Nemertide Alpha-1 as a Biopesticide: Aphid Deterrence, Antimicrobial Activity, and Safety Aspects
Abstract
1. Introduction
2. Results
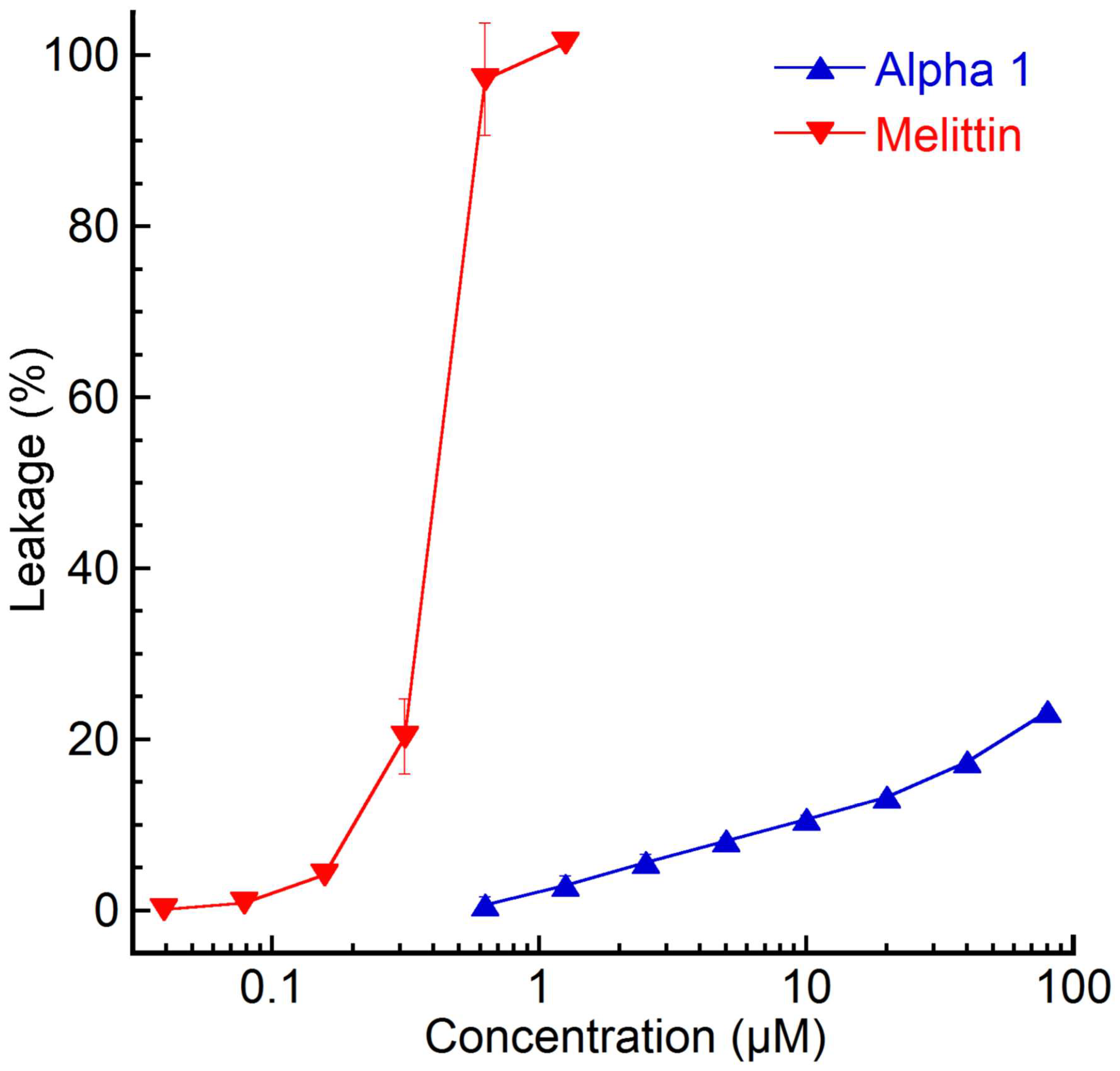
2.1. Antimicrobial Activity and Liposome Leakage Assay
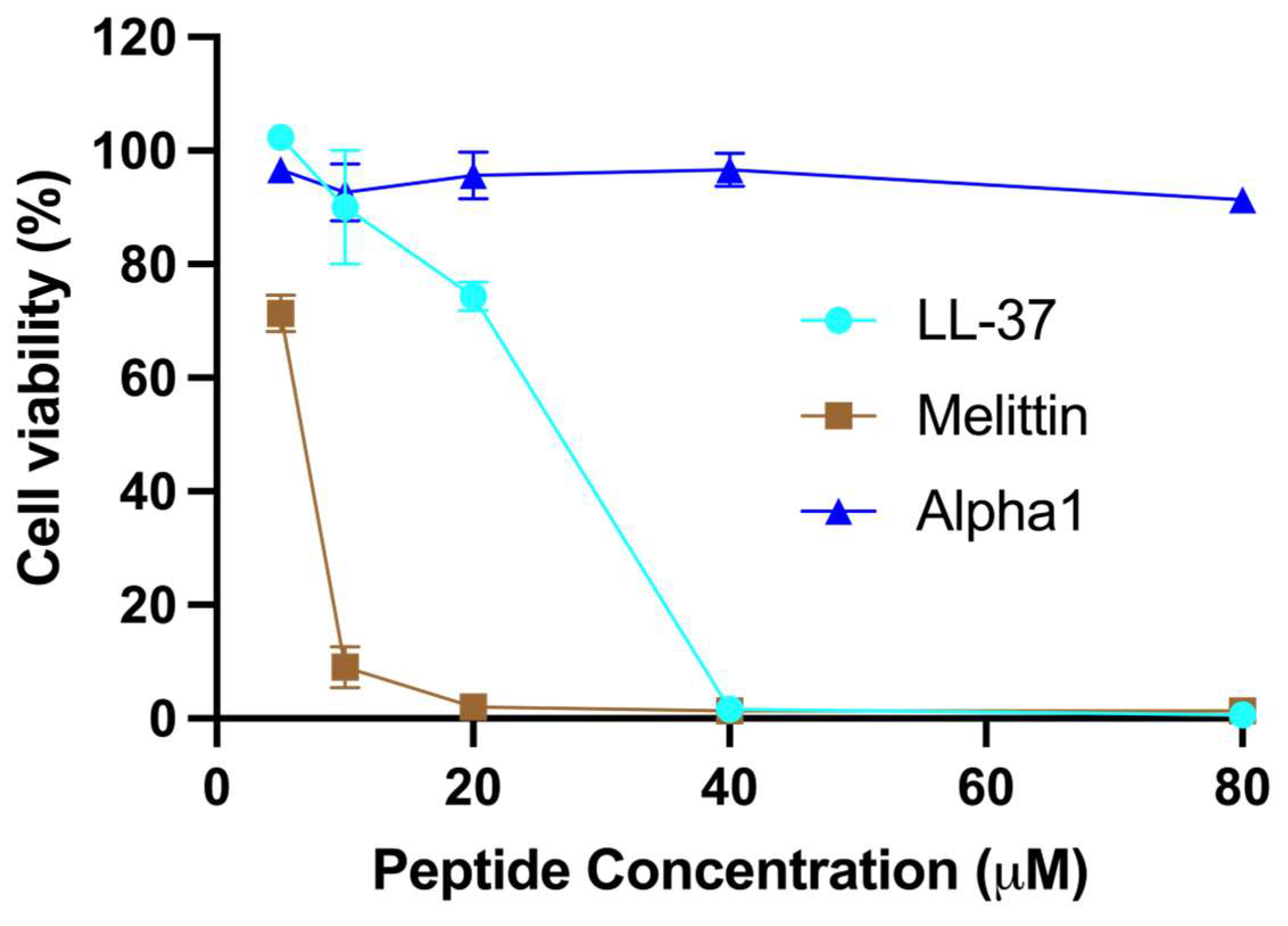
2.2. Cytotoxic Activity
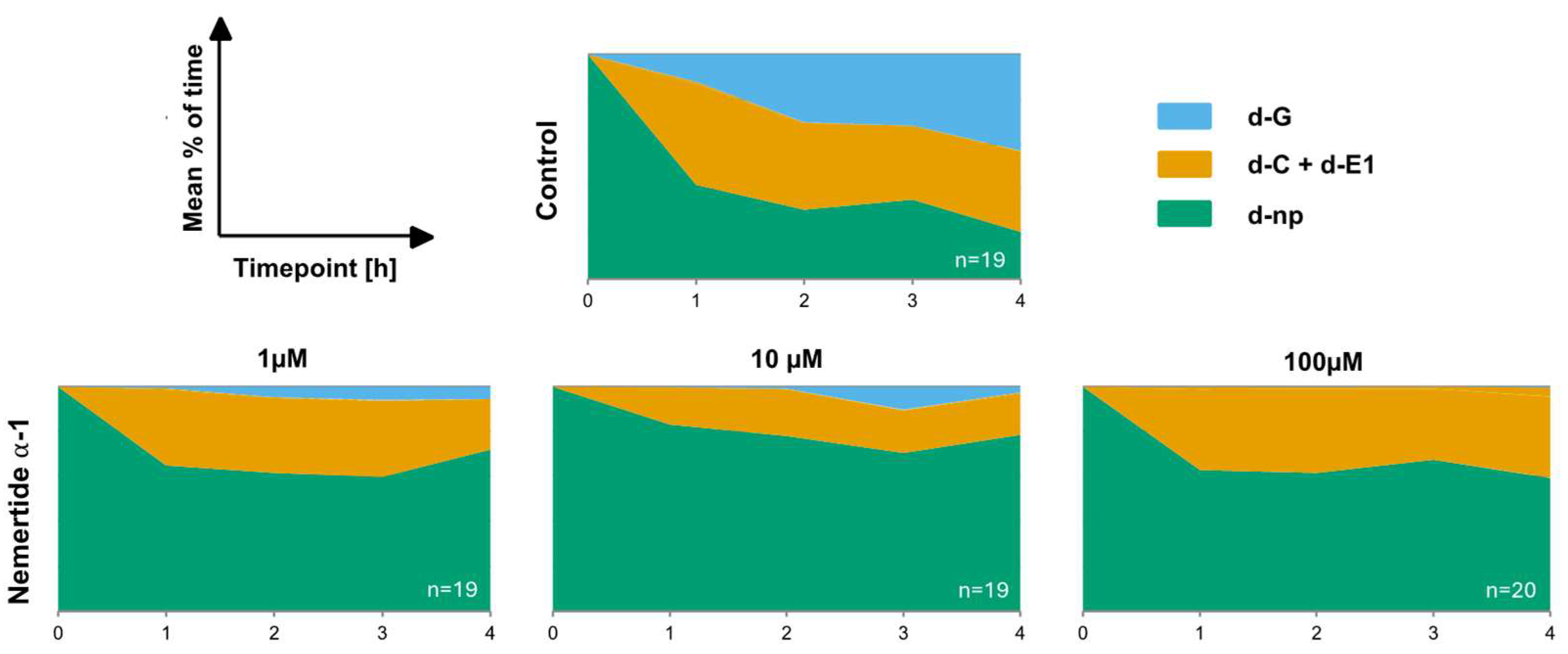
2.3. Effects on Aphids’ Feeding Behaviour
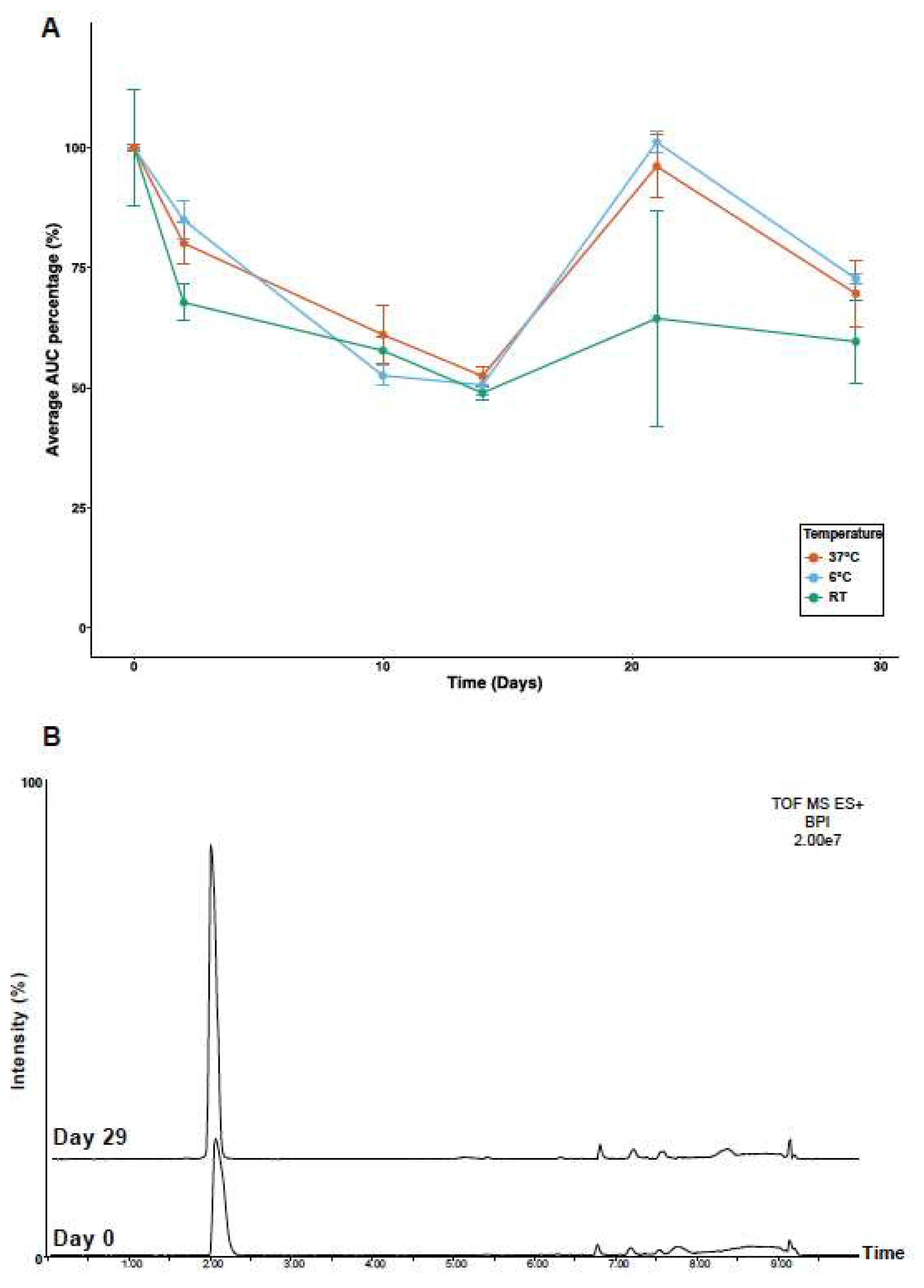
2.4. Temperature Stability
3. Discussion
3.1. Nemertide Alpha-1 Membrane Interactions
3.2. Antibacterial Potency
3.3. Membrane Disruption and Cell Toxicity
3.4. Nemertide Alpha-1 Feeding Deterrence
3.5. Nemertide Alpha-1 Thermal Stability
3.6. Limitations and Future Perspectives
4. Materials and Methods
4.1. Peptide Synthesis and Quality Assessment
4.2. Minimum Inhibitory Concentration (MIC) Assay
4.3. Bacterial Liposome Leakage Assay
4.4. Cytotoxicity Assay
4.5. Aphid EPG Measurements
4.5.1. EPG Principle
4.5.2. M. persicae Rearing Conditions Before EPG Recording
4.5.3. Preparation of Sucrose Diets for EPG Recording
4.5.4. EPG Recording Conditions
4.5.5. EPG Parameter Description
4.5.6. EPG Parameter Analysis
4.5.7. Temperature Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dedryver, C.-A.; Le Ralec, A.; Fabre, F. The Conflicting Relationships between Aphids and Men: A Review of Aphid Damage and Control Strategies. C. R. Biol. 2010, 333, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Denholm, I.; Devine, G. Insecticide Resistance. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 298–307. ISBN 978-0-12-384720-1. [Google Scholar]
- Brunner, J.F. Integrated Pest Management in Tree Fruit Crops. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Oxford, UK, 2014; pp. 15–30. ISBN 978-0-08-093139-5. [Google Scholar]
- Brosché, S. The Global Threat from Highly Hazardous Pesticides; IPEN: Stockholm, Sweden, 2024; p. 36. [Google Scholar]
