Marine-Derived Ligands of Nicotinic Acetylcholine Receptors in Cancer Research
Abstract
1. Introduction
2. Peptides of Marine Origin as Anticancer Agents Targeting Various Cancer Cell Lines and Inoculated Tumors
3. Low Molecular Weight Compounds of Marine Origin as Anticancer Agents In Vitro and In Vivo
4. Marine Compounds as Potential Analgesic Agents for Pain Relief in Cancer Therapy
| Compound | Main Target | Cancer Cell Line/Carcinoma | Observed Effects | Ref. |
|---|---|---|---|---|
| α-conotoxins MI and ImI | muscle-type and α7 nAChRs | GLCS, NCI-N592, NCI-H69 small cell lung carcinoma | inhibited serotonin release and cell proliferation evoked by nicotine or cytisine | [26,27] |
| α-conotoxin ImI | α7 nAChR | THP-1 monocytic leukemia line | increased release of TNF-α and IL-8 | [29] |
| conotoxins Cal14.1a and Cal14.1b | nAChRs | H1299, H1437, H1975, H661 lung cancer cell lines | decreased cell viability | [30,31] |
| α-conotoxin AuIB | α3β4 nAChR | DMS-53 small cell lung carcinoma cells | inhibited cell viability | [32] |
| α-conotoxin MII | α3/α6β2-containing nAChRs | A549 and H1299 lung cancer cells | increased cell migration | [35] |
| α-conotoxin MII | α3/α6β2-containing nAChRs | Erlich carcinoma | increased the cytotoxic effect of indomethacin; inhibited cell growth; increased mouse survival in vivo | [36] |
| α-conotoxin PnIA | α7 nAChR | Erlich carcinoma | increased the cytotoxic effect of baicalein; inhibited growth cells; increased mouse survival in vivo | [36] |
| α-conotoxin ArIB[L11D16] | α7 nAChRs | A549 lung cancer cells | inhibited cell proliferation | [17] |
| α-conotoxin RgIA4 | α9α10 nAChR | A549 lung cancer cells | inhibited cell proliferation | [17] |
| α-conotoxins PnIA, ArIB[L11D16], RgIA | α3β2, α7, α9α10 nAChRs | C6 glioma cells | enhanced cell proliferation, decreased the cytotoxic effect of baicalein | [38] |
| α-conotoxin TxID | α3β4 nAChR | A549 and NCI-H1299 lung cancer cells | inhibited cell growth; enhanced the inhibitory effect of adriamycin | [33] |
| α-conotoxin TxID | α3β4 nAChR | SiHa and CaSki cervical cancer cells | inhibited cell proliferation | [10] |
| αO-conotoxin GeXIVA | α9α10 nAChR | SiHa and CaSki cervical cancer cells | inhibited cell proliferation | [10] |
| αO-conotoxin GeXIVA | α9α10 nAChR | 17 different breast cancer lines | inhibited cell proliferation | [9] |
| αO-conotoxin GeXIVA | α9α10 nAChR | MDA-MB-157 breast cancer line | inhibited cell proliferation; apoptosis; decreased ability of cell migration | [40] |
| αO-conotoxin GeXIVA | α9α10 nAChR | 4T1 triple-negative breast cancer cells | suppressed the cell growth | [41] |
| α-conotoxin ImI-AFP3 | α7 nAChR | A549 and NCI-H1299 lung cancer cells | inhibited cell growth and migration; enhanced the inhibitory effect of gefitinib | [34] |
| α-conotoxin PnIA[L10] | α7 nAChR | patient-derived glioblastoma and U87MG | enhanced cell proliferation | [39] |
| α-conotoxin RgIA | α9α10 nAChR | patient-derived glioblastoma and U87MG | enhanced cell proliferation | [39] |
| conotoxin lt14a | nAChRs | HepG2 liver carcinoma cells | decreased cell viability | [75] |
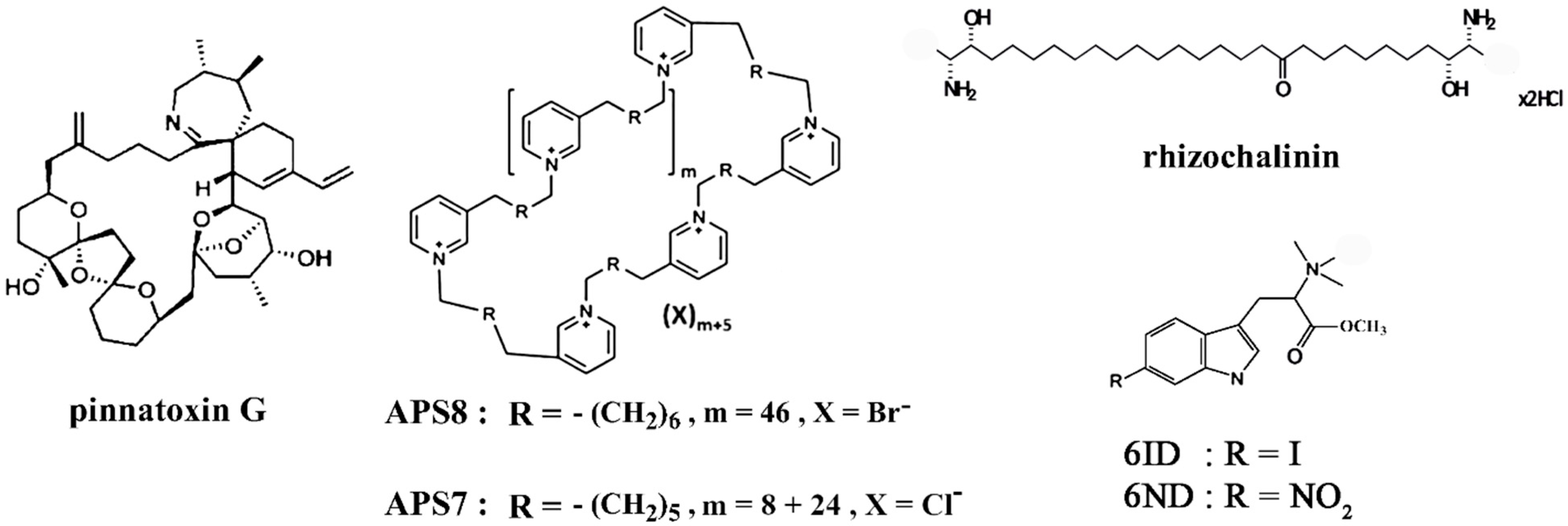
| pinnatoxin G | muscle-type, α4β2, α7 nAChRs | LN18, U87, U373 glioma cells, MDA-MB-231 breast cancer, PC3 prostate cancer, HT29 colon cancer cells | decreased cell viability | [50] |
| APS8 | α7 nAChR | A549, SKMES-1 lung carcinoma and HT29 human colon adenocarcinoma cells | inhibited growth of tumor cells, prevented regrowth of tumors, reduced the adverse anti-apoptotic effects of nicotine, impaired the cell viability, induced apoptosis | [53,54] |
| APS7 | α7 nAChR | A549 lung carcinoma cells | inhibited nicotine-induced Ca2+ influx and proliferation, inhibited nicotine-suppressed apoptosis; restored or improved the nicotine-reduced efficacy of chemotherapeutic cisplatin | [55] |
