Phytochemistry and Pharmacological Potential of the Mangrove Plant Sonneratia caseolaris: A Comprehensive Review
Abstract
1. Introduction
2. Components of Sonneratia caseolaris
2.1. Phenolic Compounds
2.1.1. Phenolic Acids and Derivatives
2.1.2. Flavonoids
2.1.3. Tannins
2.1.4. Other Phenolic Compounds
2.2. Terpenoids and Steroids
2.3. Fatty Acids and Derivatives, Fatty Alcohols, and Fatty Aldehydes
2.4. Hydrocarbons, Polysaccharides, and Other Constituents
3. Biological Activities
3.1. Safety and General Toxicity
3.1.1. Acute and Subacute Toxicological Screening
3.1.2. Brine Shrimp Lethality Assay
3.2. Antimicrobial and Antiparasitic Activities
3.2.1. Antibacterial and Antifungal Activity
3.2.2. Anthelmintic Activity
3.2.3. Antiplasmodial Assay
3.3. Antioxidant Activity
3.3.1. DPPH Scavenging Activity
3.3.2. ABTS Scavenging Activity
3.3.3. Reducing Power and Ferric-Reducing Antioxidant Power (FRAP)
3.3.4. Hydrogen Peroxide and Superoxide Scavenging
3.3.5. Metal-Chelation Assay
3.4. Anti-Inflammatory and Analgesic Effects
3.4.1. Anti-Inflammatory Activity
3.4.2. Analgesic Activity
3.5. Central Nervous System (CNS) Modulation
3.5.1. CNS-Depressant Activity
3.5.2. Anticholinesterase Activity
3.6. Anticancer Activity
3.7. Metabolic Effects
3.7.1. Anti-Hyperglycemic Activity
3.7.2. Alpha-Amylase and Alpha-Glucosidase Inhibition
3.7.3. Hypocholesterolemic Effect
3.7.4. Anti-Obesity Activity
3.8. Antidiarrheal Activity
3.9. Other Activities
3.9.1. Thrombolytic and Coagulation Modulation
3.9.2. Steroid 5a-Reductase Inhibition
3.9.3. Anti-Allergic Activity
3.9.4. Antipyretic Activity
3.9.5. Anti-Arthritic Activity
3.9.6. Melanin Inhibition
3.9.7. Anti-Collagenase Activity
4. Challenges and Future Perspectives
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eong, O.J. Mangroves-a Carbon Source and Sink. Chemosphere 1993, 27, 1097–1107. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Jayatissa, L.P.; Di Nitto, D.; Bosire, J.O.; Seen, D.L.; Koedam, N. How Effective Were Mangroves as a Defence against the Recent Tsunami? Curr. Biol. 2005, 15, R443–R447. [Google Scholar] [CrossRef]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Cerri, F.; Louis, Y.D.; Fallati, L.; Siena, F.; Mazumdar, A.; Nicolai, R.; Zitouni, M.S.; Adam, A.S.; Mohamed, S.; Lavorano, S.; et al. Mangroves of the Maldives: A Review of Their Distribution, Diversity, Ecological Importance and Biodiversity of Associated Flora and Fauna. Aquat. Sci. 2024, 86, 1–23. [Google Scholar] [CrossRef]
- Cerri, F.; Santes, B.D.; Spena, F.; Salvioni, L.; Forcella, M.; Fusi, P.; Pagliari, S.; Stahl, H.; Galli, P.; Colombo, M.; et al. Phytochemical Profiling, Antioxidant Activity, and In Vitro Cytotoxic Potential of Mangrove Avicennia Marina. Pharmaceuticals 2025, 18, 1308. [Google Scholar] [CrossRef]
- Rates, S.M.K. Plants as Source of Drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, K. Bioprospecting Potential of Mangrove Resources. In Biotechnological Utilization of Mangrove Resources; Academic Press: Cambridge, MA, USA, 2020; pp. 225–241. [Google Scholar] [CrossRef]
- Audah, K.A.; Ettin, J.; Darmadi, J.; Azizah, N.N.; Anisa, A.S.; Hermawan, T.D.F.; Tjampakasari, C.R.; Heryanto, R.; Ismail, I.S.; Batubara, I. Indonesian Mangrove Sonneratia Caseolaris Leaves Ethanol Extract Is a Potential Super Antioxidant and Anti Methicillin-Resistant Staphylococcus Aureus Drug. Molecules 2022, 27, 8369. [Google Scholar] [CrossRef]
- Cerri, F.; Giustra, M.; Anadol, Y.; Tomaino, G.; Galli, P.; Labra, M.; Campone, L.; Colombo, M. Natural Products from Mangroves: An Overview of the Anticancer Potential of Avicennia Marina. Pharmaceutics 2022, 14, 2793. [Google Scholar] [CrossRef]
- Patra, J.K.; Thatoi, H.N. Metabolic Diversity and Bioactivity Screening of Mangrove Plants: A Review. Acta. Physiol. Plant. 2011, 33, 1051–1061. [Google Scholar] [CrossRef]
- Parthiban, A.; Sivasankar, R.; Sachithanandam, V.; Khan, S.A.; Jayshree, A.; Murugan, K.; Sridhar, R. An Integrative Review on Bioactive Compounds from Indian Mangroves for Future Drug Discovery. S. Afr. J. Bot. 2022, 149, 899–915. [Google Scholar] [CrossRef]
- Kathiresan, K.; Bingham, B.L. Biology of Mangroves and Mangrove Ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, T.T.; Nguyen, H.N.; Bui, Q.T.P. Analysis of Active Compounds and Bioactivity of Leaves Extracts of Sonneratia Species. Eng. Rep. 2024, 6, e12870. [Google Scholar] [CrossRef]
- Bandaranayake, W.M. Traditional and Medicinal Uses of Mangroves. Mangroves Salt Marshes 1998, 2, 133–148. [Google Scholar] [CrossRef]
- Ghalib, R.M.; Hashim, R.; Sulaiman, O.; Awalludin, M.F.B.; Mehdi, S.H.; Kawamura, F. Fingerprint Chemotaxonomic GC–TOFMS Profile of Wood and Bark of Mangrove Tree Sonneratia caseolaris (L.) Engl. J. Saudi Chem. Soc. 2011, 15, 229–237. [Google Scholar] [CrossRef]
- Dev, S.; Acharyya, R.N.; Akter, S.; Al Bari, M.A.; Asma, K.; Hossain, H.; Sarkar, K.K.; Biswas, N.N.; Das, A.K. Toxicological Screening and Evaluation of Anti-Allergic and Anti-Hyperglycemic Potential of Sonneratia Caseolaris (L.) Engl. Fruits Clin. Phytosci. 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Asha, K.; Mathew, S.; Lakshmanan, P. Flavonoids and Phenolic Compounds in Two Mangrove Species and Their Antioxidant Property. Indian J. Geo-Mar. Sci. 2012, 41, 259–264. [Google Scholar]
- Kundu, P.; Debnath, S.L.; Devnath, H.S.; Saha, L.; Sadhu, S.K. Analgesic, Anti-Inflammatory, Antipyretic, and In Silico Measurements of Sonneratia caseolaris (L.) Fruits from Sundarbans, Bangladesh. BioMed Res. Int. 2022, 2022, 1405821. [Google Scholar] [CrossRef]
- khanh Tran, T.; Ha, P.T.T.; Henry, R.J.; Nguyen, D.N.T.; Tuyen, P.T.; Liem, N.T. Polyphenol Contents, Gas Chromatography-Mass Spectrometry (GC–; MS) and Antibacterial Activity of Methanol Extract and Fractions of Sonneratia caseolaris Fruits from Ben Tre Province in Vietnam. J. Microbiol. Biotechnol. 2023, 34, 94. [Google Scholar] [CrossRef]
- Primavera, J.; Sadaba, R.; Lebata, M.; Hazel, J.; Altamirano, J. Handbook of Mangrove in the Philippines—Panay; SEAFDEC Aquaculture Department: Iloilo, Philippines, 2004. [Google Scholar]
- Sadhu, S.K.; Ahmed, F.; Ohtsuki, T.; Ishibashi, M. Flavonoids from Sonneratia caseolaris. J. Nat. Med. 2006, 60, 264–265. [Google Scholar] [CrossRef]
- Tian, M.; Dai, H.; Li, X.; Wang, B. Chemical Constituents of Marine Medicinal Mangrove Plant Sonneratia Caseolaris. Chin. J. Oceanol. Limnol. 2009, 27, 288–296. [Google Scholar] [CrossRef]
- Yang, Y.; Duke, N.C.; Peng, F.; Li, J.; Yang, S.; Zhong, C.; Zhou, R.; Shi, S. Ancient Geographical Barriers Drive Differentiation among Sonneratia Caseolaris Populations and Recent Divergence from S. Lanceolata. Front. Plant Sci. 2016, 7, 226377. [Google Scholar] [CrossRef]
- Nabeelah Bibi, S.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Rengasamy Kannan, R.R.; Albuquerque, R.D.D.G.; Pandian, S.K. Ethnopharmacology, Phytochemistry, and Global Distribution of Mangroves—A Comprehensive Review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef]
- Chowdhury, R.; Bhuia, M.S.; Al Hasan, M.S.; Hossain Snigdha, S.; Afrin, S.; Büsselberg, D.; Habtemariam, S.; Sönmez Gürer, E.; Sharifi-Rad, J.; Ahmed Aldahish, A.; et al. Anticancer Potential of Phytochemicals Derived from Mangrove Plants: Comprehensive Mechanistic Insights. Food Sci. Nutr. 2024, 12, 6174–6205. [Google Scholar] [CrossRef]
- Beniwal, D.; Dhull, S.S.; Gulia, V.; Rani, J. Avicennia: A mangrove genus unveiled through its phytochemistry, pharmacological, and ecological importance. Rend. Lincei 2024, 35, 907–929. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, H.; Wu, X.; Feng, Z.; Li, X.; Tavakoli, S.; Wu, K.; Deng, L.; Luo, H. Botany, traditional uses, phytochemistry, pharmacological activities, and toxicity of the mangrove plant Avicennia marina: A comprehensive review. Phytochem. Rev. 2025, 1–36. [Google Scholar] [CrossRef]
- Sarkar, P.; Ahnaf, T.R.; Rouf, R.; Shilpi, J.A.; Uddin, S.J. A Review on Bioactive Phytochemical Constituents and Pharmacological Activities of Aegiceras corniculatum: A Pharmaceutically Important Mangrove Plant. J. Chem. 2024, 2024, 9992568. [Google Scholar] [CrossRef]
