In Vitro Antioxidant and Antidiabetic Effects of Atlantic Mackerel and Sardine By-Product Hydrolysates
Abstract
1. Introduction
2. Results
2.1. Proteolysis Analysis
2.2. Antioxidant Activity
2.3. Potential Antidiabetic Activity
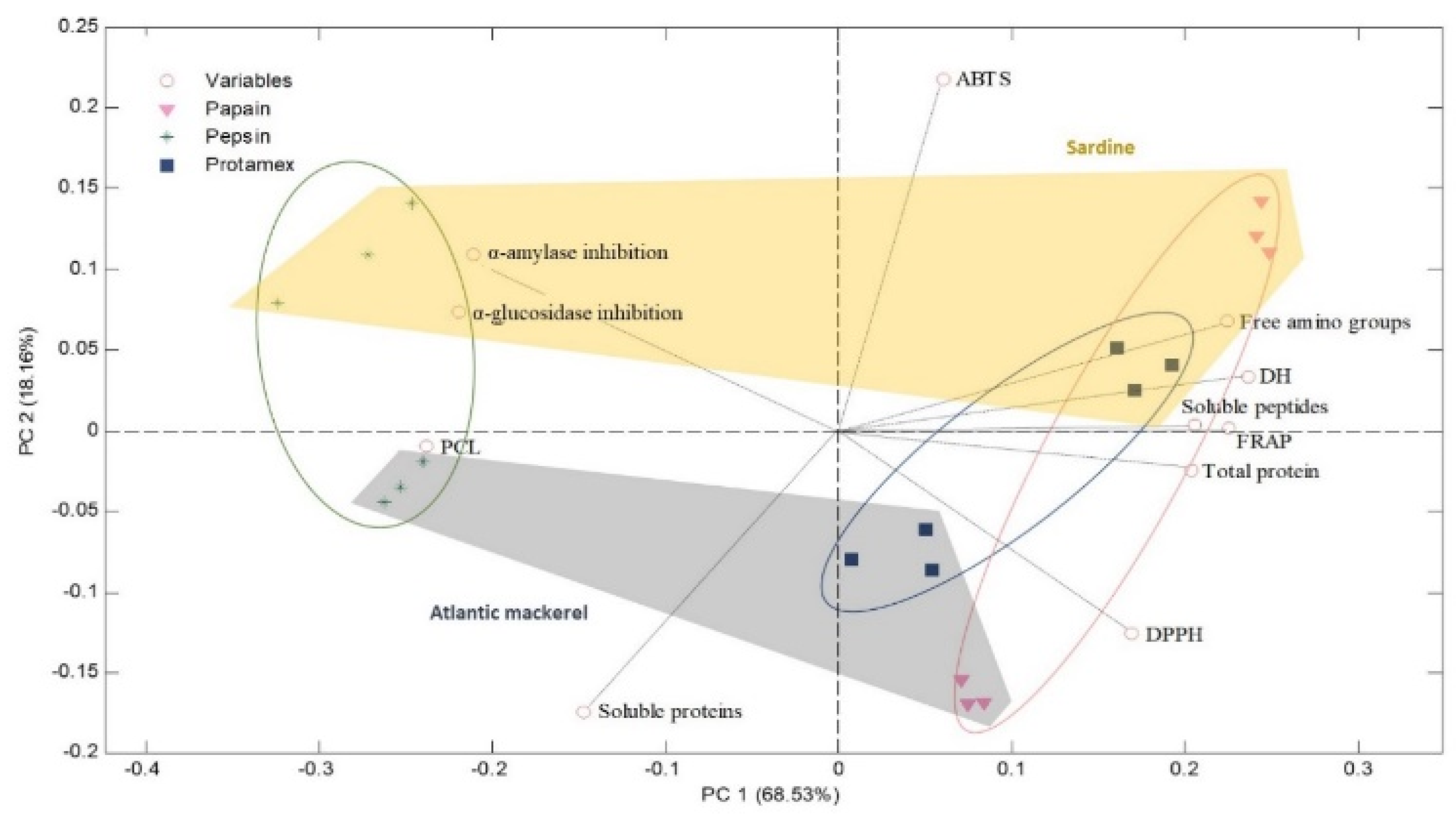
2.4. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Raw Material
4.3. Enzymatic Hydrolysis
4.4. Proteolysis Analysis
4.5. Antioxidant Activity
4.5.1. DPPH Radical Scavenging Capacity
4.5.2. ABTS Radical Scavenging Activity
4.5.3. Ferric-Reducing Antioxidant Power (FRAP)
4.6. Potential Antidiabetic Activity
4.6.1. α-Amylase Inhibition Assay
4.6.2. α-Glucosidase Inhibition Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| BSA | Bovine Serum Albumin |
| DH | Degree of hydrolysis |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| MA | Mackerel by-products hydrolysed by papain |
| FRAP | Ferric-Reducing Antioxidant Power |
| ME | Mackerel by-products hydrolysed by pepsin |
| MR | Mackerel by-products hydrolysed by Protamex |
| PCA | Principal Components Analysis |
| PCL | Peptide Chain Length |
| SA | Sardine by-products hydrolysed by papain |
| SE | Sardine by-products hydrolysed by pepsin |
| SR | Sardine by-products hydrolysed by Protamex |
| ROS | Reactive Oxygen Species |
| TCA | Trichloroacetic Acid |
| TE | Trolox Equivalents |
| TNBS | 2,4,6-trinitrobenzene sulfonic acid |
| TPTZ | 2,4,6-tri(2-pyridyl)-s-triazine |
References
- FAO. The State of World Fisheries and Aquaculture 2022; FAO: Roma, Italy, 2022. [Google Scholar]
- European Comission. Blue Bioeconomy Report; European Comission: Brussels, Belgium, 2023. [Google Scholar]
- Ferraro, V.; Carvalho, A.P.; Piccirillo, C.; Santos, M.M.; Castro, P.M.; Pintado, M.E. Extraction of high added value biological compounds from sardine, sardine-type fish and mackerel canning residues—A review. Mater. Sci. Eng. C 2013, 33, 3111–3120. [Google Scholar] [CrossRef]
- Marques, I.; Botelho, G.; Guiné, R. Comparative Study on Nutritional Composition of Fish Available in Portugal. Nutr. Food Sci. 2019, 49, 925–941. [Google Scholar] [CrossRef]
- Okyere, H.; Voegborlo, R.B.; Agorku, S.E. Human Exposure to Mercury, Lead and Cadmium through Consumption of Canned Mackerel, Tuna, Pilchard and Sardine. Food Chem. 2015, 179, 331–335. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Brennan, M.; Gangan, S.S.; Brennan, C. Utilization of Fish Waste as a Value-Added Ingredient: Sources and Bioactive Properties of Fish Protein Hydrolysate. In Sustainable Fish Production and Processing; Academic Press: Cambridge, MA, USA, 2021; pp. 203–225. ISBN 9780128242964. [Google Scholar]
- Thirukumaran, R.; Anu Priya, V.K.; Krishnamoorthy, S.; Ramakrishnan, P.; Moses, J.A.; Anandharamakrishnan, C. Resource Recovery from Fish Waste: Prospects and the Usage of Intensified Extraction Technologies. Chemosphere 2022, 299, 134361. [Google Scholar] [CrossRef]