5. Marine Compounds as Potential Cancer Cell Markers
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| nAChR | nicotinic acetylcholine receptor |
| CIPN | chemotherapy-induced peripheral neuropathy |
References
- Zhang, Y.; Liao, Q.; Wen, X.; Fan, J.; Yuan, T.; Tong, X.; Jia, R.; Chai, P.; Fan, X. Hijacking of the nervous system in cancer: Mechanism and therapeutic targets. Mol. Cancer 2025, 24, 44. [Google Scholar] [CrossRef] [PubMed]
- Batrash, F.; Shaik, A.; Rauf, R.; Kutmah, M.; Zhang, J. Paracrine Regulation and Immune System Pathways in the Inflammatory Tumor Microenvironment of Lung Cancer: Insights into Oncogenesis and Immunotherapeutic Strategies. Cancers 2024, 16, 1113. [Google Scholar] [CrossRef]
- Kaleem, M.; Azmi, L.; Shahzad, N.; Taha, M.; Kumar, S.; Mujtaba, M.A.; Hazazi, A.A.H.; Kayali, A. Epigenetic dynamics and molecular mechanisms in oncogenesis, tumor progression, and therapy resistance. Naunyn-Schmiedebergs Arch. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Song, P.; Spindel, E.R. Basic and clinical aspects of non-neuronal acetylcholine: Expression of non-neuronal acetylcholine in lung cancer provides a new target for cancer therapy. J. Pharmacol. Sci. 2008, 106, 180–185. [Google Scholar] [CrossRef]
- Español, A.; Salem, A.; Sanchez, Y.; Sales, M.E. Breast cancer: Muscarinic receptors as new targets for tumor therapy. World J. Clin. Oncol. 2021, 12, 404–428. [Google Scholar] [CrossRef] [PubMed]
- Tsimpili, H.; Zoidis, G. A New era of muscarinic acetylcholine receptor modulators in neurological diseases, cancer and drug abuse. Pharmaceuticals 2025, 18, 369. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Lindstrom, J.M. Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 2020, 168, 108021. [Google Scholar] [CrossRef]
- Lee, C.H.; Huang, C.S.; Chen, C.S.; Tu, S.H.; Wang, Y.J.; Chang, Y.J.; Tam, K.W.; Wei, P.L.; Cheng, T.C.; Chu, J.S.; et al. Overexpression and activation of the α9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J. Natl. Cancer Inst. 2010, 102, 1322–1335. [Google Scholar] [CrossRef]
- Sun, Z.; Zhangsun, M.; Dong, S.; Liu, Y.; Qian, J.; Zhangsun, D.; Luo, S. Differential expression of nicotine acetylcholine receptors associates with human breast cancer and mediates antitumor activity of αO-Conotoxin GeXIVA. Mar. Drugs 2020, 18, 61. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, J.; Sun, Z.; Zhangsun, D.; Luo, S. Cervical cancer correlates with the differential expression of nicotinic acetylcholine receptors and reveals therapeutic targets. Mar. Drugs 2019, 17, 256. [Google Scholar] [CrossRef]
- Hajiasgharzadeh, K.; Somi, M.H.; Sadigh-Eteghad, S.; Mokhtarzadeh, A.; Shanehbandi, D.; Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Baradaran, B. The dual role of alpha7 nicotinic acetylcholine receptor in inflammation-associated gastrointestinal cancers. Heliyon 2020, 6, e03611. [Google Scholar] [CrossRef]
- Ma, G.; Ji, D.; Qu, X.; Liu, S.; Yang, X.; Wang, G.; Liu, Q.; Du, J. Mining and validating the expression pattern and prognostic value of acetylcholine receptors in non-small cell lung cancer. Medicine 2019, 98, e15555. [Google Scholar] [CrossRef]
- Wu, C.H.; Lee, C.H.; Ho, Y.S. Nicotinic acetylcholine receptor-based blockade: Applications of molecular targets for cancer therapy. Clin. Cancer Res. 2011, 17, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Chen, C.H.; Wang, Y.J.; Pestell, R.G.; Albanese, C.; Chen, R.J.; Chang, M.C.; Jeng, J.H.; Lin, S.Y.; Liang, Y.C.; et al. Tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces cell proliferation in normal human bronchial epithelial cells through NFkappaB activation and cyclin D1 up-regulation. Toxicol. Appl. Pharmacol. 2005, 205, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Ho, Y.S.; Guo, H.R.; Wang, Y.J. Rapid activation of Stat3 and ERK1/2 by nicotine modulates cell proliferation in human bladder cancer cells. Toxicol. Sci. 2008, 104, 283–293. [Google Scholar] [CrossRef]