- Fang, Z.; Sun, K.; Liu, X.; Zhou, H.; Song, W.; Chen, H.; Wei, S. Structural Characterization of Ellagitannin-Rich Fractions from Leaves of Three Sonneratia Species, and Their Antioxidant Activity and α-Amylase Inhibitory Effect and Mechanism. Int. J. Food Prop. 2019, 22, 1760–1772. [Google Scholar] [CrossRef]
- Latief, M. The Characterization of Active Compound of Pedada Magrove Plants (Sonneratia Caseolaris) Which HaveThePotential as Natural Antioxidants. J. of Chem. Nat. Resour. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Tiwari, A.; Gowri, P.M.; Svs, R. Oleanolic Acid-An α-Glucosidase Inhibitory and Antihyperglycemic Active Compound from the Fruits of Sonneratia Caseolaris. Open Access J. Med. Aromat. Plants. 2010, 1, 19–23. [Google Scholar]
- Jha, N.; Madasamy, S.; Prasad, P.; Lakra, A.K.; Esakkiraj, P.; Tilwani, Y.M.; Arul, V. Optimization and Physicochemical Characterization of Polysaccharide Purified from Sonneratia Caseolaris Mangrove Leaves: A Potential Antioxidant and Antibiofilm Agent. Appl. Biochem. Biotechnol. 2023, 195, 7832–7858. [Google Scholar] [CrossRef]
- Dahibhate, N.L.; Roy, U.; Kumar, K. Phytochemical Screening, Antimicrobial and Antioxidant Activities of Selected Mangrove Species. Curr. Bioact. Compd. 2018, 16, 152–163. [Google Scholar] [CrossRef]
- Dahibhate, N.L.; Kumar, D.; Kumar, K. Determination of Bioactive Polyphenols in Mangrove Species and Their In-Vitro Anti-Candida Activities by Ultra-High-Performance Liquid Chromatography—Electrospray Ionization—Tandem Mass Spectrometry (UPLC-ESI-MS/MS). Anal. Lett. 2021, 54, 608–624. [Google Scholar] [CrossRef]
- Wu, S.B.; Wen, Y.; Li, X.W.; Zhao, Y.; Zhao, Z.; Hu, J.F. Chemical Constituents from the Fruits of Sonneratia Caseolaris and Sonneratia Ovata (Sonneratiaceae). Biochem. Syst. Ecol. 2009, 37, 1–5. [Google Scholar] [CrossRef]
- Arung, E.; Kuspradini, H.; Kusuma, I.; Bang, T.H.; Yamashita, S.; Katakura, Y.; Shimizu, K. Undefined Effects of Isolated Compound from Sonneratia Caseolaris Leaf: A Validation of Traditional Utilization by Melanin Biosynthesis and Antioxidant Assays. J. Appl. Pharmaceut. Sci. 2015, 5, 39–43. [Google Scholar] [CrossRef]
- Simlai, A.; Rai, A.; Mishra, S.; Mukherjee, K.; Roy, A. Antimicrobial and Antioxidative Activities in the Bark Extracts of Sonneratia Caseolaris, a Mangrove Plant. Excli, J. 2014, 13, 997–1010. [Google Scholar]
- Kartikaningsih, H.; Fitriana, N.; Anggraeni, I.L.; Semedi, B.; Pertiwi Koentjoro, M. The Potential of Sonneratia Caseolaris Mangrove Leaves Extract as a Bioactive Food Ingredient Using Various Water Extract. F1000Res 2025, 13, 249. [Google Scholar] [CrossRef]
- Hogg, R.W.; Gillan, F.T. Fatty Acids, Sterols and Hydrocarbons in the Leaves from Eleven Species of Mangrove. Phytochemistry 1984, 23, 93–97. [Google Scholar] [CrossRef]
- Barman, A.K.; Ahmed, T.; Das, H.; Biswas, B.; Ali, M.S.; Acharyya, R.N.; Sarkar, K.K.; Dev, S. Evaluation of Antidiabetic Potential of Extract of Sonneratia Caseolaris (L.) Engl. Leaves against Alloxan-Induced Diabetes in Mice. Trop. J. Nat. Prod. Res. 2021, 5, 77–83. [Google Scholar] [CrossRef]
- Pagarra, H.; Rahman, R.A.; Hala, Y.; Esivan, S.M.M. Phytochemical Screening, Antimicrobial and Antioxidant Activity from Sonneratia Caseolaris Leaves Extract. J. Teknol. (Sci. Eng.) 2022, 84, 59–66. [Google Scholar] [CrossRef]
- Yoong, M.H.; Rozaina, T. Effects of Mangrove Apple (Sonneratia Caseolaris) Fruit Extract on Oxidative Stability of Palm Olein under Accelerated Storage. Food Res. 2021, 5, 461–470. [Google Scholar] [CrossRef]
- Munira, S.; Islam, A.; Islam, S.; Koly, S.F.; Nesa, L.; Muhit, A. Phytochemical Screening and Comparative Antioxidant Activities of Fractions Isolated from Sonneratia Caseolaris (Linn.) Bark Extracts. Eur. J. Med. Plants 2019, 28, 1–9. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Barreiro, M.F.; Ferreira, I.C.F.R.; McPhee, D.J. Hydroxycinnamic Acids and Their De-rivatives: Cosmeceutical Significance, Challenges and Future Perspectives, a review. Molecules 2017, 22, 281. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Kundu, P.; Debnath, S.L.; Ahad, M.F.; Devnath, H.S.; Saha, L.; Karmakar, U.K.; Sadhu, S.K. Exploration of In Vivo and In Vitro Biological Effects of Sonneratia Caseolaris (L.) Fruits Supported by Molecular Docking and ADMET Study. Biomed. Res. Int. 2023, 2023, 4522446. [Google Scholar] [CrossRef] [PubMed]
- Shamsuddin, A.A.; Najiah, M.; Suvik, A.; Azariyah, M.N.; Kamaruzzaman, B.Y.; Effendy, A.W.; John, B.A. Antibacterial properties of selected mangrove plants against Vibrio species and its cytotoxicity against Artemia salina. World Appl. Sci. J. 2013, 25, 333–340. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20133378401 (accessed on 6 September 2025).
- Bokshi, B.; Zilani, M.N.H.; Hossain, H.; Ahmed, M.I.; Anisuzzman, M.; Biswas, N.N.; Sadhu, S.K. Bioactivities of Sonneratia Caseolaris (Linn) Leaf and Stem Using Different Solvent Systems. Biomed. J. Sci. Tech. Res. 2020, 31, 24578–24582. [Google Scholar] [CrossRef]
- Hosen, M.Z.; Biswas, A.; Islam, M.R.; Hossain, S.J. Anti-Bacterial, Anti-Diarrheal, and Cytotoxic Activities of Edible Fruits in the Sundarbans Mangrove Forest of Bangladesh. Prev. Nutr. Food Sci. 2021, 26, 192. [Google Scholar] [CrossRef] [PubMed]
- Yompakdee, C.; Thunyaharn, S.; Phaechamud, T. Bactericidal Activity of Methanol Extracts of Crabapple Mangrove Tree (Sonneratia Caseolaris Linn.) Against Multi-Drug Resistant Pathogens. Indian J. Pharm. Sci. 2012, 74, 230. [Google Scholar] [CrossRef]
- Kaewpiboon, C.; Lirdprapamongkol, K.; Srisomsap, C.; Winayanuwattikun, P.; Yongvanich, T.; Puwaprisirisan, P.; Svasti, J.; Assavalapsakul, W. Studies of the in Vitro Cytotoxic, Antioxidant, Lipase Inhibitory and Antimicrobial Activities of Selected Thai Medicinal Plants. BMC Complement. Altern. Med. 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mahadlek, J.; Phachamud, T.; Wessapun, C. Antimicrobial Studies of Sonneratia Caseolaris Using Different Agar Diffusion Method. Res. J. Pharm. Biol. Che. Sci. 2012, 3, 404–410. [Google Scholar]
- Thuoc, D.V.; Mai, N.T.N.; Ha, L.T.V.; Hung, L.D.; Tra, D.H.; Hung, N.K.; Hung, N.P. Evaluation of Antibacterial, Antioxidant and Antiobese Activities of the Fruit Juice of Crabapple Mangrove Sonneratia Caseolaris (Linn.). Int. J. Agric. Nat. Resour. 2018, 5, 25–29. [Google Scholar]
- Ahmad, I.; Ambarwati, N.S.S.; Lukman, A.; Masruhim, M.A.; Rijai, L.; Mun’Im, A. In Vitro Antimicrobial Activity Evaluation of Mangrove Fruit (Sonneratia Caseolaris L.) Extract. Pharmacogn. J. 2018, 10, 598–601. [Google Scholar] [CrossRef]
- Halifah, P.; Hartati, H.; Rachmawaty, R.; Yusminah, H.; Roshanida, A.R. Phytochemical screening and antimicrobial activity from Sonneratia caseolaris fruit extract. Mater. Sci. Forum. 2019, 967, 28–33. [Google Scholar] [CrossRef]
- Muhaimin, M.; Latief, M.; Putri, R.D.; Chaerunisaa, A.Y.; Aditama, A.Y.; Pravitasari, N.E.; Siregar, J.E. Antiplasmodial Activity of Methanolic Leaf Extract of Mangrove Plants against Plasmodium Berghei. Pharmacogn. J. 2019, 11, 929–935. [Google Scholar] [CrossRef]
- Ahmed, F.; Shahid, I.; Razzak, M.; Rahman, M.M.; Hoque, T.; Rahman, M.; Sadhu, S. Free Radical Scavenging Activity of Some Mangroves Available in Bangladesh. Orient. Pharm. Exp. Med. 2006, 6, 58–64. [Google Scholar] [CrossRef]
- Hosen, M.Z.; Biswas, A.; Islam, M.R.; Nazrul, M.; Bhuiyan, I.; Hossain, S.J. Comparison of physicochemical and antioxidant properties of edible fruits in the sundarbans’ mangrove forest, Bangladesh. Bangladesh J. Bot. 2020, 49, 671–678. [Google Scholar] [CrossRef]
- Syamsul, E.S.; Umar, S.; Wahyuni, F.S.; Martien, R.; Hamidi, D. Anti-Aging Activity, In Silico Modeling and Molecular Docking from Sonneratia Caseolaris. Open Access Maced. J. Med. Sci. 2022, 10, 1471–1477. [Google Scholar] [CrossRef]
- Wetwitayaklung, P.; Limmatvapirat, C.; Phaechamud, T. Antioxidant and Anticholinesterase Activities in Various Parts of Sonneratia Caseolaris (L.). Indian J. Pharm. Sci. 2013, 75, 649. Available online: https://pubmed.ncbi.nlm.nih.gov/24591739/ (accessed on 6 September 2025).