- Kandyliari, A.; Mallouchos, A.; Papandroulakis, N.; Golla, J.P.; Lam, T.K.T.; Sakellari, A.; Karavoltsos, S.; Vasiliou, V.; Kapsokefalou, M. Nutrient Composition and Fatty Acid and Protein Profiles of Selected Fish By-Products. Foods 2020, 9, 190. [Google Scholar] [CrossRef]
- Maktoof, A.A.; Elherarlla, R.J.; Ethaib, S. Identifying the Nutritional Composition of Fish Waste, Bones, Scales, and Fins. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 871, p. 012013. [Google Scholar]
- Naghdi, S.; Rezaei, M.; Tabarsa, M.; Abdollahi, M. Parallel Extraction of Sulfated Polysaccharides and Protein Hydrolysate from Skipjack Tuna Head and Their Bioactive and Functional Properties. Food Bioprocess Technol. 2023, 16, 1258–1279. [Google Scholar] [CrossRef]
- Mechri, S.; Sellem, I.; Bouacem, K.; Jabeur, F.; Chamkha, M.; Hacene, H.; Bouanane-Darenfed, A.; Jaouadi, B. Antioxidant and Enzyme Inhibitory Activities of Metapenaeus Monoceros By-Product Hydrolysates Elaborated by Purified Alkaline Proteases. Waste Biomass Valorization 2020, 11, 6741–6755. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Evaluation of the Bioactive Potential of Foods Fortified with Fish Protein Hydrolysates. Food Res. Int. 2020, 137, 109572. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.B.; Lee, K.H.; Je, J.Y. Enzymatic Production of Bioactive Protein Hydrolysates from Tuna Liver: Effects of Enzymes and Molecular Weight on Bioactivity. Int. J. Food Sci. Technol. 2010, 45, 562–568. [Google Scholar] [CrossRef]
- Saidi, S.; Saoudi, M.; Ben Amar, R. Valorisation of Tuna Processing Waste Biomass: Isolation, Purification and Characterisation of Four Novel Antioxidant Peptides from Tuna by-Product Hydrolysate. Environ. Sci. Pollut. Res. 2018, 25, 17383–17392. [Google Scholar] [CrossRef] [PubMed]
- Pezeshk, S.; Ojagh, S.M.; Rezaei, M.; Shabanpour, B. Fractionation of Protein Hydrolysates of Fish Waste Using Membrane Ultrafiltration: Investigation of Antibacterial and Antioxidant Activities. Probiotics Antimicrob. Proteins 2019, 11, 1015–1022. [Google Scholar] [CrossRef]
- Ramakrishnan, S.R.; Jeong, C.R.; Park, J.W.; Cho, S.S.; Kim, S.J. A Review on the Processing of Functional Proteins or Peptides Derived from Fish By-Products and Their Industrial Applications. Heliyon 2023, 9, 14188. [Google Scholar] [CrossRef] [PubMed]
- Herpandi, H.; Rosma, A.; Nadiah, W.A.W.; Febrianto, N.A.; Huda, N. Optimization of Enzymatic Hydrolysis of Skipjack Tuna By-Product Using ProtamexTM: A Response Surface Approach. J. Fundam. Appl. Sci. 2018, 9, 845–860. [Google Scholar] [CrossRef]
