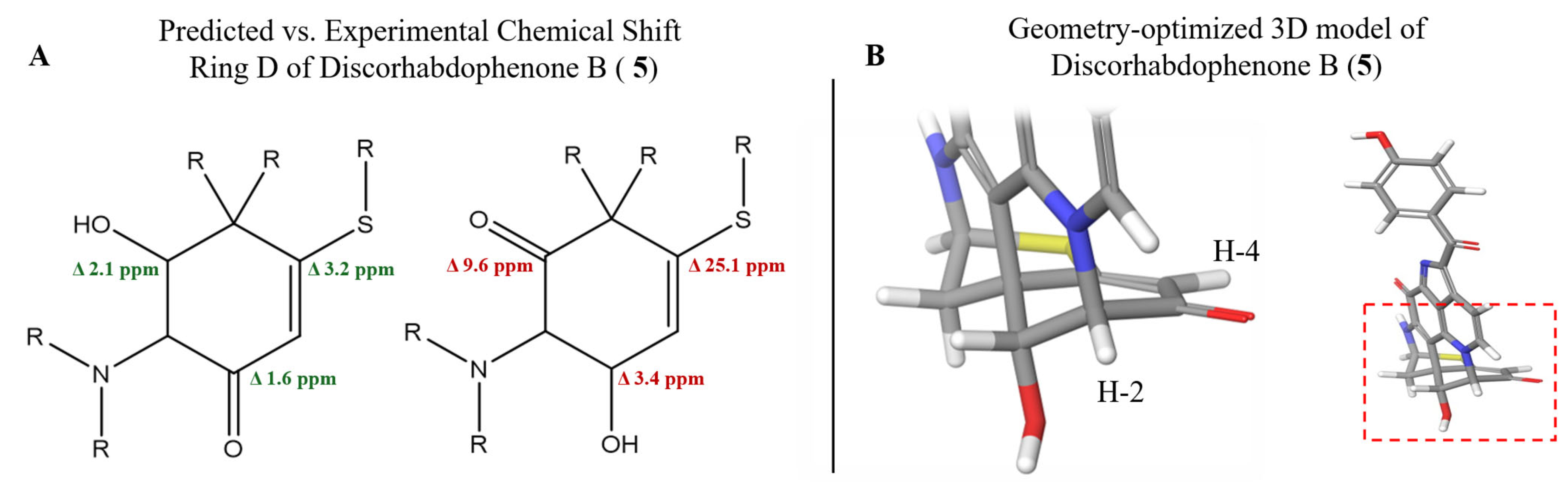
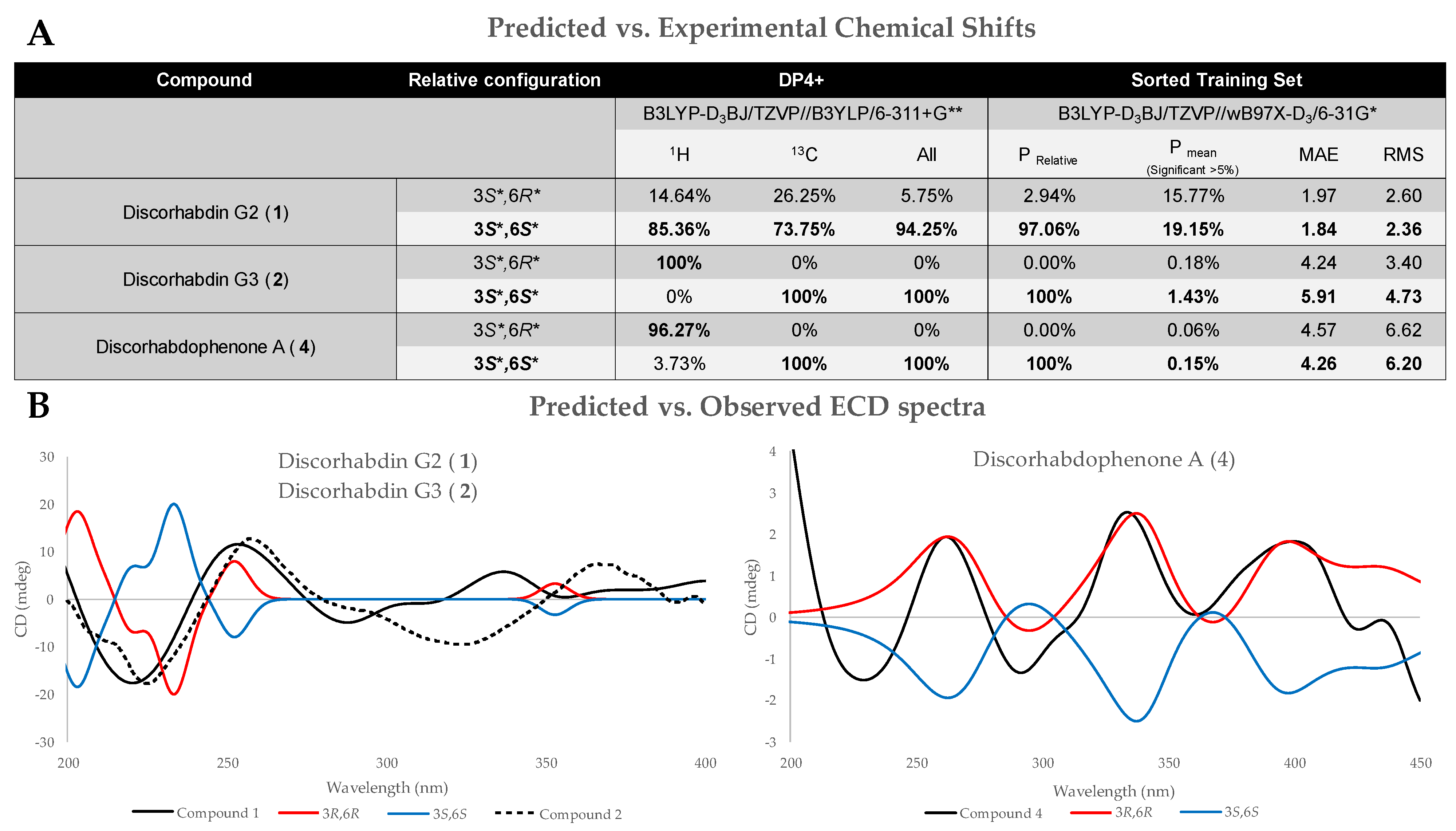
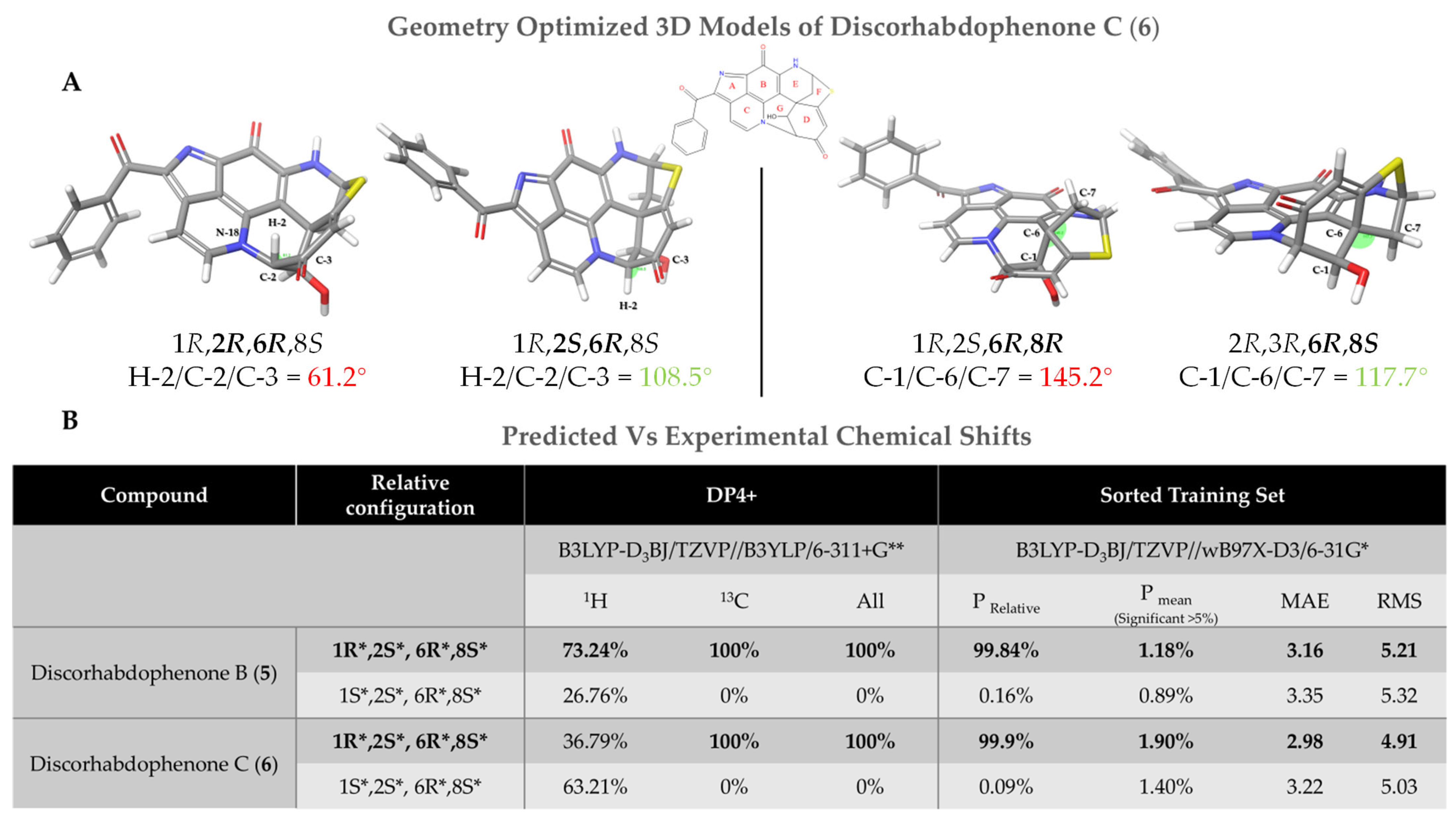
2.2.1. Relative Stereochemistry
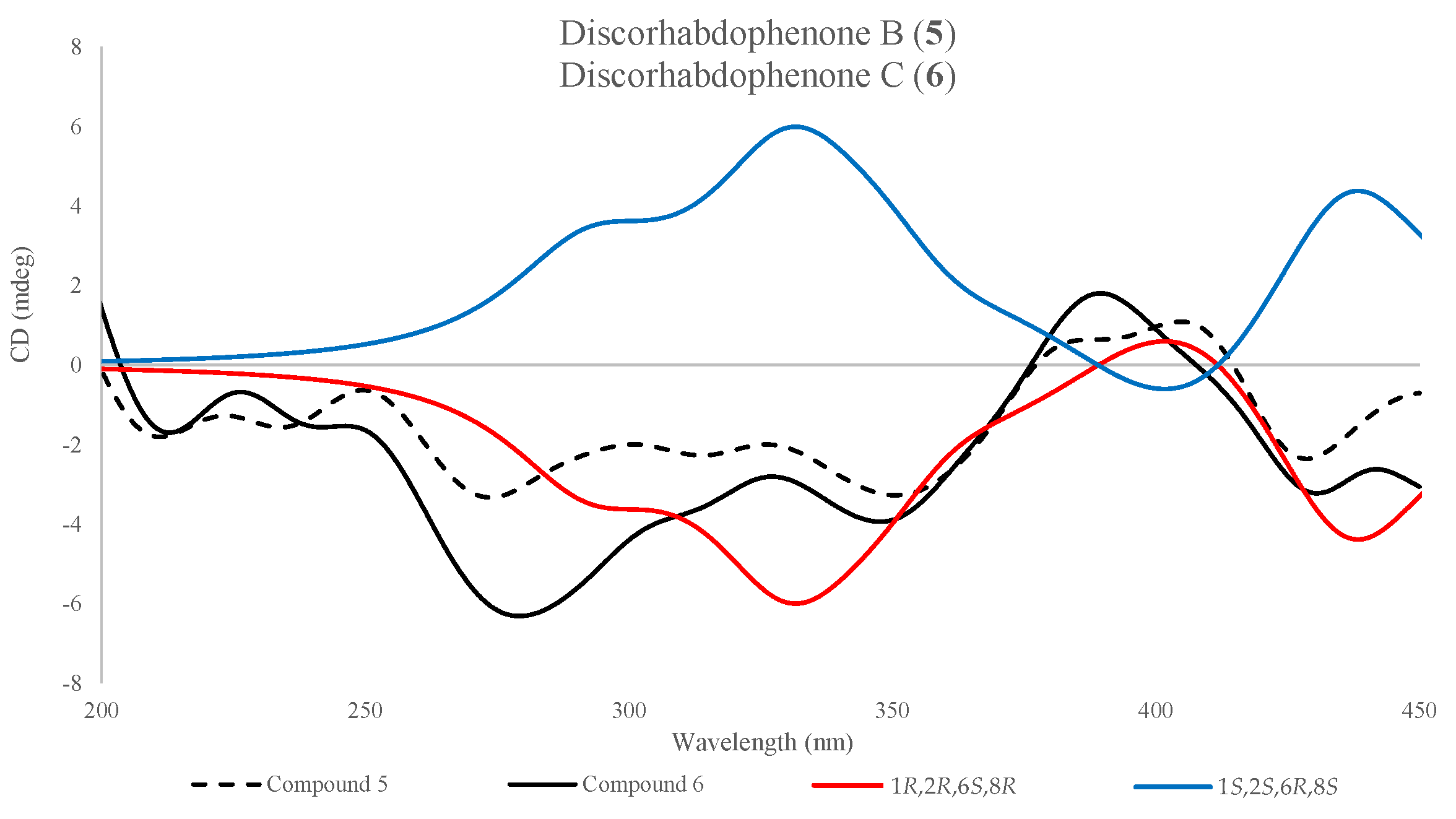
2.2.2. Absolute Stereochemistry
2.3. Bioactivity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Materials
3.3. Extraction, Isolation, and Purification
3.4. Spectroscopic Data (1, 2, and 4–6)
3.5. Computational Methods
3.5.1. Conformer Generation
3.5.2. Chemical Shift and Shielding Tensor Predictions
3.5.3. Electronic Circular Dichroism Spectral Predictions
3.6. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, J.-F.; Fan, H.; Xiong, J.; Wu, S.-B. Discorhabdins and pyrroloiminoquinone-related alkaloids. Chem. Rev. 2011, 111, 5465–5491. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, J.-C.J.; Polyzois, A.; Waterworth, S.C.; Siwe Noundou, X.; Dorrington, R.A. Current perspectives on pyrroloiminoquinones: Distribution, biosynthesis and drug discovery potential. Molecules 2022, 27, 8724. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Ding, Y.; Wang, B.; Tekwani, B.L.; Schinazi, R.F.; Franzblau, S.; Kelly, M.; Stone, R.; Li, X.-C.; Ferreira, D.; et al. Anti-infective discorhabdins from a deep-water Alaskan sponge of the senus Latrunculia. J. Nat. Prod. 2010, 73, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Peifer, C.; Janussen, D.; Tasdemir, D. New discorhabdin alkaloids from the Antarctic deep-sea sponge Latrunculia biformis. Mar. Drugs 2019, 17, 439. [Google Scholar] [CrossRef]