- Munira, M.S.; Ahmed, S.N.; Islam, M.S.; Islam, M.S.; Nesa, M.L.; Kabir, M.H.; de la Cruz, C.V. Pharmacological Screening for CNS Depression, Analgesic and Anti-Inflammatory Potentials of Sonneratia Caseolaris (Linn.). J. Pharm. Res. Int. 2019, 29, 1–11. [Google Scholar] [CrossRef]
- Munira, M.S.; Islam, M.S.; Akther, N.; Koly, S.F. Estimation of Anti-Inflammatory, Analgesic and Thrombolytic Activities of Sonneratia Caseolaris Linn. (Family: Sonneratiaceae). J. Anal. Pharm. Res. 2019, 8, 20–23. [Google Scholar] [CrossRef]
- Ahmed, F.; Shahid, I.Z.; Baksi, B.; Sadhu, S.K. Antinociceptive and Antidiarrhoeal Activities of Sonneratia Caseolaris. Orient. Pharm. Exp. Med. 2007, 7, 274–279. [Google Scholar] [CrossRef]
- Bokshi, B.; Zilani, M.N.H.; Malakar, A.; Roy, D.N.; Shilpi, J.A.; Sadhu, S.K. Study of analgesic and antidiarrhoeal activities of Sonneratia Caseolaris (Linn.) leaf and stem using different solvent system. Indones. J. Pharm. 2013, 24, 253–258. [Google Scholar]
- Rahmatullah, M.; Azam, M.N.K.; Pramanik, S.; Rahman, S.; Jahan, R. Antihyperglycemic Activity Evaluation of Rhizomes of Curcuma Zedoaria (Christm.) Roscoe and Fruits of Sonneratia Caseolaris (L.) Engl. Int. J. PharmTech Res. 2012, 4, 125–129. [Google Scholar]
- Jariyah, J.; Azkiyah, L.L.; Widjanarko, S. Hypocholesterolemic Effect of Pedada (Sonneratiacaseolaris) Fruit Flour in Wistar Rats. Int. J. PharmTech Res. 2013, 4, 1619–1627. [Google Scholar]
- Chowdhury, S.; Chowdury, M.I.A.; Alam, M.N.; Kamal, A.H.M.; Alam, S.M.A.B.; Sultana, S.; Jahan, T. Comparative Study of Potential Thrombolytic and Anti-Arthritic Activities of Pterospermum Acerifolium and Sonneratia Caseolaris Leaves. Am. J. Clin. Exp. Med. 2015, 3, 228–232. [Google Scholar] [CrossRef][Green Version]
- Liu, J.; Ando, R.; Shimizu, K.; Hashida, K.; Makino, R.; Ohara, S.; Kondo, R. Steroid 5α-Reductase Inhibitory Activity of Condensed Tannins from Woody Plants. J. Wood Sci. 2008, 54, 68–75. [Google Scholar] [CrossRef]
- Syamsul, E.S.; Umar, S.; Wahyuni, S.F.; Martien, R.; Lestari, D.; Hamidi, D. HR_LCMS-based metabolite profiling, and anti-colagenase properties of ethanolic extract of pidada merah: Computational and in vitro study. Int. J. Appl. Pharm. 2023, 15, 34–38. [Google Scholar] [CrossRef]
- Jin, J.; Koroleva, O.A.; Gibson, T.; Swanston, J.; Maganj, J.; Zhang, Y.A.N.; Rowland, I.R.; Wagstaff, C. Analysis of Phytochemical Composition and Chemoprotective Capacity of Rocket (Eruca Sativa and Diplotaxis Tenuifolia) Leafy Salad Following Cultivation in Different Environments. J. Agric. Food. Chem. 2009, 57, 5227–5234. [Google Scholar] [CrossRef]
- Nugroho, R.Y.; Hendri, M.; Fauziyah; Putri, W.A.E.; Agussalim, A. Phytochemical Profile and Toxicity of Extracts from the Leaf of Avicennia Marina (Forssk.) Vierh. Collected in Mangrove Areas Affected by Port Activities. S. Afr. J. Bot. 2022, 150, 903–919. [Google Scholar] [CrossRef]
- Abd-Elgawad, A.M.; Elshamy, A.I.; Al-Rowaily, S.L.; El-Amier, Y.A. Habitat Affects the Chemical Profile, Allelopathy, and Antioxidant Properties of Essential Oils and Phenolic Enriched Extracts of the Invasive Plant Heliotropium Curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef]
- Wang, D.H.; Du, F.; Liu, H.Y.; Liang, Z.S. Drought Stress Increases Iridoid Glycosides Biosynthesis in the Roots of Scrophularia Ningpoensis Seedlings. J. Med. Plants Res. 2010, 4, 2691–2699. [Google Scholar] [CrossRef]
- Di Ferdinando, M.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 159–179. [Google Scholar] [CrossRef]
- Neugart, S.; Krumbein, A.; Zrenner, R. Influence of Light and Temperature on Gene Expression Leading to Accumulation of Specific Flavonol Glycosides and Hydroxycinnamic Acid Derivatives in Kale (Brassica Oleracea Var Sabellica). Front. Plant. Sci. 2016, 7, 181383. [Google Scholar] [CrossRef]
- Falahi, H.; Sharifi, M.; Maivan, H.Z.; Chashmi, N.A. Phenylethanoid Glycosides Accumulation in Roots of Scrophularia Striata as a Response to Water Stress. Environ. Exp. Bot. 2018, 147, 13–21. [Google Scholar] [CrossRef]
- Franzoni, G.; Trivellini, A.; Bulgari, R.; Cocetta, G.; Ferrante, A. Bioactive Molecules as Regulatory Signals in Plant Responses to Abiotic Stresses. In Plant Signaling Molecules, 1st ed.; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N., Eds.; Woodhead Publishing: Duxford, UK, 2019; Volume 1, pp. 169–182. [Google Scholar] [CrossRef]
