Characterization of YKL-40 Binding to Extracellular Matrix Glycosaminoglycans
Abstract
1. Introduction
2. Results
2.1. Selecting Glycosaminoglycan Ligands for In Silico and In Vitro Binding Studies
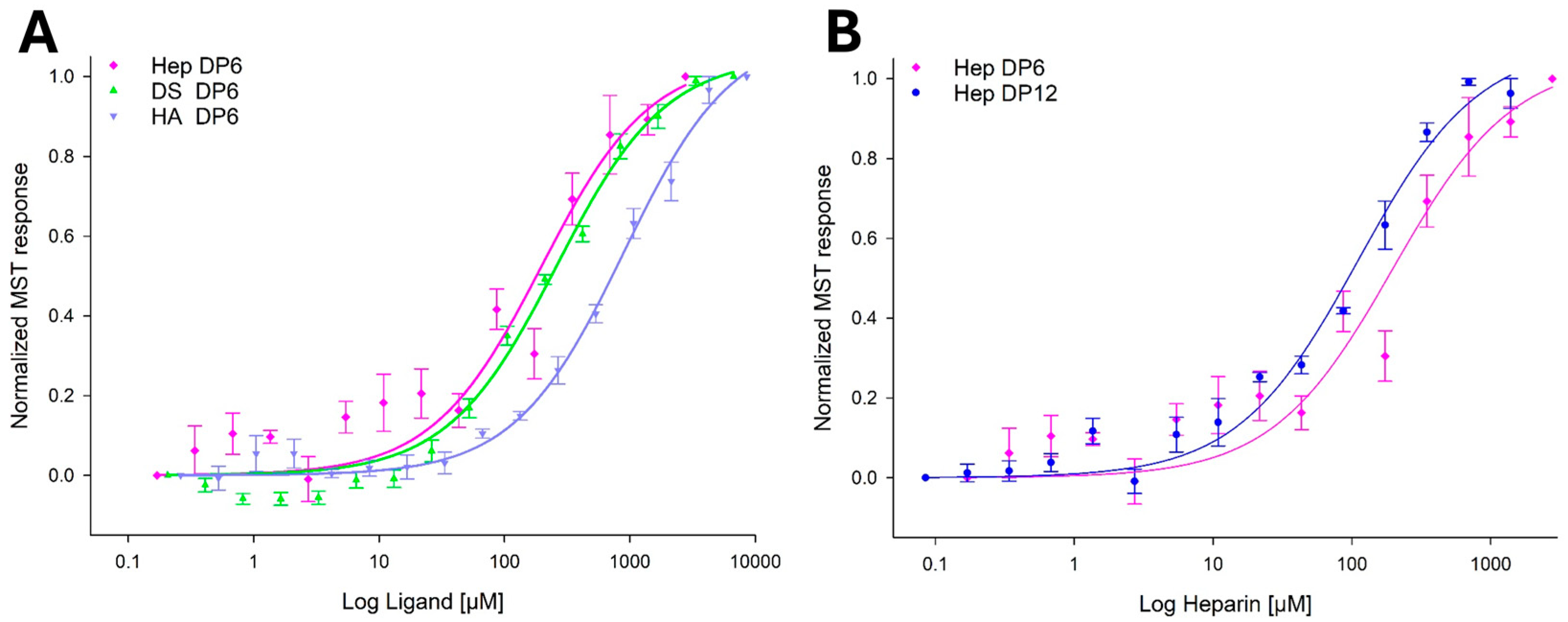
2.2. YKL-40 Exhibits Selectivity in Binding Affinities Towards Various GAGs
2.3. Molecular Docking Studies
2.3.1. Docking Analysis—YKL-40 and Chitin Tetramer
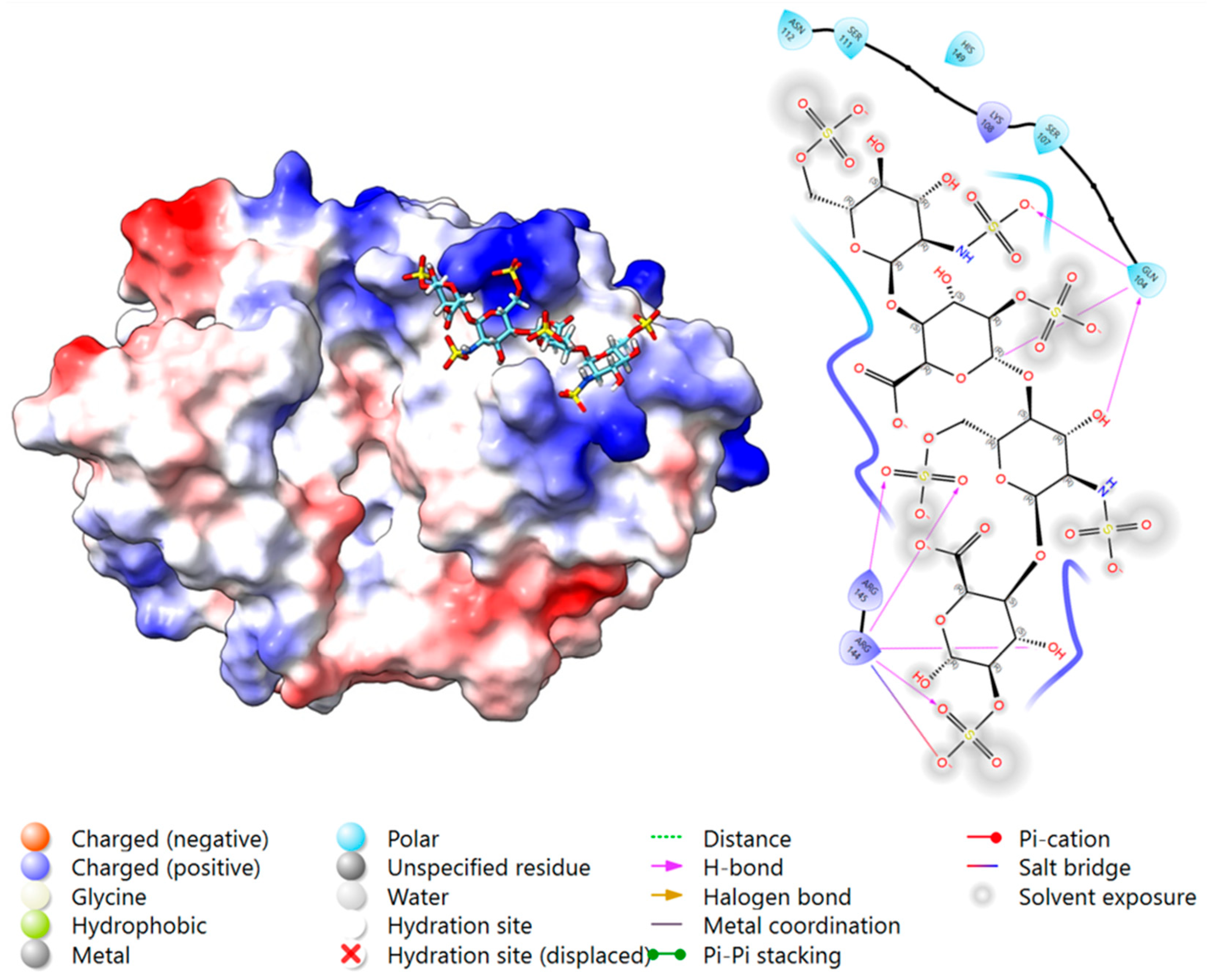
2.3.2. Docking Analysis—YKL-40 and Heparin
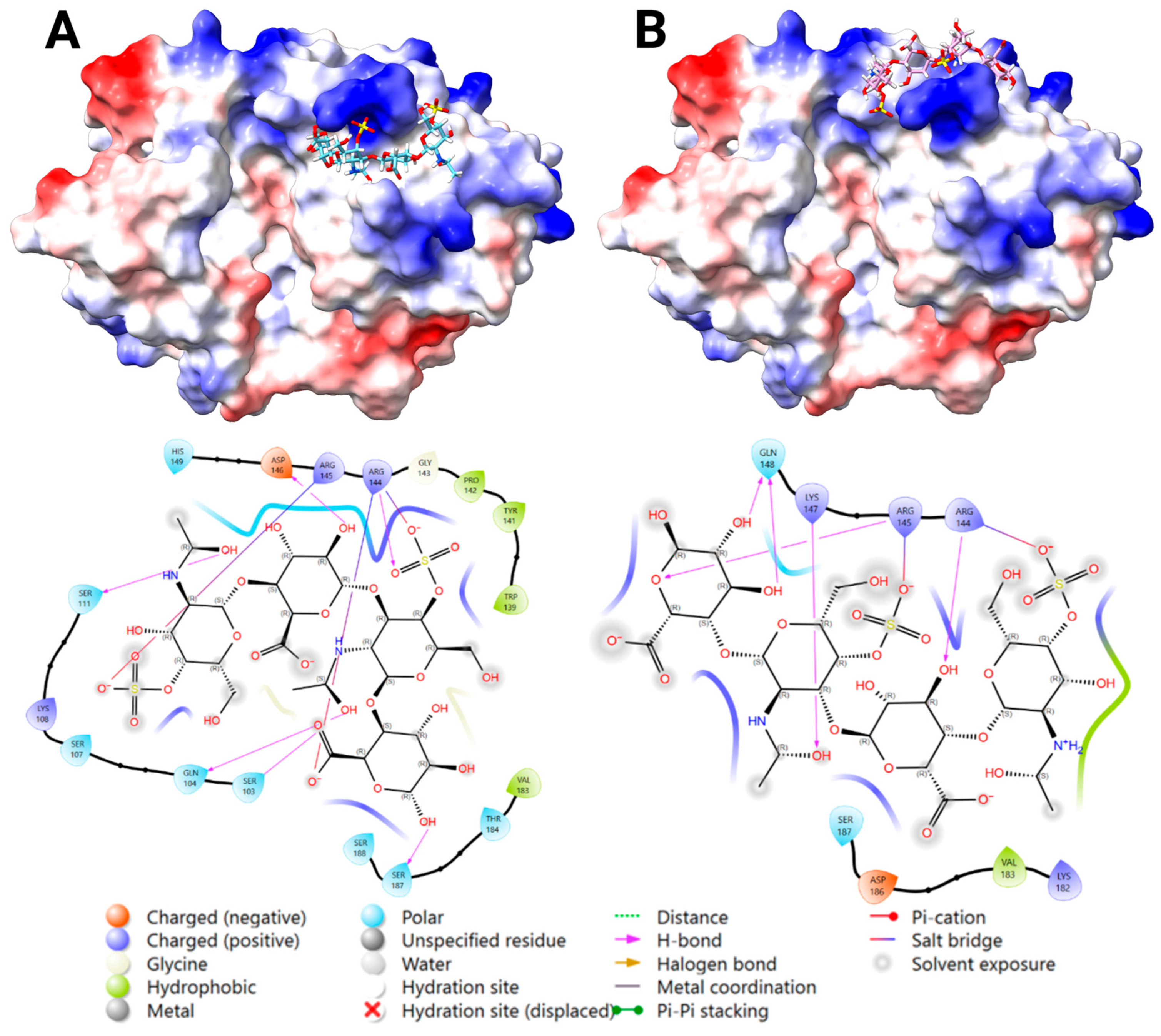
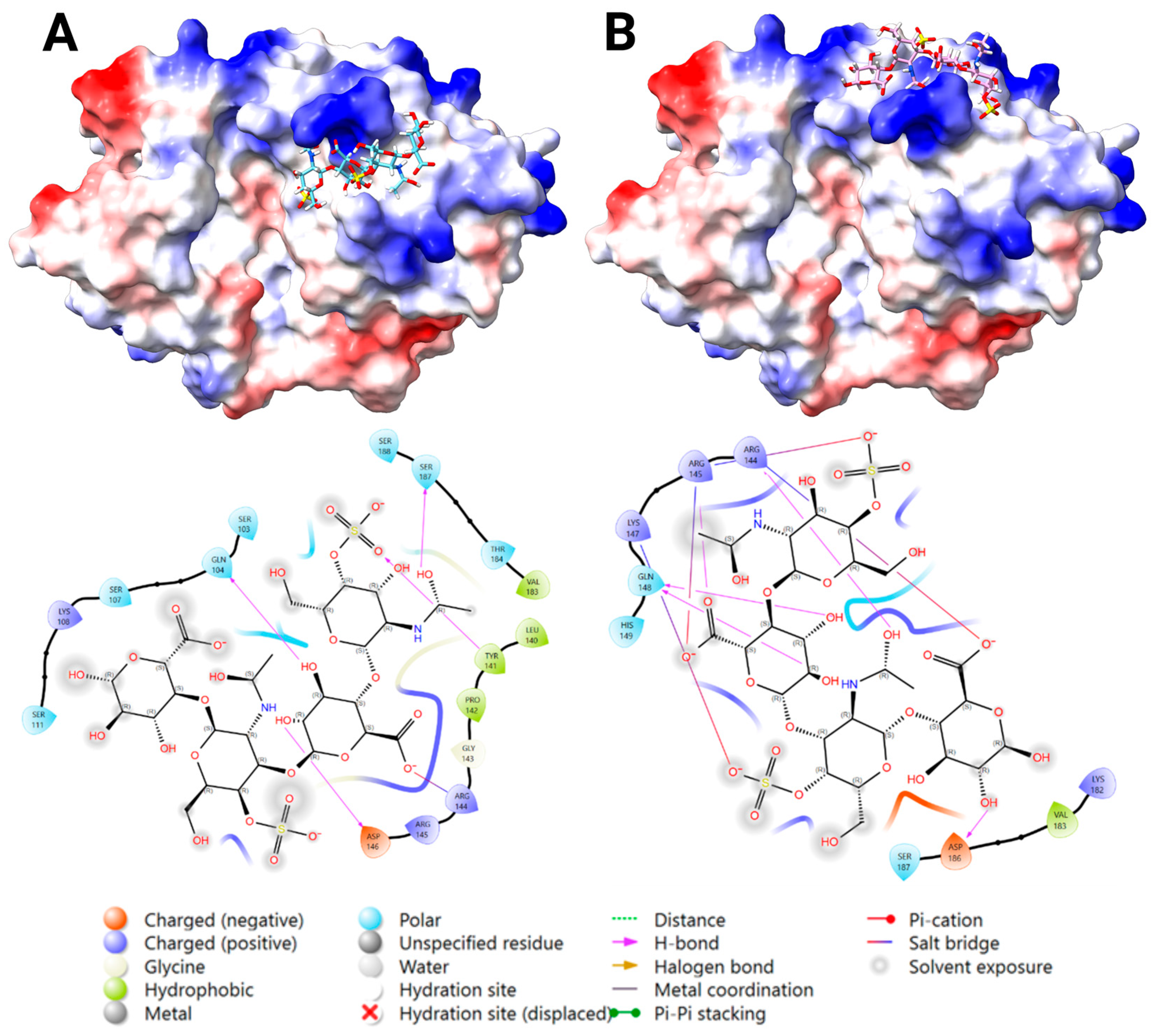
2.3.3. Docking Analysis—YKL-40 and DS
2.3.4. Docking Analysis—YKL-40 and CS
2.3.5. Docking Analysis—YKL-40 and HA
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Transfection and Production of YKL-40
4.3. YKL-40 Purification
4.4. GAG Ligand Selection for In Vitro Studies
4.5. Microscale Thermophoresis (MST)
4.6. Statistics, Data Fitting, and Graphics
4.7. In Silico Molecular Docking Analysis
4.7.1. GAG Ligand Construction
4.7.2. AutoDock Vina Docking
4.7.3. Glide Docking
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GAG | Glycosaminoglycans |
| MST | Microscale Thermophoresis |
| Hep | Heparin |
| HS | Heparan sulfate |
| DS | Dermatan sulfate |
| CS | Chondroitin sulfate |
| KS | Keratan sulfate |
| HA | Hyaluronan |
| GlcA | D-glucuronic acid |
| IdoA | L-iduronic acid |
| IdoA,2S | 2-sulfated iduronic acid |
| GlcNS | N-sulfated D-glucuronic acid |
| GlcNS,6S | 6-O-sulfated N-sulfated D-glucuronic acid |
| GlcNAc | N-acetyl D-glucosamine |
| GalNAc | N-acetyl D-galactosamine |
| GalNAc,4S | 4-O-sulfated N-acetyl D-galactosamine |
| DP | Degree of polarization |
| ChOS | Chitooligosaccharides |
| FAK | Focal adhesion kinase |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| PI3K | Phosphoinositide 3-kinase |
| AKT | Protein kinase B |
| ECM | Extracellular Matrix |
| PGs | Proteoglycans |
| HEK293T | Human embryonic kidney cells 293T |
| LID | Ligand-Interacting Diagrams |
References
- Fusetti, F.; Pijning, T.; Kalk, K.H.; Bos, E.; Dijkstra, B.W. Crystal structure and carbohydrate-binding properties of the human cartilage glycoprotein-39. J. Biol. Chem. 2003, 278, 37753–37760. [Google Scholar] [CrossRef]
- Houston, D.R.; Recklies, A.D.; Krupa, J.C.; van Aalten, D.M.F. Structure and ligand-induced conformational change of the 39-KDa glycoprotein from human articular chondrocytes. J. Biol. Chem. 2003, 278, 30206–30212. [Google Scholar] [CrossRef]
- Einarsson, J.M.; Bahrke, S.; Sigurdsson, B.T.; Ng, C.-H.; Petersen, P.H.; Sigurjonsson, O.E.; Jonsson, H.; Gislason, J.; Thormodsson, F.R.; Peter, M.G. Partially acetylated chitooligosaccharides bind to YKL-40 and stimulate growth of human osteoarthritic chondrocytes. Biochem. Biophys. Res. Commun. 2013, 434, 298–304. [Google Scholar] [CrossRef]
- Magnusdottir, U.; Thormodsson, F.R.; Kjalarsdottir, L.; Filippusson, H.; Gislason, J.; Oskarsson, K.R.; Hjorleifsson, J.G.; Einarsson, J.M. Heparin-binding of the human chitinase-like protein YKL-40 is allosterically modified by chitin oligosaccharides. Biochem. Biophys. Rep. 2025, 41, 101908. [Google Scholar] [CrossRef] [PubMed]