- Wei, P.L.; Chang, Y.J.; Ho, Y.S.; Lee, C.H.; Yang, Y.Y.; An, J.; Lin, S.Y. Tobacco-specific carcinogen enhances colon cancer cell migration through α7-nicotinic acetylcholine receptor. Ann. Surg. 2009, 249, 978–985. [Google Scholar] [CrossRef]
- Mucchietto, V.; Fasoli, F.; Pucci, S.; Moretti, M.; Benfante, R.; Maroli, A.; Di Lascio, S.; Bolchi, C.; Pallavicini, M.; Dowell, C.; et al. α9- and α7-containing receptors mediate the pro-proliferative effects of nicotine in the A549 adenocarcinoma cell line. Br. J. Pharmacol. 2018, 175, 1957–1972. [Google Scholar] [CrossRef]
- Lee, C.H.; Chang, Y.C.; Chen, C.S.; Tu, S.H.; Wang, Y.J.; Chen, L.C.; Chang, Y.J.; Wei, P.L.; Chang, H.W.; Chang, C.H.; et al. Crosstalk between nicotine and estrogen-induced estrogen receptor activation induces α9-nicotinic acetylcholine receptor expression in human breast cancer cells. Breast Cancer Res. Treat. 2011, 129, 331–345. [Google Scholar] [CrossRef]
- Chen, C.S.; Lee, C.H.; Hsieh, C.D.; Ho, C.T.; Pan, M.H.; Huang, C.S.; Tu, S.H.; Wang, Y.J.; Chen, L.C.; Chang, Y.J.; et al. Nicotine-induced human breast cancer cell proliferation attenuated by garcinol through down-regulation of the nicotinic receptor and cyclin D3 proteins. Breast Cancer Res. Treat. 2011, 125, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Pucci, S.; Zoli, M.; Clementi, F.; Gotti, C. α9-Containing Nicotinic Receptors in Cancer. Front. Cell. Neurosci. 2022, 15, 805123. [Google Scholar] [CrossRef]
- Al-Wadei, M.H.; Al-Wadei, H.A.; Schuller, H.M. Pancreatic cancer cells and normal pancreatic duct epithelial cells express an autocrine catecholamine loop that is activated by nicotinic acetylcholine receptors α3, α5, and α7. Mol. Cancer Res. 2012, 10, 239–249. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Abdel-Nabi, I.M.; El-Naggar, M.S.; Abbas, O.A.; Strong, P.N. Conus vexillum venom induces oxidative stress in Ehrlich’s ascites carcinoma cells: An insight into the mechanism of induction. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 10. [Google Scholar] [CrossRef]
- Salimi, A.; Salehian, S.; Aboutorabi, A.; Vazirizadeh, A.; Adhami, V.; Sajjadi Alehashem, S.H.; Seydi, E.; Pourahmad, J. Cytotoxicity studies of the crude venom and fractions of Persian Gulf snail (Conus textile) on chronic lymphocytic leukemia and normal lymphocytes. Asian Pac. J. Cancer Prev. 2021, 22, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Rahimitabar, N.; Vazirizadeh, A.; Adhami, V.; Pourahmad, J. Persian Gulf snail crude venom (Conus textile): A potential source of anti-cancer therapeutic agents for glioblastoma through mitochondrial-mediated apoptosis. Asian Pac. J. Cancer Prev. 2021, 22, 49–57. [Google Scholar] [CrossRef]
- Magdy, N.A.; Nafie, M.S.; El-Naggar, M.S.; Abu El-Regal, M.A.; Abdel Azeiz, A.Z.; Abdel-Rahman, M.A.; El-Zawahry, M. Cytotoxicity and apoptosis induction of the marine Conus flavidus venom in HepG2 cancer cell line. J. Biomol. Struct. Dyn. 2023, 41, 7786–7793. [Google Scholar] [CrossRef]
- Codignola, A.; Tarroni, P.; Cattaneo, M.G.; Vicentini, L.M.; Clementi, F.; Sher, E. Serotonin release and cell proliferation are under the control of α-bungarotoxin-sensitive nicotinic receptors in small-cell lung carcinoma cell lines. FEBS Lett. 1994, 342, 286–290. [Google Scholar] [CrossRef]
- Codignola, A.; McIntosh, J.M.; Cattaneo, M.G.; Vicentini, L.M.; Clementi, F.; Sher, E. α-Conotoxin Imperialis I inhibits nicotine-evoked hormone release and cell proliferation in human neuroendocrine carcinoma cells. Neurosci. Lett. 1996, 206, 53–56. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y. α7 nicotinic acetylcholine receptors in lung cancer. Oncol. Lett. 2018, 16, 1375–1382. [Google Scholar] [CrossRef]