- Sarri, E.; Termentzi, A.; Abraham, E.M.; Papadopoulos, G.K.; Baira, E.; Machera, K.; Loukas, V.; Komaitis, F.; Tani, E. Salinity Stress Alters the Secondary Metabolic Profile of M. Sativa, M. Arborea and Their Hybrid (Alborea). Int. J. Mol. Sci. 2021, 22, 4882. [Google Scholar] [CrossRef]
- More than Half of All Mangrove Ecosystems at Risk of Collapse by 2050, First Global Assessment Finds. Press Release | IUCN. Available online: https://iucn.org/press-release/202405/more-half-all-mangrove-ecosystems-risk-collapse-2050-first-global-assessment (accessed on 9 September 2025).
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Cerri, F.; Saliu, F.; Maggioni, D.; Montano, S.; Seveso, D.; Lavorano, S.; Zoia, L.; Gosetti, F.; Lasagni, M.; Orlandi, M.; et al. Cytotoxic Compounds from Alcyoniidae. An Overview of the Last 30 Years. Mar. Drugs 2022, 20, 134. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnology 2018, 16, 1–33. [Google Scholar] [CrossRef]
- Varunkumar, K.; Anusha, C.; Saranya, T.; Ramalingam, V.; Raja, S.; Ravikumar, V. Avicennia Marina Engineered Nanoparticles Induce Apoptosis in Adenocarcinoma Lung Cancer Cell Line through P53 Mediated Signaling Pathways. Process Biochem. 2020, 94, 349–358. [Google Scholar] [CrossRef]
- Tian, S.; Saravanan, K.; Mothana, R.A.; Ramachandran, G.; Rajivgandhi, G.; Manoharan, N. Anti-Cancer Activity of Biosynthesized Silver Nanoparticles Using Avicennia Marina against A549 Lung Cancer Cells through ROS/Mitochondrial Damages. Saudi J. Biol. Sci. 2020, 27, 3018–3024. [Google Scholar] [CrossRef] [PubMed]
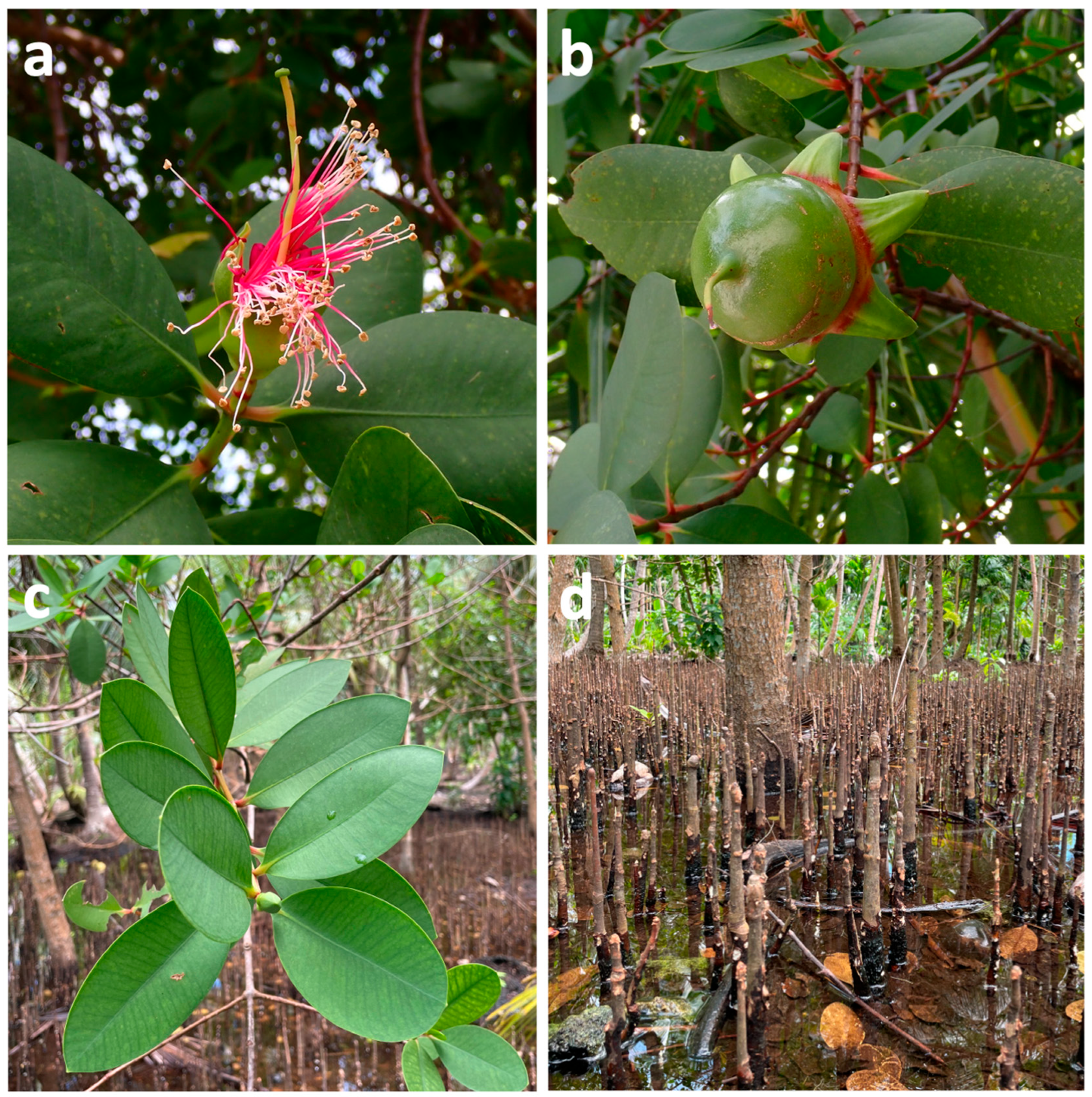
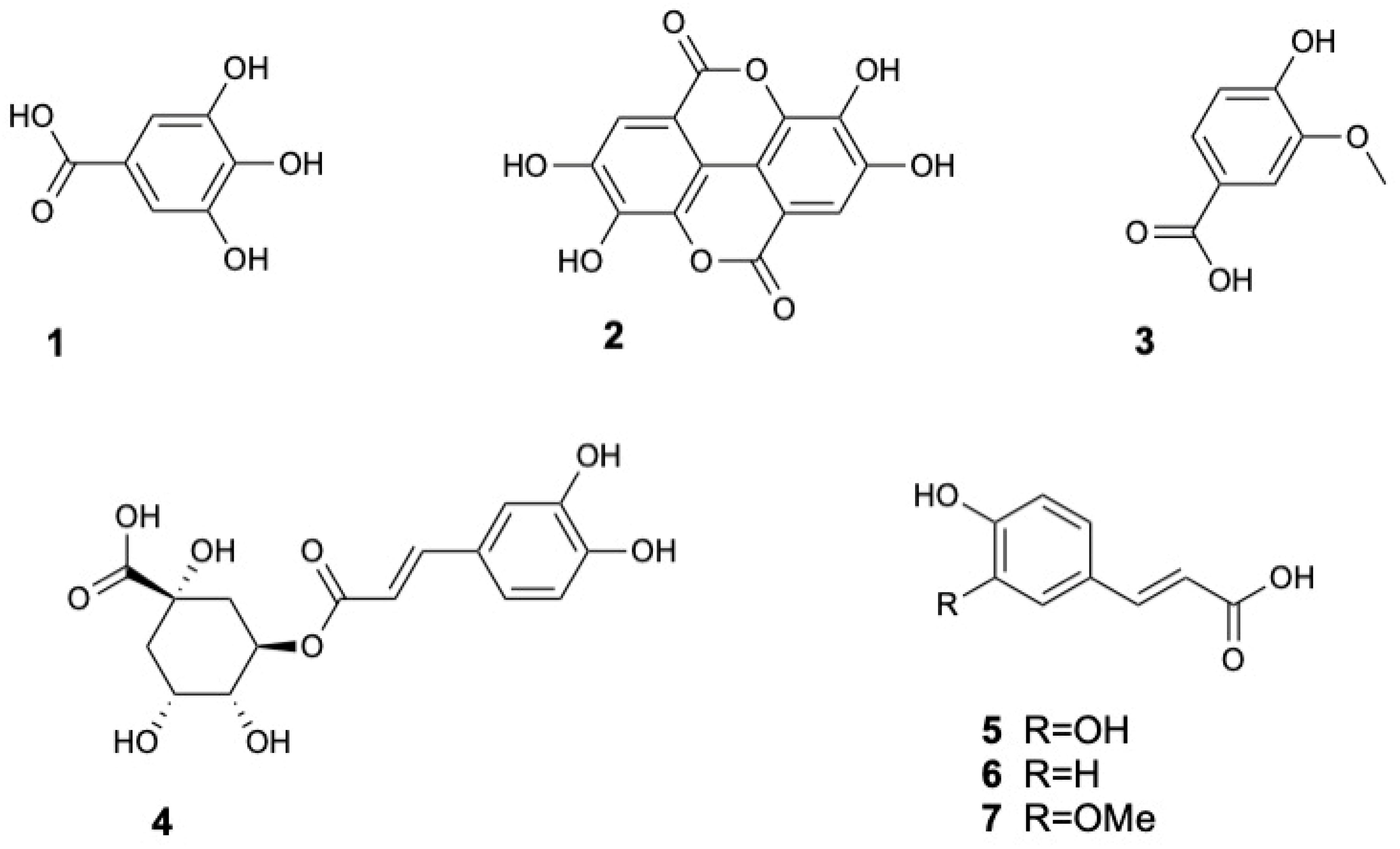
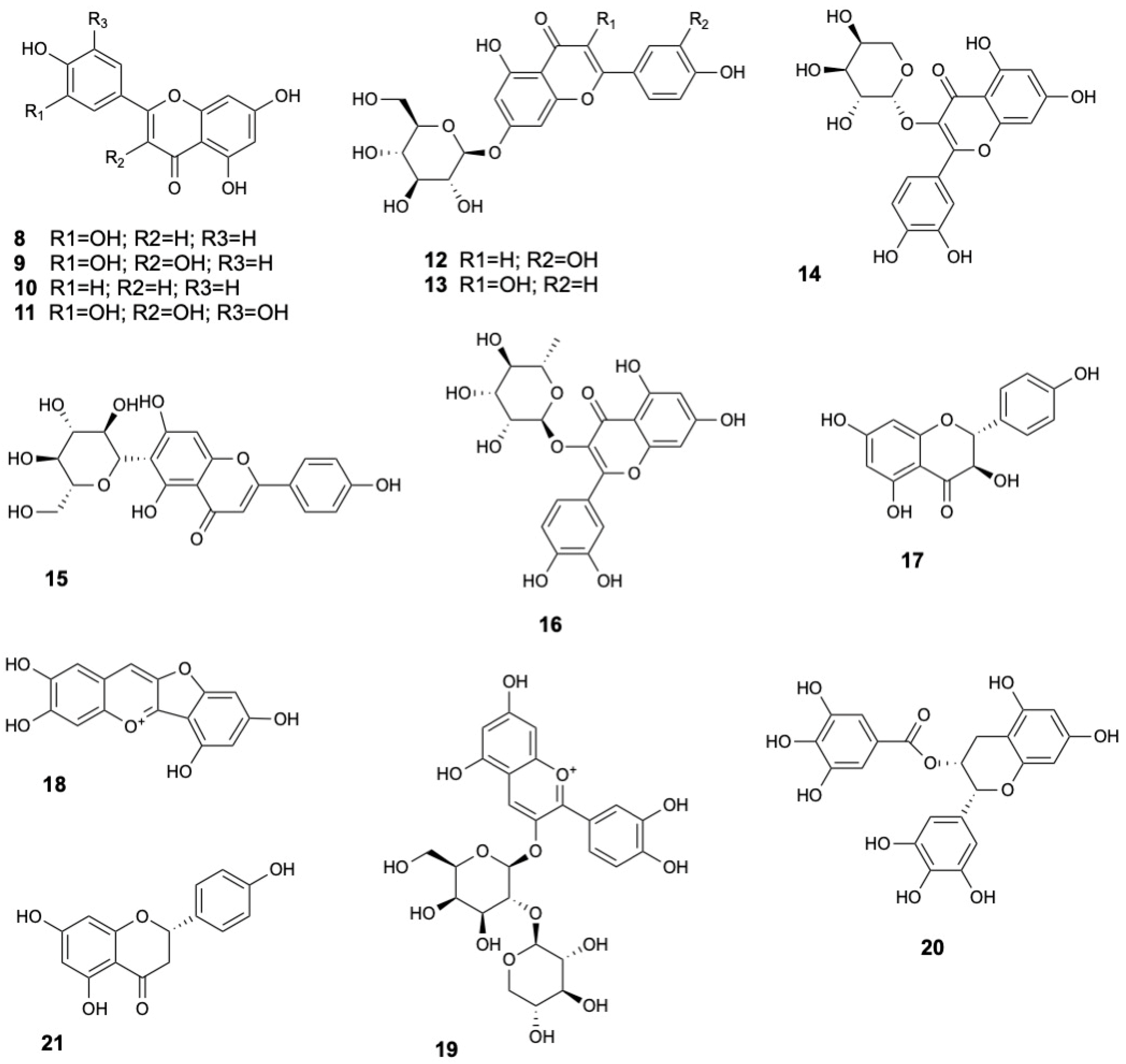
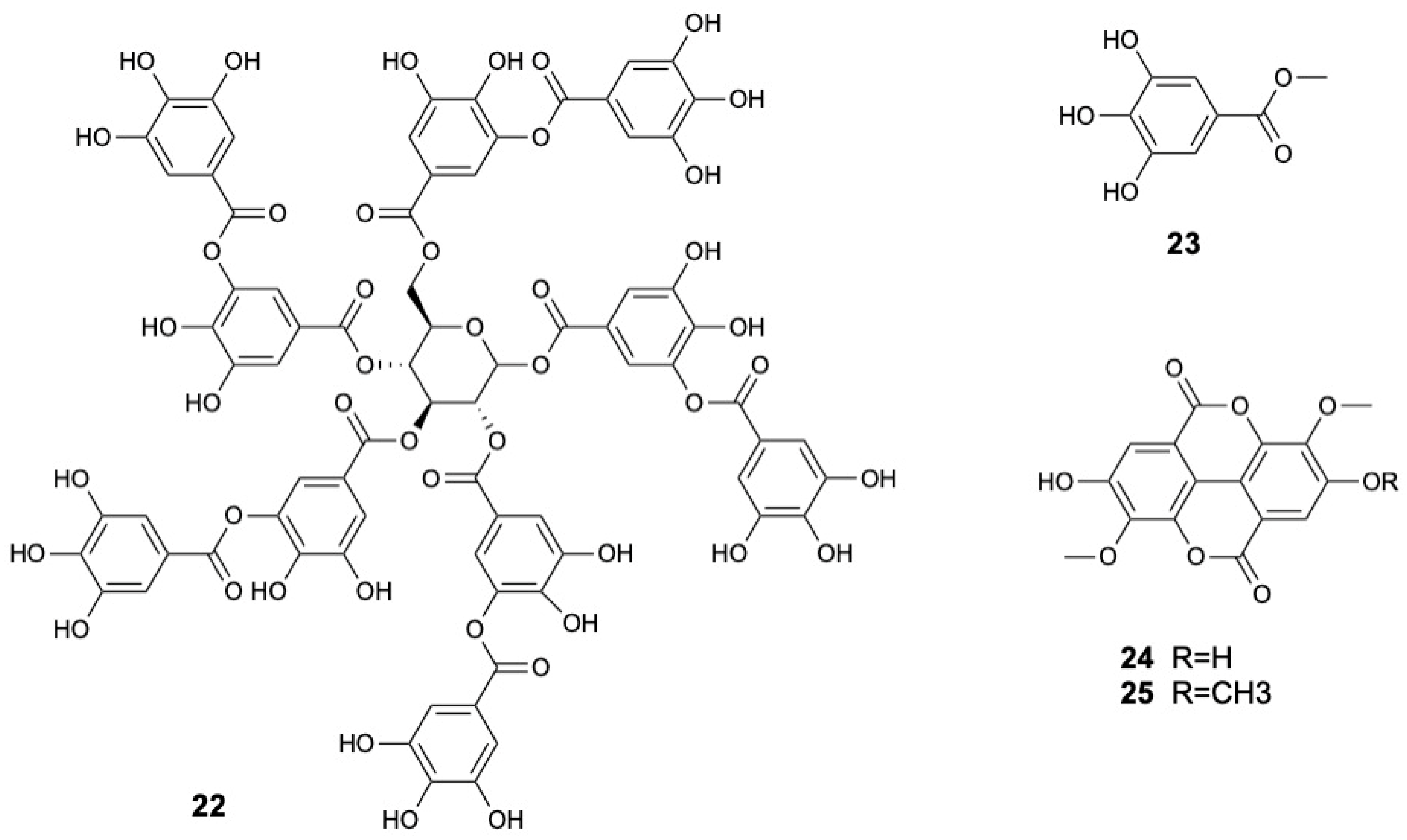
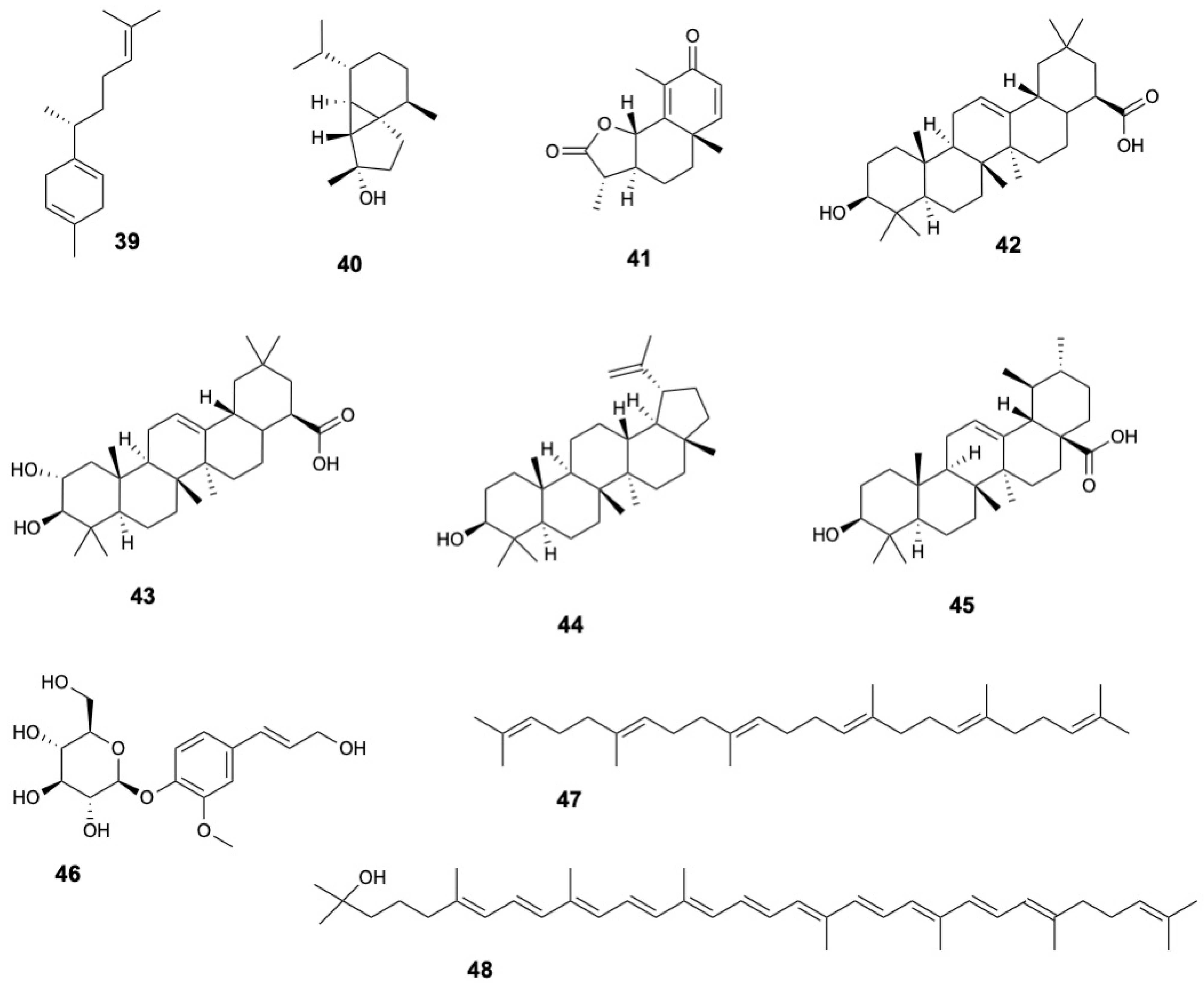
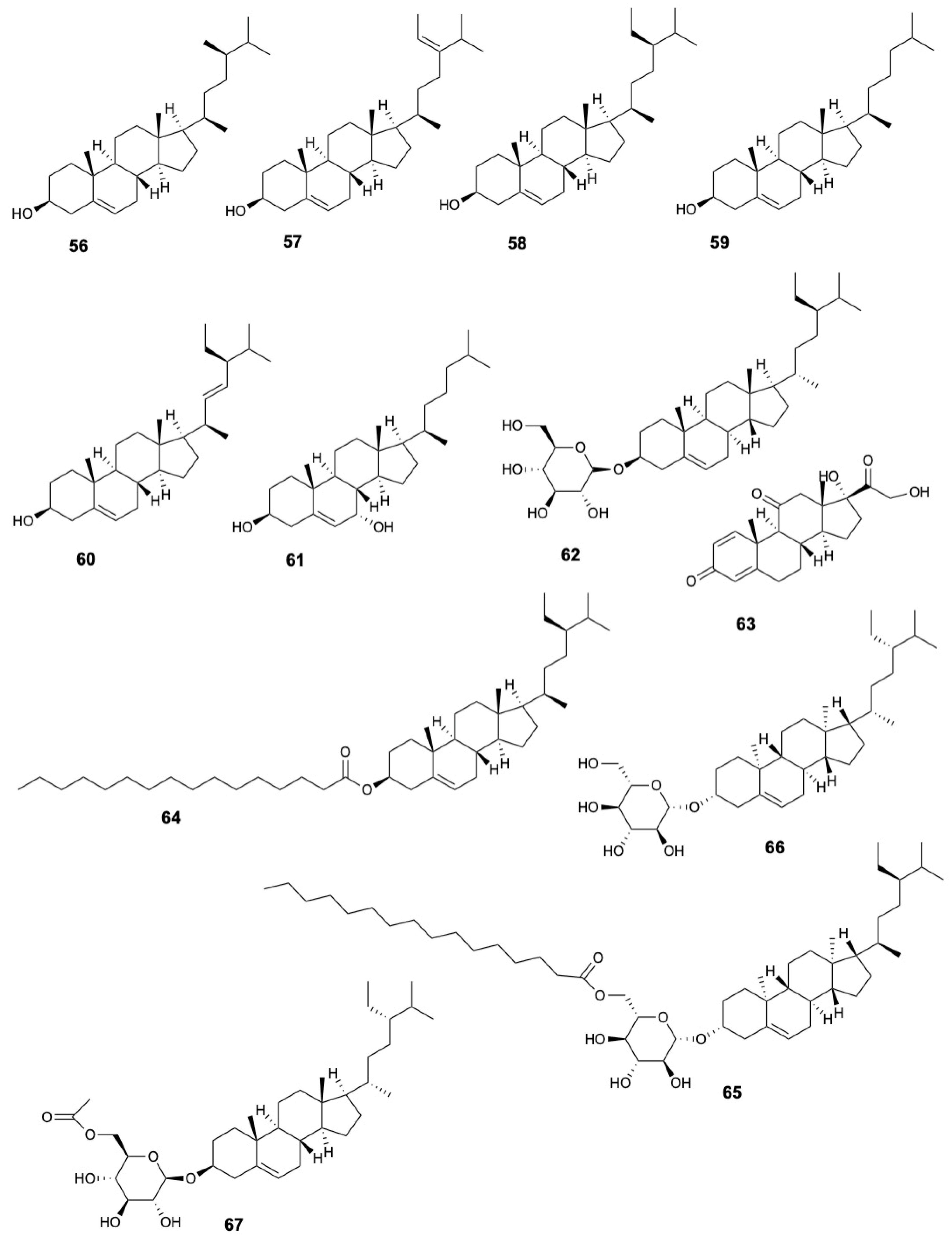
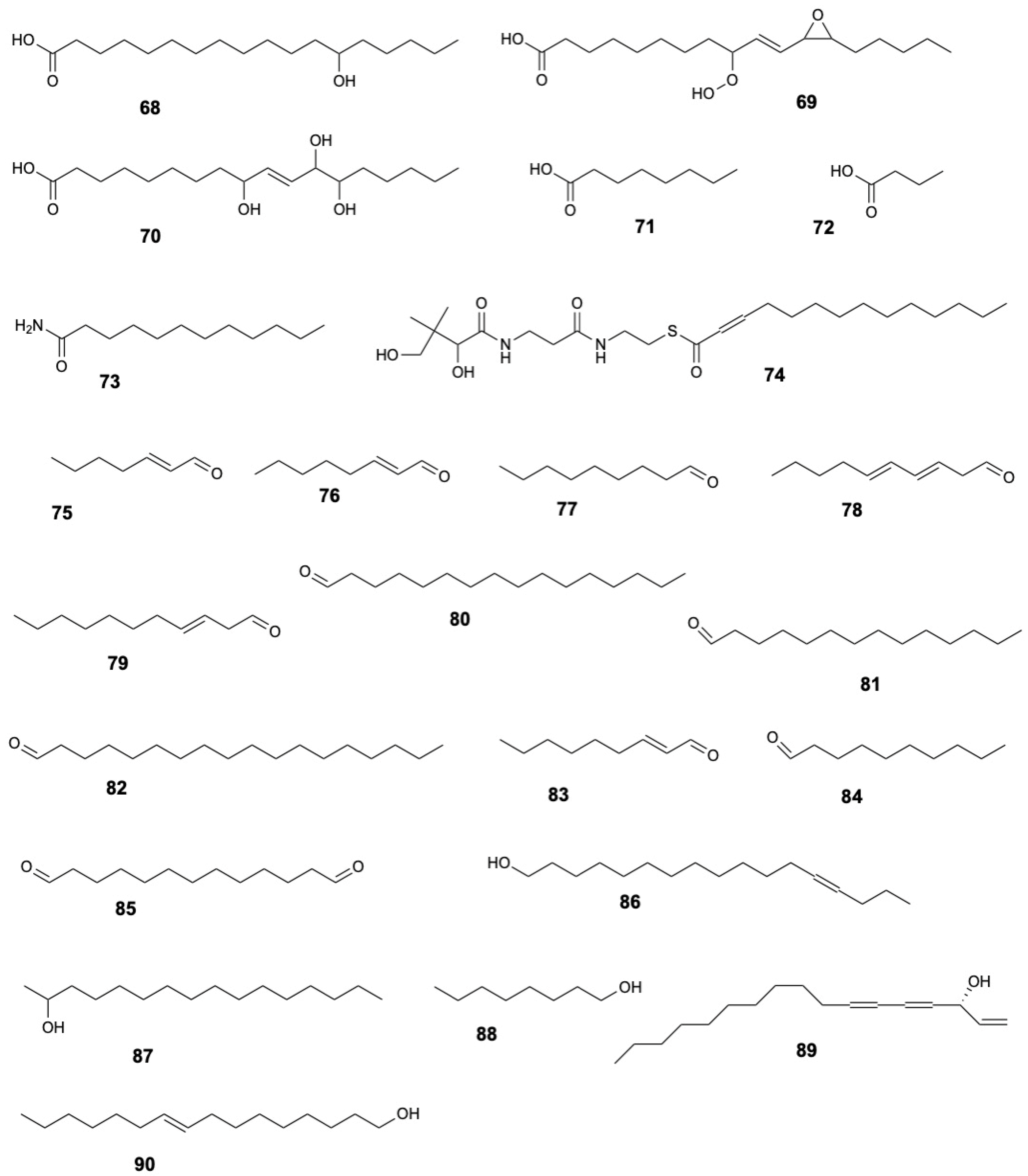
| Molecule | Plant Part | Solvent | Method | Region | Ref. |
|---|---|---|---|---|---|
| Phenolic acids and derivatives | |||||
| Gallic acid (1) | Leaf | Methanol | LC-MS | India | [31] |
| HPLC-DAD | Vietnam | [13] | |||
| Ethanol | LC-MS | India | [31] | ||
| UHPLC-HRMS | Indonesia | [8] | |||
| HPLC-DAD | Vietnam | [13] | |||
| 70% aqueous acetone | RP-HPLC | China | [32] | ||
| Ellagic acid (2) | Fruits | Ethanol | HPLC-DAD | Bangladesh | [16] |
| Leaves | 70% aqueous acetone | RP-HPLC | China | [32] | |
| Vanillic acid (3) | Fruits | Ethanol | HPLC-DAD | Bangladesh | [16] |
| Chlorogenic acid (4) | Leaves | Ethyl acetate | UPLC-ESI-MS/MS | India | [33] |
| Caffeic acid (5) | |||||
| p-Coumaric acid (6) | |||||
| Ferulic acid (7) | |||||
| Flavonoids | |||||
| Luteolin (8) | Leaves | Methanol | HPLC-DAD | Vietnam | [13] |
| Ethanol | MS; 1D- and 2D-NMR | Bangladesh | [21] | ||
| Fruits | Methanol | TLC, IR, 1H NMR | India | [29] | |
| Ethanol | 1H and 13C NMR | China | [34] | ||
| Stem and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] | |
| Quercetin (9) | Leaves | Methanol | LC-MS | India | [31] |
| Ethanol | LC-MS | India | [31] | ||
| Ethyl acetate | UPLC-ESI-MS/MS | India | [33] | ||
| Apigenin (10) | Leaves | Ethyl acetate | UPLC-ESI-MS/MS | India | [33] |
| Myricetin (11) | Fruits | Ethanol | HPLC-DAD | Bangladesh | [16] |
| Leaves | Ethyl acetate | UPLC-ESI-MS/MS | India | [33] | |
| Luteolin-7-O-glucoside (12) | Leaves | Ethanol | MS; 1D- and 2D-NMR | Bangladesh | [21] |
| 1H and 13C NMR | Indonesia | [35] | |||
| Methanol | HPLC-DAD | Vietnam | [13] | ||
| Fruits | Ethanol | 1H and 13C NMR | China | [34] | |
| Kaempferol glucoside (13) | Bark | Methanol | GC-MS | India | [36] |
| Quercetin-3-O-β-L-arabinopyranoside (14) | Stem and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] |
| Isovitexin (15) | Leaves | Ethanol | UHPLC-HRMS | Indonesia | [8] |
| Quercitrin (16) | |||||
| (+)-Dihydrokaempferol (17) | Stems and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] |
| Riccionidin A (18) | Leaves | Ethanol | UHPLC-HRMS | Indonesia | [8] |
| Cyanidin 3-O-[β-D-xylosyl-(1-2)- β-D-galactoside] (19) | |||||
| Epigallochatechin gallate (20) | Leaves | Ethyl acetate | UPLC-ESI-MS/MS | India | [33] |
| Naringenin (21) | |||||
| Tannins | |||||
| Tannic acid (22) | Leaves | Ethanol | LC-MS | India | [31] |
| Methanol | LC-MS | India | [31] | ||
| Methyl gallate (23) | Stems and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] |