- Nyirkos, P.; E Golds, E. Human synovial cells secrete a 39 kDa protein similar to a bovine mammary protein expressed during the non-lactating period. Biochem. J. 1990, 269, 265–268. [Google Scholar] [CrossRef]
- Ngernyuang, N.; Yan, W.; Schwartz, L.M.; Oh, D.; Liu, Y.-B.; Chen, H.; Shao, R. A Heparin Binding Motif Rich in Arginine and Lysine is the Functional Domain of YKL-40. Neoplasia 2018, 20, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Kognole, A.A.; Payne, C.M. Inhibition of mammalian glycoprotein YKL-40 identification of the physiological ligand. J. Biol. Chem. 2017, 292, 2624–2636. [Google Scholar] [CrossRef]
- Francescone, R.A.; Scully, S.; Faibish, M.; Taylor, S.L.; Oh, D.; Moral, L.; Yan, W.; Bentley, B.; Shao, R. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J. Biol. Chem. 2011, 286, 15332–15343. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xue, Q.; Zhao, Y.; Zheng, J. The effects of YKL-40 on angiogenic potential of HUVECs are partly mediated by syndecan-4. Int. J. Med. Sci. 2021, 18, 3759–3767. [Google Scholar] [CrossRef]
- Geng, B.; Pan, J.; Zhao, T.; Ji, J.; Zhang, C.; Che, Y.; Yang, J.; Shi, H.; Li, J.; Zhou, H.; et al. Chitinase 3-like 1-CD44 interaction promotes metastasis and epithelial-to-mesenchymal transition through β-catenin/Erk/Akt signaling in gastric cancer. J. Exp. Clin. Cancer Res. 2018, 37, 208. [Google Scholar] [CrossRef]
- Brunner, S.M.; Schiechl, G.; Kesselring, R.; Martin, M.; Balam, S.; Schlitt, H.J.; Geissler, E.K.; Fichtner-Feigl, S. IL-13 signaling via IL-13Rα2 triggers TGF-β1-dependent allograft fibrosis. Transplant. Res. 2013, 2, 16. [Google Scholar] [CrossRef]
- He, C.H.; Lee, C.G.; Cruz, C.S.D.; Lee, C.-M.; Zhou, Y.; Ahangari, F.; Ma, B.; Herzog, E.L.; Rosenberg, S.A.; Li, Y.; et al. Chitinase 3-like 1 regulates cellular and tissue responses via il-13 receptor α2. Cell Rep. 2013, 4, 830–841. [Google Scholar] [CrossRef]
- Lee, C.-M.; He, C.H.; Nour, A.M.; Zhou, Y.; Ma, B.; Park, J.W.; Kim, K.H.; Cruz, C.D.; Sharma, L.; Nasr, M.L.; et al. IL-13Rα2 uses TMEM219 in chitinase 3-like-1-induced signalling and effector responses. Nat. Commun. 2016, 7, 12752. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Z.; Zhu, B.; Jia, Z.; Luo, J.; Han, X.; Chen, H.; Shao, R. A humanized Anti-YKL-40 antibody inhibits tumor development. Biochem. Pharmacol. 2024, 225, 116335. [Google Scholar] [CrossRef]
- Tizaoui, K.; Yang, J.W.; Lee, K.H.; Kim, J.H.; Kim, M.; Yoon, S.; Jung, Y.; Park, J.B.; An, K.; Choi, H.; et al. The role of YKL-40 in the pathogenesis of autoimmune diseases: A comprehensive review. Int. J. Biol. Sci. 2022, 18, 3731–3746. [Google Scholar] [CrossRef]
- Specjalski, K.; Romantowski, J.; Niedoszytko, M. YKL-40 as a possible marker of neutrophilic asthma. Front. Med. 2023, 10, 1115938. [Google Scholar] [CrossRef]