- Klompong, V.; Benjakul, S.; Kantachote, D.; Shahidi, F. Antioxidative Activity and Functional Properties of Protein Hydrolysate of Yellow Stripe Trevally (Selaroides leptolepis) as Influenced by the Degree of Hydrolysis and Enzyme Type. Food Chem. 2007, 102, 1317–1327. [Google Scholar] [CrossRef]
- Noman, A.; Xu, Y.; AL-Bukhaiti, W.Q.; Abed, S.M.; Ali, A.H.; Ramadhan, A.H.; Xia, W. Influence of Enzymatic Hydrolysis Conditions on the Degree of Hydrolysis and Functional Properties of Protein Hydrolysate Obtained from Chinese Sturgeon (Acipenser sinensis) by Using Papain Enzyme. Process Biochem. 2018, 67, 19–28. [Google Scholar] [CrossRef]
- Nasri, M. Protein Hydrolysates and Biopeptides: Production, Biological Activities, and Applications in Foods and Health Benefits. A Review. Adv. Food Nutr. Res. 2017, 81, 109–159. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Howieson, J.; Fotedar, R.; Partridge, G.J. Enzymatic Fish Protein Hydrolysates in Finfish Aquaculture: A Review. Rev. Aquac. 2021, 13, 406–430. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-Product Protein Hydrolysates and Its Functional Properties: An Overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Fernández-Lucas, J.; Castañeda, D.; Hormigo, D. New Trends for a Classical Enzyme: Papain, a Biotechnological Success Story in the Food Industry. Trends Food Sci. Technol. 2017, 68, 91–101. [Google Scholar] [CrossRef]
- Tapal, A.; Tiku, P.K. Nutritional and Nutraceutical Improvement by Enzymatic Modification of Food Proteins. In Enzymes in Food Biotechnology Production, Applications, and Future Prospects; Academic Press: Cambridge, MA, USA, 2018; pp. 471–481. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Batista, I.; Pires, C.; Bandarra, N.M.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Antioxidant Activity of Protein Hydrolysates Obtained from Discarded Mediterranean Fish Species. Food Res. Int. 2014, 65, 469–476. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Guadix, E.M. Bioactive Fish Hydrolysates Resistance to Food Processing. LWT 2020, 117, 108670. [Google Scholar] [CrossRef]
- Stanforth, K.J.; Wilcox, M.D.; Chater, P.I.; Brownlee, I.A.; Zakhour, M.I.; Banecki, K.M.R.M.; Pearson, J.P. Pepsin Properties, Structure, and Its Accurate Measurement: A Narrative Review. Ann. Esophagus 2022, 5, 31. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Zhong, H.; Wang, S.; Sun, W.; Zhou, Q. Enzymatic Hydrolysis of Rice Dreg Protein: Effects of Enzyme Type on the Functional Properties and Antioxidant Activities of Recovered Proteins. Food Chem. 2012, 134, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G.; Rasco, B.A. Fish Protein Hydrolysates: Production, Biochemical, and Functional Properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, C.Y.; Wang, S.T.; Li, Y.Q.; Mo, H.Z.; He, J.X. Physicochemical Properties and Antioxidant Activities of Tree Peony (Paeonia suffruticosa Andr.) Seed Protein Hydrolysates Obtained with Different Proteases. Food Chem. 2021, 345, 128765. [Google Scholar] [CrossRef]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Sadeghi Mahoonak, A.R.; Rafipour, F. Development of Animal/ Plant-Based Protein Hydrolysate and Its Application in Food, Feed and Nutraceutical Industries: State of the Art. J. Clean. Prod. 2021, 278, 123219. [Google Scholar] [CrossRef]