| 3,3′-Di-O-methyl ether ellagic acid (24) | |||||
| 3,3′,4-O-Tri-O-methyl ether ellagic acid (25) | |||||
| Other phenolic compounds | |||||
| Estragole (26) | Fruits | Methanol | GC-MS | Vietnam | [19] |
| Aspirin (27) | Leaves | Ethanol | UHPLC-HRMS | Indonesia | [8] |
| 2-Phenylehtyl (28) | |||||
| Vanillin (29) | Leaves | Ethanol | LC-MS | India | [31] |
| Methanol | LC-MS | India | [31] | ||
| Bark | Hexane | GC-TOFMS | Malay Peninsula | [15] | |
| Piperonal (30) | Wood and Bark | Hexane | GC-TOFMS | Malay Peninsula | [15] |
| 2,4-Bis(1,1-dimethylethyl)-phenol (31) | |||||
| 3,7,8-Trihydroxy-5,10-dioxo-5,10-dihydrochromeno [5,4,3-cde]chromen-2-olate (32) | Fruits | Ethanol | UHPLC-HRMS | Indonesia | [8] |
| 3,5-Di-tert-Butyl-4-hydroxybenzaldehyde (33) | Leaves | Ethanol | LC-HRMS | Indonesia | [37] |
| Water | |||||
| [(–)-(R)-Nyasol (34) | Fruits | Ethanol | 1H and 13C NMR | China | [34] |
| (–)-(R)-4′-O-Methylnyasol (35) | |||||
| 3,8-Dihydroxy-6H-benzo[b,d]pyran-6-one (36) | |||||
| 3-Hydroxy-6H-benzo[b,d]pyran-6-one (37) | |||||
| Benzyl-O-β- glucopyranoside (38) | |||||
| Terpenoids | |||||
| β-Curcumene (39) | Fruits | Methanol | GC-MS | Vietnam | [19] |
| Cubedol (40) | |||||
| α-Santonin (41) | |||||
| Oleanolic acid (42) | Fruits | Methanol | TLC, IR, 1H NMR | India | [29] |
| Ethanol | 1H and 13C NMR | China | [34] | ||
| Stems and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] | |
| Bark | Methanol | GC-MS | India | [36] | |
| Maslinic acid (43) | Fruits | Ethanol | 1H and 13C NMR | China | [34] |
| Lupeol (44) | Stems and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] |
| Bark | Methanol | GC-MS | India | [36] | |
| Ursolic acid (45) | Stems and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] |
| Bark | Methanol | GC-MS | India | [36] | |
| Abietin (46) | Leaves | Ethanol | UHPLC-HRMS | Indonesia | [8] |
| Squalene (47) | Leaves | Chloroform-methanol (1:1) | GC | Australia | [38] |
| Rhodopin (48) | Fruits | Methanol | GC-MS | Vietnam | [19] |
| Lup-20(29)-en-3β,24-diol (49) | Stems and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] |
| 3β-O-(E)-Cumaroyl-alphitolinsaeure (50) | |||||
| 3β-Acetyl-oleanolic acid (51) | |||||
| 3β,13β-Dihydroxy-urs-11-en-28-oic acid-13-lactone (52) | |||||
| 3β-Hydroxy-20(29)-lupen-24-oic acid (53) | |||||
| Betulin (54) | |||||
| 1H-Cycloprop[e]azulen-4-ol, decahydro-1,1,4,7-tetramethyl-, [1ar (1aà,4á,4aá,7à,7aá,7bà)]- (55) | Wood | Hexane | GC-TOFMS | Malay peninsula | [15] |
| Steroids | |||||
| Campesterol (56) | Leaves | Chloroform-methanol (1:1) | GC | Australia | [38] |
| 28-Isofucosterol (57) | |||||
| Sitosterol (58) | |||||
| Bark | Methanol | GC-MS | India | [36] | |
| Stem and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] | |
| Cholesterol (59) | Leaves | Chloroform-methanol (1:1) | GC | Australia | [38] |
| Stem and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] | |
| Stigmasterol (60) | Leaves | Chloroform-methanol (1:1) | GC | Australia | [38] |
| Stem and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] | |
| Leaves | Acetone | UV-Vis, FTIR, 1H and 13C NMR, 2D-NMR | Indonesia | [39] | |
| Cholest-5-ene-diol (61) | Bark | Methanol | GC-MS | India | [36] |
| Stem and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] | |
| β-Sistosterol-β-D-glucopyranoside (62) | Fruits | Methanol | TLC, IR, 1H NMR | India | [29] |
| Prednisone (63) | Fruits | Methanol | GC-MS | Vietnam | [19] |
| β-Sitosterol palmitate (64) | Stem and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] |
| Stigmast-5-en-3β-O-(6-O-hexadecanoyl-β-D-glucopyranoside) (65) | |||||
| Daucosterol (66) | |||||
| 6′-O-Acetyl-β-daucosterol (67) | |||||
| Fatty acid and derivatives | |||||
| 13S-Hydroxyoctadecadienoic acid (68) | Leaves | Ethanol | UHPLC-HRMS | Indonesia | [8] |
| 9-Hydroperoxy-11-(3-pentyl-2-oxiranyl)-10-undecenoate (69) | |||||
| 9,12,13-Trihydroxy-10-octadecenoate (70) | |||||
| Octanoid acid (71) | Bark | Hexane | GC-TOFMS | Malay peninsula | [15] |
| Butanoic acid (72) | Leaves | Methanol | GC-MS | Vietnam | [19] |
| Dodecanamide (73) | Wood and Bark | Hexane | GC-TOFMS | Malay peninsula | [15] |
| Myristynoyl pantetheine (74) | Leaves | Methanol | GC-MS | Vietnam | [19] |
| Fatty aldehydes | |||||
| 2-Heptenal (75) | Wood and bark | Hexane | GC-TOFMS | Malay peninsula | [15] |