- Yeo, I.J.; Lee, C.-K.; Han, S.-B.; Yun, J.; Hong, J.T. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol. Ther. 2019, 203, 107394. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Yester, J.W.; Singh, S.K.; Biswas, D.D.; Surace, M.J.; Waters, M.R.; Hauser, K.F.; Yao, Z.; Boyce, B.F.; Kordula, T. RelB/p50 Complexes Regulate Cytokine-Induced YKL-40 Expression. J. Immunol. 2015, 194, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Lin, R.; Jin, R.; Lu, L.; Liu, X.; Hu, S.; Sun, L. Chitinase-like protein YKL-40 regulates human bronchial epithelial cells proliferation, apoptosis, and migration through TGF-β1/Smads pathway. Hum. Exp. Toxicol. 2020, 39, 451–463. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, Z.; Liu, B.; Li, X.; Li, G.; Yang, F.; Tang, H. YKL-40 mediates airway remodeling in asthma via activating FAK and MAPK signaling pathway. Cell Cycle 2020, 19, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, R.A.; García-Palmero, I.; Torres, S.; López-Lucendo, M.; Balyasnikova, I.V.; Casal, J.I. IL13 receptor α2 signaling requires a scaffold protein, FAM120A, to activate the FAK and PI3K pathways in colon cancer metastasis. Cancer Res. 2015, 75, 2434–2444. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Immunostimulatory Effects of Chitooligosaccharides on RAW 264.7 Mouse Macrophages via Regulation of the MAPK and PI3K/Akt Signaling Pathways. Mar. Drugs 2019, 17, 36. [Google Scholar] [CrossRef]
- Shao, R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front. Physiol. 2013, 4, 122. [Google Scholar] [CrossRef]
- Shao, R.; Hamel, K.; Petersen, L.; Cao, Q.J.; Arenas, R.B.; Bigelow, C.; Bentley, B.; Yan, W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 2009, 28, 4456–4468. [Google Scholar] [CrossRef]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.-J.; He, C.-H.; Takyar, S.; Elias, J.A. Role of Chitin and Chitinase/Chitinase-Like Proteins in Inflammation, Tissue Remodeling, and Injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Ghonim, M.A.; Boyd, D.F.; Flerlage, T.; Thomas, P.G. Pulmonary inflammation and fibroblast immunoregulation: From bench to bedside. J. Clin. Investig. 2023, 133, e170499. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Wang, D.; Liu, S.; Ma, Y.; Fan, H. Can YKL-40 be used as a biomarker and therapeutic target for adult asthma? Eur. Respir. J. 2018, 51, 1702194. [Google Scholar] [CrossRef]
- Sutherland, T.E. Chitinase-like proteins as regulators of innate immunity and tissue repair: Helpful lessons for asthma? Biochem. Soc. Trans. 2018, 46, 141–151. [Google Scholar] [CrossRef]
- Junker, N.; Johansen, J.S.; Andersen, C.B.; Kristjansen, P.E. Expression of YKL-40 by peritumoral macrophages in human small cell lung cancer. Lung Cancer 2005, 48, 223–231. [Google Scholar] [CrossRef]
- Ochman, B.; Mielcarska, S.; Kula, A.; Dawidowicz, M.; Robotycka, J.; Piecuch, J.; Szrot, M.; Dzięgielewska-Gęsiak, S.; Muc-Wierzgoń, M.; Waniczek, D.; et al. Do Elevated YKL-40 Levels Drive the Immunosuppressive Tumor Microenvironment in Colorectal Cancer? Assessment of the Association of the Expression of YKL-40, MMP-8, IL17A, and PD-L1 with Coexisting Type 2 Diabetes, Obesity, and Active Smoking. Curr. Issues Mol. Biol. 2023, 45, 2781–2797. [Google Scholar] [CrossRef]