- Gao, R.; Yu, Q.; Shen, Y.; Chu, Q.; Chen, G.; Fen, S.; Yang, M.; Yuan, L.; McClements, D.J.; Sun, Q. Production, Bioactive Properties, and Potential Applications of Fish Protein Hydrolysates: Developments and Challenges. Trends Food Sci. Technol. 2021, 110, 687–699. [Google Scholar] [CrossRef]
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish Viscera Protein Hydrolysates: Production, Potential Applications and Functional and Bioactive Properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef]
- Elam, E.; Feng, J.; Lv, Y.M.; Ni, Z.J.; Sun, P.; Thakur, K.; Zhang, J.G.; Ma, Y.L.; Wei, Z.J. Recent Advances on Bioactive Food Derived Anti-Diabetic Hydrolysates and Peptides from Natural Resources. J. Funct. Foods. 2021, 86, 104674. [Google Scholar] [CrossRef]
- Montoya, J.E.Z.; Sanchez, A.F. The Hydrolysates from Fish By-Product, an Opportunity Increasing. In Hydrolysates; Books on Demand: Norderstedt, Germany, 2022. [Google Scholar] [CrossRef]
- Wasowicz, E.; Gramza, A.; Hes, M.; Malecka, M.; Jelen, H.H. Oxidation of Lipids in Food Systems. Polish J. Food Nutr. Sci. 2004, 13, 87–100. [Google Scholar]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein Oxidation in Muscle Foods: A Comprehensive Review. Antioxidants 2022, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Číž, M.; Čížová, H.; Denev, P.; Kratchanova, M.; Slavov, A.; Lojek, A. Different Methods for Control and Comparison of the Antioxidant Properties of Vegetables. Food Control 2010, 21, 518–523. [Google Scholar] [CrossRef]
- Morales-Medina, R.; Tamm, F.; Guadix, A.M.; Guadix, E.M.; Drusch, S. Functional and antioxidant properties of hydrolysates of sardine (S. pilchardus) and horse mackerel (T. mediterraneus) for the microencapsulation of fish oil by spray-drying. Food Chem. 2016, 194, 1208–1216. [Google Scholar] [CrossRef]
- Sandoval-Gallardo, J.M.; Osuna-Ruiz, I.; Martínez-Montaño, E.; Hernández, C.; Hurtado-Oliva, M.Á.; Valdez-Ortiz, Á.; Rios-Herrera, G.D.; Salazar-Leyva, J.A.; Ramírez-Pérez, J.S. Influence of Enzymatic Hydrolysis Conditions on Biochemical and Antioxidant Properties of Pacific Thread Herring (Ophistonema libertate) Hydrolysates. CYTA-J. Food 2020, 18, 392–400. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and Functional Properties of Food Protein-derived Antioxidant Peptides. J. Food Biochem. 2019, 43, 1–13. [Google Scholar] [CrossRef]