| 2-Octenal (76) | |||||
| Nonanal (77) | |||||
| 2,4-Decadienal (78) | |||||
| 2-Undecenal (79) | |||||
| Hexadecanal (80) | |||||
| Tetradecanal (81) | Wood | ||||
| Octadecanal (82) | Bark | ||||
| 2-Nonenal (83) | |||||
| Decanal (84) | |||||
| Tridecanedial (85) | Leaves | Methanol | GC-MS | Vietnam | [19] |
| Fatty alcohols | |||||
| 13-Heptadecyn-1-ol (86) | Leaves | Methanol | GC-MS | Vietnam | [19] |
| 2-Hexadecanol (87) | |||||
| 1-Octanol (88) | |||||
| Falcarinol (89) | |||||
| Trans-9-hexadecen-1-ol (90) | Wood | Hexane | GC-TOFMS | Malay peninsula | [15] |
| Other lipid-derived compounds | |||||
| 4,8,12,16-Tetramethylheptadecan-4-olide (91) | Wood | Hexane | GC-TOFMS | Malay peninsula | [15] |
| Oxacycloheptadec-8-en-2-one (92) | |||||
| 13-Methyl-oxacyclotetradecane-2,11-dione (93) | |||||
| Tert-hexadecanethiol (94) | Leaves | Methanol | GC-MS | Vietnam | [19] |
| Triacetin (95) | |||||
| Azelaic acid (96) | Leaves | Ethanol | UHPLC-HRMS | Indonesia | [8] |
| Hydrocarbons | |||||
| Pentadecane (97) | Wood and Bark | Hexane | GC-TOFMS | Malay peninsula | [15] |
| 1-Hexadecene (98) | |||||
| 1-Docosene (99) | |||||
| Octacosane (100) | |||||
| Hentriacontane (101) | |||||
| Heptadecane (102) | Wood | ||||
| 2-Methyl-nonadecane (103) | |||||
| Octadecane (104) | |||||
| Tetracosane (105) | |||||
| Heptacosane (106) | |||||
| Eicosane (107) | Bark | ||||
| 17-Pentatriacontene (108) | |||||
| Isobutane (109) | |||||
| 3-Methyl-hexane (110) | |||||
| 1-Chloro-heptacosane (111) | |||||
| Polysaccharides | |||||
| Hexose (112) | Leaves | Ethanol | UHPLC-HRMS | Indonesia | [8] |
| Sorbitol (113) | |||||
| Rhamnose (114) | Water (low-molecular-weight polysaccharide fraction) | RP-HPLC | India | [30] | |
| Xylose (115) | |||||
| Mannose (116) | |||||
| Galactose (117) | |||||
| Other compounds | |||||
| Diisobutyl phthalate (118) | Leaves | Water | LC-HRMS | Indonesia | [37] |
| Bis(3,5,5 trimethylhexyl)phthalate (119) | |||||
| Monobutyl phthalate (120) | |||||
| Bis(2 ethylhexyl)phthalate (121) | |||||
| Betaine (122) | |||||
| Choline (123) | |||||
| Hexamethylenetetramine (124) | |||||
| 2,2,6,6 tetramethyl 1 piperidinol (TEMPO) (125) | |||||
| Caprolactam (126) | |||||
| 2-[(2-chlorobenzyl)sulfanyl]-4,6-dimethylnicotinonitrile (127) | |||||
| Zearalenone (128) | |||||
| Tributyl phosphate (129) | |||||
| Bis(4-ethylbenzylidene)sorbitol (130) | |||||
| DL-arginine (131) | |||||
| Bis(2-ethylhexyl)benzene-1,2-dicarboxylate (132) | Stems and twigs | Methanol | HR-ESI-MS, 1H and 13C NMR | China | [22] |
| Safrole (133) | Fruits | Methanol | GC-MS | Vietnam | [19] |
| 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester (134) | Wood and bark | Hexane | GC-TOFMS | Malay peninsula | [15] |
| 7,9-Di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione (135) | |||||
| Ethaneperoxoic acid, 1-cyano-1-[2-(2-phenyl-1,3-dioxolan-2-yl)ethyl]pentyl esterv (136) | |||||
| Trimethylamine (137) | Wood | ||||
| 1,2-Benzenedicarboxylic acid, diisooctyl ester (138) | |||||
| Propiolactone (139) | Bark | ||||
| Diethyl phthalate (140) | |||||
| 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester (141) | |||||
| Plant part | Solvent | TPC (mg GAE/g) | TFC (mg QE/g) | Ref. |
|---|---|---|---|---|
| Leaves | Methanol | 200 | - | [13] |
| 1.52 ± 0.02 | 1.98 ± 0.08 | [37] | ||
| Ethanol | 182.89 ± 1.76 | 22.70 ± 0.48 | [8] | |
| 50.03 | - | [41] | ||
| 219.53 | 454.88 | [40] | ||
| Ethanol 70% | 74.77 | - | [41] | |
| Ethyl acetate | 5.83 | - | ||
| n-Hexane | 4.67 | - | ||
| Water | 0.23–1.00 | 0.55–0.96 | [37] | |
| Fruits | Ethanol | 122 | 613 | [18] |
| 12.21 ± 1.31 | 26.06 ± 0.30 | [42] | ||
| Methanol | 82.27 ± 0.41 | 41.0 ± 0.34 | [19] | |
| Methanol (n-butanol fraction) | 82.67 ± 0.81 | 9.13 ± 0.34 | ||
| Methanol (ethyl acetate fraction) | 77.67 ± 0.32 | 26.28 ± 0.93 | ||
| Methanol (aqueous fraction) | 70.26 ± 0.35 | 1.81 ± 0.24 | ||
| Methanol (Hexane fraction) | 59.58 ± 2.70 | 16.54 ± 0.44 | ||
| Bark | Ethanol | 63.00 | - | [43] |
| 50.70 ± 0.74 | 90.04 ± 3.57 | [36] | ||
| Ethyl acetate | 60.25 | - | [43] | |
| Chloroform | 36.25 | - | ||
| Petroleum ether | 26.28 | - |
| Plant Part | Solvent | Conc. Tested | Bacterial/Fungal Species | Activity | Results | Standard | Ref. |
|---|---|---|---|---|---|---|---|