- Böckelmann, L.C.; Felix, T.; Calabrò, S.; Schumacher, U. YKL-40 protein expression in human tumor samples and human tumor cell line xenografts: Implications for its use in tumor models. Cell. Oncol. 2021, 44, 1183–1195. [Google Scholar] [CrossRef]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Target. Ther. 2020, 5, 201. [Google Scholar] [CrossRef]
- Chang, M.-C.; Chen, C.-T.; Chiang, P.-F.; Chiang, Y.-C. The Role of Chitinase-3-like Protein-1 (YKL40) in the Therapy of Cancer and Other Chronic-Inflammation-Related Diseases. Pharmaceuticals 2024, 17, 307. [Google Scholar] [CrossRef]
- De Robertis, M.; Greco, M.R.; Cardone, R.A.; Mazza, T.; Marzano, F.; Mehterov, N.; Kazakova, M.; Belev, N.; Tullo, A.; Pesole, G.; et al. Upregulation of YKL-40 Promotes Metastatic Phenotype and Correlates with Poor Prognosis and Therapy Response in Patients with Colorectal Cancer. Cells 2022, 11, 3568. [Google Scholar] [CrossRef]
- Yu, J.E.; Yeo, I.J.; Han, S.-B.; Yun, J.; Kim, B.; Yong, Y.J.; Lim, Y.-S.; Kim, T.H.; Son, D.J.; Hong, J.T. Significance of chitinase-3-like protein 1 in the pathogenesis of inflammatory diseases and cancer. Exp. Mol. Med. 2024, 56, 1–18. [Google Scholar] [CrossRef]
- Bojesen, S.E.; Johansen, J.S.; Nordestgaard, B.G. Plasma YKL-40 levels in healthy subjects from the general population. Clin. Chim. Acta 2011, 412, 709–712. [Google Scholar] [CrossRef]
- Su, P.-C.; Chen, C.-Y.; Yu, M.-H.; Kuo, I.-Y.; Yang, P.-S.; Hsu, C.-H.; Hou, Y.-C.; Hsieh, H.-T.; Chang, C.-P.; Shan, Y.-S.; et al. Fully human chitinase-3 like-1 monoclonal antibody inhibits tumor growth, fibrosis, angiogenesis, and immune cell remodeling in lung, pancreatic, and colorectal cancers. Biomed. Pharmacother. 2024, 176, 116825. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Francescone, R.; Ngernyuang, N.; Bentley, B.; Taylor, S.L.; Moral, L.; Yan, W. Anti-YKL-40 antibody and ionizing irradiation synergistically inhibit tumor vascularization and malignancy in glioblastoma. Carcinogenesis 2013, 35, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, H.; Sun, H.; Peng, X.; Tang, C.; Gan, Y.; Chen, X.; Mathur, A.; Hu, B.; Slade, M.D.; et al. Chitinase 3–like 1 suppresses injury and promotes fibroproliferative responses in mammalian lung fibrosis. Sci. Transl. Med. 2014, 6, 240ra76. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Vivès, R.R.; Schaefer, L.; Götte, M.; Merline, R.; Passi, A.; Heldin, P.; Magalhães, A.; Reis, C.A.; Skandalis, S.S.; et al. A biological guide to glycosaminoglycans: Current perspectives and pending questions. FEBS J. 2024, 291, 3331–3366. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.R.; Rudisill, J.A.; Gallo, R.L. Structural and sequence motifs in dermatan sulfate for promoting fibroblast growth factor-2 (FGF-2) and FGF-7 activity. J. Biol. Chem. 2005, 280, 5300–5306. [Google Scholar] [CrossRef]