- Padilla, A.; Keating, P.; Hartmann, J.X.; Marí, F. Effects of α-conotoxin ImI on TNF-α, IL-8 and TGF-β expression by human macrophage-like cells derived from THP-1 pre-monocytic leukemic cells. Sci. Rep. 2017, 7, 12742. [Google Scholar] [CrossRef] [PubMed]
- Oroz-Parra, I.; Navarro, M.; Cervantes-Luevano, K.E.; Álvarez-Delgado, C.; Salvesen, G.; Sanchez-Campos, L.N.; Licea-Navarro, A.F. Apoptosis activation in human lung cancer cell lines by a novel synthetic peptide derived from Conus californicus venom. Toxins 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Oroz-Parra, I.; Álvarez-Delgado, C.; Cervantes-Luevano, K.; Dueñas-Espinoza, S.; Licea-Navarro, A.F. Proapoptotic index evaluation of two synthetic peptides derived from the coneshell Californiconus californicus in lung cancer cell line H1299. Mar. Drugs 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Improgo, M.R.; Soll, L.G.; Tapper, A.R.; Gardner, P.D. Nicotinic acetylcholine receptors mediate lung cancer growth. Front. Physiol. 2013, 4, 251. [Google Scholar] [CrossRef]
- Qian, J.; Liu, Y.Q.; Sun, Z.H.; Zhangsun, D.T.; Luo, S.L. Identification of nicotinic acetylcholine receptor subunits in different lung cancer cell lines and the inhibitory effect of α-conotoxin TxID on lung cancer cell growth. Eur. J. Pharmacol. 2019, 865, 172674. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, Z.; Li, W.; Dong, X.; Chen, Y.; Lu, Y.; Zhu, M.; Li, M.; Lin, B. α-Conotoxin recombinant protein ImI-AFP3 efficiently inhibits the growth and migration of lung cancer cells. Protein Expr. Purif. 2024, 215, 106405. [Google Scholar] [CrossRef] [PubMed]
- Krais, A.M.; Hautefeuille, A.H.; Cros, M.P.; Krutovskikh, V.; Tournier, J.M.; Birembaut, P.; Thépot, A.; Paliwal, A.; Herceg, Z.; Boffetta, P.; et al. CHRNA5 as negative regulator of nicotine signaling in normal and cancer bronchial cells: Effects on motility, migration and p63 expression. Carcinogenesis 2011, 32, 1388–1395. [Google Scholar] [CrossRef]
- Osipov, A.V.; Terpinskaya, T.I.; Yanchanka, T.; Balashevich, T.; Zhmak, M.N.; Tsetlin, V.I.; Utkin, Y.N. α-Conotoxins enhance both the in vivo suppression of Ehrlich carcinoma growth and in vitro reduction in cell viability elicited by cyclooxygenase and lipoxygenase inhibitors. Mar. Drugs 2020, 18, 193. [Google Scholar] [CrossRef]
- Terpinskaya, T.I.; Osipov, A.V.; Balashevich, T.V.; Yanchanka, T.L.; Tamashionik, E.A.; Tsetlin, V.I.; Utkin, Y.N. Blockers of nicotinic acetylcholine receptors delay tumor growth and increase antitumor activity of mouse splenocytes. Dokl. Biochem. Biophys. 2020, 491, 89–92. [Google Scholar] [CrossRef]
- Terpinskaya, T.I.; Osipov, A.V.; Kryukova, E.V.; Kudryavtsev, D.S.; Kopylova, N.V.; Yanchanka, T.L.; Palukoshka, A.F.; Gondarenko, E.A.; Zhmak, M.N.; Tsetlin, V.I.; et al. α-Conotoxins and α-cobratoxin promote, while lipoxygenase and cyclooxygenase inhibitors suppress the proliferation of glioma C6 cells. Mar. Drugs 2021, 19, 118. [Google Scholar] [CrossRef]
- Gondarenko, E.; Mazur, D.; Masliakova, M.; Ryabukha, Y.; Kasheverov, I.; Utkin, Y.; Tsetlin, V.; Shahparonov, M.; Kudryavtsev, D.; Antipova, N. Subtype-selective peptide and protein neurotoxic inhibitors of nicotinic acetylcholine receptors enhance proliferation of patient-derived glioblastoma cell lines. Toxins 2024, 16, 80. [Google Scholar] [CrossRef]
- Sun, Z.; Bao, J.; Zhangsun, M.; Dong, S.; Zhangsun, D.; Luo, S. αO-Conotoxin GeXIVA inhibits the growth of breast cancer cells via interaction with α9 nicotine acetylcholine receptors. Mar. Drugs 2020, 18, 195. [Google Scholar] [CrossRef]