| Leaves (L), roots (E), flower [stamen (T) and calyx (P)], and fruit [meat of fruit (F), persistent calyx of fruit (C), and seeds (S)] | Methanol | - | AS S. aureus (+) | MIC (mg/mL) | 0.2 (L), 0.4 (E, S, P, T, F, C) | 0.0062 (CHL) | [50] |
| AS B. subtilis (+) | 0.2 (L, E), 0.4 (S, P, T, F, C) | 0.0015 (CHL) | |||||
| AS B. megaterium (+) | 0.2 (L, E, S, P), 0.4 (T, F, C) | 0.0015 (CHL) | |||||
| AS E. coli (−) | 0.2 (L, E, S, P, T, F, C) | 0.0062 (CHL) | |||||
| AS P. aeruginosa (–) | 0.2 (L, E, S, P, T, F, C) | 0.025 (CHL) | |||||
| AR S. aureus (+) | 0.4 (L, E, S, P, T, F, C) | 0.0062 (CHL) | |||||
| AR E. faecalis (+) | 0.2 (L, S), 0.4 (E, P, T, F, C) | 0.0062 (CHL) | |||||
| AR E. faecium (+) | 0.2 (L, E, S, P), 0.4 (T, F, C) | 0.0062 (CHL) | |||||
| AR E. coli (−) | 0.2 (L, E, S, P, T, F, C) | 0.0062 (CHL) | |||||
| AR P. aeruginosa (−) | 0.4 (L, E, S, P, T, F, C) | 0.1 (CHL) | |||||
| AR A. baumannii (−) | 0.2 (P, T, C), 0.4 (L, E, S, F) | n/a (CHL) | |||||
| Leaves | Ethanol (E), Water (W) | 2 mg/disc (CHL 20 μg/disc; AMB 40 μg/disc) | S. aureus (+) | ZOI (mm); MIC (µg/mL) | n/a; n/a (E), n/a; n/a (W) | 30; 7.81 (CHL) | [51] |
| B. subtilis (+) | n/a; n/a (E), n/a; n/a (W) | 30; 21.3 (CHL) | |||||
| M. luteus (+) | n/a; n/a (E), n/a; n/a (W) | 24; 31.3 (CHL) | |||||
| E. coli (−) | 15; 500 (E), 13; 250 (W) | 30; 31.3 (CHL) | |||||
| P. aeruginosa (−) | n/a; n/a (E), n/a; n/a (W) | 15; 125 (CHL) | |||||
| C. albicans | n/a; n/a (E), 15; 125 (W) | 17; 250 (AMB) | |||||
| Seeds | Methanol | -(AMP 10 μg/disc) | S. aureus (+) | ZOI (mm) | 12–14 | 29–35 (AMP) | [52] |
| E. coli (−) | n/a | 0–10 (AMP) | |||||
| C. albicans | 17–18 | n/a (AMP) | |||||
| Leaves | Water (W), Methanol (M) | 10 µg/ml | Vibrio spp. (−) | ZOI (mm); MIC (µg/mL) | n/a; 1.6–6.3 (W), n/a-18.3; 0.1–0.2 (M) | - | [47] |
| Bark | Hexane (H), Benzene (B), Chloroform (C), Mthanol (M), Water (W) | 1.5 mg/disc (AMP 3 µL/disc 125 µg/mL; CHL 3 µL/disc 10 mg/mL; FLC 3 µL/disc 10 mg/Ml) | B. subtilis (+) | ZOI (mm); MIC (mg/mL) | 9.33 ± 0.58;-(H), 8.33 ± 0.58;-(B), 7.17 ± 0.29;-(C), 18.33 ± 0.76; 3.90 (M), 15.83 ± 0.29; 15.62 (W) | 20.83 ± 0.76;-(AMP) | [36] |
| B. coagulans (+) | 9.17 ± 0.29;-(H), 9.50 ± 0.50;-(B), 10.17 ± 0.29; 7.81 (C), 19.50 ± 0.50; 7.81 (M), n/a;-(W) | 22.50 ± 0.50;-(AMP) | |||||
| E. coli (−) | n/a;-(H, B, C, M, A) | 13.00 ± 1.04;-(AMP) | |||||
| S. cerevisiae (+) | n/a;-(H, B, C, M, A) | 20.00 ± 1.73;-(FLC) | |||||
| P. vulgaris (−) | n/a;-(H, B, C), 12.67 ± 0.58; 62.50 (M), 8.67 ± 0.58; 125 (W) | 17.17 ± 1.26;-(CHL) | |||||
| Fruits | Water | - | E coli (−) | ZOI (mm) | 18–21 | - | [53] |
| V. cholerae (−) | 32–35 | - | |||||
| S. typhimurium (−) | 25–27 | - | |||||
| B. subtilis (+) | 24–29 | - | |||||
| Fruits | Methanol | 60–90% (CHL 0.025%) | S. aureus (+) | ZOI (mm) | 8.81 (80%) | 12.73 (CHL) | [54] |
| 5–20% (CHL 0.025%) | E. coli (−) | 7.17 (15%) | 11.53 (CHL) | ||||
| 20–35% (MTZ 0.15%) | C. albicans | 7.03 (30%) | 9.13 (MTZ) | ||||
| Fruits | Ethyl acetate (EA), Ethanol 70% (E) | 2.5–15% (STM 10 µg/disc) | E. coli (−) | ZOI (mm) | 14.50 (EA 15%), 13.15 (E 12.5%) | 27.61 (STM) | [55] |
| S. aureus (+) | 7.46 (EA 15%), 13.37 (E 10%) | 27.61 (STM) | |||||
| C. albicans | 11.67 (EA 15%), 28.50 (E 10%) | 31.40 | |||||
| Stems and leaves | Methanol: ethyl acetate (EAFS, EAFL), chloroform (CFS, CFL), carbon tetrachloride (CTFS, CAFL) fractions | 500 µg/disc (CIP 5 µg/mL) | E. coli (−) | ZOI (mm) | 0 (EAFS), 7.5 ± 0.08 (EAFL), 7.5 ± 0.09 (CFS), 7.5 ± 0.13 (CFL), 5.5 ± 0.07 (CTFS), 0 (CTFL) | 11.5 ± 0.07 (CIP) | [48] |
| S.aureus (+) | 6 ± 0.11 (EAFS), 7.5 ± 0.11 (EAFL), 7.5 ± 0.09 (CFS), 8 ± 0.09 (CFL), 8.5 ± 0.13 (CTFS), 0 (CTFL) | 11 ± 0.15 (CIP) | |||||
| S. dysenteriae (−) | 6.5 ± 0.07 (EAFS), 8.5 ± 0.06 (EAFL), 7.5 ± 0.16 (CFS), 5.5 ± 0.20 (CFL), 7 ± 0.09 (CTFS), 0 (CTFL) | 13.5 ± 0.05 (CIP) | |||||
| S. typhi (−) | 8.5 ± 0.11 (EAFS), 8.5 ± 0.12 (EAFL), 5.5 ± 0.06 (CFS), 7.5 ± 0.13 (CFL), 7 ± 0.13 (CTFS), 0 (CTFL) | 12 ± 0.09 (CIP) | |||||
| S. paratyphi (−) | 11.5 ± 0.1 (EAFS), 6.5 ± 0.17 (EAFL), 6 ± 0.07 (CFS), 5.5 ± 0.07 (CFL), 9 ± 0.09 (CTFS), 0 (CTFL) | 15 ± 0.15 (CIP) | |||||
| Levaes | Ethyl acetate | 0.05–50 mg/mL | C. albicans | MIC (mg/mL) | 4 | 0.5 (AMB) | [33] |
| C. tropicalis | 16 | 0.5 (AMB) | |||||
| C. auris | 32 | 1 (AMB) | |||||
| Fruits | Ethanol-methanol (1:1) | 2 mg/disc | E. coli (−) | ZOI (mm) | 16.7 | - | [49] |