- Li, F.; Kelly, M.; Tasdemir, D. Chemistry, chemotaxonomy and biological activity of the Latrunculid sponges (Order Poecilosclerida, Family Latrunculiidae). Mar. Drugs 2021, 19, 27. [Google Scholar] [CrossRef]
- Lang, G.; Pinkert, A.; Blunt, J.W.; Munro, M.H.G. Discorhabdin W, the first dimeric discorhabdin. J. Nat. Prod. 2005, 68, 1796–1798. [Google Scholar] [CrossRef]
- El-Naggar, M.; Capon, R.J. Discorhabdins revisited: Cytotoxic alkaloids from Southern Australian marine sponges of the genera Higginsia and Spongosorites. J. Nat. Prod. 2009, 72, 460–464. [Google Scholar] [CrossRef]
- Yang, A.; Baker, B.J.; Grimwade, J.; Leonard, A.; McClintock, J.B. Discorhabdin alkaloids from the Antarctic sponge Latrunculia apicalis. J. Nat. Prod. 1995, 58, 1596–1599. [Google Scholar] [CrossRef]
- Grkovic, T.; Pearce, A.N.; Munro, M.H.G.; Blunt, J.W.; Davies-Coleman, M.T.; Copp, B.R. Isolation and characterization of diastereomers of discorhabdins H and K and assignment of absolute configuration to discorhabdins D, N, Q, S, T, and U. J. Nat. Prod. 2010, 73, 1686–1693. [Google Scholar] [CrossRef]
- Grkovic, T.; Ding, Y.; Li, X.-C.; Webb, V.L.; Ferreira, D.; Copp, B.R. Enantiomeric discorhabdin alkaloids and establishment of their absolute configurations using theoretical calculations of electronic circular dichroism spectra. J. Org. Chem. 2008, 73, 9133–9136. [Google Scholar] [CrossRef]