- Guo, X.; He, L.; Xu, W.; Wang, W.; Feng, X.; Fu, Y.; Zhang, X.; Ding, R.B.; Qi, X.; Bao, J.; et al. αO-Conotoxin GeXIVA[1,2] Suppresses In vivo Tumor Growth of Triple-Negative Breast Cancer by Inhibiting AKT-mTOR, STAT3 and NF-κB Signaling Mediated Proliferation and Inducing Apoptosis. Mar. Drugs 2024, 22, 252. [Google Scholar] [CrossRef]
- Kompella, S.N.; Hung, A.; Clark, R.J.; Marí, F.; Adams, D.J. Alanine scan of α-conotoxin RegIIA reveals a selective α3β4 nicotinic acetylcholine receptor antagonist. J. Biol. Chem. 2015, 290, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Kasheverov, I.E.; Logashina, Y.A.; Kornilov, F.D.; Lushpa, V.A.; Maleeva, E.E.; Korolkova, Y.V.; Yu, J.; Zhu, X.; Zhangsun, D.; Luo, S.; et al. Peptides from the sea anemone Metridium senile with modified inhibitor cystine knot (ICK) fold inhibit nicotinic acetylcholine receptors. Toxins 2022, 15, 28. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Karthikeyan, S.; Sri Balasubashini, M.; Vijayalakshmi, S.; Somasundaram, S.T.; Balasubramanian, T. Antitumor Effect of Snake Venom (Hydrophis spiralis) on Ehrlich Ascites Carcinoma Bearing Mice. Int. J. Cancer Res. 2007, 3, 167–173. [Google Scholar] [CrossRef][Green Version]
- Karthikeyan, R.; Karthikeyan, S.; Sri Balasubashini, M.; Somasundaram, S.T.; Balasubramanian, T. Inhibition of Hep2 and HeLa cell proliferation in vitro and EAC tumor growth in vivo by Lapemis curtus (Shaw 1802) venom. Toxicon 2008, 51, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Seydi, E.; Tajarri, P.; Naserzadeh, P.; Pourahmad, J. A Search for Anti-Carcinogenic and Cytotoxic Effects of Persian Gulf Sea Snake (Enhydrina schistosa) Venom on Hepatocellular Carcinoma Using Mitochondria Isolated from Liver. Iran. J. Pharmaceut. Sci. 2020, 16, 29–38. [Google Scholar]
- Araoz, R.; Servent, D.; Molgó, J.; Iorga, B.I.; Fruchart-Gaillard, C.; Benoit, E.; Gu, Z.; Stivala, C.; Zakarian, A. Total synthesis of pinnatoxins A and G and revision of the mode of action of pinnatoxin A. J. Am. Chem. Soc. 2011, 133, 10499–10511. [Google Scholar] [CrossRef]
- Hellyer, S.D.; Indurthi, D.; Balle, T.; Runder-Varga, V.; Selwood, A.I.; Tyndall, J.D.; Chebib, M.; Rhodes, L.; Kerr, D.S. Pinnatoxins E, F and G target multiple nicotinic receptor subtypes. J. Neurochem. 2015, 135, 479–491. [Google Scholar] [CrossRef]
- Geiger, M.; Desanglois, G.; Hogeveen, K.; Fessard, V.; Leprêtre, T.; Mondeguer, F.; Guitton, Y.; Hervé, F.; Séchet, V.; Grovel, O.; et al. Cytotoxicity, fractionation and dereplication of extracts of the dinoflagellate Vulcanodinium rugosum, a producer of pinnatoxin G. Mar. Drugs 2013, 11, 3350–3371. [Google Scholar] [CrossRef]
- Clarke, M.R.; Jones, B.; Squires, C.L.M.; Imhoff, F.M.; Harwood, D.T.; Rhodes, L.; Selwood, A.I.; McNabb, P.S.; Baird, S.K. Cyclic Imine Pinnatoxin G is Cytotoxic to Cancer Cell Lines via Nicotinic Acetylcholine Receptor-Driven Classical Apoptosis. J. Nat. Prod. 2021, 84, 2035–2042. [Google Scholar] [CrossRef]
- Turk, T.; Sepčić, K.; Mancini, I.; Guella, G. 3-Akylpyridinium and 3-alkylpyridine compounds from marine sponges, their synthesis, biological activities and potential use. Stud. Nat. Prod. Chem. 2008, 35, 355–397. [Google Scholar] [CrossRef]
- Paleari, L.; Trombino, S.; Falugi, C.; Gallus, L.; Carlone, S.; Angelini, C.; Sepcic, K.; Turk, T.; Faimali, M.; Noonan, D.M.; et al. Marine sponge-derived polymeric alkylpyridinium salts as a novel tumor chemotherapeutic targeting the cholinergic system in lung tumors. Int. J. Oncol. 2006, 29, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Zovko, A.; Viktorsson, K.; Lewensohn, R.; Kološa, K.; Filipič, M.; Xing, H.; Kem, W.R.; Paleari, L.; Turk, T. APS8, a polymeric alkylpyridinium salt blocks α7 nAChR and induces apoptosis in non-small cell lung carcinoma. Mar. Drugs 2013, 11, 2574–2594. [Google Scholar] [CrossRef]