- Basnet, P.; Dhital, R.; Rakshit, A. Chapter 8—Biopesticides: A Genetics, Genomics, and Molecular Biology Perspective. In Biopesticides; Advances in Bio-Inoculant Science; Rakshit, A., Meena, V.S., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L., Parihar, M., Singh, A.K., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 107–116. ISBN 978-0-12-823355-9. [Google Scholar]
- Chen, N.; Xu, S.; Zhang, Y.; Wang, F. Animal Protein Toxins: Origins and Therapeutic Applications. Biophys. Rep. 2018, 4, 233–242. [Google Scholar] [CrossRef]
- King, G.F. Tying Pest Insects in Knots: The Deployment of Spider-Venom-Derived Knottins as Bioinsecticides. Pest Manag. Sci. 2019, 75, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Regalado, L.; Sario, S.; Mendes, R.J.; Valle, J.; Harvey, P.J.; Teixeira, C.; Gomes, P.; Andreu, D.; Santos, C. Towards a Sustainable Management of the Spotted-Wing Drosophila: Disclosing the Effects of Two Spider Venom Peptides on Drosophila Suzukii. Insects 2023, 14, 533. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, X.; Zhang, Y.; Shi, Y.; Li, H.; Zhang, Z.; Iqbal, C.; Ye, D.; Li, X.; Zhao, Y.; et al. A Novel Bee-Friendly Peptidomimetic Insecticide: Synthesis, Aphicidal Activity and 3D-QSAR Study of Insect Kinin Analogs at Phe2 Modification. Pest Manag. Sci. 2022, 78, 2952–2963. [Google Scholar] [CrossRef]
- Ikonomopoulou, M.; King, G. Natural Born Insect Killers: Spider-Venom Peptides and Their Potential for Managing Arthropod Pests. Outlooks Pest Manag. 2013, 24, 16–19. [Google Scholar] [CrossRef]
- Saez, N.J.; Herzig, V. Versatile Spider Venom Peptides and Their Medical and Agricultural Applications. Toxicon 2019, 158, 109–126. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Ye, D.-X.; Liu, Y.; Zhang, X.-Y.; Zhou, Y.-L.; Zhang, L.; Yang, X.-L. Peptides, New Tools for Plant Protection in Eco-Agriculture. Adv. Agrochem. 2023, 2, 58–78. [Google Scholar] [CrossRef]
- Göransson, U.; Jacobsson, E.; Strand, M.; Andersson, H.S. The Toxins of Nemertean Worms. Toxins 2019, 11, 120. [Google Scholar] [CrossRef]