- Jeon, J.-E.; Na, Z.; Jung, M.; Lee, H.-S.; Sim, C.J.; Nahm, K.; Oh, K.-B.; Shin, J. Discorhabdins from the Korean marine sponge Sceptrella sp. J. Nat. Prod. 2010, 73, 258–262. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.-K.; Wang, W.-X. GIAO 13C NMR calculation with sorted training sets improves accuracy and reliability for structural assignation. J. Org. Chem. 2020, 85, 11350–11358. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, M.M.; Sarotti, A.M. Sensitivity analysis of DP4+ with the probability distribution terms: Development of a universal and customizable method. J. Org. Chem. 2021, 86, 8544–8548. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.; Meyer, C.; Parker, M.; Hawk, H. Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Mol. Ecol. Resour. 2013, 13, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Zhang, C.; Idelbayev, Y.; Roberts, N.; Tao, Y.; Nannapaneni, Y.; Duggan, B.M.; Min, J.; Lin, E.C.; Gerwick, E.C.; Cottrell, G.W.; et al. Small Molecule Accurate Recognition Technology (SMART) to enhance natural products research. Sci. Rep. 2017, 7, 14243. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F. An overlap fitted chain of spheres exchange method. J. Phys. Chem. 2011, 135, 144105. [Google Scholar] [CrossRef]
- Izsák, R.; Hansen, A.; Neese, F. The resolution of identity and chain of spheres approximations for the LPNO-CCSD singles Fock term. Mol. Phys. 2012, 110, 2413–2417. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F.; Klopper, W. Robust fitting techniques in the chain of spheres approximation to the Fock exchange: The role of the complementary space. J. Chem. Phys. 2013, 139, 094111. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