- Wei, J.; Hu, M.; Huang, K.; Lin, S.; Du, H. Roles of proteoglycans and glycosaminoglycans in cancer development and progression. Int. J. Mol. Sci. 2020, 21, 5983. [Google Scholar] [CrossRef] [PubMed]
- Orlińska, K.; Komosińska-Vassev, K.; Olczyk, K.; Kowalczyk, A.; Olczyk, P. Glycosaminoglycans—Types, structure, functions, and the role in wound healing processes. Ann. Acad. Medicae Silesiensis 2023, 77, 204–216. [Google Scholar] [CrossRef]
- Silbert, J.E.; Sugumaran, G. Biosynthesis of Chondroitin/Dermatan Sulfate. IUBMB Life 2002, 54, 177–186. [Google Scholar] [CrossRef]
- Shi, D.; Sheng, A.; Chi, L. Glycosaminoglycan-Protein Interactions and Their Roles in Human Disease. Front. Mol. Biosci. 2021, 8, 639666. [Google Scholar] [CrossRef]
- Tykesson, E.; Maccarana, M.; Thorsson, H.; Liu, J.; Malmström, A.; Ellervik, U.; Westergren-Thorsson, G. Recombinant dermatan sulfate is a potent activator of heparin cofactor II-dependent inhibition of thrombin. Glycobiology 2019, 29, 446–451. [Google Scholar] [CrossRef]
- Mizumoto, S.; Fongmoon, D.; Sugahara, K. Interaction of chondroitin sulfate and dermatan sulfate from various biological sources with heparin-binding growth factors and cytokines. Glycoconj. J. 2013, 30, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Yang, Y.W.; Yanagishita, M.; Rechler, M.M. Heparin Inhibition of Insulin-Like Growth Factor-Binding Protein-3 Binding to Human Fibroblasts and Rat Glioma Cells: Role of Heparan Sulfate Proteoglycans. Endocrinology 1996, 137, 4363–4371. [Google Scholar] [CrossRef][Green Version]
- Mizumoto, S.; Yamada, S. The Specific Role of Dermatan Sulfate as an Instructive Glycosaminoglycan in Tissue Development. Int. J. Mol. Sci. 2022, 23, 7485. [Google Scholar] [CrossRef]
- Johansen, J.S. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan. Med. Bull. 2006, 53, 172–209. [Google Scholar] [PubMed]
- Clark, R.A.; Lin, F.; Greiling, D.; An, J.; Couchman, J.R. Fibroblast Invasive Migration into Fibronectin/Fibrin Gels Requires a Previously Uncharacterized Dermatan Sulfate-CD44 Proteoglycan. J. Investig. Dermatol. 2004, 122, 266–277. [Google Scholar] [CrossRef]
- Koźma, E.M.; Wisowski, G.; Olczyk, K. Platelet derived growth factor BB is a ligand for dermatan sulfate chain(s) of small matrix proteoglycans from normal and fibrosis affected fascia. Biochimie 2009, 91, 1394–1404. [Google Scholar] [CrossRef]
- Mizumoto, S.; Kosho, T.; Yamada, S.; Sugahara, K. Pathophysiological significance of dermatan sulfate proteoglycans revealed by human genetic disorders. Pharmaceuticals 2017, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Jyothsna, K.M.; Sarkar, P.; Jha, K.K.; Raghunathan, V.; Bhat, R. Differential levels of dermatan sulfate generate distinct Collagen I gel architectures. bioRxiv 2020. [Google Scholar] [CrossRef]