- Jacobsson, E.; Andersson, H.S.; Strand, M.; Peigneur, S.; Eriksson, C.; Lodén, H.; Shariatgorji, M.; Andrén, P.E.; Lebbe, E.K.M.; Rosengren, K.J.; et al. Peptide Ion Channel Toxins from the Bootlace Worm, the Longest Animal on Earth. Sci. Rep. 2018, 8, 4596. [Google Scholar] [CrossRef]
- Bell, J.; Sukiran, N.A.; Walsh, S.; Fitches, E.C. The Insecticidal Activity of Recombinant Nemertide Toxin α-1 from Lineus Longissimus towards Pests and Beneficial Species. Toxicon 2021, 197, 79–86. [Google Scholar] [CrossRef]
- Sun, D.; Jia, Z.; Zhu, J.; Liu, J.; Chen, Y.; Xu, Z.; Ma, H. Antimicrobial Peptides and Their Potential Applications in Plant Protection. Agronomy 2025, 15, 1113. [Google Scholar] [CrossRef]
- Wei, J.; Cao, X.; Qian, J.; Liu, Z.; Wang, X.; Su, Q.; Wang, Y.; Xie, R.; Li, X. Evaluation of Antimicrobial Peptide LL-37 for Treatment of Staphylococcus Aureus Biofilm on Titanium Plate. Medicine 2021, 100, e27426. [Google Scholar] [CrossRef]
- Gunasekera, S.; Muhammad, T.; Strömstedt, A.A.; Rosengren, K.J.; Göransson, U. Backbone Cyclization and Dimerization of LL-37-Derived Peptides Enhance Antimicrobial Activity and Proteolytic Stability. Front. Microbiol. 2020, 11, 168. [Google Scholar] [CrossRef]
- Tanner, J.D.; Deplazes, E.; Mancera, R.L. The Biological and Biophysical Properties of the Spider Peptide Gomesin. Molecules 2018, 23, 1733. [Google Scholar] [CrossRef]
- Wang, G. Structures of Human Host Defense Cathelicidin LL-37 and Its Smallest Antimicrobial Peptide KR-12 in Lipid Micelles*. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the Binding, Insertion and Destabilization of Phospholipid Bilayer Membranes by α-Helical Antimicrobial and Cell Non-Selective Membrane-Lytic Peptides. Biochim. Biophys. Acta (BBA) Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Chapple, D.S. Peptide Antibiotics. Antimicrob. Agents Chemother. 1999, 43, 1317–1323. [Google Scholar] [CrossRef]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A Membrane-Active Peptide with Diverse Functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.E. The Actions of Melittin on Membranes. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1990, 1031, 143–161. [Google Scholar] [CrossRef]