| Pos. | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | gCOSY | gHMBC | δC, Type | δH (J in Hz) | |
| 1 | 134.9, CH | 6.15, s | 2, 3, 5, 6, 7, 20 | 134.7, CH | 6.33, s | |
| 2 | 129.2, C | 132.1, C | ||||
| 3 | 67.3, CH | 4.42, t (7.1) | 4a, 4b | 1, 2, 4 | 67.2, CH | 4.42, t (7.8) |
| 4a | 32.7, CH2 | 2.18, qt (13.1, 4.4) | 3, 4b, 5a | 2, 3, 5, 6 | 27.4, CH2 | 1.91, dt (7.3, 3.0) |
| 4b | 2.04, dq (4.9, 7.5) | 3, 4a | 2, 3, 5, 6 | |||
| 5a | 36.9, CH2 | 2.70, td (13.6, 4.0) | 4a, 5b | 3, 4, 6, 7, 20 | 34.9, CH2 | 2.27, m |
| 5b | 2.11, dt (14.0, 3.6) | 1, 3, 4, 6, 7, 20 | 1.84, d (13.1) | |||
| 6 | 45.9, C | 40.3, C | ||||
| 7 | 104.0, C | 115.4, CH | 5.24, d (7.6) | |||
| 8 | 125.5, CH | 6.60, d (5.3) | 9 | 6, 7, 10 | 121.8, CH | 6.28, dd (3.1, 4.5) |
| 9 | 8.72, d (4.2) | 8 | 7, 11, 20 | 10.43, s | ||
| 10 | 137.9, C | 144.6, C | ||||
| 11 | 164.1, C | 166.4, C | ||||
| 12 | 118.5, C | 123.4, C | ||||
| 13 | 13.17, s | |||||
| 14 | 128.6, CH | 8.24, s | 11, 12, 15, 21 | 126.8, CH | 7.36, s | |
| 15 | 124.6, C | 119.4, C | ||||
| 16 | 113.7, CH | 7.54, d (5.8) | 17 | 14, 17, 19, 21 | 17.9, CH2 | 2.87, t (7.5) |
| 17 | 141.6, CH | 8.31, d (5.8) | 16 | 15, 16, 19, 21 | 44.8, CH2 | 3.91, dd (5.9, 7.1) |
| 18 | 8.78, s | |||||
| 19 | 147.3, C | 157.2, C | ||||
| 20 | 111.7, C | 99.6, C | ||||
| 21 | 119.5, C | 122.4, C | ||||
| Pos. | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| 1 | 137.3, CH | 67.2, CH | 4.84, s | 67.2, CH | 4.85, t (1.8) | |