- Hwang, C.F.; Chen, Y.A.; Luo, C.; Chiang, W.D. Antioxidant and Antibacterial Activities of Peptide Fractions from Flaxseed Protein Hydrolysed by Protease from Bacillus Altitudinis HK02. Int. J. Food Sci. Technol. 2016, 51, 681–689. [Google Scholar] [CrossRef]
- Prakash Nirmal, N.; Singh Rajput, M.; Bhojraj Rathod, N.; Mudgil, P.; Pati, S.; Bono, G.; Nalinanon, S.; Li, L.; Maqsood, S. Structural Characteristic and Molecular Docking Simulation of Fish Protein-Derived Peptides: Recent Updates on Antioxidant, Anti-Hypertensive and Anti-Diabetic Peptides. Food Chem. 2023, 405, 134737. [Google Scholar] [CrossRef]
- Czelej, M.; Garbacz, K.; Czernecki, T.; Wawrzykowski, J.; Waśko, A. Protein Hydrolysates Derived from Animals and Plants—A Review of Production Methods and Antioxidant Activity. Foods 2022, 11, 1953. [Google Scholar] [CrossRef]
- Badarinath, A.V.; Mallikarjuna Rao, K.; Madhu Sudhana Chetty, C.; Ramkanth, S.; Rajan, T.V.S.; Gnanaprakash, K. A Review on In-Vitro Antioxidant Methods: Comparisions, Correlations and Considerations. Int. J. PharmTech Res. 2010, 2, 1276–1285. [Google Scholar]
- Henriques, A.; Vázquez, J.A.; Valcarcel, J.; Mendes, R.; Bandarra, N.M.; Pires, C. Characterization of Protein Hydrolysates from Fish Discards and By-Products from the North-West Spain Fishing Fleet as Potential Sources of Bioactive Peptides. Mar. Drugs 2021, 19, 338. [Google Scholar] [CrossRef] [PubMed]
- Siow, H.L.; Gan, C.Y. Extraction, Identification, and Structure-Activity Relationship of Antioxidative and α-Amylase Inhibitory Peptides from Cumin Seeds (Cuminum cyminum). J. Funct. Foods 2016, 22, 1–12. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological Aspects about in Vitro Evaluation of Antioxidant Properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Gerasimova, E.; Gazizullina, E. Study of Antioxidant Properties of Agents from the Perspective of Their Action Mechanisms. Molecules 2020, 25, 4251. [Google Scholar] [CrossRef]
- Mouri, M.; Badireddy, M. Hyperglycemia [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- WHO. WHO Diabetes Mellitus; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2022. [Google Scholar]
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12, 1692. [Google Scholar] [CrossRef]
- Maralhas, A.; Monteiro, A.; Martins, C.; Kranendonk, M.; Laires, A.; Rueff, J.; Rodrigues, A.S. Genotoxicity and Endoreduplication Inducing Activity of the Food Flavouring Eugenol. Mutagenesis 2006, 21, 199–204. [Google Scholar] [CrossRef]
- Khwaja, N.U.D.; Arunagirinathan, G. Efficacy and Cardiovascular Safety of Alpha Glucosidase Inhibitors. Curr. Drug Saf. 2020, 16, 122–128. [Google Scholar] [CrossRef]