- Habermann, E. Bee and Wasp Venoms. Science 1972, 177, 314–322. [Google Scholar] [CrossRef]
- Liu, G.; Chen, S.; Hu, A.; Zhang, L.; Sun, W.; Chen, J.; Tang, W.; Zhang, H.; Liu, C.; Ke, C.; et al. The Establishment and Validation of the Human U937 Cell Line as a Cellular Model to Screen Immunomodulatory Agents Regulating Cytokine Release Induced by Influenza Virus Infection. Virol. Sin. 2019, 34, 648–661. [Google Scholar] [CrossRef]
- Irie, K.; Kitagawa, K.; Nagura, H.; Imai, T.; Shimomura, T.; Fujiyoshi, Y. Comparative Study of the Gating Motif and C-Type Inactivation in Prokaryotic Voltage-Gated Sodium Channels. J. Biol. Chem. 2010, 285, 3685–3694. [Google Scholar] [CrossRef]
- Ito, M.; Xu, H.; Guffanti, A.A.; Wei, Y.; Zvi, L.; Clapham, D.E.; Krulwich, T.A. The Voltage-Gated Na+ Channel NaVBP Has a Role in Motility, Chemotaxis, and pH Homeostasis of an Alkaliphilic Bacillus. Proc. Natl. Acad. Sci. USA 2004, 101, 10566–10571. [Google Scholar] [CrossRef] [PubMed]
- Koishi, R.; Xu, H.; Ren, D.; Navarro, B.; Spiller, B.W.; Shi, Q.; Clapham, D.E. A Superfamily of Voltage-Gated Sodium Channels in Bacteria. J. Biol. Chem. 2004, 279, 9532–9538. [Google Scholar] [CrossRef] [PubMed]
- Payandeh, J.; Minor, D.L. Bacterial Voltage-Gated Sodium Channels (BacNaVs) from the Soil, Sea, and Salt Lakes Enlighten Molecular Mechanisms of Electrical Signaling and Pharmacology in the Brain and Heart. J. Mol. Biol. 2015, 427, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Navarro, B.; Xu, H.; Yue, L.; Shi, Q.; Clapham, D.E. A Prokaryotic Voltage-Gated Sodium Channel. Science 2001, 294, 2372–2375. [Google Scholar] [CrossRef]
- Dancewicz, K.; Slazak, B.; Kiełkiewicz, M.; Kapusta, M.; Bohdanowicz, J.; Gabryś, B. Behavioral and Physiological Effects of Viola Spp. Cyclotides on Myzus Persicae (Sulz.). J. Insect Physiol. 2020, 122, 104025. [Google Scholar] [CrossRef]