| 2 | 132.8, C | 65.7, CH | 5.02, s | 65.7, CH | 5.04, d (2.4) | |
| 3 | 68.0, CH | 4.59, br s | 184.5, C | 184.5, C | ||
| 4a | 29.6, CH2 | 2.06, o/l | 109.9, CH | 5.96, s | 110.0, CH | 5.97, s |
| 4b | 1.90, o/l | |||||
| 5 | 31.1, CH2 | 2.10, o/l | 171.3, CH | 171.2, C | ||
| 1.90, o/l | ||||||
| 6 | 38.7, C | 47.5, C | 47.5, C | |||
| 7a | 31.1, CH2 | 2.06, o/l | 36.0, CH2 | 2.86, d (10.9) | 36.0, CH2 | 2.86, dd (3.6, 10.7) |
| 7b | 1.63, td (3.1, 12.6) | 2.61, d (11.2) | 2.61, d (11.8) | |||
| 8a | 37.5, CH2 | 3.55, dt (3.1, 13.3) | 63.4, CH | 5.70, s | 63.4, CH | 5.71, d (2.0) |
| 8b | 3.32, o/l | |||||
| 9 | 9.30, s | 9.34, s | ||||
| 10 | 145.6, C | 145.5, C | 145.2, C | |||
| 11 | 169.2, C | 168.7, C | 169.1, C | |||
| 12 | 133.2, C | 132.3, C | 133.2, C | |||
| 14 | 151.0, C | 130.8, C | 130.7, C | |||
| 15 | 122.1, C | 121.1, C | 121.3, C | |||
| 16 | 112.0, CH | 7.84, d (5.7) | 115.1, CH2 | 7.94, (6.5) | 114.9, CH | 7.97, d (6.7) |
| 17 | 129.9, CH | 7.91, d (5.7) | 131.5, CH2 | 8.05, (6.4) | 131.9, CH | 8.10, d (6.5) |
| 18 | ||||||
| 19 | 139.2, C | 146.0, C | 145.4, C | |||
| 20 | 101.2, C | 102.2, C | 102.4, C | |||
| 21 | 132.1, C | 132.7, C | 132.5, C | |||
| 22 | 189.1, C | 186.2, C | 188.2, C | |||
| 23 | 138.9, C | 129.2, C | 138.0, C | |||
| 24 | 130.8, CH | 8.37, d (5.6) | 133.1, CH | 8.42, d (8.2) | 130.4, CH | 8.35, d (7.8) |
| 25 | 128.4, CH | 7.56, t (7.5) | 114.8, CH | 6.90, d (8.2) | 128.0, CH | 7.55, t (7.6) |
| 26 | 132.3, CH | 7.63, t (6.9) | 161.5, C | 132.1, CH | 7.63, t (7.4) | |