- Jyothsna, K.M.; Sarkar, P.; Jha, K.K.; Krishna, A.S.L.; Raghunathan, V.; Bhat, R. A biphasic response of polymerized Type 1 collagen architectures to dermatan sulfate. J. Biomed. Mater. Res. Part A 2021, 109, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Kuwajima, M.; Sukeno, A.; Ishimaru, N.; Hayashi, Y.; Wabitsch, M.; Mizusawa, N.; Itakura, M.; Yoshimoto, K. YKL-40 secreted from adipose tissue inhibits degradation of type I collagen. Biochem. Biophys. Res. Commun. 2009, 388, 511–516. [Google Scholar] [CrossRef]
- Bigg, H.F.; Wait, R.; Rowan, A.D.; Cawston, T.E. The mammalian chitinase-like lectin, ykl-40, binds specifically to type i collagen and modulates the rate of type I collagen fibril formation. J. Biol. Chem. 2006, 281, 21082–21095. [Google Scholar] [CrossRef]
- Thelin, M.A.; Svensson, K.J.; Shi, X.; Bagher, M.; Axelsson, J.; Isinger-Ekstrand, A.; van Kuppevelt, T.H.; Johansson, J.; Nilbert, M.; Zaia, J.; et al. Dermatan sulfate is involved in the tumorigenic properties of esophagus squamous cell carcinoma. Cancer Res. 2012, 72, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Jha, K.K.; Jyothsna, K.M.; Kumar, R.V.; Raghunathan, V.; Bhat, R. Iduronate-2-Sulfatase-Regulated dermatan sulfate levels potentiate the invasion of breast cancer epithelia through collagen matrix. J. Clin. Med. 2019, 8, 1562. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, Z.; Luo, Y.; Luo, Q.; Fan, J. Knockdown of YKL-40 inhibits angiogenesis through regulation of VEGF/VEGFR2 and ERK1/2 signaling in endometrial cancer. Cell Biol. Int. 2021, 45, 2557–2566. [Google Scholar] [CrossRef]
- Areshkov, P.O.; Avdieiev, S.S.; Balynska, O.V.; Leroith, D.; Kavsan, V.M. Two Closely Related Human Members of Chitinase-like Family, CHI3L1 and CHI3L2, Activate ERK1/2 in 293 and U373 Cells but Have the Different In- fluence on Cell Proliferation. Int. J. Biol. Sci. 2012, 8, 39. [Google Scholar] [CrossRef]
- Hao, H.; Wang, L.; Chen, H.; Xie, L.; Bai, T.; Liu, H.; Wang, D. YKL-40 promotes the migration and invasion of prostate cancer cells by regulating epithelial mesenchymal transition. Am. J. Transl. Res. 2017, 9, 3749–3757. [Google Scholar]
- Rasente, R.Y.; Egitto, P.; Calabrese, G.C. Low molecular mass dermatan sulfate modulates endothelial cells proliferation and migration. Carbohydr. Res. 2012, 356, 233–237. [Google Scholar] [CrossRef]
- Day, A.J. Hyaluronan-protein interactions: Lilliput revisited. Proteoglycan Res. 2024, 2, e70007. [Google Scholar] [CrossRef]
- Vuorio, J.; Vattulainen, I.; Martinez-Seara, H. Atomistic fingerprint of hyaluronan–CD44 binding. PLoS Comput. Biol. 2017, 13, e1005663. [Google Scholar] [CrossRef]
- Vaynberg, J.; Qin, J. Weak protein–protein interactions as probed by NMR spectroscopy. Trends Biotechnol. 2006, 24, 22–27. [Google Scholar] [CrossRef] [PubMed]
- NanoTemper Technologies. Inc. MO-L018 His-Tag Labeling Kit RED-tris-NTA 2nd Generation. Version 003. Available online: https://support.nanotempertech.com/hc/en-us/articles/18013589922193-MO-L018-His-Tag-Labeling-Kit-RED-tris-NTA-2nd-Generation (accessed on 25 August 2025).