- Berne, S.; Čemažar, M.; Frangež, R.; Juntes, P.; Kranjc, S.; Grandič, M.; Savarin, M.; Turk, T. APS8 Delays Tumor Growth in Mice by Inducing Apoptosis of Lung Adenocarcinoma Cells Expressing High Number of α7 Nicotinic Receptors. Mar. Drugs 2018, 16, 367. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, V.; Joukhan, A.; Bele, T.; Križaj, I.; Kralj, S.; Turk, T.; Drobne, D. Gelatin nanoparticles loaded with 3-alkylpyridinium salt APS7, an analog of marine toxin, are a promising support in human lung cancer therapy. Biomed. Pharmacother. 2024, 177, 117007. [Google Scholar] [CrossRef] [PubMed]
- Joukhan, A.; Kononenko, V.; Bele, T.; Sollner Dolenc, M.; Peigneur, S.; Pinheiro-Junior, E.L.; Tytgat, J.; Turk, T.; Križaj, I.; Drobne, D. Attenuation of nicotine effects on A549 lung cancer cells by synthetic α7 nAChR antagonists APS7-2 and APS8-2. Mar. Drugs 2024, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Joukhan, A.; Kononenko, V.; Sollner Dolenc, M.; Hočevar, M.; Turk, T.; Drobne, D. Modulation of the effect of cisplatin on nicotine-stimulated A549 lung cancer cells using analog of marine sponge toxin loaded in gelatin nanoparticles. Nanomaterials 2024, 14, 777. [Google Scholar] [CrossRef]
- Kudryavtsev, D.; Makarieva, T.; Utkina, N.; Santalova, E.; Kryukova, E.; Methfessel, C.; Tsetlin, V.; Stonik, V.; Kasheverov, I. Marine natural products acting on the acetylcholine-binding protein and nicotinic receptors: From computer modeling to binding studies and electrophysiology. Mar. Drugs 2014, 12, 1859–1875. [Google Scholar] [CrossRef]
- Kasheverov, I.E.; Shelukhina, I.V.; Kudryavtsev, D.S.; Makarieva, T.N.; Spirova, E.N.; Guzii, A.G.; Stonik, V.A.; Tsetlin, V.I. 6-bromohypaphorine from marine nudibranch mollusk Hermissenda crassicornis is an agonist of human α7 nicotinic acetylcholine receptor. Mar. Drugs 2015, 13, 1255–1266. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Otte, K.; Alsdorf, W.H.; Hauschild, J.; Lange, T.; Venz, S.; Bauer, C.K.; Bähring, R.; Amann, K.; Mandanchi, R.; et al. Marine compound rhizochalinin shows high in vitro and in vivo efficacy in castration resistant prostate cancer. Oncotarget 2016, 7, 69703–69717. [Google Scholar] [CrossRef]
- Mollica, A.; Locatelli, M.; Stefanucci, A.; Pinnen, F. Synthesis and bioactivity of secondary metabolites from marine sponges containing dibrominated indolic systems. Molecules 2012, 17, 6083–6099. [Google Scholar] [CrossRef]
- Ivanov, I.A.; Siniavin, A.E.; Palikov, V.A.; Senko, D.A.; Shelukhina, I.V.; Epifanova, L.A.; Ojomoko, L.O.; Belukhina, S.Y.; Prokopev, N.A.; Landau, M.A.; et al. Analogs of 6-bromohypaphorine with increased agonist potency for α7 nicotinic receptor as anti-inflammatory analgesic agents. Mar. Drugs 2023, 21, 368. [Google Scholar] [CrossRef] [PubMed]
- Chalil, A.; Staudt, M.D.; Harland, T.A.; Leimer, E.M.; Bhullar, R.; Argoff, C.E. A safety review of approved intrathecal analgesics for chronic pain management. Expert Opin. Drug Saf. 2021, 20, 439–451. [Google Scholar] [CrossRef]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors: Therapeutic targets for novel ligands to treat pain and inflammation. Pharmacol. Res. 2023, 190, 106715. [Google Scholar] [CrossRef]
- Shelukhina, I.; Siniavin, A.; Kasheverov, I.; Ojomoko, L.; Tsetlin, V.; Utkin, Y. α7- and α9-Containing Nicotinic Acetylcholine Receptors in the Functioning of Immune System and in Pain. Int. J. Mol. Sci. 2023, 24, 6524. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhangsun, D.; Yu, G.; Su, R.; Luo, S. The α9α10 nicotinic acetylcholine receptor antagonist αO-conotoxin GeXIVA[1,2] alleviates and reverses chemotherapy-induced neuropathic pain. Mar. Drugs 2019, 17, 265. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Qiao, Y.; Wang, M.; Wang, W.; McIntosh, J.M.; Zhangsun, D.; Luo, S. αO-conotoxin GeXIVA[1,2] reduced neuropathic pain and changed gene expression in chronic oxaliplatin-induced neuropathy mice model. Mar. Drugs 2024, 22, 49. [Google Scholar] [CrossRef]