- Aoki, K.; Muraoka, T.; Ito, Y.; Togashi, Y.; Terauchi, Y. Comparison of Adverse Gastrointestinal Effects of Acarbose and Miglitol in Healthy Men: A Crossover Study. Intern. Med. 2010, 49, 1085–1087. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Atlantic Salmon (Salmo Salar) Co-Product-Derived Protein Hydrolysates: A Source of Antidiabetic Peptides. Food Res. Int. 2018, 106, 598–606. [Google Scholar] [CrossRef]
- Harnedy-Rothwell, P.A.; Khatib, N.; Sharkey, S.; Lafferty, R.A.; Gite, S.; Whooley, J.; O’harte, F.P.M.; Fitzgerald, R.J. Physicochemical, Nutritional and in Vitro Antidiabetic Characterisation of Blue Whiting (Micromesistius Poutassou) Protein Hydrolysates. Mar. Drugs 2021, 19, 383. [Google Scholar] [CrossRef]
- Naik, A.S.; Whitaker, R.D.; Albrektsen, S.; Solstad, R.G.; Thoresen, L.; Hayes, M. Mesopelagic Fish Protein Hydrolysates and Extracts: A Source of Novel Anti-Hypertensive and Anti-Diabetic Peptides. Front. Mar. Sci. 2021, 8, 719608. [Google Scholar] [CrossRef]
- Amini Sarteshnizi, R.; Sahari, M.A.; Ahmadi Gavlighi, H.; Regenstein, J.M.; Nikoo, M.; Udenigwe, C.C. Influence of Fish Protein Hydrolysate-Pistachio Green Hull Extract Interactions on Antioxidant Activity and Inhibition of α-Glucosidase, α-Amylase, and DPP-IV Enzymes. LWT 2021, 142, 111019. [Google Scholar] [CrossRef]
- Berraquero-García, C.; Rivero-Pino, F.; Ospina, J.L.; Pérez-Gálvez, R.; Espejo-Carpio, F.J.; Guadix, A.; García-Moreno, P.J.; Guadix, E.M. Activity, Structural Features and in Silico Digestion of Antidiabetic Peptides. Food Biosci. 2023, 55, 102954. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Hunag, S.L.; Jao, C.L.; Ho, K.P.; Hsu, K.C. Peptides Derived from Atlantic Salmon Skin Gelatin as Dipeptidyl-Peptidase IV Inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef]
- Sharkey, S.J.; Harnedy-Rothwell, P.A.; Allsopp, P.J.; Hollywood, L.E.; FitzGerald, R.J.; O’Harte, F.P.M. A Narrative Review of the Anti-Hyperglycemic and Satiating Effects of Fish Protein Hydrolysates and Their Bioactive Peptides. Mol. Nutr. Food Res. 2020, 64, 2000403. [Google Scholar] [CrossRef]
- Wang, T.Y.; Hsieh, C.H.; Hung, C.C.; Jao, C.L.; Chen, M.C.; Hsu, K.C. Fish Skin Gelatin Hydrolysates as Dipeptidyl Peptidase IV Inhibitors and Glucagon-like Peptide-1 Stimulators Improve Glycaemic Control in Diabetic Rats: A Comparison between Warm- and Cold-Water Fish. J. Funct. Foods 2015, 19, 330–340. [Google Scholar] [CrossRef]
- Roblet, C.; Akhtar, M.J.; Mikhaylin, S.; Pilon, G.; Gill, T.; Marette, A.; Bazinet, L. Enhancement of Glucose Uptake in Muscular Cell by Peptide Fractions Separated by Electrodialysis with Filtration Membrane from Salmon Frame Protein Hydrolysate. J. Funct. Foods 2016, 22, 337–346. [Google Scholar] [CrossRef]
- Cunniff, P.; Washington, D. Official methods of analysis of AOAC International. J. AOAC Int. 1997, 80, 127A. [Google Scholar]
- AOAC Method 928.08; Nitrogen in Meat, Kjeldahl Method. AOAC: Rockville, MD, USA, 1997.