- Grant, O.C.; Wentworth, D.; Holmes, S.G.; Kandel, R.; Sehnal, D.; Wang, X.; Xiao, Y.; Sheppard, P.; Grelsson, T.; Coulter, A.; et al. Generating 3D Models of Carbohydrates with GLYCAM-Web. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]

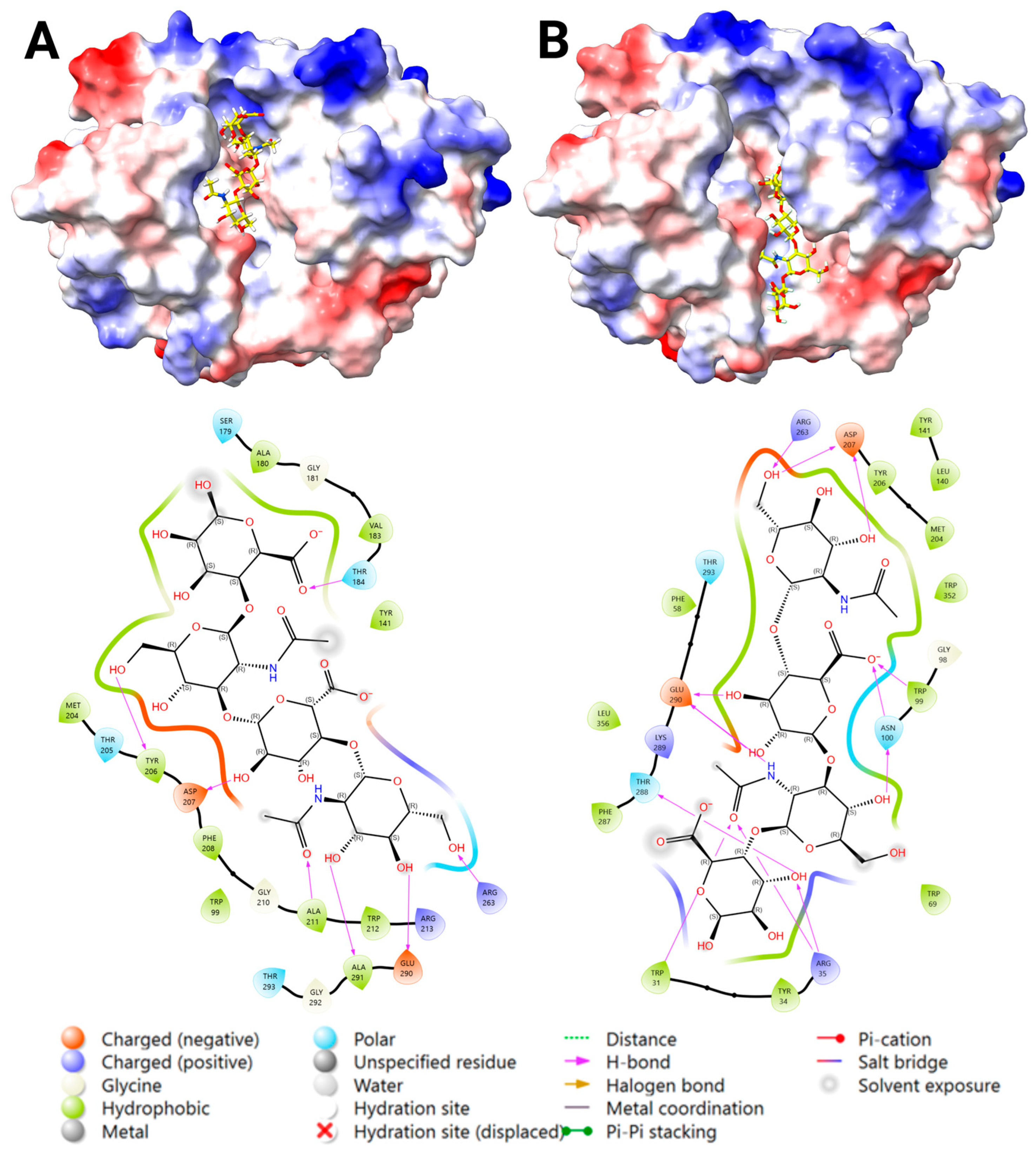

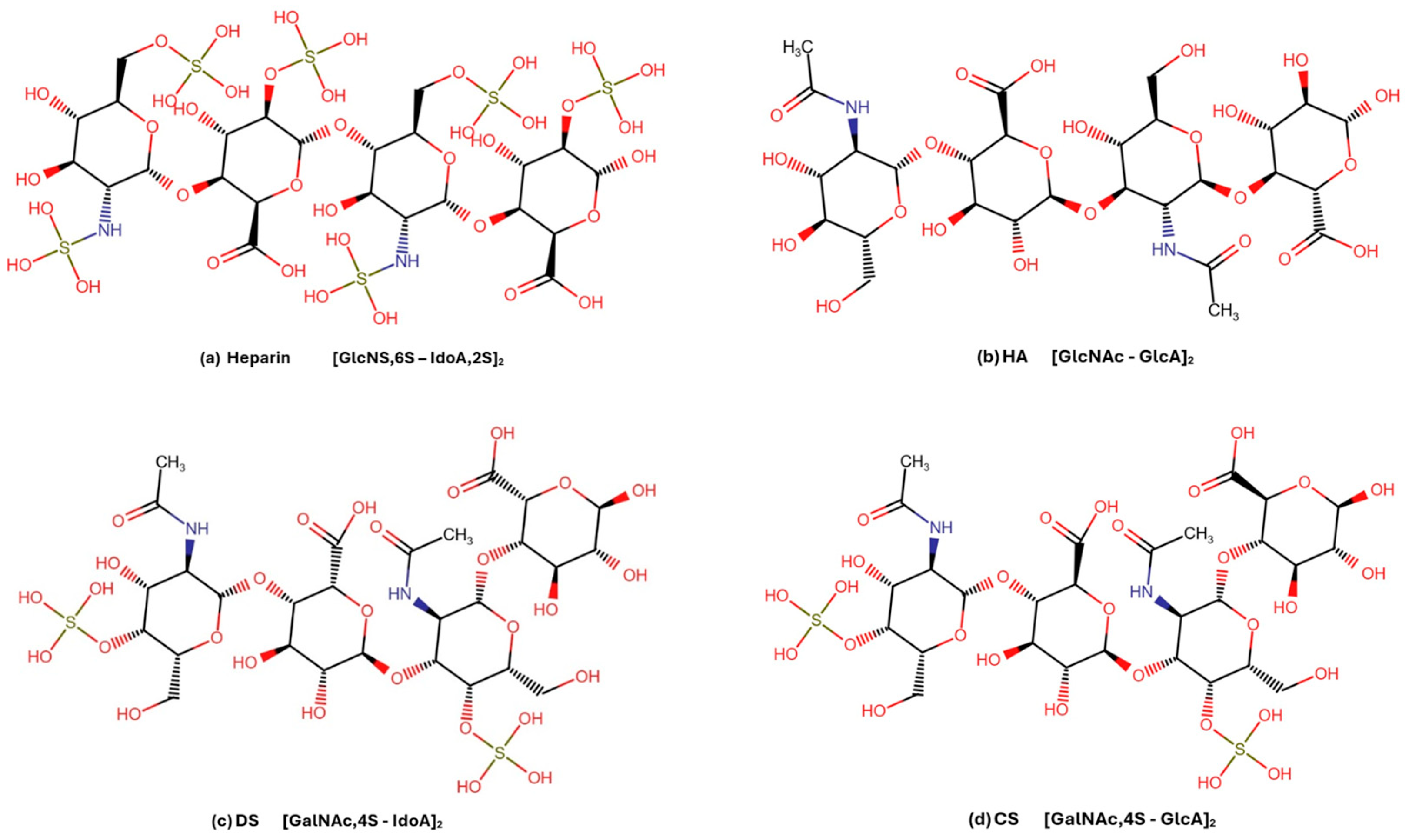
| Ligand | Main Chemical Components | Predicted Binding Site | Binding Likelihood | Justification for Binding |
|---|---|---|---|---|
| Chitin | GlcNAc (β1→4) GlcNAc | Chitin-binding site. Multiple hydrogen bonds formed and hydrophobic interactions with conserved residues in the chitin-binding groove. | Known ligand | Established experimentally; numerous crystallized structures available [1,2,3,4]. |
| Heparin | IdoA,2S (α1→4) GlcNS (α1→4) | Heparin-binding site (positively charged surface area). Highly sulfated; too negatively charged for tight chitin-binding site fit; interactions via electrostatic interactions and hydrogen bonding. | Known ligand | Binding to positively charged surface area (location not confirmed) due to high negative charge; observed binding in multiple studies; is used in YKL-40 purification processes [4,6] |
| Hyaluronan | GlcA (β1→3) GlcNAc (β1→4) | Likely chitin-binding site. Structurally similar to chitin; includes GlcNAc, negatively charged; fits chitin groove lined with polar and aromatic residues. | High | Strong hydrogen bonding and possibly some stacking compatibility to chitin-binding site; favored in simulations [7]. |
| Heparan sulfate | GlcA/IdoA (α1→4) GlcNAc/NS | Likely heparin-binding site (positively charged surface area). Similar to heparin but with lower sulfation; structural heterogeneity. | Moderate | Structurally similar to heparin, moderate binding predicted due to lower sulfation [7,8,9]. |
| Chondroitin sulfate | GlcA (β1→3) GalNAc,4S/6S (β1→4) | Likely heparin-binding site (positively charged surface area). Moderate negative charge; sulfate-mediated contacts possible. | Moderate | Plausible ionic- and hydrogen bonding but less favorable fit than heparin due to lower sulfation pattern and lower flexibility; binding supported by modeling and literature evidence [7,49]. |
| Dermatan sulfate | IdoA (β1→3) GalNAc,4S (β1→4) | Likely heparin-binding site (positively charged surface area). More flexible than chondroitin sulfate due to iduronic acid; better fit for sulfate-mediated contacts. | Moderate | Slightly improved binding over chondroitin sulfate due to increased flexibility [7,49]. |