- Pacini, A.; Micheli, L.; Maresca, M.; Branca, J.J.; McIntosh, J.M.; Ghelardini, C.; Di Cesare Mannelli, L. The α9α10 nicotinic receptor antagonist α-conotoxin RgIA prevents neuropathic pain induced by oxaliplatin treatment. Exp. Neurol. 2016, 282, 37–48. [Google Scholar] [CrossRef]
- Dyachenko, I.A.; Palikova, Y.A.; Palikov, V.A.; Korolkova, Y.V.; Kazakov, V.A.; Egorova, N.S.; Garifulina, A.I.; Utkin, Y.N.; Tsetlin, V.I.; Kryukova, E.V. α-Conotoxin RgIA and oligoarginine R8 in the mice model alleviate long-term oxaliplatin induced neuropathy. Biochimie 2022, 194, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Hone, A.J.; Roux, I.; Kniazeff, J.; Pin, J.P.; Upert, G.; Servent, D.; Glowatzki, E.; McIntosh, J.M. RgIA4 potently blocks mouse α9α10 nAChRs and provides long lasting protection against oxaliplatin-induced cold allodynia. Front. Cell. Neurosci. 2017, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Romero, H.K.; Christensen, S.B.; Di Cesare Mannelli, L.; Gajewiak, J.; Ramachandra, R.; Elmslie, K.S.; Vetter, D.E.; Ghelardini, C.; Iadonato, S.P.; Mercado, J.L.; et al. Inhibition of α9α10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2017, 114, E1825–E1832. [Google Scholar] [CrossRef]
- Huynh, P.N.; Giuvelis, D.; Christensen, S.; Tucker, K.L.; McIntosh, J.M. RgIA4 Accelerates Recovery from Paclitaxel-Induced Neuropathic Pain in Rats. Mar. Drugs 2019, 18, 12. [Google Scholar] [CrossRef]
- Zheng, N.; Christensen, S.B.; Dowell, C.; Purushottam, L.; Skalicky, J.J.; McIntosh, J.M.; Chou, D.H. Discovery of methylene thioacetal-incorporated α-RgIA analogues as potent and stable antagonists of the human α9α10 nicotinic acetylcholine receptor for the treatment of neuropathic pain. J. Med. Chem. 2021, 64, 9513–9524. [Google Scholar] [CrossRef]
- Gajewiak, J.; Christensen, S.B.; Dowell, C.; Hararah, F.; Fisher, F.; Huynh, P.N.; Olivera, B.M.; McIntosh, J.M. Selective Penicillamine Substitution Enables Development of a Potent Analgesic Peptide that Acts through a Non-Opioid-Based Mechanism. J. Med. Chem. 2021, 64, 9271–9278. [Google Scholar] [CrossRef]
- Sun, D.; Ren, Z.; Zeng, X.; You, Y.; Pan, W.; Zhou, M.; Wang, L.; Xu, A. Structure-function relationship of conotoxin lt14a, a potential analgesic with low cytotoxicity. Peptides 2011, 32, 300–305. [Google Scholar] [CrossRef]
- Liang, J.; Tae, H.S.; Zhao, Z.; Li, X.; Zhang, J.; Chen, S.; Jiang, T.; Adams, D.J.; Yu, R. Mechanism of action and structure-activity relationship of α-conotoxin Mr1.1 at the human α9α10 nicotinic acetylcholine receptor. J. Med. Chem. 2022, 65, 16204–16217. [Google Scholar] [CrossRef]
- Tan, T.; Xiang, X.; Qu, H.; Zhu, S.; Bi, Q. The study on venom proteins of Lapemis hardwickii by cDNA phage display. Toxicol. Lett. 2011, 206, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Urriola, N.; Lang, B.; Adelstein, S. Evaluation of commercially available antibodies and fluorescent conotoxins for the detection of surface ganglionic acetylcholine receptor on the neuroblastoma cell line, IMR-32 by flow cytometry. J. Immunol. Methods 2021, 498, 113124. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhu, X.; Yang, Y.; Tan, Y.; Luo, S.; Zhangsun, D. Fluorescently Labeled α-Conotoxin TxID, a New Probe for α3β4 Neuronal Nicotinic Acetylcholine Receptors. Mar. Drugs 2022, 20, 511. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Wang, N.; Gao, X.; Wang, Z.; Yu, J.; Zhangsun, D.; Zhu, X.; Luo, S. Fluorescent α-Conotoxin [Q1G, ΔR14]LvIB Identifies the Distribution of α7 Nicotinic Acetylcholine Receptor in the Rat Brain. Mar. Drugs 2024, 22, 200. [Google Scholar] [CrossRef]