- Tamone, S.L.; Harrison, J.F. Linking Insects with Crustacea: Physiology of the Pancrustacea: An Introduction to the Symposium. Integr. Comp. Biol. 2015, 55, 765–770. [Google Scholar] [CrossRef]
- van de Merbel, N.; Savoie, N.; Yadav, M.; Ohtsu, Y.; White, J.; Riccio, M.F.; Dong, K.; de Vries, R.; Diancin, J. Stability: Recommendation for Best Practices and Harmonization from the Global Bioanalysis Consortium Harmonization Team. AAPS J. 2014, 16, 392–399. [Google Scholar] [CrossRef]
- Kremsmayr, T.; Aljnabi, A.; Blanco-Canosa, J.B.; Tran, H.N.T.; Emidio, N.B.; Muttenthaler, M. On the Utility of Chemical Strategies to Improve Peptide Gut Stability. J. Med. Chem. 2022, 65, 6191–6206. [Google Scholar] [CrossRef] [PubMed]
- Pauletti, G.M.; Gangwar, S.; Siahaan, T.J.; Jeffrey Aubé, T.; Borchardt, R. Improvement of Oral Peptide Bioavailability: Peptidomimetics and Prodrug Strategies. Adv. Drug Deliv. Rev. 1997, 27, 235–256. [Google Scholar] [CrossRef]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.S.; Mitra, A.K. Approaches for Enhancing Oral Bioavailability of Peptides and Proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Thiesen, K.; BRAREN, S.; Terstegen, L. Use of Epsilon-Poly-l-Lysine for Increasing the Bioavailability of Antimicrobial Peptides in the Human Skin. European Patent EP2717965B1, 1 November 2012. [Google Scholar]
- Zhang, L.; Bulaj, G. Converting Peptides into Drug Leads by Lipidation. Curr. Med. Chem. 2012, 19, 1602–1618. [Google Scholar] [CrossRef]
- Barbeta, B.L.; Marshall, A.T.; Gillon, A.D.; Craik, D.J.; Anderson, M.A. Plant Cyclotides Disrupt Epithelial Cells in the Midgut of Lepidopteran Larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Chiou, S.-H.; Huang, Y.-W.; Lee, H.-J. Bio-Membrane Internalization Mechanisms of Arginine-Rich Cell-Penetrating Peptides in Various Species. Membranes 2022, 12, 88. [Google Scholar] [CrossRef]
- Jackson, M.A.; Yap, K.; Poth, A.G.; Gilding, E.K.; Swedberg, J.E.; Poon, S.; Qu, H.; Durek, T.; Harris, K.; Anderson, M.A.; et al. Rapid and Scalable Plant-Based Production of a Potent Plasmin Inhibitor Peptide. Front. Plant Sci. 2019, 10, 602. [Google Scholar] [CrossRef]