| Keratan sulfate | GlcNAc,6S (β1→3) Gal (β1→4) | Likely heparin-binding site (positively charged surface area); also compatible with chitin-binding site. Lacks uronic acid; fewer possible sulfation sites. | Moderate-Low | Moderate-low likelihood predicted due to lower degree of sulfation and less flexibility; not evaluated in any binding study. |
| GAG | Major Disaccharide Units | Mean Kd ± SD (μM) | p-Value | Notes |
|---|---|---|---|---|
| Hep DP12 | IdoA(2S)-GlcNS(6S) | 119 ± 36 | <0.0001 | High degree of sulfation, includes IdoA |
| Hep DP6 | IdoA(2S)-GlcNS(6S) | 234 ± 133 | 0.0037 | Shorter chain, still highly sulfated and contains IdoA |
| DS DP6 | IdoA-GalNAc(4S) | 269 ± 30 | <0.0001 | Lower degree of sulfation than heparin, contains IdoA. |
| HA DP6 | GlcA-GlcNAc | 1010 ± 244 | <0.0001 | No sulfate, no IdoA. Includes GlcNAc (same as chitin). |
| CS DP6 | GlcA-GalNAc(4S > 6S) | Not applicable | 0.5530 * | No significant binding. Lower degree of sulfation than heparin; does not contain IdoA. |
| GAG (Cat. No.) | General Formula (n = 2 for DP6, and n = 5 for DP12) | Minor Quantities |
|---|---|---|
| Heparin (HO06 and HO12) | UA,2S − (GlcNS,6S − IdoA,2S)n − GlcNS,6S | di-, mono-, and non-sulfated units |
| Dermatan sulfate (DSO06) | ∆UA − (GalNAc,4S − IdoA)n − GalNAc,4S | 6-sulfated and 2,4 di-sulfated units (5% and 7%, respectively) |
| Chondroitin sulfate (CSO06) | ∆UA − (GalNAc,4S * > 6S − GlcA)n − GalNAc,4S > 6S | GlcA,2S-GalNAc,6S (5%) |
| Hyaluronan (HA06) | ∆HexAβ1,3 − (GlcNAcβ1,4 GlcAβ1,3)n – GlcNAc | none |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnusdottir, U.; Yang Jonatansdottir, Y.; Oskarsson, K.R.; Hjorleifsson, J.G.; Einarsson, J.M.; Thormodsson, F.R. Characterization of YKL-40 Binding to Extracellular Matrix Glycosaminoglycans. Mar. Drugs 2025, 23, 379. https://doi.org/10.3390/md23100379
Magnusdottir U, Yang Jonatansdottir Y, Oskarsson KR, Hjorleifsson JG, Einarsson JM, Thormodsson FR. Characterization of YKL-40 Binding to Extracellular Matrix Glycosaminoglycans. Marine Drugs. 2025; 23(10):379. https://doi.org/10.3390/md23100379
Chicago/Turabian StyleMagnusdottir, Unnur, Yiming Yang Jonatansdottir, Kristinn R. Oskarsson, Jens G. Hjorleifsson, Jon M. Einarsson, and Finnbogi R. Thormodsson. 2025. "Characterization of YKL-40 Binding to Extracellular Matrix Glycosaminoglycans" Marine Drugs 23, no. 10: 379. https://doi.org/10.3390/md23100379
APA StyleMagnusdottir, U., Yang Jonatansdottir, Y., Oskarsson, K. R., Hjorleifsson, J. G., Einarsson, J. M., & Thormodsson, F. R. (2025). Characterization of YKL-40 Binding to Extracellular Matrix Glycosaminoglycans. Marine Drugs, 23(10), 379. https://doi.org/10.3390/md23100379