| Klebsiella sp. (−) | 17.0 | - | |||||
| S. boydii (−) | 14.7 | - | |||||
| S. sonnei (−) | 15.7 | - | |||||
| S. aureus (+) | 15.7 | - | |||||
| Leaves | Ethanol | 20,000 ppm (VAN 5 μg/disc) | Methicillin-resistant S. aureus (+) | ZOI (mm) | 11.92 | 24 (VAN) | [8] |
| Leaves | n-Hexane (H), Ethyl acetate (EA), Ethanol 70% (E70), Ethanol 90% (E90) | 2.5–15%; (STM 5 μg/disc) | S. aureus (+) | ZOI (mm) | 3.73 (H 10%), n/a (EA 12.5%), 6.98 (E70 10%), n/a (E90 10%) | 41.7–44.54 (STM) | [41] |
| E. coli (−) | 1.82 (N 10%), 1.03 (EA 10%), 1.57 (E70 15%), 2.18 (E90 15%) | 28.28–33.50 (STM) | |||||
| C. albicane | 5.70 (N 15%), n/a (EA 15%), 5.00 (E70 12.5%), n/a (E90 15%) | 16.90–24.00 | |||||
| Leaves | Water (low-molecular-weight polysaccharide fraction) | 20 mg/mL | S. pneumoniae (+) | Antibiofilm activity (%) | 78.72 ± 1.99 | - | [30] |
| P. aeruginosa (−) | 65.89 ± 1.68 | - | |||||
| S. aureus (+) | 52.04 ± 1.35 | - | |||||
| E. coli (–) | 49.54 ± 0.79 | - | |||||
| S. typhi (−) | 40.17 ± 1.53 | - | |||||
| Fruit | Methanol extract (M): hexane (HF), ethyl acetate (EAF), n-butanol (BF), and aqueous (WF) fractions | 50 mg/mL | E.coli (−) | ZOI (mm) | 21.00 ± 1.00 (M), 14.67 ± 0.58 (HF), 13.67 ± 0.58 (AEF), 17.67 ± 0.47 (BF), 17.33 ± 1.53 (AF) | - | [19] |
| S. aureus (+) | 21.00 ± 1.00 (M), 12.67 ± 0.58 (HF), 14.67 ± 0.58 (AEF), 11.33 ± 1.15 (BF), 16.33 ± 0.58 (AF) | - | |||||
| B. subtilis (+) | 18.67 ± 0.58 (M), 16.33 ± 0.58 (HF), 17.33 ± 0.58 (AEF), 18.33 ± 0.58 (BF), 16.67 ± 0.58 (AF) | - | |||||
| Leaves | Ethanol | - | E. coli (−) | ZOI (mm); MIC (mg/mL) | 26; 0.06 | - | [13] |
| S. aureus (+) | 33; 0.06 | - | |||||
| S. typhimurium (−) | 25; 0.04 | - |
| Plant Part | Solvent | Fraction | Results: IC50 (or %) | Standard: IC50 (or %) | Ref. |
|---|---|---|---|---|---|
| Leaves | Ethanol | - | 1.92 ± 0.38 μg/ml | AA: 12 ± 1.29 μg/ml | [51] |
| 4.25 ppm | AA: 5.25 ppm | [8] | |||
| 23.84 μg/mL | - | [13] | |||
| 26.30 μg/mL | AA: 3.16 μg/mL | [57] | |||
| 27.0 µg/mL | AA: 12.0 µg/mL | [40] | |||
| 171 ppm | AA: 20.5 ppm | [39] | |||
| 80.21% at 2.5 mg/mL | - | [41] | |||
| Ethyl acetate | 12.0 ± 0.12 µg/mL | AA: 8.0 ± 0.12 µg/mL | [48] | ||
| chloroform | 19.0 ± 0.07 µg/mL | ||||
| Carbon tetrachloride | 49.0 ± 0.05 µg/mL | ||||
| Ethanol 70% | - | 75.89% at 2.5 mg/mL | - | [41] | |
| Ethyl acetate | - | 16.71% at 2.5 mg/mL | |||
| n-Hexane | - | 22% at 2.5 mg/mL | |||
| Acetone | - | 166.2 ppm | - | [39] | |
| Water | - | 89 mg/mL | - | [37] | |
| 70% aqueous acetone | Ellagitannin-rich fraction | 69.39 ± 0.29 µg/mL | BHA: 116.52 ± 0.95 µg/mL | [32] | |
| Water | Low-molecular-weight polysaccharide fraction | 41.33 ± 0.82% at 3.2 mg/ml | AA: 85.26 ± 0.96% at 3.2 mg/mL | [30] | |
| Stems | Ethanol | Ethyl acetate | 138 ± 0.8 µg/mL | AA: 8.0 ± 0.12 µg/mL | [48] |
| Chloroform | 69.0 ± 0.21 µg/mL | ||||
| Carbon tetrachloride | 201.0 ± 0.15 µg/mL | ||||
| Fruits | Ethanol | - | 1.16 ± 0.76 µg/mL | AA: 5.28 ± 0.58 µg/mL | [42] |
| - | 87 μg/ml | AA: 15 μg/ml | [18] | ||
| n-Hexane | 191.31 ppm | AA: 3.70 ppm | |||
| Ethyl acetate | 96.02 ppm | ||||
| Butanol | 371.16 ppm | ||||
| Ethanol-methanol (1:1) | - | 48.1% at 50 µg/mL | - | [58] | |
| Water | - | 7.3 mg/mL | - | [53] | |
| Bark | Ethanol | - | 4.57 µg/mL | BHT: 3.25 µg/mL | [43] |
| Petroleum ether | - | 12.32 µg/mL | |||
| Ethyl acetate | - | 13.09 µg/mL | |||
| Chloroform | 192.27 µg/mL | ||||
| Methanol | 21.74 µg/mL | Quercetin: 10.14 µg/mL. | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerri, F.; Galli, P. Phytochemistry and Pharmacological Potential of the Mangrove Plant Sonneratia caseolaris: A Comprehensive Review. Mar. Drugs 2025, 23, 378. https://doi.org/10.3390/md23100378
Cerri F, Galli P. Phytochemistry and Pharmacological Potential of the Mangrove Plant Sonneratia caseolaris: A Comprehensive Review. Marine Drugs. 2025; 23(10):378. https://doi.org/10.3390/md23100378
Chicago/Turabian StyleCerri, Federico, and Paolo Galli. 2025. "Phytochemistry and Pharmacological Potential of the Mangrove Plant Sonneratia caseolaris: A Comprehensive Review" Marine Drugs 23, no. 10: 378. https://doi.org/10.3390/md23100378
APA StyleCerri, F., & Galli, P. (2025). Phytochemistry and Pharmacological Potential of the Mangrove Plant Sonneratia caseolaris: A Comprehensive Review. Marine Drugs, 23(10), 378. https://doi.org/10.3390/md23100378