- Kennedy, R.M.; Tedford, W.; Hendrickson, C.; Venable, R.; Foune, C.L.; McIntyre, J.; Carlson, A.R.; Bao, L. Insecticidal Peptide Production, Peptide Expression in Plants and Combinations of Cysteine Rich Peptides. US Patent US20200277345A1, 1 May 2020. [Google Scholar]
- Nazarian-Firouzabadi, F.; Torres, M.D.T.; de la Fuente-Nunez, C. Recombinant Production of Antimicrobial Peptides in Plants. Biotechnol. Adv. 2024, 71, 108296. [Google Scholar] [CrossRef]
- Rivera-de-Torre, E.; Rimbault, C.; Jenkins, T.P.; Sørensen, C.V.; Damsbo, A.; Saez, N.J.; Duhoo, Y.; Hackney, C.M.; Ellgard, L.; Laustsen, A.H. Strategies for Heterologous Expression, Synthesis, and Purification of Animal Venom Toxins. Front. Bioeng. Biotechnol. 2022, 9, 811905. [Google Scholar] [CrossRef]
- Strömstedt, A.A.; Park, S.; Burman, R.; Göransson, U. Bactericidal Activity of Cyclotides Where Phosphatidylethanolamine-Lipid Selectivity Determines Antimicrobial Spectra. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 1986–2000. [Google Scholar] [CrossRef]
- Strömstedt, A.A.; Kristiansen, P.E.; Gunasekera, S.; Grob, N.; Skjeldal, L.; Göransson, U. Selective Membrane Disruption by the Cyclotide Kalata B7: Complex Ions and Essential Functional Groups in the Phosphatidylethanolamine Binding Pocket. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 1317–1327. [Google Scholar] [CrossRef]
- Sundström, C.; Nilsson, K. Establishment and characterization of a human histiolytic lymphoma cell line (U-937). Int. J. Cancer 1976, 17, 565–577. [Google Scholar] [CrossRef]
- Lindhagen, E.; Nygren, P.; Larsson, R. The Fluorometric Microculture Cytotoxicity Assay. Nat. Protoc. 2008, 3, 1364–1369. [Google Scholar] [CrossRef]