| Compound | IC50 (μM) | SD a (µM) |
|---|---|---|
| 1 | 4.3 | 1.1 |
| 2 | 1.8 | 0.1 |
| 3 | 1.0 | 0.4 |
| 4 | 23.9 | 0.3 |
| 5 | >50 | ND b |
| 6 | >50 | ND b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afoullouss, S.; Olsen, S.S.H.; Morrow, S.; Cruz Rosa, E.; Geu, K.; Wilson, N.G.; Baker, B.J. Novel Discorhabdin Derivatives from Antarctic Sponges of the Genus Latrunculia: Expanding the Chemical Diversity of Polar Marine Natural Products. Mar. Drugs 2025, 23, 401. https://doi.org/10.3390/md23100401
Afoullouss S, Olsen SSH, Morrow S, Cruz Rosa E, Geu K, Wilson NG, Baker BJ. Novel Discorhabdin Derivatives from Antarctic Sponges of the Genus Latrunculia: Expanding the Chemical Diversity of Polar Marine Natural Products. Marine Drugs. 2025; 23(10):401. https://doi.org/10.3390/md23100401
Chicago/Turabian StyleAfoullouss, Sam, Stine S. H. Olsen, Sydney Morrow, Ezequiel Cruz Rosa, Kaley Geu, Nerida G. Wilson, and Bill J. Baker. 2025. "Novel Discorhabdin Derivatives from Antarctic Sponges of the Genus Latrunculia: Expanding the Chemical Diversity of Polar Marine Natural Products" Marine Drugs 23, no. 10: 401. https://doi.org/10.3390/md23100401
APA StyleAfoullouss, S., Olsen, S. S. H., Morrow, S., Cruz Rosa, E., Geu, K., Wilson, N. G., & Baker, B. J. (2025). Novel Discorhabdin Derivatives from Antarctic Sponges of the Genus Latrunculia: Expanding the Chemical Diversity of Polar Marine Natural Products. Marine Drugs, 23(10), 401. https://doi.org/10.3390/md23100401