- Pei, S.; Xu, C.; Tan, Y.; Wang, M.; Yu, J.; Zhangsun, D.; Zhu, X.; Luo, S. Synthesis, Activity, and Application of Fluorescent Analogs of [D1G, Δ14Q]LvIC Targeting α6β4 Nicotinic Acetylcholine Receptor. Bioconjug. Chem. 2023, 34, 2194–2204. [Google Scholar] [CrossRef]
- Vishwanath, V.A.; McIntosh, J.M. Synthesis of fluorescent analogs of α-conotoxin MII. Bioconjug. Chem. 2006, 17, 1612–1617. [Google Scholar] [CrossRef]
- Muttenthaler, M.; Nevin, S.T.; Inserra, M.; Lewis, R.J.; Adams, D.J.; Alewood, P. On-resin strategy to label α-conotoxins: Cy5-RgIA, a potent α9α10 nicotinic acetylcholine receptor imaging probe. Aust. J. Chem. 2020, 73, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, Y.; Zhangsun, D.; Zhu, X.; Luo, S. Design, Synthesis, and Activity of an α-Conotoxin LtIA Fluorescent Analogue. ACS Chem. Neurosci. 2021, 12, 3662–3671. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Lin, Z.; Fu, J.; He, B.; Gao, W.; Ma, L.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; et al. The use of α-conotoxin ImI to actualize the targeted delivery of paclitaxel micelles to α7 nAChR-overexpressing breast cancer. Biomaterials 2015, 42, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Zhao, L.; Chen, B.; Zhang, X.; Wang, X.; Yu, Z.; Ni, X.; Zhang, Q. α-Conotoxin ImI-modified polymeric micelles as potential nanocarriers for targeted docetaxel delivery to α7-nAChR overexpressed non-small cell lung cancer. Drug Deliv. 2018, 25, 493–503. [Google Scholar] [CrossRef]
| Name | Organism | Sequence |
|---|---|---|
| MI | Conus magus | GRCCHPACGKNYSC * |
| ImI | Conus imperialis | GCCSDPRCAWRC * |
| Cal14.1a | Conus californicus | GDCPPWCVGARCRAEKC |
| Cal14.1b | Conus californicus | GDCPPWCVGARCRAGKC |
| AuIB | Conus aulicus | GCCSYPPCFATNPDC * |
| TxID | Conus textile | GCCSHPVCSAMSPIC * |
| FITC-β-TxID[A9] | Conus textile | Fitc-(Bal)GCCSHPVCAAMSPIC |
| FITC-β-TP-2212-59 | Conus bullatus | Fitc-(Bal)GCCSHPBCFBZYC |
| FITC-β-RegIIA | Conus regius | Fitc-(Bal)GCCSHPACNVNNPHIC |
| MII | Conus magus | GCCSNPVCHLEHSNLC * |
| PnIA | Conus pennaceus | GCCSLPPCAANNPDYC * |
| PnIA[L10] | Conus pennaceus | GCCSLPPCALANNPDYC * |
| RgIA | Conus regius | GCCSDPRCRYRCR |
| RgIA4 | Conus regius | GCCTDPRC(Cyt)(Yio)QCY |
| ArIB[L11D16] | Conus arenatus | DECCSNPACRLNNPHDCRRR |
| GeXIVA | Conus generalis | TCRSSGRYCRSPYDRRRRYCRRITDACV * |
| lt14a | Conus litteratus | MCPPLCKPSCTNC * |
| lt14a[A7] | Conus litteratus | MCPPLCAPSCTNC * |
| Mr1.1[Dap4] | Conus marmoreus | GCC(Dap)HPACSVNNPDIC * |
| RegIIA | Conus regius | GCCSHPACNVNNPHIC * |
| RegIIA[A11A12] | Conus regius | GCCSHPACNVAAPHIC * |
| 1 Ms11a-3 | Metridium senile | GCKKLNSYCTRQHRECCHGLVCRRPDYGIGRGILWKCTRARK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasheverov, I.E.; Shelukhina, I.V.; Utkin, Y.N.; Tsetlin, V.I. Marine-Derived Ligands of Nicotinic Acetylcholine Receptors in Cancer Research. Mar. Drugs 2025, 23, 389. https://doi.org/10.3390/md23100389
Kasheverov IE, Shelukhina IV, Utkin YN, Tsetlin VI. Marine-Derived Ligands of Nicotinic Acetylcholine Receptors in Cancer Research. Marine Drugs. 2025; 23(10):389. https://doi.org/10.3390/md23100389
Chicago/Turabian StyleKasheverov, Igor E., Irina V. Shelukhina, Yuri N. Utkin, and Victor I. Tsetlin. 2025. "Marine-Derived Ligands of Nicotinic Acetylcholine Receptors in Cancer Research" Marine Drugs 23, no. 10: 389. https://doi.org/10.3390/md23100389
APA StyleKasheverov, I. E., Shelukhina, I. V., Utkin, Y. N., & Tsetlin, V. I. (2025). Marine-Derived Ligands of Nicotinic Acetylcholine Receptors in Cancer Research. Marine Drugs, 23(10), 389. https://doi.org/10.3390/md23100389