- McLean, D.L.; Kinsey, M.G. A Technique for Electronically Recording Aphid Feeding and Salivation. Nature 1964, 202, 1358–1359. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electronic Recording of Penetration Behaviour by Aphids. Entomol. Exp. Appl. 1978, 24, 721–730. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical Nature of Recorded Signals during Stylet Penetration by Aphids. Entomol. Exp. Appl. 1985, 38, 177–186. [Google Scholar] [CrossRef]
- Halarewicz, A.; Gabryś, B. Probing Behavior of Bird Cherry-Oat Aphid Rhopalosiphum padi (L.) on Native Bird Cherry Prunus padus L. and Alien Invasive Black Cherry Prunus serotina Erhr. in Europe and the Role of Cyanogenic Glycosides. Arthropod-Plant Interact. 2012, 6, 497–505. [Google Scholar] [CrossRef]
- Sauvion, N.; Rahbé, Y.; Peumans, W.J.; VAN Damme, E.J.M.; Gatehouse, J.A.; Gatehouse, A.M.R. Effects of GNA and Other Mannose Binding Lectins on Development and Fecundity of the Peach-Potato Aphid Myzus Persicae. Entomol. Exp. Appl. 1996, 79, 285–293. [Google Scholar] [CrossRef]
- Hewer, A.; Will, T.; van Bel, A.J.E. Plant Cues for Aphid Navigation in Vascular Tissues. J. Exp. Biol. 2010, 213, 4030–4042. [Google Scholar] [CrossRef]
- Sadeghi, A.; Van Damme, E.J.M.; Smagghe, G. Evaluation of the Susceptibility of the Pea Aphid, Acyrthosiphon Pisum, to a Selection of Novel Biorational Insecticides Using an Artificial Diet. J. Insect Sci. 2009, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Cid, M.; Fereres, A. Characterization of the Probing and Feeding Behavior of Planococcus Citri (Hemiptera: Pseudococcidae) on Grapevine. Ann. Entomol. Soc. Am. 2010, 103, 404–417. [Google Scholar] [CrossRef]
- Sauvion, N.; Charles, H.; Febvay, G.; Rahbé, Y. Effects of Jackbean Lectin (ConA) on the Feeding Behaviour and Kinetics of Intoxication of the Pea Aphid, Acyrthosiphon Pisum. Entomol. Exp. Appl. 2004, 110, 31–44. [Google Scholar] [CrossRef]
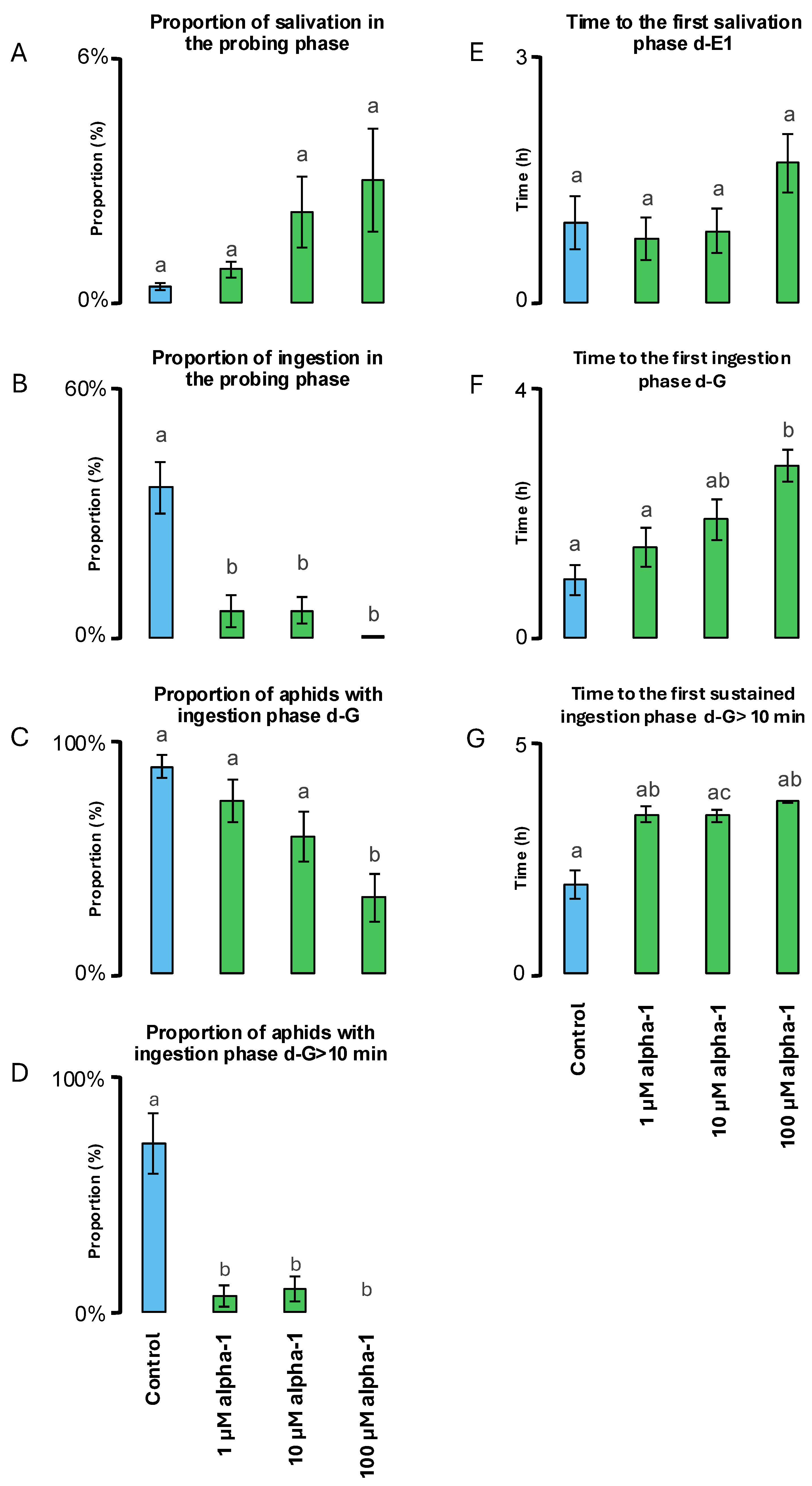
| EPG Parameters | Total Duration of Non-Penetration Phase (h) | Total Duration of Penetration Phase † (h) | Total Duration of Salivation Phase ‡ (min) | Total Duration of Ingestion Phase § (min) | Number of Probes (#) | Number of Probes with Ingestion Phases (#) | Number of Ingestion Phases (#) | Number of Sustained Ingestion Phases ¶ (#) |