- Gallego, M.; Arnal, M.; Barat, J.M.; Talens, P. Effect of Cooking on Protein Digestion and Antioxidant Activity of Different Legume Pastes. Foods 2020, 10, 47. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the Degree of Hydrolysis of Food Protein Hydrolysates by Trinitrobenzenesulfonic Acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Gallego, M.; Arnal, M.; Talens, P.; Toldrá, F.; Mora, L. Effect of Gelatin Coating Enriched with Antioxidant Tomato By-Products on the Quality of Pork Meat. Polymers 2020, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Arnal, M.; Gallego, M.; Talens, P.; Mora, L. Impact of Thermal Treatments and Simulated Gastrointestinal Digestion on the α-Amylase Inhibitory Activity of Different Legumes. Food Chem. 2023, 418, 135884. [Google Scholar] [CrossRef] [PubMed]




| Raw Material | Enzyme | TP (%) | TSP (mg/g) | TCA-SP (mg/g) | FAG (mg/g) | DH (%) | PCL |
|---|---|---|---|---|---|---|---|
| Papain Pepsine ProtamexTM | 52.55 (0.83) aA | 3.13 (0.11) aA | 141.92 (3.60) aA | 166.57 (3.56) aA | 46.65 (1.00) aA | 2.14 (0.05) aA | |
| Mackerel | 48.83 (0.17) aB | 3.19 (0.10) aB | 64.65 (1.43) aB | 134.63 (4.45) aB | 34.45(1.14) aB | 2.90 (0.10) aB | |
| 57.05 (0.37) aC | 2.92 (0.17) aA | 92.24 (3.56) aC | 199.07 (8.19) aC | 44.61 (1.84)aA | 2.24 (0.09) aA | ||
| Papain Pepsine ProtamexTM | 58.51 (0.77) bA | 2.17 (0.12) bA | 138.83 (6.14) bA | 256.63 (2.64) bA | 55.39 (0.57) bA | 1.81 (0.02) bA | |
| Sardine | 45.26 (0.29) bB | 2.83 (0.18) bB | 90.00 (1.15) bB | 127.72 12.07) bB | 34.74 (3.28) bB | 2.90 (0.29) bB | |
| 52.41 (1.52) bC | 2.63 (0.18) bA | 145.41 11.81) bC | 252.25 (6.80) bC | 55.61 (1.50) bA | 1.80 (0.05) bA |
| F | E | F × E | |
|---|---|---|---|
| Proteolysis analysis | |||
| Total protein | 7.83 * | 410.75 *** | 159.41 *** |
| TCA-soluble proteins | 60.63 *** | 9.38 ** | 9.37 ** |
| Soluble peptides | 82.81 *** | 179.36 ** | 34.56 *** |
| Free amino groups | 186.77 *** | 313.40 *** | 72.20 *** |
| Degree of Hydrolysis (DH) | 63.33 *** | 161.40 *** | 15.09 *** |
| Peptide Chain Length (PCL) | 17.95 ** | 93.38 *** | 4.45 * |
| Antioxidant capacity | |||
| ABTS radical scavenging activity | 103.79 *** | 6.31 * | 12.67 ** |
| DPPH radical scavenging activity | 47.42 *** | 3.06 ns | 9.90 ** |
| FRAP activity | 225.36 *** | 860.68 *** | 18.95 *** |
| Potential antidiabetic activity | |||
| α-Amylase Inhibition Assay (1 mg/mL) | - | - | - |
| α-Amylase Inhibition Assay (10 mg/mL) | - | - | - |
| α-Amylase Inhibition Assay (100 mg/mL) | 287.23 *** | 1716.82 *** | 147.46 *** |
| α-Glucosidase Inhibition Assay (1 mg/mL) | 0.61 ns | 44.78 *** | 3.92 ns |
| α-Glucosidase Inhibition Assay (10 mg/mL) | 14.73 ** | 230.03 *** | 6.24 * |
| α-Glucosidase Inhibition Assay (100 mg/mL) | 4.94 * | 2273.75 *** | 229.86 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, C.; Verdú, S.; Grau, R.; Barat, J.M.; Fuentes, A. In Vitro Antioxidant and Antidiabetic Effects of Atlantic Mackerel and Sardine By-Product Hydrolysates. Mar. Drugs 2025, 23, 393. https://doi.org/10.3390/md23100393
Fuentes C, Verdú S, Grau R, Barat JM, Fuentes A. In Vitro Antioxidant and Antidiabetic Effects of Atlantic Mackerel and Sardine By-Product Hydrolysates. Marine Drugs. 2025; 23(10):393. https://doi.org/10.3390/md23100393
Chicago/Turabian StyleFuentes, Cristina, Samuel Verdú, Raúl Grau, José Manuel Barat, and Ana Fuentes. 2025. "In Vitro Antioxidant and Antidiabetic Effects of Atlantic Mackerel and Sardine By-Product Hydrolysates" Marine Drugs 23, no. 10: 393. https://doi.org/10.3390/md23100393
APA StyleFuentes, C., Verdú, S., Grau, R., Barat, J. M., & Fuentes, A. (2025). In Vitro Antioxidant and Antidiabetic Effects of Atlantic Mackerel and Sardine By-Product Hydrolysates. Marine Drugs, 23(10), 393. https://doi.org/10.3390/md23100393