|---|---|---|---|---|---|---|---|---|
| Control n = 19 | 1.3 ± 0.2 a | 2.7 ± 0.2 a | 0.7 ± 0.2 a | 71.6 ± 14.2 a | 36.2 ± 5.2 a | 6.4 ± 1.2 a | 8.2± 1.3 a | 1.2 ± 0.2 a |
| Nemertide alpha-1 | ||||||||
| 1 µM n = 19 | 2.58 ± 0.18 b | 1.4 ± 0.2 b | 0.7 ± 0.1 a | 9.9 ± 7.4 b | 41.4 ± 5.2 ac | 3.8 ± 1.5 ab | 4.5 ± 1.8 ab | 0.1 ± 0.07 b |
| 10 µM n = 19 | 3.12 ± 0.2 b | 0.9 ± 0.2 b | 0.8 ± 0.4 a | 8.3 ± 4.7 b | 18.6 ± 3.0 ab | 2.5 ± 0.6 a | 2.8 ± 0.8 b | 0.2 ± 0.09 b |
| 100 µM n = 20 | 2.5 ± 0.2 b | 1.5 ± 0.2 b | 4.1 ± 1.8 a | 0.4 ± 0.2 b | 44.8 ± 5.2 ab | 0.6 ± 0.2 b | 0.9 ± 0.3 b | 0.0 ± 0.0 b |
| p | <0.001 | <0.001 | 0.8256 | <0.001 | 0.0016 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laborde, Q.; Dancewicz, K.; Jacobsson, E.; Strömstedt, A.A.; Muhammad, T.; Eriksson, C.; Slazak, B.; Göransson, U.; Andersson, H.S. Nemertide Alpha-1 as a Biopesticide: Aphid Deterrence, Antimicrobial Activity, and Safety Aspects. Mar. Drugs 2025, 23, 388. https://doi.org/10.3390/md23100388
Laborde Q, Dancewicz K, Jacobsson E, Strömstedt AA, Muhammad T, Eriksson C, Slazak B, Göransson U, Andersson HS. Nemertide Alpha-1 as a Biopesticide: Aphid Deterrence, Antimicrobial Activity, and Safety Aspects. Marine Drugs. 2025; 23(10):388. https://doi.org/10.3390/md23100388
Chicago/Turabian StyleLaborde, Quentin, Katarzyna Dancewicz, Erik Jacobsson, Adam A. Strömstedt, Taj Muhammad, Camilla Eriksson, Blazej Slazak, Ulf Göransson, and Håkan S. Andersson. 2025. "Nemertide Alpha-1 as a Biopesticide: Aphid Deterrence, Antimicrobial Activity, and Safety Aspects" Marine Drugs 23, no. 10: 388. https://doi.org/10.3390/md23100388
APA StyleLaborde, Q., Dancewicz, K., Jacobsson, E., Strömstedt, A. A., Muhammad, T., Eriksson, C., Slazak, B., Göransson, U., & Andersson, H. S. (2025). Nemertide Alpha-1 as a Biopesticide: Aphid Deterrence, Antimicrobial Activity, and Safety Aspects. Marine Drugs, 23(10), 388. https://doi.org/10.3390/md23100388






